Abstract
Insomnia is an adverse cancer outcome impacting mood, pain, quality of life, and mortality in cancer patients. Cognitive Behavioral Therapy (CBT) is an evidence-based treatment for diverse psychophysiological disorders, including pain and insomnia. Primarily studied in breast cancer, there is limited research on CBT within gynecology oncology. This study examined CBT effects on subjective and behavioral sleep outcomes: Sleep Efficiency (SE), Sleep Quality (SQ), Total Wake Time (TWT), Sleep Onset Latency (SOL), and Wake After Sleep Onset (WASO). Thirty-five women with insomnia status/post-surgery for gynecologic cancer were randomized to CBT for insomnia and pain (CBTi.p., N=18) or Psychoeducation (N=17). Sleep was assessed via sleep diaries and wrist-worn actigraphy at baseline (T1), post-intervention (T2), and two-month follow-up (T3). Intent-to-treat analyses utilizing mixed linear modeling examined longitudinal group differences on sleep controlling for age and advanced cancer. All participants demonstrated improved (1) subjective SE (0.5, p<0.01), SOL (−1.2, p<0.01), TWT (−1.2, p<0.01), and (2) behavioral SE (0.1, p=0.02), TWT (−1.2, p=0.03), WASO (−0.8, p<0.01) across time. Group-level time trends were indicative of higher subjective SE (6.8, p=0.02), lower TWT (−40.3, p=0.01), and lower SOL (−13.0, p=0.05) in CBTi.p. compared to Psychoeducation. Supplemental analyses examining clinical significance and acute treatment effects demonstrated clinical improvements in SE (T1), TWT (T2, T3), and SOL (T3). Remaining effects were not significant. Despite lacking power to detect interaction effects, CBTi.p. clinically improved sleep in women with gynecologic cancers and insomnia during the active treatment phase. Future research will focus on developing larger trials within underserved populations.
Keywords: Insomnia, sleep, cognitive behavioral therapy, gynecologic cancer
Introduction
Affecting female reproductive organs, gynecologic malignancies are estimated to account for over 113,000 new cancer diagnoses and 33,000 cancer-related deaths among women in the United States in 2020 (American Cancer Society [ACS], 2020). Adverse outcomes associated with gynecologic cancers include sleep dysfunctions (Roscoe et al., 2007), fatigue (Schrepf et al., 2013), pain (Honerlaw et al., 2016), and adverse mood symptoms (Bergerot et al., 2018).
Insomnia is a prevalent cancer outcome associated with mood disorders, low quality of life (QOL), inflammation, immune dysregulations, and mortality (Clevenger et al., 2012, 2013; Peoples et al., 2017). Davidson and colleagues (2002) found that 29% of gynecology cancer patients endorsed insomnia while 21.3% endorsed other sleep-related difficulties. Recently replicated (Palesh et al., 2010), these findings underscore the pervasiveness of insomnia within oncology. Findings further support a bidirectional relationship between pain and insomnia. Specifically, insomnia worsens pain outcomes while chronic pain heightens severity of sleep dysregulations (Husak & Bair, 2020).
Grounded in behavioral modification and cognitive restructuring, Cognitive Behavioral Therapy (CBT) can improve coping skills for management of diverse mental health disorders (Hofmann et al., 2012). CBT for insomnia (CBTi) is a first-line intervention for sleep shown to be efficacious within breast cancer and cancer survivors (Johnson et al., 2016; Quaseem et al., 2016; Savard et al., 2016). CBT for pain (CBTp) is validated for managing distress and functional limitations related to pain disorders. Knoerl and colleges (2016) reported reductions in pain intensity and sleep disturbance within a chronic pain population following CBTp.
Despite being a first-line intervention for insomnia, particularly when used in conjunctions with behavioral modifications for pain, there is a paucity of research examining CBTi effects within gynecology oncology. The present study examined effects of CBT for insomnia and pain (CBTi.p.) on sleep outcomes in women with insomnia status/post-surgery for gynecologic cancer. The study utilized a novel adaptation of an evidence-based intervention within a historically underrepresented disease group reporting significant and impairing needs.
Materials and Methods
This was a randomized controlled trial (RCT) examining effects of 6 weekly sessions of CBTi.p. compared to a time- and attention-matched Psychoeducation program (PE) among women with gynecologic cancers and insomnia. Outcome variables were subjective sleep quality (SQ) and subjective and behavioral sleep efficiency (SE), total wake time (TWT), sleep onset latency (SOL), and wake after sleep onset (WASO). This trial was registered at ClinicalTrials.gov (NCT02609880) and approved by the University of Florida (UF) Institutional Review Board-01.
Participants were recruited and enrolled from the UF Health Cancer Center’s Gynecologic Oncology Clinic at their surgical consultation visit. Patients with suspected gynecologic cancers without a medical-record history of seizure disorder, Psychotic Disorders, Bipolar Disorder, or Neurocognitive Disorders were screened for insomnia with the PSQI (Buysse et al., 1989) or a 4-item Brief Insomnia Screener based on the Research Diagnostic Criteria “A” for Insomnia Disorder (Edinger et al., 2004). Patients screening positive for insomnia (i.e., obtained PSQI Global Score > 5 or endorsed at least 1 item on the Brief Insomnia Screener) were invited to participate in the study. Interested patients underwent informed consent procedures. They then completed additional assessment to exclude participants with suicidality (BSS), Psychotic Disorders (SCID Psychotic Screening Module), Bipolar Disorder (SCID), or Neurocognitive Disorders (MMSE). Participants who continued to be eligible then underwent pre-surgical (T0) assessment of patient-reported outcomes, salivary cortisol (to assess hypothalamic-pituitary adrenal (HPA) axis functioning), and serum cytokine (to assess pro-inflammatory and pro-angiogenic processes).
Within one week of the pre-surgical assessment, participants underwent clinically necessary surgical resection. Six to eight weeks following surgery, those with confirmed gynecologic cancer underwent post-surgical/pre-intervention (T1) assessment procedures, including: patient-reported outcomes, salivary cortisol, serum cytokine, overnight ambulatory polysomnography to screen out obstructive sleep apnea (OSA) and/or periodic limb movement disorders (PLMDs), 14 days of paper-based sleep diary and wrist-worn actigraphy monitoring, and a 60-minute clinical sleep and pain interview. Given findings indicative of non-correlated actigraph and sleep diary measures among individuals with insomnia (Aili et al., 2017), subjective and behavioral sleep measures were expected to provide analogous yet unique dimensions of sleep outcomes.
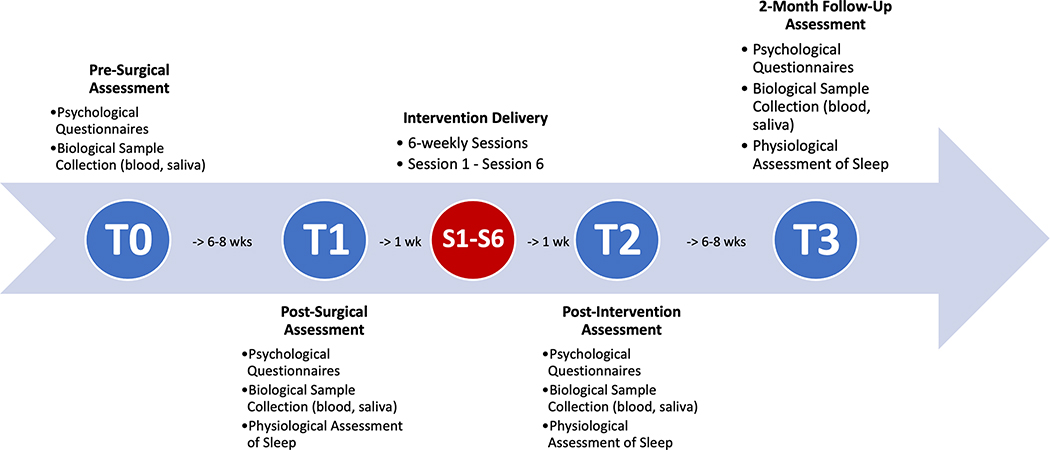
Participants with sleep diary confirmed insomnia who were negative for OSA and PLMD were randomized to either CBTi.p. or PE at a 1:1 ratio. Pre-intervention assessment procedures were repeated at post-intervention (T2) and 2-month follow-up (T3). An overview of the study timeline is depicted in Figure 1. Participants were compensated $150 for each completed timepoint and $10 for each completed intervention session.
Figure 1.
Study Timeline
Study participants
Recruitment occurred from 2010 through 2017. Eligibility checks were conducted at enrollment/pre-surgery, post-surgery, and pre-randomization. Pre-surgery inclusion criteria were: (1) planned surgical resection for suspected/confirmed gynecologic cancer, (2) subjective sleep complaints, and (3) English proficiency. Post-surgery inclusion criterion included the presence of surgically confirmed gynecologic cancer or borderline ovarian tumors of any stage. Pre-randomization inclusion criteria were: (1) willingness to be randomized, (2) (a) 6+ days of sleep diary reported WASO > 30 minutes and/or SOL > 30 minutes or (b) 1 to 5 days of sleep diary reported WASO > 30 minutes and/or SOL > 30 minutes and SE < 85% on the PSQI at post-surgery, (3) Apnea/Hypopnea Index (AHI) < 15 on post-surgical, study-administered PSG, (4) periodic leg movement (PLM) Arousal Index < 15 on post-surgical, study-administered PSG, and (5) (a) discontinuation of prescribed sleep medications for at least 1 month, or (b) stabilized on prescribed sleep medication for at least 6 months.
Interventions
CBTi.p. and PE facilitation were manualized, comprising of six weekly 90-minute sessions (S1-S6; Figure 1). Sessions were conducted in private spaces of participants’ choosing, including their home (N[sessions]=102), PI’s research office (N[sessions]=38), ACS local office (N[sessions]=6), and other locations (e.g., participant’s work office, study-arranged hotel room) (N[sessions]=23). A total of 9 therapists facilitated treatment delivery, the majority (67%) of whom facilitated both CBTi.p. and PE sessions.
CBTi.p. was grounded in empirically supported CBT for insomnia, CBT for pain, CBT for anxiety/depression and Cognitive Behavioral Stress Management interventions for individuals with cancer (Antoni, 2003; McCrae, 2014; McCrae et al., 2019; Waxenberg, 2008). Treatment components are listed in Table 1. Strategies unique to CBT for insomnia included psychoeducation on insomnia, sleep hygiene, stimulus control, and sleep restriction; strategies unique to CBT for pain included psychoeducation on the gate control theory of pain and activity pacing; strategies common among all three interventions included relaxation, cognitive restructuring, pleasant activity scheduling, and self-monitoring/homework. PE was developed utilizing peer-reviewed and expert-verified materials available publicly (e.g., LIVESTRONG Foundation, 2015). While PE was matched to CBTi.p. on level of attention and time, it did not incorporate cognitive behavioral approaches integral for improved psychophysiological outcomes.
Table 1.
Treatment Plan for CBTi.p. and PE
| CBTi.p. | PE | |
|---|---|---|
| Session 1 | ||
| Topic/Skill | • Psychoeducation about sleep, pain, and mood in context of cancer • Sleep hygiene • Stimulus control |
• Relationships during cancer treatment • Home healthcare • Employment issues |
| Relaxation | • Diaphragmatic breathing | |
| Homework | • Sleep diaries | |
| Session 2 | ||
| Topic/Skill | • Review of prior week’s session and homework • Sleep restriction • Activity pacing |
• Evaluating health information • Healthcare teams and settings • Effective communication and problem resolution |
| Relaxation | • Progressive muscle relaxation | |
| Homework | • Sleep diaries | |
| Session 3 | ||
| Topic/Skill | • Review of prior week’s session and homework • Adjust sleep prescription, as needed • Identifying negative thoughts about sleep and pain secondary to cancer |
• Physical concerns of treatment • Common treatment side effects • Coping with treatment side effects • Practical changes to treatment |
| Relaxation | • Mindfulness meditation | |
| Homework | • Sleep diaries | |
| Session 4 | ||
| Topic/Skill | • Review of prior week’s session and homework • Adjust sleep prescription, as needed • Cognitive therapy for insomnia and pain secondary to cancer, Part I |
• Late effects of cancer treatment • Developing survivorship care and healthcare follow-up plans • Recommendations for screening • Fear of cancer recurrence |
| Relaxation | • Visual imagery relaxation | |
| Homework | • Sleep diaries | |
| Session 5 | ||
| Topic/Skill | • Review of prior week’s session and homework • Adjust sleep prescription, as needed • Cognitive therapy for insomnia and pain secondary to cancer, part II |
• Education on cancer epidemiology, risk, symptoms, evaluation, treatment, and survivorship specific to the participant’s diagnosis |
| Relaxation | • Participant’s choice | |
| Homework | • Sleep diaries | |
| Session 6 | ||
| Topic/Skill | • Review of skills and long-term maintenance | • Reliable and accurate cancer-specific resources |
| Relaxation | • Participant’s choice | |
| Homework | • Sleep diaries | |
Treatment delivery
Clinicians were masters’ level clinicians who underwent six weeks of training in providing CBT.i.p. and six weeks of training in providing PE. Trainers were the PI (DBP), licensed psychologist and Attending Psychologist for the UF Health Cancer Center, and a Co-I (CSM), licensed psychologist board certified in Behavioral Sleep Medicine. Training involved observing recorded sessions and facilitating mock group sessions with real-time feedback from DBP and CSM. Clinicians were cleared to facilitate sessions upon achieving ≥95% treatment integrity ratings by the PI for each mock session and received weekly individual supervision with the PI throughout intervention delivery.
Nine total clinicians facilitated treatment delivery. Of those nine, six facilitated both CBTi.p. and PE individual sessions, two facilitated only CBTi.p. individual sessions, and one facilitated only PE individual sessions. Of 172 CBTi.p. and PE facilitated sessions, 157 (91%) were audio-recorded while 15 (9%) were not due to equipment failure or participant decline. The first 13 participants had sessions facilitated by 6 therapists. For these first 13 participants, all recorded sessions were rated for treatment integrity by trained, non-intervention study staff. For the remaining participants, one already-rated therapist and four new therapists facilitated sessions. For the four new therapists, the PI selected 25% of their sessions at random for treatment integrity ratings. Overall, 85 sessions (54%) were rated for treatment integrity. The average treatment integrity rating was 96.9% (SD=7.0%).
Treatment receipt
To ensure comprehension of CBT.i.p., participants were given workbooks containing instructions and rationale for session-taught techniques. CBT.i.p. participants were asked about experiences with home practice of techniques; modifications were adopted as necessary. Participants completed a 10-question quiz at the beginning of S2 to ensure treatment mastery; incorrect items were reviewed with participants. PE participants also received workbooks but were not assigned homework or encouraged to review materials in between sessions.
Treatment enactment
CBT.i.p. workbooks facilitated adherence to home practice of techniques. Therapists further encouraged adherence of home practice while emphasizing its importance. Participants maintained daily adherence logs, which were reviewed in session. Difficulties with adherence were problem-solved to optimize home practice across subsequent sessions.
Treatment credibility
A treatment credibility questionnaire was administered at the end of S2 and S6 of CBTi.p. in the absence of the therapist. To promote honest self-expression while limiting bias, the questionnaire was sealed until participants completed the study.
Primary outcomes
SE
Subjective SE was assessed via 14-days of sleep diaries completed at T1, T2, and T3. CBTi.p. participants completed sleep diaries throughout the intervention period. Participants recorded daily in-bed time, SOL in minutes, WASO in minutes, final wake time, and out-of-bed time. Time in Bed (TIB) was calculated in minutes as in-bed time minus out-of-bed time. Time Attempting to Sleep after Final Awakening (TASAFA) was calculated in minutes as out-of-bed time minus wake-up time. Total Sleep Time (TST) was calculated as TIB – [SOL+WASO+TASAFA). SE was calculated as the ratio of TST to TIB multiplied by 100 and expressed as a percentage. Subjective SE was calculated for each day at each timepoint and averaged for that timepoint.
All behavioral sleep outcomes were measured via wrist-worn Actiwatch-L® actigraphs, which participants wore on their non-dominant wrist for 24-hours per day (except while bathing or swimming) throughout the same 14-day period they completed sleep diaries across T1-T3. Actigraphs provided behavioral sleep measurements based on ambient light recording as well as limb movement and acceleration patterns. Actiware-Sleep v.3.3 software was used to analyze actigraphic data. Specifically, behavioral SE was calculated for each night at each timepoint and averaged for that timepoint.
SQ
Subjective SQ was also assessed across T1-T3 using sleep diaries. Participants rated their SQ using a 5-point Likert scale (1=Very Poor; 5=Excellent). Daily ratings were averaged to provide a mean SQ rating per timepoint.
Secondary outcomes
Secondary outcomes included subjective (sleep diary) and behavioral (actigraph) measures of TWT (TIB – TST) and its components (WASO and SOL).
Random assignment
Block randomization with 3 block sizes of 10 and 1 block size of 5 were used to allocate participants to either CBTi.p. or PE at a 1:1 ratio. DBP maintained sole possession of the randomization schedule, revealing participant assignment only to that participant’s interventionist.
Blinding
Participants and therapists were not blinded to study aims or condition. Pre-intervention assessors and participants were blinded to condition. In addition, post-intervention assessors were blinded to condition; participants were instructed not to reveal group assignment to assessors. Data analysts and manuscript writers could not be blinded to condition given unique data management requirements of CBTi.p.
Statistical methods
Intent-to-treat analyses were conducted using IBM® Statistical Package for the Social Sciences (SPSS®) 25.0 statistical software. Controlling for age and advanced disease, mixed linear modeling (MLM) using maximum likelihood estimation and variance components structure for random effects were used to analyze intervention effects across outcomes.
Group was dichotomized, with “0” representing PE and “1” representing CBTi.p. Advanced disease was dichotomized, with “0” representing stages I-II and “1” representing stages III-IV. Age was continuous. Outcome variable were continuous, with: (1) higher SE percentiles indicative of higher SE, (2) higher TWT indicative of greater time spent awake at night, (3) higher SOL indicative of greater time attempting to fall asleep, (4) higher WASO indicative of greater time awake following initial sleep onset. All predictors were grand-mean centered to reduce collinearity of higher-order time trends and interactions while aiding result interpretability (Aiken & West, 1991). Analyses evaluating −2 Log Likelihood (2LL) fit statistics were conducted to determine the best fitting model. Chi-square analyses were run to identify significant changes in 2LL.
To expound upon primary findings and refine result interpretation, clinical significance testing assessing clinically relevant changes to sleep outcomes was conducted. Outcomes were percent of participants with (1) SE > 85%, (2) SOL < 30 minutes, and (3) WASO < 30 minutes. These tests were followed by MLM analyses assessing CBTi.p. effects on sleep outcomes across an amalgam of timepoints and intervention sessions (T1, S1-S6, T2, T3).
Results
Participants
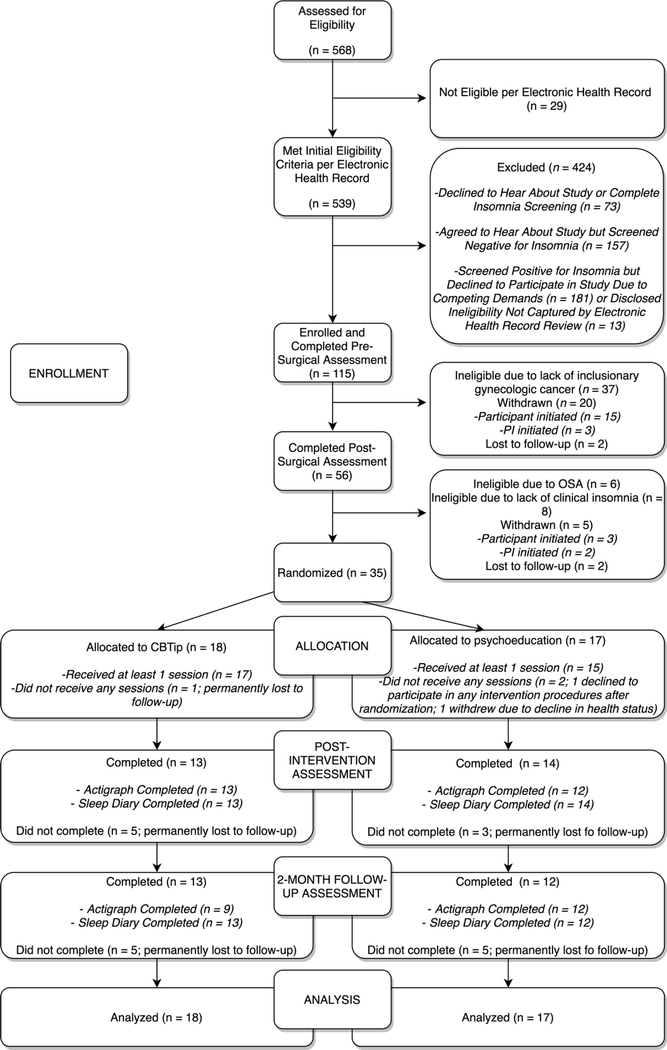
A total of 115 women scheduled to undergo surgery for gynecologic cancer screening positive for insomnia were enrolled to the study (Figure 2). Of these, 51 were ineligible for randomization primarily due to surgically confirmed lack of inclusionary gynecologic cancer (n=37); 25 were self- or PI-withdrawn and 4 were lost to follow-up. Thirty-five participants were randomized to either CBTi.p. (N=18) or PE (N=17). Among participants who were not lost to follow-up, adherence to sleep diary and actigraphy procedures across T2 and T3 were 69.2% (i.e., for completion of actigraphy by CBTi.p. participants at T3), 85.7% (i.e., for completion of actigraphy for PE participants at T2), and 100% (e.g., completion of actigraphy and sleep diaries among CBTi.p. participants at T2 and among PE participants at T3) (Figure 2). Table 2 depicts baseline characteristics and descriptive statistics. There were no significant differences in baseline characteristics between participants who completed the intervention and those who did not (data not shown). There were no significant differences in session completion rates across CBTi.p. and PE [t(33)=−0.894,p=0.378]. Consistent with previous findings (Aili et al., 2017), actigraph and sleep diary measures were not correlated (data not shown).
Figure 2.
Consort Flow Diagram
Table 2.
Baseline Characteristics and Descriptive Statistics by Treatment Group
| Variable Name | PE (N=17) |
CBTi.p. (N=18) |
|---|---|---|
| Demographic Characteristics [N(%)] | ||
| Age [years; M(SD)] | 59.9(10.3) | 58.9(12.2) |
| Education (years) | 14.7(2.4) | 13.4(2.4) |
| Marital Status (married) | 10(58.8) | 13(72.2) |
| Race (Caucasian) | 16(94.1) | 15(83.3) |
| Ethnicity (Non-Hispanic) | 15(88.2) | 18(100.0) |
| Physiological Characteristics [N(%)] | ||
| Body Mass Index (kg/m2; M(SD)] | 32.8(8.5) | 33.4(7.2) |
| Chemotherapy (yes) | 7(41.2) | 7(38.9) |
| Radiotherapy (yes) | 5(29.4) | 2(11.1) |
| Invasive Surgery (yes) | 10(58.8) | 7(38.9) |
| Surgical Complications (yes) | 4(23.5) | 2(11.1) |
| Length of Hospital Stay [days; M(SD)] | 3.7(2.7) | 2.9(2.4) |
| Cancer Stage | ||
| Stage I | 9(52.9) | 11(61.1) |
| Stage II | 2(11.8) | 1(5.6) |
| Stage III | 4(23.5) | 5(27.8) |
| Stage IV | 1(5.9) | 0 |
| Unknown Stage | 1(5.9) | 1(5.6) |
| Tumor Site | ||
| Endometrial | 14(82.4) | 12(66.7) |
| Ovarian | 2(11.8) | 2(11.1) |
| Fallopian Tube | 2(5.9) | 1(5.6) |
| Cervical | 0 | 1(5.6) |
| Vulvar | 0 | 2(11.1) |
| Descriptive Statistics | ||
| Completed Intervention Sessions | 4.6(2.3) | 5.2(1.8) |
| Sleep Medication Use (yes) | ||
| Rx Sedatives | ||
| T1 | 1(5.6) | 3(17.6) |
| T2 | 1(7.7) | 1(5.9) |
| T3 | 1(7.7) | 1(5.9) |
| OTC Sleep Aids | ||
| T1 | 2(11.1) | 1(5.9) |
| T2 | 1(7.7) | 0(0) |
| T3 | 1(7.7) | 0(0) |
| Rx Antidepressants | ||
| T1 | 0(0) | 1(5.9) |
| T2 | 1(7.7) | 2(14.3) |
| T3 | 1(7.7) | 3(25.0) |
| Pain Medication Use (yes) | ||
| Opioids | ||
| T1 | 7(38.9) | 8(47.1) |
| T2 | 4(30.8) | 4(28.6) |
| T3 | 4(30.8) | 3(25.0) |
| Non-Opioid Analgesics | ||
| T1 | 14(77.8) | 10(58.8) |
| T2 | 7(53.8) | 10(71.4) |
| T3 | 7(53.8) | 8(66.7) |
| Neuroleptics | ||
| T1 | 0(0) | 3(17.6) |
| T2 | 2(15.4) | 5(35.7) |
| T3 | 2(15.4) | 4(33.3) |
| AHI (per hour) | ||
| Mild Apnea (AHI 5–15; yes) | ||
| T1 | 6(35.3) | 5(29.4) |
| T2 | 6(50.0) | 3(25.0) |
| T3 | 1(11.1) | 2(16.7) |
| Moderate Apnea (AHI 16–30; yes) | ||
| T1 | 0(0) | 0(0) |
| T2 | 1(8.3) | 1(8.3) |
| T3 | 0(0) | 0(0) |
| Sleep Diary Variables [M(SD)] | ||
| Total Wake Time (minutes) | ||
| T1 | 126.9(67.9) | 88.1(45.3) |
| T2* | 93.9(44.7) | 53.7(35.3) |
| T3* | 102.4(65.9) | 44.6(30.1) |
| Sleep Onset Latency (minutes) | ||
| T1 | 49.8(25.9) | 37.4(27.3) |
| T2 | 33.0(20.3) | 23.1(24.3) |
| T3* | 36.7(25.3) | 18.3(13.1) |
| Wake After Sleep Onset (minutes) | ||
| T1 | 45.4(37.0) | 42.0(26.2) |
| T2 | 38.4(25.3) | 21.0(18.0) |
| T3 | 32.0(26.3) | 17.6(17.3) |
| Sleep Efficiency (%) | ||
| T1 | 76.0(11.4) | 81.7(9.2) |
| T2 | 81.8(7.4) | 88.0(9.1) |
| T3* | 80.0(11.6) | 90.0(6.1) |
| Sleep Quality (total score) | ||
| T1 | 2.1(0.5) | 2.0(0.5) |
| T2 | 2.2(0.6) | 2.3(0.6) |
| T3 | 2.2(0.6) | 2.3(0.5) |
| Actigraphy Variables [M(SD)] | ||
| Total Wake Time (minutes) | ||
| T1 | 107.2(45.2) | 97.9(41.3) |
| T2 | 95.4(32.9) | 82.7(24.0) |
| T3 | 88.8(35.0) | 80.3(30.7) |
| Sleep Onset Latency (minutes) | ||
| T1 | 33.6(22.3) | 29.3(22.5) |
| T2 | 29.3(17.3) | 22.4(14.2) |
| T3 | 26.7(14.9) | 24.5(15.0) |
| Wake After Sleep Onset (minutes) | ||
| T1 | 53.4(18.4) | 50.5(16.2) |
| T2 | 46.9(19.3) | 44.4(14.6) |
| T3 | 44.4(22.3) | 41.9(19.9) |
| Sleep Efficiency (%) | ||
| T1 | 79.4(7.3) | 80.8(5.4) |
| T2 | 81.8(4.6) | 82.1(5.1) |
| T3 | 82.6(5.0) | 82.4(5.6) |
| PSG Variables [M(SD)] | ||
| Total Wake Time (minutes) | ||
| T1 | 133.9(68.0) | 119.0(67.9) |
| T2 | 82.4(63.8) | 120.5(45.0) |
| T3 | 83.0(67.2) | 83.0(33.7) |
| Sleep Onset Latency (minutes) | ||
| T1 | 36.2(37.5) | 24.7(38.5) |
| T2 | 20.9(21.4) | 48.9(50.7) |
| T3 | 21.6(36.1) | 5.6(40.2) |
| Wake After Sleep Onset (minutes) | ||
| T1 | 94.8(54.1) | 91.1(51.3) |
| T2 | 82.5(65.9) | 70.6(37.5) |
| T3 | 71.6(68.2) | 49.6(61.5) |
| Sleep Efficiency (%) | ||
| T1 | 71.0(16.5) | 75.5(15.3) |
| T2 | 79.5(13.2) | 75.8(7.8) |
| T3 | 81.1(14.7) | 63.0(57.4) |
Note. Rx = Prescription; OTC = Over-the-counter; AHI = Apnea-Hypoapnea Index; REM = rapid eye movement sleep; PSG = Polysomnography.
Independent samples t-test (2-tailed) indicative of a significant (p<0.05) difference in group means.
Treatment enactment
Across all CBTi.p. participants and treatment sessions (S1-S6), the following home practice logs were completed: 82.3% of relaxation logs, 88.7% of stimulus control logs, 89.2% of thought record logs, 89.6% of activity pacing logs, 94.1% of pleasant activity scheduling logs, 93.5% of sleep hygiene logs, and 98.7% of sleep diaries. The average percentage of assigned logs completed on a weekly basis across subjects was 90.4% (SD = 22.4%).
Treatment credibility
CBT.i.p. participants rated treatment credibility and therapist likeability/confidence highly at both Session 2 and Session 6 (average ratings of all questionnaire items ≥ 7.3). CBTi.p. participants also rated treatment satisfaction highly at Session 6 (M=9.9, SD=0.3).
Primary sleep outcomes
SE
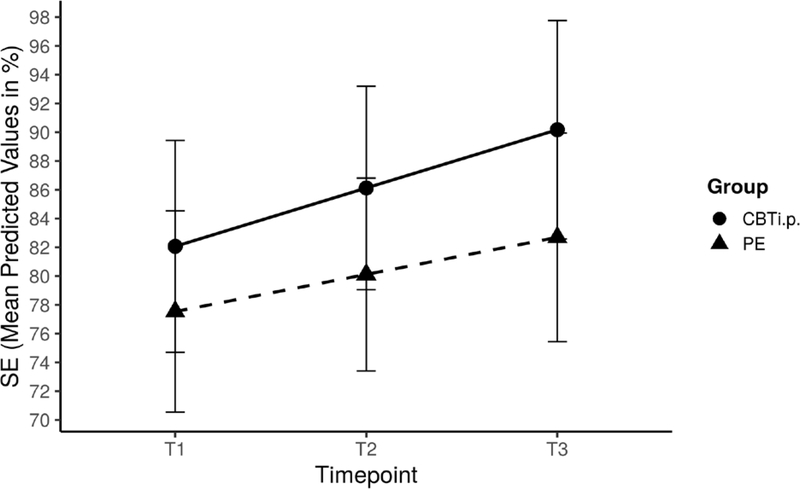
A significant main effect of time revealed that all participants reported improved SE across timepoints as assessed by both sleep diaries (0.5, SE=0.1, 95% CI=0.2–0.7) and actigraphy (0.1, SE=0.1, 95% CI=0.02–0.3; Table 4). Furthermore, significant group effects were indicative of higher SE in CBTi.p. participants (6.8, SE=2.6, 95% CI=1.4–12.2; Table 3) compared to PE participants (Figure 3). Older participants reported significantly higher SE (0.3, SE=0.1, 95% CI=−0.01–0.5). All other effects were non-significant.
Table 4.
Results for Primary and Secondary Behavioral Sleep Outcomes
| Sleep Efficiency (SE) | Total Wake Time (TWT) | Sleep Onset Latency (SOL) | Wake After Sleep Onset (WASO) | |
|---|---|---|---|---|
| Fixed Effects | ||||
| Intercept | 81.6(0.8)** | 90.3(4.9)** | 27.8(2.9)** | 45.6(2.6)** |
| Group | −0.8(1.6) | −0.5(9.8) | −1.5(5.8) | −0.5(5.2) |
| Age | 0.1(0.1) | −0.5(0.5) | −0.2(0.3) | −0.1(0.2) |
| Stage | −1.4(1.8) | 13.9(10.6) | 7.6(6.2) | −1.2(5.7) |
| Rate of Change | ||||
| Occasion (Linear) | 0.1(0.1)* | −1.2(0.5)* | −0.2(0.2) | −0.8(0.2)** |
| xGroup | −0.1(0.1) | −0.1(0.9) | −0.1(0.5) | <0.1(0.4) |
| Random Effects | ||||
| Residual | 7.4(2.4)** | 201.4(65.3)** | 117.8(26.7)** | 71.2(22.3)** |
| Intercept | 16.3(5.5)** | 603.8(224.9)** | 194.0(71.7)** | 169.1(53.0)** |
| Occasion (Linear) | <0.1(<0.1) | 2.9(1.9) | *** | 0.4(0.4) |
| Fit Statistics | ||||
| 2LL (null) | 479.4 | 782.2 | 693.1 | 670.5 |
| 2LL (final) | 421.9 | 695.8 | 619.0 | 600.9 |
| Δχ2 | 57.5** | 86.4** | 74.2** | 69.6** |
Note. Behavioral sleep data was derived from participant actigraphy data collected at each timepoint.
p≤0.05.
p≤0.01.
Unable to calculate due to resulting problems with the final hessian matrix. Standard errors are in parentheses.
Table 3.
Results for Primary and Secondary Subjective Sleep Outcomes
| Sleep Efficiency (SE) | Sleep Quality (SQ) | Total Wake Time (TWT) | Sleep Onset Latency (SOL) | Wake After Sleep Onset (WASO) | |
|---|---|---|---|---|---|
| Fixed Effects | |||||
| Intercept | 83.2(1.3)** | 2.2(0.1)** | 82.3(6.6)** | 32.2(3.1)** | 31.4(3.1)** |
| Group | 6.9(2.6)** | <0.1(0.1) | −40.3(13.4) | −13.0(6.4)* | −6.0(6.3) |
| Age | 0.3(0.1)* | <0.1(<0.1) | −1.2(0.6) | −0.5(0.3) | 0.01(0.3) |
| Stage | −2.6(2.9) | −0.3(0.1) | 21.7(14.5) | 9.6(6.9) | 8.1(6.8) |
| Rate of Change | |||||
| Occasion (Linear) | 0.5(0.1)** | <0.1(<0.1) | 2.8(0.7)** | −1.2(0.3)** | −1.7(0.6)** |
| xGroup | 0.2(0.2) | <0.1(<0.1) | −0.8(1.5) | −0.3(0.7) | −0.3(1.1) |
| Random Effects | |||||
| Residual | 36.5(7.4)** | 0.1(<0.1)** | 819.23(324.9)** | 261.7(75.7)** | 202.1(66.6)** |
| Intercept | 38.2(13.5)** | 0.1(<0.1)** | 1001.9(338.5)** | 200.3(77.8)** | 192.0(72.6)** |
| Occasion (Linear) | *** | <0.1(<0.1) | 5.9(7.9) | 0.3(1.1) | 6.6(2.9)* |
| Fit Statistics | |||||
| 2LL (null) | 613.2 | 112.3 | 925.0 | 792.9 | 821.7 |
| 2LL (final) | 548.5 | 96.3 | 831.6 | 717.2 | 738.0 |
| Δχ2 | 64.7** | 16.0** | 93.4** | 75.7** | 83.8** |
Note. Subjective sleep data was derived from two-week sleep diaries completed by participants at each study timepoint.
p≤0.05.
p≤0.01.
Unable to calculate due to resulting problems with the final hessian matrix. Standard errors are in parentheses.
Figure 3.
Effects of Cognitive Behavioral Therapy for Insomnia and Pain (CBTi.p.) on Sleep Efficiency (SE) Controlling for Age and Advanced Cancer. Controlling for age and advanced disease, significant main effects of time were indicative of improvements in subjective SE across all participants, regardless of group allocation. Furthermore, group effects demonstrated higher SE in CBTi.p. participants compared to PE participants not otherwise accounted for by baseline differences in SE (see text). There were no group-by-time effects, possibly due to low statistical power. In the pictured line graph, the x-axis represents the three study timepoints, each six to eight weeks apart, while the y-axis represents SE sores in percentages.
SQ
There were no significant effects of time, group, or group-by-time on SQ as assessed by sleep diaries (Table 3).
Secondary sleep outcomes
TWT
A significant main effect of time revealed that all participants experienced decreases in TWT as assessed by both sleep diaries [−2.8, SE=0.7, 95% CI=(−4.4)-(−1.3)] and actigraphy [−1.2, SE=0.5, 95% CI=(−2.1)-(−0.2); Table 4] across time. Furthermore, significant group effects indicated that CBTi.p. participants reported an overall lower TWT as assessed by sleep diaries compared to PE participants [−40.3, SE=13.4, 95% CI=(−67.5)-(−13.0); Table 3]. All other effects were non-significant.
SOL
A significant main effect of time revealed that all participants experienced improvements in sleep-diary assessed SOL across study visits [−1.2, SE=0.3, 95% CI=(−1.8)-(−0.5)]. In addition, significant group effects indicated that CBTi.p. participants demonstrated lower sleep-diary assessed SOL compared to PE participants [−13.0, SE=6.4, 95% CI=(−25.9)-(−0.1); Table 3]. All other sleep-diary assessed effects were non-significant. There were no significant effects on actigraphy-assessed SOL (Table 4).
WASO
All participants experienced decreases in WASO as assessed by sleep diaries [−1.7, SE=0.6, 95% CI=(−2.8)-(−0.5); Table 3] and actigraphy [−0.8, SE=0.2, 95% CI=(−1.2)-(−0.3); Table 4] across time. All other effects were non-significant.
Clinical significance
SE
At T1, 47% of CBTi.p. participants reported subjective SE > 85% compared to 24% of PE participants. By T3, 85% of CBTi.p. participants reported a SE > 85% compared to 50% of PE participants. While there were no group differences at T1 or T2, CBTi.p. participants reported greater subjective SE at T3 compared to PE participants [t(23)=−2.68, p=0.01; Table 2].
At T1, 29% of CBTi.p. participants had behavioral SE > 85% compared to 25% of PE participants. By T3, 36% of CBTi.p. participants had SE > 85% compared to 38% of PE participants. There were no longitudinal group differences.
SOL
At T1, 56% of CBTi.p. participants reported subjective SOL < 30 minutes compared to 47% of PE participants. At T3, 70% of CBTi.p. participants reported a SOL < 30 minutes compared to 50% of PE participants. While there were no group differences at T1 or T2, CBTi.p. participants reported lower subjective SOL at T3 compared to PE participants [t(23)=2.28 p=0.03]. (Table 2).
At T1, 76% of CBTi.p. participants had behavioral SOL < 30 minutes compared to 50% of PE participants. At T3, 73% of CBTi.p. participants had SOL < 30 minutes compared to 62% of PE participants. There were no longitudinal group differences.
WASO
At T1, 39% of CBTi.p. participants reported subjective WASO < 30 minutes compared to 18% of PE participants. At T3, 92% of CBTi.p. participants reported WASO < 30 minutes compared to 58% of PE participants. While there were no group differences in WASO at T1 and T3, there was a non-significant trend at T2 indicative of lower WASO within CBTi.p. [t(25)=2.04, p=0.05; Table 2].
At T1, 6% of CBTi.p. participants had behavioral WASO < 30 minutes compared to 13% of PE participants. At T3, 27% of CBTi.p. participants had WASO < 30 minutes compared to 31% of PE participants. There were no longitudinal group differences.
Exploratory intervention-specific outcomes
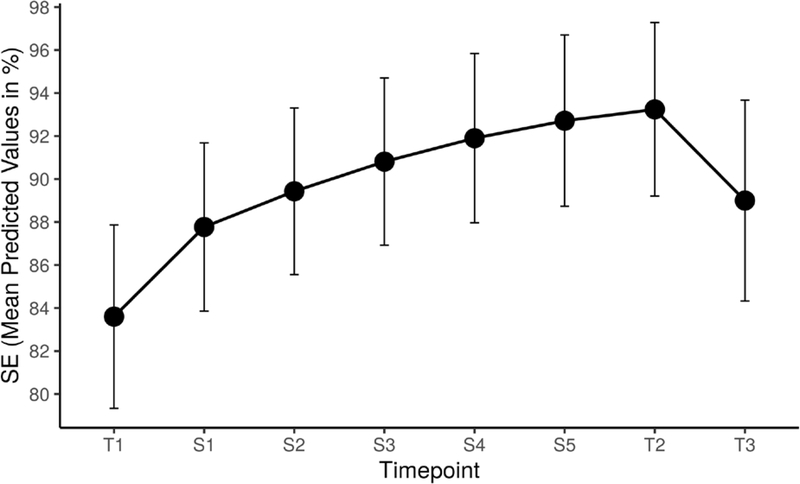
MLM analyses were conducted to examine CBTi.p. effects on subjective sleep outcomes across an amalgam of study timepoints and intervention sessions. Controlling for age and advanced disease, results were indicative of significant linear and quadratic effects of time on all outcomes across timepoints and embedded treatment sessions. CBTi.p. participants reported significant improvements in SE (0.9, SE=0.2, 95% CI=0.6 – 1.2) and SQ (0.04, SE=0.01, 95% CI=0.02 – 0.1) along with reductions in TWT [−4.8, SE=0.7, 95% CI=(−6.3) – (−3.3)], SOL [−2.1, SE=0.5, 95% CI=(−3.0) – (−1.2)], and WASO [−3.0, SE=0.5, 95% CI=(−4.1) – (−2.0)]. Participants further demonstrated: (1) initial reductions in SOL (0.2, SE=0.1, 95% CI=0.1 – 0.3) and WASO (1.1, SE=.4, 95% CI=0.2 – 2.0) that continued to gradually improve, (2) moderate improvements in SE [−0.1, SE=0.02, 95% CI=(−0.2) – (−0.1); Figure 4] and SQ [<−0.01, SE<0.01, 95% CI=(−0.01) – (<−0.01)] from T1-S6 followed by an expected decline between T2 and T3, and (3) reductions in TWT (0.3, SE=0.1, 95% CI=0.1 – 0.4) across T1-S6 followed by an expected loss of intervention effect between T2 and T3. There were no significant effects of age and advanced disease across sleep outcomes.
Figure 4.
Supplemental Analysis Examining the Effects of Cognitive Behavioral Therapy for Insomnia and Pain (CBTi.p.) on Sleep Efficiency (SE). Exploratory analyses were conducted to examine CBTi.p. effects on subjective SE (and other sleep outcomes) across an amalgam of study timepoints and intervention sessions (i.e., T1, S1-S6, T2, T3; see text). Controlling for age and advanced disease, significant main effects of linear and quadratic time demonstrated that CBTi.p. participants experienced significant improvements in subjective SE that then declined between T2 and T3. In the pictured line graph, the x-axis represents the study (T1-T3) and session (S1-S5) timepoints while the y-axis represents SE sores in percentages. Of note, S6 is not represented on the x-axis given that data from this final intervention session is fully captured in T2.
Discussion
This RCT examined effects of CBTi.p. versus PE on sleep outcomes in women with insomnia status/post-surgery for gynecologic cancer. Given extensive corroboration of empirical findings outlining the unique yet analogous dimensions measured across subjective and behavioral measures of sleep outcomes (findings which were replicated in the current study) (Aili et al., 2017), both sleep diary (subjective) and actigraphy (behavioral) data were utilized in efforts to maintain adherence to validated methods and strengthen interpretation of outcomes through the implementation of correspondent comprehensive measures. Results were indicative of (1) longitudinal improvements to subjective SOL and subjective and behavioral SE, SQ, TWT, and WASO and (2) group differences on subjective SE, TWT, and SOL, with CBTi.p. participants demonstrating higher SE and lower TWT and SOL compared to PE participants. There were no significant group-by-time effects.
Significant improvements in sleep across time and between groups throughout the acute post-operative phase of cancer treatment is a key finding warranting consideration given previous research highlighting the prevalence, persistence, and adverse impacts of sleep dysregulations in gynecologic cancer populations across the diagnostic and treatment spectrum (Clevenger et al., 2013). These trends may reflect a regression to the mean given a sample of women with early stage, good prognosis cancers and/or both CBTi.p. and PE confer benefits on sleep outcomes.
Consistent with previous findings that corroborate CBTi as an effective treatment for insomnia in medical populations (Espie et al., 2008), group main effects revealed that CBTi.p. participants had higher SE and lower TWT and SOL across timepoints compared to PE participants. Although there were no significant group differences in rate of change across time, group main effects are encouraging of additional investigation.
Clinical significance testing indicated lower TWT at T2 and T3, lower SOL at T3, and higher SE at T3 for CBTi.p. participants compared to PE participants. Within CBTi.p, findings revealed significant linear and quadratic time effects across all outcomes, with greater change magnitudes following the initial CBTi.p. session. Participants demonstrates ongoing gradual improvements across sessions followed by worsening sleep outcomes about seven weeks following S6.
Regarding the lack of significant intervention effects, results were impacted by low power given the study’s small sample size and utilization of complex statistical modelling. Loss of treatment effects at T3 may have blunted longitudinal CBTi.p. trends, restricted between-group variability in rates of change across outcomes and overall detectability of intervention effects when modeling primary analyses that do not incorporate individual session data.
Previous RCTs examining CBTi within cancer populations primarily enrolled patients at posttreatment (Garland et al., 2014). Given that current study participants were enrolled pretreatment and many underwent adjuvant chemotherapy (N=14) and/or radiotherapy (N=7) throughout the active trial period, cancer treatment may have equally impacted sleep across groups resulting in similar outcomes. CBTi.p. effects may have further attenuated by clinical benefits derived by PE participants from individualized education on managing cancer outcomes from master’s level clinicians. Similar findings were described by Dirksen and colleagues (2020) after examining CBTi effects versus sleep education on insomnia in breast cancer survivors.
This trial had several limitations. Sample size adversely impacted power, increasing the probability of a Type II error. Sample size and power were further impacted by stringent preliminary and post-enrollment eligibility criteria. Participant homogeneity limited generalizability to Caucasian, non-Hispanic women with endometrial cancer. The absence of diagnostic data on chronicity of insomnia may have impacted time-dependent results given susceptibility of patients with acute insomnia to time-sensitive/cause-specific sleep difficulties. Between-group treatment credibility analyses could not be conducted given that treatment credibility was only evaluated within the CBTi.p. group. However, the fact that there were no significant differences in attrition and number of sessions attended between the CBTi.p. and PE participants lends some support to the notion that treatment credibility may not have been substantially lower in the PE group. Furthermore, although flexibility in treatment location may have enhanced external validity, it is possible that certain treatment locations may have been associated with differential treatment effects. This should be explored in future research. Finally, participants were not blinded to study aims or conditions; thus, it is possible that placebo and/or nocebo responses may have influenced participants’ outcomes in some systematic way.
Study strengths included the effective implementation of a first-line intervention within a hard-to-reach population reporting unmet needs. The study followed these patients throughout diagnosis and treatment which, while a novel approach within psycho-oncology literature, presented significant recruitment and retention challenges. The intervention was credible to CBTi.p. participants, who reported heightened subjective views of the intervention as a logical and effective treatment for insomnia and other psychophysiological factors. There was also high treatment integrity amongst CBTi.p. interventionists, who were rated by participants as highly competent and likable. The trial employed an active control group and single-blind measures to assess treatment efficacy while controlling for confounds. The study utilized PSG as a physiological sleep measure to rule out OSA across T1-T3. Overall, the study incorporated rigorous eligibility criteria alongside cutting-edge methodology to examine adverse outcomes in an underserved, hard-to-reach oncology population.
This study provided support for the clinical benefits of CBTi.p. on insomnia within gynecology oncology. Given evidence for sleep dysfunctions as an adverse cancer outcome and the concomitant ameliorative effects of CBTi on comorbid mood and physiological symptoms associated with insomnia (Manber et al., 2008), CBTi.p. has the potential to meet clinical needs of oncology patients while helping improve health-related QOL. Future studies should implement large, multi-arm RCTs that include a standard care group to further elucidate findings. Studies may benefit from a multi-site approach and inclusion of patients with chronic insomnia. Research should focus on increased enrollment of marginalized, underrepresented groups.
Acknowledgements:
We would like to thank all participants, corresponding authors, and clinicians and staff members at the UF Health Cancer Center’s Gynecologic Oncology Clinic for their dedication and helpful collaboration.
Funding:
This work was supported by the National Institutes of Health under Grant R01 CA138808.
Footnotes
Competing interests: The authors declare that there are no conflicts of interest.
References
- American Cancer Society (ACS). 2020. Cancer facts & figures. Atlanta: American Cancer Society. [Google Scholar]
- Aiken LS, & West SG (1991). Multiple regression: Testing and interpreting interactions. Sage. [Google Scholar]
- Aili K, Åström-Paulsson S, Stoetzer U, Svartengren M, & Hillert L (2017). Reliability of Actigraphy and Subjective Sleep Measurements in Adults: The Design of Sleep Assessments [research-article]. 13(1), 39–47. 10.5664/jcsm.6384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH (2003). Stress Management Intervention for Women With Breast Cancer: Training Manual & Participant’s Workbook. American Psychological Association. [Google Scholar]
- Bergerot CD, Clark KL, Obenchain R, Philip EJ, & Loscalzo M (2018). Breast and gynecological cancer patients’ risk factors associated with biopsychosocial problem-related distress. Psychooncology, 27(3), 1013–1020. 10.1002/pon.4607 [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- Clevenger L, Schrepf A, Christensen D, DeGeest K, Bender D, Ahmed A, Goodheart MJ, Penedo F, Lubaroff DM, Sood AK, & Lutgendorf SK (2012). Sleep disturbance, cytokines, and fatigue in women with ovarian cancer. Brain, Behavior, and Immunity, 26(7), 1037–1044. 10.1016/j.bbi.2012.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevenger L, Schrepf A, Degeest K, Bender D, Goodheart M, Ahmed A, Dahmoush L, Penedo F, Lucci J, Thaker PH, Mendez L, Sood AK, Slavich GM, & Lutgendorf SK (2013). Sleep Disturbance, Distress, and Quality of Life in Ovarian Cancer Patients During the First Year After Diagnosis. Cancer, 119(17), 3234–3241. 10.1002/cncr.28188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson JR, MacLean AW, Brundage MD, & Schulze K (2002). Sleep disturbance in cancer patients. Social Science & Medicine, 54(9), 1309–1321. https://doi.org/0277-9536/02/$ [DOI] [PubMed] [Google Scholar]
- Dirksen SR, & Epstein DR (2020). Efficacy of an insomnia intervention on fatigue, mood and quality of life in breast cancer survivors. Journal of Advanced Nursing, 61(6), 664–675. 10.1111/j.1365-2648.2007.04560.x [DOI] [PubMed] [Google Scholar]
- Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, Jamieson AO, McCall WV, Morin CM, & Stepanski EJ (2004). Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep, 27(8), 1567–1596. [DOI] [PubMed] [Google Scholar]
- Espie CA, Fleming L, Cassidy J, Samuel L, Taylor LM, White CA, Douglas NJ, Engleman HM, Kelly HL, & Paul J (2008). Randomized Controlled Clinical Effectiveness Trial of Cognitive Behavior Therapy Compared With Treatment as Usual for Persistent Insomnia in Patients With Cancer. Journal of Clinical Oncology, 26(28), 4651–4658. 10.1200/JCO.2007.13.9006 [DOI] [PubMed] [Google Scholar]
- Garland EL, Manusov EG, Froeliger B, Kelly A, Williams JM, & Howard MO (2014). Mindfulness-oriented Recovery Enhancement for Chronic Pain and Prescription Opioid Misuse: Results From an Early-Stage Randomized Controlled Trial. Journal of Consulting and Clinical Psychology, 82(3), 448–459. 10.1037/a0035798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Asnaani A, Vonk IJ, Sawyer AT, & Fang A (2012). The Efficacy of Cognitive Behavioral Therapy: A Review of Meta-analyses. Cognitive Therapy and Research, 36(5), 427–440. 10.1007/s10608-012-9476-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honerlaw KR, Rumble ME, Rose SL, Coe CL, & Costanzo ES (2016). Biopsychosocial predictors of pain among women recovering from surgery for endometrial cancer. Gynecologic Oncology, 140(2), 301–306. 10.1016/j.ygyno.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husak AJ, & Bair MJ (2020). Chronic Pain and Sleep Disturbances: A Pragmatic Review of Their Relationships, Comorbidities, and Treatments. Pain Medicine, pnz343. 10.1093/pm/pnz343 [DOI] [PubMed] [Google Scholar]
- Johnson JA, Rash JA, Campbell TS, Savard J, Gehrman PR, Perlis M, Carlson LE, & Garland SN (2016). A Systematic Review and Meta-Analysis of Randomized Controlled Trials of Cognitive Behavior Therapy for Insomnia (CBT-I) in Cancer Survivors. Sleep Medicine Reviews, 27, 20–28. 10.1016/j.smrv.2015.07.001 [DOI] [PubMed] [Google Scholar]
- Knoerl R, Lavoie Smith EM, & Weisberg J (2016). Chronic Pain and Cognitive Behavioral Therapy: An Integrative Review. Western Journal of Nursing Research, 38(5), 596–628. 10.1177/0193945915615869 [DOI] [PubMed] [Google Scholar]
- LIVESTRONG Guidebook. (2015). LIVESTRONG Foundation. [Google Scholar]
- Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, & Kalista T (2008). Cognitive Behavioral Therapy for Insomnia Enhances Depression Outcome in Patients with Comorbid Major Depressive Disorder and Insomnia. Sleep, 31(4), 489–495. 10.1093/sleep/31.4.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae CS (2014). Cognitive Behavioral Therapy for Insomnia (CBTi) University of Florida. [Google Scholar]
- McCrae CS, Williams J, Roditi D, Anderson R, Mundt JM, Miller MB, Curtis AF, Waxenberg LB, Staud R, Berry RB, & Robinson ME (2019). Cognitive behavioral treatments for insomnia and pain in adults with comorbid chronic insomnia and fibromyalgia: Clinical outcomes from the SPIN randomized controlled trial. Sleep, 42(3), zsy234. 10.1093/sleep/zsy234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palesh OG, Roscoe JA, Mustian KM, Roth T, Savard J, Ancoli-Israel S, Heckler C, Purnell JQ, Janelsins MC, & Morrow GR (2010). Prevalence, Demographics, and Psychological Associations of Sleep Disruption in Patients With Cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. Journal of Clinical Oncology, 28(2), 292–298. 10.1200/JCO.2009.22.5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples AR, Garland SN, Perlis ML, Savard J, Heckler CE, Kamen CS, Ryan JL, Mustian KM, Janelsins MC, Peppone LJ, Morrow GR, & Roscoe JA (2017). Effects of Cognitive Behavioral Therapy for Insomnia and Armodafinil on Quality of Life in Cancer Survivors: A Randomized Placebo-Controlled Trial. Journal of Cancer Survivorship, 11(3), 401–409. 10.1007/s11764-017-0597-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscoe JA, Kaufman ME, Matteson-Rusby SE, Palesh OG, Ryan JL, Kohli S, Perlis ML, & Morrow GR (2007). Cancer-related fatigue and sleep disorders. Oncologist, 12(Suppl 1), 35–42. 10.1634/theoncologist.12-S1-35 [DOI] [PubMed] [Google Scholar]
- Savard J, Ivers H, Savard MH, & Morin CM (2016). Long-Term Effects of Two Formats of Cognitive Behavioral Therapy for Insomnia Comorbid with Breast Cancer. Sleep, 39(4), 813–823. 10.5665/sleep.5634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrepf A, Clevenger L, Christensen D, DeGeest K, Bender D, Ahmed A, Goodheart MJ, Dahmoush L, Penedo F, Lucci JA 3rd, Ganjei-Azar P, Mendez L, Markon K, Lubaroff DM, Thaker PH, Slavich GM, Sood AK, & Lutgendorf SK (2013). Cortisol and inflammatory processes in ovarian cancer patients following primary treatment: relationships with depression, fatigue, and disability. Brain, Behavior, and Immunology, 30 Suppl, S126–S134. 10.1016/j.bbi.2012.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JA, Mancl L, & Aaron LA (2006). Short- And Long-Term Efficacy of Brief Cognitive-Behavioral Therapy for Patients With Chronic Temporomandibular Disorder Pain: A Randomized, Controlled Trial. Pain, 121(3), 181–194. 10.1016/j.pain.2005.11.017 [DOI] [PubMed] [Google Scholar]
- Waxenberg LB (2008). Pain Management Workbook: A Guide for Behavioral Pain Management Techniques. University of Florida. [Google Scholar]