Abstract
Introduction
Pulmonary atypical carcinoid (PAC) is a rare subtype of pulmonary neuroendocrine neoplasm. Although EML4-ALK fusion has been detected in PAC, EGFR mutations have not been reported before.
Methods
We performed hematoxylin and eosin staining, immunohistochemistry, and next-generation sequencing on tissues at baseline and after surgery.
Results
The patient was diagnosed with having advanced PAC harboring the EGFR L858R mutation and then received a combination of icotinib and irinotecan plus cisplatin chemotherapy, achieving a partial response before the operation. Postoperative histology results revealed SCLC harboring the EGFR L858R mutation. Surprisingly, both the KRAS amplification and the RB1 deletion disappeared.
Conclusions
EGFR tyrosine inhibitors plus irinotecan plus cisplatin chemotherapy might be a potential treatment option for advanced pulmonary neuroendocrine neoplasms harboring EGFR mutations.
Keywords: Pulmonary atypical carcinoid, Neuroendocrine tumor, EGFR L858R, Icotinib, Chemotherapy
Introduction
The 2021 WHO tumor classification (fifth edition) classified pulmonary neuroendocrine neoplasms (NENs) into four subtypes, typical carcinoid, atypical carcinoid (AC), large cell neuroendocrine carcinoma (LCNEC), and SCLC, according to some morphologic and protein expression immunohistochemistry (IHC) features.1 Among NENs, AC accounts for only approximately 2.5% and 0.2% of lung carcinomas, making them rare lesions.2,3 In addition, cancer-related gene mutations are rare.4 Nevertheless, as reported by Rickman et al.,5 ErbB3 and ErbB4 receptors can be expressed in AC tumors, and there were no activating mutations in the EGFR kinase domain from AC tumor tissue in the Mayo Clinic Lung Cancer Specimen Registry from 2001 to 2006. The EML4-ALK fusion gene was detected in patients with AC.6 Nevertheless, no patients with pulmonary AC harboring EGFR mutations have been reported.
Materials and Methods
Patient Information
A patient with advanced pulmonary AC also with the EGFR L858R mutation was treated and evaluated at Guangdong Provincial People’s Hospital. The patient signed the informed consent form and gave permission for the use of their tumor tissues.
Pathological Characteristics and IHC Imaging
Pathology was confirmed by hematoxylin and eosin staining and tissue-specific markers according to the 2021 WHO tumor classification (fifth edition). Tissues were fixed in 4% formaldehyde, and 5-μm sections were stained with hematoxylin and eosin reagent after embedding in paraffin (×100 and ×200 magnification). Immunohistochemical staining markers included Ki67, Syn, CgA, TTF1, and CD56. Each section was examined under a ×200 power field. Positive cells were scored as expressing less than 10% (−), 10% to 25% (+), 25% to 75% (++), and more than 75% (+++).
Next-Generation Sequencing
Next-generation sequencing (NGS) of baseline and postsurgical tissues was conducted using baseline and postsurgical tissues, respectively, without plasma at Guangdong Provincial People’s Hospital using 196-gene panel (MiSeqDx, Illumina).
Results
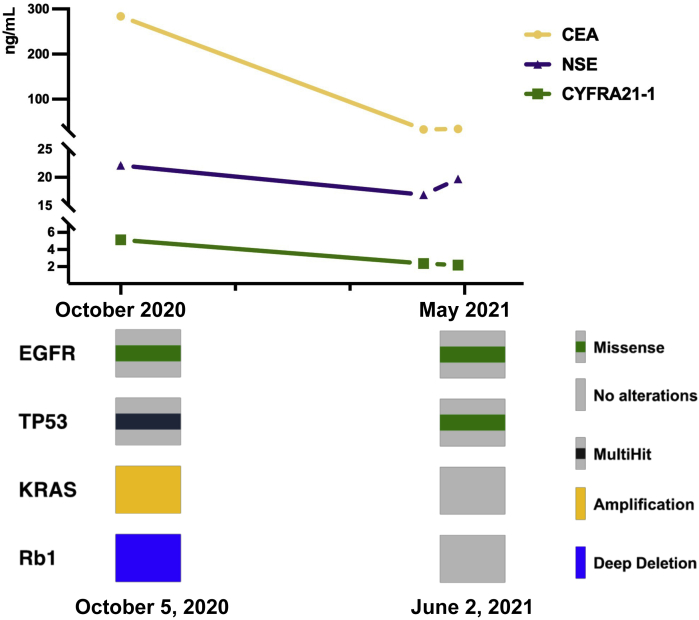
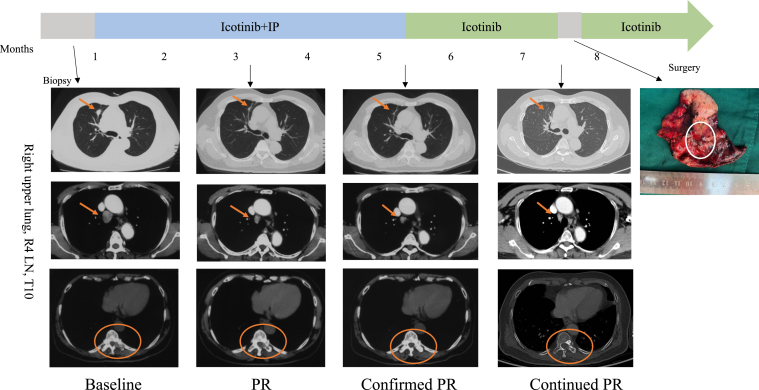
The patient, a 72-year-old man who was a heavy smoker, presented with a physical examination of the chest and abdominal computed tomography revealing a 21-mm lesion in the right upper lung with multiple mediastinal lymph node (LN) metastases and bone destruction of the tenth thoracic vertebral body and left vertebral arch. He had no fever, cough, chest pain, or any other symptoms. Results of the endobronchial ultrasound-guided transbronchial needle aspiration of the right lower paratracheal (R4) LN revealed pulmonary AC with IHC staining results as Ki-67 (25%+), Syn (++), CgA (++), TTF-1 (+), and CD56 (+++) (Fig. 1A). NGS results revealed EGFR L858R mutation, TP53 mutation, KRAS amplification, and RB1 deletion (Fig. 2). He received icotinib (125 mg thrice a d) combined with four cycles of irinotecan plus cisplatin (IP) chemotherapy (irinotecan 60 mg/m2 on d1, d8, and d15, cisplatin 60 mg/m2 on d1), achieving partial response according to the Response Evaluation Criteria in Solid Tumors version 1.1 at 2 months and confirmed partial response at 4 months after the initiation of treatment (Fig. 3). The level of serum carcinoembryonic antigen was considerable decreased (Fig. 2).
Figure 1.
Pathology images of HE staining and IHC (×200). A: (a): HE staining result of LN as AC at baseline. (b–g): IHC staining revealed Ki-67 (25%+), Syn (++), CgA (++), TTF-1 (+), and CD56 (+++). B: (a): HE staining result of LNs after surgery for SCLC. (b–g): IHC staining revealed Ki-67 (60%+), Syn (+), Cg A (+), TTF-1 (++), and CD56 (+++). C: (a): HE staining result of the lung lesion after surgery, which was diagnosed with SCLC. (b–g): IHC staining revealed Ki-67 (60%+), Syn (+), CgA (+), TTF-1 (+), and CD56 (+). Scale bar = 100 μm. AC, atypical carcinoid; HE, hematoxylin and eosin; IHC, immunohistochemistry; LN, lymph node; R4, right lower paratracheal.
Figure 2.
Dynamic changes in serum tumor markers and cancer-related gene variant changes of tumor tissues by NGS. The levels of CEA, CYFRA21-1, and NSE at baseline were 283.53 ng/mL, 5.14 ng/mL, and 22.11 ng/mL, respectively. After 6 months, the levels of CEA, CYFRA21-1, and NSE were 33.13 ng/mL, 2.36 ng/mL, and 16.91 ng/mL, respectively. The preoperative levels of CEA, CYFRA21-1, and NSE were 34.19 ng/mL, 2.17 ng/mL, and 19.71 ng/mL, respectively. NGS results revealed that the variant allele frequencies of the EGFR L858R missense mutation were 31.37% and 13.30% at baseline and after surgery, respectively. Both the KRAS amplification and the Rb1 deletion had disappeared. NGS, next-generation sequencing.
Figure 3.
Timeline of treatment with clinical responses to a combination of icotinib and IP chemotherapy. The patient achieved a PR after 2 months of treatment with a combination of icotinib and chemotherapy (IP). PR was confirmed 2 months after. After 6 months of treatment, he continued to achieve PR, and the T10 vertebral body had an osteogenesis change, and we performed a wedge resection. The arrows and circles indicate lesions. IP, irinotecan plus cisplatin; PR, partial response; LN, lymph node; R4, right lower paratracheal.
After 5 months of treatment, repeated positron emission tomography-computed tomography results revealed no increase in fludeoxyglucose (F-18) in the primary lesion of the right upper lung, whereas the R4 LN and the tenth thoracic vertebral body, including the left vertebral arch, were still F-18 positive. To further confirm the pathologic diagnosis and to achieve better disease control, we conducted wedge resection of the anterior segment of the right upper lung lobe and the mediastinal LNs followed by the intended palliative radiation of the tenth thoracic vertebrae. Intraoperative frozen results revealed poorly differentiated cancer. Interestingly, the postoperative pathology and IHC results of the LNs and the lung primary lesion were Ki-67 (60%+), Syn (+), Cg A (+), TTF-1 (++), and CD56 (+++) and Ki-67 (60%+), Syn (+), CgA (+), TTF-1 (+), and CD56 (+), respectively. (Fig. 1B and 1C) Furthermore, the NGS results still revealed the EGFR L858R mutation, whereas both the KRAS amplification and the RB1 deletion had disappeared (Fig. 2).
Discussion
Pulmonary AC has a low frequency of cancer-related mutations, and patients with AC harboring EGFR mutations have not been reported.5 The low mutation frequency was related to the low detected frequency to some extent among patients with AC. Thus, performing NGS to detect any targetable mutations, particularly in patients with rare diagnosis, is of vital importance that may give more patients opportunity to receive tyrosine kinase inhibitors (TKIs) that may be a better choice for them. Among NENs, the malignant degree of AC is between typical carcinoid and LCNEC, and LN metastasis is common.3,7 Approximately 20% of patients with pulmonary AC were first diagnosed with distant metastases; therefore, we tend to administer the chemotherapy recommended for SCLC.2 A Japanese study revealed both a longer median overall survival time and a higher two-year survival rate in the IP group than in the etoposide plus cisplatin group (p < 0.005) among patients with advanced-stage SCLC.8 A meta-analysis suggested that icotinib achieved better efficacy in patients with EGFR 21 exon L858R mutation than in those with EGFR wild type (progression-free survival [PFS] = 8.7 versus 2.6 mo).9 In our previous study, published in the 2020 World Conference on Lung Cancer, the median PFS of patients with transformed SCLC with EGFR mutations receiving EGFR TKI plus chemotherapy was significantly longer than the PFS times of those treated with chemotherapy alone.10 Thus, this patient was treated with a combination of icotinib and IP chemotherapy. To the best of our knowledge, this was the first patient with advanced pulmonary AC with EGFR mutation who responded to EGFR TKI plus chemotherapy.
Regarding histology, this patient was first diagnosed with having pulmonary AC, and according to a survival analysis of patients with AC, receiving surgery could reduce the risk of death (hazard ratio = 0.19, 95% confidence interval: 0.137–0.264, p < 0.001).2 To our surprise, this patient was diagnosed with having SCLC after surgery. For this changed pathology, we have two hypotheses. First, this might be due to the spatial heterogeneity of the tumor tissue, as we observed that the postoperative pathology indicated a large spatial heterogeneity, whereas the baseline sample was too small to reveal heterogeneity. Meanwhile, it also suggested that more tissue or multispot biopsy would do more help for diagnosis and may reveal the spatial heterogeneity of tumors to a certain extent, thus providing more information for the precise treatment decision made at the time of diagnosis. Second, a two-way clustering analysis of NGS data of patients with NENs suggested an innovative view, as low- and middle-grade NENs have the potential to evolve into high-grade tumors, indicating most high-grade pulmonary NENs are likely to develop from pre-existing carcinoids.11 Radiotherapy of the tenth thoracic vertebrae is currently conducted, with close follow-up.
Rubino et al.12 proposed the concept of lung carcinoids with high proliferative activity, defined as mitotic count greater than 10/2 mm2 and Ki-67 index greater than 20%. They indicated that the recurrence-free survival times of patients with carcinoids on the basis of pathological characteristics who underwent primary tumor surgery by AC and lung carcinoids with high proliferative activity were 178 and 24 months, respectively (p < 0.01). In addition, patients harboring the EGFR L858R mutation achieved a lower efficacy with EGFR TKIs than those with the EGFR 19del mutation.13 The INCREASE study revealed that icotinib had a significantly longer median PFS in the L858R high-dose group (250 mg, thrice a d) than in the L858R routine-dose group (125 mg, thrice a d) (12.9 versus 9.2 mo, hazard ratio = 0.75, 95% confidence interval: 0.53–1.05, p < 0.05) and was comparable to that in the 19del group at 12.5 months.14 Thus, how to individualize the postoperative follow-up and when to double the dose of icotinib for maintenance treatment are questions that need to be further investigated.
EGFR TKI plus IP chemotherapy administered successfully in the first patient with pulmonary AC with EGFR mutation may provide a potential treatment mode for advanced NENs harboring EGFR mutations. Nevertheless, further investigations of treatment regimens and genomic specificity of patients with AC are warranted.
CRediT Authorship Contribution Statement
Yu-Qing Chen: Investigation, Data curation, Writing - original draft, Visualization.
Yu-Fa Li: Investigation, Data curation.
Chan-Yuan Zhang: Investigation.
Shi-Ling Zhang: Visualization.
Zhi-Yi Lv, Xu-Chao Zhang: Resources.
Song Dong, Hua-Jun Chen: Validation.
Yi-Long Wu: Supervision.
Jin-Ji Yang: Conceptualization, Methodology, Writing - review & editing.
Acknowledgments
This study was supported by funding from the National Natural Science Foundation of China (grant number 81972164, to Dr. JJ Yang), the Provincial Natural Science Foundation of Guangdong Province, China (grant number 2019A1515010931, to Dr. JJ Yang), and the High-Level Hospital Construction Project (grant number DFJH201809, to Dr. JJ Yang). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors owe thanks to the patient and his family. The authors thank the staff at Guangdong Lung Cancer Institute, Guangdong Provincial People's Hospital, and Guangdong Academy of Medical Sciences.
Footnotes
Ms. Yu-Qing Chen and Dr. Yu-Fa Li contributed equally to this work.
Disclosure: The authors declare no conflict of interest.
Cite this article as: Chen YQ, Li YF, Zhang CY, et al. Response to icotinib plus chemotherapy in pulmonary atypical carcinoid harboring the EGFR L858R mutation: a brief report. JTO Clin Res Rep. 2021;2:100258.
References
- 1.Derks J.L., Rijnsburger N., Hermans B.C.M., et al. Clinical-pathological challenges in the classification of pulmonary neuroendocrine neoplasms and targets on the horizon for future clinical practice. J Thorac Oncol. 2021;16:1632–1646. doi: 10.1016/j.jtho.2021.05.020. [DOI] [PubMed] [Google Scholar]
- 2.Steuer C.E., Behera M., Kim S., et al. Atypical carcinoid tumor of the lung: a surveillance, epidemiology, and end results database analysis. J Thorac Oncol. 2015;10:479–485. doi: 10.1097/JTO.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 3.Marquez-Medina D., Popat S. Systemic therapy for pulmonary carcinoids. Lung Cancer. 2015:139–147. doi: 10.1016/j.lungcan.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Asiedu M.K., Thomas C.F., Dong J., et al. Pathways impacted by genomic alterations in pulmonary carcinoid tumors. Clin Cancer Res. 2018;24:1691–1704. doi: 10.1158/1078-0432.CCR-17-0252. [DOI] [PubMed] [Google Scholar]
- 5.Rickman O.B., Vohra P.K., Sanyal B., et al. Analysis of ErbB receptors in pulmonary carcinoid tumors. Clin Cancer Res. 2009;15:3315–3324. doi: 10.1158/1078-0432.CCR-08-2549. [DOI] [PubMed] [Google Scholar]
- 6.Liu N., Wang J., Fu X., et al. A case of primary pulmonary atypical carcinoid with EML4-ALK rearrangement. Cancer Biol Ther. 2020;21:12–16. doi: 10.1080/15384047.2019.1665957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filosso P.L., Rena O., Guerrera F., et al. Clinical management of atypical carcinoid and large-cell neuroendocrine carcinoma: a multicentre study on behalf of the European Association of Thoracic Surgeons (ESTS) Neuroendocrine Tumours of the Lung Working Group. Eur J Cardio Thorac Surg. 2015;48:55–64. doi: 10.1093/ejcts/ezu404. [DOI] [PubMed] [Google Scholar]
- 8.Noda K., Nishiwaki Y., Kawahara M., et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85–91. doi: 10.1056/NEJMoa003034. [DOI] [PubMed] [Google Scholar]
- 9.Pan H., Liu R., Li S., et al. Effects of icotinib on advanced non-small cell lung cancer with different EGFR phenotypes. Cell Biochem Biophys. 2014;70:553–558. doi: 10.1007/s12013-014-9955-y. [DOI] [PubMed] [Google Scholar]
- 10.Zhang C., Zhang S., Yao Y., et al. MA12.08 Chemotherapy plus EGFR TKIs or bevacizumab versus chemotherapy alone in SCLC-transformed EGFR-mutant lung adenocarcinoma. J Thorac Oncol. 2021;16(3 suppl):S178–S179. [Google Scholar]
- 11.Pelosi G., Bianchi F., Dama E., et al. Most high-grade neuroendocrine tumours of the lung are likely to secondarily develop from pre-existing carcinoids: innovative findings skipping the current pathogenesis paradigm. Virchows Arch. 2018;472:567–577. doi: 10.1007/s00428-018-2307-3. [DOI] [PubMed] [Google Scholar]
- 12.Rubino M., Scoazec J.Y., Pisa E., et al. Lung carcinoids with high proliferative activity: further support for the identification of a new tumor category in the classification of lung neuroendocrine neoplasms. Lung Cancer. 2020;148:149–158. doi: 10.1016/j.lungcan.2020.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Jackman D.M., Yeap B.Y., Sequist L.V., et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non–small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:3908–3914. doi: 10.1158/1078-0432.CCR-06-0462. [DOI] [PubMed] [Google Scholar]
- 14.Li X., Zhang L., Jiang D., et al. Routine-dose and high-dose icotinib in advanced non-small cell lung cancer patients harboring EGFR exon 21 L858R mutation: the randomized, phase II, INCREASE trial. Clin Cancer Res. 2020;26:3162–3171. doi: 10.1158/1078-0432.CCR-19-3064. [DOI] [PubMed] [Google Scholar]