Abstract
When B cells are exposed to antigens, they use their B cell receptors (BCRs) to transduce this external signal into internal signaling cascades and uptake antigen, which activate transcriptional programs. Signaling activation requires complex cytoskeletal remodeling initiated by BCR signaling. The actin cytoskeletal remodeling drives B cell morphological changes, such as spreading, protrusion, contraction, and endocytosis of antigen by mechanical forces, which in turn affect BCR signaling. Therefore, the relationship between the actin cytoskeleton and BCR signaling is a two-way feedback loop. These morphological changes represent the indirect ways by which the actin cytoskeleton regulates BCR signaling. Recent studies using high spatiotemporal resolution microscopy techniques have revealed that actin also can directly influence BCR signaling. Cortical actin networks directly affect BCR mobility, not only during the resting stage by serving as diffusion barriers, but also at its activation stage by altering BCR diffusivity through enhanced actin flow velocities. Furthermore, the actin cytoskeleton along with myosin enable B cells to sense the physical properties of its environment and generate and transmit forces through the BCR. Consequently, the actin cytoskeleton modulates the signaling threshold of BCR to antigenic stimulation. This review discusses the latest research on the relationship between BCR signaling and actin remodeling, and the research techniques. Exploration of the role of actin in BCR signaling will expand the fundamental understanding of the relationship between cell signaling and the cytoskeleton and the mechanisms underlying cytoskeleton related immune disorders and cancer.
Keywords: B cell, B cell receptor, actin, antigen, signaling, antigen presenting cell
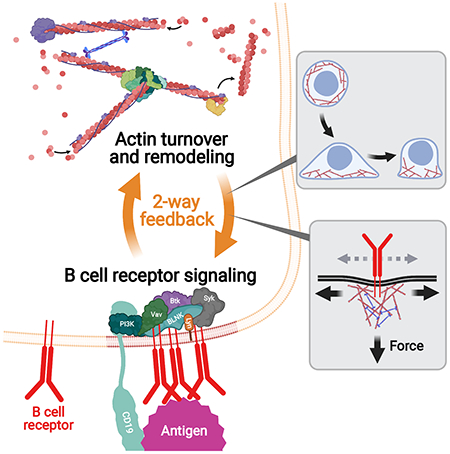
Graphical Abstract
Antigen-induced B-cell receptor (BCR) signaling leads to actin reorganization in B cells. Actin turnover and remodeling in turn regulate BCR signaling by altering B-cell morphology, regulating BCR mobility, and generating mechanical forces transmitted through the BCR. This bidirectional feedback between BCR signaling and the actin cytoskeleton allows B-cells to activate upon encountering antigen beyond certain concentration and affinity thresholds, amplify BCR signaling and then downregulate it, and endocytose antigen fragments.
Introduction
B lymphocytes play a critical role in the human adaptive immune system. The main function of B cells is to mount antibody responses against foreign antigens. They also function as regulators of other immune cells by presenting antigens and secreting cytokines. Immune deficiency diseases, like x-linked Severe Combined Immuno-Deficiency (SCID) and Wiskott-Aldrich syndrome, and autoimmune diseases, like Systemic Lupus Erythematosus (SLE), can be linked to aberrant or defective B cell activation that results from altered BCR signaling [1–4]. Complex mechanisms tightly regulate the activity of B cells, since they play such an important role in conferring adaptive immunity.
B cells originate in the bone marrow and migrate to secondary lymphoid organs like the spleen and lymph nodes, where they encounter foreign antigens for the first time [5,6]. These naïve B cells can be activated directly by antigen on the surface of pathogens, antigen presented by an antigen presenting cells (APCs), or soluble antigen [7,8]. B cells use their clonally specific B cell receptors (BCRs) to recognize and bind the antigenic epitopes. Since different B cell clones express unique BCRs, only those that recognize a given foreign antigen are activated and allowed to generate immune responses, ensuring the selection of B cell clones specific to that antigen. Following activation, the BCR endocytose and deliver antigen to endosomes where antigen is fragmented and loaded on to MHCII molecules for presentation to T helper cells, leading to T cell activation. The activated T helper cells provide cytokines and growth factors for the antigen-presenting B cells, providing essential signals for B cell clonal expansion and differentiation into memory B cells and antibody-secreting plasma cells [9,10]. In germinal centers, activated B cells undergo repeated rounds of somatic hypermutation and antigen-based selection by interacting with antigen presenting cells and T helper cells. As a result, only B cells with high affinity for antigens present within germinal centers survive and differentiate into antibody-secreting cells, leading to high affinity antibody responses [11,12].
The B cell response to surface-bound antigens involves not only signal transduction, but also changes in cell morphology as B cells interact with the antigen-presenting surface. B cells spread over antigen presenting cells, forming immunological synapses, which is the signaling center and the site of antigen extraction [7]. On artificial antigen presenting surfaces, such as antigen-coated supported planar lipid bilayers (PLBs), naïve B cells have been shown to spread and then contract, followed by attempts to extract antigen [7,13,14]. B cells that are unable to spread well enough cannot mount a strong B cell response, which contributes to immune deficiencies [15]. On the other hand, perturbing or inhibiting B cell contraction results in higher and more sustained BCR signaling, which is associated with increases in auto-antibody production [13]. These morphological transformations (B cell spreading and contraction) are largely dependent on the actin cytoskeleton. In addition to its role in driving contact formation, it is being increasingly recognized that actin dynamics plays an important role in direct modulation of signaling. Recent studies on BCR mobility have shown that key actin nucleators and regulatory proteins affect BCR dynamics through actin remodeling [16,17]. Furthermore, studies using antigen-presenting surfaces with different stiffnesses have shown that the actin cytoskeleton helps the B cell sense its environment and regulate signaling and transcriptional programs [18].
Although the involvement of the actin cytoskeleton in the B cell signaling response has been shown using knockout and inhibitor studies, direct visualization of actin structures has been challenging due to their highly dynamic nature and small size. Recent advancements in single molecule imaging, super-resolution microscopy, and high throughput data analysis have made it possible to study molecular interactions from a completely different perspective. This review discusses the different mechanisms by which BCR signaling regulates the actin cytoskeleton and how signaling-induced actin remodeling and dynamics regulates the BCR signaling. The review also discusses a range of methods used to visualize and analyze actin structures and dynamics during BCR activation.
Antigen binding to BCR initiates B cell activation
The BCR initiates B cell activation upon binding antigen that it can recognize using its BCRs (cognate antigen). The BCR consists of an immunoglobulin with transmembrane anchors, non-covalently associated with Igα/Igβ heterodimer. The Igα/β chains, also known as CD79a/CD79b, have Immunoreceptor Tyrosine-based Activation Motifs (ITAMs) in their cytoplasmic domains, which are phosphorylated upon receptor activation [19]. Multivalent soluble antigen, or surface-bound (insoluble) antigen, but not soluble monovalent antigen, can induce a BCR signaling response [20–22].
Super-resolution imaging studies have shown that the BCR resides as monomers or nanometer-sized clusters in the unstimulated cell [23–25]. Multiple identical epitopes on multivalent antigens or membranes cause surface BCRs to physically cluster to form BCR-antigen microclusters [26]. These BCR microclusters become associated with lipid rafts on the plasma membrane, which contain raft-resident Src kinases like Lyn, which phosphorylate the BCR’s ITAMs [27–29]. Phosphorylation is followed by the recruitment of downstream kinases like Spleen tyrosine kinase (Syk) and Bruton’s tyrosine kinase (Btk), and adaptor proteins like B cell Linker (BLNK) to these receptor clusters, forming signalosomes [30–32]. This leads to signal activation at BCR microclusters and allows propagation of signaling cascades to the cytoplasm and nucleus through calcium signaling, Rho family kinases, Mitogen Activated Protein (MAP) kinases, as well as transcription factors, like Nuclear Factor κB (NF-κB) (Fig. 1).
Fig. 1. BCR engagement by multivalent antigen activates actin remodeling in B cells.
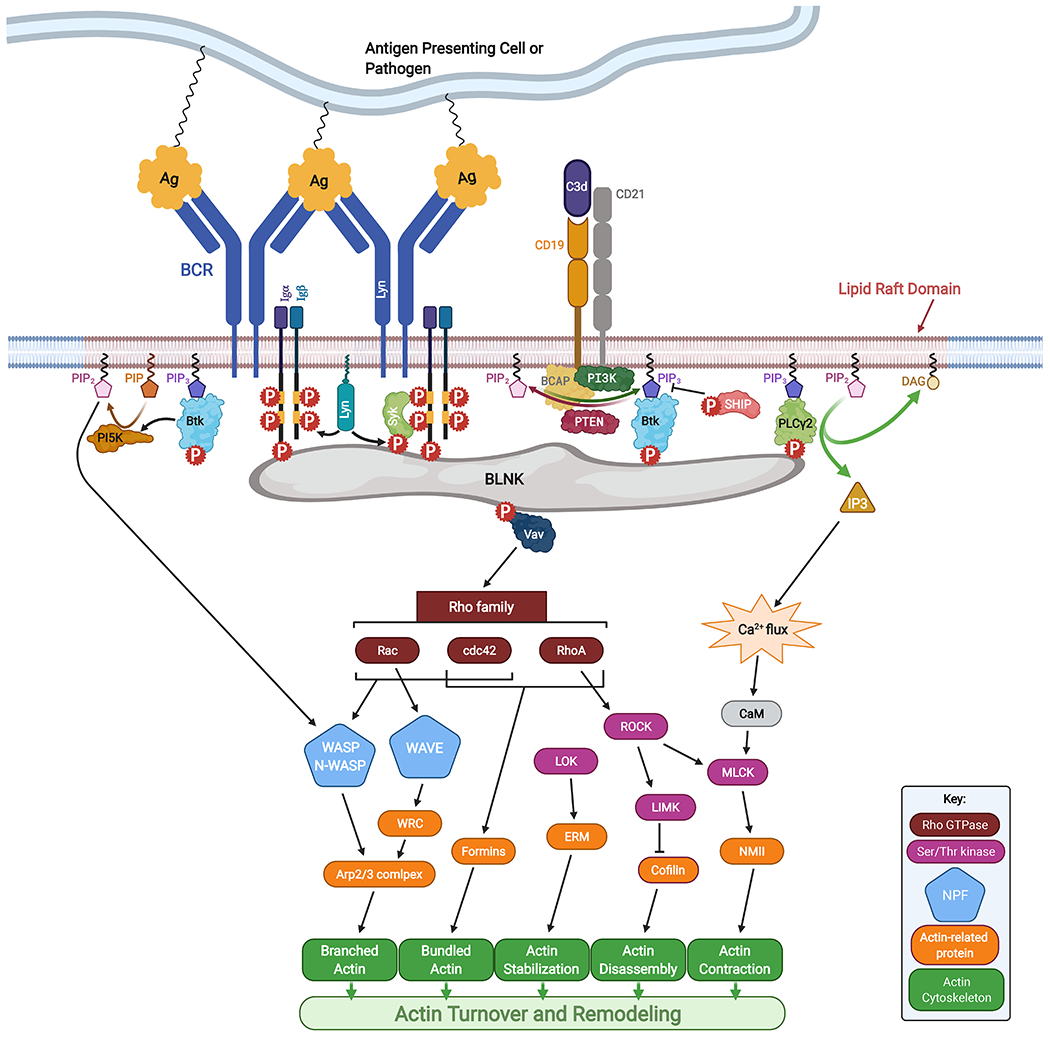
BCR signaling is initiated by BCR binding to antigen, which activates actin turnover and remodeling through Rho family GTPases, nucleation promoting factors, Ser/Thr kinases and motor proteins. Coreceptor molecules like CD19 also influence key steps in BCR signaling. Created with BioRender.com.
When exposed to membrane-bound antigen, B cells spread on the surface, generating more BCR microclusters, which then coalesce into larger clusters by moving centripetally towards the center of the contact zone between the B cell and the antigen-presenting surface [22,33,34]. When activated by soluble multivalent antigen (or anti-BCR antibodies which can also crosslink BCR), BCR microclusters polarize to form a BCR “cap” on one pole of the B cell [34,35]. Cluster coalescence and cap formation both lead to a dramatic reduction in BCR lateral mobility. The formation of such BCR caps has been shown to depend on the actin cytoskeleton [34]. During this stage, phosphatases like Src Homology 2 domain tyrosine Phosphatase-1 (SHP-1) and Src Homology 2 domain-containing Inositol 5-Phosphatase (SHIP-1) are recruited to the larger BCR clusters, dephosphorylating activated signaling molecules, leading to a downregulation of BCR signaling, which reverses the initial signaling amplification. Such a signaling balance has been illustrated by the interplay between the stimulatory kinase Btk and inhibitory phosphatase SHIP-1, wherein SHIP-1 knockout B cells displayed enhanced Btk phosphorylation and enhanced BCR signaling. Inhibition of Btk using a small molecule inhibitor in SHIP-1 knockout B cells rescued this phenotype, bringing BCR signaling back down to normal levels [36,37]. Thus, Btk and SHIP-1 fine tune the timing and levels of BCR signaling, with amplification of BCR signaling first, followed by BCR signaling attenuation.
In resting B cells, tonic BCR signaling, a relatively low-level of signaling, is necessary for naïve B cells to survive [38]. However, exposure to cognate antigen primes the B cell for proliferation and affinity maturation. The entry of B cells into germinal centers to competitively acquire antigen for affinity maturation appears to require strong signaling [8,39,40]. The BCR detects extracellular signals in the form of antigen, soluble or tethered, and forms signalosomes, leading to a cellular response. However, the mechanism underlying BCR activation upon antigen binding is not completely understood. A few models have been proposed, describing the possible mechanisms [41]. One of these models is the BCR clustering-activation model, according to which monomeric surface BCRs are clustered into oligomers upon antigen binding, triggering ITAM phosphorylation by raft-resident Src kinases [20]. The binding to surface antigen induces a conformational change in the BCR molecule, which enhances BCR oligomerization [22,42]. BCRs with different isotype of mIgs, IgM and IgG, undergo distinct conformational changes, leading to different force-sensitivity between IgM-expressing naïve B cells and mIgG expressing effector B cells, which would explain why IgG BCR have a lower threshold for activation than IgM BCR [43–45]. A different model states that surface BCRs are present as oligomers in resting B cells, and dissociate from the oligomers when spaced out by antigen binding, making the ITAMs accessible for phosphorylation [46,47]. Evidence for both these models exists and the exact mechanism for BCR activation is yet to be objectively defined.
BCR signaling activates actin nucleators and actin-related proteins
Upon BCR engagement, downstream signaling through the adaptor protein BLNK and Btk recruits and activates the guanine nucleotide exchange factors (GEFs) like Vav, which activate Rho family GTPases like RhoA, Rac1/2, and Cdc42 [32,48,49] (Fig. 1). These GTPases and phosphatidylinositol-4,5-biphosphate [PI(4,5)P2] activate actin nucleation promoters like Wiskott-Aldrich Syndrome Protein (WASP) expressed exclusively in immune cells and Neuronal-WASP (N-WASP), a ubiquitously expressed homolog of WASP. Proteins of the WASP family are well known actin regulators that activate the Actin-Related Protein complex (Arp2/3) and its nucleation of branched actin filaments [50,51]. Wiskott-Aldrich Syndrome (WAS), caused by WASP-deficiency, is a severe immune disorder, characterized by immune deficiency and high incidences of autoimmune diseases and lymphoid cancers [52]. Another Arp2/3 activator, WASP-family verprolin-homologous protein (WAVE), may also function downstream of the BCR. Knocking out the HEM-1 protein, an essential component of the WAVE regulatory complex (WRC), resulted in defects in B cell activation in response to antigen [53,54]. In addition to the Arp2/3 complex that nucleates branched actin sheets and lamellae, B cells express another type of actin nucleators, formins, that nucleate bundled actin filaments [55–57]. Polarized actin reorganization and morphological changes in B cells predominantly occur in response to a polarized array of antigens, as opposed to soluble antigen. This is likely due to the activation of specific actin remodeling proteins near sites of BCR signaling. The activation of these actin nucleators, triggered by antigen-induced BCR signaling at lipid rafts, directs actin polymerization and remodeling at sites of BCR signaling, resulting in a cellular response polarized in the direction of antigenic stimulation [27]. We refer the reader to some recent review articles that discuss the function of different actin-regulatory proteins in B cell activation in more detail [58] and describe how defects in actin-regulatory proteins cause immune dysfunction [59].
Btk has been shown to be essential for activating actin remodeling by BCR signaling. Btk induces actin reorganization by activating WASP and N-WASP through phosphorylating Vav and phosphatidylinositol-5 kinase [60]. Btk also recruits Phosphatidylinositol-4-phosphate 5-kinase (PIP5K) to lipid rafts, generating phosphatidylinositol-4,5-bisphosphate (PIP2), which recruits WASP and N-WASP [60,61]. WASP and N-WASP activate Arp2/3 mediated actin polymerization at BCR-antigen interaction sites. This leads to polarized actin accumulation, causing the B cell to spread over the antigen-coated surface [33]. Interestingly, besides its involvement in B cell spreading, N-WASP was found to have a unique role in B cell contraction. In B cells stimulated with antigen tethered to lipid bilayers, both WASP and N-WASP are required for F-actin accumulation at the B cell-antigen contact zone and cell spreading, while activated N-WASP curtails F-actin accumulation and promotes B cell contraction [13]. Furthermore, WASP and N-WASP negatively regulate each other’s activation and likely compete for the available activation machineries and Arp2/3 in the cell. They are also differentially regulated by BCR signaling. Btk promotes WASP activation and inhibits N-WASP activation, while SHIP-1 promotes N-WASP activation and inhibits WASP activation by inhibiting Btk [36]. The mechanisms by which WASP and N-WASP are differentially regulated by BCR signaling and influence the activation of each other remain unknown. The activation mechanism, timing, and localization of formins in B cells is also not well understood. While formins have been shown to generate actomyosin arcs in T cells, propelling TCR clusters centripetally [62], its role in BCR activation remain to be explored.
BCR activation also recruits cofilin and gelsolin, which are actin remodeling proteins involved in severing F-actin. F-actin severing can induce a transient depolymerization of the cortical F-actin network, freeing up BCR, allowing them to move laterally and form BCR microclusters [34,63]. The adaptor protein BLNK recruits Phospholipase C-γ2 (PLCγ2), which cleaves phosphatidylinositol-3,4,5-triphosphate (PIP3) to generate inositol triphosphate (IP3) and diacylglycerol (DAG) [31]. IP3 triggers intracellular Ca2+ flux, which activates calmodulin and Myosin Light Chain Kinase (MLCK), which in turn phosphorylates and activates non-muscle myosin II (NMII) [64]. NMII has been shown to regulate actin polymerization and generate contractile forces by pulling actin filaments together [64]. In B cells, NMII was shown to affect actin remodeling, generate contractile forces and enhance antigen extraction [65,66].
The actin cytoskeleton regulates key steps in BCR-mediated B cell activation
The role of the actin cytoskeleton in the initiation of BCR signaling
In resting B cells, IgM and IgG BCRs are predominantly present as monomers or nanoclusters, with some highly heterogeneous protein islands, up to 500 BCR molecules per island, shown using direct Stochastic Optical Reconstruction Microscopy (dSTORM) [24]. These BCR typically have low lateral mobility due to their lateral containment by the cortical F-actin network beneath the plasma membrane [67]. This cortical actin network is tethered to the plasma membrane by Ezrin/Radixin/Moesin (ERM) family of proteins, which can bind both F-actin and transmembrane proteins [68], and are phosphorylated by lymphocyte-oriented kinase (LOK) in B cells [69]. Cortical actin can thus regulate the spatial distribution and dynamics of membrane receptors via ERM proteins [70]. BCR lateral mobility is enhanced in actin and ezrin poor regions, and the deletion of BCR cytoplasmic tails also increases its lateral mobility [67,71]. Disruption of cortical actin can induce BCR signaling [34]. Thus, the cortical actin network forms a physical barrier around BCRs, constraining their motion [23,67] (Fig. 2A).
Fig. 2. Actin influences BCR signaling at multiple stages of early B cell activation by using distinct mechanisms.
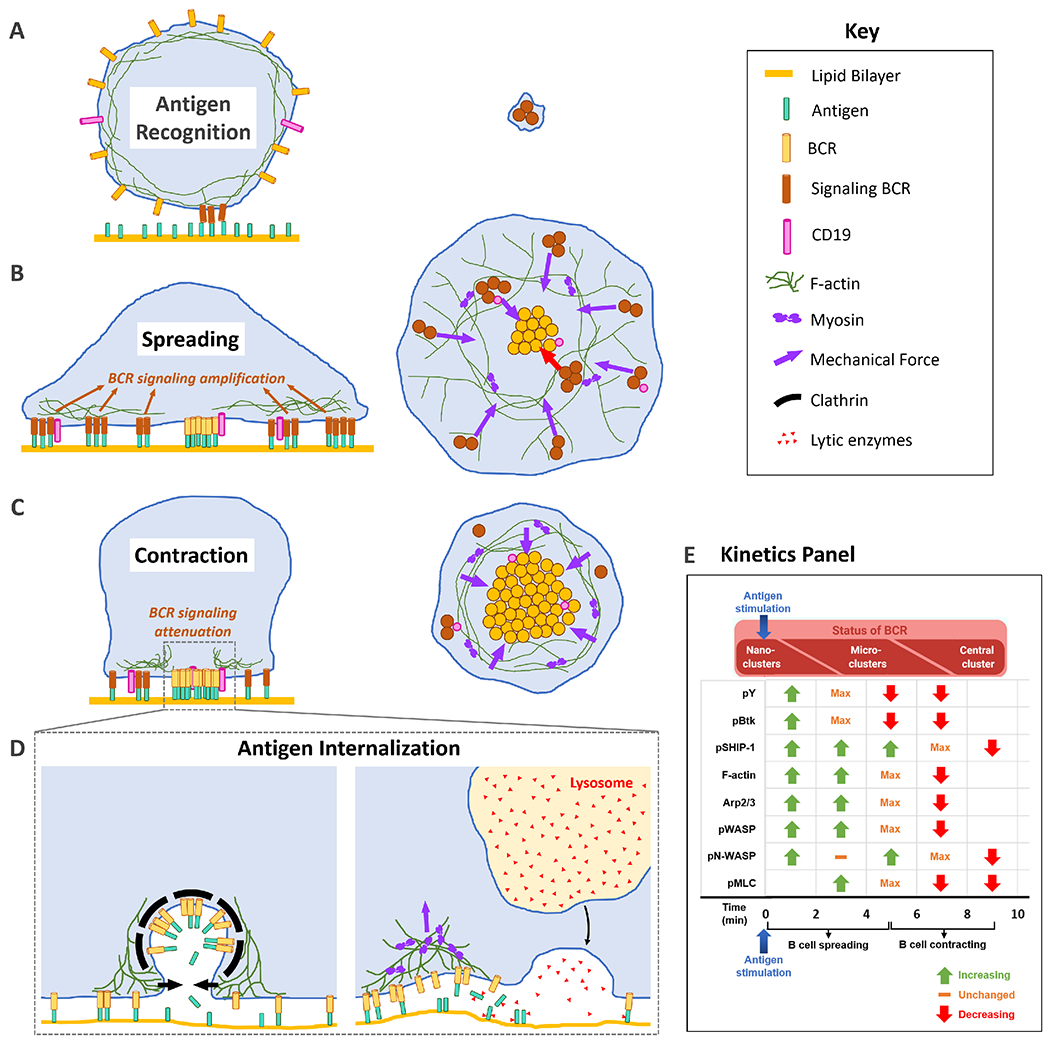
(A) Transient depolymerization of cortical actin induced by BCR-antigen interaction releases BCR from actin-mediated confinement. (B) Actin drives B cell spreading and enhances the mobility and inter-mixing of BCR and CD19, leading to amplification of BCR signaling. (C) Actin drives B cell contraction, merging BCR microclusters and reducing their mobility, which downregulates BCR signaling. (D) BCR internalization of antigen via the clathrin-dependent pathway requires F-actin at the membrane fission step. Clathrin-independent antigen uptake requires actomyosin mediated mechanical force and extracellular enzymatic cleavage. (E) The Kinetics Panel shows an approximation of the kinetics (increasing and decreasing mean fluorescence intensity in the B cell contact zone) of some signaling molecules and actin-related proteins in mouse splenic B cells activated on anti-Ig coated PLBs. The kinetics scheme is based on data that utilize the same model and mode of stimulation [13, 20, 36].
Upon BCR engagement and activation by multivalent or membrane antigen, ERM proteins are rapidly dephosphorylated and detach from the cortical actin network, followed by a rapid depolymerization of F-actin mediated by cofilin and gelsolin [34,63]. Toll-like receptor stimulation also induces cofilin activation, and cofilin-mediated actin severing reduces the spatial confinement of BCR, sensitizing B cells and enhances signaling [72]. The release of these constraints increases BCR lateral mobility, leading to the reorganization of BCR-antigen complexes into microclusters, with up to ~5000 BCR molecules per cluster [24]. Transient depolymerization of cortical actin was shown to be necessary and sufficient for initiating a BCR signaling response, that typically occurs as a result of exposure to cognate antigen. Depolymerization of actin in resting B cells using Latrunculin led to slow but spontaneous BCR clustering and signaling activation [34]. In contrast, stabilization of cortical F-actin in resting B cells using Jasplakinolide prohibited BCR clustering and signaling activation even in the presence of multivalent antigen and membrane-bound antigen [34]. These studies showed that the cortical actin cytoskeleton plays a pivotal role in the initiation of BCR signaling by forming a mechanical barrier and thereby impeding BCR lateral mobility prior to antigenic stimulation. Upon exposure to cognate antigen, the increase in BCR lateral mobility and intermixing with stimulatory co-receptors enhances antigen-induced BCR signaling, which drives the priming of naïve B cells. This actin-mediated increase in BCR mobility is absent in resting B cells and differentiates tonic BCR signaling from B cell-priming BCR signaling in response to antigen.
The role of the actin cytoskeleton in BCR signaling amplification
Subsequent to its role as a mechanical barrier in the absence of BCR stimulation, the actin cytoskeleton plays an important role in the amplification of BCR signaling. One mechanism by which the actin cytoskeleton influences BCR signaling is controlling cell morphology to determine the size and shape of the contact zone between the B cell and antigen-presenting surface. Following the transient depolymerization of cortical actin upon antigen recognition, F-actin is rapidly polymerized at the BCR-antigen interaction sites [73], driving B cell spreading over the antigen presenting surface. Remodeling of F-actin at the periphery of the contact zone enables continued B cell spreading and antigen engagement via the BCR. The enhanced contact increases the number of BCR-antigen microclusters which recruit more signaling molecules, thus amplifying BCR signaling [33,52] (Fig. 2B). The relative kinetics of the activation of some signaling molecules and actin-related proteins, observed in primary mouse B cells stimulated on antigen-coated PLBs, are shown in Fig. 2E. Actin polymerization is not uniform but forms dynamic filaments and foci in the contact zone, which is likely initiated at and associated with signaling BCR microclusters [74], further amplifying signaling. The reduced cell spreading and signaling in mice with B cell specific gene knockout of WASP and/or N-WASP confirms the involvement of Arp2/3 and branched actin in signaling amplification [13]. Similarly, the reduced B cell spreading caused by HEM-1 mutations that impair WAVE activity implicates the WAVE regulatory complex, Arp2/3 complex, and branched actin in BCR signaling amplification [54].
Actin’s role in amplifying BCR signaling is not limited to its effect on B cell morphology. Ketchum et al. have demonstrated that actin dynamics directly increases BCR cluster mobility on the B cell membrane interacting with PLBs, where the antigen are laterally mobile, but not on glass, where the antigens are laterally immobile [16]. This is in contrast to the role of actin before antigenic stimulation, when it acts as a mechanical barrier constraining BCR mobility. Single molecule tracking of individual BCRs in B cells activated on PLBs showed that inhibition of the actin nucleating factors Arp2/3 and formins dramatically reduces BCR diffusivity [17]. Furthermore, single particle tracking (SPT) of surface BCRs on WASP and N-WASP knockout B cells revealed that the loss of both these Arp2/3 activators reduced BCR diffusivity, but N-WASP knockout has a much larger effect than WASP [17]. N-WASP was also found to modulate the diffusivity of the coreceptor CD19 [17]. The reduction in diffusivity was correlated with disorganized actin dynamics and reduced actin flow speeds, indicative of a direct link between actin dynamics and nano-scale mobility of BCRs.
While Arp2/3 nucleates branched actin, formins are known to nucleate actin bundles [56]. Accordingly, the foci in the B cell contact zone were found to predominantly contain Arp2/3 nucleated branched actin, while formins were found to nucleate filaments that interconnect the foci [74]. TIRF-Structured Illumination Microscopy (TIRF-SIM) was used to show that dynamic arc-like actin structures control the spatial patterning of BCR microclusters and drive their centralization [75]. These arc-like structures were disrupted upon Arp2/3 inhibition. Stimulated Emission Depletion (STED) Microscopy was used to show that the arc-like structures were associated with myosin IIA [75]. The above studies show that dynamic branched as well as bundled actin structures enhance receptor diffusivity, spatially re-organize BCR-antigen clusters, increase their signaling, and enhance transcriptional activation in B cells. Although BCR diffusivities have been shown to be dependent on actin accumulation, the nanoscale architecture and dynamics of actin responsible for controlling BCR lateral mobility and spatial patterning are yet to be visualized.
BCR signaling has also been shown to be influenced by the presence of co-receptor molecules like CD19, CD21 and CD81 in BCR-antigen clusters. Actin regulators downstream of the BCR also modulate BCR interaction with these coreceptors. In resting cells, the actin cortex has been found to control the activation of the BCR and its interaction with coreceptors like CD19 by defining barriers that limit receptor mobility [23,25]. The presence of CD19 in BCR microclusters reduces the threshold for BCR signaling by recruiting phosphatidylinositol 3-kinase (PI3K) [76–78]. PI3K-generated phophatidylinositides around BCR clusters form the major scaffold for downstream signaling proteins, enhancing BCR signaling. Thus, the composition and dynamics of BCR clusters affects BCR signaling. Arp2/3-generated branched actin has been shown to affect the lateral mobility of the co-receptor CD19 and its interaction with BCR, wherein Arp2/3 inhibition decreased the colocalization of CD19 with BCR as well as CD19 phosphorylation [75].
BCR signaling is tightly regulated by the local balance of signaling kinases and phosphatases recruited to BCR signalosomes. While kinases like Syk and Btk are phosphorylated earlier during the B cell response and amplify BCR signaling, phosphatases like SHP-1 and SHIP-1 are phosphorylated later and attenuate BCR signaling by dephosphorylating the BCR ITAMs, downstream signaling molecules, and phosphatidylinositol phosphates that are the plasma membrane docking sites of many signaling molecules [13,36]. The signaling state of BCR clusters was found to be related to their extent of clustering. Smaller BCR microclusters generated soon after antigen engagement showed higher levels of tyrosine phosphorylation (pY), while larger BCR clusters formed later from the merging of BCR microclusters had lower levels of pY but higher levels of phosphatases like SHIP-1 [36,79]. Therefore, the activation/attenuation state of a given BCR in a cluster depends upon the number of BCRs in the cluster to a certain extent. Even though it remains to be proven, the retrograde flow of actin likely drives the centripetal flow and merging of BCR microclusters, regulating the BCR signaling state.
The role of the actin cytoskeleton in BCR signaling down regulation
Regulation of BCR signaling is of paramount importance for proper immune responses. During naïve B cell activation, BCR proximal signaling was found to rapidly increase until it reaches a maximum and then drops steadily. Notably, the maximal BCR signaling is achieved in primary B cells at the time of maximum spreading. Following maximal spreading and BCR microcluster formation, the cell morphology switches to contraction, which together with retrograde actin flows, promotes BCR microclusters to move inwards towards the center of the contact zone. The mechanism by which the actin cytoskeleton switches B cells’ behavior from spreading to contraction remains unexplored, but it is likely caused by a combination of actin turnover and dynamic redistribution of F-actin structures at the end of B cell spreading. Patients having a mutation in the cofilin cofactor protein WD repeat-containing protein 1 (WDR1) displayed enhanced B cell spreading and increased F-actin accumulation (reduced actin turnover), as well as increased apoptosis of stimulated B cells, which shows that actin turnover is important for B cell contraction and subsequent B cell activation [80].
As B cells contract, BCR signaling goes down [28,52,81] (Fig. 2C). This reduction in protein tyrosine phosphorylation is concurrent with the recruitment of phosphatases like SHIP-1 [36] (Fig. 2E). Knocking out these negative regulators of BCR signaling leads to impairment in the selection of antigen-specific B cells, leading to the generation of autoreactive B cells and auto-antibody [82]. Interestingly, knocking out actin regulatory proteins (N-WASP and NMII) involved in B cell contraction not only impaired contraction, but also delayed BCR signaling downregulation and led to auto-antibody production and elevated levels of surface activation markers, respectively [13,65]. Furthermore, knocking out phosphatases, like SHIP-1, delays B cell contraction [36]. These results suggest direct links between B cell contraction and BCR signaling downregulation. Lack of B cell contraction and BCR signaling downregulation leads to continued BCR signaling, also referred to as chronic active BCR signaling, a typical characteristic of B lymphomas and B cells from autoimmune patients [4,83,84]. However, the underlying biochemical interactions that trigger and control contraction and signaling downregulation are unknown.
There are multiple pathways linking BCR signaling and actin dynamics. The ubiquitin ligase Cbl and adaptor proteins Grb2 and Dok-3 are involved in the centripetal movement of BCR clusters in the contact zone [85]. The adaptor molecule Actin-binding protein 1 (Abp1) links the actin cytoskeleton and BCR signal attenuation by binding to both F-actin and Hematopoietic Progenitor Kinase 1 (HPK1) that inhibits BLNK, which promotes B-cell contraction, BCR microcluster merger, and SHIP-1 recruitment to BCR microclusters [86]. Activated RhoA in response to BCR signaling leads to the activation of Rho-associated protein kinase (ROCK), which then activates LIM domain kinase (LIMK) [87,88]. LIMK has been shown to reduce cofilin-mediated actin disassembly and actin turnover [89]. This ROCK-mediated LIMK activation likely stabilizes centripetally moving actin structures at the B cell contact zone during B cell contraction.
During B cell spreading, activated WASP co-localizes with BCR microclusters and at the leading edge of the cell [34,60], promoting actin polymerization associated with cell spreading and BCR microcluster motility. WASP-deficiency also reduces BCR internalization, one of the mechanisms for signal attenuation, suggesting that WASP can contribute to positive and negative regulation of BCR signaling [13]. On the other hand, B cells from B-cell-specific N-WASP knockout mice display delayed contraction, impaired formation of BCR central clusters, and a drastic reduction in BCR internalization [13], indicating a dominant role of N-WASP in BCR signaling attenuation. As WASP and N-WASP negatively regulate each other, a balance between WASP and N-WASP’s branched actin nucleating activity appears to support the balance of B cell spreading and contraction. Distinct roles of WASP and N-WASP in B cell spreading and contraction is surprising because the two proteins share greater than 50% sequence homology and activate the same actin nucleation factor, Arp2/3 complex. While the underlying cause for the different functions of WASP- and N-WASP-activated branched actin nucleation remains unknown, these studies suggest that sustained actin dynamics are necessary for effective signal downregulation.
The role of the actin cytoskeleton in antigen internalization
B cells expressing high-affinity BCRs are competitive for antigen engagement, capture, endocytosis, process, and presentation to acquire T cell help, which is essential for the selection of high affinity B cell clones to expand and differentiate. In addition to antigen presentation, antigen-induced BCR internalization may also help clear antigen. The actin reorganization is required for BCR internalization of both soluble and surface-bound antigen [34] (Fig. 2D). The kinetics of BCR-mediated antigen internalization appear to depend on BCR-antigen binding affinity and the type of antigen: faster for internalization of soluble multivalent antigen than surface-bound antigen [60,90]. Upon binding to soluble multivalent antigen, B cells gather antigen-bound BCRs to “caps” in an actin-dependent manner, and subsequently, BCRs endocytose antigen through a clathrin-mediated pathway [34,91]. Actin has been shown to be required for the fission of clathrin-coated pits that contain BCR-antigen complexes [91,92]. FCH and double SH3 domain-containing protein 2 (FCHSD2), a protein that generates F-actin at sites of clathrin-mediated endocytosis, selectively co-localizes with relatively larger nanoscale BCR-Ag clusters, generating F-actin around them, which enhances their endocytosis. In contrast, relatively smaller nanoscale BCR clusters (induced by lowering the concentration of soluble antigen) were only associated with classical clathrin-mediated endocytic pits [93]. The BCR can internalize antigen through clathrin independent pathways (Fig. 2D). An actin-dependent clathrin-independent BCR internalization pathway has been suggested [94]. Upon exposure to antigen-coated plasma membrane sheets (PMS), B cells generate dynamic actin foci that co-localize with clathrin at sites of antigen uptake. Inhibiting actin polymerization and specifically inhibiting Arp2/3 that nucleates branched actin by an inhibitor, CK-666, resulted in a significant reduction in antigen uptake, but inhibiting formin that nucleates bundled actin by an inhibitor, SMIFH2, only had a minor effect [74]. Extraction of membrane-associated antigen relies on NMII-mediated contractile forces [95]. B cells extract antigen either by applying mechanical forces through the BCR or by releasing lytic enzymes into the site of interaction between the B cell and the antigen presenting surface to enzymatically cleave antigen from the presenting surface [18,95,96]. Our recent data show that BCR engaging antigen tightly associated to surfaces in high-affinity permeabilizes the B-cell plasma membrane at antigen binding sites in a NMII-dependent manner. The membrane permeabilization triggers a repair response, including lysosomal exocytosis, which provides lytic enzymes for antigen cleavage from presenting surfaces [90]. Therefore, the actin cytoskeleton is involved in multiple antigen capture pathways in an affinity dependent manner.
The physical properties of antigen presenting cells modulate B cell actin dynamics and BCR signaling
The physical properties of antigen that B cells encounter in vivo are much more complex than the model antigen used for research so far. The plasma membrane of antigen presenting cells is a two-dimensional fluid with heterogeneous lateral mobility and various morphologies, forming a complex nanotopography. The lateral mobility of antigen on the presenting surface has been shown to influence B cell actin dynamics and surface BCR clustering [16], similar to what was reported for T cells [97]. In the lymph nodes and spleen, B cells encounter antigens mainly on APCs, such as marginal zone macrophages and follicular dendritic cells (FDCs). The surfaces of dendritic cells are highly convoluted with folds that have a radius of curvature on the order of 200 to 300 nm. The dendrites of FDCs found in B cell follicles form intricate three-dimensional networks that are several microns in length and 100-300 nm in diameter [98]. Recent studies have used nanofabrication to study the influence of topography on B cell activation. Antigen-coated parallel nanoridges, with dimensions similar to in vivo structures, were shown to induce different B cell morphological changes from flat surfaces, with enrichment of actin and BCRs near the nanoridges. The spacing between nanoridges also modulate actin dynamics and calcium signaling in B cells [99]. The presence of the integrin ligand intercellular cell adhesion molecule-1 (ICAM-1) on antigen presenting substrates leads to improved B cell responses through LFA-1/ICAM-1 interaction, which has been shown to reduce the threshold for B cell activation by reducing the level of antigen required to activate B cells [100]. Furthermore, integrins, like LFA-1, are sensitive to mechanical forces and actomyosin-generated force-dependent conformational changes in LFA-1 may influence the transduction of costimulatory signals in T cells [101,102]. In B cells, actomyosin-generated force-dependent conformational changes in integrins might be involved in BCR affinity maturation.
The antigen presenting surfaces encountered by B cells in vivo are much softer (with stiffness in the range of 0.5-5 kPa) than surfaces commonly used in most studies. Recent studies have examined how the mechanical compliance of the antigen presenting substrate affects B cell signaling activation using hydrogels with tunable stiffness to activate B cells. These studies show that stiffer hydrogels, having an elastic modulus of around 1100 kPa, are more effective in triggering BCR clustering and phosphorylation than softer hydrogels [103]. Such hydrogel substrates also enable the measurement of forces exerted by B cells using a technique called traction force microscopy. Cellular forces have been shown to be critical for the BCR to discriminate antigen binding affinity and antigen extraction. Antigen-coated hydrogels of known stiffness are embedded with fluorescent beads as fiducial markers. As B cells exert forces on the surface during antigen engagement, the gel deforms. The displacement field of the embedded beads can be used to determine the magnitude and direction of sub-cellular forces exerted by B cells during activation. Using this technique, studies have shown that B cells generate forces on the order of 10-20 nN on substrates with stiffness similar to APCs (0.5 to 1kPa) [104]. These forces increase during the first 5 minutes following B cell interaction with the antigen presenting surface and then plateaued [66]. Cellular forces are known to be generated by actomyosin and dynein-mediated contractility. Forces have been detected at myosin and Arp2/3 dependent protrusive actin patches [65,66,95]. Such forces have been shown to be required for BCR conformational changes, which facilitate BCR-BCR interaction and signaling activation, and the internalization of surface-associated antigen by the BCR, pulling antigen from presenting surfaces. The activation of IgM-BCR expressed on naïve B cells requires a much higher force threshold than the activation of isotype-switched IgG- or IgE-BCR expressed on germinal center and memory B cells [43]. This threshold difference at the molecular level may explain the differential morphological changes, force generation, and BCR signaling between germinal center B cells and naïve B cells [40,104]. While traction force microscopy allows measurement of forces at physiological stiffness, it has a limited spatial resolution. DNA-based sensors [105,106] offer considerable promise for measuring forces associated with BCR activation with higher spatial resolution. However, the latter technique requires the careful design of sensors and only provides the range of forces applied by the cell, depending on the threshold force required to turn on the DNA-based sensors. Both these techniques can be readily adapted to concurrently monitor signaling in live cells.
Emerging microscopy and analysis techniques provide new insights into BCR signaling and actin dynamics
Accumulated studies have demonstrated the impact of the physical properties of antigen and antigen presenting surfaces on B cell actin cytoskeletal dynamics and signaling activation. However, the connections between specific actin structures, forces generated by these actin structures, and BCR signaling remain elusive. Advanced fluorescence microscopy techniques, most of which have been developed in the last couple of decades, have provided cell biologists and biophysicists with opportunities to tackle these challenging questions. Single molecule localization microscopy (SMLM) techniques can resolve structures with resolution on the order of 10-30 nm [107,108]. SMLM breaches the diffraction limit by exciting only a few fluorophores during each camera exposure. While this technique provides the highest resolution, thousands of sequential frames are required to sample the structures, making this technique best suited for fixed samples. Total Internal Reflection Fluorescence (TIRF) microscopy is the most commonly used technique used in combination with SMLM. TIRF uses illumination at a critical angle which produces an evanescent wave that excites the fluorophores closest to the glass slide (cell-substrate) (~100 nm) [109,110]. The high axial resolution that TIRF provides together with the vast lateral resolution improvement achieved using single molecule localizations have provided biologists a great tool to investigate cell membrane proteins and underlying cytoplasmic structures [111,112]. Using direct Stochastic Optical Reconstruction Microscopy (dSTORM), a SMLM technique, Roper et al. showed that the B cell immune synapse has a thin lamellipodia and is filled with numerous dense actin foci interspersed by a sparse network of fibers [74]. SMLM techniques have also been adapted for live cell imaging and provided new insights into BCR signaling. Using instantaneous cross-correlation on live two-color SMLM data, Stone et al. [113] showed that Lyn kinase is recruited to BCRs soon after activation and that the co-localized Lyn molecules diffuse more slowly suggesting binding to components within BCR clusters. Similar approaches using fast two-color super-resolution imaging have allowed the identification of TCR and signaling protein nanodomains after T cell activation [114] and the dynamics of individual molecules within these nanoclusters [115]. Further improvements in the fluorophores and equipment employed for SMLM will help continue to fill the gap between molecular resolution and real time imaging in living cells.
Structured illumination microscopy (SIM) is another technique that breaks the diffraction barrier (lateral resolution of ~120 nm) [116] and is fast enough to be used regularly in live cells. In SIM the sample is illuminated with an excitation pattern generated by a grid, which is rotated by 60 and 120 degrees to obtain nine images which are then combined to generate a single image of the sample with a resolution about twice of the one obtained with diffraction-limited techniques [116]. The resolution gain comes at the cost of overall longer exposure times per image and increased exposure of the sample to light, which increases photobleaching. More recent implementations like iSIM (instant Structured Illumination Microscopy) overcome this limitation through an analog implementation [117], and the technique has been improved to allow multicolor and 3D imaging [118]. Rey et al. showed using iSIM (instant Structured Illumination Microscopy) that chemical inhibition of actin nucleators and the actin nucleation promoting factor N-WASP results in a reduction of actin dynamics as well as BCR motility [17]. Wang et al. used TIRF-SIM to describe the lamellipodial and adjacent actin fiber network in primary B cells [119], reminiscent of the actin architecture in T cells, as described by Murugesan et al. [62]. Complementing these improvements in imaging techniques, a series of image analysis techniques have been developed that allow a quantitative analysis of the dynamics of actin and associated proteins during BCR signaling (Figure 3). Image analysis methods, such as kymographs and optical flow, can be applied to datasets obtained with almost any fluorescence microscope (widefield, TIRF, confocal, SIM), while Spatiotemporal Image Correlation Spectroscopy (STICS) [120] (Figure 3) works best with higher resolution data obtained with TIRF-SIM or iSIM. Several actin dynamics analysis techniques (see Figure 3) generate vector maps with precise information of the local spatial and temporal dynamics of the actin cytoskeleton. These actin flow maps can be used together with the traction force maps to find the correlation between force generation and actin dynamics. While the techniques described above can be used to analyze actin dynamics in any type of cell, the B cell biology field could benefit from sophisticated analysis techniques that allow a better understanding of the spatial and temporal variations in actin dynamics during BCR activation and its connection to the regulation of BCR signaling. However, further studies that combine actin dynamics with fluorescence resonance energy transfer (FRET)-based sensors or other methods to monitor signaling will be needed to establish the role that specific actin structures play during the initiation, amplification and down-regulation of signaling in B cells, and their modulation by physical parameters.
Fig. 3. Image analysis techniques for the study of actin dynamics.
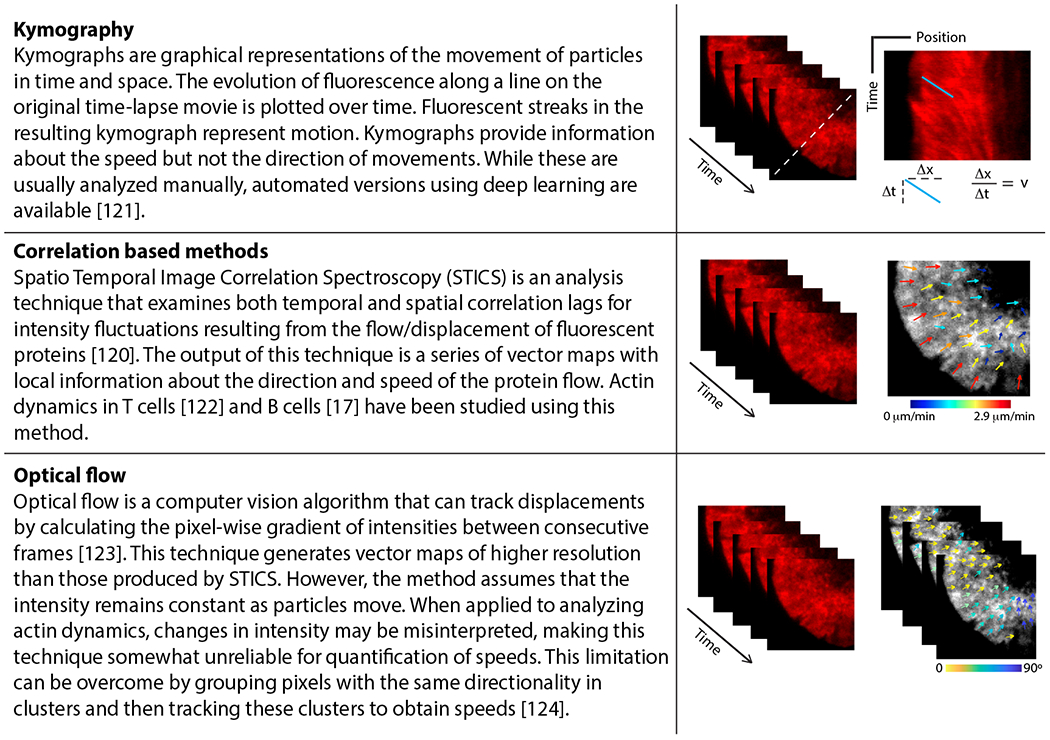
Spatial and temporal changes in the fluorescence intensity of F-actin markers can be tracked using several techniques as described here. Top panel: Kymography. Slope of fluorescent streaks in the kymograph represents the speed (v) of the moving particle; Middle panel: Correlation-based methods. Color-coded arrows represent local speed (color and length of arrows) and direction of flows; Bottom panel: Optical flow. Color-coded arrows represent directionality of clusters. These methods enable quantification of the speed and directionality of actin flows, allowing a more complete characterization of actin dynamics during B-cell immune synapse formation.
Perspectives and Concluding Remarks
This review has discussed recent research progress on the relationship between BCR-initiated B cell activation and the actin cytoskeleton. Accumulated evidence demonstrates that the crosstalk between BCR signaling and actin is reciprocal. BCR signaling induces actin remodeling, which in turn regulates BCR signaling and endocytosis. The actin cytoskeleton has been shown to be involved in almost every step of BCR activation following the engagement of BCR with antigen, starting with actin depolymerization which frees BCR from lateral restrictions, to driving B cell spreading, BCR movement and clustering, and B cell contraction, which enhances BCR activation and facilitates BCR signaling attenuation sequentially. The actin cytoskeleton is also required for BCR-mediated endocytosis of antigen by driving the fission of clathrin-coated vesicles and extracting antigen from antigen presenting surfaces. The actin cytoskeleton plays versatile roles in BCR activation, serving as a barrier to reduce BCR motility in resting B cells, a mixer by dynamically remodeling BCR and signaling molecules into BCR clusters, a driver moving BCR clusters to the center of the B cell contact zone, and a force generator for B cell spreading and antigen extraction depending on the properties of the antigen and the antigen presenting surface. In addition, the actin cytoskeleton also helps modulate the mobility of BCR co-receptors, influencing their interactions with the BCR. With this plasticity and versatility, the actin cytoskeleton provides bridges connecting the physical and biochemical responses within B cells, ensuring a tight regulation of B cell activation.
Depending on its level and duration, BCR signaling can be broadly classified into three types: tonic, antigen-induced, and chronic-active BCR signaling. While tonic BCR signaling, a continuous low-level of signaling, is required for the survival of unstimulated B cells, upon exposure to antigen, a relatively higher level but shorter duration of BCR signaling results in the selection of high-affinity antigen-specific B cells. Continuous high level BCR signaling (chronic active) potentially leads to the survival of auto-reactive B cells. Therefore, by controlling BCR signaling, the actin cytoskeleton can influence the fate of B cells.
Despite recent research progress, many questions remain. The structure and dynamics of the actin cytoskeleton and the mechanisms by which these actin structures function in each stage of BCR activation and internalization are largely unknown. How WASP and N-WASP-promoted branched actin polymerization functions distinctly in BCR signaling amplification and attenuation is still an open question. Finally, how the actin cytoskeleton functions in B cells in vivo within lymphoid organs remains to be elucidated. Innovations in the field of microscopy and live imaging provide new opportunities to pursue these questions.
Acknowledgements
A.U. acknowledges NSF grants PHY 1607645 and PHY 1806903. I. R-S. acknowledges support from the Fulbright-Colciencias scholarship. W.S. acknowledges NIH grant R01 GM064625.
Abbreviations:
- Abp-1
actin-binding protein 1
- Ag
antigen
- APC
antigen presenting cell
- Arp2/3
actin related protein complex
- BCAP
B cell adaptor for phosphatidylinositol 3-kinase
- BCR
B cell receptor
- BLNK
B cell linker
- Btk
Bruton’s tyrosine kinase
- C3d
complement component 3d
- CaM
calmodulin
- Cbl
Casitas B-lineage lymphoma
- Cdc42
cell division control protein 42 homolog
- DAG
diacyl glycerol
- Dok-3
docking protein 3
- dSTORM
direct stochastic optical reconstruction microscopy
- ERM
ezrin/radixin/moesin
- FCHSD2
FCH double SH3 domain-containing protein 2
- FDC
follicular dendritic cell
- FRET
fluorescence resonance energy transfer
- GEF
guanidine exchange factor
- Grb2
growth factor receptor-bound protein 2
- GTP
guanidine triphosphate
- HEM-1
hematopoietic protein-1
- HPK1
hematopoietic progenitor kinase 1
- ICAM-1
intercellular cell adhesion molecule-1
- Ig
immunoglobulin
- IP3
inositol triphosphate
- iSIM
instant structured illumination microscopy
- ITAM
immunoreceptor tyrosine-based activation motif
- LFA-1
lymphocyte function-associated antigen-1
- LIMK
LIM domain kinase
- LOK
lymphocyte oriented kinase
- MAP
mitogen activated protein
- MHCII
major histocompatibility complex II
- MLCK
myosin light chain kinase
- NF-κB
nuclear factor κB
- NMII
non-muscle myosin II
- N-WASP
neuronal Wiskott-Aldrich syndrome protein
- PI(4,5)P2
phosphatidylinositol-4,5-biphosphate
- PI3K
phosphatidylinositol 3-kinase
- PIP2,
phosphatidylinositol-4,5-bisphosphate
- PIP3
phosphatidylinositol-3,4,5-triphosphate
- PIP5K
Phosphatidylinositol-4-phosphate 5-kinase
- PLB
planar lipid bilayer
- PLCγ2
phospholipase C-γ2
- PMS
plasma membrane sheet
- PTEN
phosphatase and tensin homolog
- pY
phosphorylated tyrosine
- Rho
ras homology family
- RhoA
ras homology family member A
- ROCK
Rho-associated protein kinase
- SCID
severe combined immuno-deficiency
- SHIP-1
Src homology 2 domain-containing inositol 5-phosphatase-1
- SHP-1
Src homology 2 domain tyrosine phosphatase-1
- SIM
structured illumination microscopy
- SLE
systemic lupus erythematosus
- SMIFH2
small molecule inhibitor of formin homology-2 domain
- SMLM
single molecule localization microscopy
- SPT
single particle tracking
- STED
stimulated emission depletion
- STICS
spatiotemporal image correlation spectroscopy
- Syk
spleen tyrosine kinase
- TCR
T cell receptor
- TIRF
total internal reflection fluorescence
- WAS
Wiskott-Aldrich syndrome
- WASP
Wiskott-Aldrich syndrome protein
- WAVE
WASP and WASP-family verprolin-homologous protein
- WDR1
WD repeat-containing protein 1
- WRC
WAVE regulatory complex
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Ochs H & Thrasher A (2006) The Wiskott-Aldrich syndrome. J Allergy Clin Immunol 117, 725–738. [DOI] [PubMed] [Google Scholar]
- 2.Recher M, Burns SO, de la Fuente MA, Volpi S, Dahlberg C, Walter JE, Moffitt K, Mathew D, Honke N, Lang PA, Patrizi L, Falet H, Keszei M, Mizui M, Csizmadia E, Candotti F, Nadeau K, Bouma G, Delmonte OM, Frugoni F, Fomin ABF, Buchbinder D, Lundequist EM, Massaad MJ, Tsokos GC, Hartwig J, Manis J, Terhorst C, Geha RS, Snapper S, Lang KS, Malley R, Westerberg L, Thrasher AJ & Notarangelo LD (2012) B cell-intrinsic deficiency of the Wiskott-Aldrich syndrome protein (WASp) causes severe abnormalities of the peripheral B-cell compartment in mice. Blood 119, 2819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cleland SY & Siegel RM (2011) Wiskott-Aldrich Syndrome at the nexus of autoimmune and primary immunodeficiency diseases. FEBS Lett 585, 3710–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dörner T, Giesecke C & Lipsky PE (2011) Mechanisms of B cell autoimmunity in SLE. Arthritis Res Ther 13, 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pereira JP, Kelly LM & Cyster JG (2010) Finding the right niche: B-cell migration in the early phases of T-dependent antibody responses. Int Immunol 22, 413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wykes M, Pombo A, Jenkins C & MacPherson GG (1998) Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J Immunol 161, 1313–9. [PubMed] [Google Scholar]
- 7.Batista FD, Iber D & Neuberger MS (2001) B cells acquire antigen from target cells after synapse formation. Nature 411, 489–494. [DOI] [PubMed] [Google Scholar]
- 8.Nowosad CR, Spillane KM & Tolar P (2016) Germinal center B cells recognize antigen through a specialized immune synapse architecture. Nat Immunol 17, 870–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanzavecchia A (1985) Antigen-specific interaction between T and B cells. Nature 314, 537–539. [DOI] [PubMed] [Google Scholar]
- 10.Schwickert TA, Victora GD, Fooksman DR, Kamphorst AO, Mugnier MR, Gitlin AD, Dustin ML & Nussenzweig MC (2011) A dynamic T cell—limited checkpoint regulates affinity-dependent B cell entry into the germinal center. J Exp Med 208, 1243–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisen HN (2014) Affinity enhancement of antibodies: how low-affinity antibodies produced early in immune responses are followed by high-affinity antibodies later and in memory B-cell responses. Cancer Immunol Res 2, 381–92. [DOI] [PubMed] [Google Scholar]
- 12.Allen CDC, Okada T, Tang HL & Cyster JG (2007) Imaging of germinal center selection events during affinity maturation. Science 315, 528–31. [DOI] [PubMed] [Google Scholar]
- 13.Liu C, Bai X, Wu J, Sharma S, Upadhyaya A, Dahlberg CIM, Westerberg LS, Snapper SB, Zhao X & Song W (2013) N-WASP Is Essential for the Negative Regulation of B Cell Receptor Signaling. PLoS Biol 11, e1001704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harwood NE & Batista FD (2010) Early Events in B Cell Activation. Annu Rev Immunol 28, 185–210. [DOI] [PubMed] [Google Scholar]
- 15.Westerberg L, Larsson M, Hardy SJ, Fernández C, Thrasher AJ & Severinson E (2005) Wiskott-Aldrich syndrome protein deficiency leads to reduced B-cell adhesion, migration, and homing, and a delayed humoral immune response. Blood 105, 1144–1152. [DOI] [PubMed] [Google Scholar]
- 16.Ketchum C, Miller H, Song W & Upadhyaya A (2014) Ligand mobility regulates B cell receptor clustering and signaling activation. Biophys J 106, 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rey-Suarez I, Wheatley BA, Koo P, Bhanja A, Shu Z, Mochrie S, Song W, Shroff H & Upadhyaya A (2020) WASP family proteins regulate the mobility of the B cell receptor during signaling activation. Nat Commun 11, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spillane KM & Tolar P (2017) B cell antigen extraction is regulated by physical properties of antigen-presenting cells. J Cell Biol. 216.1, 217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao XR, Flaswinkel H, Reth M & Scott DW (1995) Immunoreceptor tyrosine-based activation motif is required to signal pathways of receptor-mediated growth arrest and apoptosis in murine B lymphoma cells. J Immunol 155, 652–61. [PubMed] [Google Scholar]
- 20.Tolar P, Sohn HW & Pierce SK (2005) The initiation of antigen-induced B cell antigen receptor signaling viewed in living cells by fluorescence resonance energy transfer. Nat Immunol 6, 1168–1176. [DOI] [PubMed] [Google Scholar]
- 21.Mongini PKA, Vilensky MA, Highet PF & Inman JK (1998) Membrane IgM-stimulated human B lymphocytes succumb to activation- related apoptosis at a G1 → S transition: Influence of ligand affinity and valency. Cell Immunol 188, 137–150. [DOI] [PubMed] [Google Scholar]
- 22.Tolar P, Hanna J, Krueger PD & Pierce SK (2009) The Constant Region of the Membrane Immunoglobulin Mediates B Cell-Receptor Clustering and Signaling in Response to Membrane Antigens. Immunity 30, 44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattila PK, Feest C, Depoil D, Treanor B, Montaner B, Otipoby KL, Carter R, Justement LB, Bruckbauer A & Batista FD (2013) The Actin and Tetraspanin Networks Organize Receptor Nanoclusters to Regulate B Cell Receptor-Mediated Signaling. Immunity 38, 461–474. [DOI] [PubMed] [Google Scholar]
- 24.Lee J, Sengupta P, Brzostowski J, Lippincott-Schwartz J & Pierce SK (2017) The nanoscale spatial organization of B-cell receptors on immunoglobulin M- and G-expressing human B-cells. Mol Biol Cell 28, 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maity PC, Blount A, Jumaa H, Ronneberger O, Lillemeier BF & Reth M (2015) B cell antigen receptors of the IgM and IgD classes are clustered in different protein islands that are altered during B cell activation. Sci Signal 8.394, pp. ra93. [DOI] [PubMed] [Google Scholar]
- 26.Harwood NE & Batista FD (2009) Visualizing the Molecular and Cellular Events Underlying the Initiation of B-Cell Activation. Visualizing Immunity 153–177. [DOI] [PubMed] [Google Scholar]
- 27.Cheng PC, Dykstra ML, Mitchell RN & Pierce SK (1999) A role for lipid rafts in B cell antigen receptor signaling and antigen targeting. J Exp Med 190, 1549–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sohn HW, Tolar P & Pierce SK (2008) Membrane heterogeneities in the formation of B cell receptor-Lyn kinase microclusters and the immune synapse. J Cell Biol 182, 367–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dykstra M, Cherukuri A, Sohn HW, Tzeng SJ & Pierce SK (2003) Location is everything: Lipid rafts and immune cell signaling. Annu Rev Immunol 21, 457–481. [DOI] [PubMed] [Google Scholar]
- 30.Weber M, Treanor B, Depoil D, Shinohara H, Harwood NE, Hikida M, Kurosaki T & Batista FD (2008) Phospholipase C-γ2 and Vav cooperate within signaling microclusters to propagate B cell spreading in response to membrane-bound antigen. J Exp Med 205, 853–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurosaki T (2011) Regulation of BCR signaling. Mol Immunol 48, 1287–1291. [DOI] [PubMed] [Google Scholar]
- 32.Dalporto J, Gauld SB, Merrell KT, Mills D, Pugh-Bernard AE & Cambier J (2004) B cell antigen receptor signaling 101. Mol Immunol 41, 599–613. [DOI] [PubMed] [Google Scholar]
- 33.Fleire SJ, Goldman JP, Carrasco YR, Weber M, Bray D & Batista FD (2006) B Cell Ligand Discrimination Through a Spreading and Contraction Response. Science (80- ) 312, 738–741. [DOI] [PubMed] [Google Scholar]
- 34.Liu C, Miller H, Orlowski G, Hang H, Upadhyaya A & Song W (2012) Actin Reorganization Is Required for the Formation of Polarized B Cell Receptor Signalosomes in Response to Both Soluble and Membrane-Associated Antigens. J Immunol 188, 3237–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schreiner GF & Unanue ER (1977) Capping and the lymphocyte: models for membrane reorganization. J Immunol 119, 1549–51. [PubMed] [Google Scholar]
- 36.Liu C, Miller HM, Lam Hui K, Grooman B, Bolland S, Upadhyaya A & Song W (2011) A balance of Bruton’s tyrosine kinase and SHIP activation regulates B-cell receptor cluster formation by controlling actin remodelling. J Immunol 187.1, 230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brauweiler AM, Tamir I & Cambier JC (2000) Bilevel control of B-cell activation by the inositol 5-phosphatase SHIP. Immunol Rev 176, 69–74. [DOI] [PubMed] [Google Scholar]
- 38.Monroe JG (2004) Ligand-independent tonic signaling in B-cell receptor function. Curr Opin Immunol 16, 288–295. [DOI] [PubMed] [Google Scholar]
- 39.Mesin L, Ersching J & Victora GD (2016) Germinal Center B Cell Dynamics. Immunity 45, 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwak K, Quizon N, Sohn H, Saniee A, Manzella-Lapeira J, Holla P, Brzostowski J, Lu J, Xie H, Xu C, Spillane KM, Tolar P & Pierce SK (2018) Intrinsic properties of human germinal center B cells set antigen affinity thresholds. Sci Immunol 3, eaau6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Treanor B (2012) B-cell receptor: From resting state to activate. Immunology 136, 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tolar P & Pierce SK (2010) A Conformation-Induced Oligomerization Model for B cell Receptor Microclustering and Signaling. Immunological Synapse pp. 155–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wan Z, Chen X, Chen H, Ji Q, Chen Y, Wang J, Cao Y, Wang F, Lou J, Tang Z & Liu W (2015) The activation of IgM- or isotype-switched IgG- and IgE-BCR exhibits distinct mechanical force sensitivity and threshold. Elife 4, e06925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wan Z, Xu C, Chen X, Xie H, Li Z, Wang J, Ji X, Chen H, Ji Q, Shaheen S, Xu Y, Wang F, Tang Z, Zheng JS, Chen W, Lou J & Liu W (2018) PI(4,5)P2 determines the threshold of mechanical force-induced B cell activation. J Cell Biol 217, 2565–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen Z, Liu S, Li X, Wan Z, Mao Y, Chen C & Liu W (2019) Conformational change within the extracellular domain of B cell receptor in B cell activation upon antigen binding. Elife 8, e42271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang J & Reth M (2010) Oligomeric organization of the B-cell antigen receptor on resting cells. Nature 467, 465–469. [DOI] [PubMed] [Google Scholar]
- 47.Yang J & Reth M (2010) The dissociation activation model of B cell antigen receptor triggering. FEBS Lett 584, 4872–4877. [DOI] [PubMed] [Google Scholar]
- 48.Fischer KD, Tedford K & Penninger JM (1998) Vav links antigen-receptor signaling to the actin cytoskeleton. Semin Immunol 10, 317–327. [DOI] [PubMed] [Google Scholar]
- 49.Abe K, Rossman KL, Liu B, Ritola KD, Chiang D, Campbell SL, Burridge K & Der CJ (2000) Vav2 is an activator of Cdc42, Rac1, and RhoA. J Biol Chem 275, 10141–10149. [DOI] [PubMed] [Google Scholar]
- 50.Alekhina O, Burstein E & Billadeau DD (2017) Cellular functions of WASP family proteins at a glance. J Cell Sci 130, 2235–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T & Kirschner MW (1999) The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97, 221–31. [DOI] [PubMed] [Google Scholar]
- 52.Song W, Liu C, Seeley-Fallen MK, Miller H, Ketchum C & Upadhyaya A (2013) Actin-mediated feedback loops in B-cell receptor signaling. Immunol Rev 256, 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park H, Chan MM & Iritani BM (2010) Hem-1: Putting the “WAVE” into actin polymerization during an immune response. FEBS Lett 584, 4923–4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salzer E, Zoghi S, Kiss MG, Kage F, Rashkova C, Stahnke S, Haimel M, Platzer R, Caldera M, Ardy RC, Hoeger B, Block J, Medgyesi D, Sin C, Shahkarami S, Kain R, Ziaee V, Hammerl P, Bock C, Menche J, Dupré L, Huppa JB, Sixt M, Lomakin A, Rottner K, Binder CJ, Stradal TEB, Rezaei N & Boztug K (2020) The cytoskeletal regulator HEM1 governs B cell development and prevents autoimmunity. Sci Immunol 5.49, eabc3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stradal TE & Scita G (2006) Protein complexes regulating Arp2/3-mediated actin assembly. Curr Opin Cell Biol 18, 4–10. [DOI] [PubMed] [Google Scholar]
- 56.Evangelista M, Zigmond S, Boone C, Martin PR, Schnabel H, Schnabel R & Bowerman B (2003) Formins: signaling effectors for assembly and polarization of actin filaments. J Cell Sci 116, 2603–11. [DOI] [PubMed] [Google Scholar]
- 57.Pollard TD (2007) Regulation of Actin Filament Assembly by Arp2/3 Complex and Formins. Annu Rev Biophys Biomol Struct 36, 451–477. [DOI] [PubMed] [Google Scholar]
- 58.Tolar P (2017) Cytoskeletal control of B cell responses to antigens. Nat Rev Immunol 17, 621–634. [DOI] [PubMed] [Google Scholar]
- 59.Sprenkeler EGG, Webbers SDS & Kuijpers TW (2021) When Actin is Not Actin’ like It Should: A New Category of Distinct Primary Immunodeficiency Disorders. J Innate Immun 13, 3–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma S, Orlowski G & Song W (2009) Btk Regulates B Cell Receptor-Mediated Antigen Processing and Presentation by Controlling Actin Cytoskeleton Dynamics in B Cells. J Immunol 182, 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saito K, Tolias KF, Saci A, Koon HB, Humphries LA, Scharenberg A, Rawlings DJ, Kinet JP & Carpenter CL (2003) BTK regulates PtdIns-4,5-P2 synthesis: Importance for calcium signaling and PI3K activity. Immunity 19, 669–677. [DOI] [PubMed] [Google Scholar]
- 62.Murugesan S, Hong J, Yi J, Li D, Beach JR, Shao L, Meinhardt J, Madison G, Wu X, Betzig E & Hammer JA (2016) Formin-generated actomyosin arcs propel t cell receptor microcluster movement at the immune synapse. J Cell Biol 215, 383–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Freeman SA, Lei V, Dang-Lawson M, Mizuno K, Roskelley CD & Gold MR (2011) Cofilin-mediated F-actin severing is regulated by the Rap GTPase and controls the cytoskeletal dynamics that drive lymphocyte spreading and BCR microcluster formation. J Immunol 187, 5887–900. [DOI] [PubMed] [Google Scholar]
- 64.Vicente-Manzanares M, Ma X, Adelstein RS & Horwitz AR (2009) Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 10, 778–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoogeboom R, Natkanski EM, Nowosad CR, Malinova D, Menon RP, Casal A & Tolar P (2018) Myosin IIa Promotes Antibody Responses by Regulating B Cell Activation, Acquisition of Antigen, and Proliferation. Cell Rep 23, 2342–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumari A, Pineau J, Sáez PJ, Maurin M, Lankar D, San Roman M, Hennig K, Boura VF, Voituriez R, Karlsson MCI, Balland M, Lennon Dumenil A-M & Pierobon P (2019) Actomyosin-driven force patterning controls endocytosis at the immune synapse. Nat Commun 10, 2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Treanor B, Depoil D, Gonzalez-Granja A, Barral P, Weber M, Dushek O, Bruckbauer A & Batista FD (2010) The Membrane Skeleton Controls Diffusion Dynamics and Signaling through the B Cell Receptor. Immunity 32, 187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pore D & Gupta N (2015) The ezrin-radixin-moesin family of proteins in the regulation of B-cell immune response. Crit Rev Immunol 35, 15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Belkina NV, Liu Y, Hao JJ, Karasuyama H & Shaw S (2009) LOK is a major ERM kinase in resting lymphocytes and regulates cytoskeletal rearrangement through ERM phosphorylation. Proc Natl Acad Sci U S A 106, 4707–4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ponuwei GA (2016) A glimpse of the ERM proteins. J Biomed Sci 23, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Treanor B, Depoil D, Bruckbauer A & Batista FD (2011) Dynamic cortical actin remodeling by ERM proteins controls BCR microcluster organization and integrity. J Exp Med 208, 1055–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Freeman SA, Jaumouillé V, Choi K, Hsu BE, Wong HS, Abraham L, Graves ML, Coombs D, Roskelley CD, Das R, Grinstein S & Gold MR (2015) Toll-like receptor ligands sensitize B-cell receptor signalling by reducing actin-dependent spatial confinement of the receptor. Nat Commun 6, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu C, Miller H, Sharma S, Beaven A, Upadhyaya A & Song W (2012) Analyzing actin dynamics during the activation of the B cell receptor in live B cells. Biochem Biophys Res Commun 427, 202–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roper SI, Wasim L, Malinova D, Way M, Cox S & Tolar P (2019) B cells extract antigens at Arp2/3-generated actin foci interspersed with linear filaments. Elife 8, e48093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bolger-Munro M, Choi K, Scurll JM, Abraham L, Chappell RS, Sheen D, Dang-Lawson M, Wu X, Priatel JJ, Coombs D, Hammer JA & Gold MR (2019) Arp2/3 complex-driven spatial patterning of the BCR enhances immune synapse formation, BCR signaling and B cell activation. Elife 8, e44574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tedder TF, Haas KM & Poe JC (2002) CD19-CD21 complex regulates an intrinsic Src family kinase amplification loop that links innate immunity with B-lymphocyte intracellular calcium responses. In Biochemical Society Transactions pp. 807–811. [DOI] [PubMed] [Google Scholar]
- 77.Fujimoto M, Poe JC, Inaoki M & Tedder TF (1998) CD19 regulates B lymphocyte responses to transmembrane signals. Semin Immunol 10, 267–277. [DOI] [PubMed] [Google Scholar]
- 78.Rickert RC (2005) Regulation of B lymphocyte activation by complement C3 and the B cell coreceptor complex. Curr Opin Immunol 17, 237–243. [DOI] [PubMed] [Google Scholar]
- 79.Bolland S, Pearse RN, Kurosaki T & Ravetch JV (1998) SHIP modulates immune receptor responses by regulating membrane association of Btk. Immunity 8, 509–16. [DOI] [PubMed] [Google Scholar]
- 80.Pfajfer L, Mair NK, Jiménez-Heredia R, Genel F, Gulez N, Ardeniz Ö, Hoeger B, Bal SK, Madritsch C, Kalinichenko A, Chandra Ardy R, Gerçeker B, Rey-Barroso J, Ijspeert H, Tangye SG, Simonitsch-Klupp I, Huppa JB, van der Burg M, Dupré L & Boztug K (2018) Mutations affecting the actin regulator WD repeat—containing protein 1 lead to aberrant lymphoid immunity. J Allergy Clin Immunol 142, 1589–1604.e11. [DOI] [PubMed] [Google Scholar]
- 81.Sohn HW, Tolar P, Jin T & Pierce SK (2006) Fluorescence resonance energy transfer in living cells reveals dynamic membrane changes in the initiation of B cell signaling. Proc Natl Acad Sci 103, 8143–8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Akerlund J, Getahun A & Cambier JC (2015) B cell expression of the SH2-containing inositol 5-phosphatase (SHIP-1) is required to establish anergy to high affinity, proteinacious autoantigens. J Autoimmun 62, 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Davis RE, Ngo VN, Lenz G, Tolar P, Young RM, Romesser PB, Kohlhammer H, Lamy L, Zhao H, Yang Y, Xu W, Shaffer AL, Wright G, Xiao W, Powell J, Jiang J-K, Thomas CJ, Rosenwald A, Ott G, Muller-Hermelink HK, Gascoyne RD, Connors JM, Johnson NA, Rimsza LM, Campo E, Jaffe ES, Wilson WH, Delabie J, Smeland EB, Fisher RI, Braziel RM, Tubbs RR, Cook JR, Weisenburger DD, Chan WC, Pierce SK & Staudt LM (2010) Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature 463, 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Niemann CU & Wiestner A (2013) B-cell receptor signaling as a driver of lymphoma development and evolution. Semin Cancer Biol 23, 410–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schnyder T, Castello A, Feest C, Harwood NE, Oellerich T, Urlaub H, Engelke M, Wienands J, Bruckbauer A & Batista FD (2011) B Cell Receptor-Mediated Antigen Gathering Requires Ubiquitin Ligase Cbl and Adaptors Grb2 and Dok-3 to Recruit Dynein to the Signaling Microcluster. Immunity 34, 905–918. [DOI] [PubMed] [Google Scholar]
- 86.Seeley-Fallen MK, Liu LJ, Shapiro MR, Onabajo OO, Palaniyandi S, Zhu X, Tan T-H, Upadhyaya A & Song W (2014) Actin-binding protein 1 links B-cell antigen receptors to negative signaling pathways. Proc Natl Acad Sci 111, 9881–9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ricker E, Chowdhury L, Yi W & Pernis AB (2016) The RhoA-ROCK pathway in the regulation of T and B cell responses. F1000Research 5 Rev-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lou Z, Billadeau DD, Savoy DN, Schoon RA & Leibson PJ (2001) A Role for a RhoA/ROCK/LIM-Kinase Pathway in the Regulation of Cytotoxic Lymphocytes. J Immunol 167, 5749–5757. [DOI] [PubMed] [Google Scholar]
- 89.Scott RW & Olson MF (2007) LIM kinases: Function, regulation and association with human disease. J Mol Med 85, 555–568. [DOI] [PubMed] [Google Scholar]
- 90.Maeda FY, van Haaren JJH, Langley DB, Christ D, Andrews NW & Song W (2020) Surface-bound antigen induces B-cell permeabilization and lysosome exocytosis facilitating antigen uptake and presentation to T-cells. bioRxiv, 2020.07.24.220418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brown BK & Song W (2001) The Actin Cytoskeleton is Required for the Trafficking of the B Cell Antigen Receptor to the Late Endosomes. Traffic 2, 414–427. [DOI] [PubMed] [Google Scholar]
- 92.Stoddart A, Dykstra ML, Brown BK, Song W, Pierce SK & Brodsky FM (2002) Lipid rafts unite signaling cascades with clathrin to regulate BCR internalization. Immunity 17, 451–462. [DOI] [PubMed] [Google Scholar]
- 93.Roberts AD, Davenport TM, Dickey AM, Ahn R, Sochacki KA & Taraska JW (2020) Structurally distinct endocytic pathways for B cell receptors in B lymphocytes. Mol Biol Cell 31, 2826–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stoddart A, Jackson AP & Brodsky FM (2005) Plasticity of B cell receptor internalization upon conditional depletion of clathrin. Mol Biol Cell 16, 2339–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Natkanski E, Lee WY, Mistry B, Casal A, Molloy JE & Tolar P (2013) B cells use mechanical energy to discriminate antigen affinities. Science 340.6140, 1587–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yuseff MI, Reversat A, Lankar D, Diaz J, Fanget I, Pierobon P, Randrian V, Larochette N, Vascotto F, Desdouets C, Jauffred B, Bellaiche Y, Gasman S, Darchen F, Desnos C & Lennon-Duménil AM (2011) Polarized Secretion of Lysosomes at the B Cell Synapse Couples Antigen Extraction to Processing and Presentation. Immunity 35, 361–374. [DOI] [PubMed] [Google Scholar]
- 97.Hsu C-J, Hsieh W-T, Waldman A, Clarke F, Huseby ES, Burkhardt JK & Baumgart T (2012) Ligand Mobility Modulates Immunological Synapse Formation and T Cell Activation. PLoS One 7, e32398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Felts RL, Narayan K, Estes JD, Shi D, Trubey CM, Fu J, Hartnell LM, Ruthel GT, Schneider DK, Nagashima K, Bess JW, Bavari S, Lowekamp BC, Bliss D, Lifson JD & Subramaniam S (2010) 3D visualization of HIV transfer at the virological synapse between dendritic cells and T cells. Proc Natl Acad Sci U S A 107, 13336–13341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ketchum CM, Sun X, Suberi A, Fourkas JT, Song W & Upadhyaya A (2018) Subcellular topography modulates actin dynamics and signaling in B-cells. Mol Biol Cell 29, 1732–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carrasco YR, Fleire SJ, Cameron T, Dustin ML & Batista FD (2004) LFA-1/ICAM-1 interaction lowers the threshold of B cell activation by facilitating B cell adhesion and synapse formation. Immunity 20, 589–599. [DOI] [PubMed] [Google Scholar]
- 101.Alon R & Dustin ML (2007) Force as a Facilitator of Integrin Conformational Changes during Leukocyte Arrest on Blood Vessels and Antigen-Presenting Cells. Immunity 26, 17–27. [DOI] [PubMed] [Google Scholar]
- 102.Arana E, Harwood NE & Batista FD (2008) Regulation of integrin activation through the B-cell receptor. J Cell Sci 121, 2279–2286. [DOI] [PubMed] [Google Scholar]
- 103.Zeng Y, Yi J, Wan Z, Liu K, Song P, Chau A, Wang F, Chang Z, Han W, Zheng W, Chen Y-H, Xiong C & Liu W (2015) Substrate stiffness regulates B-cell activation, proliferation, class switch, and T-cell-independent antibody responses in vivo. Eur J Immunol 45, 1621–1634. [DOI] [PubMed] [Google Scholar]
- 104.Wang J, Lin F, Wan Z, Sun X, Lu Y, Huang J, Wang F, Zeng Y, Chen Y-H, Shi Y, Zheng W, Li Z, Xiong C & Liu W (2018) Profiling the origin, dynamics, and function of traction force in B cell activation. Sci Signal 11.542, eaai9192. [DOI] [PubMed] [Google Scholar]
- 105.Ma R, Kellner AV., Ma VPY, Su H, Deal BR, Brockman JM & Salaita K (2019) DNA probes that store mechanical information reveal transient piconewton forces applied by T cells. Proc Natl Acad Sci U S A 116, 16949–16954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brockman JM, Su H, Blanchard AT, Duan Y, Meyer T, Quach ME, Glazier R, Bazrafshan A, Bender RL, Kellner AV., Ogasawara H, Ma R, Schueder F, Petrich BG, Jungmann R, Li R, Mattheyses AL, Ke Y & Salaita K (2020) Live-cell super-resolved PAINT imaging of piconewton cellular traction forces. Nat Methods 17, 1018–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, Davidson MW, Lippincott-Schwartz J & Hess HF (2006) Imaging Intracellular Fluorescent Proteins at Nanometer Resolution. Science (80- ) 313, 1642–1645. [DOI] [PubMed] [Google Scholar]
- 108.Rust MJ, Bates M & Zhuang X (2006) Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat Meth 3, 793–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Axelrod D (1981) Cell-substrate contacts illuminated by total internal reflection fluorescence. J Cell Biol 89, 141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stout AL & Axelrod D (1989) Evanescent field excitation of fluorescence by epi-illumination microscopy. Appl Opt 28, 5237. [DOI] [PubMed] [Google Scholar]
- 111.Shashkova S & Leake MC (2017) Single-molecule fluorescence microscopy review: Shedding new light on old problems. Biosci Rep 37.4, BSR20170031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Coelho S, Baek J, Graus MS, Halstead JM, Nicovich PR, Feher K, Gandhi H, Gooding JJ & Gaus K (2020) Ultraprecise single-molecule localization microscopy enables in situ distance measurements in intact cells. Sci Adv 6, eaay8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stone MB & Veatch SL (2015) Steady-state cross-correlations for live two-colour super-resolution localization data sets. Nat Commun 6, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lillemeier BF, Mörtelmaier MA, Forstner MB, Huppa JB, Groves JT & Davis MM (2010) TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat Immunol 11, 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sherman E, Barr V, Manley S, Patterson G, Balagopalan L, Akpan I, Regan CK, Merrill RK, Sommers CL, Lippincott-Schwartz J & Samelson LE (2011) Functional nanoscale organization of signaling molecules downstream of the T cell antigen receptor. Immunity 35, 705–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gustafsson MGL (2000) Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J Microsc 198, 82–87. [DOI] [PubMed] [Google Scholar]
- 117.York AG, Chandris P, Nogare DD, Head J, Wawrzusin P, Fischer RS, Chitnis A & Shroff H (2013) Instant super-resolution imaging in live cells and embryos via analog image processing. Nat Methods 10, 1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ma Y, Wen K, Liu M, Zheng J, Chu K, Smith ZJ, Liu L & Gao P (2021) Recent advances in structured illumination microscopy. JPhys Photonics 3, 24009. [Google Scholar]
- 119.Wang JC & Hammer JA (2020) The role of actin and myosin in antigen extraction by B lymphocytes. Semin Cell Dev Biol 102, 90–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hebert B, Costantino S & Wiseman PW (2005) Spatiotemporal image correlation spectroscopy (STICS) theory, verification, and application to protein velocity mapping in living CHO cells. Biophys J 88, 3601–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jakobs MAH, Dimitracopoulos A & Franze K (2019) KymoButler, a deep learning software for automated kymograph analysis. Elife 8, e42288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ashdown GW, Burn GL, Williamson DJ, Pandžić E, Peters R, Holden M, Ewers H, Shao L, Wiseman PW & Owen DM (2017) Live-Cell Super-resolution Reveals F-Actin and Plasma Membrane Dynamics at the T Cell Synapse. Biophys J 112, 1703–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Horn BKP & Schunck BG (1981) Determining Optical Flow. Artificial Intelligence 17.1-3, 185–203. [Google Scholar]
- 124.Lee R, Campanello L, Hourwitz M, Alvarez P, Omidvar A, Fourkas J & Losert W (2020) Quantifying topography-guided actin dynamics across scales using optical flow. Mol Biol Cell, mbc.E19–11-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]


