1. Introduction
Tissue injury produces nociceptive pain that gradually subsides as the injury heals and local inflammation resolves. However, as in chronic pain conditions such as complex regional pain syndrome (CRPS) type I and chronic post-surgical pain, pain can persist long after the inciting injury has healed, without apparent organic abnormalities underlying the pain [33,50]. This type of chronic pain, recently termed ‘nociplastic pain’ by the International Association for the Study of Pain (IASP) taskforce, is a unique category of chronic pain disorders distinct from idiopathic and neuropathic pain [24]. In such disorders, nociplastic pain originates from “altered nociception despite no clear evidence of actual or threatened tissue damage causing the activation of peripheral nociceptors or evidence for disease or lesion of the somatosensory system” [24]. Owing to its heterogeneous etiology and a lack of mechanistic insight, the development and implementation of effective therapeutics to prevent and treat nociplastic pain conditions have been greatly hindered.
There are multiple critical questions which must be addressed in nociplastic pain studies. Notably, females are disproportionately affected by nociplastic pain conditions [19,35,43]. The female overrepresentation in chronic pain conditions in general has been attributed to both biological and psychosocial factors. However, the potential contribution of inherent female susceptibility or sexually dimorphic pain mechanisms to the incidence of nociplastic pain is unclear. Aside from sex, it is noteworthy that in some nociplastic pain conditions such as CRPS type I [6], fibromyalgia [17], post-infectious irritable bowel syndrome, and chronic post-surgical pain, potential or obvious inciting injuries (trauma, infection, etc.) could be identified. Since such injuries do not always result in nociplastic pain conditions, it is of interest and significance to understand how the injury-induced initial pain transitions to nociplastic pain, and how the nociplastic pain state is maintained, once established, in the absence of ongoing tissue damage.
With respect to such transition, chronic post-surgical pain provides valuable information about risk factors. Surgery being considered a tissue injury, clinical findings indicate that the magnitude of post-surgical (i.e., post-injury) pain itself may be predictive of its ‘chronification’ [54]. Designing experimental approaches for modeling nociplastic pain in animals to address the abovementioned questions, we used these clinical findings to experimentally trigger the transition from an injury-induced, normally resolving pain to nociplastic pain in an animal model of acute injury. Specifically, we enhanced post-injury pain by stimulating the injured area. Our previous studies show that capsaicin-induced mechanical hypersensitivity in rats is transiently enhanced and prolonged by stimulation of the capsaicin-injected paw even at a normally innocuous intensity [21,22]. Using this paradigm, we established here that mechanical hypersensitivity can be significantly prolonged after post-injury stimulation in both male and female mice, without apparent persistent tissue damage, and that this prolonged mechanical hypersensitivity is maintained by sexually dimorphic mechanisms. Portions of these studies have been reported in abstract form [11,34].
2. Methods
2.1. Animals
Adult male and female C57BL/6N mice (aged 7-8 weeks) were purchased from Charles River (Houston, TX, USA) or bred inhouse. Mice were housed in groups of five in plastic cages with a 12-12 hour light-dark cycle and fed ad libitum. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch (UTMB) and in accordance with the National Institutes of Health (NIH) guidelines.
2.2. Experimental injury
All procedures were conducted while animals were under 1.5-2.5% isoflurane anesthesia.
Intraplantar capsaicin injection:
As an experimental chemical injury, capsaicin (0.1% in 10% ethanol, 10% Tween-20, and 80% saline; Sigma-Aldrich, St. Louis, MO, USA) was injected intradermally at the (plantar side) base of the third and fourth digits of the left hindpaw (3 μg in 3 μL) using a 30G needle.
Plantar incision:
A group of mice underwent a plantar incision procedure instead of intraplantar capsaicin injection. The skin incision (~4.0 mm) was made along the (plantar side) base of the 2nd-4th digits and sutured (9-0 microsurgical needle; Fine Science Tools, Foster City, CA, USA).
2.3. Post-injury stimulation
All procedures were conducted while animals were under 1.5-2.5% isoflurane anesthesia.
Post-injury thermal stimulation:
In some groups of mice that received intraplantar capsaicin injection, the injured paw (i.e. the capsaicin injection area and surrounding tissues) was stimulated 2, 6, 24, or 72 hr after the injury. Post-injury thermal stimulation (30°C or 40°C) was applied in the following manner: the distal half of hindpaw (including the toes, injury area, and surrounding tissues, but not the von Frey testing site) was repeatedly submerged into temperature controlled sterile water for 30 sec at 30 sec intervals over a period of 10 min (10 times of 30 sec in and 30 sec out). When the experimenter’s fingers were immersed in 40°C water, a sensation of warmth was elicited; no definitive cooling or warmth was sensed in 30°C water. In experiments when treatment timepoints coincided with behavioral tests, post-injury thermal stimulation was administered 30 min prior to behavioral tests. The timeline of these manipulations and behavioral testing is depicted in Fig. 1.
Fig. 1. Timeline for behavioral experiments using capsaicin injection followed by post-injury thermal (warmth) stimulation paradigm.
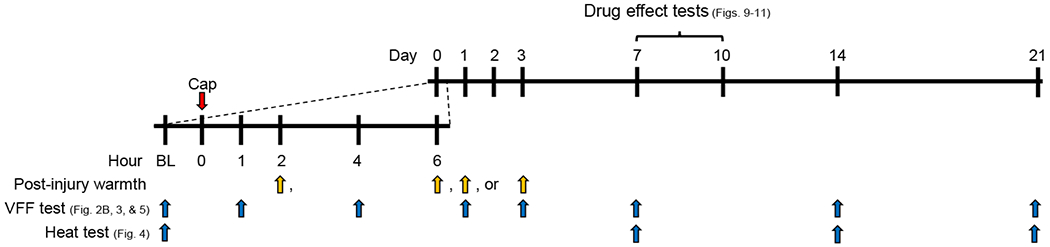
After determining baseline (BL) behaviors, capsaicin (Cap, red arrow) was injected at the plantar side of hindpaw. The capsaicin-injected paw was immersed into warm water at time points marked by yellow arrows; at days 1 and 3 post-Cap, the warmth stimulation was delivered 30 min before von Frey filament (VFF) testing. VFF tests and radiant heat tests were conducted at predetermined times (blue arrows) in separate groups of mice. Effects of various drugs on persistent mechanical hypersensitivity were determined days 7-10 post-Cap.
As a control for the hindpaw manipulation associated with water immersion after intraplantar capsaicin injection, a subset of mice were treated with capsaicin followed by ’air immersion’ 2 hr after capsaicin injection. Mice subjected to ‘air immersion’ underwent the same dipping motion into an empty water-bath instrument.
In some mice that received plantar incision instead of capsaicin injection, 40°C post-injury thermal stimulation was performed 23 hr after the incision (i.e., 1 hr before von Frey assay at 24 hr post-incision) in the manner described above.
Post-injury vibratory stimulation:
In experiments utilizing vibration (92 Hz) rather than water immersion, vibration was applied focally to the capsaicin-injected hindpaw using a Hitachi HV-1 mini massager (2.25 mm2 contact surface with 13-22 mN/mm2 of pressure). Vibration was applied for 10 sec at 30 sec intervals over 10 minutes. When tested on the experimenter’s fingers, a mild but definite vibratory sensation was elicited. Sham vibration was used as a control, applying the vibration probe to the paw without turning on the vibrator.
2.3. Behavioral test
Mechanical sensitivity test:
Mice were habituated to behavioral test conditions (including experimenters) for four days prior to conducting behavioral procedures. Mice were placed into acrylic chambers (14 cm length x 5 cm width x 4.5 cm height) on a raised metal grid-floor platform and were acclimated to testing conditions for 30 minutes prior to testing on the day of experiment. Mechanical sensitivity of the capsaicin-injected hindpaw was tested using a von Frey filament (0.98 mN) that evokes only 0-20% withdrawal responses in naïve mice. This mechanical force is below the mechanical thresholds of hindpaw-innervating Aδ/C fibers, determined in ex-vivo skin nerve preparations from C57BL/6 mice (2.0-13.9 mN, [37,48]) or in vivo from C3H/HeJ mice (the interquartile range 10-25 mN [3]), and therefore unlikely to be a ‘threatened tissue damage causing the activation of peripheral nociceptors’ in normal conditions. Considering the possibility that direct repeated probing of the injured area (e.g., capsaicin injection site) over time could be a confounding factor in experimentally defining post-injury stimulation, we stimulated the area outside the injury (4-5 mm proximal to the injured area; mid-hindpaw) with the 0.98 mN von Frey filament. Mechanical hypersensitivity is known to develop outside the injured area (commonly called secondary mechanical hypersensitivity) due to the injury-induced sensitization of nociceptive system at a central level [36,44,47,52]. The percent of withdrawal responses out of ten probing trials was recorded.
Thermal sensitivity test:
After habituation as described above, mice were placed into acrylic chambers on a glass platform. A mobile laser emitter under the glass platform was placed beneath the middle of the hindpaw and turned on. When the mouse withdrew the hindpaw from the radiant heat of the laser, the emitter was automatically turned off and the latency to withdrawal was recorded. It should be noted here that the radiant heat could not be restricted to the outside of injured area, which therefore, could confound the ‘post-injury stimulation of injured area’ paradigm when used before capsaicin-induced thermal hypersensitivity substantially abates (see Results 3.2). Therefore, except for the experiment determining the resolution time course of capsaicin-induced thermal hypersensitivity (i.e., Fig. 1A and Supplemental Fig. 1), we performed this radiant heat test at baseline and after persistent mechanical hypersensitivity was established.
2.4. Drug administration
For all injections, mice were anesthetized by 1.5-2.5% isoflurane. Seven to ten days after the capsaicin injection, mice received 1) either a single intraplantar injection of 0.75% bupivacaine (3 μL; Sigma-Aldrich) or saline (0.9%; Baxter Healthcare Corporation, Deerfield, IL, USA) at the capsaicin injection area; 2) an intraperitoneal injection of morphine (5 mg/kg; Westward, Eatontown, NJ, USA), gabapentin (100 mg/kg; Spectrum Chemical Mfg Corporation, New Brunswick, NJ, USA) or saline (0.9%, Baxter Healthcare Corporation); or 3) a single intrathecal injection of unconjugated saporin or Mac-1-saporin (8.85 μM, 5 μL; Advanced Targeting Systems, San Diego, CA, USA) using a 30G needle.
2.5. Evans Blue extravasation
Under 2% isoflurane anesthesia, Evans Blue (50 mg/mL; Sigma Aldrich) was intravenously administered (50 mg/kg) via the tail vein either 2 hr, 1 day, or 7 days after capsaicin injection with or without 40°C water immersion at 2 hr post-capsaicin; for 2 hr post-capsaicin time point data in the water immersion group, Evans Blue injection was done immediately after the immersion. Thirty minutes after Evans Blue injection, mice were perfused with saline, and glabrous skin samples (2 mm x 2 mm) from the capsaicin injection area and corresponding area on the contralateral hindpaw were collected. Samples were dried in a 37°C oven for 72 hr. Evans Blue dye deposits were extracted in formamide (16 mL per 1.0 g dry weight tissue; Sigma Aldrich) at 37°C for 72 hr. The concentration of Evans Blue was quantified using a Nanodrop 2000C (Thermo Fisher, Waltham, MA, USA) and analyzed as described in the literature [32].
2.6. Quantification of proinflammatory cytokine gene transcripts
On post-capsaicin injection day 1 or day 7, skin samples (2 mm x 2 mm) from the capsaicin injection area and corresponding area of the contralateral hindpaw were collected and flash-frozen on dry ice. Skin tissue was diced finely, then transferred to an Eppendorf tube containing TRIzol reagent (Thermo Fisher) with 1 mg/mL collagenase type I (≥ 125 U/mg, Gibco, Waltham, MA, USA). Using a bead mill homogenizer with micro glass beads (0.5 mm, Biospecs Products, Bartlesville, OK, USA), tissue was processed for 10–15 cycles of 120 sec at speed 5. The supernatant was isolated and 400 μL pheno-chloroform (Amresco, Solon, OH, USA) per 1.0 mL TRIzol reagent was added. After centrifuging the mixture at 12,000 x g for 15 min, the supernatant was transferred to a fresh Eppendorf tube containing an equal volume of isopropanol, incubated on wet ice for 10 min, and centrifuged at 10,000 x g for 10 min. Finally, the RNA pellet was rinsed three times with 1.0 mL 70% ethanol and centrifuged at 5,000 x g for 5 min prior to resuspension in nuclease-free water. RNA quality and purity were checked using a Nanodrop 2000C (Thermo Scientific) prior to being transcribed into cDNA using the QuantiTect Reverse Transcription Kit (Qiagen, Hilden, Germany) per manufacturer instructions. SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA) was mixed with 10 ng of cDNA and β-2-microglobulin primer (forward: 5’-TGGTCTTTCTGGTGCTTGTC-3’, 100 μM; reverse: 5’-GCAGTTCAGTATGTTCGGCT-3’, 100 μM), IL-1β primer (forward: 5’-CTGGTGTGTGACGTTCCCATTA-3’, 100 μM; reverse: 5’-CCACAGCACGAGGCTTT-3’, 100 μM), IL-6 primer (forward: 5’-AAGAACAAAGCCAGAGTCCTTC-3’, 300 μM; reverse: 5’-TAGGAGAGCATTGGAAATTGGG-3’, 300 μM), or TNF-α primer (forward: 5’-CCCTCACACTCACAAACCAC-3’, 300 μM; reverse: 5’-TTTGAGATCCATGCCGTTGG-3’, 300 μM). qPCR was conducted at 95°C for 10 min, followed by 40 cycles of 95°C for 10 sec, 55.6°C for 30 sec, and 60°C for 30 sec. Single amplicon was confirmed by melt curve. As a readout of proinflammatory cytokine gene transcript amount, we first calculated a difference in quantification cycle number (ΔCq), detected using LinRegPCR software [41], between the reference gene (β-2-microglobulin) and the proinflammatory cytokine gene transcripts in each sample. Next, we calculated ΔΔCq by subtracting each ΔCq value from the mean of corresponding contralateral ΔCq values; the greater the ΔΔCq value, the greater the amount of proinflammatory cytokine gene transcript in the ipsilateral side relative to that in the contralateral side.
2.7. Immunohistochemistry
Twenty-four hours after treatment with intrathecal unconjugated saporin or Mac-1-saporin, mice were perfusion-fixed using phosphate buffered saline (PBS) followed by Lana’s fixative (4% paraformaldehyde and 14% picric acid in PBS). The lumbar spinal cord (L3–L5) was resected and post-fixed in Lana’s fixative for 4 hr, and then dehydrated in 30% sucrose solution overnight. Samples were then embedded in Tissue-Plus Optimal Cutting Temperature (O.C.T.) Compound (Fisher Health Care, Norwich, UK). Lumbar spinal cord sections (12 μm thick) were stained for Iba1 using an anti-Iba1 primary antibody (1:2,000, rabbit; Wako, Japan), followed by AlexaFluor 488-conjugated anti-rabbit secondary antibody (1:300; Thermo Fisher). Stained sections were mounted with DAPI-containing media (Vectashield; Vector Laboratories, Burlingame, CA, USA). ImageJ was used to analyze confocal microscope images by percent area florescence [25,55] within the medial dorsal horn.
2.8. Statistical analyses
In experiments assessing thermal sensitivity by measuring paw withdrawal latency at multiple time points, mean±SD or individual values are presented, and data were analyzed using linear mixed model (LMM) with the first order autoregressive (AR1) covariance structure for repeated measures (Time) followed by Sidak multiple comparison tests. To delineate the resolution time course of capsaicin-induced thermal hypersensitivity, the difference between withdrawal latencies at baseline and each time point (ΔLt) was normalized to the peak difference at 2 hr post-capsaicin (ΔL2h) in individual mice. Curve fitting was performed using a single-phase exponential decay function in each sex and statistically tested to determine whether different curves fit for male and female data (Prism ver. 8, GraphPad, CA, USA). In experiments assessing mechanical sensitivity by counting the number of paw withdrawals out of 10 trials at multiple experimental time points, data are presented as median with interquartile range (IQR) and were analyzed using generalized linear mixed model (GLMM) with a logistic link function for binomial distribution and AR1 covariance structure for repeated measures (Group x Time [repeated] in each sex; sequential Sidak procedure for multiple comparisons between groups at each time point); degrees of freedom were allowed to vary across tests (SPSS ver. 25, IBM, NY, USA). In an Evans Blue extravasation assay, data were log-transformed to resolve heteroscedasticity and analyzed using LMM (Paw side [nested within an animal] x Model x Time in each sex; Bonferroni test for multiple comparisons). The levels of gene expression were analyzed by comparing ΔΔCq value between groups using LMM (Paw side [nested within an animal] x Model x Time in each sex; Bonferroni test for multiple comparisons). Iba1-immunoreactive density values from multiple sections were averaged per animal. Differences in Iba1-immunoreactivity between treatment groups were analyzed in each sex using LMM (Doral horn side [nested within an animal] x Treatment; Bonferroni test for multiple comparisons). For Bonferroni tests, we took an a priori approach by predetermining comparison pairs (e.g., ipsi- vs. contra-lateral sides at each time point within a model, and between models at each time point only in the ipsilateral side); p-value was adjusted accordingly. In LMM or GLMM analysis, random intercepts accounting for individual animal variances were included. However, this random effect was removed from the statistical model when final Hessian matrix was not positive definite despite satisfying all convergence criteria.
3. Results
3.1. Intraplantar capsaicin produced pain hypersensitivity that resolved in different time courses between sexes.
Our model utilizes 0.1% capsaicin injection as an experimental injury, followed by post-injury thermal stimulation. This concentration of capsaicin is commonly used as a chemical injury for producing acute pain by directly activating nociceptors (primarily producing the pain of burning sensation) [46] and inducing both peripheral [2] and central sensitization [9,26]. Prior to developing a nociplastic pain animal model using a post-injury thermal stimulation to increase post-injury pain, we first characterized capsaicin-induced sensitization, focusing on sex differences in the magnitude of capsaicin-induced thermal and mechanical hypersensitivity. In our radiant heat test condition, males showed a longer latency to withdrawal at baseline and a steeper trajectory back to the baseline level after capsaicin injection (Fig. 2A). Curve fitting of the resolution time course of capsaicin-induced thermal hypersensitivity (with the hypersensitivity at 2 hr post-capsaicin being set as the 100% peak for each mouse) revealed slower resolution in females than in males (Suppl. Fig. 1: R2=0.73 for females, 0.84 for males; F(2,90)=24.8, p<0.001). A resolution time constant (tau) was 3.1 days for females (95% CI: 2.3-4.1 days) and 1.0 day for males (95% CI: 0.8-1.2 days), suggesting a slower subsidence of capsaicin-induced thermal nociception in females.
Fig. 2. Sex difference in capsaicin-induced thermal and mechanical hypersensitivity.
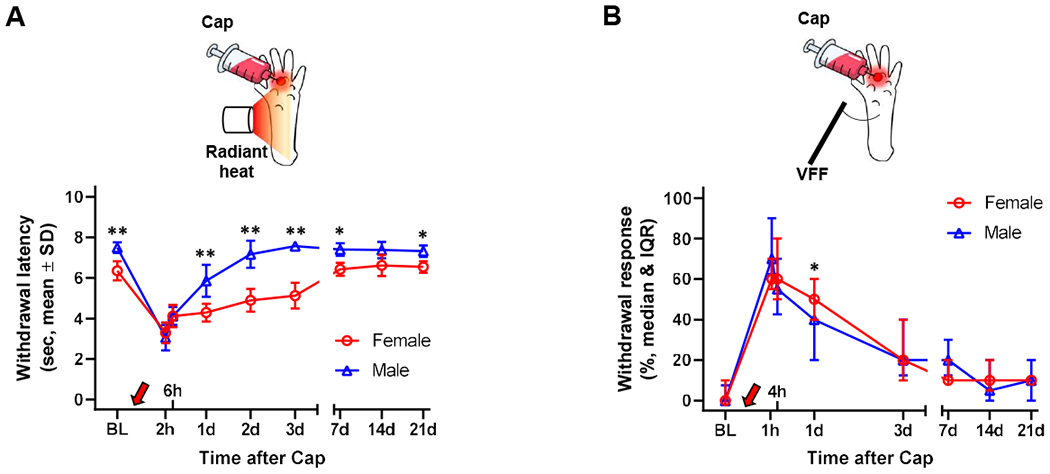
(A) Mice developed thermal hypersensitivity after intraplantar capsaicin (Cap, red arrow) injection, showing decreased latency to withdraw from radiant heat. Females (n=6) showed a slower recovery to the baseline (BL) level than males (n=8 until day 1, n=6 on day 2, n=4 in days 3-21). (B) Mice developed mechanical hypersensitivity after intraplantar Cap injection, showing increased hindpaw withdrawals from von Frey filament (VFF, 0.98 mN) probing of an area outside the Cap injection site, which gradually resolved in ~7 days. Female (n=9) showed greater mechanical hypersensitivity at 1 day post-capsaicin than males (n=8). *p<0.05, **p<0.01 between males and females by Sidak multiple comparison test.
We also noted differences in the resolution of capsaicin-induced mechanical hypersensitivity. While both sexes showed a similar degree of mechanical hypersensitivity at 1 hr post-capsaicin and a gradual decrease over the following 3 days, females manifested significantly greater mechanical hypersensitivity than males at 1 day post-capsaicin (Fig. 2B: t(105)=2.60 by sequential Sidak test, p=0.011).
We next determined whether the movement of the capsaicin-injected hindpaw for post-injury thermal stimulation itself would affect capsaicin-induced mechanical hypersensitivity in the area outside the capsaicin injection site. This ‘air immersion’ was done 2 hr after capsaicin injection. As shown in Supplemental Fig. 2A and B, the magnitude of mechanical hypersensitivity was not significantly different between the capsaicin alone and the ‘capsaicin plus air dip’ groups in each sex (Cap vs. Cap+air dip across the experimental time points: F(1,27)=0.33, p=0.57 in females; F(1,13)=0.051, p=0.82 in males by GLMM analysis). As the results indicated that the hindpaw immersing movement itself does not change capsaicin-induced mechanical hypersensitivity, we pooled the ‘capsaicin plus air immersion’ group with the ‘capsaicin alone’ group and regarded them as ‘capsaicin controls’ throughout this study.
3.2. Post-injury stimulation prolonged injury-induced mechanical hypersensitivity
Having determined that the hindpaw immersing movement itself does not affect capsaicin-induced mechanical hypersensitivity, we next assessed whether the hypersensitivity could be prolonged by stimulating the capsaicin-injected paw with 40°C water immersion (30 sec per min for 10 min at 2 hr after capsaicin; the von Frey testing site was not immersed). This temperature is normally innocuous but reported to cause discomfort in humans after development of capsaicin-induced thermal hypersensitivity [38]. While the 40°C stimulation of the vehicle-injected hindpaw did not induce mechanical hypersensitivity (data not shown), this thermal stimulation applied to the capsaicin-injected hindpaw significantly prolonged capsaicin-induced mechanical hypersensitivity (Fig. 3); capsaicin plus 40°C group showed greater mechanical hypersensitivity than capsaicin control in both sexes from day 1 (male) or day 3 (female) and on up to day 21 post-capsaicin. This prolonged mechanical hypersensitivity was no longer present by day 28 in both sexes (males: median=10%, IQR=0-10%, n=9; females: median=10%, IQR=0-17.5%, n=8). We next asked whether chronification of capsaicin-induced mechanical hypersensitivity would depend on the intensity of post-injury thermal stimulation. When the capsaicin-injected hindpaw was stimulated with 30°C water at 2 hr post-capsaicin, capsaicin-induced mechanical hypersensitivity was still significantly prolonged in females, but not in males. Based on the results that 40°C water immersion reliably prolongs capsaicin-induced mechanical hypersensitivity in both sexes, we chose this as the intensity of post-injury thermal stimulation for all future experiments. Of note, capsaicin-induced thermal hypersensitivity was not prolonged by the 40°C post-injury thermal stimulation; the latency to withdraw from radiant heat at 7-21 days post-capsaicin did not differ from the baseline values in both sexes (Fig. 4). Therefore, we focused our later studies on chronification of mechanical hypersensitivity.
Fig. 3. Chronification of capsaicin-induced mechanical hypersensitivity by post-injury thermal stimulation.
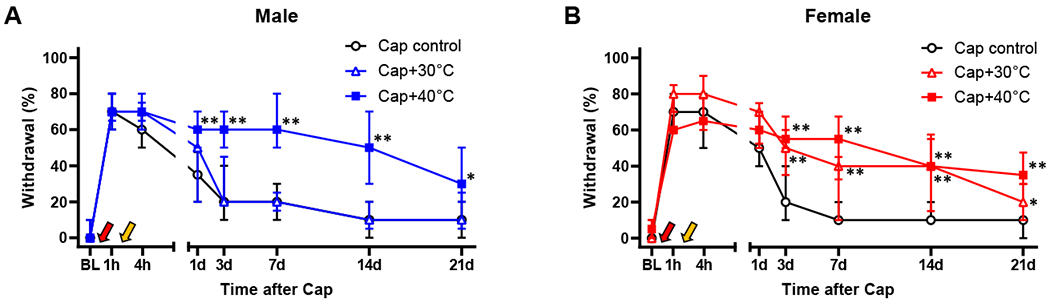
In both males (A) and females (B), stimulation of capsaicin (Cap, red arrow)-injected area by 40°C water (yellow arrow) at 2 hr post-capsaicin significantly prolonged capsaicin-induced mechanical hypersensitivity. However, when 30°C water was used instead of 40°C water, only females showed a significant chronification. Males: n=16 in Cap control, n=9 in Cap+30°C, and n=11 in Cap+40°C. Females: n=19 in Cap control, n=9 in Cap+30°C, and n=8 in Cap+40°C. *p<0.05, **p<0.01 vs. Cap control by sequential Sidak multiple comparison tests; as the same Cap control and Cap+40 °C (at 2h) groups were used for statistical comparisons in Fig. 5, differences between Cap control and all the other groups in Figs. 3 and 5 were analyzed in the same statistical test. BL, baseline.
Fig. 4. Chronification of capsaicin-induced thermal hypersensitivity does not occur by 40°C post-injury thermal stimulation.
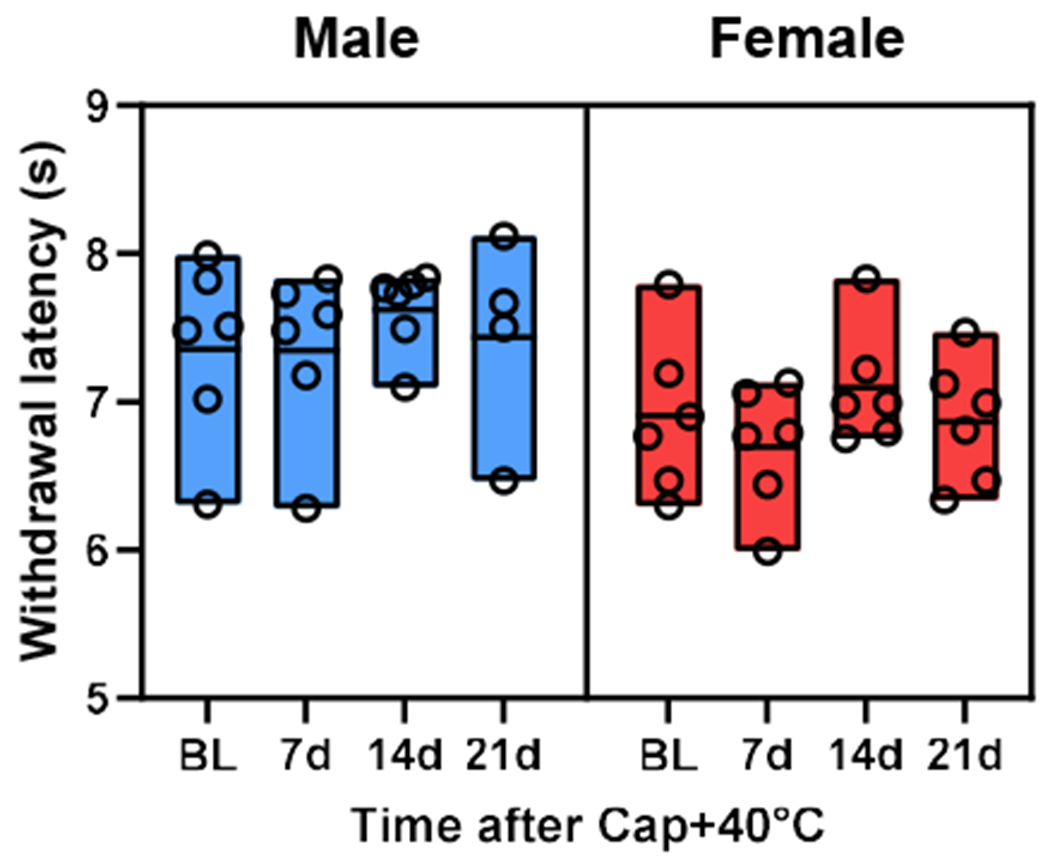
In both sexes, the heat sensitivity, measured as the latency to withdraw from radiant heat, did not differ between baseline (BL) and 7-21 days after the stimulation of capsaicin (Cap)-injected area with 40°C water at 2 hr post-capsaicin. The bottom, middle line, and top of each bar indicate the minimum, mean, and maximum values in each group, respectively. Males: n=6 until day 14 and n=4 at day 21. Females, n=6 throughout.
In our modeling approach, post-injury stimulation was used to increase pain after injury, based on clinical findings indicating that greater pain after surgery (i.e., surgery being an injury) increases the risk of chronification of post-surgical (i.e., post-injury) pain. Therefore, in our experimental design using capsaicin as an experimental injury, we hypothesized that the effect of post-injury 40°C stimulation, which serves to increase post-injury pain, will diminish as the injury-induced thermal hypersensitivity abates, consequently reducing the likelihood of mechanical hypersensitivity chronification. Thus, we next tested whether the 40°C post-injury stimulation would still effectively induce the chronification of mechanical hypersensitivity when the capsaicin-induced thermal hypersensitivity decreased to approximately 70% or 35% of the peak thermal hypersensitivity (100% at 2 hr post-capsaicin). Because the thermal hypersensitivity resolves differentially between sexes as shown in Supplemental Fig. 1, we chose sex-specific time points for the two abating phases: 6 hr vs. 1 day post-capsaicin for males and 1 day vs. 3 day post-capsaicin for females to represent the 70% vs. 35%, respectively based on curve fitting results. In mice that receive this ‘delayed’ 40°C stimulation, no difference vs. capsaicin control would be expected prior to the post-injury stimulation. When capsaicin-induced thermal hypersensitivity abated to approximately 70% (i.e., 6 hr in males and 1 day in females), the 40°C stimulation still significantly increased mechanical hypersensitivity at time points later than the time point of 40°C stimulation (Fig. 5A and B). When the thermal hypersensitivity subsided to approximately 35% (i.e., 1 day in males and 3 day in females), mechanical hypersensitivity was not prolonged in males (Fig. 5C); however, in females, the hypersensitivity was still significantly increased at time points later than day 3 (Fig. 5D; note that the post-injury stimulation was applied 30 min before the behavioral tests on day 3). These data suggest that the magnitude of capsaicin-induced thermal hypersensitivity at the time of post-injury thermal stimulation is predictive of mechanical hypersensitivity chronification and females have a wider timeframe than males, in which post-injury stimulation can trigger such chronification.
Fig. 5. Magnitude of capsaicin-induced thermal hypersensitivity at the time of post-injury thermal stimulation is predictive of mechanical hypersensitivity chronification.
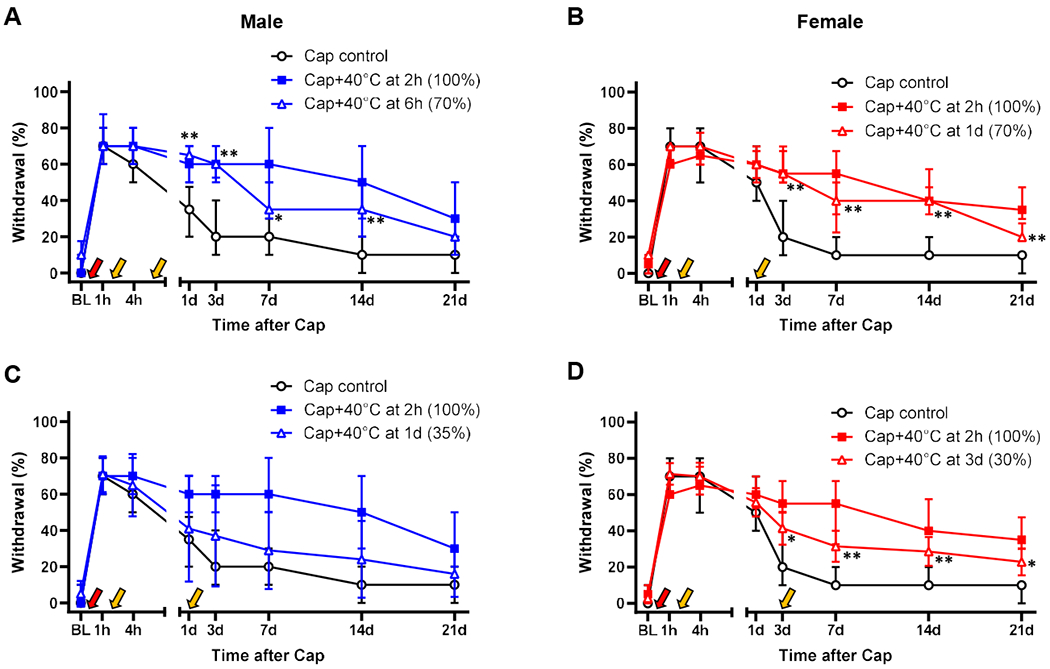
In both males (A) and females (B), mechanical hypersensitivity was significantly prolonged by the post-injury thermal stimulation (yellow arrow) when capsaicin (Cap, red arrow)-induced thermal hypersensitivity has abated to ~70% of the peak (6 hr in males and 1 day in females). However, when the thermal hypersensitivity decreased to ~35% of the peak (1 day in males and 3 day in females), the 40°C post-injury thermal stimulation did not produce chronification of capsaicin-induced mechanical hypersensitivity in males (C); females still showed significantly greater mechanical hypersensitivity at time points ≥ day 3 post-Cap (D). *p<0.05, **p<0.01 vs. Cap control by sequential Sidak multiple comparison tests. Males: n=16 in Cap control, n=11 in Cap+40°C at 2h, n=8 in Cap+40°C at 6h, and n=10 in Cap+40°C at 1d. Females: n=19 in Cap control, n=8 in Cap+40°C at 2h, n=8 in Cap+40°C at 1d, and n=7 in Cap+40°C at 3d. *p<0.05, **p<0.01 vs. Cap control by sequential Sidak multiple comparison tests; as the same Cap control and Cap+40 °C at 2h groups were used for statistical comparisons in Fig. 3, differences between Cap control and all the other groups in Figs. 3 and 5 were analyzed in the same statistical test. BL, baseline.
Next, we determined whether a different type of post-injury stimulation would also be able to prolong mechanical hypersensitivity. When vibration (92 Hz, 10 sec at 30 sec intervals over 10 min) was used instead of thermal stimulation at 2 hr post-capsaicin, mechanical hypersensitivity was effectively prolonged as shown in Fig. 6A & B. This result suggested that chronification of mechanical hypersensitivity may be induced by different modalities of post-injury stimulation.
Fig. 6. Confirmation of mechanical hypersensitivity chronification using different modeling paradigms.
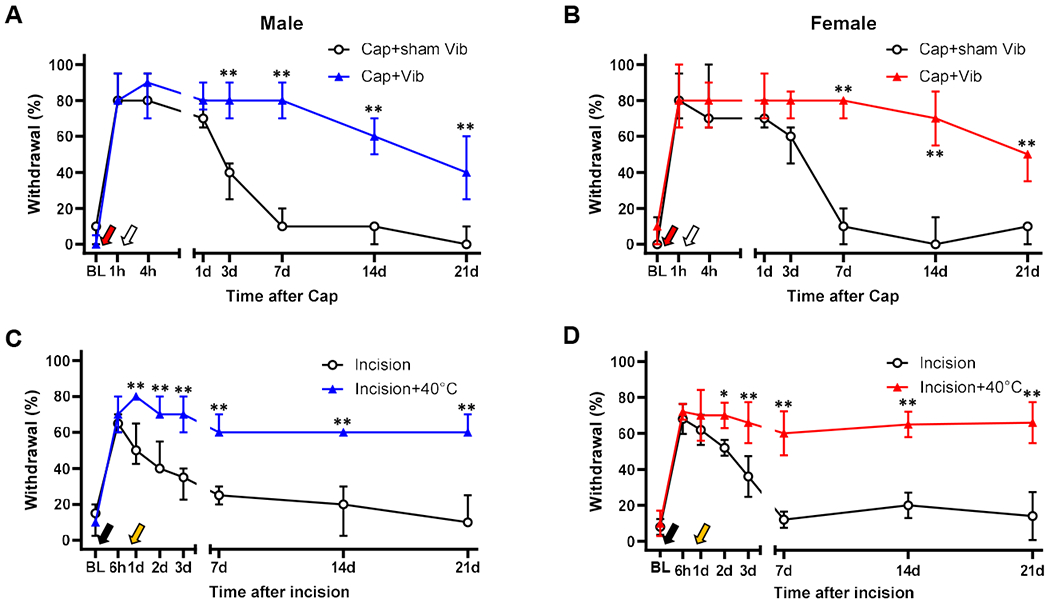
(A & B) When the capsaicin (Cap)-injected hindpaw was stimulated with vibration (92 Hz, 10 sec at a 30-sec interval for 10 min, white arrow) at 2 hr post-capsaicin, Cap-induced mechanical hypersensitivity was significantly prolonged in both sexes. The vibrator probe was placed on the paw but not turned on in Cap+sham Vib group. In both males and females, n=5 in each group. (C & D) When the hindpaw was immersed in 40°C water (30 sec/min for 10 min, yellow arrow) 23 hr after plantar incision, the incision-induced mechanical hypersensitivity was significantly prolonged in both sexes. Males: n=4 in incision and n=7 in incision+40°C. Females: n=5 in each group. **p<0.01 vs. corresponding control. BL, baseline. Vib, vibration.
We next investigated the potential limitation of the ability for post-injury thermal stimulation to prolong mechanical hypersensitivity in other types of injuries. In this experiment, we applied the 40°C thermal stimulation to an incision injury area at 23 hr post-incision (i.e., 1 hr before behavioral tests on day 1). We found that the incision-induced mechanical hypersensitivity was significantly prolonged as shown in Fig. 6C & D. This data indicated that mechanical hypersensitivity chronification by post-injury thermal stimulation is not restricted to the capsaicin model. Observing that these different modeling paradigms commonly demonstrate that post-injury stimulation prolongs post-injury mechanical hypersensitivity beyond the normal resolution time, we chose to use capsaicin injection followed by 40°C thermal post-injury stimulation at 2 hr (capsaicin plus 40°C) throughout the rest of this study.
3.3. No clear evidence of persistent inflammation at the previous injury area to account for the persistent mechanical hypersensitivity
Having found that post-injury stimulation of the capsaicin-injected paw at 2 hr post-capsaicin made the capsaicin-induced mechanical hypersensitivity persistent, we next examined whether this persistent hypersensitivity could be accounted for by persistent inflammation at the capsaicin-injected paw, as capsaicin produces neurogenic inflammation [16,29]. Visual examination of the footpad during our experiments did not reveal obvious tissue damage such as skin discoloration or edema. As inflammation may be present without overt abnormalities, we next used an Evans Blue extravasation assay to assess whether plasma extravasation, an indicator of inflammation, was augmented at the capsaicin injection area [32]. Two hours after capsaicin injection, plasma extravasation was significantly increased at the injection area compared to the corresponding contralateral area (Fig. 7: t(38)=6.66, p<0.001 in males; t(37)=8.43, p<0.001 in females by Bonferroni test for 9 pairwise comparisons). A similar increase was detected in the capsaicin plus 40°C group at 2 hr post-capsaicin (t(38)=6.73, p<0.001 in males; t(37)=7.52, p<0.001 in females). Plasma extravasation at the capsaicin injection area was still increased in both sexes at day 1 in capsaicin control (t(38)=4.09, p<0.005 in males; t(37)=6.45, p<0.001 in females ) and capsaicin plus 40°C groups (t(38)=3.82, p=0.004 in males; t(37)=7.62, p<0.001 in females ). However, at day 7, plasma extravasation was not significantly different from that of the corresponding contralateral area in all groups. These data suggested that capsaicin-induced local inflammation has resolved by day 7 in both capsaicin control and capsaicin plus 40°C group.
Fig. 7. No evidence of increased vascular leakage at the capsaicin-injected area 7 days after capsaicin plus 40°C post-injury thermal stimulation.
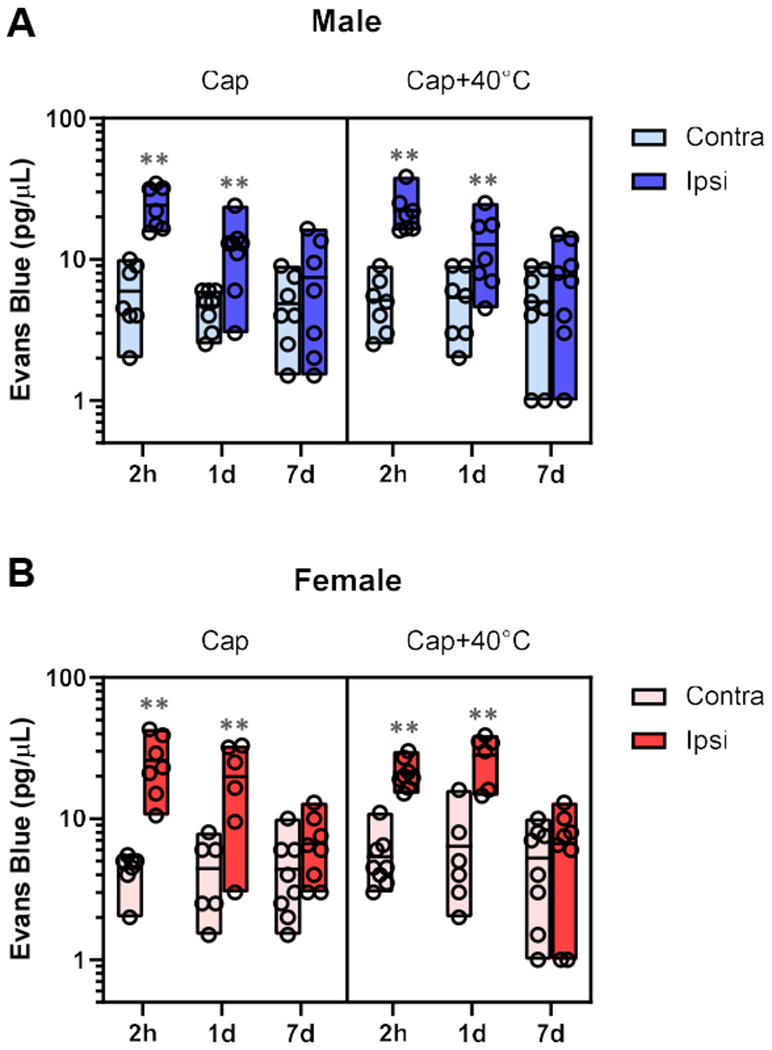
In both males (A) and females (B), Evans Blue extravasation, an indicator of vascular leakage associated with tissue inflammation, was significantly greater in the capsaicin (Cap)-injected area than in the corresponding contralateral area at 2 hr and 1 day post-capsaicin. However, such increased vascular leakage was not detected 7 days after Cap or Cap plus 40°C post-injury stimulation (Cap+40°C). **p<0.01 between paired paw sides by Bonferroni tests for 9 pairwise comparisons. The bottom, middle line, and top of each bar indicate the minimum, mean, and maximum values in each group, respectively. Males: at 2 hr, n=7 in Cap and in Cap+40°C; at 24 hr, n= 8 in Cap and n=7 in Cap+40°C; at day 7, n=7 in Cap and n=8 in Cap+40°C. Females: at 2hr, n=7 in Cap and n=8 in Cap+40°C; at 24hr, n=6 in Cap and Cap+40°C; at day 7, n=8 in Cap and Cap+40°C.
In addition, we quantified proinflammatory cytokine gene transcripts at the capsaicin injection area. As shown in Fig. 8, tissue contents of IL-1β mRNA in the capsaicin injection area were greater in the capsaicin control (t(18.5)=4.20, p=0.003 in males; t(16.7)=2.84, p=0.07 in females by Bonferroni test for 6 pairwise comparisons) and capsaicin plus 40°C group (t(7.8)=4.48, p=0.017 in males; t(6.43)=5.0, p=0.015 in females) than in corresponding contralateral areas at 1 day post-capsaicin. At this time point, gene expression of other proinflammatory cytokines were also elevated in the capsaicin injected area. TNF-α mRNA level was high in male capsaicin control (t(26)=2.77, p=0.06) and female capsaicin plus 40°C groups (t(10.3)=5.15, p=0.003). Of note, statistically significant increases in the quantities of IL-6 (t(23.9)=4.44, p=0.001) and TNF-α mRNAs (t(23.7)=3.82, p=0.005) were detected only in female capsaicin plus 40°C group, compared with their capsaicin control counterparts.
Fig. 8. No evidence of increased gene expression of proinflammatory cytokines at the capsaicin-injected area 7 days after capsaicin plus 40°C post-injury thermal stimulation.
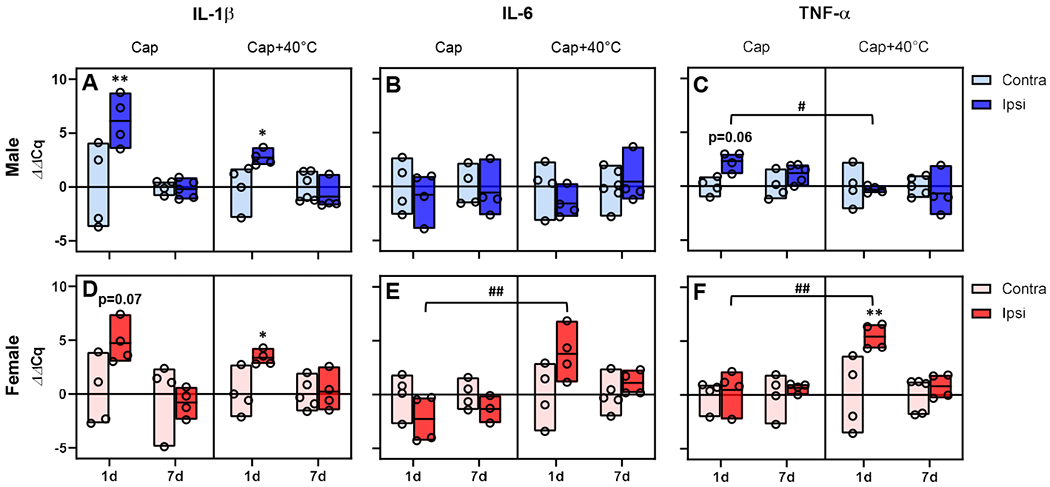
In both males (A) and females (D), the quantity of IL-1β gene transcript was greater in capsaicin (Cap)-injected area (Ipsi) than in the contralateral counterparts (Contra) at 24 hr post-capsaicin. However, such upregulation was not detected 7 days after Cap or Cap plus 40°C post-injury stimulation (Cap+40°C). On day 7, the gene expression of IL-6 (B & E) and TNF-α (C & F) also did not differ between Contra- and ipsi-lateral sides. Of note, the TNF-α gene expression in the ipsilateral side (C) was significantly lower at 24 hr post-capsaicin in male Cap+40°C group, compared with that in Cap control. In female Cap+40°C group, IL-6 (E) and TNF-α (F) mRNAs were significantly increased in the ipsilateral side at 24 hr post-capsaicin, compared with those in female Cap control. *p<0.05 and **p<0.01 between paw sides; #p<0.05 and ##p<0.01 between Cap and Cap+40°C groups in the ipsilateral side by Bonferroni tests for 6 pairwise comparisons. The bottom, middle line, and top of each bar indicate the minimum, mean, and maximum values in each group, respectively. Males: in IL-1β, n=4 in contra and n=5 in ipsi for Cap; n=6 in contra and n=4 in ipsi for Cap+40°C; in IL-6, n=4 in contra and n=5 in ipsi for Cap; n=5 in contra and n=4 in ipsi for Cap+40°C; for TNF-α, n=4 in contra and n=5 in ipsi for Cap; n=5 in contra and n=4 in ipsi for Cap+40°C. Females: in IL-1β, n=4 in both sides for Cap; n=5 in contra and n=4 in ipsi for Cap+40°C; in IL-6, n=4 in both sides for Cap; n=5 in contra and n=4 in ipsi for Cap+40°C; for TNF-α, n=4 in both sides for Cap; n=5 in contra and n=4 in ipsi for Cap+40°C.
In line with the above plasma extravasation results, the quantities of these mRNAs in the previously capsaicin-injected area 7 days post-capsaicin did not differ either between the ipsi- and contra-lateral sides or between the capsaicin control and capsaicin plus 40°C group. Taken together, these data show that persistent mechanical hypersensitivity in the capsaicin plus 40°C group arises despite no clear evidence of ongoing inflammation at the previous injury site, suggesting that this animal model is in the nociplastic pain state. Therefore, henceforth, we called this model a ‘nociplastic pain’ model.
3.4. Persistent mechanical hypersensitivity in our nociplastic pain model was alleviated by pain medications
Human subjects who receive intradermal capsaicin injection report increased pain to mechanical stimulation of areas around the injection site [27,28,30]. Capsaicin-induced mechanical hypersensitivity in animals is also regarded as increased mechanical nociception. In fact, this hypersensitivity in animals is effectively alleviated by known pain medications such as morphine and gabapentin [18]. Thus, we determined whether the same pain medications would inhibit the prolonged capsaicin-induced mechanical hypersensitivity in our model. As shown in Fig. 9, both morphine and gabapentin immediately and robustly inhibited the persistent mechanical hypersensitivity present at days 7-10 post-capsaicin in both males and females. These results also indicated the nociceptive quality of the observed persistent mechanical hypersensitivity in our nociplastic pain model.
Fig. 9. Alleviation of persistent mechanical hypersensitivity by pain medications.
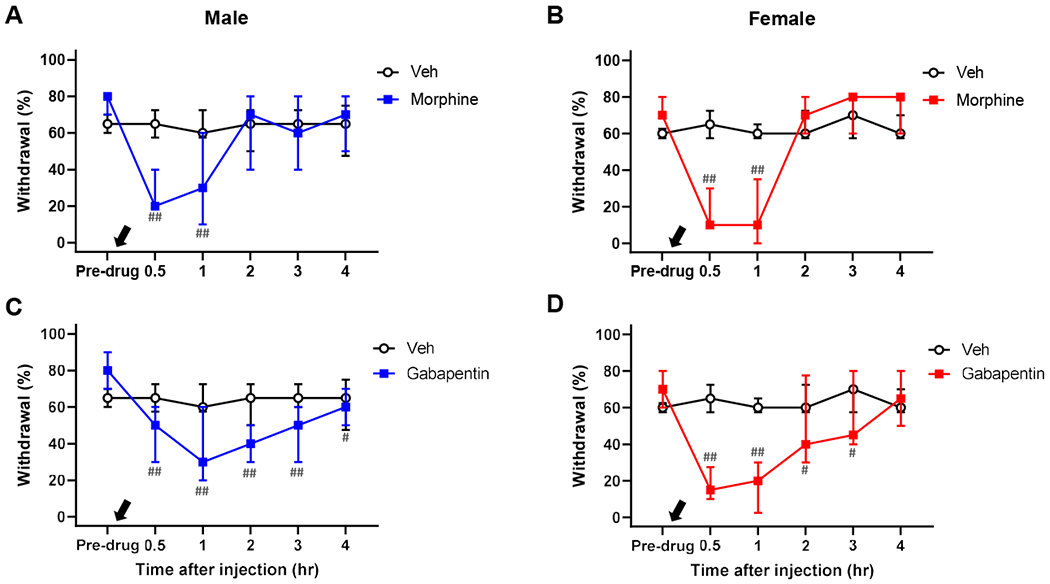
In both males and females, morphine (5 mg/kg, black arrow, A & B) and gabapentin (100 mg/kg, black arrow, C & D) significantly alleviated the persistent mechanical hypersensitivity at 7-10 days after stimulating the capsaicin-injected area with 40°C water. Males: n=6 in vehicle (Veh), n=7 in morphine, and n=7 in gabapentin. Females: n=6 in Veh, n=5 in morphine, and n=8 in gabapentin. #p<0.05, ##p<0.01 vs. pre-drug level in each group by sequential Sidak multiple comparison tests.
3.5. Persistent mechanical hypersensitivity in females was maintained by ongoing afferent activity at the previous injury site
Having determined that our nociplastic pain model manifests persistent mechanical hypersensitivity in the absence of ongoing inflammation in both males and females, we further tested whether this hypersensitivity outside the capsaicin injection area was maintained by ongoing afferent activity at the capsaicin injection (i.e., previous injury) area. It was reported that activity of peripheral afferents at the previously injured area in CRPS patients was critical for maintaining chronic mechanical allodynia remote from the injured area [10]. To determine whether persistent mechanical hypersensitivity was similarly maintained by such afferent activity in our model, we locally injected bupivacaine at the previously capsaicin-injected area 7-10 days post-capsaicin to silence afferents innervating the area. As shown in Fig. 10, bupivacaine treatment significantly attenuated persistent mechanical hypersensitivity outside of the treatment area in females but not in males. These data indicate the involvement of sexually dimorphic mechanisms in our nociplastic pain model; specifically, that ongoing activity of afferents innervating the previous injury site plays a critical role in maintaining the nociceptive system sensitization underlying persistent mechanical hypersensitivity outside the injury area in females but not in males.
Fig. 10. Female-specific alleviation of persistent mechanical hypersensitivity by local anesthesia of previously capsaicin-injected area.
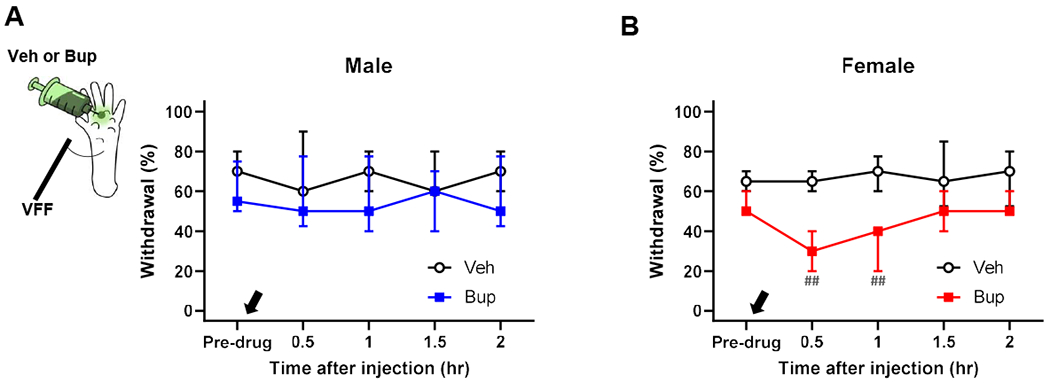
While a local injection of bupivacaine (Bup, 0.75%, black arrow) at the previous injury (i.e., capsaicin injection) area had no effect on persistent mechanical hypersensitivity outside the previous injury area in males (A), it significantly alleviated the hypersensitivity in females (B) at 7-10 days after stimulating the capsaicin-injected area with 40°C water. Males: n=7 in vehicle (Veh) and n=8 in Bup. Females: n=8 in Veh and n=7 in Bup. ##p<0.01 vs. pre-drug level in each group by sequential Sidak multiple comparison tests. VFF, von Frey filament.
3.6. Persistent mechanical hypersensitivity in males is maintained by activated microglia in the spinal cord
Recent studies revealed that activated microglia play a male-specific role in other chronic pain models [31,49,51]. To obtain insights into the maintenance mechanism of the persistent mechanical hypersensitivity in the male nociplastic pain model, we next investigated the role of microglia in the spinal cord. To this end, we determined the effect of Mac-1-saporin, a microglia targeting toxin, on the persistent mechanical hypersensitivity 7-10 days post-capsaicin. We observed that Mac-1-saporin treatment significantly attenuated persistent mechanical hypersensitivity in males, but not in females (Fig. 11A & B). We immunostained spinal cord samples from these mice and quantified the immunoreactivity of Iba1, a protein upregulated in activated microglia [15]. As shown in Fig. 11C & D, in the male nociplastic pain model treated with control saporin, Iba1-immunoreactivity in the ipsilateral dorsal horn was greater than in the paired contralateral side (t(10)=6.45, p<0.001 by Bonferroni test for 4 pairwise comparisons), but not in the female model. Such a difference in Iba1-immunoreactivity between ipsi- and contra-lateral dorsal horns were not detected in the nociplastic pain models treated with Mac-1-saporin, suggesting the effectiveness of Mac-1-saporin treatment on microglial inhibition in our experiments. Of note, the dose of Mac-1-saporin used in this experiment appeared to ‘normalize’ the upregulated Iba1 expression rather than to inhibit the expression below the control level. Together, these data indicate that in males, but not in females, our nociplastic pain model leads to activation of spinal microglia, which maintain the persistent mechanical hypersensitivity.
Fig. 11. Male-specific alleviation of persistent mechanical hypersensitivity by inhibition of spinal microglia.
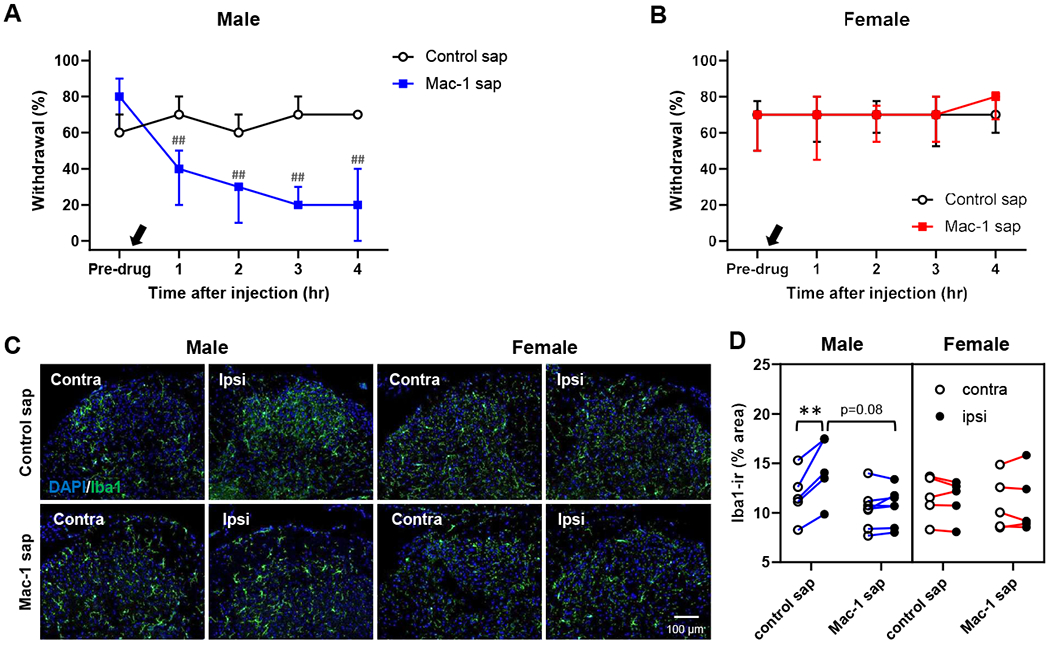
An intrathecal injection of the microglia-targeting toxin Mac-1-saporin (Mac-1-sap, 8.85 μM, black arrow) significantly alleviated persistent mechanical hypersensitivity in males (A) but not in females (B) at 7-10 days after stimulating the capsaicin-injected area with 40°C water. ##p<0.01 vs. pre-drug level in each group by sequential Sidak multiple comparison tests. C & D: The immunoreactivity of Iba1, a marker of activated microglia, was significantly higher in the ipsilateral side in the male nociplastic pain model treated with unconjugated saporin (control sap); the increased Iba1 immunoreactivity (Iba1-ir) in the male nociplastic pain model was effectively decreased by intrathecal Mac-1-sap. No difference was detected between either dorsal horn sides or treatment groups in the female nociplastic pain model. Males: n=7 in each group. Females: n=8 in control sap and n=9 in Mac-1-sap. **p<0.01 by Bonferroni tests for 4 pairwise comparisons in each sex.
4. Discussion
In the present study, we introduce a novel mouse model to facilitate elucidation of mechanisms for the transition to and maintenance of a nociplastic pain state. In this model, post-injury stimulation (40°C water or vibration) was applied to the injured area created by either intraplantar capsaicin injection or plantar incision to trigger the transition from normally resolving pain to persistent nociplastic pain. Using these experimental paradigms, we significantly prolonged the injury-induced mechanical hypersensitivity, modeling pain chronification and thus providing a platform for understanding its mechanisms. Importantly, we did not detect clear evidence of ongoing tissue damage (inflammation) accounting for this persistent mechanical hypersensitivity. Hence, the phenotypes of our model reflect the transition to a nociplastic pain state as opposed to a persistent nociceptive pain state due to chronic inflammation.
Similar to the hyperalgesic priming (type I) model that has been used for studying mechanisms of pain chronification [39], we also used a paradigm of an initial injury (to ’prime/sensitize’ the nociceptive system) followed by a post-injury stimulus to establish pain chronification. However, our model differs from the hyperalgesic priming model in multiple aspects. First, in our nociplastic pain model, sensitization of the nociceptive system by an acute injury ’transitions’ to a persistent state (as a continuum) by post-injury stimulation, as the post-injury stimulation must be given before the initial injury-induced hypersensitivity substantially abates to ensure the transition to the nociplastic pain state. By contrast, in the hyperalgesic priming model, chronification is ’precipitated’ by the post-injury stimulation (most commonly an injection of inflammatory mediators) given after the initial injury-induced hypersensitivity completely resolves. Additionally, while the injury area is probed for detecting mechanical hypersensitivity in the hyperalgesic priming model (i.e., mainly focusing on peripheral nociceptor sensitization), areas outside the initial injury are probed in our nociplastic pain model. This approach makes it possible to infer the involvement of central sensitization in nociplastic pain as central sensitization mediates such ‘secondary mechanical hypersensitivity’. We acknowledge, however, that definitive evidence of a lack of peripheral sensitization in the probed area is necessary to attribute the persistent mechanical hypersensitivity in our model solely to central sensitization. With these similar and dissimilar features to hyperalgesic priming models, our nociplastic pain model provides novel and complementary systems to investigate the mechanisms of pain chronification.
One of the interesting behavioral phenotypes of this nociplastic pain model (capsaicin plus 40°C group) is that only mechanical, not thermal, hypersensitivity can be made persistent by the post-injury stimulation. This finding suggests that pain hypersensitivity in the nociplastic pain state may not be due to a generalized sensitization of the nociceptive system. Although an original injury can cause such generalized sensitization, as indicated by the observation that capsaicin induces both mechanical and thermal hypersensitivity, chronification of pain hypersensitivity seems to occur rather specifically at nociceptive circuits of a given sensory modality. This notion is consistent with the fact that chronic pain patients can be stratified by their sensory profiles (e.g., mechanical hyperalgesia- vs. thermal hyperalgesia-predominant patient groups) [1,53]. In this regard, it would be an interesting question whether the nature of post-injury stimulation would be a factor for such a ‘circuit-specific’ chronification of nociceptive system sensitization. In this study, we found both warmth and vibration similarly yielded chronification of capsaicin-induced mechanical hypersensitivity. Therefore, at least in the capsaicin model, it could be that the two different stimulation modalities commonly activate polymodal afferents (sensitized by the initial injury) to persistently sensitize central circuits for mechanical nociception. Alternatively, two different (modality-wise) peripheral afferent pathways may converge on the same central targets (sensitized by the initial injury) that drive persistent sensitization of mechanical nociceptive circuits. Interestingly, the absence of persistent thermal hypersensitivity in our nociplastic pain model corresponds to the absence of local inflammation in the affected hindpaw. If there were persistent inflammation, this model would likely show persistent thermal hypersensitivity as do other inflammatory pain models [42]. It should be noted that in this study we did not measure thermal hypersensitivity in mice treated with a different type of initial injury (i.e., plantar incision) or post-injury stimulation (i.e., vibration). Thus, it remains to be investigated whether these two additional models would also manifest a circuit-specific chronification of nociceptive system sensitization.
Clinically, women are disproportionately affected by nociplastic pain [35]. We found that females are more susceptible than males to pain chronification in at least two ways. First, pain chronification can be triggered by relatively lower intensity of post-injury stimulation in females than in males, as 30°C thermal stimulation post-capsaicin prolonged the capsaicin-induced mechanical hypersensitivity only in female mice. Second, compared with males, females have a wider timeframe in which post-injury stimulation can trigger pain chronification. Therefore, if any post-injury events stimulating an injured area occur after a while at low intensity, more females than males are likely to develop a nociplastic pain condition. Our nociplastic pain model is expected to be a valuable tool to further understand the mechanisms underlying these sex differences in the stimulus responsiveness and resolution of sensitized nociceptive system.
In addition to the abovementioned sex differences, we have identified sexually dimorphic mechanisms maintaining the nociplastic pain state. We found that silencing afferents innervating the previous capsaicin injection area significantly attenuated persistent mechanical hypersensitivity outside the injection site only in females. This ‘peripherally-maintained’ mechanical hypersensitivity observed in our model is reminiscent of the clinical report on 4 Complex Regional Pain Syndrome cases (3 women and 1 man) whereby local anesthesia of previous injury sites abolished chronic mechanical allodynia in areas remote from the injury sites [10]. It remains to be identified how and what afferents are persistently active at the previous injury site to mediate the maintenance of the nociplastic pain state in females. With respect to this, it is noteworthy that 1 day after capsaicin injection, the amounts of IL-6 and TNF-α mRNAs in the injection area were significantly greater in female capsaicin plus post-injury thermal stimulation group than in capsaicin controls. Considering that peripheral injection of IL-6 or TNF-α induces hyperalgesic priming in nociceptors [7,13,40], it could be that these cytokines (elevated by post-injury thermal stimulation only in females) prime or sensitize nociceptors innervating the capsaicin injection site by mechanisms similar to hyperalgesic priming. As to the identity of such sensitized nociceptors maintaining the nociplastic pain state in female mice, they likely belong to afferent populations not critical for cutaneous heat nociception in the mouse because thermal sensitivity was normal in this nociplastic pain model. Additionally, as female sex hormones, notably estrogen, are implicated as mechanistically important in hyperalgesic priming [20], future studies are warranted to investigate the hormone’s potential role in the ’peripherally maintained’ nociplastic pain state.
Unlike in females, persistent mechanical hypersensitivity in males is ’centrally maintained’ by activated spinal microglia. This finding corroborates previous studies using chronic neuropathic pain models, in which microglia mediates pain in males [8,23], but not in females [31,49]. However, those neuropathic pain models and our nociplastic pain model differ in the upregulation of microglial activation markers in ‘females’. In chronic neuropathic pain models, microglia in the spinal dorsal horn of female mice are also activated as determined by the upregulation of microglial markers such as Iba1 [49]. However, in females, activated microglia appear not to contribute functionally to neuropathic pain per se. In our female nociplastic pain model, by contrast, microglial activation was not detected, suggesting that spinal microglia in female mice may respond differently in neuropathic and nociplastic pain conditions. Conversely, it will be interesting to determine whether detailed cellular properties of spinal microglia in male animals are similar in these two different persistent pain conditions.
Spinal microglia can be activated by factors released by afferent activity [4,5,14,45]. For example, a brief intense electrical stimulation of C-fibers, but not Aβ/δ-fibers, led to the activation of microglia in the spinal dorsal horn of male rats, inducing mechanical hypersensitivity which lasted longer than that produced by intraplantar capsaicin injection [12]. These observations indicate the possibility that, in our male nociplastic pain model, peripheral sensitization by capsaicin injection allows the sensitized C-fibers to directly activate spinal microglia in response to post-injury stimulation at ‘normally innocuous’ intensity. Alternatively, central sensitization induced by the capsaicin injection may prime male spinal microglia to be readily activated by Aβ/δ-fiber inputs generated by post-injury stimulation.
In conclusion, we have developed a novel mouse model that meets the criteria of IASP’s nociplastic pain definition: persistent pain arising from altered nociception despite no clear evidence of actual or threatened tissue damage. This model recapitulates that severe pain after injury and female sex are risk factors for pain chronification, suggesting the significance of intensive pain management after an injury for the prevention of chronic nociplastic pain development. We expect that this model will be a useful tool for providing mechanistic insight to the transition to and maintenance of the nociplastic pain state and will assist in the development of therapeutic interventions for both male and female nociplastic pain syndromes.
Supplementary Material
Acknowledgments:
We thank Dr. Heidi Spratt for statistical consultation and the UTMB Optical Microscopy Core for use of their facilities. This study was supported by NIH R01 NS112344 and NS112344-02S1 Supplement to Promote Diversity (JHL), R01 DA050530 (SJT and JMC), F99 NS120636 (KEM), and the Jeane B. Kempner Predoctoral Fellowship (KMH).
Footnotes
Conflict of interest statement: The authors declare no competing financial interests.
References
- [1].Baron R, Maier C, Attal N, Binder A, Bouhassira D, Cruccu G, Finnerup NB, Haanpää M, Hansson P, Hüllemann P, Jensen TS, Freynhagen R, Kennedy JD, Magerl W, Mainka T, Reimer M, Rice ASC, Segerdahl M, Serra J, Sindrup S, Sommer C, Tölle T, Vollert J, Treede R-D. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. Pain 2017;158:261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Baumann TK, Simone DA, Shain CN, LaMotte RH. Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol 1991;66:212–227. [DOI] [PubMed] [Google Scholar]
- [3].Cain DM, Khasabov SG, Simone DA. Response properties of mechanoreceptors and nociceptors in mouse glabrous skin: an in vivo study. J Neurophysiol 2001;85:1561–1574. [DOI] [PubMed] [Google Scholar]
- [4].Calvo M, Zhu N, Grist J, Ma Z, Loeb JA, Bennett DLH. Following nerve injury neuregulin-1 drives microglial proliferation and neuropathic pain via the MEK/ERK pathway. Glia 2011;59:554–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Clark AK, Malcangio M. Fractalkine/CX3CR1 signaling during neuropathic pain. Front Cell Neurosci 2014;8. doi: 10.3389/fncel.2014.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Complex Regional Pain Syndrome Fact Sheet. 2020. Available: https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Complex-Regional-Pain-Syndrome-Fact-Sheet. Accessed 3 May 2021.
- [7].Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience 2008;152:521–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Echeverry S, Shi XQ, Yang M, Huang H, Wu Y, Lorenzo L-E, Perez-Sanchez J, Bonin RP, De Koninck Y, Zhang J. Spinal microglia are required for long-term maintenance of neuropathic pain. PAIN 2017;158:1792. [DOI] [PubMed] [Google Scholar]
- [9].Gazerani P, Andersen OK, Arendt-Nielsen L. A human experimental capsaicin model for trigeminal sensitization. Gender-specific differences. Pain 2005;118:155–163. [DOI] [PubMed] [Google Scholar]
- [10].Gracely RH, Lynch SA, Bennett GJ. Painful neuropathy: altered central processing maintained dynamically by peripheral input. Pain 1992;51:175–194. [DOI] [PubMed] [Google Scholar]
- [11].Hankerd KM, La J-H, Chung JM. Female-Specific Mechanisms of Central Sensitization Underlying Chronic Pain. Program No. 218.23. 2019 Neuroscence Meeting Planner. Chicago, IL, 2019. [Google Scholar]
- [12].Hathway GJ, Vega-Avelaira D, Moss A, Ingram R, Fitzgerald M. Brief, low frequency stimulation of rat peripheral C-fibres evokes prolonged microglial-induced central sensitization in adults but not in neonates. PAIN® 2009;144:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hendrich J, Alvarez P, Joseph EK, Chen X, Bogen O, Levine JD. Electrophysiological correlates of hyperalgesic priming in vitro and in vivo. Pain 2013;154:2207–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Inoue K, Tsuda M, Koizumi S. ATP receptors in pain sensation: Involvement of spinal microglia and P2X4 receptors. Purinergic Signal 2005;1:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Molecular Brain Research 1998;57:1–9. [DOI] [PubMed] [Google Scholar]
- [16].Jancsó N, Jancsó-Gábor A, Szolcsányi J. The role of sensory nerve endings in neurogenic inflammation induced in human skin and in the eye and paw of the rat. Br J Pharmacol Chemother 1968;33:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jiao J, Vincent A, Cha SS, Luedtke CA, Kim CH, Oh TH. Physical Trauma and Infection as Precipitating Factors in Patients with Fibromyalgia. Am J Phys Med Rehabil 2015;94:1075–1082. [DOI] [PubMed] [Google Scholar]
- [18].Joshi SK, Hernandez G, Mikusa JP, Zhu CZ, Zhong C, Salyers A, Wismer CT, Chandran P, Decker MW, Honore P. Comparison of antinociceptive actions of standard analgesics in attenuating capsaicin and nerve-injury-induced mechanical hypersensitivity. Neuroscience 2006;143:587–596. [DOI] [PubMed] [Google Scholar]
- [19].Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. The Lancet 2006;367:1618–1625. [DOI] [PubMed] [Google Scholar]
- [20].Khomula EV, Ferrari LF, Araldi D, Levine JD. Sexual Dimorphism in a Reciprocal Interaction of Ryanodine and IP3 Receptors in the Induction of Hyperalgesic Priming. J Neurosci 2017;37:2032–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kim HK, Schattschneider J, Lee I, Chung K, Baron R, Chung JM. Prolonged maintenance of capsaicin-induced hyperalgesia by brief daily vibration stimuli. Pain 2007;129:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kim HT, Park SK, Lee SE, Chung JM, Lee DH. Non-noxious A fiber afferent input enhances capsaicin-induced mechanical hyperalgesia in the rat. PAIN 2001;94:169–175. [DOI] [PubMed] [Google Scholar]
- [23].Kohno K, Kitano J, Kohro Y, Tozaki-Saitoh H, Inoue K, Tsuda M. Temporal Kinetics of Microgliosis in the Spinal Dorsal Horn after Peripheral Nerve Injury in Rodents. Biol Pharm Bull 2018;41:1096–1102. [DOI] [PubMed] [Google Scholar]
- [24].Kosek E, Cohen M, Baron R, Gebhart GF, Mico J-A, Rice ASC, Rief W, Sluka AK. Do we need a third mechanistic descriptor for chronic pain states? PAIN 2016;157:1382. [DOI] [PubMed] [Google Scholar]
- [25].La J-H, Feng B, Kaji K, Schwartz ES, Gebhart GF. Roles of isolectin B4-binding afferents in colorectal mechanical nociception. Pain 2016;157:348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].La J-H, Wang J, Bittar A, Shim HS, Bae C, Chung JM. Differential involvement of reactive oxygen species in a mouse model of capsaicin-induced secondary mechanical hyperalgesia and allodynia. Mol Pain 2017;13:1744806917713907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].LaMotte RH, Lundberg LE, Torebjörk HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. The Journal of Physiology 1992;448:749–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].LaMotte RH, Shain CN, Simone DA, Tsai EF. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. Journal of Neurophysiology 1991;66:190–211. [DOI] [PubMed] [Google Scholar]
- [29].Lin Q, Wu J, Willis WD. Dorsal root reflexes and cutaneous neurogenic inflammation after intradermal injection of capsaicin in rats. J Neurophysiol 1999;82:2602–2611. [DOI] [PubMed] [Google Scholar]
- [30].Magerl W, Fuchs PN, Meyer RA, Treede R-D. Roles of capsaicin-insensitive nociceptors in cutaneous pain and secondary hyperalgesia. Brain 2001;124:1754–1764. [DOI] [PubMed] [Google Scholar]
- [31].Mapplebeck JCS, Dalgarno R, Tu Y, Moriarty O, Beggs S, Kwok CHT, Halievski K, Assi S, Mogil JS, Trang T, Salter MW. Microglial P2X4R-evoked pain hypersensitivity is sexually dimorphic in rats. PAIN 2018;159:1752–1763. [DOI] [PubMed] [Google Scholar]
- [32].Martin Y, Avendaño C, Piedras MJ, Krzyzanowska A. Evaluation of Evans Blue extravasation as a measure of peripheral inflammation. Protocol Exchange 2010. doi: 10.1038/protex.2010.209. [DOI] [Google Scholar]
- [33].McCabe CS, Blake DR. An embarrassment of pain perceptions? Towards an understanding of and explanation for the clinical presentation of CRPS type 1. Rheumatology (Oxford) 2008;47:1612–1616. [DOI] [PubMed] [Google Scholar]
- [34].McDonough KE, Hankerd KM, La J-H, Chung JM. Maintenance Mechanism of Central Sensitization in Male Nociplastic Pain Model. Program No. 218.22. 2019 Neuroscience Meeting Planner. Chicago, IL, 2019. [Google Scholar]
- [35].Melchior M, Poisbeau P, Gaumond I, Marchand S. Insights into the mechanisms and the emergence of sex-differences in pain. Neuroscience 2016;338:63–80. [DOI] [PubMed] [Google Scholar]
- [36].Melzack R, Wall PD. Pain Mechanisms: A New Theory. Science 1965;150:971–979. [DOI] [PubMed] [Google Scholar]
- [37].Milenkovic N, Wetzel C, Moshourab R, Lewin GR. Speed and temperature dependences of mechanotransduction in afferent fibers recorded from the mouse saphenous nerve. J Neurophysiol 2008;100:2771–2783. [DOI] [PubMed] [Google Scholar]
- [38].Moulton EA, Pendse G, Morris S, Strassman A, Aiello-Lammens M, Becerra L, Borsook D. Capsaicin-induced thermal hyperalgesia and sensitization in the human trigeminal nociceptive pathway: an fMRI study. Neuroimage 2007;35:1586–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Parada CA, Reichling DB, Levine JD. Chronic hyperalgesic priming in the rat involves a novel interaction between cAMP and PKCepsilon second messenger pathways. Pain 2005;113:185–190. [DOI] [PubMed] [Google Scholar]
- [40].Parada CA, Yeh JJ, Joseph EK, Levine JD. Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. The European Journal of Neuroscience 2003;17:1847–1852. [DOI] [PubMed] [Google Scholar]
- [41].Ramakers C, Ruijter JM, Deprez RHL, Moorman AFM. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neuroscience Letters 2003;339:62–66. [DOI] [PubMed] [Google Scholar]
- [42].Ren K, Dubner R. Inflammatory Models of Pain and Hyperalgesia. ILAR journal 1999;40:111–118. [DOI] [PubMed] [Google Scholar]
- [43].Sandroni P, Benrud-Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study. PAIN® 2003;103:199–207. [DOI] [PubMed] [Google Scholar]
- [44].Sang CN, Gracely RH, Max MB, Bennett GJ. Capsaicin-evoked Mechanical Allodynia and Hyperalgesia Cross Nerve Territories Evidence for a Central Mechanism. Anesthesiology 1996;85:491–496. [DOI] [PubMed] [Google Scholar]
- [45].Sawada M, Suzumura A, Yamamoto H, Marunouchi T. Activation and proliferation of the isolated microglia by colony stimulating factor-1 and possible involvement of protein kinase C. Brain Research 1990;509:119–124. [DOI] [PubMed] [Google Scholar]
- [46].Schmelz M, Schmid R, Handwerker HO, Torebjörk HE. Encoding of burning pain from capsaicin-treated human skin in two categories of unmyelinated nerve fibres. Brain 2000;123:560–571. [DOI] [PubMed] [Google Scholar]
- [47].Simone DA, Sorkin LS, Oh U, Chung JM, Owens C, LaMotte RH, Willis WD. Neurogenic hyperalgesia: central neural correlates in responses of spinothalamic tract neurons. Journal of Neurophysiology 1991;66:228–246. [DOI] [PubMed] [Google Scholar]
- [48].Smith AK, O’Hara CL, Stucky CL. Mechanical sensitization of cutaneous sensory fibers in the spared nerve injury mouse model. Mol Pain 2013;9:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sorge RE, Mapplebeck JCS, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin J-S, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji R-R, Zhang J, Salter MW, Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nature Neuroscience 2015;18:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Steegers MAH, Snik DM, Verhagen AF, van der Drift MA, Wilder-Smith OHG. Only Half of the Chronic Pain After Thoracic Surgery Shows a Neuropathic Component. The Journal of Pain 2008;9:955–961. [DOI] [PubMed] [Google Scholar]
- [51].Taves S, Berta T, Liu D-L, Gan S, Chen G, Kim YH, Van de Ven T, Laufer S, Ji R-R. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: Sex-dependent microglial signaling in the spinal cord. Brain, Behavior, and Immunity 2016;55:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Torebjörk HE, Lundberg LE, LaMotte RH. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. The Journal of Physiology 1992;448:765–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Vollert J, Maier C, Attal N, Bennett DLH, Bouhassira D, Enax-Krumova EK, Finnerup NB, Freynhagen R, Gierthmühlen J, Haanpää M, Hansson P, Hüllemann P, Jensen TS, Magerl W, Ramirez JD, Rice ASC, Schuh-Hofer S, Segerdahl M, Serra J, Shillo PR, Sindrup S, Tesfaye S, Themistocleous AC, Tölle TR, Treede R-D, Baron R. Stratifying patients with peripheral neuropathic pain based on sensory profiles: algorithm and sample size recommendations. Pain 2017;158:1446–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best L-A, Granot M. Prediction of chronic post-operative pain: Pre-operative DNIC testing identifies patients at risk. PAIN 2008;138:22–28. [DOI] [PubMed] [Google Scholar]
- [55].Zhang F, Zhou H, Wilson BC, Shi J-S, Hong J-S, Gao H-M. Fluoxetine protects neurons against microglial activation-mediated neurotoxicity. Parkinsonism & Related Disorders 2012;18:S213–S217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


