Summary
Macrophages are key innate immune cells involved in a broad spectrum of physiological and pathological processes. Macrophage depletion with clodronate-liposomes is commonly used to investigate in vivo functions of macrophages in mice. Here, we describe a protocol that combines the depletion of resident macrophages with the reconstitution of the mice with in vitro differentiated, lentivirus-transduced bone marrow-derived macrophages (BMDMs) in the context of an experimental sepsis model. This experimental strategy is easily adapted to other experimental designs.
For complete details on the use and execution of this protocol, please refer to Du et al. (2020).
Subject areas: Cell Biology, Flow Cytometry/Mass Cytometry, Immunology, Model Organisms, Cell Differentiation
Graphical abstract
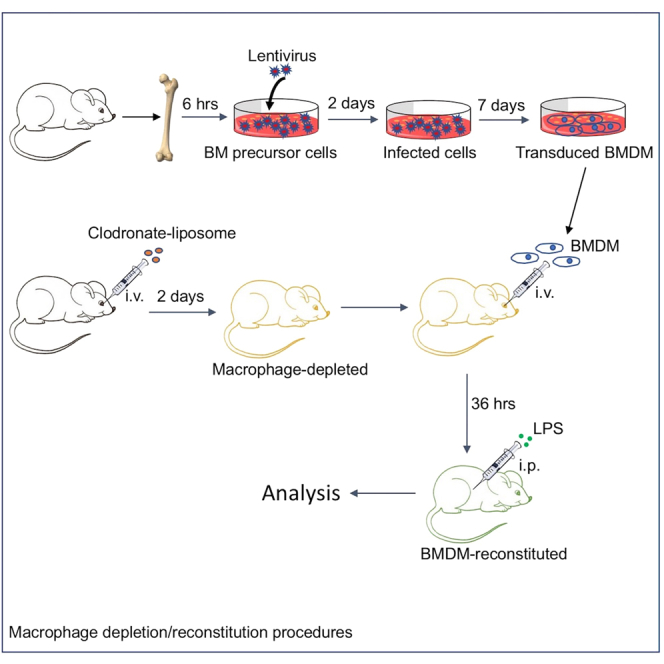
Highlights
-
•
Differentiation and lentiviral transduction of bone marrow-derived macrophages
-
•
Depletion of macrophages from mice and its validation
-
•
Reconstitute macrophage-depleted mice with lentivirus-transduced macrophages
-
•
Induce sepsis in mice with lipopolysaccharide
Macrophages are key innate immune cells involved in a broad spectrum of physiological and pathological processes. Macrophage depletion with clodronate-liposomes is commonly used to investigate in vivo functions of macrophages in mice. Here, we describe a protocol that combines the depletion of resident macrophages with the reconstitution of the mice with in vitro differentiated, lentivirus-transduced bone marrow-derived macrophages (BMDMs) in the context of an experimental sepsis model. This experimental strategy is easily adapted to other experimental designs.
Before you begin
The principle of macrophage depletion by clodronate-containing liposomves is that macrophages undergo apoptosis upon phagocytosis of clodronate liposomes (Naito et al., 1996; van Rooijen et al., 1996). This is a very effective method. Substantial depletion of macrophages can usually be achieved within 24–48 h with a single dose of clodronate-liposome injection. This method is frequently used to explore the role of macrophages in vivo under various conditions. On the other hand, the macrophage-depleted mice can be reconstituted with exogenous macrophages that have been genetically modified or pharmacologically treated, so that the reconstituted mice are used as an in vivo model to study these new macrophages. As macrophages will repopulate the mice within 1–2 weeks following their depletion (van Rooijen et al., 1989), timing is crucial in the experimental design in order to generate meaningful results.
The depletion/reconstitution protocol that we describe here has several steps, including bone marrow (BM) cell preparation, lentiviral transduction of the BM cells, in vitro differentiation of the BM cells into BMDMs, liposome-induced macrophage depletion in mice, reconstitution of the mice with lentivirus-transduced BMDMs, and lipopolysaccharide (LPS) challenge of the reconstituted mice. Because every step is time-sensitive, a careful coordination of each step is crucial for the success of the experiment.
Because mice are used in this procedure, institutional approval of all animal use protocols should be obtained before the experimental planning.
Similar protocols for macrophage depletion and reconstitution can be found elsewhere (Kozicky and Sly, 2019).
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-mouse MHCII FITC | BioLegend | Cat#: 107605, RRID: AB_313320 |
| Anti-mouse F4/80 APC | BioLegend | Cat#: 123115, RRID: AB_893493 |
| Chemicals, peptides, and recombinant proteins | ||
| RPMI 1640 | Corning | Ref#: 10-040-CV |
| DMEM | Corning | Ref#: 10-013-CV |
| Penicillin/Streptomycin (5,000 U/mL) | Thermo Fisher Scientific | Cat#: 15070063 |
| 200 mM Glutamine | GEMINI | Cat#: 400-106 |
| Trypsin-EDTA solution | Corning | Ref#: 25-053-Cl |
| Fetal bovine serum (FBS) | Gibco | Cat#: 10-082-147 |
| Sterile phosphate buffered saline (PBS) (pH 7.4) | Thermo Fisher Scientific | Cat#: AM9624 |
| Red blood cell lysis buffer (NH4Cl) | Thermo Fisher Scientific | Cat#: 00-4333-57 |
| 99.9% isoflurane | Baxter | Cat#: 10019-773-60 |
| Ethanol | Decon Labs | Cat#: 2701 |
| LPS from E coli O111:B4 | Sigma-Aldrich | Cat#: L2630 |
| Polybrene Transfection Reagent | Merck Millipore | Cat#: TR-1003-G |
| Live and Dead Violet Viability Kit | Invitrogen | L34963 |
| Critical commercial assays | ||
| Standard Macrophage Depletion Kit | Encapsula NanoSciences | Cat#: CLD-8901 |
| Experimental models: Cell lines | ||
| L929 | ATCC | Cat#: CCL-1 |
| Experimental models: Organisms/strains | ||
| C57BL/6J mice, males and females, 2-3 months of age | The Jackson Laboratory | Stock No: 000664 |
| YTHDF1(-/-) mice, males and females, 2-3 months of age | Chuan He | https://pubmed.ncbi.nlm.nih.gov/33220174/ |
| Recombinant DNA | ||
| pLV-Neo-EF1A-GFP (Control) (packaged into lentivirus at ∼108 PFU/mL) | VectorBuilder: Plasmid construction; Northwestern University Gene Editing Transduction and Nanotechnology Core (https://www.feinberg.northwestern.edu/research/cores/units/skin-disease.html): Virus package |
N/A |
| pLV-Neo-EF1A-hSOCS1 (packaged into lentivirus at ∼108 PFU/mL) | VectorBuilder: Plasmid construction; Northwestern University Gene Editing Transduction and Nanotechnology Core (https://www.feinberg.northwestern.edu/research/cores/units/skin-disease.html): Virus package |
ID: VB191018-1238kwr |
| Software and algorithms | ||
| FlowJo software V10 | BD | https://www.flowjo.com/ |
| ImageJ | National Institutes of Health (NIH) | https://imagej.nih.gov/ij/ |
| Other | ||
| Microtubes, 1.7 mL and 2 mL | Fisher Scientific or other qualified suppliers | N/A |
| Pipette tips, 10 μL, 200 μL and 1000 μL | Fisher Scientific or other qualified suppliers | N/A |
| Syringes, 1 mL and 3 mL; Needle, 28G |
Fisher Scientific or other qualified suppliers | N/A |
| Falcon centrifuge tubes, 15 mL and 50 mL, sterile | Fisher Scientific or other qualified suppliers | N/A |
| Serological pipettes, 5 mL and 10 mL | Fisher Scientific or other qualified suppliers | N/A |
| Cell culture plates, 6-well | Fisher Scientific or other qualified suppliers | N/A |
| Cell culture dishes, 10cm | Fisher Scientific or other qualified suppliers | N/A |
| Petri dishes, 10cm | Fisher Scientific or other qualified suppliers | N/A |
| Membrane filter system, 0.22 μm | Fisher Scientific | Cat#: 09-761-126 |
| Cell strainers, 70 μm | Fisher Scientific | Cat#: 08-771-2 |
| Hemocytometer | Fisher Scientific | Cat#: 02-671-54 |
| Surgical scissors, forceps and blades | Kent Scientific or other qualified suppliers | N/A |
| Legend RT Plus refrigerated tabletop centrifuge | Thermo Scientific Sorvall | N/A |
| LSRFortessa Cell Analyzer | BD Biosciences | N/A |
Reagent substitution
Most reagents can be substituted with a similar product of equal quality from a different supplier. L929 conditioned medium can be substituted with recombinant M-CSF.
Step-by-step method details
Preparation of L929 conditioned medium
Timing: 7 days
L929 is a mouse fibroblast line that secretes macrophage colony stimulating factor (M-CSF), which is required for the differentiation of BMDMs from hematopoietic stem cells. Thus, L929 cell conditioned media are used to differentiate BMDMs (see BMDM differentiation step).
-
1.
Culture L929 cells in complete DMEM (Table 1) in 10-cm cell culture dishes at 37°C and 5% CO2. At about 80% confluency, replace the culture medium with fresh complete DMEM.
Note: If cells are seeded at 20% confluency initially, it usually takes 3 days to reach 80% confluency.
-
2.
Three days after complete confluency, harvest the medium and then filtrate the medium through a 0.22 μm filter. The conditioned media can be aliquoted and stored at −80°C.
Note: Some cell death may occur at this time, but dead cells do not affect the quality of the conditioned medium. All cells and cell debris will be removed through filtration. The conditioned medium is yellowish at this time.
Table 1.
Complete DMEM medium
| Reagent | Final concentration | Amount for 500 mL |
|---|---|---|
| FBS | 10% | 50 mL |
| Penicillin-Streptomycin | 1% | 5 mL |
| DMEM | n/a | to 500 mL |
Store at 4°C for up to 1 month
The concentration of M-CSF in the conditioned medium is critical for the successful differentiation of BMDMs in the following steps. Therefore, it is recommended that the quality of each batch of conditioned media to support BMDM differentiation be tested by pilot experiments.
The L929 conditioned medium can be substituted with recombinant M-CSF.
Bone marrow isolation
Timing: 6 h
-
3.Sacrifice the mouse using a CO2 chamber followed by cervical dislocation.
-
a.Immediately spray the mouse with 70% EtOH before dissection.
-
b.Use surgical scissors and forceps and dissect out the whole leg, from hip to ankle, and immediately soak the leg in 70% EtOH for 5 min in a 10-cm petri dish.
-
a.
Note: Dissection and isolation should be performed in a sterile environment.
-
4.Use forceps and scissors to remove the skin and skeletal muscle tissue from the bone and wash the bone with cold PBS in a 10 cm petri dish.
-
a.Place the bone in a new 10 cm petri dish containing PBS, and separate femur and tibia by cutting at the knee joint.
-
b.Clean away any remaining tissue, and wash again with PBS in a new dish.
-
a.
Note: Bones must be thoroughly cleaned of skeletal muscle to avoid contamination from the tissue cells.
-
5.Fill up a 3-mL syringe with cold PBS containing 2% FBS.
-
a.Cut off the ends of the bone, gently insert a 28-gauge needle attached to the syringe into the marrow cavity, and flush out the bone marrow into an empty 10-cm petri dish by expelling the PBS solution through the cavity.
-
b.Refill the syringe with PBS/FBS solution and repeat this step until all bone marrow materials have been flushed out into the dish.
-
a.
Note: The PBS/FBS solution is made fresh each time before bone marrow isolation.
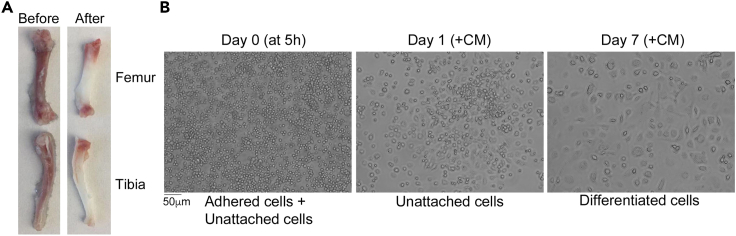
The color of the bone changes from dark red to white when the bone marrow is completely flushed out (see Figure 1A).
Figure 1.
Preparation of bone marrow derived macrophages (BMDMs)
(A) Mouse long bones before and after the marrow is flushed out.
(B) Representative cell images at several critical time points.
The amount of bone marrow cells to be obtained is dependent on age and genetic background of the mouse. Usually 6–8 week-old mice are ideal BM donors.
-
6.
Gently pipette the PBS/FBS/BM mixture up and down several time using a 5 mL serological pipette to disburse the bone marrow. Then transfer the solution into a 15 mL conical tube and continue to pipette up and down several times. Place the tube on ice.
-
7.Lyse red blood cells.
-
a.Centrifuge (1400 rpm) for 5 min at 25°C to pellet cells.
-
b.Aspirate the supernatant. Add 5 mL Red Blood Cell Lysis Buffer and 1 mL FBS. Resuspend the cells and incubate for 5 min at room temperature to lyse red blood cells.
-
a.
Note: Red Blood Cell Lysis Buffer is toxic to bone marrow cells. Including FBS can reduce cytotoxicity and increase cell viability.
-
8.Wash the cells with cold PBS.
-
a.Centrifuge (1400 rpm) for 5 min at 4°C to pellet the cells.
-
b.Resuspend the cells in 10 mL cold PBS, and centrifuge again (1400 rpm) for 5 min to pellet cells.
-
c.Repeat the wash two more times.
-
a.
-
9.
Resuspend the cells in 1 mL of complete RPMI 1640 medium pre-warmed at 37°C (Table 2) before adding to a 10-cm cell culture dish containing 6 mL of warm complete RPMI 1640 medium.
-
10.
Incubate 5 h at 37°C and 5% CO2 to remove stromal cells.
Note: After 5 h, the stromal cells will attach to the dish surface whereas the bone marrow stem cells and precursor cells remain unattached or suspended (Figure 1B).
CRITICAL: This step is required to remove the stromal cells, which attach to the dish and are indistinguishable from differentiated BMDMs if allowed to grow. This step is crucial for the purity of BMDMs.
-
11.After 5 h, carefully collect the media that contain unattached cells in 15 mL falcon tubes. Take care not to disturb the adhered cells.
-
a.Centrifuge (1400 rpm) at 4°C for 5 min to pellet the cells, and resuspend the cells in 1 mL of complete RPMI 1640 media.
-
b.Count the cell number using a hemocytometer.
-
a.
Table 2.
Complete RPMI 1640 medium
| Reagent | Final concentration | Amount for 500 mL |
|---|---|---|
| FBS | 10% | 50 mL |
| Penicillin-Streptomycin | 1% | 5 mL |
| RPMI 1640 | n/a | to 500 mL |
Store at 4°C for up to 1 month
Lentiviral transduction
Timing: 2 days
In this protocol, we use lentivirus-mediated DNA transfer to modify the BM cells before they are differentiated to BMDMs (Miller and Blystone, 2015). In our experiment, we transduced the BM cells with lentivirus that carries SOCS1 cDNA (SOCS1-Lentivirus), using GFP-lentivirus as control (Du et al., 2020). We found that transducing BM precursor cells is more effective to deliver the gene into BMDMs than directly transducing fully differentiated BMDMs.
-
12.
Add the 1 mL BM cells (up to 2 × 106 cells) into a new 10-cm cell culture dish containing 6 mL of complete RPMI 1640.
Note: Usually 1–5 × 106 bone marrow cells can be obtained from one mouse, depending on the age and size of the mouse.
-
13.
Thaw lentivirus stock solutions. To the lentivirus solution (0.1–0.2 mL) add polybrene to a final concentration of 6 μg/mL. Incubate at 25°C for 5 min.
CRITICAL: Polybrene is required to increase lentiviral transduction efficiency. The underlying principle is that Polybrene can neutralize the charge repulsion between the cell surface and virions.
-
14.
Add the lentivirus-polybrene complex to the BM cells in the dish at a M.O.I. of 10. Incubate the cells at 37°C and 5% CO2 for 48 h.
BMDM differentiation
Timing: 7 days
The transduced BM cells are differentiated into BMDMs using the differentiation medium (Table 3).
-
15.
After 48 h of culture, carefully aspirate the medium containing lentivirus and replace with the differentiation medium.
Note: Most of the BM cells become attached to the plate at this time.
-
16.
Continue the culture for 7 days at 37°C and 5% CO2. Change fresh differentiation media every 2 days.
Note: After 7 days the shape of the cultured cells (BMDMs) appears longer and more oval-like (Figure 1B).
Table 3.
BMDM differentiation medium
| Reagent | Final concentration | Amount for 500 mL |
|---|---|---|
| Complete RPMI 1640 Medium | 70% | 350 mL |
| L929 conditioned medium | 30% | 150 mL |
Store at 4°C for up to 1 month
Transduced BMDMs are GFP positive. The transduction efficiency can be estimated by observing the cells under a fluorescent microscope.
The expression of the delivered gene (such as SOCS1) can be assessed by Western blotting.
Liposome injection
Timing: 30 minto1 h
To deplete macrophages, the clodronate-containing liposomes need to be delivered to the mice intravenously. We injected these liposomes through the retro-orbital venous sinus (Yardeni et al., 2011).
-
17.
Two hours prior to injection, take out clodronate liposomes and control liposomes to equilibrate to 25°C.
Note: Clodronate liposomes and PBS liposomes (control liposomes) are stored at 4°C
-
18.
Invert the liposome tube several times (8–10 times) until the liquid becomes homogenized.
-
19.
Use a 1-mL syringe with a 28-gauge needle to take 0.2 mL of clodronate liposomes or control liposomes.
CRITICAL: Make sure to remove any air bubbles before injecting the mouse. Injecting air into the vein can lead to death.
-
20.Anesthetize the mouse with isoflurane using a precision vaporizer in a Class II Type B2 Biological Safety Cabinet.
-
a.Place the mouse inside the chamber and tightly close the lid.
-
b.Adjust oxygen flowmeter and isoflurane vaporizer setting to 1 L and 0.05–4%, respectively.
-
c.Wait until the mouse becomes deeply unconscious and then remove the mouse from the chamber. Complete anesthesia can be confirmed by loss of pedal reflex.
-
a.
Note: When the mouse is appropriately anesthetized, its breathing becomes rhythmic when it is lying on its side. Do not overexpose the mouse to the isoflurane as excessive exposure can lead to death. As the anesthetized mouse can wake up within 15 seconds, the injection should be completed within 6–7 s.
-
21.
Immediately before injection, resuspend the liposome solution by inverting the syringe containing liposomes 6 times to homogenize the liposome liquid.
-
22.Slowly inject 0.2 mL liposomes into the retro-orbital sinus.
-
a.For injection, position the anesthetized mouse onto one side and pull back skin above and below the eye such that the eye slightly protrudes.
-
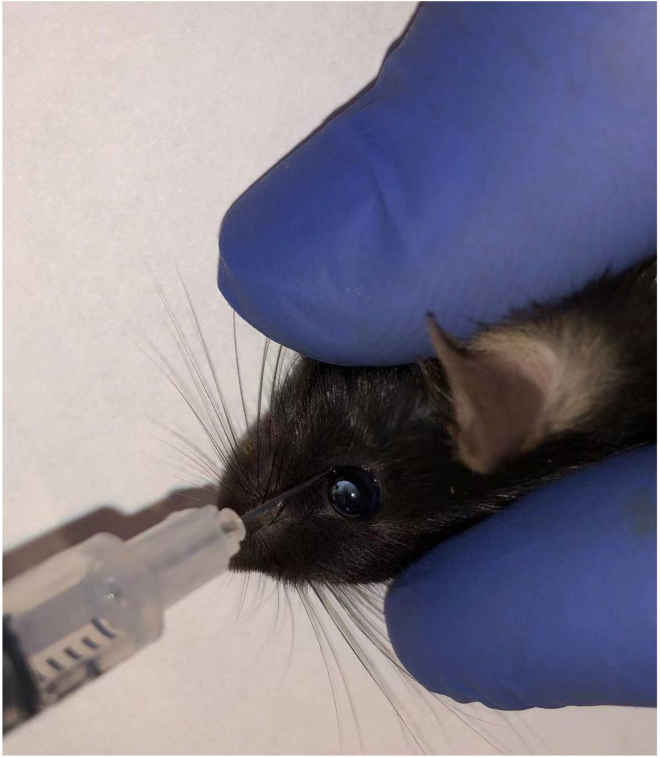
b.At the base of the eye, insert the needle at the medial canthus at about a 30° angle into the retro-orbital sinus (see Figure 2). For more detailed information about retro-orbital injection, please see this reference (Yardeni et al., 2011).
-
a.
Note: There should be no resistance felt if the needle is inserted into the correct position and little to no bleeding should occur post-injection.
Figure 2.
Mouse retro-orbital injection with 1 mL syringe.
Liposome solution can also be injected from the tail vein.
-
23.
Once injection is completed, place the mouse back into its cage.
Note: Observe the mouse to make sure it has fully recovered from anesthesia before leaving it unattended. It usually takes 15–20 s for the mouse to recover.
Validation of macrophage depletion
Timing: 2 days
Elimination of macrophages can be confirmed by analyzing F4/80+ resident macrophages in the spleen using fluorescence-activated cell sorting (FACS) assays after 48 h. This validation step is needed to establish the experimental condition. Once an optimal experimental condition is established, this step is not always necessary.
-
24.At 48 h following liposome injection, kill the mouse using a CO2 chamber followed by cervical dislocation.
-
a.Immediately sanitize the skin with 70% ethanol, and open the peritoneal cavity with surgical scissors and harvest the spleen.
-
b.Place the spleen into a 10-cm petri dish filled with cold RPMI1640 containing 10% FBS.
-
a.
-
25.Place the spleen onto a 70-μm cell strainer and use the end of a 1-mL syringe to smash the spleen through the cell strainer. Collect the filtered cell suspension into a 15 mL tube.
-
a.Spin down the cells (1400 rpm for 5 min at 4°C). Resuspend the cells in 5 mL of Red Blood Cell Lysis Buffer and incubate at 25°C for 5 min to lyse the red blood cells.
-
b.Add 10 mL of cold PBS. Centrifugation at 1400 rpm for 5 min to collect the cells.
-
c.Wash the cells again with cold PBS by repeating this step. Keep the cells on ice.
-
a.
-
26.FACS analysis.
-
a.Incubate the cells in 0.1 mL of the Live and Dead Violet Viability solution on ice for 30 min. Wash the cells with cold PBS once and spin down the cells (1400 rpm for 5 min at 4°C).
-
b.Stain the cells with anti-mouse MHCII FITC (1:200 dilution, clone M5/114.15.2, BioLegend) and anti-mouse F4/80 APC (1:200 dilution, clone BM8, BioLegend) on ice for 30 min in the dark. Wash the cells with cold PBS once.
-
c.Perform FACS analyses using a BD LSRFortessa unit (BD Biosciences).
-
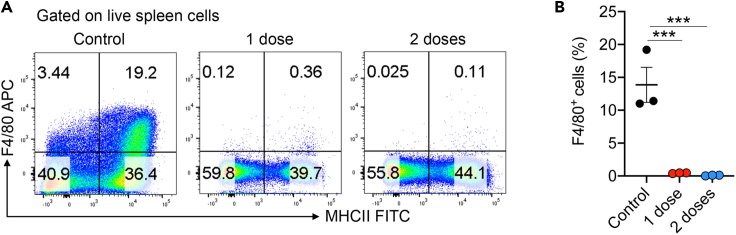
d.Analyze the FACS data using FlowJo software (V10) (Figure 3).
-
a.
Note: Successful depletion of resident macrophages (>90% reduction) can usually be observed after 48 h following one dose of liposome injection. Figure 3 shows an example of spleen macrophage depletion following one or two doses of clodronate liposome delivery at 48 h (Du et al., 2020).
Figure 3.
Validation of macrophage depletion by FACS
(A) Representative FACS plots of mouse spleen macrophage analyses at 48 h after treatment with control liposomes, one dose and two doses of clodronate liposomes. ∗∗∗p<0.001, by one-way ANOVA.
(B) Quantitative data of the FACS analyses (adapted from Du et al., 2020, with permission from Elsevier).
Macrophage reconstitution
Timing: 1–2 h
Two days after clodronate-liposome treatment, the macrophage-depleted mouse can be reconstituted with new macrophages. In this protocol, we used lentivirus-transduced BMDMs. These BMDMs are delivered by intravenous injection through the retro-orbital sinus. Because endogenous macrophages will repopulate the depleted mouse within 1–2 weeks (van Rooijen et al., 1989), there is a window of only 4–5 days in which the exogenous macrophages can be assayed in the mouse. Therefore, it is crucial that the experiment is planned so that the in vitro differentiation of BMDMs is completed and ready for use within this window.
-
27.
Aspirate the culture media of BMDMs and wash the cells with 10 mL PBS. Add 5 mL of Trypsin-EDTA and incubate at 37°C for 5 min to dissociate cells from the plate.
Note: BMDMs are usually ready for use by day 7 of culture in the conditioned medium.
An example of fully differentiated BMDMs is shown in Figure 1B.
-
28.Use a sterile cell scraper to scrape the cells off the plate.
-
a.Transfer the cells into a 50 mL conical tube. Rinse the culture dish with 5 mL of PBS (pH 7.0) 2–3 times and transfer rinse solution into the tube containing the harvested cells.
-
b.Gently pipette the cell solution several times to disburse cell clumps.
-
a.
-
29.
Centrifuge (1400 rpm) for 5 min at 25°C to pellet cells. Wash the cells with sterile PBS (pH 7.0) 2–3 times.
-
30.
Resuspend the cells with 5 mL of sterile PBS. Count cells with a hemocytometer.
-
31.
Centrifuge (1400 rpm) for 5 min at 25°C to pellet cells. Remove the supernatant, and resuspend the cells in sterile PBS at a concentration of 107 cells/mL.
-
32.
Anesthetize the recipient mouse (macrophage-depleted) using isoflurane as described above.
-
33.
Fill a 1 mL syringe with 0.2 mL cell solution. Use a 28-gauge needle to slowly inject this 0.2 mL of BMDM solution through the retro-orbital venous sinus.
Note: Each mouse receives 2 × 106 BMDMs. 0.2 mL of PBS can be injected as a control. The BMDM solution can also be injected through the tail vein.
-
34.
After injection is complete, place the mouse back into its cage.
Note: Observe the mouse to make sure it has fully recovered from anesthesia before leaving it unattended.
LPS-induced sepsis and analysis
Timing: 3–4 days
The reconstituted mouse is an in vivo platform to study the exogenous macrophages. Thirty-six hours after BMDM injection, the mouse is ready to use for experiment. In this protocol, we used the LPS-induced sepsis model to study the in vivo function of the reconstituted BMDMs (Du et al., 2020).
-
35.
Dissolve LPS in sterile PBS (pH 7.0) at 10 mg/mL.
-
36.
Weigh the mouse. Based on the weight, calculate the volume of the LPS solution to be injected to the mouse at 20 mg/kg body weight.
Note: For the LPS model, the ideal body weight of the mouse should be >18 grams.
-
37.
Load a 1-mL syringe with appropriate LPS solution, and inject the mouse intraperitoneally using a 28-gauge needle.
Note: For injection, insert the needle at a 30°–40° angle with the mouse’s abdomen in the lower right quadrant. Once the needle has pierced through skin and peritoneal lining, angle parallel with the stomach to avoid puncturing organs. PBS can be used as control for injection.
-
38.
Analyze the mice.
Note: The mice will develop hypothermia within a few hours following LPS injection, and up to 50% of C57BL/6 mice may die within 72 h at this LPS dose. LPS injection causes severe systemic inflammation and multi-organ injury. The mice can be euthanized at different time points for analysis.
Expected outcomes
This protocol describes the procedure of macrophage depletion and reconstitution for a mouse model of experimental sepsis. There are several key factors that will determine the outcome of this protocol: the quality of the differentiated BMDMs (including the transduction efficiency of the BMDMs), the extent of macrophage depletion in the mouse, and the quick repopulation of the exogenous BMDMs in the recipient mouse.
If the quality of the L929 conditioned media is good, differentiation of BMDMs is expected to be achieved within 7 days. The cell morphology can usually give some hints (see Figure 1B). If the cells are not differentiated after 7 days, it suggests that the conditioned medium is not functional and the cells should be abandoned. In our hand, the lentiviral transduction protocol can usually generate very high transduction efficiency (>80–90%) in the differentiated BMDMs.
The clodronate-liposome is very effective to deplete resident macrophages from a mouse. One dose of intravenous injection is expected to reduce spleen macrophages by >90% within 1–2 days (See Figure 3).
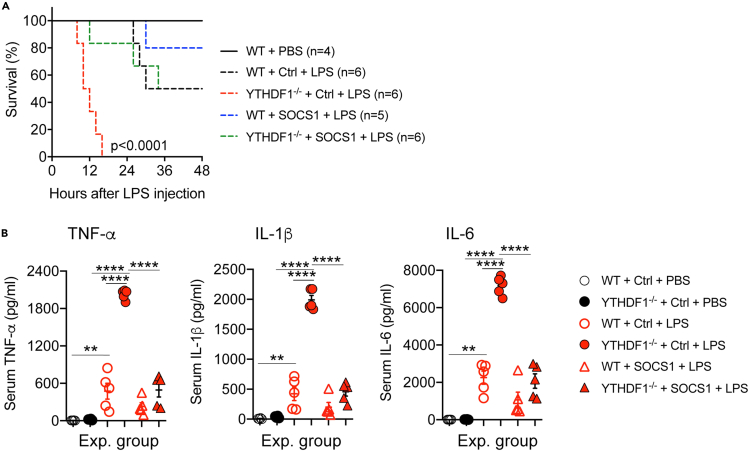
At the end, whether the depletion/reconstitution procedure is successful has to be validated by experiments. Here we use our study as an example. In the study we aimed to assess whether SOCS1 functions downstream of YTHDF1 in the regulation of macrophage activation. To this end, we derived BMDMs from wild-type (WT) and YTHDF1−/− mice, and then transduced these WT and YTHDF1−/− BMDMs with SOCS1-lentivirus or GFP (Ctrl)-lentivirus, respectively. We depleted macrophages from WT mice, and then reconstituted these mice with the SOCS1- or GFP (Ctrl)-transduced WT or YTHDF1−/− BMDMs. Finally, we subjected these reconstituted mice to the LPS-induced sepsis model. As shown in Figure 4, LPS challenge led to much higher mortality (100% death within 24 h) in the mice receiving Ctrl-transduced YTHDF1−/− BMDMs compared with the mice receiving Ctrl-transduced WT BMDMs (Figure 4A), and this is expected. The mice receiving Ctrl-transduced YTHDF1−/− BMDMs produced much higher levels of serum inflammatory cytokines than the mice receiving Ctrl-transduced WT BMDMs (Figure 4B), and this is also expected. These observations indicate that the lentivirus transduction and the depletion/reconstitution procedure were all successful (As all recipient mice were WT mice, if the procedures were not working, the phenotype of the recipient mice should be the same). When the mice receiving SOCS1-transduced YTHDF1−/− BMDMs were treated with LPS, the high mortality rate was prevented (Figure 4A), and the serum inflammatory cytokine levels were reduced to the Ctrl levels (Figure 4B). These data indicate that the depletion/reconstitution procedure indeed is an excellent system to study the in vivo function of exogenous macrophages. For detailed information about these experiments please refer to our publication (Du et al., 2020).
Figure 4.
Forced expression of SOCS1 corrects the hyper inflammatory phenotype of YTHDF1−/− macrophages in mice
(A) Survival curves of macrophage-depleted mice reconstituted with WT or YTHDF1−/− BMDMs transduced with SOCS1-lentivirus or Ctrl-lentivirus after LPS challenge; p < 0.0001 YTHDF1−/− + Ctrl + LPS vs. the rest.
(B) Serum cytokine concentrations in macrophage-depleted mice reconstituted with WT or YTHDF1−/− BMDMs transduced with SOCS1-lentivirus or Ctrl lentivirus after LPS challenge. ∗p < 0.05, ∗∗p < 0.01; ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, by one-way ANOVA. All data are presented as mean ± SD (adapted from Du et al., 2020, with permission from Elsevier).
Macrophage-depleted mice provide an experimental platform to study in vivo functions of macrophages - allowing for comparisons of mouse phenotypes in the presence and absence of macrophages. The macrophage depletion/reconstitution protocol described here moves a step further – allowing for study of in vivo functions of “exogenous” macrophages in the absence of the endogenous macrophages. The exogenous macrophages can be modified genetically or manipulated pharmacologically, so that the biological impact of the modification(s) in these macrophages can be evaluated in mice under different experimental conditions or disease models. As such, this protocol can be adapted to various experimental designs based on specific research purposes.
Limitations
Macrophages are differentiated from bone marrow stem cells and precursor cells within tissues. Following clodronate-liposome-mediated macrophage depletion, the endogenous macrophages can repopulate the mouse within 1–2 weeks. As such, the entire experiment has to be completed within several days. Therefore, this protocol is only applicable to acute experimental models (such as the sepsis model). To sustain macrophage depletion for long-term studies, repeated clodronate-liposome injections may be used.
Troubleshooting
Problem 1
Cell contamination (Bone marrow isolations and BMDM differentiation steps)
Potential solution
Fully sterilize the surgical instrument by autoclave. Perform the whole procedure under a sterile environment. Cell contamination is a very common problem in this protocol.
Problem 2
Macrophages were not depleted (Liposome injection and Validation of macrophage deletion steps)
Potential solution
Use fresh liposomes to inject mice. If stored properly at 4°C–8°C, liposomes can be effective for at least 6 weeks.
Problem 3
Mice died after liposome injection (Liposome injection steps)
Potential solution
Make sure to properly homogenize the liposome solution before loading the syringe and immediately before injection. If the mixture is not homogenous, the mouse can die after injection. Air bubbles in the syringe can also cause death.
Problem 4
Low yield of BMDMs (BMDM differentiation steps)
Potential solution
Select a mouse at least 8-week old for bone marrow isolation. Make sure to completely flush out the bone marrow to collect all BM cells. Macrophage numbers are dependent on mouse age and genetic background. Approximately 5 × 106 BMDMs can be derived from an 8–12 week old C57BL/6 mouse. BMDM yield is considered low if less than 1 × 106 BMDMs are obtained from one mouse.
Problem 5
Mice died after macrophage reconstitution injection (Macrophage reconstitution steps)
Potential solution
Make sure to suspend BMDMs in PBS well before intravenous injection. This is best done just prior to injection as BMDMs can clump quickly. Cell clumps can block blood flow leading to death.
Problem 6
No difference is seen in experimental outcomes after BMDM reconstitution (Macrophage reconstitution steps)
Potential solution
This suggests some procedure is not working. Use PBS as a reconstitution control to sort out whether BMDM reconstitution is working. Use unmodified BMDMs as a control to figure out whether lentiviral transduction is working.
Problem 7
Efficiency of lentivirus-transduction is low (Lentiviral transduction steps)
Potential solution
Make sure that the ratio of lentivirus:cell is 10:1. Use of polybrene should improve transduction efficiency.
Resource availability
Lead contact
Further information and requests regarding resources and reagents should be directed to and will be fulfilled by the lead contact, Yan Chun Li (cyan@medicine.bsd.uchicago.edu).
Materials availability
This study did not generate new materials or reagents.
Acknowledgments
This work was supported in part by National Institutes of Health grants R21AI140152 and R01AI151162.
Author contributions
Conceptualization: Y.C.L.; methodology: T.N., J.D., Y.C.L; investigation: T.N., J.D.; writing – original draft, T.N.; writing – review & editing, J.D. and Y.C.L.; funding acquisition: Y.C.L.; supervision: Y.C.L.
Declaration of interests
The authors declare no competing interests.
Data and code availability
This study did not generate new datasets or codes.
References
- Du J., Liao W., Liu W., Deb D.K., He L., Hsu P.J., Nguyen T., Zhang L., Bissonnette M., He C., et al. N(6)-adenosine methylation of Socs1 mRNA is required to sustain the negative feedback control of macrophage activation. Dev. Cell. 2020;55:737–753.e7. doi: 10.1016/j.devcel.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozicky L.K., Sly L.M. Depletion and reconstitution of macrophages in mice. Methods Mol. Biol. 2019;1960:101–112. doi: 10.1007/978-1-4939-9167-9_9. [DOI] [PubMed] [Google Scholar]
- Miller M.R., Blystone S.D. Reliable and inexpensive expression of large, tagged, exogenous proteins in murine bone marrow-derived macrophages using a second generation lentiviral system. J. Biol. Methods. 2015;2:e23. doi: 10.14440/jbm.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito M., Nagai H., Kawano S., Umezu H., Zhu H., Moriyama H., Yamamoto T., Takatsuka H., Takei Y. Liposome-encapsulated dichloromethylene diphosphonate induces macrophage apoptosis in vivo and in vitro. J. Leukoc. Biol. 1996;60:337–344. doi: 10.1002/jlb.60.3.337. [DOI] [PubMed] [Google Scholar]
- van Rooijen N., Kors N., Kraal G. Macrophage subset repopulation in the spleen: differential kinetics after liposome-mediated elimination. J. Leukoc. Biol. 1989;45:97–104. doi: 10.1002/jlb.45.2.97. [DOI] [PubMed] [Google Scholar]
- van Rooijen N., Sanders A., van den Berg T.K. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J. Immunol. Methods. 1996;193:93–99. doi: 10.1016/0022-1759(96)00056-7. [DOI] [PubMed] [Google Scholar]
- Yardeni T., Eckhaus M., Morris H.D., Huizing M., Hoogstraten-Miller S. Retro-orbital injections in mice. Lab Anim. (Ny) 2011;40:155–160. doi: 10.1038/laban0511-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate new datasets or codes.