Abstract
Introduction and importance
Ischemic colitis, also rare, is the most common ischemic pathology of the digestive system. It usually affects the ageing population and those suffering from end-stage renal disease (ESRD), hypertension, and heart failure. Its incidence varies from 4.5 to 44 cases per 100,000 annually.
Case presentation
We have reported a case of gangrenous colitis in a 62-year-old man suffering from acute promyelocytic leukaemia (APL) and myelofibrosis. He had hypertension and ESRD due to obstructive uropathy from seven years ago in his past medical history. His recurrent constitutional symptoms and persistent leukocytosis of more than 20,000 in μL was always treated as catheter-related infection or sepsis until acute abdomen emerged. The surgical team encountered a vast gangrenous right hemicolon. The leukocytosis did not resolve. Thus, the haematological investigations proved APL with myelofibrosis. The affected colon was free of leukemic infiltration.
Clinical discussion
Hypoperfusion due to ESRD and hemodialysis accompanied with malignancy induced hypercoagulative state provided a context in which small vessels of the bowel were obstructed.
Conclusion
Malignancies are associated with thrombophilia, and colonic involvement is not always related to lymphatic infiltration in leukaemia patients.
Abbreviations: ESRD, End-stage renal disease; APL, Acute promyelocytic leukaemia; GI, Gastrointestinal; ED, Emergency department; RLQ, Right lower quadrant; OR, Operating room; PBS, Peripheral blood smear; MF, Myelofibrosis; TGF-β, Transforming growth factor β; PDGF, Platelet-derived growth factor; bFGF, Basic fibroblast growth factor; AML, Acute myeloid leukaemia
Keywords: Ischemic colitis, Acute promyelocytic leukaemia, Myelofibrosis, ESRD
Highlights
-
•
Ischemic colitis, also rare, is the most common ischemic pathology of the digestive system.
-
•
Not every leukocytosis should be viewed as sepsis.
-
•
Although rare, acute promyelocytic leukaemia can be accompanied by myelofibrosis.
-
•
Ischemic colitis may be a result of a hypercoagulable state caused by leukaemias, not necessarily leukaemic invasion.
-
•
The cecum is within a watershed of the colon in which incomplete anastomoses of the marginal arteries make its blood supply poor and vulnerable to ischemia.
1. Introduction
Ischemic colitis, in general, is a rare entity, however, constituting the most common ischemic pathology of the gastrointestinal (GI) tract [1]. Ischemic colitis affects more men than women in the general population, and its incidence varies from 4.5 to 44 cases per 100,000 annually [2]. Congestive heart failure, hemodialysis, vasculitis, etc., with the pathophysiology of hypoperfusion, can be underlying factors. Due to the condition of blood supply to the right colon, ischemia of the right colon is generally more common. Malignancies may induce thrombosis by activation of blood coagulation via procoagulant substances, impairment of fibrinolytic pathways, and alterations of endothelium toward a thrombogenic state [1]. We have reported a case of gangrenous colitis in a 62-year-old man on regular hemodialysis that his periodic leukocytosis was treated as recurrent sepsis. Finally, haematological investigations illustrated he had APL beside myelofibrosis. The pathology study also revealed that the gangrenous colon was free of leukaemic infiltration. It is thought that the malignant nature of the APL and its releasing mediators created a hypercoagulable state in the body affecting the colon’s vasculature. This work has been reported in line with the SCARE criteria [2].
2. Presentation of case
A 62-year-old man was presented to the general emergency department (ED) complaining of severe malaise and general myalgia. He was suffering from hypertension and ESRD due to obstructive uropathy for seven years ago and on regular three times weakly hemodialysis. However, these had become his constant complaints since his diseases initiated. Although it was not his dialysis time yet, he underwent dialysis. After conservative care, some degrees of improvement were obtained until a dull, severe, colicky pain focused more on the suprapubic and right lower quadrant (RLQ), along with nausea and vomiting, persuades him to refer to the surgical ED. With the initial diagnosis of acute appendicitis, the patient was scheduled in the operating room (OR) list. The RLQ region ultrasonography ruled out typical appendicitis or collection. However, focal tenderness in the McBurney area and decreased regional intestinal peristalsis are considered retrocecal or atypical appendicitis. As an incidental finding, massive splenomegaly attracted the examiner. The spleen was approximately 300 in 120 mm in diameters. The kidneys were atrophic, appearing end-stage. Before surgery, the CT scan showed a local fat stranding in the paracecal region and confirmed the massive splenomegaly (Fig. 1). In the OR, after McBurney incision and appendectomy, the surgical team noted an inflamed cecum turned with gradual colour changing to gangrene in the whole of the right colon (Fig. 2). The surgery was ended with the right hemicolectomy and initial anastomosis. The specimens were sent to the pathology. Five days after surgery, a more severe weakness overwhelmed him. For a leukocytosis of 52,800 in μL, a hemoglobin level of 10.6 g/dL, and a platelet count of 275,000 in μL, a haematological consultation was requested. In the peripheral blood smear (PBS) performed, band cells, metamyelocytes, and myelocytes were seen. As for the clinic of the patient, haematological and biochemical analysis, particularly a left shift in leukocytes, myelocytes, and metamyelocytes found in the peripheral blood, differential diagnosis of toxic granulation of neutrophils, and myelodysplastic syndromes were made. Smears derived from bone marrow biopsy and aspiration under light microscopy demonstrated only a few marrow cells composed mainly of myeloid stems compatible with myelofibrosis (MF) (Fig. 3A & B). The pathology report was ready to reveal acute, chronic inflammation with necrosis and haemorrhage with no pieces of evidence of malignancies besides viable surgical margins (Fig. 4). Acute periappendicitis was also reported. The post-operation period was uneventful, and then the chemotherapy was initiated soon after recovery.
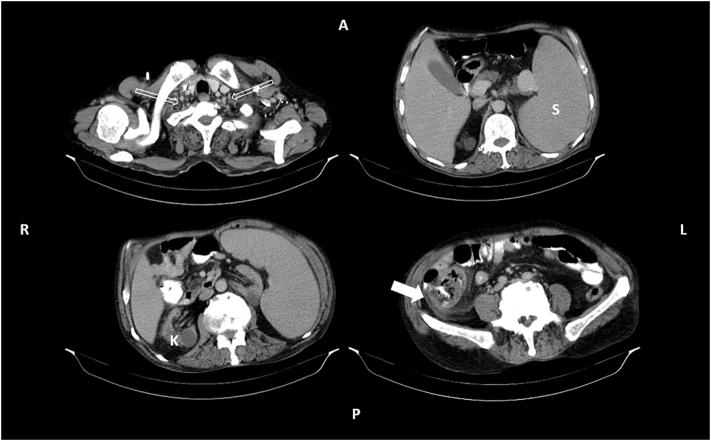
Fig. 1.
The CT scan of the patient with oral contrast showing multiple cervicothoracic lymphadenopathies (hollow arrows) in the left above, massive splenomegaly (S) in the right above, degenerated right kidney (K) with a cortical cyst in the left below, and the ischemic cecum (solid arrow) with the fat stranding around in the right below.
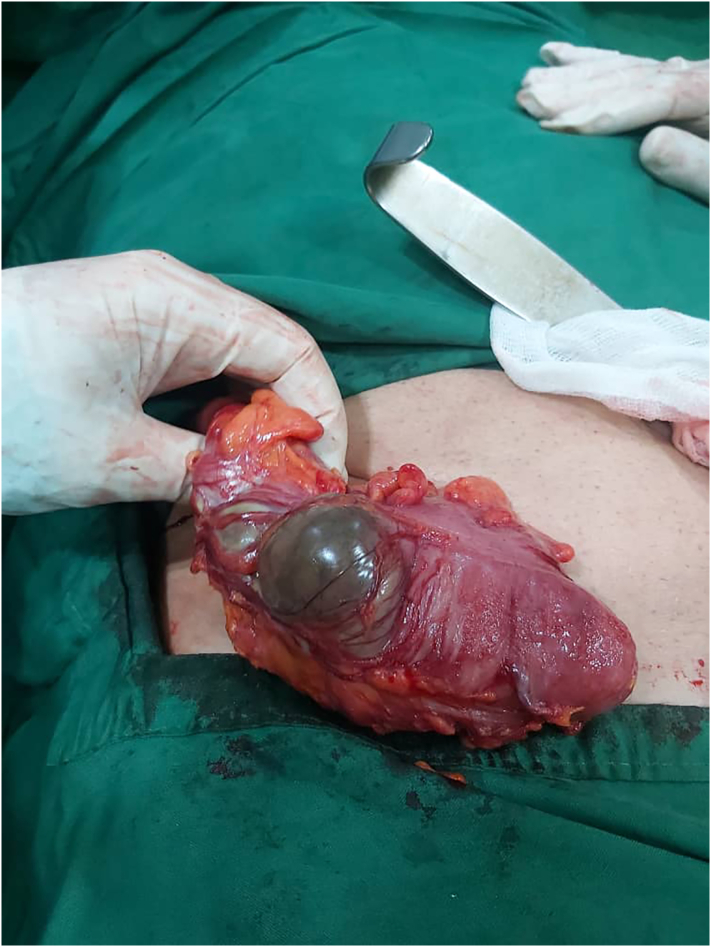
Fig. 2.
The gangrenous cecum with inflamed fatty tissue.
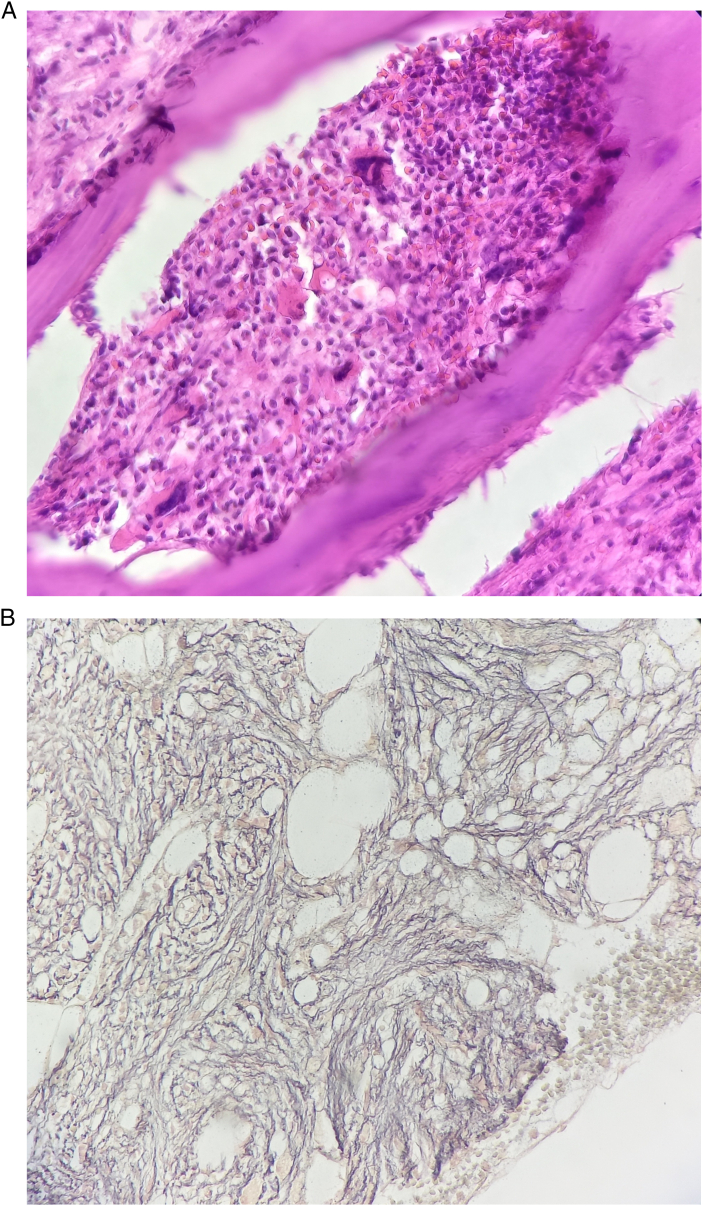
Fig. 3.
Hypocellular bone marrow in the study of H&E in biopsy specimen and myelofibrosis (×1000 magnification) (A). The reticulin stain confirms the fibrosis (B).
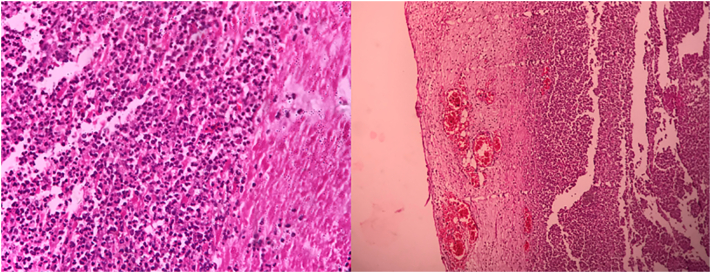
Fig. 4.
Hematoxylin and eosin (H&E) study of the colon under ×100 and ×1000 magnification revealing neutrophil infiltration of mucosal, submucosal, and muscular layers. There is no lymphatic involvement.
3. Discussion
If we assume a case of ischemic colitis, the patient probably would be a man in his 7th decade of life suffering from congestive heart disease and hypertension, taking anti-inflammatory drugs, or is under intensive care for the reasons of surgery. Two conditions are predisposing to ischemic colitis: occlusive and nonocclusive. In the occlusive category, thrombosis and emboli stand at the top, and the next common ones are vasculitis, thrombophilia, and iatrogenic conditions. The non-occlusive pathophysiology involves hypoperfusion due to cardiac failure, septic or hemorrhagic shock for hemodialysis, anaphylaxis, pancreatitis, drugs, and mechanical colonic obstruction. The drugs are responsible for the process of ischemic colitis, including diuretics, antibiotics, antihypertensive, and chemotherapeutic agents (especially vinca alkaloids and taxanes), appetite suppressants (phentermine), digoxin, nonsteroidal anti-inflammatory drugs, oral contraceptives, pseudoephedrine, sumatriptan, cocaine and alosetron [3], [4], [5].
Meta-analyses demonstrate that up to 80% of ischemic colitis cases were localized in the left colon and 25% in splenic flexure, where the two nutritional arterial watersheds come together [3]. Although highly unspecific like any intra-abdominal pathologies, the chief complaint of patients with ischemic colitis is abdominal pain, in almost more than 67% of the cases with its particular characteristics. The pain is acutely cramping with severity varying from mild to moderate, typically accompanied by an urge for defecation [4], [5], [6]. In the case we have reported, however, the patient presented with an acute abdomen. At first sight, the surgical team encountered a gangrenous colon that was not perforated. For the nature of visceral pain, the localization cannot be done correctly. Changing the colouration of the stool from bright red to maroon red, hematochezia mixed with faeces to overt lower gastrointestinal bleeding has been extensively reported in the cases of left colon involvement [7]. He had no complaint of GI bleeding in his history. Tenderness over the affected region or even peritonitis signs is observed when full-thickness necrosis has been occurred [3], [4], [5], as in our case. However, rarely leukaemic involvement of the GI system and especially colon has already been observed, the ischemic colitis in leukaemic patients has not been reported. Ischemic colitis is rather common among immunocompromised patients; thus, immunosuppressive agents involve a medication-related risk factor [5], [8]. Furthermore, Cytomegalovirus-induced ischemic colitis, especially in those with renal transplantation, is well known [9]. Frequent hypotensive episodes caused by hemodialysis induce vasoconstriction of the vasa recta, particularly in the right colon, leading to colonic ischemia [10]. Moreover, the longer the vasa recta of the right colon in one hand and originating further away from the bowel than those on the left side in the other hand, may increase the more resistance to reperfusion after an episode of hypotension. This indicates the vulnerability of the right colon to vascular events. Furthermore, the coincidence of promyelocytic leukaemia and MF is such a rare phenomenon that has already been reported in less than 10 cases [11], [12], [13], [14], [15], [16], [17], [18], [19]. There is a hypothesis that MF is a defence mechanism response to increased hematopoietic activity. Cytokines such as transforming growth factor β [20], (TGF-β), platelet-derived growth factor (PDGF), and basic fibroblast growth factor (bFGF) are known to play essential roles in the development of bone marrow fibrosis [20]. It is proved that TGF- β, PDGF, and bFGF to be produced by a spectrum of cells, including leukaemic cells besides megakaryoblasts [21], while in all stages, the platelet counts of our patient were in normal ranges. Mori et al. has reported the overexpression of the TGF-β gene in the leukaemic cells of APL patients with MF [18]. Accompanying MF with acute leukaemia has been observed with a poor prognosis attributed to possible interference with marrow regeneration after chemotherapy. The association between cancer and thrombosis has been known so far. A clear example of it is deep vein thrombosis and pulmonary embolism due to solid malignancies. An increased risk of thrombosis, as well as haemorrhage, threatens a patient with acute leukaemia. The thrombosis incidence in these patients varies between 2 and 36% [22]. Further risk factors for venous thromboembolism in acute myeloid leukaemia (AML) patients include the overexpression of tissue factors in leukaemic cells, its activation on cellular surfaces, and hyperleukocytosis [23]. Thrombosis in acute promyelocytic leukaemia patients is probably an underestimated complication [24]. Thrombosis is a less detected and probably underestimated life-threatening manifestation in patients with APL. However, the incidence of thrombotic events in large cohorts of patients with APL has been rarely reported [25], [26].
4. Conclusion
The nature of the malignancies is associated with thrombophilia. A clear example of it is venous thromboembolism in cancerous patients. The case we have reported had three aspects: leukocytoses of the patient were considered as various infections like urinary tract infections, catheter induced, etc. whereas leukocyte count of higher than 20,000 should provoke the possibility of haematological pathology; Leukaemias may also induce hypercoagulable state, and rarely APL can cause myelofibrosis.
Consent
The consent in which the patient has allowed to use medical records and therapeutic information is attached to the medical document. The authors testify the patient privacy maintenance. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
The authors ensure that all the images/figures/photos are suitably anonymised with no patient information or means of identifying the patient.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Ethical approval
This issue has been raised and approved in the ethics committee of Ardabil University of Medical Sciences, Iran, with the code “IR.ARUMS.REC.1400.260”.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Guarantor
Ali Samady Khanghah accepts full responsibility for the work and approves the whole process from designing the study to publish.
Research registration number
None.
CRediT authorship contribution statement
Mohammad Vakili Ojarood was the surgeon who operated on the patient, suggested publishing the method of surgery, and in the role of supervision and conceptualization.
Ali Samady Khanghah, a member of the research committee of the hospital prepared the manuscript and pursuits the submission process.
Mahdieh Belalzadeh did the pathological investigations.
Declaration of competing interest
The authors declare there are no conflicts of interest.
Acknowledgments
We thank the hospital staff and all the people who helped us write this manuscript.
Contributor Information
Mohammad Vakili Ojarood, Email: mohammad.vakili@arums.ac.ir.
Ali Samady Khanghah, Email: alisamady89@yahoo.com.
References
- 1.Rickles F.R., Falanga A. Molecular basis for the relationship between thrombosis and cancer. Thromb. Res. 2001;102(6):V215–V224. doi: 10.1016/s0049-3848(01)00285-7. [DOI] [PubMed] [Google Scholar]
- 2.Agha R.A., et al. The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 3.Washington C., Carmichael J.C. Management of ischemic colitis. Clin. Colon Rectal Surg. 2012;25(04):228–235. doi: 10.1055/s-0032-1329534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green B.T., Tendler D.A. Ischemic colitis: a clinical review. South Med. J. 2005;98(2):217–223. doi: 10.1097/01.SMJ.0000145399.35851.10. [DOI] [PubMed] [Google Scholar]
- 5.Stamatakos M., et al. Ischemic colitis: surging waves of update. Tohoku J. Exp. Med. 2009;218(2):83–92. doi: 10.1620/tjem.218.83. [DOI] [PubMed] [Google Scholar]
- 6.Paterno F., et al. Ischemic colitis: risk factors for eventual surgery. Am. J. Surg. 2010;200(5):646–650. doi: 10.1016/j.amjsurg.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Doulberis M., et al. Update on ischemic colitis: from etiopathology to treatment including patients of intensive care unit. Scand. J. Gastroenterol. 2016;51(8):893–902. doi: 10.3109/00365521.2016.1162325. [DOI] [PubMed] [Google Scholar]
- 8.Rezende-Neto J.B., Rotstein O.D. Abdominal catastrophes in the intensive care unit setting. Crit. Care Clin. 2013;29(4):1017–1044. doi: 10.1016/j.ccc.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Veroux M., et al. Cytomegalovirus and Clostridium difficile ischemic colitis in a renal transplant recipient: a lethal complication of anti-rejection therapy? Urol. Int. 2007;79(2):177–180. doi: 10.1159/000106334. [DOI] [PubMed] [Google Scholar]
- 10.Flobert C., et al. Right colonic involvement is associated with severe forms of ischemic colitis and occurs frequently in patients with chronic renal failure requiring hemodialysis. Am. J. Gastroenterol. 2000;95(1):195–198. doi: 10.1111/j.1572-0241.2000.01644.x. [DOI] [PubMed] [Google Scholar]
- 11.Abou Dalle I., Nassif S., Bazarbachi A. Acute promyelocytic leukemia with increased bone marrow reticulin fibrosis: description of three cases and review of the literature. Hematol. Oncol. Stem Cell Ther. 2018;11(2):99–104. doi: 10.1016/j.hemonc.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Batlle M., et al. Acute promyelocytic leukemia in a patient with idiopathic myelofibrosis. Leukemia. 1999;13(3):492–494. doi: 10.1038/sj.leu.2401340. [DOI] [PubMed] [Google Scholar]
- 13.Dutta P., et al. Acute promyelocytic leukemia with secondary myelofibrosis—case report and review of the literature. Am. J. Hematol. 2006;81(6):476–477. doi: 10.1002/ajh.20607. [DOI] [PubMed] [Google Scholar]
- 14.Fukuno K., et al. A variant form of acute promyelocytic leukemia with marked myelofibrosis. Int. J. Hematol. 2001;74(3):322–326. doi: 10.1007/BF02982068. [DOI] [PubMed] [Google Scholar]
- 15.Kwong J.H., et al. Primary myelofibrosis presenting in acute promyelocytic transformation. Br. J. Haematol. 2017;178(3) doi: 10.1111/bjh.14738. (349-349) [DOI] [PubMed] [Google Scholar]
- 16.Lu Q., Chen Y., Li Z. Long-term remission in a case of acute promyelocytic leukemia patient with marked myelofibrosis treated with arsenic trioxide, all-trans retinoic acid and consolidation therapy with daunorubicin plus cytarabine. Leuk. Res. 2012;6(36):e119–e121. doi: 10.1016/j.leukres.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Xiao M., et al. Acute promyelocytic leukemia with myelofibrosis: a case report and literature review. Medicine. 2021;100(13) doi: 10.1097/MD.0000000000024567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mori A., et al. Acute promyelocytic leukemia with marrow fibrosis at initial presentation: possible involvement of transforming growth factor-β1. Acta Haematol. 2000;103(4):220–223. doi: 10.1159/000041054. [DOI] [PubMed] [Google Scholar]
- 19.Nadiminti K., et al. T (15; 17) associated with primary myelofibrosis: a case report of an unusual clinical presentation and diagnostic dilemma. OncoTargets Ther. 2019;12:5449. doi: 10.2147/OTT.S208290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Bousse-Kerdiles M.-C. 1996. Differential expression of transforming growth factor-beta, basic fibroblast growth factor, and their receptors in CD34+ hematopoietic progenitor cells from patients with myelofibrosis and myeloid metaplasia. [PubMed] [Google Scholar]
- 21.Allouche M., et al. Expression of basic fibroblast growth factor (bFGF) and FGF-receptors in human leukemic cells. Leukemia. 1995;9(1):77–86. [PubMed] [Google Scholar]
- 22.De Stefano V., et al. The risk of thrombosis in patients with acute leukemia: occurrence of thrombosis at diagnosis and during treatment. J. Thromb. Haemost. 2005;3(9):1985–1992. doi: 10.1111/j.1538-7836.2005.01467.x. [DOI] [PubMed] [Google Scholar]
- 23.Oehadian A., Iqbal M., Sumantri R. Deep vein thrombosis in acute myelogenous leukemia. Acta Med. Indones. 2009;41(4):200–204. [PubMed] [Google Scholar]
- 24.Sanz M.A., Montesinos P. Open issues on bleeding and thrombosis in acute promyelocytic leukemia. Thromb. Res. 2010;125:S51–S54. doi: 10.1016/S0049-3848(10)70013-X. [DOI] [PubMed] [Google Scholar]
- 25.Breccia M., et al. Occurrence of thrombotic events in acute promyelocytic leukemia correlates with consistent immunophenotypic and molecular features. Leukemia. 2007;21(1):79–83. doi: 10.1038/sj.leu.2404377. [DOI] [PubMed] [Google Scholar]
- 26.Montesinos P., et al. Incidence and risk factors for thrombosis in patients with acute promyelocytic leukemia. Experience of the PETHEMA LPA96 and LPA99 protocols. Blood. 2006;108(11):1503. [Google Scholar]