Abstract
Migraine patients suffer from high morbidity related to the repeated headache attacks characteristic of the disorder, poor sleep and a high prevalence of co-morbid psychosocial disorders. Current pharmacological therapies do not address these aspects of migraine, but non-pharmacological treatments such as Mindfulness Based Stress Reduction (MBSR) have been shown to improve both pain and psychological well-being. In this secondary analysis, we examined the change over time in sleep quality and psychosocial outcomes from the MRI Outcomes for Mindfulness Meditation Clinical Trial and assessed how these mediated treatment response (50% reduction in headache frequency post-intervention). We also examined the relationship between baseline values and treatment response.
The trial (primary outcomes previously reported) included 98 episodic migraine patients randomized to either enhanced MBSR (MBSR+) or stress management for headache (SMH). They completed psychosocial questionnaires and headache diaries at baseline (pre-intervention), mid-intervention (10 weeks post baseline) and post-intervention (20 weeks post baseline). There was a significant improvement in sleep quality from baseline to post-intervention (p=0.0025), in both groups. There were no significant changes from baseline or between groups in anxiety, depression and stress. There was also no significant association between baseline scores and treatment response. Mediation analysis showed a significant indirect effect of 6% for sleep: in other words, small improvements in sleep may have contributed to the efficacy of MBSR+.
Trial registration:
Introduction
Migraine is a chronic, neurological disorder affecting 10–15% of the general population [20]. Anxiety and depression are more prevalent in migraine patients compared to the general population [8], stress is one of the most common migraine triggers [26,47], and sleep disturbances are common in migraine [16,27,45]. While the biological mechanisms associating these factors with migraine are unclear, the relationship is likely bi-directional, as patients with anxiety and depression report greater migraine frequency [17,25,27,38,49].
Co-morbid conditions complicate treating migraine. Current migraine therapies either reduce pain or prevent migraines from occurring [38]. However, relief from migraine is a combination of the physical components (e.g. pain, nausea), and the affective factors, all of which may be improved by non-pharmacological interventions such as mindfulness based stress reduction (MBSR) [2,18]. Though some mindfulness studies in headache patients report improvements in headache frequency [2,6], pain intensity and quality of life compared to usual care, findings are inconsistent. Two recent reviews found psychological therapies, including mindfulness, did not significantly improve headache frequency compared to controls [1,36]. Similarly, a recent clinical trial for MBSR compared to headache education showed no treatment group differences [46]. Though evidence for the efficacy of mindfulness for migraine is mixed, heterogeneity in results might be due to small sample sizes and differences in study design, including a lack of an active control arm [11,21,22,29,44].
Considering psychological endpoints, MBSR is associated with reductions of stress, anxiety and depression in healthy populations, pain and cancer patients [13,35,41], but there is variability and small to moderate effect sizes depending on the pain population [10,29]. In migraine, Wells et al. found that patients who received MBSR had greater improvement in depression compared to headache education [46]. Others have shown improved anxiety in migraine patients but lack of statistical power limits interpretation [37,47]. MBSR shows only moderate evidence for sleep improvement and only in studies with non-specific active controls [30]. Evidence in other populations shows that individual differences in these psychological factors can play a role in the treatment success of MBSR [9,14,15,24]. Distress tolerance and social anxiety have been shown to be moderators of MBSR [9,15]. Given that MBSR may improve psychosocial factors and increased stress, anxiety, depression and sleep can trigger migraines, it is plausible that MBSR operates through one or several of these pathways to reduce headache frequency. Neither treatment moderating factors nor potential mediators of treatment response have been examined in the headache literature on mindfulness.
In this study, we leverage the sample size, the use of an active control arm and a more targeted intervention we call enhanced MBSR (MBSR+) [32], which we previously reported has efficacy for migraine [32]. We hypothesized: a) compared to SMH, patients randomized to MBSR+ have greater improvement in sleep quality, anxiety, depression and stress symptoms over time; b) higher values (i.e. poor sleep, worse anxiety etc.) of baseline sleep quality, anxiety, depression and stress symptoms are associated with treatment response to MBSR+ and; c) sleep quality, anxiety, depression and stress symptoms mediate the MBSR+ treatment response.
Methods
Clinical Trial Design
This secondary analysis utilized data from the MRI Outcomes of Mindfulness Meditation for Migraine clinical trial (NCT02133209) [32]. Participants were enrolled from June 2014 to February 2017. The primary objective of the clinical trial was to determine the short and long-term efficacy of MBSR+ on headache frequency, brain structure and function. This was a randomized placebo-controlled trial with single masking. Participants were excluded if they reported severe or unstable psychiatric conditions, but patients with anxiety and or depression were eligible for inclusion. All migraine patients had episodic migraine (EM) and were randomly assigned to receive either MBSR+ or SMH. Patients were randomized 1:1 to either treatment in pre-specified blocks, using a web-based randomization system. Randomization was stratified by the presence or absence of another chronic pain disorder and by headache frequency: 4–8 (low frequency) or 9–14 (high frequency) headache days per 28 days. The clinical trial also enrolled 30 healthy pain free controls, matched to the migraine patients on age (±5 years), sex, body-mass index (BMI) (±5), education (college and no college) and race. Data from healthy controls were not utilized in these analyses. Each treatment was administered weekly for 8 weeks with an additional half day retreat for the MBSR+ arm and then both treatments were administered for an additional 4 sessions over 8 weeks. Full inclusion and exclusion criteria have been previously reported [32].
Outcome Measures
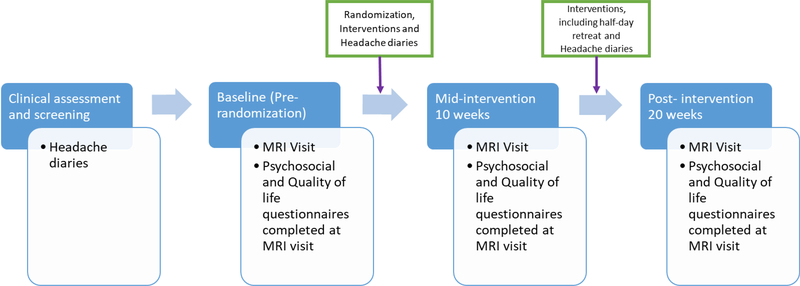
Patients were assessed at three time points: baseline (pre-randomization), mid-intervention (week 10) and post-intervention (week 20). The mid-intervention assessment occurred following the first 8 weeks of intervention and the post-intervention assessment occurred following the second 8 weeks (Figure 1).
Figure 1: Flow diagram to illustrate the ordering of study activities.
Blue rectangles represent the time periods in the study from pre-baseline when patients were enrolled to post-intervention when patients had completed the last intervention session. Boxes outlined in blue at the bottom highlight activities that take place at the MRI visits at each time point. Boxes with a green outline detail activities that take place between each MRI session. All participants completed headache diaries prior to randomization and the commencement of intervention. Headache diaries were collected over a 28-day period prior to randomization. After the initial 8 weeks of intervention, participants competed their second 28 day headache diary. After the completion of the second 8 weeks of intervention, participants completed their post-intervention 28 day headache diary. All participants completed an MRI visit and the associated questionnaires at baseline prior to the randomization and intervention. The second MRI visit was conducted after the initial 8 weeks of intervention and the third was conducted after the second 8 weeks of intervention.
Headache Frequency
Daily headache diaries were completed electronically (online via a link sent through email) and collected detailed information on headaches over a 28-day period (e.g. pain intensity, headache duration and other symptoms). Headache diaries were used to ascertain clinical endpoints including headache frequency at baseline and over time. Given the nature of headache diaries not all patients completed a full 28 days. To address this, we calculated a proportion for everyone, by dividing the number of headache days reported by the total number of diary days collected in that period. For any given individual the maximum denominator was 28. Additionally, we multiplied the proportion by 28 to get a continuous variable for headache days. In final models, we utilized treatment response as the main clinical headache end-point. To calculate the treatment response in the sample we used the above proportion and the standardized headache days variable and calculated the difference between baseline and the post-intervention time point. Treatment responders were defined as achieving at least a 50% reduction in headache frequency from baseline to post-intervention (week 20). This end point is consistent with several studies for migraine preventative medications such as topiramate and non-pharmacological treatments like acupuncture [12,19,40].
Sleep quality and psychosocial questionnaires
Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI) questionnaire [3], a 23 item scale that assesses the quality of sleep over the last month with possible scores ranging from 0–21 [3]. Anxiety was assessed using the Generalized Anxiety Disorder (GAD-7) questionnaire [28,33], a 7 item questionnaire that measures common symptoms of anxiety with scores ranging from 0–21. Depression was measured with the Patient Health Questionnaire-9 (PHQ-9) [34], a nine item questionnaire for depressive symptom severity over the last two weeks with scores ranging from 0–27. Stress was assessed with the Perceived Stress Scale (PSS) [5,23], a 10 item questionnaire that measures an individual’s perceived stress to everyday life situations over the last month with scores ranging from 0–40. Questionnaire scores were also categorized according to validated cut points. PSQI total scores ≥5 indicate poor sleep quality [3]. GAD-7 was categorized into four groups: 0–4 (no anxiety symptoms), 5–9 (mild anxiety symptoms), 10–14 (moderate anxiety symptoms), 15≤ (severe anxiety symptoms) [28,33]. Similarly, PHQ-9 was separated into 5 categories: (0–4, 5–9, 10–14, 15–19 and ≥20) to represent no depression symptoms, mild, moderate, moderately severe and severe depression symptoms [34]. PSS was categorized into three groups where 0–13 is average, 14–26 is moderate stress and high stress is 27–40 [5,23]. Finally, patients were assessed as either having symptoms or not for all of the above questionnaires (e.g. depression symptoms yes/no) using the minimum cut point for each (e.g. score of 5 on the PSQI questionnaire).
Statistical Analysis
Demographic and Clinical Variables
Distributions of baseline patients’ characteristics between those randomized to MBSR+ and SMH and differences between treatment responders and non-responders were assessed utilizing Chi-square, t-tests, and nonparametric Wilcoxon and Kruskal-Wallis tests. Baseline characteristics included headache frequency, presence of an additional idiopathic pain condition, education, age, sex, race and BMI. In addition to sleep quality and psychosocial factors, the following clinical headache characteristics were examined: headache days, mean headache pain and headache impact test (HIT-6) scores. All analyses utilized intention to treat (ITT) analysis, therefore patients were analyzed as randomized.
Change in sleep quality and psychosocial symptom scores over time
Exploratory data analysis examined the distributions of baseline continuous scores and the change in scores over time (baseline, 10 and 20 weeks) of all questionnaires (Supplementary Table 1). Wilcoxon, ANOVA and repeated measures ANOVA were used to assess differences in group, time and group by time interactions. Several regression diagnostic measures were utilized to examine the linearity of variables as outcomes and to assess the correlation of these variables as predictors. Using variance inflation factor (VIF), Cook’s D, plots of both the raw and scaled residuals and predicted values we examined the assumptions of normality, linearity and independence of these variables as both outcome and predictive measures. Given the skewed nature of PSQI, GAD-7 and PHQ-9 we also assessed the utility of log transformation on these factors. Using mixed effects model diagnostics it was determined to use the untransformed continuous scores in all final models.
All final analyses used mixed effect models and a random intercept was included in the final model for depression only as this was the only outcome where this approach improved the model fit. All models were corrected for multiple comparisons, utilized an unstructured covariance and assessed differences between MBSR+ and SMH groups, change from baseline and group by time interactions. Models were assessed using the Akaike Information Criterion (AIC) as well as the impact of additional variables on the standard error.
Baseline sleep quality and psychosocial scores and the relationship with treatment response
PSQI, GAD-7, PHQ-9 and PSS scores are known to be related and initial analyses assessed the statistical correlation using Spearman’s Rho as well as their impact on each other by examining collinearity diagnostics. Rho values equal to or more then 0.5 as well as a p value ≤0.05 were assessed further. We assessed condition index, variance inflation factor, tolerance and proportion of variance values. It was determined that each predictor would be modeled separately to assess their individual association with treatment response.
Individual models assessed continuous baseline sleep and psychosocial variables as the predictors, adjusting for treatment group. We assessed interactions between treatment group and baseline values for sleep and psychosocial factors but there were none and thus final models do not stratify by group. Confounders of interest at baseline were BMI, education, race, sex, age, idiopathic pain condition and HIT-6. We also assessed the role of baseline mindfulness and pain catastrophizing as potential confounders but inclusion did not significantly affect the model or change the size of the estimates for the psychosocial variables of interest. Final models were assessed as described above. All statistical analyses were conducted using SAS (v.9.4, SAS Institute Inc. Cary, NC). Testing was two-sided and done at the 0.05 level of significance.
Mediation of Treatment Response by Change Sleep Quality over Time
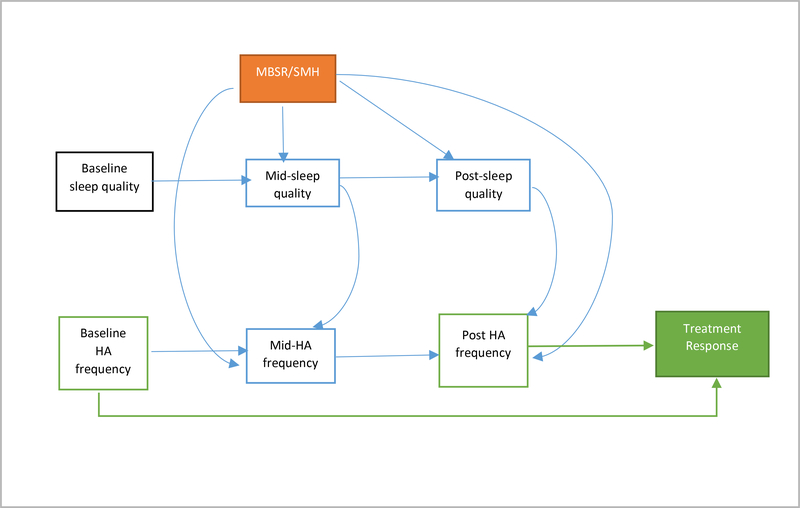
We examined the mediation of treatment effects (MBSR+ vs. SMH) on treatment response through changes in sleep. To assess the presence of mediation we employed the mediation analysis methods for binary outcomes and multiple mediators as outlined by VanderWeele [42] and VanderWeele and Vansteelandt [43]. Figure 2 depicts the directed acyclic graph (DAG) that guided the development of the final models. The weighting method developed by VanderWeele and Vansteelandt utilizes an inverse probability of treatment weighting approach to estimate the direct and indirect effects even when mediators impact each other [43].
Figure 2: Causal diagram to represent plausible mediation relationship between MBSR+ and treatment response.
Arrows assume direction of causal relationships. Model assumes no unmeasured confounding. Black boxes indicate baseline covariates. Solid black arrows represent adjusted confounding. The orange box indicates the randomized treatment MBSR+ vs. SMH. The solid green box is the outcome which is treatment response. This variable is created from baseline and post-intervention headache frequency (green outlined boxes) Blue boxes represent possible mediators of the relationship between exposure and outcome. Baseline covariates were measured prior to randomization and therefore are not affected by the treatment. Mid-point (10 weeks post baseline) sleep quality, anxiety, depression and stress were measured at the scan visit directly after the completion of the first block of treatment. Headache diaries were completed for 28 days post the first block of treatment and therefore are captured after the psychosocial measurements. Post-treatment (20 weeks post baseline) psychosocial measurements were taken at the scan visit directly after the final treatment block had been completed. These visits usually occur over a two-week period post study completion. Headache diaries are completed for 28 days post treatment.
This approach estimates three weighted averages which correspond to the following counterfactuals: E[Treatment response a*Ma*], E[Treatment response aMa] and E[Treatment response aMa*] where a= MBSR+ and a*= SMH and M = mediator vector. Each is calculated in a three step process. For E[Treatment response a*Ma*], the first step fits a logistic regression model using a mediator vector (this included sleep and headache frequency reported at 10 weeks) and baseline covariates to estimate predicted probabilities of P(SMH|ci). Secondly a weighted average of the predicted values for subjects is calculated by P(A=SMH)/P(A=SMH|ci). The final step utilizes the weight in a regression model to estimate the value of E[Treatment response a*Ma*]. The process is repeated for each counterfactual component. The direct effect is given by E[Treatment responseaMa*]−E[Treatment responsea*Ma*] and the indirect effect: E[Treatment responseaMa] − E[Treatment responseaMa*].
We adjusted for the baseline values of the anxiety, stress and depression. To account for mediator-outcome confounding, headache frequency at mid-intervention is included in the mediator vector. Exposure-mediator (treatment group*sleep) interactions and interactions between sleep and other psychosocial factors were assessed, however these models did not differ significantly from those without interaction. Given that no interactions existed, the natural direct effect is assumed equivalent to the controlled direct effect. To calculate standard errors and 95% confidence intervals for each estimate a bootstrapping approach was employed. This process generated 1000 samples with replacement after which counterfactual estimates and confidence intervals were generated for all samples. Confidence intervals that did not include the null were significant. Analyses were conducted using SAS (v.9.4, SAS Institute Inc. Cary, NC).
Power for this analysis was calculated using the powerMediation package in R™ for dichotomous outcomes. Assuming a sample size of 98 (49 per group), power 0.8, standard deviation of the mediator 0.4, correlation between the predictor and mediator 0.3, alpha 0.05 and 30% marginal prevalence of the outcome, the minimal detectable difference attributable to the mediator will be an OR of 1.62.
Results
Description of baseline demographic and clinical measures in episodic migraine patients
Of the 583 patients screened, 122 completed baseline assessments and 98 were eligible and randomized to receive either MBSR+ (n = 50) or SMH (n = 48). All 98 patients were included in the analyses examining change in sleep and psychosocial metrics over time. Treatment response analyses were reduced to the 95 patients with baseline and post-intervention follow-up data that was needed to assess the outcome.
Migraine patients randomized to MBSR+ or SMH were of comparable age, race, sex, BMI and educational attainment. Both groups were predominantly female, White, college educated with a median age of 36 (Table 1). Clinical characteristics, particularly those related to migraine, were also similar between the randomized groups. The proportion of headache days ranged from 0.11–0.5 in both groups with an average value of 0.25 in the SMH group and 0.29 in the MBSR+ group. Overall 29% of patients reported poor sleep quality, 13% of all patients met criteria for anxiety symptoms, 20% for depression symptoms, and 39% for stress. There was no significant difference in the proportions between MBSR+ and SMH groups (Supplementary Table 2).
Table 1:
Baseline clinical and demographic variables in episodic migraine patients randomized to either MBSR+ or SMH
| Variable | MBSR+(n=50) | SMH (n=48) | p valuea |
|---|---|---|---|
| Sex (%) | |||
| Male | 3 (6) | 6 (12.5) | 0.31 |
| Female | 47 (94) | 42 (87.5) | |
| Age/median (range) | 36 (18–65) | 35.5 (21–63) | 0.44 |
| Race/n (%) | |||
| Black | 10 (20) | 7 (14.58) | 0.79 |
| White | 35 (70) | 36 (75) | |
| Other | 4 (8) | 5 (10.42) | |
| Unknown | 1(2) | 0 | |
| Education/ n (%) | |||
| Some College or Less | 13 (26) | 7 (14.58) | 0.16 |
| College and Above | 37 (74) | 41 (85.42) | |
| BMI/median (range) | 27.30 (15.66–47.44) | 25.71 (16.63–45.45) | 0.79 |
| Migraine Qualities at Baseline | |||
| Low Frequency (4–8 days)/n (%) | 25 (50) | 25 (52.08) | 0.84 |
| High Frequency (9–14 days)/n (%) | 25 (50) | 23 (47.92) | |
| Headache Days/median (range) | 8 (3–14) | 7 (3–14) | 0.83 |
| Headache Pain/mean±SD | 4.72±1.65 | 4.31±1.57 | 0.22 |
| HIT-6 Total mean±SD | 61±5.83 | 60.85±5.586 | 0.52 |
| Idiopathic Pain/n (%) | |||
| Yes | 15 (30) | 13 (27.08) | 0.75 |
| No | 35 (70) | 35 (72.92) | |
| Preventative Migraine Medication Use/n (%) | |||
| Yes | 11 (22) | 4 (8.33) | 0.06 |
| No | 39 (78) | 44 (91.67) | |
| Sleep quality and psychosocial measures/ Median (range) | |||
| PSQI | 3.5(0– 11.67) | 4.67 (0–14) | 0.77 |
| GAD-7 | 1 (0–16) | 1.5 (0–16) | 0.08 |
| PHQ-9 | 2 (0–13) | 3 (0–7) | 0.07 |
| PSS | 9 (2–32) | 13 (1–26) | 0.18 |
Abbreviations: BMI: body mass index, HIT-6: headache impact test, PSS: perceived stress score, GAD-7: generalized anxiety disorder, PHQ-9: patient healthy questionnaire, PSQI: Pittsburgh sleep quality index
p values calculated from T tests, Chi Square, Wilcoxon, Kruskal Wallis tests.
Change over time in MBSR+ and SMH groups
Sleep Quality
Sleep quality results show that though patients randomized to MBSR+ on average had lower scores (better sleep quality) than those randomized to SMH, the difference between the groups was not significant (Table 2). However, the change in sleep quality across groups over time was significant. Patients reported improved sleep quality from baseline to week 10, as impairment was reduced by −0.13 (95% CI: −0.70, 0.45) and further at 20 weeks (−0.67, 95% CI: −1.10, −0.24) (Table 2). (See Supplementary Tables 1 and 2).
Table 2:
Adjusted analyses assessing effects of group and time on sleep and psychosocial factors in episodic migraine patients
| Variable | Mean Difference (95% Confidence Interval) | p valuea |
|---|---|---|
| Sleep Quality (PSQI) b | ||
| Group effect | ||
| MBSR+ vs. SMH | −0.42 (−1.39, 0.55) | 0.39 |
| Time effect | ||
| Δ 10 weeks | −0.13 (−0.70, 0.45) | 0.66 |
| Δ 20 weeks | −0.67 (−1.10, −0.24) | 0.0025 |
| Anxiety (GAD-7) c | ||
| Group Effect | ||
| MBSR+ vs. SMH | −0.13 (−0.71, 0.46) | 0.67 |
| Time Effect | ||
| Δ 10 weeks | −0.20 (−0.66, 0.25) | 0.38 |
| Δ 20 weeks | 0.14 (−0.26, 0.55) | 0.48 |
| Depression (PHQ-9) d | ||
| Random effects variance parameter | ||
| Intercept | 2.62 (1.85, 3.98) | |
| Residual | 2.23 (1.83, 2.78) | |
| Group Effect | ||
| MBSR+ vs. SMH | −0.14 (−0.88, 0.61) | 0.72 |
| Time Effect | ||
| Δ 10 weeks | 0.35 (−0.09, 0.80) | 0.12 |
| Δ 20 weeks | −0.11 (−0.57, 0.35) | 0.64 |
| Stress (PSS) e | ||
| Group Effect | ||
| MBSR+ vs. SMH | −0.93 (−2.49, 0.62) | 0.24 |
| Time Effect | ||
| Δ 10 weeks | 0.09 (−0.84, 1.03) | 0.85 |
| Δ 20 weeks | −0.49 (−1.65, 0.67) | 0.40 |
Abbreviations: PSS: perceived stress score, GAD-7: generalized anxiety disorder, PHQ-9: patient healthy questionnaire, PSQI: Pittsburgh sleep quality index
p value from linear mixed model
Model adjusted for stress, BMI and headache pain
Model adjusted for stress and depression.
Model adjusted for anxiety, headache impact score and additional idiopathic pain condition. Model includes random intercept.
Model adjusted for depression, anxiety, sleep and headache days
Psychosocial factors
Although patients randomized to MBSR+ had lower scores across all metrics, the difference between groups was not statistically significant (Table 2). With the exception of anxiety symptoms (an increase at 20 weeks), the scores for all other metrics had declined at 20 weeks. However, these were not significant (Table 2). (See Supplementary Tables 1 and 2).
Baseline sleep quality, anxiety, depression and stress symptom scores and the association with treatment response
Table 3 provides a description of the patients included in this analysis. There was no significant association between any baseline symptom score and treatment response (Table 4). There was no interaction between intervention arm and baseline psychosocial score. Therefore, results are not stratified by intervention arm.
Table 3:
Baseline clinical and demographic variables in episodic migraine patients by treatment responder status
| Variable | Treatment Responder | p valuea | |
|---|---|---|---|
| Yes (n=37) | No (n=58) | ||
| Sex (%) | |||
| Male | 2 (5.41) | 6 (10.34) | 0.48 |
| Female | 35 (94.59) | 52 (89.66) | |
| Age/median (range) | 35 (22–65) | 37 (18–63) | 0.77 |
| Race/n (%) | |||
| Black | 22 (59.46) | 47 (81.03) | 0.06 |
| White | 8 (21.62) | 8 (13.79) | |
| Other | 6 (16.22) | 3 (5.17) | |
| Unknown | 1 (2.7) | 0 | |
| Education/ n (%) | |||
| Some College or Less | 11 (29.73) | 8 (13.79) | 0.06 |
| College and Above | 26 (70.27) | 50 (86.21) | |
| BMI/median (range) | 28.64 (16.75–47.44) | 25.03 (15.66–45.45) | 0.05 |
| Migraine Qualities at Baseline | |||
| Low Frequency (4–8 days)/n (%) | 16 (43.24) | 32 (55.17) | 0.26 |
| High Frequency (9–14 days)/n (%) | 21 (56.76) | 26 (44.83) | |
| Headache Days/median (range) | 9 (5–13) | 8 (3–14) | 0.15 |
| Baseline Headache Pain/mean±SD | 4.74±1.38 | 4.35±1.59 | 0.22 |
| HIT-6 Total mean±SD | 60.59± 5.86 | 61± 5.40 | 0.73 |
| Idiopathic Pain/n (%) | |||
| Yes | 15 (40.54) | 12 (20.69) | 0.04 |
| No | 22 (59.46) | 46 (79.31) | |
| Preventative Migraine Medication Use b /n (%) | |||
| Yes | 8 (21.62) | 6 (10.34) | 0.13 |
| No | 29 (73.38) | 52 (89.66) | |
| Sleep quality and psychosocial measures/ Median (range) | |||
| PSQI | 4.67 (1.17–11.67) | 3.5 (0–14) | 0.07 |
| GAD-7 | 1 (0–16) | 1 (0–16) | 0.63 |
| PHQ-9 | 3 (0–13) | 2 (0–10) | 0.38 |
| PSS | 12 (2–28) | 11.5 (1–32) | 0.28 |
Abbreviations: HIT-6: headache impact test, PSS: perceived stress score, GAD-7: generalized anxiety disorder, PHQ-9: patient healthy questionnaire, PSQI: Pittsburgh sleep quality index
p values calculated from T tests, Chi Square, Wilcoxon, Kruskal Wallis tests.
Preventative medications cover four categories of medications which have been combined in this table.
Psychological medication covered three categories, which have been combined in this table.
Table 4:
Adjusted models to examine the association between continuous values of baseline sleep quality, psychosocial scores and treatment response
| Variable | Odds Ratio (95% CI)a | p valuea |
|---|---|---|
| Sleep Quality b | ||
| PSQI | 1.10 (0.94, 1.30) | 0.24 |
| Anxiety c | ||
| GAD-7 | 1.11 (0.95, 1.30) | 0.19 |
| Depression c | ||
| PHQ-9 | 1.13 (0.92, 1.38) | 0.25 |
| Stress c | ||
| PSS | 1.03 (0.96, 1.11) | 0.39 |
Abbreviations: PSQI: Pittsburgh Sleep Quality Index; GAD-7: generalized anxiety disorder; PHQ-9: patient healthy questionnaire; PSS: perceived stress score
p value from adjusted logistic regression model
Model adjusted for randomization group (MBSR+ vs. SMH), race and BMI
Models adjusted for randomization group (MBSR+ vs. SMH), additional idiopathic pain condition and BMI
Mediation of Treatment Response by Change in Sleep Quality over Time
Results from the preceding analyses indicate that there was no significant change in anxiety, depression or stress, and that baseline values were not associated with treatment response. Thus, mediation analyses were restricted to sleep quality, which showed a significant change over time. The total causal effect, that is the total difference in treatment response between those randomized to MBSR+ and SMH is 28% (Supplementary Table 3). The direct effect of MBSR+, that is, the effect of MBSR+ independent of any other pathways ranged from 22% (Table 5).The direct effect for the sleep model was 0.19 (95% CI 0.18, 0.2), indicating that 19% of the effect of MBSR+ is through pathways which do not include sleep. The indirect effect, the effect of MBSR+ mediated through pathways that included sleep, was 0.06 (95% CI: 0.05, 0.08). This shows that 6% of the effect of MBSR+ was through the improvement of sleep. This suggests that an additional 6% in treatment response could be seen in those randomized to SMH if their sleep quality had been set to that of those who received MBSR+ (Table 5).
Table 5:
Direct and indirect effects of MBSR+ on treatment response
| Effecta | Estimate | 95% CIb |
|---|---|---|
| Direct effect of MBSR | 0.19 | 0.18, 0.20 |
| Indirect effect through sleep | 0.06 | 0.05, 0.08 |
Model adjusted for baseline values of sleep, anxiety, stress and depression. Models includes headache frequency at mid-intervention in the mediator matrix to account for mediator outcome confounding.
Confidence intervals are highlighted in bold to identify that direct and indirect effects are significant
Discussion
We examined the longitudinal relationship between MBSR+ treatment, sleep quality and psychosocial symptoms in the context of a randomized trial using an active control for headache management. We found that sleep quality was significantly improved in both treatment groups at 20 weeks. There was no significant association between baseline sleep disturbance, anxiety, depression or stress and treatment response regardless of treatment condition. The total effect of MBSR+ on treatment response, operationalized as a 50% reduction in headaches at 20 weeks, included the indirect effect of improved sleep quality, in addition to direct effects of MBSR+.
The impact of MBSR on psychological well-being is inconsistent and, at best, modest. A meta-analysis of mindfulness studies with specific and non-specific control arms, across several patient groups reported an effect size of mindfulness on anxiety of 0.38 (95% CI 0.12–0.64) and 0.30 (95% CI 0.00–0.59) for depression at 8 weeks, both of which declined by 6 months [10]. Within pain populations (fibromyalgia and chronic back pain), these effects on depression and anxiety, were not significant [10]. When compared to active treatments, like acceptance based therapy, the effects of MBSR in chronic pain patients on anxiety and depression are small and not significant [44]. In chronic lower back pain patients randomized to MBSR, cognitive behavioral therapy or usual care, Cherkin and colleagues reported significant improvements in depression and anxiety up to 26 weeks post intervention [4]. Effects were seen at 8 weeks post intervention and group differences were most notable comparing MBSR to usual care. In our study, though not significant, group differences in GAD-7 score between MBSR+ and SMH was −0.13 in our migraine patients, which is similar to that observed comparing MBSR and CBT in chronic lower back pain patients at 8 week follow up (−0.18) [4]. A recent study by Wells et al. reported that migraine patients who received a standard MBSR had greater improvement in depression but not anxiety compared to education [46]. While this differs from our findings, patients in our study had baseline values for PHQ-9 and GAD-7 that were much lower than in the Wells study. This would suggest that there was less room for improvement and may explain why anxiety and depression showed no significant improvements over time in our study.
We do, however, see effects of treatment on sleep quality, yet no differential benefit of MBSR+ over SMH in reducing sleep disturbance. As with indices of psychological well-being, the impact of MBSR on sleep quality is varied. A systematic review assessing the effects of MBSR on sleep disturbance reported significant improvements in sleep quality in several clinical populations, including fibromyalgia [48]. Importantly significant improvements were only observed in uncontrolled studies, but was absent in those with an active control arm [31,48]. Similarly we observed significant improvements in sleep quality but there was no difference between our two active arms at 20 weeks, indicating that both treatments improved sleep quality. One possibility is that the improvement in sleep quality we observed was related to the reduction in headache frequency, rather than a direct effect of intervention. Findings from the main outcomes paper show that though MBSR+ patients had a significantly greater decrease in headache frequency, those in the SMH arm also saw long-term improvements [32]. Studies suggest that preventative migraine treatments and behavioral interventions such as CBT, and relaxation training also improve sleep in migraine patients [39]. Though patients in the SMH arm did not learn any specific mindfulness techniques, the program did provide discussions about lifestyle factors that can help modify stress and migraine triggers such as the importance of diet, exercise and sleep hygiene. The causal relationship between sleep and migraine continues to be investigated and while research suggest that it might be bi-directional, active behavioral interventions likely share components that improve both.
We observed an indirect effect of 6%, suggesting that some of the effect of MBSR+ in reducing headache frequency, may be mediated through pathways that include improvements in sleep quality. Other studies that have explored the mechanisms involved in mindfulness based cognitive therapy (MBCT) and the effect on depressive disorder found that the main predictive and mediating components were alterations in mindfulness, rumination, worry and self-compassion [41]. Patients who were more mindful saw greater improvement and improvement in depression was mediated by other negative affect components such as rumination and worry [41]. In headache patients, Day and colleagues assessed headache pain via the brief pain inventory score, and reported that pathways through pain acceptance were responsible for small reductions of pain scores [7]. These effects are comparable to our findings in that though the indirect effects were small, they still made up a significant proportion of the success of treatment. This small mediation effect and the absence of predictive value of baseline sleep and non-significant group differences over time are likely because our sample generally reported good sleep quality leaving little room for improvement. Previous studies have reported that, compared to chronic pain patients with co-morbid psychosocial symptomatology, patients with clinically diagnosed psychiatric disorders report greater symptom reduction after receipt of MBSR [13,35,41]. It is plausible that MBSR+ for migraine might be most effective in patients with high baseline levels of these co-morbid factors. Though there were some patients with anxiety and depression enrolled in the study, those with unstable or uncontrolled psychological disorders were excluded.
Strengths and Limitations
This is the largest RCT assessing mindfulness in migraine patients to date, providing the first comprehensive look at MBSR+ in episodic migraine. The active control treatment arm allowed us to make comparisons that are more appropriate and provided evidence that though MBSR+ does significantly reduce headache frequency, other active interventions such as SMH can be useful and likely share mechanisms of action. Stratified block randomization mitigated possible imbalances in risk factors between intervention groups and patient retention was excellent. All analyses used ITT, preserving the benefits of randomization. While patients randomized to SMH may have tried alternative stress management techniques or attempted MBSR on their own, the benefits of ITT outweigh the harms and the likelihood of patients trying both treatments is slim given the time burden associated.
However, our study does have some limitations. It is possible the study attracted migraine patients who are not representative of the general migraine population given their interest in non-pharmacological therapy. We recruited from two large health systems and academic campuses, and a large metropolitan community. This limited geographic scope, the demands of weekly and bi-weekly treatment sessions and the requirement to complete imaging likely limit the representativeness of the patient sample.
Through meditation analysis we show that small changes in sleep may have contributed to treatment response. One drawback is that negative affect and stress, while highly correlated, may also affect sleep. However, in separate models (not reported) we assessed the effect of each symptom - anxiety, depression, stress, separately and together and the effect was similar. This may suggest that MBSR+ works through the improvement of sleep quality; however given the small effect size, and the fact that there were no group differences in sleep quality over time, this mediation effect is likely only partially responsible for the change that we observe. Finally, there is always the concern of unmeasured confounding especially in light of the strict assumptions which must be employed with these analyses. The randomized design incorporated a rich collection of baseline covariates, diminishing this risk in this study.
Conclusions
MBSR+ may be a comprehensive therapeutic approach in migraine patients. Benefits may differ based on a patient’s baseline sleep quality, but that cannot be definitively concluded in this study. Further work incorporating a wider distribution of sleep profiles is needed. We also show that the active control arm, SMH, leads to improvement and opens the door for further exploration into the specific components of behavioral interventions that might be most effective. Importantly, MBSR+ provides patients with the opportunity to take responsibility and control over their care eliminating some of the barriers associated with traditional pharmacological options. Patients acquire lifelong skills which once maintained can improve their overall quality of life.
Supplementary Material
Acknowledgements
Funding: NCCIH/NIH R01 AT007176 to DAS.
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to declare.
Data availability:
Data available by request to corresponding author.
References
- 1.Anheyer D, Leach MJ, Klose P, Dobos G, Cramer H. Mindfulness-based stress reduction for treating chronic headache: A systematic review and meta-analysis. Cephalalgia. 2019;39(4):544–555. doi: 10.1177/0333102418781795 [doi]. [DOI] [PubMed] [Google Scholar]
- 2.Bakhshani NM, Amirani A, Amirifard H, Shahrakipoor M. The effectiveness of mindfulness-based stress reduction on perceived pain intensity and quality of life in patients with chronic headache. Glob J Health Sci. 2015;8(4):142–151. doi: 10.5539/gjhs.v8n4p142 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 0165-1781(89)90047-4 [pii]. [DOI] [PubMed] [Google Scholar]
- 4.Cherkin DC, Sherman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: A randomized clinical trial. JAMA. 2016;315(12):1240–1249. doi: 10.1001/jama.2016.2323 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 6.Day MA, Thorn BE, Rubin NJ. Mindfulness-based cognitive therapy for the treatment of headache pain: A mixed-methods analysis comparing treatment responders and treatment non-responders. Complement Ther Med. 2014;22(2):278–285. doi: 10.1016/j.ctim.2013.12.018 [doi]. [DOI] [PubMed] [Google Scholar]
- 7.Day MA, Thorn BE, Ward LC, et al. Mindfulness-based cognitive therapy for the treatment of headache pain: A pilot study. Clin J Pain. 2014;30(2):152–161. doi: 10.1097/AJP.0b013e318287a1dc [doi]. [DOI] [PubMed] [Google Scholar]
- 8.Fuller-Thomson E, Jayanthikumar J, Agbeyaka SK. Untangling the association between migraine, pain, and anxiety: Examining migraine and generalized anxiety disorders in a canadian population based study. Headache. 2017;57(3):375–390. doi: 10.1111/head.13010 [doi]. [DOI] [PubMed] [Google Scholar]
- 9.Gawrysiak MJ, Leong SH, Grassetti SN, Wai M, Shorey RC, Baime MJ. Dimensions of distress tolerance and the moderating effects on mindfulness-based stress reduction. Anxiety Stress Coping. 2016;29(5):552–560. doi: 10.1080/10615806.2015.1085513 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goyal M, Singh S, Sibinga EM, et al. Meditation programs for psychological stress and well-being: A systematic review and meta-analysis. JAMA Intern Med. 2014;174(3):357–368. doi: 10.1001/jamainternmed.2013.13018 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. J Psychosom Res. 2004;57(1):35–43. doi: S0022399903005737 [pii]. [DOI] [PubMed] [Google Scholar]
- 12.Ha H, Gonzalez A. Migraine headache prophylaxis. Am Fam Physician. 2019;99(1):17–24. doi: 14075 [pii]. [PubMed] [Google Scholar]
- 13.Hilton L, Hempel S, Ewing BA, et al. Mindfulness meditation for chronic pain: Systematic review and meta-analysis. Ann Behav Med. 2017;51(2):199–213. doi: 10.1007/s12160-016-9844-2 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu SH, Collins SE, Marlatt GA. Examining psychometric properties of distress tolerance and its moderation of mindfulness-based relapse prevention effects on alcohol and other drug use outcomes. Addict Behav. 2013;38(3):1852–1858. doi: 10.1016/j.addbeh.2012.11.002 [doi]. [DOI] [PubMed] [Google Scholar]
- 15.Jazaieri H, Lee IA, Goldin PR, Gross JJ. Pre-treatment social anxiety severity moderates the impact of mindfulness-based stress reduction and aerobic exercise. Psychol Psychother. 2016;89(2):229–234. doi: 10.1111/papt.12060 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Cho SJ, Kim WJ, Yang KI, Yun CH, Chu MK. Insufficient sleep is prevalent among migraineurs: A population-based study. J Headache Pain. 2017;18(1):50–8. Epub 2017 Apr 28. doi: 10.1186/s10194-017-0756-8 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koffel E, Kroenke K, Bair MJ, Leverty D, Polusny MA, Krebs EE. The bidirectional relationship between sleep complaints and pain: Analysis of data from a randomized trial. Health Psychol. 2016;35(1):41–49. doi: 10.1037/hea0000245 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lengacher CA, Johnson-Mallard V, Post-White J, et al. Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psychooncology. 2009;18(12):1261–1272. doi: 10.1002/pon.1529 [doi]. [DOI] [PubMed] [Google Scholar]
- 19.Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016;2016(6):CD001218. doi: 10.1002/14651858.CD001218.pub3 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipton RB, Silberstein SD. Episodic and chronic migraine headache: Breaking down barriers to optimal treatment and prevention. Headache. 2015;55 Suppl 2:103–6. doi: 10.1111/head.12505_2 [doi]. [DOI] [PubMed] [Google Scholar]
- 21.MacCoon DG, Imel ZE, Rosenkranz MA, et al. The validation of an active control intervention for mindfulness based stress reduction (MBSR). Behav Res Ther. 2012;50(1):3–12. doi: 10.1016/j.brat.2011.10.011 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mccubbin T, Dimidjian S, Kempe K, Glassey MS, Ross C, Beck A. Mindfulness-based stress reduction in an integrated care delivery system: One-year impacts on patient-centered outcomes and health care utilization. Perm J. 2014;18(4):4–9. doi: 10.7812/TPP/14-014 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moon HJ, Seo JG, Park SP. Perceived stress in patients with migraine: A case-control study. J Headache Pain. 2017;18(1):73–8. Epub 2017 Jul 21. doi: 10.1186/s10194-017-0780-8 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nyklicek I, Irrmischer M. For whom does mindfulness-based stress reduction work? moderating effects of personality. Mindfulness (N Y). 2017;8(4):1106–1116. doi: 10.1007/s12671-017-0687-0 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh K, Cho SJ, Chung YK, Kim JM, Chu MK. Combination of anxiety and depression is associated with an increased headache frequency in migraineurs: A population-based study. BMC Neurol. 2014;14:238–4. doi: 10.1186/s12883-014-0238-4 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pancheri C, Maraone A, Roselli V, et al. The role of stress and psychiatric comorbidities as targets of non-pharmacological therapeutic approaches for migraine. Riv Psichiatr. 2020;55(5):262–268. doi: 10.1708/3457.34458 [doi]. [DOI] [PubMed] [Google Scholar]
- 27.Rains JC. Sleep and migraine: Assessment and treatment of comorbid sleep disorders. Headache. 2018;58(7):1074–1091. https://pubmed.ncbi.nlm.nih.gov/30095163. doi: 10.1111/head.13357. [DOI] [PubMed] [Google Scholar]
- 28.Robinson CM, Klenck SC, Norton PJ. Psychometric properties of the generalized anxiety disorder questionnaire for DSM-IV among four racial groups. Cogn Behav Ther. 2010;39(4):251–261. doi: 10.1080/16506073.2010.486841 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenzweig S, Greeson JM, Reibel DK, Green JS, Jasser SA, Beasley D. Mindfulness-based stress reduction for chronic pain conditions: Variation in treatment outcomes and role of home meditation practice. J Psychosom Res. 2010;68(1):29–36. doi: 10.1016/j.jpsychores.2009.03.010 [doi]. [DOI] [PubMed] [Google Scholar]
- 30.Rusch HL, Rosario M, Levison LM, et al. The effect of mindfulness meditation on sleep quality: A systematic review and meta-analysis of randomized controlled trials. Ann N Y Acad Sci. 2019;1445(1):5–16. doi: 10.1111/nyas.13996 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt S, Grossman P, Schwarzer B, Jena S, Naumann J, Walach H. Treating fibromyalgia with mindfulness-based stress reduction: Results from a 3-armed randomized controlled trial. Pain. 2011;152(2):361–369. doi: 10.1016/j.pain.2010.10.043 [doi]. [DOI] [PubMed] [Google Scholar]
- 32.Seminowicz DA, Burrowes SAB, Kearson A, et al. Enhanced mindfulness-based stress reduction in episodic migraine: A randomized clinical trial with magnetic resonance imaging outcomes. Pain. 2020. doi: 10.1097/j.pain.0000000000001860 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seo JG, Park SP. Validation of the generalized anxiety disorder-7 (GAD-7) and GAD-2 in patients with migraine. J Headache Pain. 2015;16:97–8. Epub 2015 Nov 23. doi: 10.1186/s10194-015-0583-8 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seo JG, Park SP. Validation of the patient health questionnaire-9 (PHQ-9) and PHQ-2 in patients with migraine. J Headache Pain. 2015;16:65–2. Epub 2015 Jul 15. doi: 10.1186/s10194-015-0552-2 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serpa JG, Taylor SL, Tillisch K . Mindfulness-based stress reduction (MBSR) reduces anxiety, depression, and suicidal ideation in veterans. Med Care. 2014;52(12 Suppl 5):19. doi: 10.1097/MLR.0000000000000202 [doi]. [DOI] [PubMed] [Google Scholar]
- 36.Sharpe L, Dudeney J, Williams ACC, et al. Psychological therapies for the prevention of migraine in adults. Cochrane Database Syst Rev. 2019;7(7):CD012295. doi: 10.1002/14651858.CD012295.pub2 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simshäuser K, Lüking M, Kaube H, Schultz C, Schmidt S. Is mindfulness-based stress reduction a promising and feasible intervention for patients suffering from migraine? A randomized controlled pilot trial. Complement Med Res. 2020;27(1):19–30. doi: 10.1159/000501425 [doi]. [DOI] [PubMed] [Google Scholar]
- 38.Smelt AF, Louter MA, Kies DA, et al. What do patients consider to be the most important outcomes for effectiveness studies on migraine treatment? results of a delphi study. PLoS One. 2014;9(6):e98933. doi: 10.1371/journal.pone.0098933 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tiseo C, Vacca A, Felbush A, et al. Migraine and sleep disorders: A systematic review. The Journal of Headache and Pain. 2020;21(1):126. 10.1186/s10194-020-01192-5. doi: 10.1186/s10194-020-01192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torres-Ferrus M, Gallardo VJ, Alpuente A, Pozo-Rosich P. Influence of headache pain intensity and frequency on migraine-related disability in chronic migraine patients treated with OnabotulinumtoxinA. J Headache Pain. 2020;21(1):88–8. doi: 10.1186/s10194-020-01157-8 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Velden AM, Kuyken W, Wattar U, et al. A systematic review of mechanisms of change in mindfulness-based cognitive therapy in the treatment of recurrent major depressive disorder. Clin Psychol Rev. 2015;37:26–39. doi: 10.1016/j.cpr.2015.02.001 [doi]. [DOI] [PubMed] [Google Scholar]
- 42.VanderWeele TJ. Mediation analysis: A practitioner’s guide. Annu Rev Public Health. 2016;37:17–32. doi: 10.1146/annurev-publhealth-032315-021402 [doi]. [DOI] [PubMed] [Google Scholar]
- 43.VanderWeele TJ, Vansteelandt S. Mediation analysis with multiple mediators. Epidemiol Methods. 2014;2(1):95–115. doi: 10.1515/em-2012-0010 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veehof MM, Trompetter HR, Bohlmeijer ET, Schreurs KM. Acceptance- and mindfulness-based interventions for the treatment of chronic pain: A meta-analytic review. Cogn Behav Ther. 2016;45(1):5–31. doi: 10.1080/16506073.2015.1098724 [doi]. [DOI] [PubMed] [Google Scholar]
- 45.Vgontzas A, Pavlović J, M. Sleep disorders and migraine: Review of literature and potential pathophysiology mechanisms. Headache. 2018;58(7):1030–1039. https://pubmed.ncbi.nlm.nih.gov/30091160 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6527324/. doi: 10.1111/head.13358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wells RE, O’Connell N, Pierce CR, et al. Effectiveness of mindfulness meditation vs headache education for adults with migraine: A randomized clinical trial. JAMA Intern Med. 2020. doi: 10.1001/jamainternmed.2020.7090 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wells RE, Seng EK, Edwards RR, et al. Mindfulness in migraine: A narrative review. Expert Rev Neurother. 2020;20(3):207–225. doi: 10.1080/14737175.2020.1715212 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winbush NY, Gross CR, Kreitzer MJ. The effects of mindfulness-based stress reduction on sleep disturbance: A systematic review. Explore (NY). 2007;3(6):585–591. doi: S1550–8307(07)00274–1 [pii]. [DOI] [PubMed] [Google Scholar]
- 49.Zebenholzer K, Lechner A, Broessner G, et al. Impact of depression and anxiety on burden and management of episodic and chronic headaches - a cross-sectional multicentre study in eight austrian headache centres. J Headache Pain. 2016;17:15–3. Epub 2016 Feb 27. doi: 10.1186/s10194-016-0603-3 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available by request to corresponding author.