Abstract
The increasing attention on personalized breast cancer care has resulted in an explosion of new interactive, tailored, web-based clinical decision tools for guiding treatment decisions in clinical practice. The goal of this study was to review, compare, and discuss the clinical implications of current tools, and highlight future directions for tools aiming to improve personalized breast cancer care. We searched PubMed, Embase, PsychInfo, Cochrane Database of Systematic Reviews, Web of Science, and Scopus to identify web-based decision tools addressing breast cancer treatment decisions. There was a total of 17 articles associated with 21 unique tools supporting decisions related to surgery, radiation therapy, hormonal therapy, bisphosphonates, HER2-targeted therapy, and chemotherapy. The quality of the tools was assessed using the International Patient Decision Aid Standard instrument. Overall, the tools considered clinical (e.g., age) and tumor characteristics (e.g., grade) to provide personalized outcomes (e.g., survival) associated with various treatment options. Fewer tools provided the adverse effects of the selected treatment. Only one tool was field-tested with patients, and none were tested with healthcare providers. Future studies need to assess the feasibility, usability, acceptability, as well as the effects of personalized web-based decision tools on communication and decision making from the patient and clinician perspectives.
Keywords: Web-based decision tools, Treatment, Breast cancer
Highlights
-
•
There are 21 web-based interactive decision tools for guiding breast cancer treatment decisions in current clinical practice.
-
•
The tools guided decisions related to neoadjuvant, radiation, and adjuvant therapies, and adverse effects of treatments.
-
•
There is limited data on the tools’ usability, feasibility, acceptability, and their impact on shared decision making.
1. Introduction
Over the last two decades an increasing number of clinical trials have helped improve and increase treatment options for women diagnosed with breast cancer [1]. However, translation of new trial data to clinical practice will remain a challenge if trial findings do not apply to individual women seen in real-world clinical settings. Personalized cancer treatment is broadly defined as the process of creating tailored treatment plans for patients considering individual differences in disease severity, clinical presentation, natural history, preferences, and treatment tolerance [2]. In clinical practice, personalized care is facilitated by information on different treatment outcomes associated with patient demographic characteristics (e.g., age), clinical (e.g., comorbidities) and tumor (e.g., tumor grade, size) features, molecular profiles (e.g., 21- gene recurrence score), and preferences. [2] In this context, interactive web-based clinical decision tools that provide personalized outcomes based on individual characteristics have shown to be useful in delivering tailored recommendations to guide personalized cancer care in clinical practice [[3], [4], [5]].
The use of web-based clinical tools in cancer care can be traced back to the 1980's [6]. However, these initial web-based tools were time intensive, and were often limited to academic research [7]. At the time, the use of computers in medicine also raised significant ethical and legal issues while questioning physician autonomy. There were concerns relating to who will bear the responsibility of the recommendations generated from a computer system [8]. However, over the past three decades, studies have managed to clarify the role of web-based clinical decision tools in oncology care [[9], [10], [11], [12]]. Clinical decision tools for cancer screening [13,14], breast cancer prevention, and breast cancer genetic testing [[15], [16], [17]] have shown that these tools could help patients understand their choices, increase knowledge, reduce patient's anxiety, distress, and fear, help patients appreciate the scientific uncertainties inherent in their choices, clarify their personal values or desirability of potential benefits relative to potential harms, help communicate patient's values to clinicians, and increase patient involvement in shared decision making. Shared decision making is a process in which clinicians and patients work together to make decisions and select treatment plans based on clinical evidence that balances the risks and benefits with patient preferences and values [18]. Web-based clinical decision tools could facilitate shared decision making by providing a vehicle to present personalized evidence on the risks and benefits of the various treatment options available to the patient. However, there is limited data on the impact of interactive, web-based, breast cancer treatment-related, clinical decision tools on such outcomes. Current clinical tools are often integrated with electronic health records (EHRs) or computerized provider order entry (COPE) systems [19]. These tools are also accessible through desktops, tablets, and smartphones.
However, there are several challenges in using web-based clinical decision tools to guide personalized treatment decisions in breast cancer care [12,20]. First, many women and their healthcare providers have limited knowledge on the currently available decision tools that can be used to guide specific breast cancer treatment decisions in clinical settings [21]. Second, we do not know if current breast cancer treatment decision tools have been tested for usability, feasibility, and acceptability or if these tools have been shown to improve patient communication and shared decision making in clinical settings. In clinical tool development, usability testing is conducted to evaluate the tool's ease of use and the user's ability to understand the tool content [22]. Feasibility tests are used to evaluate the recruitment and retention of users as well as the likelihood of the decision tool in enhancing patient-provider interaction [23].Acceptability generally refers to the user's degree of satisfaction, such as whether the tool was practical or whether the user experienced any enjoyment and/or frustration when using the tool [24]. Third, current personalized interactive web-based decision tools only target one decision choice at a time [25]. As a result, a woman may have to use several tools that consider different individual and tumor characteristics to cover all relevant breast cancer treatment decisions following diagnosis. There is limited data on how personalized outcomes from separate tools could complement each other to provide women with complete coverage of all the relevant breast cancer treatment options [25]. Fourth, most healthcare providers are concerned about the validity of these tools in informing treatment decisions for patients seen in real-world settings, and the amount of time required to convey and discuss personalized information from a decision tool [26,27]. Finally, previous reviews on breast cancer treatment decision aids [25,28,29] have not specifically focused on personalized web-based clinical tools with interactive features. An updated review on this subject is necessary as new tools have become available (e.g., RSClin [30]), while several tools are now no longer available (e.g., ‘Adjuvant! Online’ [31]) for clinical use.
In this study, we aimed to fill these gaps by reviewing, comparing, and discussing the current, English-language, interactive, web-based, clinical decision tools available to support personalized breast cancer treatment in clinical practice. Further, we aimed to evaluate the validity, usability, feasibility, and acceptability, as well as the impact of these tools on patient-provider communication, shared decision making, patient knowledge, distress, and other patient-related outcomes in women diagnosed with breast cancer. We also aimed to identify breast cancer treatment domains that could benefit from the development of personalized web-based decision tools in the future. The overarching goal of this study was to help facilitate better integration of web-based clinical decision tools across modalities into breast cancer treatment decision making in clinical practice.
2. Methods
This scoping review was designed and conducted according to the guidelines included in the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist (See Appendix A for the study checklist) [32]. The study was registered in the International Prospective Register of Systematic Reviews (PROSPERO, ID 272094). We used study-level results; therefore, this study was exempt from institutional review board approval at Georgetown University.
2.1. Data sources and search strategy
A systematic search of published literature was conducted to identify tailored web-based clinical decision tools or clinical outcome calculators aimed to inform treatment decisions for clinicians and breast cancer patients. We searched PubMed, Embase, PsychInfo, Cochrane Database of Systematic Reviews, Web of Science, and Scopus databases. We also screened the reference lists of the relevant reviews and systematic reviews. Then the additional publications identified from the reference lists were further reviewed for study inclusion/exclusion. The date of the most recent search is Nov. 10, 2021. Our comprehensive search strategy included a combination of keywords, synonyms, Medical Subject Headings (MeSH) terms, and Emtree terms relating to concepts of decision tools, treatment, web-based, and breast cancer (see Appendix B).
2.2. Inclusion and exclusion criteria
For articles identified through database searching, the inclusion criteria included, (1) reported in a peer-reviewed scientific journal; (2) written in English; and (3) published between Jan 1, 2008, and July 1, 2021. We excluded study protocols.
Personalized web-based clinical decision tools often use statistical and/or mathematical models to predict treatment outcomes associated with individual characteristics. These models need to be validated in different cohorts by comparing the predicted outcomes with actual patient outcomes to evaluate model performance (e.g., area under the receiver-operator curve (AUC)) [33]. In our review, we included the original publications associated with the development and validation of the tools. However, most tools also had multiple validation studies published following the original publication. So, we reviewed these additional validation studies and incorporated the most updated information regarding the validation status of each tool.
For web decision tools identified through the articles, the inclusion criteria included (1) written in English; (2) (publicly) available; and (3) fully accessible with no monetary associations. The exclusion criteria included (1) tools with no English language version; (2) tools on topics other than breast cancer treatment (for example, breast cancer screening or prevention); (3) tools that did not target breast cancer specifically; (4) tools not in a web-based format; and (5) tools lacking an interactive tailoring or personalization feature.
2.3. Selection and assessment of articles and tools
Three authors (AZ, JJ, ML) screened titles and abstracts of all articles retrieved from database searching for initial eligibility. Full texts of potentially eligible articles were reviewed for final eligibility based on the inclusion and exclusion criteria described above. Any disagreements between the authors were resolved through conferencing.
For tools with publicly available websites, we visited each website and tested the tools with pseudo patient characteristics to review available input parameters and outcomes. For tools with websites that were not publicly available, we closely examined the tool-development section of the associated articles, and reviewed any given pictures or screenshots provided by the author. Data points collected and summarized included name of the tool (if given), main purpose, applicable population, intervention discussed, input characteristics, outcomes evaluated, target users, validation status, and the date of the last update of the tool.
We also searched for clinical trials testing the effects of the tool on outcomes such as patient-provider communication, shared decision making, patient's knowledge, distress, decisional conflict, and other related outcomes. We first reviewed the authors' names and publication lists for studies related to the decision tool of interest. Then, we read the “About” or “Publications” sections on the tool's website to find any relevant information on the development or recent updates. Lastly, we searched PubMed, Embase, PsychInfo, Cochrane Database of Systematic Reviews, Web of Science, and Scopus for articles that may have been missed in the above steps.
2.4. Quality assessment
We evaluated the web-based clinical decision tools according to the IPDASi checklist [43]. The checklist consists of eight dimensions including ‘Information about options’, ‘Outcome probabilities’, ‘Clarifying values’, ‘Decision guidance’, ‘Development process’, ‘Using evidence’, ‘Disclosure and transparency’, and ‘Plain language’.
3. Results
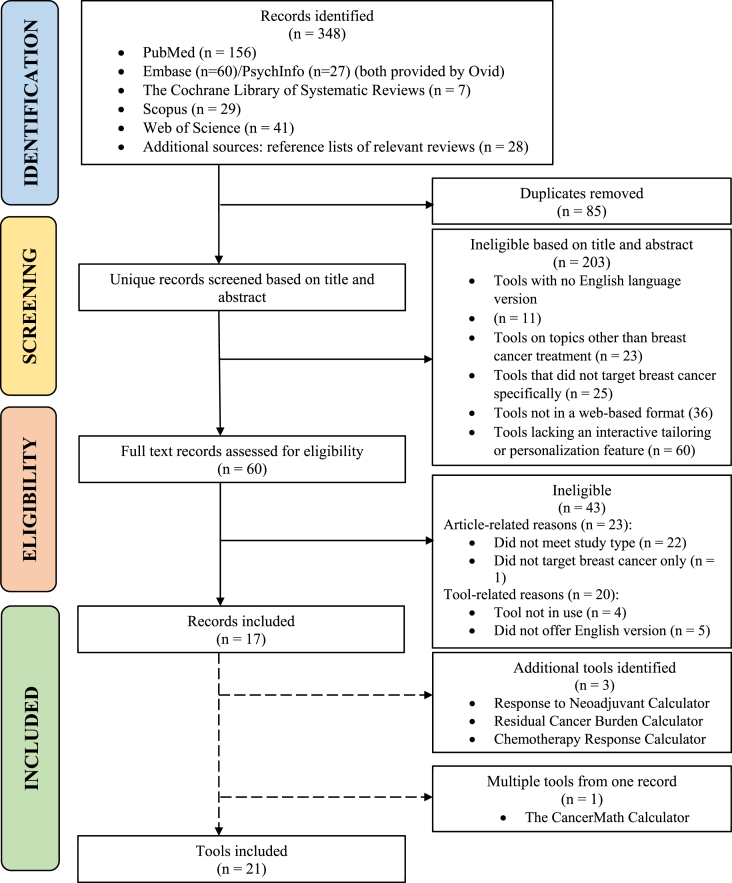
A total of 249 records were identified through PubMed, Embase, PsychInfo, Cochrane Database of Systematic Reviews, Web of Science, Scopus, and additional sources. After removing ineligible or duplicate articles, we included a total of 17 articles associated with 21 unique web decision tools (Fig. 1). Seven tools were developed for guiding neoadjuvant treatment decisions; two for radiation therapy; ten for surgery and adjuvant therapy such as mastectomy, chemotherapy, and endocrine therapy; two for adverse effects including surgical complications after mastectomy and risk of ischemic heart disease after radiation therapy. Fourteen tools were developed in the United States, five in the United Kingdom, and two in the Netherlands.
Fig. 1.
Article identification process.
Table 1 presents the name, main purpose, applicable population, intervention discussed, input characteristics, outcomes evaluated, target users, validation status, and the date of the last update of the tool.
Table 1.
Summary of the English language, interactive, web-based clinical decision tools available to support breast cancer treatment in clinical practice.
| Tool | Purpose | Population | Intervention | Characteristics | Outcome | Target User/s | Validation | Date of Last Update |
|---|---|---|---|---|---|---|---|---|
| Neoadjuvant Therapy | ||||||||
| Breast Cancer Nomogram to Predict Positive SLNs, after Neoadjuvant Chemotherapy [34,35] | The probability of finding positive SLNs in clinically node-negative breast cancer patients who have been treated with neoadjuvant chemotherapy | Women diagnosed with invasive breast cancer from 27 to 87 years who have undergone (preoperative) neoadjuvant chemotherapy | Neoadjuvant chemotherapy | Age, histologic type, nuclear grade, preoperative tumor size, percent decrease in tumor size, location of tumor in the breast (multifocal or multicentric), lymphovascular invasion, ER/PR/HER2 | Probability of positive SLNs | Physicians | Unknown/Not Externally Validated | Unknown |
| Breast Cancer Nomogram to Predict Positive SLNs, without Neoadjuvant Chemotherapy [36,37] | The probability of finding positive SLNs in breast cancer patients who have not undergone neoadjuvant chemotherapy | Women diagnosed with invasive breast cancer from 22 to 99 years who have not undergone (preoperative) neoadjuvant chemotherapy | Neoadjuvant chemotherapy | Age, location of tumor in the breast (upper inner quadrant & multifocal or multicentric), histologic type, preoperative tumor size, lymphovascular invasion, ER/PR/HER2 | Probability of positive SLNs | Physicians | Unknown/Not Externally Validated | Unknown |
| Breast Cancer Nomogram to Predict Additional Positive Non-SLN, without Neoadjuvant Chemotherapy [38,39] | The probability of finding additional positive non-SLNs in breast cancer patients found to have disease on SLN biopsy and have not undergone neoadjuvant chemotherapy | Women diagnosed with invasive breast cancer with disease on SLN biopsy who have not undergone neoadjuvant chemotherapy | Neoadjuvant chemotherapy | Histologic type, tumor size on surgical pathology, # of lymph nodes removed, # of positive SLNs with cancer, size of largest focus of metastasis in the SLN, extranodal extension in positive lymph nodes, lymphovascular invasion | Probability of positive non-SLNs | Physicians | External | Unknown |
| Breast Cancer Non-SLN Nomogram Calculator, with Neoadjuvant Chemotherapy [40,41] | The probability of finding additional positive non-SLNs in breast cancer patients found to have disease on SLN biopsy and have completed neoadjuvant chemotherapy | Women diagnosed with invasive breast cancer on SLN biopsy after neoadjuvant chemotherapy with primary tumor size <14 cm | Neoadjuvant chemotherapy | Lymphovascular invasion, detection method of SLN, multicentric primary tumor, nodal disease prior to neoadjuvant chemotherapy, pathologic tumor size | Probability of positive non-SLNs | Physicians | External [41] | Unknown |
| Response to Neoadjuvant Chemotherapy [42] | The probability of having no invasive cancer left in the breast and lymph nodes after completion of neoadjuvant chemotherapy | Women diagnosed invasive breast cancer | Anthracycline-based chemotherapy/Paclitaxel/FAC | Age, tumor size, initial diameter, histologic type, histologic grade, ER status, multicentricity | 1. Probability of achieving pathologic complete response 2. Probability of residual invasive tumor less than 3 cm 3. Probability of breast conserving surgery |
Physicians and patients | Unknown | Unknown |
| Residual Cancer Burden Calculator [43] |
The probability of residual cancer burden after neoadjuvant treatment | Women diagnosed with invasive breast cancer | Neoadjuvant treatment | Histologic assessment of primary tumor bed area, overall cancer cellularity, histologic estimate of the % of cancer that is in situ, # of positive metastatic lymph nodes, diameter of largest nodal metastasis | 1. Residual cancer burden 2. Residual cancer burden class |
Physicians and patients | Unknown | Unknown |
| Chemotherapy Response Calculator [44] | 5–10-year disease-free survival after receiving 3–4 courses of preoperative anthracycline based chemotherapy. | Women diagnosed with DCIS or invasive breast cancer who have undergone 3–4 courses of preoperative anthracycline based chemotherapy. | Preoperative anthracycline chemotherapy | Histologic type, histologic grade, ER status, tumor size, number of axillary metastatic nodes | 5/10-year disease-free probability | Physicians and patients | Unknown | Unknown |
| Radiation Therapy | ||||||||
| Breast Cancer Nomogram to Predict Benefit of Radiation for Older Patients who have undergone Breast Conserving Surgery [45,46] | 5- and 10-year risk of mastectomy with and without any radiation therapy for older women with breast cancer after breast conserving surgery | Women diagnosed with breast cancer from 66 to 79 years who have undergone breast conserving surgery | 1. Breast conserving surgery with any radiation therapy 2. Breast conserving surgery without any radiation therapy |
Age, race, tumor size, ER status, pathological nodal status | 5/10-year risk of mastectomy | Physicians and patients | Internal | Unknown |
| IBTR! Version 2.0 [47] | 10-year ipsilateral breast tumor recurrence risk with and without the addition of whole breast radiation therapy | Women diagnosed with invasive, non-metastatic breast cancer who have undergone breast conserving surgery and axillary evaluation. | 1. Whole breast radiation therapy 2. No radiation therapy |
Age, tumor size, tumor grade, margin status, lymphovascular invasion, chemotherapy, tamoxifen/aromatase inhibitor | 10-year risk of ipsilateral breast tumor recurrence | Physicians | External (Only one arm has been validated) | June 2018 |
| Surgery/Adjuvant Treatment | ||||||||
| BTxChoice [48](Limited availability (Currently only accessible through PI) | Predicted probability of 21-gene score, 10-year risk of distant recurrence, breast cancer specific mortality, and lifeyears gained with and without chemotherapy | Women diagnosed with node-negative, invasive, hormone receptor-positive, HER2-negative breast cancer |
1. Endocrine therapy alone 2. Endocrine + Chemotherapy |
Age, comorbidities, tumor size, histologic tumor grade, ER/PR status, with and without 21-gene recurrence score | 1. 10-year risk of distant recurrence 2. Breast cancer specific mortality 3. Life-years gained with and without chemotherapy |
Physicians and patients | External [48] | May 2021 |
| RSClin [30] (Limited availability: Accessible through OncotypeIQ®/Exact Sciences website) | 10-year risk of distant recurrence with and without chemotherapy | Women diagnosed with node-negative, hormone receptor-positive, HER2-negative breast cancer |
1. Endocrine therapy alone 2. Endocrine + Chemotherapy |
Age, tumor size, histologic tumor grade, ER/PR status, 21-gene recurrence score | 10-year risk of distant recurrence with and without chemotherapy | Physicians and patients | External [30] | December 2020 |
| Age Gap Decision Tool: Primary Endocrine Therapy with and without Surgery [49] | 2- and 5-year risk of breast cancer specific mortality, other-cause mortality, and all-cause mortality with endocrine therapy+/-surgery | Women from 70 to 99 years diagnosed with primary operable invasive breast cancer, tumor size (T1–4), nodes 0–2, no distant metastasis | 1. Primary endocrine therapy alone 2. Surgery + Primary endocrine therapy |
Age, tumor grade, tumor size, nodal status, individual comorbidities, frailty (Activities of Daily Living) | 1. 2/5-year risk of overall mortality 2. 2/5-year risk of breast cancer related mortality 3. 2/5-year risk of other-cause mortality |
Physicians and patients | External [50] | August 2019 |
| Age Gap Decision Tool: Surgery with and without chemotherapy [50] | 2- and 5-year risk of breast cancer specific mortality, other-cause mortality, and all-cause mortality with surgery+/-chemotherapy | Women from 70 to 99 years diagnosed with primary operable invasive breast cancer, tumor size (T1–4), nodes 0–2, no distant metastasis | 1. Surgery only 2. Surgery + Chemotherapy |
Age, tumor grade, tumor size, nodal status, ER status, HER2 status, individual comorbidities, frailty (Activities of Daily Living) | 1. 2/5-year risk of overall mortality 2. 2/5-year risk of breast cancer related mortality 3. 2/5-year risk of other-cause mortality |
Physicians and patients | Unknown | August 2019 |
| INFLUENCE [51,52] | The 1–5-year risk of locoregional recurrence in early breast cancer patients treated with radiotherapy, chemotherapy, or hormone therapy | Women from 18 to 100 years diagnosed with invasive, non-metastatic breast cancer who have undergone surgery | Hormone therapy/Chemotherapy/Radiotherapy | Age, tumor size, nodal involvement, differentiation, ER status, PR status, multifocality | 1 to 5-year risk of locoregional recurrence | Physicians and patients | External [52] | May 2021 |
| Breastconservation.com [53,54] | The preoperative risk of positive margins after breast-conserving surgery (Only for training and educational purposes) | Women diagnosed with invasive, T1-2, Nodes 0–2, non-metastatic breast cancer considering breast conserving surgery | Breast conserving surgery | Preoperative MRI availability, microcalcifications, preoperative N-stage/T-stage, remaining % of fibro glandular tissue on mammography, palpability of tumor, suspicion of multifocality, ER status, presence of DCIS in biopsy, histological type, histological grade | Risk of positive surgical margins following breast conserving surgery | Physicians | External [53,55] | October 2019 |
| PREDICT [56] | The 5-, 10- or 15-year overall, breast cancer-specific, and other-cause survival after endocrine therapy/chemotherapy trastuzumab/bisphosphonates/surgery | Women diagnosed with invasive, non-metastatic breast cancer from 25 to 85 years. Results may be less accurate for women over 80 years old | Combinations of Surgery/Endocrine therapy/Bisphosphonates/Chemotherapy/Trastuzumab | Age, menopausal stage, ER status, HER2 status, Ki-67 status, size of largest invasive tumor (before neoadjuvant treatment), tumor grade, primary breast cancer detection type, # of positive nodes, micrometastases status | 5/10/15-year overall, breast cancer-specific, and other-cause survival | Physicians and patients | External [56] | March 2020 |
| CancerMath: Therapy calculator [57] | The 15-year breast cancer-specific survival and life expectancy for hormonal therapy+/-chemotherapy | Women diagnosed with hormone receptor-positive DCIS or invasive breast cancer | Hormone therapy/Chemotherapy | Age, tumor size, # of positive nodes, nodal detail (optional), ER status, PR status, HER2 status, histological type, grade | 1. 15-year breast cancer death rate 2. Life expectancy with and without therapy |
Physicians | External [58,59] | April 2009 |
| CancerMath: Nipple involvement calculator [57] | The risk of cancer in the nipple for assistance in deciding on nipple-sparing mastectomy | Women diagnosed with early-stage breast cancer | Nipple-sparing mastectomy | Tumor size, tumor distance | Probability of nipple involvement | Physicians | External [60] | April 2009 |
| Nottingham Prognostic Index [6,61] | The 5-year overall survival which allows physicians to select those patients with an excellent prognosis after surgery alone, in whom adjuvant therapies are inappropriate | Women diagnosed with invasive early-stage node negative breast cancer who have received surgery only and are inappropriate to receive adjuvant therapies | Surgery | Tumor size, lymph node stage, histological grade | 1. 1 to 5-year overall survival 2. Annual percentage overall mortality rate |
Physicians | External [6] | June 2017 |
| Adverse Effects | ||||||||
| BRA Score [62] | A woman's 30-day and/or 1-year risk for surgical or medical complications following mastectomy with immediate breast reconstruction | Women diagnosed with breast cancer who have undergone mastectomy with immediate tissue expander or autologous reconstruction | 1. Tissue expander (30-day, 1 year) 2. TRAM flap (30 day) 3. Latissimus flap (30-day) 4. Microvascular reconstruction (30-day) 5. Single-stage implant (1-year) |
Height, weight, age, comorbidities, medication history, procedures history, American Society of Anesthesiologists physical status, radiation therapy, smoking status | 1. Overall surgical complications (surgical site infection, seroma, dehiscence, flap loss, explanation) 2. Risk of reoperation 3. 30-day surgical complications |
Physicians and patients | Internal [62] | Unknown |
| Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer [63,64] | The absolute and cumulative risk of radiation related ischemic heart disease and death by age 80 years (Designed for training and educational purposes only) | Women diagnosed with invasive, non-metastatic breast cancer from 40 to 80 years who have undergone radiation therapy | Radiation therapy | Age, mean radiation dose, laterality of breast cancer, history of ischemic heart disease, history of other circulatory disease, history of diabetes, history of COPD, current smoker, BMI, analgesic medication, hormone replacement therapy | 1.Cumulative/absolute risk of ischemic heart disease after radiation by age 80 2. Cumulative/absolute risk of death by ischemic heart disease after radiation by age 80 |
Physicians | Unknown | November 2018 |
CMF, Cyclophosphamide Methotrexate Fluorouracil; FAC, Fluorouracil, Adriamycin, and Cyclophosphamide; SLN, sentinel-lymph node; UIQ, upper inner quadrant.
3.1. Neoadjuvant therapy
There were seven web decision tools for guiding neoadjuvant treatment decisions in invasive breast cancer [[34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44]]. The nomograms developed by Allen et al. [34] and Veerapong et al. [37] provided estimates of the probability of finding positive sentinel lymph nodes (SLNs) with and without neoadjuvant chemotherapy, respectively. Both tools considered age as an input characteristic and allowed an age range from 20 to 89 years. Other characteristics included histologic tumor type, tumor size, location, lymphovascular invasion, and hormone receptor status. The nomograms developed by Mittendorf [39] and Jeruss [41] provided estimates of the probability of finding additional positive non-SLNs with and without neoadjuvant chemotherapy, but these tools did not include age as an input characteristic which is a determinant of advanced disease. None of the four tools described above allowed specification of the generation, type, or number of cycles of the neoadjuvant chemotherapy treatment.
The ‘Residual Cancer Burden Calculator’ [43] provided estimates for residual cancer burden and residual cancer burden class after neoadjuvant treatment. Input characteristics were the area of primary tumor bed, overall cancer cellularity, percentage of in situ cancer disease, number of positive lymph nodes, and diameter of largest metastasis. The tool website provided detailed descriptions of how to measure the characteristics obtained from pathologic examinations and included supporting documents and a video for guidance.
Of the seven tools, the ‘Response to Neoadjuvant Chemotherapy Nomogram’ [42] was the most comprehensive tool in providing outcomes associated with neoadjuvant treatment such as the probability of achieving pathologic complete response, probability of residual invasive tumor <3 cm, and the probability of needing breast conserving surgery. Age was an input characteristic along with histologic tumor size, histologic type, tumor grade, estrogen receptor (ER) status, and multicentricity. Additionally, this tool allowed users to specify the type of chemotherapy and the number of cycles used.
The ‘Chemotherapy Response Calculator’ [44] is the only tool that estimated survival outcomes associated with neoadjuvant treatment. It provides the 5 and 10-year disease-free survival after anthracycline-based chemotherapy using histologic tumor type, tumor grade, tumor size, ER status, and the number of axillary metastatic nodes.
Five of the seven tools were designed for physician-use only, while the ‘Response to Neoadjuvant Chemotherapy Calculator’ [42] and the ‘Chemotherapy Response Calculator’ [44] were designed for both clinician and patient use. Only two tools were known to be externally validated [39,41]. None of the tools reported usability, feasibility, or acceptability in clinical settings. We did not find any clinical trials evaluating the impact of these tools on treatment-related knowledge, levels of distress, decisional conflict, patient-physician communication, shared decision making or patient outcomes.
3.2. Radiation therapy
Two web-based decision tools estimated outcomes related to radiation therapy following breast conserving surgery. The nomogram developed by Albert et al. [45,46] predicted the benefit of radiation therapy by comparing the risk of mastectomy with and without radiation therapy after breast conserving surgery. This nomogram was developed for guiding treatment decisions for older women between 66 and 79 years. In addition to age, tumor size, ER status, and nodal status, this tool also incorporated race as an input parameter.
‘IBTR! Version 2.0’ [47] compared the 10-year ipsilateral breast tumor local recurrence risk with and without the addition of whole breast radiation therapy for women who have undergone breast conserving surgery and axillary evaluation. Unlike the nomogram by Albert et al. [45,46], ‘IBRT! Version 2.0’ [47] was designed for women of all ages. In addition, it provides outcomes associated with chemo and hormonal therapies.
The nomogram by Albert et al. [45,46] was designed for physician and patient-use, while ‘IBTR! Version 2.0’ [47] was only intended for physicians who are familiar with the complexity of treatment decisions. However, the nomogram by Albert et al. [45,46] has not been externally validated yet, therefore the accuracy and reliability are unknown. For ‘IBTR! Version 2.0’ [47], the arm that calculates local recurrence risk with radiation has undergone rigorous validation testing, but the other arm that calculates the risk without radiation has not been validated due to the lack of a large cohort of patients who have not received radiation therapy. Neither tool was evaluated for usability, feasibility, or acceptability. We also did not find any clinical trials evaluating the impact of these tools on knowledge, communication, shared decision making or other patient outcomes.
3.3. Surgery and adjuvant therapy
The highest number of tools were available for guiding treatment decisions related to surgery and/or adjuvant therapy [6,30,[48], [49], [50], [51], [52], [53], [54],56,57,61]. ‘IBTR! Version 2.0’ [47] also included a module for surgery and adjuvant therapy decisions. Two newly developed tools, ‘BTxChoice’ [48] and ‘RSClin’ [30] considered 21-gene recurrence scores to guide chemotherapy treatment decisions. Both tools estimated the 10-year risk of distant recurrence, but ‘BTxChoice’ [48] additionally provides life-years gained with chemotherapy which could help guide treatment decisions among older women. The ‘BTxChoice’ [48] tool also provided the probability distribution of 21-gene recurrence score and chemotherapy outcomes without 21-gene recurrence score test results for women who have not yet undergone recurrence score testing.
The ‘Age Gap Decision Tools’ [49,50] were designed for the comparison of breast cancer treatments for older women from 70 to 99 years. The first model compared primary endocrine therapy (PET) and surgery [49] and the second model compared surgery with and without chemotherapy [50]. Outcomes calculated were the 2 and 5-year overall mortality, breast cancer-related mortality, and other-cause mortality. In addition to age, tumor grade, tumor size, and nodal status, these models also consider individual comorbidities and frailty. Frailty was assessed using the Activity of Daily Living (ADL) [65] where users can rate the level of difficulty of the listed tasks from 0 to 3 points to automatically calculate the ADL stage.
The ‘Age Gap Decision Tools’ [49,50] were intended for use by both physicians and patients with a carefully designed user interface. Users can download an instruction manual from the website that guides usage of the tools. In addition, two decision aid booklets were written by experts to provide background information on the disease and treatment options. The model comparing PET and surgery was externally validated [49]. ‘The Age Gap Decision Tools’ [49,50] were tested in a multicenter cluster randomized trial to evaluate the effects of the tools on quality of life, survival, treatment choice, decision regret, anxiety, perception of cancer, knowledge, and preferences of treatment choices [66]. The trial included 1339 women aged 70 years or above diagnosed with primary operable invasive breast cancer. The women were randomized to an intervention arm consisting of ‘The Age Gap Decision Tools’ [49,50] together with a booklet containing background information and guidance on how to use the tool. The control arm included normal decision-making practices. The outcomes were measured using several validated surveys including the European Organization for the Research and Treatment of Cancer (EORTC) QoL questionnaire (QLQ) C30 [67], and Brief COPE questionnaire [68]. The trial found that the ‘Age Gap Decision Tools’ [49,50] significantly increased women's knowledge of breast cancer treatment options compared to the control arm (94% vs. 74%). Treatment choice between the two arms were significantly different, where women in the intervention arm selected primary endocrine therapy at a higher rate compared to the women in the control arm (difference = 5.5%, 95% CI: (1.1–10.0)). This indicates that the tool had a differential effect on treatment choice. However, there was no significant difference in the proportion of participants stating that they knew their preferred option (96% vs. 91%) or felt ready to make an informed decision (99% vs. 90%) [66].
‘Breastconservation.com’ [53] provided a nomogram for predicting the risk of positive surgical margins following lumpectomy. The tool identified high-risk patients who might benefit from preoperative MRI and/or oncoplastic surgery [53]. The tool considered various pathological characteristics including stage, remaining breast density, tumor palpability, multifocality, and ER status to predict positive surgical margins after breast conserving surgery. The result calculated in this tool belonged to three risk intervals – low risk, intermediate risk, and high risk. Detailed written descriptions were provided on whether the patients in each risk interval were suitable for breast conserving therapy, pre-operative MRI, or more extensive surgical excisions. Apart from its original validation in a Dutch cohort, this tool has also undergone two external validations in a US cohort [69] and another Dutch cohort [55]. The validation testing done in the US population did not yield promising results, where no significant correlation between the calculated risk values and the presence of positive surgical margins were found [69].
‘PREDICT’ [56] and ‘CancerMath’ [57] are two of the most widely used web decision tools in current clinical practice. ‘PREDICT’ [56] was first proposed in 2010 and has been updated five times during the past eleven years. HER2 status and Ki-67 status have been added through its updates into existing input characteristics including age, menopausal stage, size of largest invasive tumor, tumor grade, detection type, number of positive nodes, and micro metastases status. The newest version, ‘PREDICT v2.2’ [70], estimates the 5, 10, and 15-year overall breast cancer-related and other-cause survival with benefits of endocrine therapy, chemotherapy with and without trastuzumab, and biphosphates for post-menopausal women in addition to surgery. The length and type of hormone and chemotherapy could also be specified by users. ‘PREDICT’ [56] was intended for physician and patient-use, therefore its output format was more comprehensive and carefully designed, where results were presented in not only tables, but also in curves, charts, texts, and icons.
‘CancerMath’ [57] consists of five web calculators. Only two calculators, the ‘Therapy Calculator’ [57] and the ‘Nipple Involvement Calculator’ [57] were included in this review. The remaining three tools were excluded as they did not include a treatment component. The ‘Therapy Calculator’ [57] provided the 15-year breast cancer death rate and life expectancy with and without adjuvant therapy. Compared to ‘PREDICT’ [56], the ‘CancerMath, Therapy Calculator’ [57] offered outcomes associated with a wider range of therapy types. For example, users could only select from 2nd and 3rd generation chemotherapy in ‘PREDICT’ [56], while in the ‘CancerMath, Therapy Calculator’ [57], the types of drugs and number of cycles could also be specified. However, it must be noted that ‘CancerMath’ [57] has not been updated regularly since its development in 2009. Therefore, its content maybe outdated and should be considered with caution. Moreover, its output is only presented in text form and the other formats including curves, bar charts, pie charts, and pictograms are unavailable. Both ‘PREDICT’ [56] and ‘CancerMath, Therapy Calculator’ [57] have been externally validated in various populations [[58], [59], [60],[71], [72], [73], [74], [75]]. A study performed by Karapanagiotis et al. [72] directly compared the validity of ‘PREDICT’ [56] and ‘CancerMath’ [57] using a European validation cohort and concluded that ‘PREDICT’ [56] outperformed ‘CancerMath’ [57] in discriminatory accuracy, calibration, and clinical utility.
‘CancerMath, Nipple Involvement Calculator’ was the only tool that could assist decisions on nipple-sparing mastectomy [57]. The largest lesion size and the shortest distance between the lesion and nipple from imaging could be entered to calculate the probability of cancer in the nipple. Hwang et al. [60] performed a validation study on this calculator in a Korean population and found relatively poor performance.
The ‘Nottingham Prognostic Index’ (NPI) [6] was first developed to provide a prognostic score considering lymph node stage, tumor size and pathological grade in breast cancer. NPI was later implemented into a web-based calculator [61] to provide 1 to 5-year overall survival and annual percentage mortality rate to support the selection of patients for adjuvant treatment.
We did not find studies reporting the usability, feasibility, or acceptability of the clinical decision tools in this category. With the exception of ‘The Age Gap Decision Tools’ [49,50], we also did not find trials evaluating the impact of these tools on treatment-related knowledge, levels of distress, decisional conflict, patient-physician communication, shared decision making or other patient outcomes.
3.4. Adverse effects
Two web-based decision tools evaluated adverse effects of breast cancer treatment. The ‘Breast Reconstruction Risk Assessment Score’ (BRA score) [62] provided estimates of the risk of overall surgical complications including surgical site infection, seroma, dehiscence, and flap loss after mastectomy with immediate reconstruction. The 30-day and/or one year risk of the complications associated with the reconstructive modalities were listed in a table. The risk of reoperation following each modality was also calculated. This tool has only been internally validated in its original research study [62].
The final tool we evaluated was the ‘Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer’ [63]. This tool was developed for women aged 40–80 years. The model used patients' age, mean radiation dose, and risk factors of heart disease such as laterality of breast cancer, history of various types of circulatory disease, smoking status, body mass index, and medication history to determine the cumulative and absolute risk of ischemic heart disease and risk of death by ischemic heart disease after radiation. The validation status of this tool is not known. Neither tool was evaluated for usability, feasibility, or acceptability, and we did not find any trials evaluating the impact of these tools on any patient outcomes, shared decision making or communication.
3.5. Quality assessment
Table 2 provides the sum of the scores for each dimension for each tool. Appendix C provides the individual items included in each dimension. All the tools clearly described the health conditions, the decision that needs to be considered, the outcome probabilities associated with the options, and the patients for which the probabilities apply. Most tools also clearly laid out the options available for the decision. The tools that received the highest scores were the ‘Age Gap Decision Tools’ (26/36) [49,50] and ‘PREDICT’ [56] (22/36). These tools scored well on several dimensions in the IPDASi checklist. For example, ‘The Age Gap Decision Tools’ [49,50] received the highest score for decision guidance (2/2) because the tool provided step-by-step user guidance and worksheets for patients to discuss with practitioners. ‘PREDICT’ [56] received the highest score (5/5) for describing how research evidence was synthesized as the tool provided citations to studies, list of publications, update policy, and quality of research evidence.
Table 2.
Results from the quality assessment of the interactive, web-based clinical decision tools for personalized breast cancer treatment using the ‘International Patient Decision Aids Standards instrument (IPDASi) checklist.
| Tool | Information about options (8) | Outcome probabilities (8) | Clarifying values (4) | Decision guidance (2) | Development process (6) | Using evidence (5) | Disclosure and transparency (2) | Plain language (1) | Total (36) |
|---|---|---|---|---|---|---|---|---|---|
| Neoadjuvant Therapy | |||||||||
| Breast Cancer Nomogram to Predict Positive SLNs, after Neoadjuvant Chemotherapy [34,35] | 3 | 3 | 0 | 1 | 1 | 2 | 1 | 1 | 12 |
| Breast Cancer Nomogram to Predict Positive SLNs, without Neoadjuvant Chemotherapy [36,37] | 3 | 3 | 0 | 1 | 1 | 2 | 1 | 1 | 12 |
| Breast Cancer Nomogram to Predict Additional Positive Non-SLN, without Neoadjuvant Chemotherapy [38,39] | 3 | 3 | 0 | 1 | 1 | 2 | 1 | 1 | 12 |
| Breast Cancer Non-SLN Nomogram Calculator, with Neoadjuvant Chemotherapy [40,41] | 3 | 3 | 0 | 1 | 1 | 4 | 1 | 1 | 14 |
| Response to Neoadjuvant Chemotherapy [42] | 3 | 6 | 0 | 1 | 2 | 0 | 0 | 0 | 12 |
| Residual Cancer Burden Calculator [43] |
2 | 2 | 0 | 1 | 2 | 0 | 0 | 0 | 7 |
| Chemotherapy Response Calculator [44] | 3 | 4 | 0 | 1 | 2 | 0 | 0 | 0 | 10 |
| Radiation Therapy | |||||||||
| Breast Cancer Nomogram to Predict Benefit of Radiation for Older Patients who have undergone Breast Conserving Surgery [45,46] | 4 | 6 | 0 | 1 | 1 | 4 | 2 | 0 | 18 |
| IBTR! Version 2.0 [47] | 4 | 6 | 0 | 1 | 1 | 4 | 2 | 0 | 18 |
| Surgery/Adjuvant Treatment | |||||||||
| BTxChoice [48] | 5 | 6 | 0 | 1 | 1 | 4 | 2 | 0 | 19 |
| Age Gap Decision Tool: Primary Endocrine Therapy with and without Surgery [49] | 7 | 6 | 1 | 2 | 3 | 5 | 2 | 0 | 26 |
| Age Gap Decision Tool: Surgery with and without chemotherapy [50] | 7 | 6 | 1 | 2 | 3 | 5 | 2 | 0 | 26 |
| INFLUENCE [51,52] | 6 | 6 | 0 | 1 | 2 | 4 | 2 | 0 | 21 |
| Breastconservation.com [53,54] | 4 | 3 | 0 | 1 | 1 | 4 | 2 | 0 | 15 |
| PREDICT [56] | 6 | 6 | 0 | 1 | 2 | 5 | 2 | 0 | 22 |
| CancerMath: Therapy calculator [57] | 6 | 6 | 0 | 1 | 1 | 4 | 2 | 0 | 20 |
| CancerMath: Nipple involvement calculator [57] | 2 | 1 | 0 | 1 | 1 | 4 | 2 | 0 | 11 |
| Nottingham Prognostic Index [6,61] | 3 | 4 | 0 | 1 | 1 | 3 | 2 | 0 | 14 |
| Adverse Effects | |||||||||
| BRA Score [62] | 4 | 6 | 0 | 1 | 2 | 3 | 2 | 0 | 18 |
| Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer [63,64] | 4 | 4 | 0 | 1 | 1 | 3 | 2 | 0 | 15 |
Information about options: The tool describes the health condition, options available, natural course of the condition, and compares positive/negative features of each option.
Outcome probabilities: The tool provides information about the outcome probabilities for each option, specifies the defined reference class, event rates, and time period over which the outcomes apply, compares probabilities across the same time periods, provides information about uncertainty, and provides more than one way to view the probability.
Clarifying values: The tool describes features of options to help patients imagine what it is like to experience physical, psychological, and social effects.
Decision guidance: The tool provides a step-by-step way to decide and includes tools for discussion with practitioner.
Development process: The tool (or associated paper) mentions the development process including finding out what health professionals/patients need to prepare, whether the tool has been field-tested with professionals/patients, and any expert views.
Using evidence: The tool (or associated paper) describes how research evidence was selected or synthesized, provides a publication rate, and proposes update policy.
Disclosure and transparency: The tool (or associated technical documentation) provides information about funding used and includes author/developer credentials.
Plain Language: The tool (or associated paper) reports readability levels using available scales.
While all the tools provided information on funding and author credentials, none of them included features to help patients understand the physical, psychological, and social impact of treatment. Except for ‘Age Gap Decision Tools’ [49,50], there were no data on the review of the tools by patients or healthcare professionals, or whether the tools had been field-tested for usability, acceptability, and feasibility. None of the tools reported readability levels using standard scales.
Table 3 presents a summary of key strengths and weaknesses of the current web-based clinical decision tools.
Table 3.
Key strengths and weaknesses of current web-based clinical decision tools.
| Strengths | Weaknesses |
|---|---|
|
|
4. Discussion
Treatment decision making for breast cancer often involves complex choices. Personalized decision tools are useful in synthesizing existing evidence on harms and benefits of treatment with patient characteristics, preferences, and values to support decisions about various treatment options available in clinical practice. Previous studies [20,25,28,29,76] have reviewed the role of decision guides such as booklets, workbooks, audiotapes, and videos in supporting clinical decisions. However, none of these studies have evaluated and compared web-based clinical decision tools that provide personalized outcomes for individual women seen in clinical practice. In this scoping review, we identified 21 currently available individualized web-based clinical tools to guide treatment decisions for four breast cancer treatment modalities – neoadjuvant therapy, radiation therapy, surgery and adjuvant therapy, as well as tools that will help incorporate the adverse effects of breast cancer treatment into decision making.
All tools reviewed were developed using rigorous mathematical and/or statistical models. Input parameters were carefully selected, and results were validated in 14 out of the 21 tools. Common input characteristics considered for personalization included age, tumor characteristics (tumor size, tumor grade), and nodal status. However, these tools did not frequently include comorbidities (3/21) or race (1/21) as key determinants of treatment effectiveness due to the unavailability of these variables in the data sources used to develop the predictive algorithms. Studies have shown that comorbidities such as cardiovascular disease, diabetes, and other chronic diseases can be significantly associated with increased mortality for women with breast cancer [77]. Comorbidities are especially useful in guiding treatment decisions in older women, where the relative benefit of treatment may reduce with increasing age and severity of comorbidities. Moreover, clinical dilemmas are increased in caring for diverse groups of women, since there are important differences in the distributions of biological risk factors and access to screening by race/ethnicity [[78], [79], [80], [81], [82], [83]]. There are also differences in comorbidity by race, where comorbidities like diabetes and obesity are seen more often in Black vs. White women [83]. Since US minority populations are growing at a faster rate than Whites [84,85] there is a need to develop clinical decision support tools that are applicable to minority women. Moreover, most tools also did not include the method of breast cancer detection or the type and frequency of chemotherapy, all of which have been shown to be strongly associated with breast cancer mortality.
The web decision tools included in our study, clearly stated the target audience – whether they were specifically designed for physicians who are familiar with the complexity of treatment options, or if they can be safely used by patients. ‘Response to Neoadjuvant Chemotherapy Nomogram’ [42] provided separate written instructions for physicians and patients on the webpage. It is intuitive that tools designed for trained physicians would incorporate more medical language and provide less background information, while tools designed for patients pay closer attention to providing knowledge and clarifications on medical terms in plain language. However, only the ‘Age Gap Decision Tools’ [49,50] were evaluated in a cluster randomized trial and found that the use of this tool increased knowledge of breast cancer treatment options and facilitated shared decision making in its target user population [66].
To our knowledge, there are no data on the usability, feasibility, or acceptability of the web-based clinical decision tools included in this scoping review. Except for ‘The Age Gap Decision Tools’ [49,50] none of the other web-tools had been assessed for its impact on communication, decision making or patient outcomes in clinical trials. Therefore, we do not know if these tools are used by healthcare providers or patients as intended by the tool developers. Personalized nomograms were developed to supplement a physician's decision-making process, yet there is limited data on how physicians use these tools or if they improve clinical decisions in practice. There is a need to test the efficacy, implementation, and dissemination of widely used clinical decision tools such as ‘CancerMath’ [57] and ‘PREDICT’ [56].
Breast cancer treatment decisions require the consideration of several treatment options at a given time [25]. For example, an older woman who has undergone breast conserving surgery may have to decide about radiation therapy, adjuvant chemotherapy, as well as the side effects of surgery and treatment. Yet, we did not find any web decision tools that addressed treatment options across multiple modalities. The development of multimodality tools in the future could help women facing more complex treatment decisions. Also, current tools addressing similar populations on different modalities could be integrated into one comprehensive tool. For example, the MD Anderson Calculators [35,36,38,40,[42], [43], [44]] for guiding neoadjuvant treatment can be combined into a single tool. Integrating multiple prediction calculators into one comprehensive decision tool may increase acceptability and convenience for users.
We did not find tools addressing decisions for newer breast cancer treatments such as immunotherapy, cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors, tyrosine kinase inhibitors, and poly (ADP-ribose) polymerase (PARP) inhibitors. Future studies should consider the development of tools addressing such advancement in breast cancer treatment. Furthermore, breast cancer has become a chronic condition rather than a life-threatening illness owing to advances in screening, diagnosis, and treatment [86]. There is increased attention on the quality of life and long-term outcomes of survivors. Therefore, tools addressing decision making in breast cancer survivorship could be useful to patients, their family members, and healthcare providers.
Our evaluation of the web-based, interactive decision tools using IPDASi found several improvements that could be made on the format and presentation of the existing tools. First, few tools presented the outcome probabilities in more than one way. Most nomograms provided a single percentage only, without any options for visualizing the outcomes in graphs or charts. This might be acceptable for tools for physician use but is less informative for communicating statistical information to patients. ‘PREDICT’ [56], on the other hand, provided outcomes in five different ways – tables, curves, charts, graphs, and icons. Second, most tools did not provide information on the level of uncertainty around the calculated probabilities. Confidence intervals derived from sample data or prediction intervals derived from prediction models should be presented to users with explanations using plain non-statistical language. Finally, although tools communicated information in plain language without jargon, none of them evaluated readability using a standard scale as recommended by IPDASi.
4.1. Implications for clinical practice
It is critical to address the lack of data on the usability, acceptability, and feasibility of the current web-based clinical decision tools used to guide breast cancer treatment decisions. Clinicians could collaborate with behavioral scientists and health services researchers to design studies that will help evaluate the use and impact of these tools on communication and decision quality in clinical practice. It is important for clinicians to be involved in such studies, as clinical input is essential in developing trials that include clinically-relevant endpoints that will help capture the utility of these tools in supporting treatment decisions and cancer care.
Current quality assessment checklists for clinical decision tools primarily evaluate the performance of the tools from the patient's perspective. These checklists have limited items to capture the healthcare provider's perspectives on clinical decision tools. For example, there are no items in IPDASi to evaluate how the tools help the clinicians with communication or shared decision making. New quality assessment tools could be developed in collaboration with clinicians to evaluate the current clinical decision tools.
Clinicians have a keen understanding of current challenges, evidence gaps, and other clinical dilemmas related to modern breast cancer treatment, whereas patients know their values and preferences that influence treatment decisions. Therefore, it is important to involve both clinicians and patients in web tool development from conception to implementation to create novel tools that could be useful for both patients and healthcare providers.
4.2. Limitations
There are several limitations that should be considered in evaluating our study findings. First, we were unable to include clinical decision tools that were no longer publicly accessible [87,88] as well as those tools that did not have English language versions [89,90]. Therefore, we may have underestimated the clinical decision tools eligible for this study. Second, we did not compare the statistical/mathematical models used in these tools as it was beyond the scope of our study.
Despite these limitations, we conducted a comprehensive search and clearly laid out the steps we followed to identify a significant number of tools and other pertinent information. To our knowledge, this is the first review and compilation of information on interactive, personalized, web-based clinical decision tools that are currently available for breast cancer treatment.
5. Conclusion
There are several interactive, web-based clinical decision tools that could support personalized breast cancer treatment decisions in clinical practice. These tools could potentially help patients become more involved in the decision-making process by making their decisions clear, providing information about the options and outcomes, and by clarifying personal values. It is important to note that these decision tools were designed to complement, rather than replace recommendations from a healthcare provider. However, there is limited data on end-user evaluations and the effects of these tools on breast cancer care. Therefore, future studies should focus on assessing the impact of interactive, web-based personalized breast cancer treatment decision tools on patient-provider communication, the clinician's decision-making process, shared decision making, and patient outcomes such as knowledge, anxiety, fear, and distress.
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number K99CA241397 and R03CA259896 to Dr. Jayasekera.
The study funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Author contributions
Conception and design: Jinani Jayasekera. Financial support: Jinani Jayasekera. Administrative support: Jinani Jaysekera and Amy Zhao. Collection and assembly of data: Amy Zhao, Jinani Jayasekera, and Maya Larbi. Data analysis and interpretation: Amy Zhao and Jinani Jayasekera. Manuscript writing: Amy Zhao and Jinani Jayasekera. Final approval of manuscript: All authors. Accountable for all aspects of the work: All authors.
Declaration of competing interest
Jinani Jayasekera, Maya Larbi, Amy Zhao, and Kristen Miller have nothing to disclose. Suzanne O'Neill has received research funding from Pfizer.
Appendix A.
Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist.
| SECTION | ITEM | PRISMA-ScR CHECKLIST ITEM | REPORTED ON PAGE # |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a scoping review. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary that includes (as applicable): background, objectives, eligibility criteria, sources of evidence, charting methods, results, and conclusions that relate to the review questions and objectives. | 2 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. Explain why the review questions/objectives lend themselves to a scoping review approach. | 3–4 |
| Objectives | 4 | Provide an explicit statement of the questions and objectives being addressed with reference to their key elements (e.g., population or participants, concepts, and context) or other relevant key elements used to conceptualize the review questions and/or objectives. | 5 |
| METHODS | |||
| Protocol and registration | 5 | Indicate whether a review protocol exists; state if and where it can be accessed (e.g., a Web address); and if available, provide registration information, including the registration number. | 6 |
| Eligibility criteria | 6 | Specify characteristics of the sources of evidence used as eligibility criteria (e.g., years considered, language, and publication status), and provide a rationale. | 6–7 |
| Information sources∗ | 7 | Describe all information sources in the search (e.g., databases with dates of coverage and contact with authors to identify additional sources), as well as the date the most recent search was executed. | 6 |
| Search | 8 | Present the full electronic search strategy for at least 1 database, including any limits used, such that it could be repeated. | Appendix B (page 45) |
| Selection of sources of evidence† | 9 | State the process for selecting sources of evidence (i.e., screening and eligibility) included in the scoping review. | 7–8 |
| Data charting process‡ | 10 | Describe the methods of charting data from the included sources of evidence (e.g., calibrated forms or forms that have been tested by the team before their use, and whether data charting was done independently or in duplicate) and any processes for obtaining and confirming data from investigators. | 8 |
| Data items | 11 | List and define all variables for which data were sought and any assumptions and simplifications made. | Table 1 (pages 11–17) |
| Critical appraisal of individual sources of evidence§ | 12 | If done, provide a rationale for conducting a critical appraisal of included sources of evidence; describe the methods used and how this information was used in any data synthesis (if appropriate). | 8 |
| Synthesis of results | 13 | Describe the methods of handling and summarizing the data that were charted. | Table 1 (pages 11–17) |
| RESULTS | |||
| Selection of sources of evidence | 14 | Give numbers of sources of evidence screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally using a flow diagram. | Fig. 1 (page 10) |
| Characteristics of sources of evidence | 15 | For each source of evidence, present characteristics for which data were charted and provide the citations. | 18–25 |
| Critical appraisal within sources of evidence | 16 | If done, present data on critical appraisal of included sources of evidence (see item 12). | Table 2 (pages 27–28) |
| Results of individual sources of evidence | 17 | For each included source of evidence, present the relevant data that were charted that relate to the review questions and objectives. | 18–25 |
| Synthesis of results | 18 | Summarize and/or present the charting results as they relate to the review questions and objectives. | 18–25 |
| DISCUSSION | |||
| Summary of evidence | 19 | Summarize the main results (including an overview of concepts, themes, and types of evidence available), link to the review questions and objectives, and consider the relevance to key groups. | 30–34 |
| Limitations | 20 | Discuss the limitations of the scoping review process. | 34–35 |
| Conclusions | 21 | Provide a general interpretation of the results with respect to the review questions and objectives, as well as potential implications and/or next steps. | 35 |
| FUNDING | |||
| Funding | 22 | Describe sources of funding for the included sources of evidence, as well as sources of funding for the scoping review. Describe the role of the funders of the scoping review. | 36 |
JBI = Joanna Briggs Institute; PRISMA-ScR = Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews.
∗ Where sources of evidence (see second footnote) are compiled from, such as bibliographic databases, social media platforms, and Web sites.
† A more inclusive/heterogeneous term used to account for the different types of evidence or data sources (e.g., quantitative and/or qualitative research, expert opinion, and policy documents) that may be eligible in a scoping review as opposed to only studies. This is not to be confused with information sources (see first footnote).
‡ The frameworks by Arksey and O'Malley (6) and Levac and colleagues (7) and the JBI guidance (4, 5) refer to the process of data extraction in a scoping review as data charting.
§ The process of systematically examining research evidence to assess its validity, results, and relevance before using it to inform a decision. This term is used for items 12 and 19 instead of “risk of bias” (which is more applicable to systematic reviews of interventions) to include and acknowledge the various sources of evidence that may be used in a scoping review (e.g., quantitative and/or qualitative research, expert opinion, and policy document).
From: Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D et al. PRISMA Extension for Scoping Reviews (PRISMAScR): Checklist and Explanation. Ann Intern Med. 2018; 169:467–473. https://doi.org/10.7326/M18-0850.
Appendix B.
Search strategy.
-
1.
clinical decision tools.mp.
-
2.
Decision aids.mp.
-
3.
Decision algorithms.mp.
-
4.
exp clinical decision rules/
-
5.
exp Clinical Decision-Making/
-
6.
treatment decisions.mp.
-
7.
treatment outcome.mp.
-
8.
treatment outcome.mp.
-
9.
exp treatment outcome/
-
10.
web-based.mp.
-
11.
Internet-based.mp.
-
12.
Online.mp.
-
13.
exp Internet-Based Intervention/
-
14.
breast cancer.mp.
-
15.
exp Breast Neoplasms/
-
16.
1 or 2 or 3 or 4 or 5
-
17.
6 or 7 or 8 or 9
-
18.
10 or 11 or 12 or 13
-
19.
14 or 15
-
20.
16 and 17 and 18 and 19
Appendix C.
International Patient Decision Aids Standards Instrument (IPDASi) Checklist.
| Item | IPDAS dimension | Item description |
|---|---|---|
| 1 | Information about options | The DST describes the health condition or problem (intervention, procedure, or investigation) for which the index decision is required |
| 2 | The DST described the decision that needs to be considered (the index decision) | |
| 3 | The DST describes the options available for the index decision | |
| 4 | The DST describes the natural course of the health condition or problem, if no action is taken | |
| 5 | The DST describes positive features (benefits or advantages) of each option | |
| 6 | The DST describes negative features (harms, side effects or disadvantages) of each option | |
| 7 | The DST makes it possible to compare the positive and negative features of the available options | |
| 8 | The DST shows the negative and positive features of options with equal detail | |
| 9 | Outcome probabilities | The DST provides information about outcome probabilities associated with the options (i.e, the likely consequences of decisions) |
| 10 | The DST specifies the defined group (reference class) of patients for which the outcome probabilities apply | |
| 11 | The DST specifies the event rates for the outcome probabilities | |
| 12 | The DST specifies the time period over which the outcome probabilities apply | |
| 13 | The DST allows the user to compare outcome probabilities across options using the same denominator and time period | |
| 14 | The DST provides information about the levels of uncertainty around event or outcome probabilities | |
| 15 | The DST provides more than one way of viewing the probabilities | |
| 16 | The DST provides balanced information about event or outcome probabilities to limit framing bias | |
| 17 | Clarifying values | The DST describes the features of options to help patients imagine what it is like to experience physical effects |
| 18 | The DST describes the features of options to help patients imagine what it is like to experience the psychological effects | |
| 19 | The DST describes the features of options to help patients imagine what it is like to experience social effects | |
| 20 | The DST asks patients to think about which positive and negative features of the options matters most to them | |
| 21 | Decision guidance | The DST provides a step-by-step way to make a decision |
| 22 | The DST includes tools like worksheets or lists of questions to use when discussing options with a practitioner | |
| 23 | Development process | The DST (or associated paper) mentions that the development process included finding out what clients or patients need to prepare them to discuss a decision |
| 24 | The DST (or associated paper) mentions that the development process included finding out what health professionals need to prepare them to discuss a specific decision with patients | |
| 25 | The DST (or associated paper) mentions that the development process included expert review by clients/patients not involved in producing the DST | |
| 26 | The DST (or associated paper) mentions that the development process included expert review by health professionals not involved in producing the DST | |
| 27 | The DST (or associated paper) mentions that the DST was field tested with patients who were facing the decision | |
| 28 | The DST (or associated paper) mentions that the DST was field tested with practitioners who counsel patients who face the decision | |
| 29 | Using evidence | The DST (or associated paper) provides citations to the studies selected |
| 30 | The DST (or associated paper) describes how research evidence was selected or synthesized | |
| 31 | The DST (or associated paper) provides a production or publication rate | |
| 32 | The DST (or associated paper) provides information about the proposed update policy | |
| 33 | The DST (or associated paper) describes the quality of the research evidence used | |
| 34 | Disclosure and transparency | The DST (or associated technical documentation) provides information about the funding used for development |
| 35 | The DST includes author/developer credentials or qualifications | |
| 36 | Plain language | The DST (or associated paper) reports readability levels (using one or more of the available scales) |
Note. DST = Decision support technology.
References
- 1.Krzyszczyk P., Acevedo A., Davidoff E.J., et al. The growing role of precision and personalized medicine for cancer treatment. Technology (Singap World Sci) Sep-Dec 2018;6(3–4):79–100. doi: 10.1142/S2339547818300020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho S.-H., Jeon J., Kim S.I. Personalized medicine in breast cancer: a systematic review. J Breast Cancer. 2012;15(3):265–272. doi: 10.4048/jbc.2012.15.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baptista S., Teles Sampaio E., Heleno B., Azevedo L.F., Martins C. Web-based versus usual care and other formats of decision aids to support prostate cancer screening decisions: systematic review and meta-analysis. J Med Internet Res. Jun 26. 2018;20(6):e228. doi: 10.2196/jmir.9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tong G., Geng Q., Wang D., Liu T. Web-based decision aids for cancer clinical decisions: a systematic review and meta-analysis. Support Care Cancer. Apr 8 2021 doi: 10.1007/s00520-021-06184-y. [DOI] [PubMed] [Google Scholar]
- 5.Yu L., Li P., Yang S., et al. Web-based decision aids to support breast cancer screening decisions: systematic review and meta-analysis. J Comp Eff Res. Oct 2020;9(14):985–1002. doi: 10.2217/cer-2020-0052. [DOI] [PubMed] [Google Scholar]
- 6.Haybittle J.L., Blamey R.W., Elston C.W., et al. A prognostic index in primary breast cancer. Br J Cancer. Mar 1982;45(3):361–366. doi: 10.1038/bjc.1982.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Dombal F.T. Computers, diagnoses and patients with acute abdominal pain. Arch Emerg Med. Sep 1992;9(3):267–270. doi: 10.1136/emj.9.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dias D., Paulo Silva Cunha J. Wearable health devices-vital sign monitoring, systems and technologies. Sensors. Jul 25 2018;18(8) doi: 10.3390/s18082414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belkora J.K., Miller M.F., Dougherty K., Gayer C., Golant M., Buzaglo J.S. The need for decision and communication aids: a survey of breast cancer survivors. J Community Support Oncol. Mar 2015;13(3):104–112. doi: 10.12788/jcso.0116. [DOI] [PubMed] [Google Scholar]
- 10.Collins I.M., Steel E., Mann G.B., et al. Assessing and managing breast cancer risk: clinicians' current practice and future needs. Breast. Oct 2014;23(5):644–650. doi: 10.1016/j.breast.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Vickers A.J. Prediction models in cancer care. Ca - Cancer J Clin. Sep-Oct 2011;61(5):315–326. doi: 10.3322/caac.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stacey D., Légaré F., Lewis K., et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017:4. doi: 10.1002/14651858.CD001431.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elkin E.B., Pocus V.H., Mushlin A.I., Cigler T., Atoria C.L., Polaneczky M.M. Facilitating informed decisions about breast cancer screening: development and evaluation of a web-based decision aid for women in their 40s. BMC Med Inform Decis Mak. Mar 21. 2017;17(1):29. doi: 10.1186/s12911-017-0423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathieu E., Barratt A.L., McGeechan K., Davey H.M., Howard K., Houssami N. Helping women make choices about mammography screening: an online randomized trial of a decision aid for 40-year-old women. Patient Educ Counsel. Oct 2010;81(1):63–72. doi: 10.1016/j.pec.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Green M.J., Biesecker B.B., McInerney A.M., Mauger D., Fost N. An interactive computer program can effectively educate patients about genetic testing for breast cancer susceptibility. Am J Med Genet. Sep 15. 2001;103(1):16–23. doi: 10.1002/ajmg.1500. [DOI] [PubMed] [Google Scholar]
- 16.Fagerlin A., Dillard A.J., Smith D.M., et al. Women's interest in taking tamoxifen and raloxifene for breast cancer prevention: response to a tailored decision aid. Breast Cancer Res Treat. Jun 2011;127(3):681–688. doi: 10.1007/s10549-011-1450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozanne E.M., Annis C., Adduci K., Showstack J., Esserman L. Pilot trial of a computerized decision aid for breast cancer prevention. Breast J. Mar-Apr 2007;13(2):147–154. doi: 10.1111/j.1524-4741.2007.00395.x. [DOI] [PubMed] [Google Scholar]
- 18.Elwyn G., Laitner S., Coulter A., Walker E., Watson P., Thomson R. Implementing shared decision making in the NHS. BMJ. Oct 14 2010;341:c5146. doi: 10.1136/bmj.c5146. [DOI] [PubMed] [Google Scholar]
- 19.Klifto K.M., Khan H., Manahan M.A., et al. Decision aid for women with newly diagnosed breast cancer seeking breast reconstruction surgery: a prospective, randomized, controlled, single-blinded, pilot study. J Plast Reconstr Aesthetic Surg. Oct 2021;74(10):2519–2526. doi: 10.1016/j.bjps.2021.03.029. [DOI] [PubMed] [Google Scholar]
- 20.Vromans R., Tenfelde K., Pauws S., et al. Assessing the quality and communicative aspects of patient decision aids for early-stage breast cancer treatment: a systematic review. Breast Cancer Res Treat. Nov 2019;178(1):1–15. doi: 10.1007/s10549-019-05351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Neill S.C., Taylor K.L., Clapp J., et al. Multilevel influences on patient-oncologist communication about genomic test results: oncologist perspectives. J Health Commun. 2018;23(7):679–686. doi: 10.1080/10810730.2018.1506836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tark R., Metelitsa M., Akkermann K., Saks K., Mikkel S., Haljas K. Usability, acceptability, feasibility, and effectiveness of a gamified mobile health intervention (triumf) for pediatric patients: qualitative study. JMIR Serious Games. Sep 30 2019;7(3) doi: 10.2196/13776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jayasekera J., Vadaparampil S.T., Eggly S., et al. Question prompt list to support patient-provider communication in the use of the 21-gene recurrence test: feasibility, acceptability, and outcomes. JCO Oncol Pract. Oct 2020;16(10):e1085–e1097. doi: 10.1200/jop.19.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaughn J., Summers-Goeckerman E., Shaw R.J., Shah N. A protocol to assess feasibility, acceptability, and usability of mobile technology for symptom management in pediatric transplant patients. Nurs Res. Jul/Aug 2019;68(4):317–323. doi: 10.1097/nnr.0000000000000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholas Z., Butow P., Tesson S., Boyle F. A systematic review of decision aids for patients making a decision about treatment for early breast cancer. Breast. Apr 2016;26:31–45. doi: 10.1016/j.breast.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Ankolekar A., Dekker A., Fijten R., Berlanga A. The benefits and challenges of using patient decision aids to support shared decision making in health care. JCO Clin Cancer Inform. Dec 2018;2:1–10. doi: 10.1200/cci.18.00013. [DOI] [PubMed] [Google Scholar]
- 27.Koon S. Important considerations for design and implementation of decision aids for shared medical decision making. Perm J. 2020;24doi doi: 10.7812/tpp/19.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muhlbauer V., Berger-Hoger B., Albrecht M., Muhlhauser I., Steckelberg A. Communicating prognosis to women with early breast cancer - overview of prediction tools and the development and pilot testing of a decision aid. BMC Health Serv Res. Mar 15. 2019;19(1):171. doi: 10.1186/s12913-019-3988-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rabin B.A., Gaglio B., Sanders T., et al. Predicting cancer prognosis using interactive online tools: a systematic review and implications for cancer care providers. Cancer Epidemiol Biomarkers Prev. Oct 2013;22(10):1645–1656. doi: 10.1158/1055-9965.EPI-13-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sparano J.A., Crager M.R., Tang G., Gray R.J., Stemmer S.M., Shak S. Development and validation of a tool integrating the 21-gene recurrence score and clinical-pathological features to individualize prognosis and prediction of chemotherapy benefit in early breast cancer. J Clin Oncol. Feb 20 2021;39(6):557–564. doi: 10.1200/JCO.20.03007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravdin P.M., Siminoff L.A., Davis G.J., et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. Feb 15. 2001;19(4):980–991. doi: 10.1200/jco.2001.19.4.980. [DOI] [PubMed] [Google Scholar]
- 32.Tricco A.C., Lillie E., Zarin W., et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. Oct 2 2018;169(7):467–473. doi: 10.7326/m18-0850. [DOI] [PubMed] [Google Scholar]
- 33.van Steenbeek C.D., van Maaren M.C., Siesling S., Witteveen A., Verbeek X., Koffijberg H. Facilitating validation of prediction models: a comparison of manual and semi-automated validation using registry-based data of breast cancer patients in The Netherlands. BMC Med Res Methodol. Jun 8 2019;19(1):117. doi: 10.1186/s12874-019-0761-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen L., Bassett R., Gadgil P., et al. Presented at: the American society of clinical oncology (ASCO) breast cancer symposium. 2012. Breast cancer nomogram to predict positive sentinel lymph nodes after neoadjuvant chemotherapy. [Google Scholar]
- 35.Breast cancer nomogram to predict positive sentinel lymph nodes, after neoadjuvant chemotherapy. http://www3.mdanderson.org/app/medcalc/bc_nomogram4/index.cfm?pagename=sln
- 36.Breast cancer nomogram to predict positive sentinel lymph nodes, without neoadjuvant chemotherapy. http://www3.mdanderson.org/app/medcalc/bc_nomogram3/index.cfm?pagename=sln
- 37.Veerapong J., Boughey J., Mittendorf E., et al. Presented at: the society of surgical oncology annual cancer symposium. 2011. A validated risk assessment of sentinel lymph node involvement in breast cancer patients. [Google Scholar]
- 38.Breast cancer nomogram to predict additional positive non-SLN, without neoadjuvant chemotherapy. http://www3.mdanderson.org/app/medcalc/bc_nomogram2/index.cfm?pagename=nsln
- 39.Mittendorf E., Boughey J., Degnim A., et al. 2010. The san Antonio breast cancer Symposium. presented at: [Google Scholar]
- 40.Breast cancer non-SLN nomogram calculator, with neoadjuvant chemotherapy. http://www3.mdanderson.org/app/medcalc/bc_nomogram/index.cfm?pagename=nsln
- 41.Jeruss J.S., Newman L.A., Ayers G.D., et al. Factors predicting additional disease in the axilla in patients with positive sentinel lymph nodes after neoadjuvant chemotherapy. Cancer. Jun 15 2008;112(12):2646–2654. doi: 10.1002/cncr.23481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Response to neoadjuvant chemotherapy. http://www3.mdanderson.org/app/medcalc/bc_nomogram/index.cfm?pagename=nsln
- 43.Residual cancer burden calculator. MD Anderson cancer center web site. http://www3.mdanderson.org/app/medcalc/index.cfm?pagename=jsconvert3
- 44.Chemotherapy response calculators. 2021. http://www3.mdanderson.org/app/medcalc/index.cfm?pagename=jsconvert1 [Google Scholar]
- 45.Breast cancer nomogram to predict benefit of radiation for older patients with breast cancer treated with conservative surgery. http://www3.mdanderson.org/app/medcalc/bc_nomogram5/index.cfm?pagename=opcs [DOI] [PMC free article] [PubMed]
- 46.Albert J.M., Liu D.D., Shen Y., et al. Nomogram to predict the benefit of radiation for older patients with breast cancer treated with conservative surgery. J Clin Oncol. Aug 10. 2012;30(23):2837–2843. doi: 10.1200/JCO.2011.41.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanghani M., Truong P.T., Raad R.A., et al. Validation of a web-based predictive nomogram for ipsilateral breast tumor recurrence after breast conserving therapy. J Clin Oncol. Feb 10. 2010;28(5):718–722. doi: 10.1200/JCO.2009.22.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jayasekera J., Sparano J.A., O'Neill S., et al. Development and validation of a simulation model-based clinical decision tool: identifying patients where 21-gene recurrence score testing may change decisions. J Clin Oncol. Sep 10 2021;39(26):2893–2902. doi: 10.1200/JCO.21.00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ward S.E., Holmes G.R., Morgan J.L., et al. Bridging the Age Gap: a prognostic model that predicts survival and aids in primary treatment decisions for older women with oestrogen receptor-positive early breast cancer. Br J Surg. Nov 2020;107(12):1625–1632. doi: 10.1002/bjs.11748. [DOI] [PubMed] [Google Scholar]
- 50.Ward S.E., Holmes G.R., Ring A., et al. Adjuvant chemotherapy for breast cancer in older women: an analysis of retrospective English cancer registration data. Clin Oncol. Jul 2019;31(7):444–452. doi: 10.1016/j.clon.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 51.INFLUENCE: locoregional recurrence risk in breast cancer patients. https://www.evidencio.com/models/show/721
- 52.Witteveen A., Vliegen I.M., Sonke G.S., Klaase J.M., Mj I.J., Siesling S. Personalisation of breast cancer follow-up: a time-dependent prognostic nomogram for the estimation of annual risk of locoregional recurrence in early breast cancer patients. Breast Cancer Res Treat. Aug 2015;152(3):627–636. doi: 10.1007/s10549-015-3490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pleijhuis R.G., Kwast A.B., Jansen L., et al. A validated web-based nomogram for predicting positive surgical margins following breast-conserving surgery as a preoperative tool for clinical decision-making. Breast. Oct 2013;22(5):773–779. doi: 10.1016/j.breast.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 54.Preoperative risk of positive surgical margins in breast-conserving surgery. https://www.evidencio.com/models/show/73/
- 55.Barentsz M.W., Postma E.L., van Dalen T., et al. Prediction of positive resection margins in patients with non-palpable breast cancer. Eur J Surg Oncol. Jan 2015;41(1):106–112. doi: 10.1016/j.ejso.2014.08.474. [DOI] [PubMed] [Google Scholar]
- 56.Wishart G.C., Azzato E.M., Greenberg D.C., et al. PREDICT: a new UK prognostic model that predicts survival following surgery for invasive breast cancer. Breast Cancer Res. 2010;12(1):R1. doi: 10.1186/bcr2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen L.L., Nolan M.E., Silverstein M.J., et al. The impact of primary tumor size, lymph node status, and other prognostic factors on the risk of cancer death. Cancer. Nov 1. 2009;115(21):5071–5083. doi: 10.1002/cncr.24565. [DOI] [PubMed] [Google Scholar]
- 58.Hoveling L.A., van Maaren M.C., Hueting T., et al. Validation of the online prediction model CancerMath in the Dutch breast cancer population. Breast Cancer Res Treat. Dec 2019;178(3):665–681. doi: 10.1007/s10549-019-05399-2. [DOI] [PubMed] [Google Scholar]
- 59.Miao H., Hartman M., Verkooijen H.M., et al. Validation of the CancerMath prognostic tool for breast cancer in Southeast Asia. BMC Cancer. Oct 21 2016;16(1):820. doi: 10.1186/s12885-016-2841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hwang H., Park S., Koo J.S., et al. Factors predictive of occult nipple-areolar complex involvement in patients with carcinoma in situ of the breast. J Surg Oncol. Dec 2017;116(8):1046–1055. doi: 10.1002/jso.24768. [DOI] [PubMed] [Google Scholar]
- 61.Nottingham prognostic index. https://www.evidencio.com/models/show/633
- 62.Kim J.Y.S., Khavanin N., Jordan S.W., et al. Individualized risk of surgical-site infection: an application of the breast reconstruction risk assessment score. Plast Reconstr Surg. Sep 2014;134(3):351e–362e. doi: 10.1097/PRS.0000000000000439. [DOI] [PubMed] [Google Scholar]
- 63.Darby S.C., Ewertz M., McGale P., et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. Mar 14. 2013;368(11):987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 64.Risk of ischemic heart disease in women after radiotherapy for breast cancer. https://www.evidencio.com/models/show/1584 [DOI] [PubMed]
- 65.Stineman M.G., Xie D., Pan Q., et al. All-cause 1-, 5-, and 10-year mortality in elderly people according to activities of daily living stage. J Am Geriatr Soc. Mar 2012;60(3):485–492. doi: 10.1111/j.1532-5415.2011.03867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wyld L., Reed M.W.R., Collins K., et al. Bridging the age gap in breast cancer: cluster randomized trial of two decision support interventions for older women with operable breast cancer on quality of life, survival, decision quality, and treatment choices. Br J Surg. May 27 2021;108(5):499–510. doi: 10.1093/bjs/znab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aaronson N.K., Ahmedzai S., Bergman B., et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. Mar 3. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 68.Carver C.S. You want to measure coping but your protocol's too long: consider the brief COPE. Int J Behav Med. 1997;4(1):92–100. doi: 10.1207/s15327558ijbm0401_6. [DOI] [PubMed] [Google Scholar]
- 69.Agostinho J.L., Zhao X., Sun W., et al. Prediction of positive margins following breast conserving surgery. Breast. Feb 2015;24(1):46–50. doi: 10.1016/j.breast.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 70.Predict breast. https://breast.predict.nhs.uk/about/technical/history
- 71.Gray E., Marti J., Brewster D.H., Wyatt J.C., Hall P.S., Group S.A. Independent validation of the PREDICT breast cancer prognosis prediction tool in 45,789 patients using Scottish Cancer Registry data. Br J Cancer. Oct 2018;119(7):808–814. doi: 10.1038/s41416-018-0256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Karapanagiotis S., Pharoah P.D.P., Jackson C.H., Newcombe P.J. Development and external validation of prediction models for 10-year survival of invasive breast cancer. Comparison with PREDICT and CancerMath. Clin Cancer Res. May 1 2018;24(9):2110–2115. doi: 10.1158/1078-0432.CCR-17-3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Polchai N., Sa-Nguanraksa D., Numprasit W., Thumrongtaradol T., E Oc, P Oc A comparison between the online prediction models CancerMath and PREDICT as prognostic tools in Thai breast cancer patients. Cancer Manag Res. 2020;12:5549–5559. doi: 10.2147/CMAR.S258143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Maaren M.C., van Steenbeek C.D., Pharoah P.D.P., et al. Validation of the online prediction tool PREDICT v. 2.0 in the Dutch breast cancer population. Eur J Cancer. Nov 2017;86:364–372. doi: 10.1016/j.ejca.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 75.Zaguirre K., Kai M., Kubo M., et al. Validity of the prognostication tool PREDICT version 2.2 in Japanese breast cancer patients. Cancer Med. Mar 2021;10(5):1605–1613. doi: 10.1002/cam4.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stacey D., Legare F., Lewis K., et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. Apr 12 2017;4:CD001431. doi: 10.1002/14651858.CD001431.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nechuta S., Lu W., Zheng Y., et al. Comorbidities and breast cancer survival: a report from the shanghai breast cancer survival study. Breast Cancer Res Treat. May 2013;139(1):227–235. doi: 10.1007/s10549-013-2521-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Colditz G.A., Willett W.C., Hunter D.J., et al. Family history, age, and risk of breast cancer. Prospective data from the Nurses' Health Study. J Am Med Assoc. Jul 21 1993;270(3):338–343. [PubMed] [Google Scholar]
- 79.Palmer J.R., Boggs D.A., Adams-Campbell L.L., Rosenberg L. Family history of cancer and risk of breast cancer in the Black Women's Health Study. Cancer Causes Control. Nov 2009;20(9):1733–1737. doi: 10.1007/s10552-009-9425-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Agurs-Collins T., Rosenberg L., Makambi K., Palmer J.R., Adams-Campbell L. Dietary patterns and breast cancer risk in women participating in the Black Women's Health Study. Am J Clin Nutr. Sep 2009;90(3):621–628. doi: 10.3945/ajcn.2009.27666. doi:10.3945/ajcn.2009.27666 ajcn.2009.27666 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weiss S.E., Tartter P.I., Ahmed S., et al. Ethnic differences in risk and prognostic factors for breast cancer. Cancer. Jul 15 1995;76(2):268–274. doi: 10.1002/1097-0142(19950715)76:2<268::aid-cncr2820760217>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 82.Chlebowski R.T., Chen Z., Anderson G.L., et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. Mar 16 2005;97(6):439–448. doi: 10.1093/jnci/dji064. 97/6/439 [pii] 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 83.Kochanek K.D., Arias E., Anderson R.N. How did cause of death contribute to racial differences in life expectancy in the United States in 2010? NCHS data brief. Jul 2013;(125):1–8. [PubMed] [Google Scholar]
- 84.Lee Smith J., Hall I.J. Advancing health equity in cancer survivorship: opportunities for public health. Am J Prev Med. Dec 2015;49(6 Suppl 5):S477–S482. doi: 10.1016/j.amepre.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith B.D., Smith G.L., Hurria A., Hortobagyi G.N., Buchholz T.A. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. Jun 10. 2009;27(17):2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 86.Bodai B.I., Tuso P. Breast cancer survivorship: a comprehensive review of long-term medical issues and lifestyle recommendations. Perm J. Spring. 2015;19(2):48–79. doi: 10.7812/tpp/14-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ozanne E.M., Schneider K.H., Soeteman D., et al. onlineDeCISion.org: a web-based decision aid for DCIS treatment. Breast Cancer Res Treat. Nov 2015;154(1):181–190. doi: 10.1007/s10549-015-3605-y. [DOI] [PubMed] [Google Scholar]
- 88.Sivell S., Edwards A., Manstead A.S., et al. Increasing readiness to decide and strengthening behavioral intentions: evaluating the impact of a web-based patient decision aid for breast cancer treatment options (BresDex: www.bresdex.com) Patient Educ Counsel. Aug 2012;88(2):209–217. doi: 10.1016/j.pec.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 89.Benoit A., Grynberg M., Morello R., Sermondade N., Grandazzi G., Moutel G. Does a web-based decision aid improve informed choice for fertility preservation in women with breast cancer (DECISIF)? Study protocol for a randomised controlled trial. BMJ Open. Feb 10 2020;10(2) doi: 10.1136/bmjopen-2019-031739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Garvelink M.M., ter Kuile M.M., Fischer M.J., et al. Development of a Decision Aid about fertility preservation for women with breast cancer in The Netherlands. J Psychosom Obstet Gynaecol. Dec 2013;34(4):170–178. doi: 10.3109/0167482x.2013.851663. [DOI] [PubMed] [Google Scholar]