Summary
We describe a protocol for identifying cellular thiol metabolites such as cysteine and cystine in adherent cells using high performance liquid chromatography (HPLC) tandem mass spectrometry-based metabolomics. We applied a modified extraction and sample derivatization protocol to accurately quantify the intracellular levels of labile thiol species and to inhibit oxidation prior to analysis.
For complete details on the use and execution of this protocol, please refer to Liu et al. (2020) and Koppula et al. (2021).
Subject areas: Cancer, Metabolism, Mass Spectrometry
Graphical abstract
Highlights
-
•
LC-MS-based quantification of labile thiol species
-
•
Modified extraction and derivatization procedure to prevent sample oxidation
-
•
Protocol for quantification of reduced and oxidized thiol species
We describe a protocol for identifying cellular thiol metabolites such as cysteine and cystine in adherent cells using high performance liquid chromatography (HPLC) tandem mass spectrometry-based metabolomics. We applied a modified extraction and sample derivatization protocol to accurately quantify the intracellular levels of labile thiol species and to inhibit oxidation prior to analysis.
Before you begin
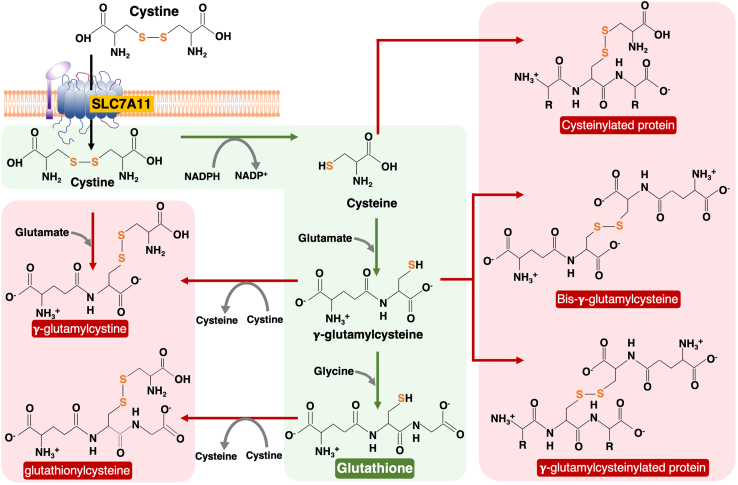
Several analytical approaches have been developed to quantify thiols in biological samples such as combining high-performance liquid chromatography (HPLC) and capillary electrophoresis (CE) with ultraviolet (UV) detection (Kuśmierek et al., 2009; Toyo’oka 2009) , fluorescence detection (McMenamin et al. 2009; Carlucci and Tabucchi 2009) and electrochemical detection (Sporea et al., 2006; Inoue and Kirchhoff 2002) . However, these methods typically employ uncommon or customized equipment, toxic or otherwise difficult reagents, and may require complete reduction of disulfide bonds prior to analysis and so will not preserve information about thiol-disulfide ratios. This protocol uses a liquid chromatography coupled to mass spectrometry (LC-MS) platform to perform a quantitative analysis of thiol metabolite species (Figure 1), such as glutathione (GSH), cystine, cysteine, γ-glutamylcystine, glutathionylcysteine, and oxidized glutathione (GSSG), from cultured cell lines.
Figure 1.
Metabolism of cystine and cystine-derived metabolites. Schematic shows cystine uptake via SLC7A11
Imported cystine is reduced to cysteine by utilizing NADPH (the reduced form of nicotinamide adenine dinucleotide phosphate). Cysteine is used to synthesize glutathione by combining with glutamate and glycine (shown in the green background). Intracellular cystine can combine with other molecules such as γ-glutamylcysteine (an intermediate of the glutathione pathway) to form γ-glutamylcystine; similarly, cystine can also donate one cysteine to combine with glutathione to form glutathionylcysteiene (shown in the left red background). In addition, these metabolites can be linked to proteins via disulfide bonds (shown in the right red background).
Cell line selection is a critical factor that will determine the success of this experiment as abundance of intracellular metabolites is significantly influenced by expression levels of specific nutrient transporters and/or metabolic enzymes. For instance, SLC7A11high cancer cell lines generally exhibit high levels of intracellular cysteine and/or glutathione due to increased cystine uptake (Figure 1). In this protocol, we primarily used the UMRC6 cell line, which has high SLC7A11 expression (Koppula et al., 2017; Liu et al., 2020).
Standard solution preparation
Timing: 0–1 h
-
1.
Prepare the dissolution solution (at least 1 mL per 10 mg of each internal standard compound).
Dissolution solution
| Reagent | Final Concentration | Notes |
|---|---|---|
| Milli-Q or HPLC grade water | 99.42% | N/A |
| Formic acid (HPLC grade) | 100 mM | Pure formic acid (i.e., Fisher #A117-50) is 26.5 M. This lowers the pH to prevent the reactive thiolate anion from forming. |
| 0.5 M EDTA | 1 mM | Add from standard 500 mM EDTA stock (pH 8.0). This prevents oxidation due to metal ions. |
Store at 4°C for up to 12 months.
-
2.
Prepare the 1 M HCl solution (at least 1 mL per 50 mg of U-13C-15N-cystine).
1 M HCl solution
| Reagent | Final concentration | Notes |
|---|---|---|
| Milli-Q or HPLC grade water | 90% | N/A |
| Hydrochloric acid (10 N) | 1 M | This lowers the pH so that cystine can be dissolved at a high concentration. |
Store at 25°C up to 12 months
CRITICAL: Hydrochloric acid is highly caustic, and can cause severe burns upon exposure to skin and eyes. It can also cause respiratory irritation. A laboratory coat, goggles, and gloves should be worn when working with this solvent. A chemical fume hood should be used when working with large volumes.
-
3.
Dissolve U-13C-15N-cysteine, 2×13C-U-15N-glutathione, and 4×13C-U-15N-glutathione disulfide individually into dissolution solution at 10 mg/mL each. Dissolve U-13C-15N-cystine into 1 M HCl solution at 50 mg/mL.
-
4.
Dilute each stock solution into extraction solution and mix to make a combined internal standard solution with the final concentrations: 2×13C-U-15N-glutathione, 50 μg/mL; 4×13C-U-15N-glutathione disulfide, 10 μg/mL; U-13C-15N-cysteine, 1 μg/mL; U-13C-15N-cystine, 10 μg/mL.
Note: U-13C-15N-cystine will need to be diluted at least 100-fold (i.e., from 50 mg/mL to 500 μg/mL) into an extraction solution in order to stay dissolved.
-
5.
Aliquot the internal standard mixture into individual tubes and store at −80°C. They should be stable for at least 6 months when stored in these conditions (up to 12 months). The aliquot volume should be determined by the number of samples expected per run, assuming 10 μL is needed per sample.
Standard curve
Timing: 1 h
-
6.
Prepare stock solutions of unlabeled glutathione, glutathione disulfide, and cysteine by dissolving directly into dissolution solution at 10 mg/mL each. Dissolve unlabeled cystine into 1 M HCl solution at 50 mg/mL.
-
7.
Dilute these stock solutions into extraction solution and mix to make a combined internal standard solution with the final concentrations: 2×13C-U-15N-glutathione, 500 μg/mL; 4×13C-U-15N-glutathione disulfide, 100 μg/mL; U-13C-15N-cysteine, 10 μg/mL; U-13C-15N-cystine, 100 μg/mL.
Note: U-13C-15N-cystine will need to be diluted at least 100-fold (i.e., from 50 mg/mL to 500 μg/mL) into an extraction solution in order to stay dissolved.
-
8.
Serially dilute this mixture into an extraction solution using two-fold steps, for a total of 9 dilutions plus one blank consisting of the extraction solution. Do this in duplicate.
-
9.
Thaw one or more aliquots of the internal standard mix on ice (total of at least 200 μL). Place 20 pre-labeled Eppendorf tubes on ice to cool.
-
10.
Transfer 10 μL of internal standard mix to each tube on ice.
-
11.
Transfer 90 uL of each dilution in the dilution series into the corresponding labeled tube on ice.
-
12.
Add 10 μL of triethylamine to each reaction tube, cap, briefly vortex to mix, and return to ice.
CRITICAL: triethylamine is highly flammable, a suspected fetal toxin, an eye irritant, and is considered acutely and chronically toxic. A laboratory coat, goggles, and gloves should be worn when working with this chemical. A chemical fume hood should be used when working with this chemical.
-
13.
Add 1 μL of benzyl chloroformate to each reaction tube, cap, briefly vortex to mix, and return to ice.
CRITICAL: benzyl chloroformate is considered acutely and chronically toxic. A laboratory coat, goggles, and gloves should be worn when working with this chemical. A chemical fume hood should be used when working with this chemical.
-
14.
Place the reaction tubes in a heating block or water bath at 37°C and incubate for 10 min.
-
15.
Spin the tubes (5 min, 16000 × g, 4°C).
-
16.
Transfer 100 μL of the derivatized samples to autosampler vials analyzed by LC-MS as described in the section Sample analysis by LC-MS. Use an initial injection volume of 5 μL.
-
17.
For each sample, determine the ratio of the unlabeled analyte peak to that of the isotope-labeled internal standard peak in that same sample.
-
18.
Plot the ratios for each analyte against the concentration of that analyte in the dilution series sample.
Note: inspect the plots to determine (a) the linear range for each analyte, and (b) the limit of detection. Given the large difference in concentrations between these analytes you may find that, at a given injection volume, high-abundance analytes such as glutathione have a limited linear range due to saturating the detector, while low-abundance analytes such as cysteine have an unacceptably high limit of detection. In this case you can optimize the quantitative ranges by running the standard curves at two different injection volumes, such as 1 and 20 μL. You will then need to run each sample at the same two injection volumes.
-
19.
Fit a line to the data for each analyte and determine the parameters of the linear equation. You will use these to calculate the concentrations of the analytes in cell extracts.
Analyte formulae and m/z values after derivatization
| Analyte | Formula | m/z ([M-H]-) |
|---|---|---|
| Cysteine | C19H19NO6S | 388.08603 |
| Cystine | C22H24N2O8S2 | 507.09012 |
| Glutathione | C26H29N3O10S | 574.15009 |
| Glutathione disulfide | C36H44N6O16S2 | 879.21826 |
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| U-13C-15N-cysteine | Cambridge Isotope Laboratories | Cat# CNLM-3871-H-PK |
| U-13C-15N-cystine | Cambridge Isotope Laboratories | Cat# CNLM-4244-H-PK |
| 2×13C-U-15N-glutathione | Cambridge Isotope Laboratories | Cat# CNLM-6245-HP-PK |
| 4×13C-U-15N-glutathione disulfide | Cambridge Isotope Laboratories | Cat# CNLM-8782-PK |
| Benzyl choloroformate | ACROS ORGANICS | Cat# 152945000 |
| 0.5M EDTA | Lonza | Cat# 51234 |
| Acetonitrile HPLC grade | Sigma-Aldrich | Cat# 439134-1L |
| Methanol HPLC grade | Sigma-Aldrich | Cat# 34860-1L-R |
| Formic Acid | Sigma-Aldrich | Cat# 33015-500ML |
| Dialyzed Fetal Bovine Serum | Sigma-Aldrich | Cat# F0392 |
| KL-11743 | Kadmon Corporation | N/A |
| DMEM without glucose | Thermo Fisher Scientific | Cat# 11966 |
| Penicillin-Streptomycin | Life Technologies | Cat# 15140-122 |
| DMEM | Sigma-Aldrich | Cat# D6429 |
| 0.25% Trypsin EDTA | Life Technologies | Cat# 25200056 |
| DPBS | Sigma-Aldrich | Cat# D8537 |
| Water LC-MS grade | Fisher Scientific | Cat# W64 |
| Methanol LC-MS grade | Fisher Scientific | Cat# A456-4 |
| Tributylamine | Sigma-Aldrich | Cat# 90781 |
| Acetic acid LC-MS grade | Fisher Scientific | Cat# A11350 |
| Hydrochloric acid | Fisher Scientific | Cat# A508 |
| Triethylamine | Fisher Scientific | Cat# AC219510500 |
| Negative Ion Calibration Solution | Thermo Fisher Scientific | Cat# 88324 |
| Experimental models: Cell lines | ||
| UMRC6 | Laboratory of W. G. Kaelin Lab | N/A |
| Software and algorithms | ||
| GraphPad | GraphPad | https://www.graphpad.com, RRID:SCR_002798 |
| Xcalibur 3.0.63 | Thermo Scientific |
https://www.thermofisher.com/, RRID:SCR_014593 |
| El-MAVEN | Elucidata | https://resources.elucidata.io/elmaven |
| ProteoWizard | SourceForge | https://proteowizard.sourceforge.io/index.shtml |
| Other | ||
| Refrigerated micro-centrifuge | Eppendorf | Cat# EPP-5424R |
| CO2 Incubator | Thermo Scientific | Cat# TH-370N |
| BSC Cabinet | NuAire | Cat# ES NU-427 |
| Plate Rocker | Corning | Cat# 6780-FP |
| PCV Cell Counting Tubes | Sigma-Aldrich | Cat# Z760986-50EA |
| Plastic Cell Scrapers | Corning | Cat# 08-771-1B |
| Cold Block | Thermo Scientific | Cat# 88-870-104 |
| Eppendorf tubes | Thermo Scientific | Cat# MCT-175C |
| 35 mm dish | Thermo Scientific | Cat# 353001 |
| Synergi Hydro-RP C18 column | Phenomenex | Cat# 00D-4387-B0 |
| Autosampler vials | Thermo Scientific | Cat# 03-377-299 |
| Autosampler vial caps | Thermo Scientific | Cat# 14-823-399 |
Materials and equipment
Extraction solution for extracting thiol species from cells
Extraction solution
| Reagent | Final concentration | Notes |
|---|---|---|
| Methanol (HPLC grade) | 40% | N/A |
| Acetonitrile (HPLC grade) | 40% | N/A |
| Milli-Q or HPLC grade water | 20% | N/A |
| Formic acid (HPLC grade) | 100 mM | Pure formic acid (i.e., Fisher #A117-50) is 26.5 M. This lowers the pH to prevent the reactive thiolate anion from forming. |
| 0.5 M EDTA | 1 mM | Add from standard 500 mM EDTA stock (pH 8.0). This prevents oxidation due to metal ions. |
CRITICAL: Methanol is highly flammable, a suspected fetal toxin, an eye irritant, and is considered an acutely and chronically toxic solvent. A laboratory coat, goggles, and gloves should be worn when working with this solvent. A chemical fume hood should be used when working with large volumes of this solvent.
CRITICAL: Acetonitrile is highly flammable and is considered acutely toxic solvent on skin exposure. A laboratory coat, goggles, and gloves should be worn when working with this solvent. A chemical fume hood should be used when working with large volumes of this solvent.
CRITICAL: Formic Acid is highly flammable, highly corrosive and can cause severe skin burns and eye damage. A laboratory coat, goggles, and gloves should be worn when working with this solvent. A chemical fume hood should be used when working with large volumes of this solvent.
LC-MS platform and solvent for thiol analysis
LC-MS analysis was performed essentially according to the method described in Lu et al. (2010). The complete platform consists of an Accela 1250 HPLC system, Accela Open Autosampler, MayLab Mistraswitch column oven and Exactive Orbitrap mass spectrometer, controlled by the Xcalibur 3.0.63 software package. Samples were analyzed by single-stage mass spectrometry and peaks identified on the basis of their accurate mass.
Chromatography was performed with a Phenomenex Synergi Hydro-RP C18 column (100 × 2 mm, 2.5 μm particle size) at a column oven temperature of 40°C.
Solvent A
| Reagent | Final concentration | Amount |
|---|---|---|
| tributylamine | 10 mM | 9.5 mL |
| acetic acid | 15 mM | 3.4 mL |
| ddH2O | n/a | 3987.1 mL |
| Total | n/a | 4000 mL |
Solvent A can be prepared and stored directly in the 4 L amber glass bottle that the LC-MS-grade water is stored in. Remove 12.9 mL of water from the bottle, add the tributylamine and acetic acid, and place a clean stir bar in the bottle. Mix the solution by vigorously stirring for at least 1 h.
Note: tributylamine readily adsorbs to the interior surfaces of the HPLC apparatus (tubing, valves, etc.) and can be difficult to fully remove except with extensive washing. While this does not pose an issue with HPLC-MS analyses in negative ionization mode, the strong propensity of tributylamine to form positive ions in solution tends to result in persistent background signal when run in positive ionization mode. Therefore this method should only be employed on an HPLC platform dedicated to negative-mode analyses or one that can be subject to extensive washing prior to positive mode analysis. An example wash protocol is to flow a solution of 0.1% formic acid in 50% methanol / 50% water through the HPLC system at 20 μL/in for 24–48 h.
Note on storage conditions: Solvent A should be stored at 4°C when not in use, and is stable for at least 6 months (up to 12 months).
CRITICAL: tributylamine and acetic acid are both irritants to skin and mucous membranes, and can be toxic by inhalation. Use nitrile gloves and eye protection when handling, and use a fume hood.
Alternatives: other brands of LC-MS grade water or formic acid are acceptable. Tributylamine should always be of the highest purity available.
Solvent B
| Reagent | Final concentration | Amount |
|---|---|---|
| Methanol | n/a | n/a |
LC-MS settings for thiol analysis
Untargeted analysis is performed using a Thermo Accela HPLC system coupled to an Exactive mass spectrometer. The MS parameters are listed in the following table.
Exactive MS parameter settings
| Parameters | Values |
|---|---|
| Ionization | Electrospray ionization |
| MS mass range | m/z 80 – m/z 1000 |
| Sheath gas flow rate | 30 (arbitrary units) |
| Aux gas flow rate | 10 (arbitrary units) |
| Sweep gas flow rate | 3 (arbitrary units) |
| Spray voltage | 3 kV |
| Capillary temperature | 325°C |
| Capillary voltage | −25 V |
| Tube lens voltage | −50 V |
| Maximum inject time | 250 ms |
| Resolution (@ 1 Hz) | 100,000 |
| Automatic gain control target | 1e6 |
HPLC gradient and parameters
| Time (min) | Gradient (% B) |
|---|---|
| 0.0 | 0 |
| 2.5 | 0 |
| 5.0 | 20 |
| 7.5 | 20 |
| 13.0 | 55 |
| 18.5 | 95 |
| 19.0 | 0 |
| 25.0 | 0 |
The flow rate is set to 0.2 mL/min for the duration of the run. The column oven temperature is set to 40°C. These conditions typically result in a maximum backpressure of 200–220 bars, with pressures in excess of 275 bar indicating a clog in the solvent line. The method can be run at 25°C, without a solvent oven, but this will result in higher baseline pressure readings and may lead to variability in retention times.
Step-by-step method details
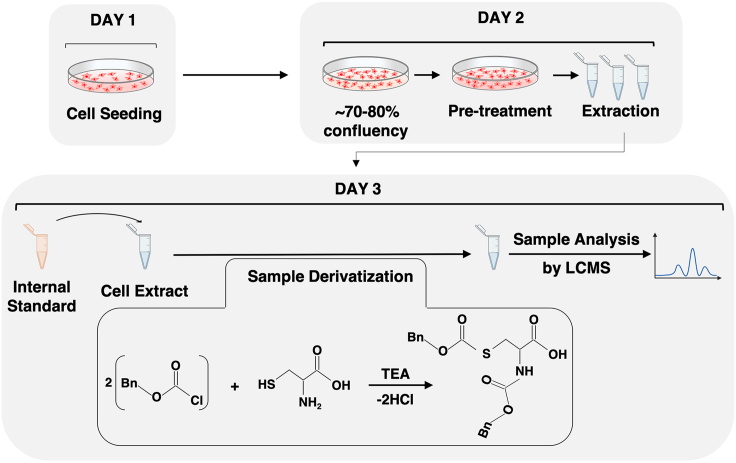
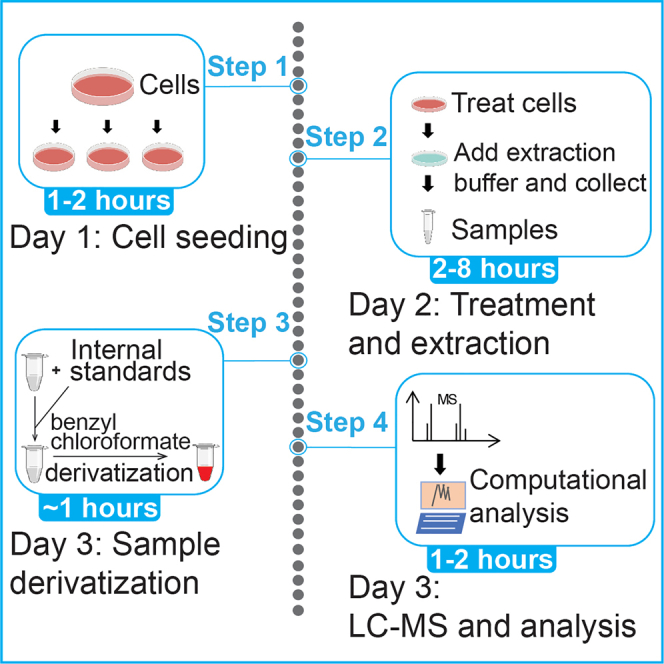
An overview of the procedure is summarized in Figure 2. The cells are plated on Day 1 (Step 1). The samples for thiol analyses are extracted from the cells on Day 2 (steps 2–4). The samples may be stored at this point, and are spiked with internal standards, derivatized and analyzed on Day 3.
Figure 2.
An overview of the procedures
Day 1: Cell seeding
Timing: 1–2 h
-
1.
Prepare relevant growth medium using dialyzed FBS. The DMEM medium for culturing UMRC6 cells contains glutamine and 1% (v/v) penicillin/streptomycin.
-
2.
Plate 5 × 105 UMRC6 cells in a 35 mm dish at a density that will ensure ∼70–80% confluence at the time of extraction.
Note: We recommend using dialyzed FBS-containing medium for all the relevant steps in this protocol, as this allows better control of the metabolite levels the cells are exposed to during growth and treatment phases. The protocol is optimized for adherent mammalian cells grown on 6-well plates or 35 mm dishes. 60 mm dishes have also been used successfully, but require scaling up some of the volumes.
Day 2: Pretreatment and treatment
Timing: 2–6 h
-
3.For short-term treatments (0.25 to 4-h drug treatments, for example), on the day of the experiment, the cells should be transferred into fresh medium for 1–2 h prior to beginning the treatment.
-
a.This controls for the nutrients that were depleted during overnight culture, as well as any waste products that may have accumulated in the medium.
-
a.
Preparation for extraction
Timing: 0–1 h
-
4.
Prepare the extraction solution (at least 500 μL per sample).
-
5.
Cool a tube of extraction solution on ice.
-
6.
Cool a tube of PBS on ice (at least 1 mL per sample).
-
7.
Place the cold block in a box or bin filled with ice.
Note: If cold block is unavailable, the relevant steps can be performed on dry ice with caution.
-
8.
Label all Eppendorf tubes.
-
9.
For media samples, fill Eppendorf tubes with 450 μL of extraction solution.
-
10.
Trypsinize parallel plates/dishes of cells and measure the packed cell volume (PCV) using PCV measurement tubes.
Optional: count cells.
Extraction protocol
Timing: 0–2 h
-
11.
Move the ice bin, DPBS, extraction solution, and media collection tubes into the TC hood.
-
12.
Transfer culture plates/dishes from incubator to TC hood.
-
13.
Harvest 50 μL media directly into media collection tubes containing extraction solution, and place on ice.
Note: steps 14 and 15 should be performed one plate/dish at a time.
-
14.
Aspirate the medium from a culture plate, then quickly add 1 mL cold DPBS and place on the cold block.
-
15.
Aspirate the PBS, then quickly add 500 μL of cold extraction solution and place the plate back on the cold block.
Note: all subsequent steps can be performed outside the TC hood
-
16.
Gently rock the cold block with the plates (still on ice) for 5 min on a rocker.
-
17.
Scrape each plate with a cell scraper and transfer the suspended cell debris into a labeled 2 mL Eppendorf tube on ice.
-
18.
Spin the tubes (5 min, 5000 × g, 4 C).
-
19.
Transfer the supernatants into new, labeled 2 mL tubes on ice.
-
20.
The samples can be stored at this point at −80°C for a maximum of 48 h. The samples need to be on dry ice if they need to be shipped. Note these extracts will freeze on ice.
Day 3: Sample derivatization
Timing: 0.5–1 h
-
21.
If the samples are frozen, thaw them on ice or on a cold block at ice-cold temperatures. Alternatively, you can proceed directly to this step from sample collection. Also thaw one or more aliquots of internal standard solution on ice (10 μL per sample to be analyzed).
-
22.
Label a fresh set of Eppendorf tubes (one per sample to be analyzed) and place them on ice to cool.
-
23.
Transfer 10 μL of internal standard solution to each of the pre-labeled tubes.
-
24.
Transfer 90 μL of extracts from the sample tubes to the corresponding reaction tubes.
-
25.
Add 10 μL of triethylamine to each reaction tube, cap, briefly vortex to mix, and return to ice.
CRITICAL: Triethylamine is highly flammable, a suspected fetal toxin, an eye irritant, and is considered acutely and chronically toxic. A laboratory coat, goggles, and gloves should be worn when working with this chemical. A chemical fume hood should be used when working with this chemical.
-
26.
Add 1 μL of benzyl chloroformate to each reaction tube, cap, briefly vortex to mix, and return to ice.
CRITICAL: Benzyl chloroformate is considered acutely and chronically toxic. A laboratory coat, goggles, and gloves should be worn when working with this chemical. A chemical fume hood should be used when working with this chemical.
-
27.
Place the reaction tubes in a heating block or water bath at 37°C and incubate for 10 min.
-
28.
Spin the tubes (5 min, 16000 × g, 4°C).
-
29.
Transfer 100 μL of the derivatized samples to autosampler vials and proceed to sample analysis.
Sample analysis by LC-MS
Timing: 0.5–1 h per sample
The derivatized samples are analyzed by LC-MS. The method detailed here uses reversed-phase chromatography with a C18 column and tributylamine as an ion-pairing reagent with an Orbitrap-based mass spectrometer, but other column chemistries and mass spectrometers can be used as well. In that case you will need to develop the chromatography method and confirm separation and detection with standard solutions.
-
30.
Prepare mobile phase Solvent A as described in the “materials and equipment” section.
-
31.
Fill the mobile phase reservoirs of the LC pump with Solvent A and methanol.
-
32.
Purge the solvent lines of the LC pump to remove any air bubbles.
-
33.
Calibrate the mass spectrometer using negative ion calibration solution according to the manufacturer’s instruction.
-
34.
Set up the sequence to run your samples, using the injection volumes determined in the Standard curve section. Run at least one blank first to ensure the LC column is fully conditioned, and to establish baseline values.
Note: due to the large difference in intracellular concentrations between some of these analytes, you may need to run each sample twice, with two different injection volumes, to ensure that all the analytes are within the linear range of the mass spectrometer.
Note: see Troubleshooting section if the LC reports a high- or low-pressure error.
-
35.
Once the sample sequence has finished, convert the Thermo data files (∗.raw) to the mzXML format (∗.mzxml) using the msConvert or msConvertGUI tools in the ProteoWizard package.
-
36.
Open the mzXML files in the El-MAVEN software package.
-
37.
Find the peaks for the analytes of interest and their respective internal standards, and extract the peak area values to a comma-separated values (∗.csv) file.
Data analysis and quantitation
-
38.
For each sample, determine the ratio of the unlabeled analyte peak to that of the isotope-labeled internal standard peak in that same sample.
Note: if you had to run each sample twice, with different injection volumes, be sure to use only the data from the run with signals in the linear range.
-
39.
Using the standard curve data from the Standard curves section, convert each ratio to a molar concentration. This is the concentration of the analyte in the extract.
-
40.
Using the PCV values determined in the Preparation for extraction section, convert the extract concentrations to intracellular concentrations using the following formula:
| Ccell = Cextract × 500 × PCV |
Where Ccell is the intracellular concentration, Cextract is the extract concentration, PCV is the packed cell volume for that sample group in μL.
Linear concentration ranges in extracts and intracellular space
| Analyte | Linear range in extract (μM) | Linear range in cells (μM) |
|---|---|---|
| Cysteine | 0.2–10 | 20–1,000 |
| Cystine | 0.1–50 | 10–5,000 |
| Glutathione | 0.5–200 | 50–20,000 |
| Glutathione disulfide | 0.05–20 | 5–2,000 |
Note: the linear range in the intracellular space is estimated from the linear range in extracts by assuming a packed cell volume of 5 μL. Extracting different volumes of cells may shift the range.
Expected outcomes
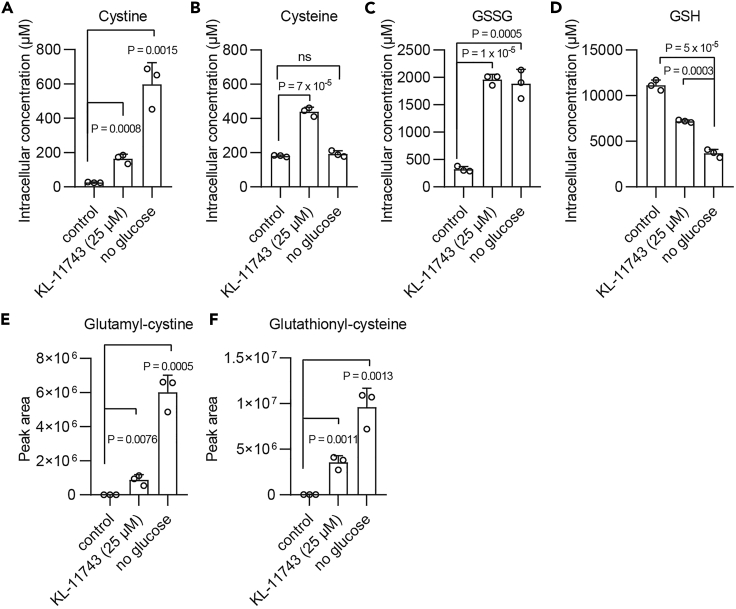
Based on this protocol described above, we have successfully conducted thiol analysis in our recent publications (Liu et al., 2020; Koppula et al., 2021). The RSD/CV for these analytes are typically below 15% for biological replicates, and under 5% for technical replicates. For example, we showed that glucose starvation, by limiting the supply of intracellular NADPH (through the pentose phosphate pathway) for cystine reduction, markedly increased intracellular cystine concentrations in UMRC6 cells (a SLC7A11high cell line); likewise, treatment of UMRC6 cells with GLUT inhibitor KL-11743 also significantly increased intracellular cystine levels, although cystine accumulation by KL-11743 was less potent than that by glucose starvation (Figure 3A). It should be noted that glucose starvation or GLUT inhibitor treatment typically did not cause a corresponding decrease of intracellular cysteine levels (Figure 3B), likely because cysteine utilization for protein synthesis is also dramatically suppressed under glucose starvation (which is known to inhibit mTORC1 signaling). Our methods also revealed that glucose starvation or KL-11743 treatment significantly increased intracellular levels of other disulfide molecules such as GSSG, glutamyl-cystine, glutathionyl-cysteine, with a concomitant decrease of intracellular GSH levels (Figures 3C–3F). We further showed that these thiol level changes caused by glucose starvation or GLUT inhibition are dependent on high SLC7A11 expression in UMRC6 cells (data not shown). Together, using these methods, we demonstrate that, in SLC7A11high cancer cells, high rates of cystine import and subsequent cystine reduction to cysteine promote glucose dependency due to high NADPH demand; consequently, limiting glucose supply (by either glucose starvation or GLUT inhibitor treatment) restrains cystine reduction to cysteine, resulting in marked accumulation of cystine and other disulfide molecules. (In studies described above, the relative standard deviations for these analytes were typically below 15% for biological replicates, and under 5% for technical replicates.) Another recent study revealed similar results (Joly et al., 2020).
Figure 3.
Thiol analysis in UMRC6 cells cultured in glucose-free medium or treated with GLUT inhibitor KL-11743
(A–D) Measured intracellular concentrations of cystine (A), cysteine (B), GSSG (C), and GSH (D) in UMRC6 cells cultured in DMEM with or without KL-11743 or glucose-free DMEM medium.
(E and F) Peak area measured by HPLC-MS of glutamyl-cystine (E) and glutathionyl-cysteine (F) in UMRC6 cells cultured in DMEM with or without KL-11743 or glucose free DMEM medium. All p values were calculated using two-tailed unpaired Student’s t-test. All data are mean ± s.d., n = 3 independent experiments. The “ns” means not significant (p > 0.05).
Limitations
Low cell number may lead to output lower than detection range. Since this method calculates intracellular concentrations using the total cell volume, but only a fraction of that volume consists of water, these concentrations will not strictly reflect the concentration of the analyte in solution. For example, common estimates for mammalian cells suggest that water constitutes 70% of the volume of the cytosol, which itself is approximately 70% of the total cell volume. Since the aqueous fraction of volume varies between cell types, and is difficult to determine experimentally, we use the total cell volume as an approximation that is not likely to differ from the aqueous volume by much more than a factor of two. The whole-cell extracts result in the mixing of intracellular compartments and so do not allow for organelle-specific concentrations to be determined. However, this technique could be adapted to measure thiol and disulfide concentrations in isolated organelles (Chen et al., 2016).
Troubleshooting
Problem 1
No difference in positive and negative controls (step by step method details, step 2–3).
Potential solution
It is possible that the cell density at the time of collection was too high. This creates an environment where the cells are competing with each other for resources. It is possible that the pre-treatment with media wasn’t done properly to restore depleted nutrients. It is possible that the cell line was with low-expression of SLC7A11/SLC3A2 or was with wild-type KEAP1 gene if significant accumulation of cystine or GSSG weren’t shown upon glucose starvation. For more detail, please refer to our recent publications (Liu et al., 2020; Koppula et al., 2021).
Problem 2
Too much variation in signals from intra-assay replicates of the same sample (step by step method details, step 2–20).
Potential solution
It is possible that the seeding wasn’t consistent between the replicates. If treating the cells to a chemical agent such as a drug, please make sure to use that same working stock solution. Make sure to perform the relevant steps as recommended to maintain the samples at ambient temperatures to avoid sample degradation.
Problem 3
High- or low-pressure error when attempting to run the LC system (step by step method details, step 30–37).
Potential solution
A high-pressure error indicates that there is a clog somewhere in the solvent line between the LC pump and the ESI source. Locate the clog by disconnecting each individual section of tubing, in series, while continuing to pump solvent (be sure to collect the solvent in a waste container). After disconnecting each section, monitor the pressure to see if it is above baseline. If not, reconnect the tubing and move on to the next section. Once you have found the location of the clog you will need to remove it either by cleaning the clogged piece of the pump or replacing the piece/tubing. A low-pressure error indicates a leak somewhere in the solvent line between the LC pump and the ESI source. Inspect the entire solvent line for liquid residue to find the leak. Replace any damaged tubing or equipment and tighten any loose fittings to stop the leak.
Problem 4
No signal detected, even for the internal standards (Step by Step method details, step 37).
Potential solution
Make sure to perform the relevant steps as recommended to maintain the samples and avoid sample degradation. Examine the HPLC-MS platform to ensure that it is operating properly. Check for leaks in the sample flow line and confirm that the liquid flow is reaching the ion source. Directly infuse calibration standard mixture into the MS and confirm that the signals are within normal ranges. If the problem persists, contact tech support.
Problem 5
One or more analytes give signals outside the linear range of the assay (step by step method details, step 38–40).
Potential solution
If the signals are too low, try to extract a larger volume of cells. If the signals are too high, either extract a lower volume of cells or dilute the extracts in the extraction solution.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Boyi Gan (bgan@mdanderson.org).
Materials availability
This study did not generate new unique materials.
Acknowledgements
This research was supported by Institutional Research Fund and Bridge Fund from The University of Texas MD Anderson Cancer Center, Emerson Collective Cancer Research Fund and R01CA181196, R01CA244144, and R01CA247992 from the National Institutes of Health (to B.G.). B.G. was an Andrew Sabin Family Fellow. P.K. was supported by CPRIT Research Training Grant (RP170067) and the Dr. John J. Kopchick Research Award from The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences. This research has also been supported by the National Institutes of Health Cancer Center Support Grant P30CA016672 to The University of Texas MD Anderson Cancer Center.
Author contributions
P.K., X.L., K.O., and B.G. organized the protocol design. P.K., X.L., and B.G. wrote the sample preparation protocol. K.O. wrote the HPLC-MS method protocol for thiol analysis. B.G. initiated the manuscript and provided funding support for the research described in this protocol.
Declaration of interests
K.O. is a full-time employee of the Barer Institute. The other authors declare no competing interests.
Contributor Information
Kellen Olszewski, Email: kellen.olszewski@barerinstitute.com.
Boyi Gan, Email: bgan@mdanderson.org.
Data and code availability
This study did not generate unique datasets or codes.
References
- Carlucci F., Tabucchi A. Capillary electrophoresis in the evaluation of aminothiols in body fluids. J. Chromatogr. B. 2009;877:3347–3357. doi: 10.1016/j.jchromb.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Chen W.W., Freinkman E., Wang T., Birsoy K., Sabatini D.M. Absolute quantification of matrix metabolites reveals the dynamics of mitochondrial metabolism. Cell. 2016;166:1324–1337.e11. doi: 10.1016/j.cell.2016.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T., Kirchhoff J.R. Determination of thiols by capillary electrophoresis with amperometric detection at a coenzyme pyrroloquinoline quinone modified electrode. Anal. Chem. 2002;74:1349–1354. doi: 10.1021/ac0108515. [DOI] [PubMed] [Google Scholar]
- Joly J.H., Delfarah A., Philip S.P., Parrish S., Graham N.A. A synthetic lethal drug combination mimics glucose deprivation–induced cancer cell death in the presence of glucose. J. Biol. Chem. 2020;295:1350–1365. doi: 10.1074/jbc.RA119.011471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppula P., Olszewski K., Zhang Y., Kondiparthi L., Liu X., Lei G., Das M., Fang B., Poyurovsky M.V., Gan B. KEAP1 deficiency drives glucose dependency and sensitizes lung cancer cells and tumors to GLUT inhibition. iScience. 2021;24:102649. doi: 10.1016/j.isci.2021.102649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppula P., Zhang Y., Shi J., Li W., Gan B. The glutamate/cystine antiporter SLC7A11/xCT enhances cancer cell dependency on glucose by exporting glutamate. J. Biol. Chem. 2017;292:14240–14249. doi: 10.1074/jbc.M117.798405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuśmierek K., Chwatko G., Głowacki R., Bald E. Determination of endogenous thiols and thiol drugs in urine by HPLC with ultraviolet detection. J. Chromatogr. B. 2009;877:3300–3308. doi: 10.1016/j.jchromb.2009.03.038. [DOI] [PubMed] [Google Scholar]
- Liu X., Olszewski K., Zhang Y., Lim E.W., Shi J., Zhang X., Zhang J., Lee H., Koppula P., Lei G. Cystine transporter regulation of pentose phosphate pathway dependency and disulfide stress exposes a targetable metabolic vulnerability in cancer. Nat. Cell Biol. 2020;22:476–486. doi: 10.1038/s41556-020-0496-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W., Clasquin M.F., Melamud E., Amador-Noguez D., Caudy A.A., Rabinowitz J.D. Metabolomic analysis via reversed-phase ion-pairing liquid chromatography coupled to a stand alone orbitrap mass spectrometer. Anal. Chem. 2010;82:3212–3221. doi: 10.1021/ac902837x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin M.E., Himmelfarb J., Nolin T.D. Simultaneous analysis of multiple aminothiols in human plasma by high performance liquid chromatography with fluorescence detection. J. Chromatogr. B. 2009;877:3274–3281. doi: 10.1016/j.jchromb.2009.05.046. [DOI] [PubMed] [Google Scholar]
- Sporea I., Popescu A., Şirli R., Golea O., Totolici C., Dănilă M., Vernic C. Pegylated-interferon alpha 2a treatment for chronic hepatitis C in patients on chronic haemodialysis. World J. Gastroenterol. 2006;12:4191. doi: 10.3748/wjg.v12.i26.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyo’oka T. 'Recent advances in separation and detection methods for thiol compounds in biological samples. J. Chromatogr. B. 2009;877:3318–3330. doi: 10.1016/j.jchromb.2009.03.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate unique datasets or codes.