Abstract
Objective:
To examine the association between the duration of HIV infection and the stage of cervical cancer in Lusaka, Zambia.
Methods:
This retrospective case-case study included 1583 cervical cancer patients from the Cancer Diseases Hospital in Lusaka, Zambia. A sub-population of HIV-positive patients with additional clinical HIV information was identified following linkage of cancer and HIV databases. Logistic regression models examined the relationship between HIV status and early-onset cervical cancer diagnosis, and between HIV infection duration and initial diagnosis of metastatic cervical cancer.
Results:
The study population had an average age of 49 years and 40.9% had an initial diagnosis of metastatic cancer. HIV-positive women were more than two times as likely to be diagnosed at early-onset cervical cancer compared to HIV-negative women. Among the sub-population of HIV-positive patients, a longer duration of HIV infection was associated with 20% lowered odds of initial metastatic cancer diagnosis.
Conclusion:
The availability, accessibility, and impact of the cervical screening program in this population should be further examined to elucidate the relationship between cervical screening, age and duration of HIV infection, and the stage of diagnosis of cervical cancer.
Keywords: age, cervical cancer, HIV, stage, Zambia
1. INTRODUCTION
Cervical cancer is the fourth most commonly diagnosed cancer and fourth leading cause of cancer death among women globally.[1] There were an estimated 570,000 incident cervical cancer cases and 311,000 deaths worldwide in 2018.[1–3] In developing countries, cervical cancer is the second most diagnosed and second leading cause of cancer mortality.[1, 4] In Zambia, the cervical cancer incidence rate of about 43.1 per 100,000 per year and mortality of 20.0 per 100,000 per year are among the highest in the world.[1, 3, 5] The human papillomavirus (HPV) is the main causal agent of cervical cancer. HPV is responsible for 99.7% of all cervical carcinomas with high-risk HPV types 16 and 18 accounting for 75% of all cases.[4] Both high-risk HPV oncogenic types 16 and 18 each have a prevalence of 21.6% in Zambia.[6]
Cervical cancer is one of three acquired immunodeficiency syndrome (AIDS)-defining cancers.[7] Co-infection with the human immunodeficiency virus (HIV) is a major risk factor for developing HPV-caused pre-cancerous lesions and the progression to invasive cervical carcinoma.[8, 9] Women with HIV are more than three times more likely to be diagnosed with cervical cancer.[7] Persistent infection with HPV is associated with advanced HIV infection characterized by a weakened immune system that results from reduction in the number of CD4 T-helper lymphocytes, a symptom of AIDS.[8, 10] In Zambia, there is an adult HIV prevalence of 16% and an estimated 21% of women of reproductive age (15 to 49 years) are living with HIV.[2, 3] Because of the association with AIDS, cervical cancer has been classified as an AIDS-defining illness by the Centers for Disease Control and Prevention since 1993.[11] For these reasons, cervical cancer in HIV-positive populations in low-resources settings, like Zambia, presents a major public health challenge regarding women’s health. Cervical cancer must not be allowed to continue to cause high mortality in women in low and middle-income countries.
Although the interplay between HPV and HIV infection is complex, their synergistic interaction in advancing the pathology of cancer has been well studied. There has been evidence that suggests that immunosuppression among women with HIV leads to more aggressive cervical cancers with advanced stage at presentation.[11, 12] The HIV care and treatment infrastructure has dramatically improved over the recent years in Zambia, in a large part, as the result of funding from the Global Fund for AIDS, TB, and Malaria and the US President’s Emergency Fund for AIDS Relief (PEPFAR).[12]Specifically, the Cervical Cancer Prevention Program in Zambia (CCPPZ) which initially targeted HIV-infected women for cervical cancer screening has expanded services to all women, regardless of HIV status.[13] Furthermore, through a low-cost vertical scale-up of the current HIV/AIDS care infrastructure with antiretroviral therapy (ARV) being available in the public sector since 2004, a public sector cervical cancer prevention program in Zambia was launched in 2006 to provide cervical cancer screening using visual inspection with acetic acid and with immediate treatment of pre-cancer lesions with cryotherapy or thermocoagulation.[5]
Our previous study, also conducted in Lusaka, documented the relationship between duration of HIV infection and cervical cancer progression among cervical cancer patients.[14] The purpose of this study was to assess the effect of HIV infection on age of initial cervical cancer diagnosis and to examine the effects of length of HIV infection on diagnosis of cervical cancer diagnosis at metastatic stages between 2008 and 2012 in Lusaka, Zambia.
2. METHODS
Data Collection and Linkage
This retrospective case-case study was conducted during the period of May 2017-August 2018 and included 2628 cervical cancer cases diagnosed at the Cancer Diseases Hospital (CDH) in Lusaka, Zambia between 2008 and 2012 which were identified in our previous study at the same hospital.[3] The data included HIV status, cervical cancer diagnosis information, cancer treatment information, and details of patient demographics. Cervical cancer cases with missing HIV status were excluded (n=1045). A total of 1583 cases was included in the final analysis (Figure 1).
Figure 1.
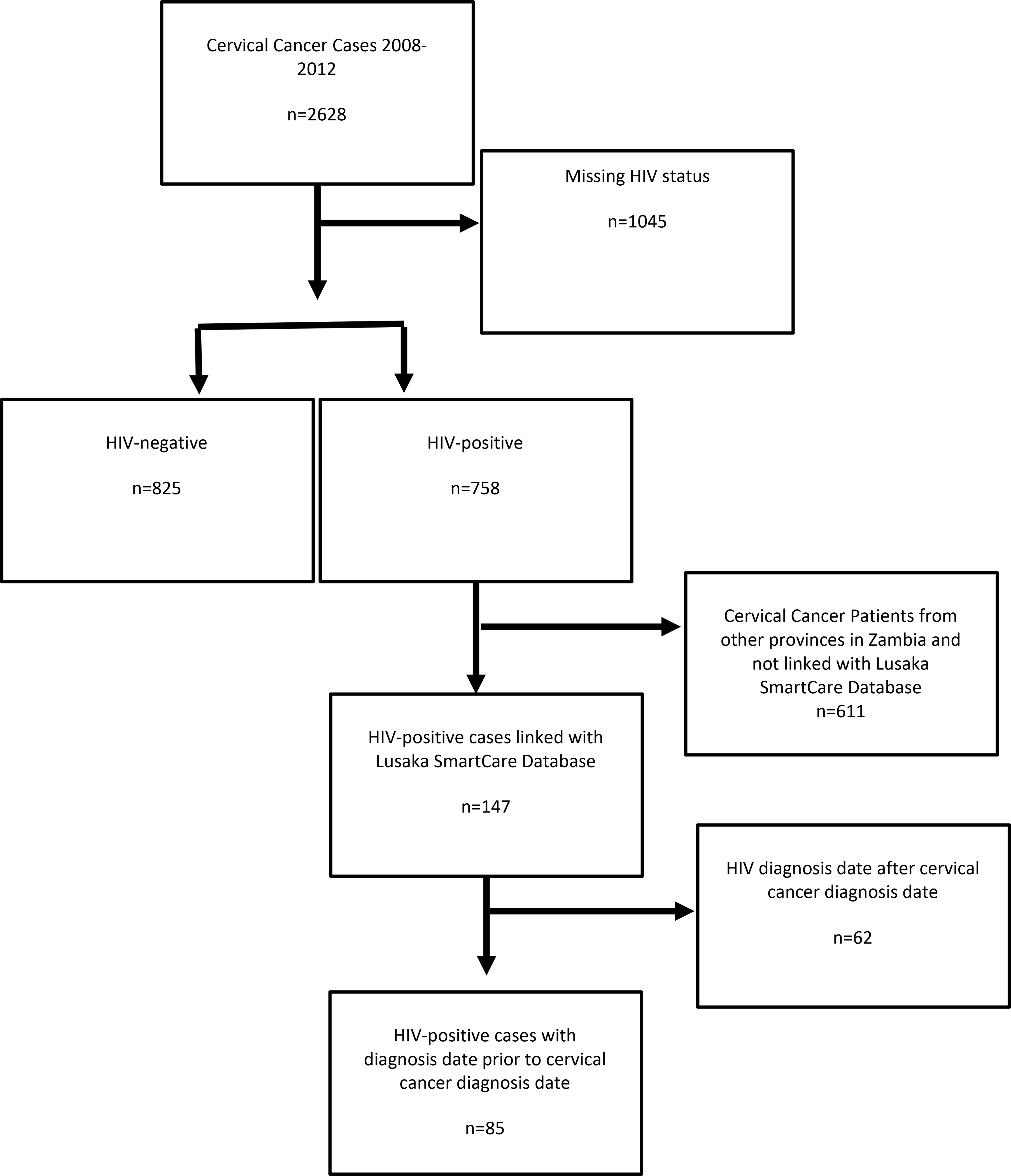
Flow Diagram for cervical cancer patients seen at the Cancer Diseases Hospital, Lusaka, Zambia.
To obtain HIV infection and treatment details, a linkage was conducted between the CDH cervical cancer cases and the SmartCare database, Zambia’s national electronic HIV database. Cases were matched on first name, last name, date of birth or age (allowing for 5 years older or younger), cancer diagnosis date, and National Registration Card number. Only data from Lusaka had been in SmartCare, therefore, only patients who were from Lusaka were linked.
Among the 758 cases that were HIV-positive, a subgroup of 147 cases were matched with SmartCare, and 85 of which were confirmed to have an HIV test date prior to cancer diagnosis date. Only cases with non-missing HIV status were included in the analysis to assess the relationship between length of HIV infection and metastatic cancer(Figure 1). All linkage and data abstraction from SmartCare were performed using Microsoft SQL Server Management Studio 17 (Redmond, WA).
Outcomes
Our primary outcomes of interest were metastatic cervical cancer, which was defined as FIGO stages III/IV (non-metastatic was defined as stages I/II), and age (40 years and younger vs over 40 years). Age was used as a binary variable because cervical cancer diagnosed younger than 40 are considered early-onset diagnosis. [15, 16]
Covariates and potential confounders
HIV status (positive, negative), baseline CD4 count (<200 cells/mL3, 200 to <500 cells/mL3, and ≥500 cells/mL3 ),[17, 18] metastatic cancer at initial visit (non-metastatic was defined as FIGO stages I or II, metastatic was defined as FIGO staged III or IV), age (dichotomized as either 40 and younger or greater than 40) at cervical cancer diagnosis, marital status (single, married, divorced, widowed), occupation (farmer, housewife, business, unemployed, other), and tribe (Bemba, Chewa, Lozi, Ngoni/lla, Nsenga, Tonga/Toka-Leya, Tumbuka, Other), length of HIV infection (number of years since first HIV-positive test date to cancer diagnosis date), age in years of the patient at start of antiretroviral therapy, length in years of antiretroviral therapy use. Covariates were included as confounders if they were independently associated with the outcome and the exposure with a significance threshold at P≤0.1, and if it produced a 10% or greater change in the beta estimate of the crude and adjusted models.
Statistical Analysis
Descriptive statistics were compared using Chi-Square, T-tests. Pearson correlation coefficient was used to assess the relationship between the outcomes and covariates as well as testing for multicollinearity. We used unadjusted and adjusted logistic regression models to assess the relationship between HIV status and cancer diagnosis at age 40 or younger.
Among HIV-positive women only, we assessed the relationship between duration of HIV infection and initial metastatic cervical cancer diagnosis. All analysis was conducted using a complete case scenario, removing cases with any missing variable information. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Ethics approval
The study was approved by the Institutional Review Board of the University of Colorado School of Public Health and the CDH in Zambia. Due the retrospective nature of the study, it was exempt from informed consent.
3. RESULTS
Participants
Descriptive statistics for 2628 (following removal of 74 with missing stage) cervical cancer cases by HIV status and summarized are in Table 1. There were 1045 (39.8%) cervical cancer cases that had missing HIV status, 758 (28.8%) were HIV-positive and 825 (31.4%) were HIV-negative. The overall average age at diagnosis was 49.3 years with a statistically significantly (P <0.001) older average age at diagnosis among HIV-negative women (52.6 years) compared to HIV-negative women (41.7 years). One thousand and seventy-five (41%) of all women were diagnosed with metastatic cancer (360 [47.5%] among HIV-positive women and 381 [46.2%] among HIV-negative women), 1,327 (50.5%) of the women with cervical cancer were married and 821 (31.2%) were unemployed.
TABLE 1.
Patient characteristics by HIV-status for cervical cancer cases at the Cancer Diseases Hospital from 2008–2012.
| N (%) | Total n=2628 |
Unknown HIV n=1045 (39.8) |
HIV-positive n=758 (28.8) |
HIV-negative n=825 (31.4) |
P valuea |
|---|---|---|---|---|---|
| Age at Diagnosis | - | - | - | < 0.001* | |
| Mean Age in Years (SD) | 49.3 (12.7) | 52.2 (13.2) | 41.7 (8.4) | 52.6 (12.3) | - |
| <40 years (%) | 771 (29.3) | 226 (29.3) | 392 (51.7) | 153 (18.6) | - |
| >40 years (%) | 1857 (70.7) | 819 (78.4) | 366 (48.2) | 672 (81.4) | - |
| Stage | - | - | - | 0.665 | |
| Non-metastatic (%) | 899 (34.2) | 239 (22.6) | 313 (41.3) | 347 (42.1) | - |
| Metastatic (%) | 1075 (40.9) | 334 (31.9) | 360 (47.5) | 381 (46.2) | - |
| Unknown (%) | 654 (24.9) | 472 (45.2) | 85 (11.2) | 97 (11.7) | - |
| Marital Status b | - | - | - | < 0.001* | |
| Single (%) | 581 (22.1) | 213 (20.4) | 201 (26.5) | 167 (20.2) | - |
| Married (%) | 1327 (50.5) | 445 (42.6) | 368 (48.5) | 514 (62.3) | - |
| Divorced (%) | 137 (5.2) | 37 (3.5) | 60 (7.9) | 40 (4.8) | - |
| Widowed (%) | 267 (10.2) | 77 (7.4) | 111 (14.6) | 79 (9.6) | - |
| Occupation c | - | < 0.001* | |||
| Farmer (%) | 442 (16.8) | 166 (15.9) | 82 (10.8) | 194 (23.5) | - |
| Housewife (%) | 130 (4.9) | 45 (4.3) | 40 (5.3) | 45 (5.5) | - |
| Business (%) | 197 (7.5) | 39 (3.7) | 116 (15.3) | 42 (5.1) | - |
| Unemployed (%) | 821 (31.2) | 272 (26.0) | 237 (31.3) | 312 (37.8) | - |
| Other (%) | 239 (9.1) | 52 (5.0) | 136 (17.9) | 51 (6.2) | - |
| Tribe d | - | - | - | 0.667 | |
| Bemba (%) | 371 (14.1) | 102 (9.8) | 127 (16.8) | 142 (17.2) | - |
| Chewa (%) | 172 (6.5) | 47 (4.5) | 61 (8.0) | 64 (7.8) | - |
| Lozi (%) | 105 (4.0) | 32 (3.1) | 42 (5.5) | 31 (3.8) | - |
| Ngoni/Ila (%) | 93 (3.5) | 23 (2.2) | 37 (4.9) | 33 (4.0) | - |
| Nsenga (%) | 135 (5.1) | 31 (3.0) | 51 (6.7) | 53 (6.4) | - |
| Tonga/Toka (%) | 236 (9.0) | 83 (7.9) | 66 (8.7) | 87 (10.5) | - |
| Tumbuka (%) | 104 (4.0) | 39 (3.7) | 32 (4.2) | 33 (4.0) | - |
| Other (%) | 520 (19.8) | 176 (16.8) | 164 (21.6) | 180 (21.8) | - |
Statistically significant at ≤0.05
Note: All percentages are column percentages.
P values for t-tests and chi-square tests comparing HIV-positive and HIV-negative individuals.
12% of values missing
30% of values missing
25% of values missing
Among the cervical cancer cases with unknown HIV status, 472 (45.2%) were also missing cancer staging data (Table 1). Six hundred and fifty four (24.9%) of cervical cancer cases had missing cancer staging, of which, 472 (72.2%) were also missing HIV status. Furthermore, the proportion of those missing cancer staging data that had a known HIV status, there seem to be an even distribution of cases that are HIV-positive (85 [13.0%]) compared to HIV-negative (97 [14.8%]).
HIV status and Age at Cervical Cancer Diagnosis
Next, we examined the relationship between HIV status and age at cervical cancer diagnosis. HIV status was associated with the outcome age at diagnosis (392 [51.7%] of HIV-positive cases diagnosed ≤40 years of age compared to 153 [18.6%] of HIV-negative cases), marital status (368 [48.5%] for HIV-positive cases and 514 [62.3%] for HIV-negative cases) and occupation. Marital status and occupation were found to be highly correlated (P = 0.003, statistically significant) and to prevent multicollinearity, we removed occupation from further analysis. After adjusting for marital status, the odds of being diagnosed with cervical cancer at age 40 or younger was 2.36 (95% CI: 2.09 to 2.67) times higher among HIV-positive women compared to HIV-negative women (Table 2).
TABLE 2.
Odds ratios for age at cancer diagnosis by HIV-status among patients at the Cancer Diseases Hospital.
| Outcome | Crude Model | Adjusted Modela | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P value | |
| Age <40 at diagnosis (HIV+ vs HIV−) | 2.17 (1.94, 2.43) | < 0.0001** | 2.36 (2.09, 2.67) | < 0.001* |
Statistically significant at ≤0.05
Adjusted for marital status
HIV-positive Sub-Population
Among the subpopulation of HIV-positive cases linked with HIV test and treatment details, the average length of having been HIV-positive was 2.5 years with a mean baseline CD4 count of 236 cells/mL3 (Table 3). Women who were diagnosed with non-metastatic were more likely to have had a longer duration of HIV-infection (3.4 years among non-metastatic versus 2.1 years among metastatic). The average ARV start age of 41 years for those diagnosed with non-metastatic cervical cancer and 38 for those diagnosed with metastatic cervical cancer. Overall, the mean age of cervical cancer diagnosis among this subgroup was 42 years (Table 3).
TABLE 3.
Descriptive statistics of demographics and HIV infection measures of severity with respect to outcome measures among HIV-positive cases seen at the Cancer Diseases Hospital in Luska, Zambia.
| N | Overall | Stage at Diagnosis | Age at Diagnosis | |||||
|---|---|---|---|---|---|---|---|---|
| Non-metastatic | Metastatic | P-value | < 40 years | >40 years | P value | |||
| Overall | 85 | - | 37 (48.68%) | 39 (51.32%) | - | 47 (55.29%) | 38 (44.71%) | - |
| Age | 85 | - | - | - | - | - | - | - |
| Mean Age (SD) | - | 41.9 (8.4) | 42.6 (8.1) | 41.3 (9.4) | 0.491 | 35.8 (3.6) | 49.4 (6.2) | - |
| Years on ARV | 44 | 3.2 (2.5) | 3.7 (2.8) | 2.9 (2.2) | 0.305 | 3.0 (2.5) | 3.4 (2.5) | 0.606 |
| Average Age started ARV | 48 | 39.7 (9.4) | 41.0 (8.0) | 38.4 (11.2) | 0.388 | 32.7 (4.2) | 46.2 (8.1) | < 0.001* |
| Length of HIV infection | 84 | 2.5 (2.5) | 3.39 ± 2.75 | 2.05 ± 2.15 | 0.021* | 2.4 ± 2.06 | 2.7 ± 2.93 | 0.676 |
| Baseline CD4 (cells/mL3) | 82 | - | - | - | 0.210 | - | - | 0.716 |
| Mean CD4 (SD) | - | 236.1 (178.3) | 258.3 (217.5) | 219.7 (148.0) | 0.368 | 211.8 (138.9) | 267.1 (216.8) | 0.165 |
| 0 to < 200 (%) | - | 39 (47.56%) | 18 (50.00%) | 17 (43.59%) | - | 23 (50.00%) | 16 (44.44%) | - |
| 200 to <500 (%) | - | 38 (46.34%) | 14 (38.89%) | 21 (53.85%) | - | 21 (45.65%) | 17 (47.22%) | - |
| >=500 (%) | - | 5 (6.10%) | 4 (11.11%) | 1 (2.56%) | - | 2 (4.35%) | 3 (8.33%) | - |
| Marital Status | 84 | - | - | - | 0.721 | - | - | 0.068 |
| Single (%) | - | 21 (25.00%) | 11 (29.73%) | 8 (21.05%) | - | 7 (14.89%) | 14 (37.84%) | - |
| Married (%) | - | 42 (50.00%) | 16 (43.24%) | 21 (55.26%) | - | 27 (57.45%) | 15 (40.54%) | - |
| Divorced (%) | - | 5 (5.95%) | 3 (8.11%) | 2 (5.26%) | - | 2 (4.26%) | 3 (8.11%) | - |
| Widowed (%) | - | 16 (19.05%) | 7 (18.92%) | 7 (18.92%) | - | 11 (23.40%) | 5 (13.51%) | - |
| Occupation | 65 | - | - | - | 0.388 | - | - | 0.637 |
| Farmer (%) | - | 10 (15.38%) | 5 (16.13%) | 4 (13.79%) | - | 5 (14.71%) | 5 (16.13%) | - |
| Housewife (%) | - | 4 (6.15%) | 0 (0.00%) | 3 (10.34%) | - | 3 (8.82%) | 1 (3.23%) | - |
| Business (%) | - | 7 (10.77%) | 4 (12.90%) | 2 (6.90%) | - | 2 (5.88%) | 5 (16.13%) | - |
| Unemployed (%) | - | 27 (41.54%) | 14 (45.16%) | 11 (37.93%) | - | 15 (44.12%) | 12 (38.71%) | - |
| Other (%) | - | 17 (26.15%) | 8 (25.81%) | 9 (31.03%) | - | 9 (26.47%) | 8 (25.81%) | - |
| Tribe | 67 | - | - | - | 0.671 | - | - | 0.766 |
| Bemba (%) | - | 14 (20.90%) | 4 (12.50%) | 6 (20.00%) | - | 6 (18.75%) | 8 (22.86%) | - |
| Chewa (%) | - | 13 (19.40%) | 6 (18.75%) | 6 (20.00%) | - | 6 (18.75%) | 7 (20.00%) | - |
| Lozi (%) | - | 5 (7.46%) | 2 (6.25%) | 3 (10.00%) | - | 3 (9.38%) | 2 (5.71%) | - |
| Ngoni/Ila (%) | - | 5 (7.46%) | 3 (10.00%) | 2 (6.25%) | - | 1 (3.13%) | 4 (11.43%) | - |
| Nsenga (%) | - | 7 (10.45%) | 6 (18.75%) | 1 (3.33%) | - | 5 (5.63%) | 2 (5.71%) | - |
| Tonga/Toka (%) | - | 5 (7.46%) | 2 (6.25%) | 3 (10.00%) | - | 3 (9.38%) | 2 (5.71%) | - |
| Tumbuka (%) | - | 3 (4.48%) | 1 (3.13%) | 2 (6.67%) | - | 1 (3.13%) | 2 (5.71%) | - |
| Other (%) | - | 15 (22.39%) | 8 (25.00%) | 7 (23.33%) | - | 7 (21.88%) | 8 (22.86%) | - |
statistically significant at ≤0.05
Note: percentages in the HIV-positive and HIV-negative columns are column percentages.
Duration of HIV infection and Metastatic Cancer at Diagnosis
Among the subpopulation of HIV-positive women linked with HIV test and treatment details, we assessed the independent effects of length of HIV infection, age started on ARV, years on ARV, and baseline CD4 count on the primary outcome metastatic cancer. The length of HIV infection was found to be statistically significantly associated with metastatic cancer diagnosis, all other exposures and covariates relationships were not statistically significantly associated with metastatic cancer diagnosis. From the crude logistic regression model, the odds of having a metastatic cancer diagnosis were 20% lower odds (OR = 0.80; 95% CI: 0.65, 0.97) for every one-year increase in the length of HIV infection (Table 4). Adjusted models were not assessed due to the lack of relationship between metastatic cancer diagnosis and possible the covariates, and the small sample size.
TABLE 4.
Odds ratio for metastatic cancer diagnosis at initial visit and length of HIV infection among HIV-positive cases seen at the Cancer Diseases Hospital in Luska, Zambia.
| Outcome: | Exposures | OR (95% CI) | P value |
|---|---|---|---|
| Metastatic cancer diagnosis | Length of HIV infection | 0.80 (0.65, 0.97) | 0.026* |
statistically significant at ≤0.05
4. DISCUSSION
Cervical cancer is often diagnosed at late stages in low-resource settings due to lack of access to care and treatment facilities.[19] In our study, a large proportion of cervical cancer cases were diagnosed with metastatic cancer, independent of their HIV status. Although some studies have suggested that immunosuppression among HIV-positive individuals lead to more aggressive cancers [11, 20, 21], we did not find that the odds of having an initial metastatic cervical cancer diagnosis was associated with HIV status. However, increased access to health care infrastructure among HIV-positive individuals may facilitate an earlier diagnosis. Due to international funding for HIV care and treatment, HIV-positive patients have improved access to clinical care and can benefit from additional vertical programmatic efforts to improve other health outcomes.[22] Although not all sub-Saharan countries have seen benefits in their overall healthcare systems as a result of HIV care and treatment funding [23], some countries have showed spill-over effects of the PEPFAR funded HIV care and treatment program on the overall health care system.[22, 24, 25]
In this study, we found that the mean age at cancer diagnosis was approximately 11 years lower among HIV-positive women compared to HIV-negative women. This is the bimodal peak introduced by the HIV coinfection making women living with HIV present at a younger age as compared to the HIV negative cohort. Also, HIV-positive women were more likely to be diagnosed with early-onset cervical cancer compared to HIV-negative women. Among HIV-positive cervical cancer patients, our study suggests that a longer time of HIV infection is associated with decreased odds of metastatic cancer diagnosis, which supports our previous finding by Trejo et al.[14] The longer length of HIV infection may lead to higher exposure to health care services leading to the detection of disease at an earlier stage and longer disease management. These two findings support the hypothesis that increased access to the health care infrastructure afforded by the scale-up of the HIV care and treatment program may lead to down staging of the disease at presentation owing to the fact that the immunity has had a chance to repair and therefore clearance of HPV may have started making the disease less aggressive. Further support for this hypothesis comes from the fact that women, regardless of HIV-status (CD4 cell count permitting) received the same care at the CDH.[14]
The CCPPZ is a public-sector cervical cancer screening program funded by PEPFAR to provide VIA screening and cryotherapy treatment services set up in government led clinics including antiretroviral therapy clinics. Evaluating the role of cervical cancer screening in future studies will greatly improve our understanding of the role of HIV infection on cervical cancer prognosis and may provide insights on how to best counteract the high mortality and morbidity associated with the co-burden of HIV and cervical cancer.
There were some limitations to this study that should be noted. First, our data is not generalizable to patients outside of Lusaka province. CDH is the only cancer treatment center in Zambia providing free cancer treatment to those that can access its facilities. However, women who reside in locations outside of Lusaka province have an increased difficulty in attending CDH for care and treatment due to many factors including the financial cost of traveling and access to transportation. Therefore, our results may not reflect cancer patients outside of this geographic region. Secondly, we were able to link about 20% of HIV-positive cervical cancer cases with the national HIV database. One reason for this is the time constraints in collecting data from the HIV clinics around the province. As more data is being collected from additional HIV care centers, this should improve our linkage as well as inflating the sample size of our study. Although missing data is limitation to this study, the distribution of missing HIV data across cancer stages was consistent with the overall distribution of cancer staging. Furthermore, there were a relatively equal proportion of individuals missing cancer staging data from by HIV status. A final limitation to this study is in the study design. Because this is a case-case analysis, we do not have a true disease-free comparison group. One barrier to conducting a case-control study was that cervical Cancer cases identified for this study were abstracted from paper medical records. The lack of an electronic health record system at CDH makes identifying non-case controls a real challenge.
Despite some challenges and limitations of this study, there are many strengths as well. First, a case-case design, like a case-control study, allows us to ascertain more cases than would a cohort study and are well suited for common exposures. Although cervical cancer incidence is high in Zambia, the overall prevalence of disease is still rare. On the other hand, HIV is a common exposure with one in five women of reproductive age infected with HIV.[2, 3] A second strength of this study were the availability HIV infection and treatment data from SmartCare, although data completeness is a limitation.
5. CONCLUSION
HIV status was found to be associated with early-onset diagnosis of cervical cancer. However, a longer duration of HIV infection was associated with decreased odds of initial metastatic cervical cancer at diagnosis. Future studies should examine both clinical and non-clinical factors related to HIV infection and stage of cervical cancer diagnosis, such as: access to and results of cervical cancer screening, and healthcare access to further elucidate this relationship.
Synopsis.
HIV status was associated with early-onset cervical cancer. However, a longer duration of HIV infection was associated with decreased odds of metastatic cervical cancer.
Acknowledgements
We thank all of the staff at the Cancer Diseases Hospital and the University Teaching Hospital who made this work possible.
Funding
Mario Trejo and Yuli Chen were supported by the Cancer Epidemiology Education in Special Populations (CEESP) Program through funding from the National Cancer Institute Grant (R25 CA112383- PI Amr Soliman, MD, PhD). The funding body had no role in the design of the study, collection, analysis, interpretation of the data, or in writing the manuscript. Dr Kennedy Lishimpi was the local mentor for both Mario Trejo and Yuli Chen.
Footnotes
Conflicts of Interest
The authors declare that they have no competing interests.
Ethics approval
The study was approved by the Institutional Review Board of the University of Colorado School of Public Health and the CDH in Zambia.
Availability of Data
The deidentified participant data used and analyzed during the current study are available upon reasonable request from the corresponding author.
REFERENCES
- 1.Bray F, et al. , Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 2018. 68(6): p. 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Bateman AC, et al. , The burden of cervical pre-cancer and cancer in HIV positive women in Zambia: a modeling study. BMC Cancer, 2015. 15(1): p. 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalima M, et al. , Observed and expected incidence of cervical cancer in lusaka and the southern and Western provinces of Zambia, 2007 to 2012. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society, 2015. 25(1): p. 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nour NM, Cervical cancer: a preventable death. Reviews in obstetrics & gynecology, 2009. 2(4): p. 240–244. [PMC free article] [PubMed] [Google Scholar]
- 5.Parham GP, et al. , Population-Level Scale-Up of Cervical Cancer Prevention Services in a Low-Resource Setting: Development, Implementation, and Evaluation of the Cervical Cancer Prevention Program in Zambia. PLOS ONE, 2015. 10(4): p. e0122169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng’andwe C, et al. , The distribution of sexually-transmitted Human Papillomaviruses in HIV positive and negative patients in Zambia, Africa. BMC Infectious Diseases, 2007. 7(1): p. 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernández-Ramírez RU, et al. , Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. The Lancet HIV, 2017. 4(11): p. e495–e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heard I, et al. , Increased risk of cervical disease among human immunodeficiency virus–infected women with severe immunosuppression and high human papillomavirus load11The authors are grateful to J.-D. Poveda (Centre de Biologie Médicale Spécialisée, Institut Pasteur) for his help with human papillomavirus typing. Obstetrics & Gynecology, 2000. 96(3): p. 403–409. [DOI] [PubMed] [Google Scholar]
- 9.Sun X-W, et al. , Human Papillomavirus Infection in Women Infected with the Human Immunodeficiency Virus. New England Journal of Medicine, 1997. 337(19): p. 1343–1349. [DOI] [PubMed] [Google Scholar]
- 10.De Vuyst H, et al. , HIV, human papillomavirus, and cervical neoplasia and cancer in the era of highly active antiretroviral therapy. European Journal of Cancer Prevention, 2008. 17(6). [DOI] [PubMed] [Google Scholar]
- 11.Shiels MS, et al. , Cancer stage at diagnosis in patients infected with the human immunodeficiency virus and transplant recipients. Cancer, 2015. 121(12): p. 2063–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biesma RG, et al. , The effects of global health initiatives on country health systems: a review of the evidence from HIV/AIDS control. Health Policy and Planning, 2009. 24(4): p. 239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mwanahamuntu MH, et al. , Advancing cervical cancer prevention initiatives in resource-constrained settings: insights from the Cervical Cancer Prevention Program in Zambia. PLoS Med, 2011. 8(5): p. e1001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trejo MJ, et al. , Effects of HIV status on non-metastatic cervical cancer progression among patients in Lusaka, Zambia. Int J Gynecol Cancer, 2020. 30(5): p. 613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meanwell CA, et al. , Young age as a prognostic factor in cervical cancer: analysis of population based data from 10 022 cases. British Medical Journal (Clinical research ed.), 1988. 296(6619): p. 386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benard VB, et al. , Cervical carcinoma rates among young females in the United States. Obstetrics and gynecology, 2012. 120(5): p. 1117–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xia Q, Braunstein SL, and Torian LV, Using the Revised Centers for Disease Control and Prevention Staging System to Classify Persons Living With Human Immunodeficiency Virus in New York City, 2011–2015. Sexually Transmitted Diseases, 2017. 44(11): p. 653–655. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention, 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep, 1992. 41(Rr-17): p. 1–19. [PubMed] [Google Scholar]
- 19.Ghebre RG, et al. , Cervical cancer control in HIV-infected women: Past, present and future. Gynecologic Oncology Reports, 2017. 21: p. 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coghill AE, et al. , Elevated Cancer-Specific Mortality Among HIV-Infected Patients in the United States. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 2015. 33(21): p. 2376–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suneja G, et al. , Cancer treatment disparities in HIV-infected individuals in the United States. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 2014. 32(22): p. 2344–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menon MP, et al. , Association Between HIV Infection and Cancer Stage at Presentation at the Uganda Cancer Institute. J Glob Oncol, 2018. 4(4): p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luboga SA, et al. , Did PEPFAR investments result in health system strengthening? A retrospective longitudinal study measuring non-HIV health service utilization at the district level. Health Policy and Planning, 2016. 31(7): p. 897–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grépin KA, HIV Donor Funding Has Both Boosted And Curbed The Delivery Of Different Non-HIV Health Services In Sub-Saharan Africa. Health Affairs, 2012. 31(7): p. 1406–1414. [DOI] [PubMed] [Google Scholar]
- 25.Kruk ME, et al. , PEPFAR Programs Linked To More Deliveries In Health Facilities By African Women Who Are Not Infected with HIV. Health Affairs, 2012. 31(7): p. 1478–1488. [DOI] [PubMed] [Google Scholar]


