Abstract
The therapeutic effectiveness of anticancer drugs with a selective target for the nucleus of cancer cells may be improved by experimental approaches. In this regard, the formulation of anticancer drugs is considered one of the best ways to improve their effectiveness in targeting cancerous tissues. To enhance the anticancer activity of 2-methoxy-estradiol (2 ME) for breast cancer, 2-methoxyestradiol loaded alpha lipoic acid nanoparticles have been formulated. The prepared formula was observed to be spherical with a nanometer-scale and low PDI size (.234). The entrapment efficiency of the 2ME-ALA NPs was 87.32 ± 2.21% with > 85% release of 2 ME within 24 h. There was a 1.2-fold increase in apoptosis and a 3.46-fold increase in necrosis of the MCF-7 cells when incubated with 2ME-ALA NPs when compared to control cells. This increased apoptosis was also associated with increased ROS and increased p53 expression in 2ME-ALA NPs treated cells compared to the raw-2 ME group. Evaluation of cell-cycle data showed a substantial arrest of the G2-M phase of the MCF-7 cells when incubated with 2ME-ALA NPs. At the same time, a dramatically increased number of pre-G1 cells showed the increased apoptotic potential of the 2 ME when administered via the proposed formulation. In the end, the differential upregulation of caspase-3, p53, and ROS in MCF-7 cells established the superiority of the 2ME-ALA-Ms approach in targeting breast cancer. In summary, these results demonstrate that 2ME-ALA NPs are an efficient delivery tool for controlling the growth of breast cancer cells.
Keywords: 2-methoxy-estradiol, alpha lipoic acid, cell-cycle assay, P53 expression, nanoparticles, molecular markers
Introduction
As the name suggests, breast cancer arises in the cells of the breast tissues. Following skin cancer, breast cancer is the most commonly diagnosed cancer in women in the United States. Despite the fact that both men and women are susceptible to breast cancer, women are significantly more susceptible to this disease. There is a growing body of literature focusing on the prevention and treatment of this type of cancer with natural compounds. In this regard, a biologically active estrogen metabolite, 2-methoxy-estradiol (2 ME), has shown its anti-proliferative and anti-angiogenic efficacy against cancer cells. Furthermore, 2 ME, a nonpolar endogenous metabolite of 17beta-estradiol, has also been shown to induce apoptosis selectively in a variety of cancer cell lines in comparison to regular cells. In the various cell lines studied, the mechanism of 2 ME-induced apoptosis seems to differ considerably (Harrison et al, 2011). 2-methoxy-estradiolhas displayed antitumor ability by the induction of apoptosis through suppressing hypoxia-inducible factor 1 and activating p53 (Harrison et al, 2011). Other researches showed that the binding of 2 ME to the tubulin promotes the arrest of the mitotic cell cycle by blocking the development of microtubules (Attalla et al, 1996). The advancements in the nanoparticle’s colloidal formulation methods of 2 ME demonstrated enhanced pharmacokinetic profile of the drug and increased antitumor activity in animal models (Harrison et al, 2011; Sweeney et al, 2005; Tevaarwerk et al, 2009). Some plants and animals, including humans, naturally synthesize alpha lipoic acid (ALA), a form of thioctic acid. Endogenous ALA serves as a cofactor that covalently linked to essential mitochondrial enzymes and a number of multienzyme complexes containing branched-chain alpha-keto acid dehydrogenases, for example, pyruvate dehydrogenase, alpha-ketoglutarate dehydrogenase, and glycine decarboxylase. It is also bearing a negative charge in comparison to the lipoamide neutral charge. Both ALA and its reduced form, dihydrolipoic acid (DHLA), have been thought to have free radical scavenging properties; acting as antioxidants (Gorain et al, 2018).
Alhakamy et al, previously reported a similar nanoparticulate system of 2 ME Loaded Nanocarrier for Prostate Cancer (Alhakamy et al, 2021b, 2021a). A review by Lakhani details about the anticancer potential of 2 ME (Lakhani et al, 2003). Pillai and associates formulated PLGA nanoparticles of 2 ME and stabilized by PEG or Casein and evaluated pharmacokinetics and tumor accumulation property (Pillai et al, 2017). To the best of our knowledge there is no nanocarrier system of 2 ME developed yet with alpha lipoic acid for the management of breast cancer. Thus, the present research has focused on loading 2 ME on ALA NPs for effective and safe delivery of 2 ME. The incorporation of 2 ME within the NPs was characterized in vitro for size, zeta potential, entrapment efficiency, and release characteristics. To establish the anticancer potential of the formulation, assays regarding cell viability, apoptosis potential, cell cycle, and the expression of molecular markers were performed.
Methodology
Materials
2-methoxy-estradiol (> 99% purity), and ALA were procured from Sigma, USA. HPLC grade acetonitrile and methanol were procured from Sigma, USA. Trizol was purchased from Invitrogen, Paisley, UK. The rest of the chemicals used in this project were of analytical grade. The human tumor cell line MCF-7 used in this study was obtained from the VACSERA (Giza, Egypt) cell culture unit that were originally acquired from ATCC (Manassas, VA, USA).
Method of Preparation of 2 ME-ALA NPs
2ME-ALA NPs were prepared by dissolving 30 mg of 2 ME and 100 mg ALA in 99% ethanol and 1% dimethyl sulfoxide (DMSO) with a magnetic stirrer (400 r/min) for 5 minutes. The 2 ME organic solution was dropped by syringe in distilled water over 30 minutes under stirring at 800 r/min. The formed suspension was allowed to stir (400 r/min) overnight to evaporate the ethanol. The formed dispersion was subjected to a rotary evaporator (BÜCHI Labortechnik AG, Flawil, Switzerland) for 1 h at 35oC. The prepared dispersion was centrifuged for 45 minutes at 30 000 r/min at 4°C. The residue was washed with double distilled water and subjected to centrifuged for 45 minutes at 30 000 r/min at 4°C. Next, lyophilization process was applied to the residue using a freeze dryer (alpha 1-2 LD plus lyophilizer; Christ, Osterode am Harz, Germany) for 48 hours and processed for further characterization.
Evaluation of 2 ME -ALA NPs
Determination of Particle Size, Polydispersity Index and Zeta Potential
Evaluation of the particle size, PDI and zeta potential of the produced 2ME-ALA NPs was performed using a laser diffraction particle size analyzer (Zetasizer Nano ZSP, Malvern Panalytical, UK) where the size and polydispersity were estimated at 25C after a 2 minute balancing time.
Entrapment Efficiency
The entrapment efficiency of the produced 2ME-ALA NPs was calculated by dissolving the formulated NPs in ethanol: DMSO (99:1) and the quality of 2 ME was analyzed by the RP-HPLC method using column C18 (4.6 mm x 150 mm, 5 μm). The separation of 2 ME was accomplished using a mobile process composed of acetonitrile and methanol at a ratio of 55:45 (v/v) at a wavelength of 285 nm (Du et al, 2010). Finally, the percentage of intrusion efficiency was calculated using the following equation
In Vitro Release
The release pattern of 2 ME from ALA NPs and 2 ME aqueous suspension was determined by the dialysis bag method (Chin et al, 2021) at which each sample containing 5 mg of 2 ME was inserted into the triggered dialysis bag (12 kDa cut-off) and the ends were hermetically sealed. The liberation of 2 ME from the setup was then calculated in the paddle-type dissolution apparatus, where the saline phosphate buffer (pH 7.4) was used as a release medium. The paddle was set at 100 r/min and the machine temperature was held at 37 ± .5°C. The 10 mL sample volume was removed at each time point at 0, 2, 4, 6, 8, 10, 12, 18, and 24 h. The study of 2 ME in the withdrawn sample was conducted using the HPLC method as described above.
Cell-Based Evaluation of the 2ME-ALA NPs Formulation
Cytotoxicity Assay
The cytotoxicity analysis of the free 2 ME and the formulated 2 ME in the MCF-7 cell line was evaluated following the MTT assay (1). Establishing various concentrations of drugs in a 96-well plate containing 3×103 cells in each well was incubated at 37°C for 24 h. Following the incubation period, the wells were washed with phosphate buffer saline (pH 7.4). Afterward, .5% (w/v) of MTT solution was added to the wells and further incubated for another 4 h. Next, the cells were washed again to extract the MTT entry solution from the cells and 150 μL of DMSO was applied to each well to dissolve the formed formazan crystals. Finally, the viability of the cells was calculated by the results of the 490 nm optical density of the wells tested in the microplate reader.
Apoptotic assay
We also implemented a double staining strategy to evaluate the apoptosis-inducing potential of the blank micelle, the free 2 ME and 2ME-ALA NPs in the MCF-7 cell line. In brief, MCF-7 cells were exposed to samples in a 12-well plate, where the initial density of the seed cells was preserved at 105 cells per well. (Zhang et al, 2020). A 10 μM concentration of 2 ME was maintained in the wells, which were incubated at 37°C for 24 h. Thereafter, the cells were washed with phosphate buffer in order to remove the micelles and free drug from the wells, and the apoptotic potential of the agents was measured using the conjugate of Annexin V–fluorescein isothiocyanate (FITC) and propidium iodide following the instruction provided with the apoptotic detection kit (BD Bioscience, USA). Finally, the flow cytometer (FACS Calibur, BD Bioscience) was used to analyze the cells, and the obtained data were analyzed using the Multicycle software (Phoenix Flow Systems, San Diego, CA).
Cell-cycle analysis
Cell-cycle analysis was performed by the treatment of blank NPs, free 2 ME and formulated 2ME-ALA NPs with 1 × 106 MCF-7 cell counts per well. After 24 h of therapy, the treated cells were trypsinized to isolate the cells from the respective wells. The cells were extracted and cleaned with phosphate buffer and re-fixed in a different well plate. Subsequently, the cells were tested for arrest in the cell cycle using the TESTTM PLUS DNA reagent kit (BD Biosciences, San Jose, CA) following the manufacturer instructions in the cell cycle. Finally, the testing of cell cycle distribution was conducted using a flow cytometer (FACS Calibur, BD Bioscience) and analyzed using the Multicycle software (Phoenix Flow Systems, San Diego, CA).
Molecular Marker Estimation Using ELISA Method
In determining the molecular markers inside the treated cells, the MCF-7 cells of various groups were collected for 5 minutes after application, trypsinization and centrifugation at 3000 r/min. Cells were first washed twice with phosphate buffer saline (pH 7.4) and then lysed using an ice-cold radio immune precipitation buffer following the insertion of a protease inhibitor. (Balachandran et al, 2014). The levels of caspase-3, p53, and ROS within the MCF-7cells were measured following the manufacturer’s instructions in the commercially available kits (Invitrogen, USA) (Mohamed et al, 2020).
Statistical Analysis
Using the SPSS software kit, the obtained data analysis was completed. The statistical data analysis was carried out using the one-way analysis of the variance (ANOVA) test, where the P-value less than .05 was regarded as significant.
Results
2ME-ALA NPs Characterization
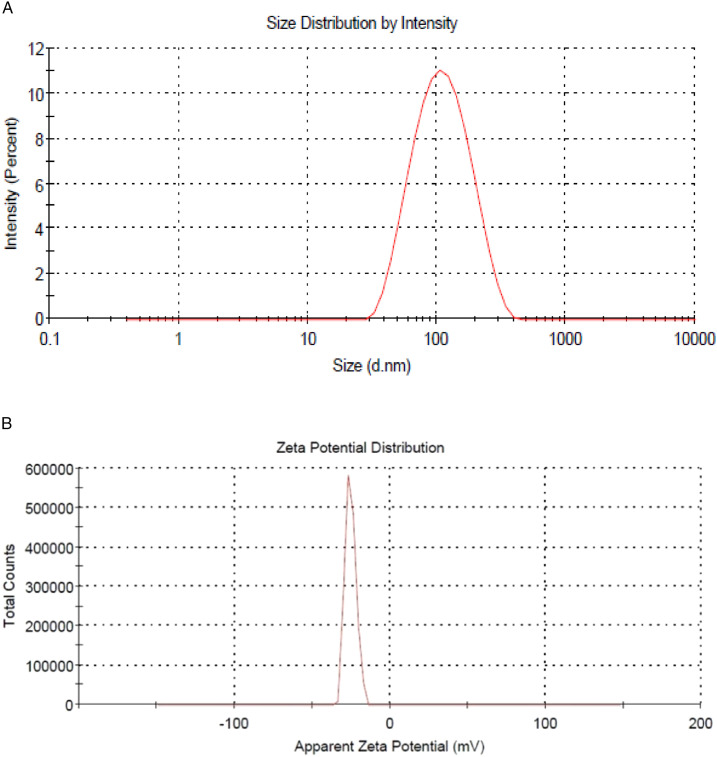
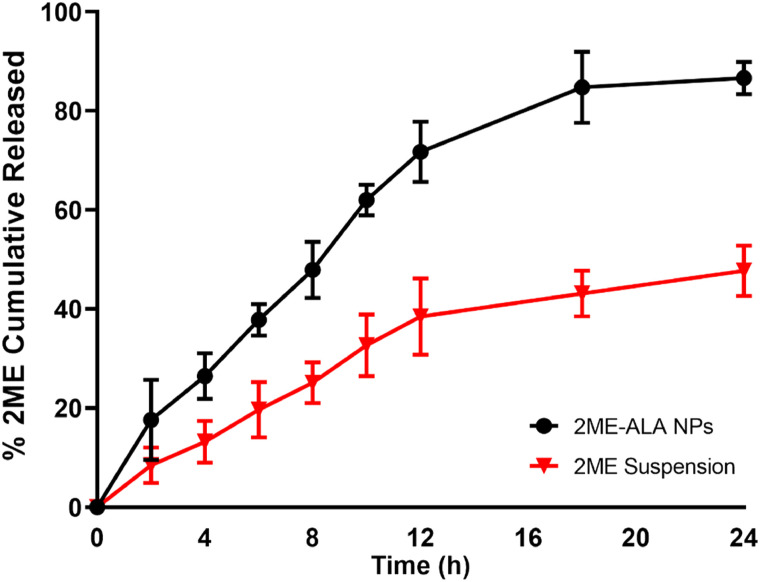
The size and polydispersity index of the prepared formula that are homogenous in size. The prepared 2ME-ALA NPs particle size was 102.15 ± 2.58 nm, with a polydispersity index of .219±.01 (Figure 1(A)) and a zeta potential of −25.1 ± 3.4 mV (Figure 1(B)). The 2 ME %EE of the prepared NPs was 88.11 ± 2.21%. Figure 2 shows the 2 ME release from ALA NPs and aqueous suspension. 2ME-ALA NPs experienced improved release pattern compared with raw-2 ME suspension. The results revealed that 2ME-ALA NPs released 86.65 ± 2.3% of 2 ME content within 24 h compared to 47.70 ± 5.1% of 2 ME released from the 2 ME suspension during the same period.
Figure 1.
Particle size distribution (A) and zeta potential (B) of 2ME-ALA NPs.
Figure 2.
2 ME release from 2ME-ALA NPs within 24h.
Cytotoxicity Assay
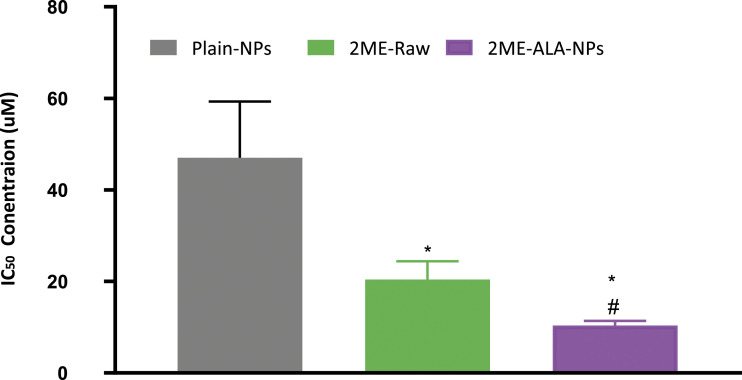
The results of the MTT assay on the MCF-7 cell line following the treatment with plain, raw- 2 ME and 2ME-ALA NPs are shown in Figure 3. It is seen that there is a dose-dependent reduction in cell viability for all treatments; however, the significant benefits of the drug-loaded NPs are clearly visible. The results also demonstrate a substantial decrease (P < .05) in the IC50 value of 2ME-ALA NPs (10.41± 1.2uM) relative to raw-2 ME (20.44± 4.1uM) in the MCF-7 cell line. The cytotoxicity of the blank formulation may be explained by the existence of ALA, as the literature on the anticancer capacity of ALA against breast cancer is established.
Figure 3.
Cytotoxicity (IC 50) values of 2ME-ALA NPs in MCF-7 cells. Values are expressed as mean ± SD (n = 3). *Significantly different from plain- NPs at P < .05. # Significantly different from 2ME-raw at P < .05.
Apoptotic Assay
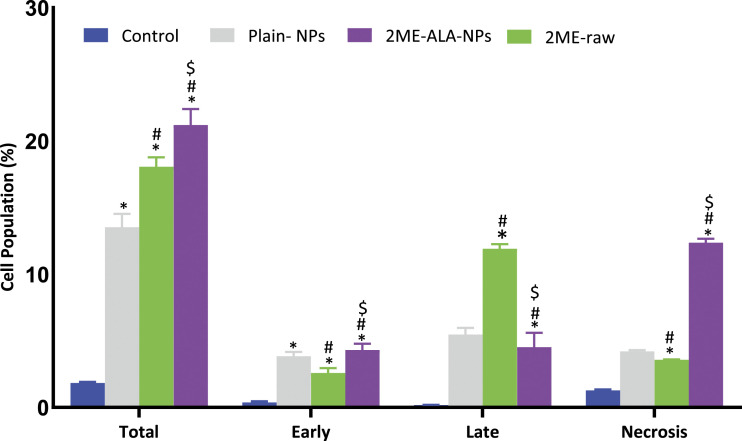
The mechanism of apoptosis is a genetically engineered, controlled process of cell death to regulate and sustain a stable state of the body, while the process of premature cell death is known as necrosis. Early apoptotic cells are Annexin V-positive and PI-negative (Annexin V-FITC+/PI−), whereas late (end-stage) apoptotic cells are Annexin V/PI-double-positive (Annexin V-FITC+/PI+). Figures 4 presents the apoptotic potential of the plain-NPs, the 2ME-ALA NPs and the raw-2 ME. Incubation of breast cancer cells with all samples examined for 24 h revealed a significant increase (P < .05) in apoptotic activity relative to control cells (1.83± .09%). When the influence of 2ME-ALA NPs (21.19± 1.22%) was compared to raw-2 ME (18.06 ± .71per cent), there was also a substantial difference in apoptosis between the 2 groups (Choudhury et al, 2019; Faramarzi et al, 2019). The difference in apoptotic activity may be as a result of improved solubilty of 2 ME in the culture media, as solubility determines the contact time between the drug and cancer cells (Alhakamy et al, 2020; Alhakamy and Md, 2019). These results support the potential anticancer activity of 2 ME and the capability of ALA NPs to enhance in vitro dissolution.
Figure 4.
Apoptotic and necrotic assessment of plain-NPs, raw-2 ME and 2ME-ALA NPs in MCF-7 cell line. The cells were exposed to the samples for 24 h and stained with Annexin-V/FITC and propidium iodide. Total = Apoptosis+ necrosis; early = Apoptotic phase; late = late apoptotic phase. Values are expressed as mean ± SD (n =3). *Significantly different from untreated cells at P < .05. # Significantly different from plain-NPs at P < .05. $ Significantly different from plain-NPs at P < .05.
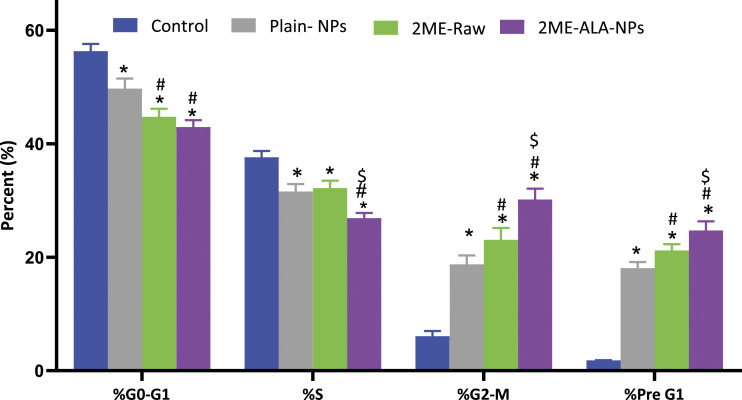
Cell Cycle Analysis
The effect of the raw- 2 ME and 2ME-ALA NPs on the growth of MCF-7 cells was determined by the DNA content of the cytometric assay. The results are set out in Figure 5. At a concentration of 5 μM, 2ME-ALA NPs was observed to cause an arrest of the G2-M phase of the cell cycle. The reduction of G2-M from 23.33% attributable to free drug therapy was raised by 56.93, which may be associated with the increased apoptotic ability.
Figure 5.
Cell cycle analysis of plain-NPs, raw-2 ME, and 2ME-ALA NPs in MCF-7 cell line. The cells were exposed to the samples for 24 h and stained with Annexin-V/FITC and propidium iodide. Values are expressed as mean ± SD (n = 3). *Significantly different from untreated cells at P < .05. # Significantly different from plain-NPs at P < .05. $ Significantly different from plain-NPs at P < .05.
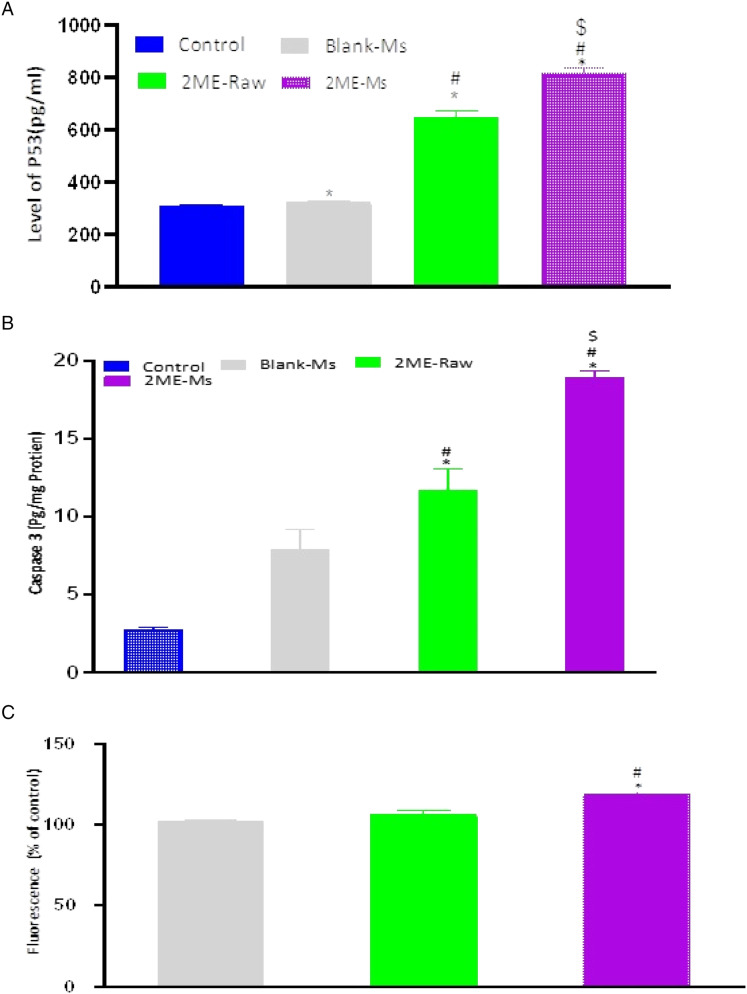
Molecular Marker Expression as Measured via ELISA
In order to establish the molecular mechanism of the improved anticancer potential of the 2ME-ALA NPs, further analysis was conducted using the ELISA to determine the expression of several molecular markers. According to the results illustrated in Figure 6, there is an upregulation of caspase-3, p53, and ROS.
Figure 6.
P53 gene expression (A), Caspase-3 expression (B) and ROS (C) evaluation in MCF-7 cells after treatment with plain-NPs, raw-2 ME and 2ME-ALA-NPs. Values are expressed as mean ± SD (n =3). *Significantly different from untreated cells at P < .05. # Significantly different from plain-NPs at P < .05. $ Significantly different from plain-NPs at P < .05.
Discussion
The increased cytotoxic ability of the micellar method of 2 ME could be explained by the improved release of 2 ME from the micelles, better penetration of the drug across the cell membrane due to the nanometric size scale (Choudhury et al, 2019; Faramarzi et al, 2019). Furthermore, the cytotoxic potential of TPGS against breast cancer cells would be also considered (Neophytou and Constantinou, 2015). Improvements in cell penetration and cytotoxicity of 2 ME have been also reported in the literature when administered as a nanoformulation (Wang et al, 2011). Similarly, micellar deliveries including drug-free formulations in MCF-7 cells revealed a significant increase in necrosis (P < .05) when compared to the necrosis observed in control cells (2.14 ± .12%). This highlights the apoptosis-inducing activity of these treatments against cancer cells (Figure 5(B)).
Our results on the arrest of the G2-M phase by the action of 2 ME is similar to the reported literature (Lee et al, 2008). Yet, an increased level of cell cycle arrest in the G2-M phase was associated with the novel formulation developed in this study. In addition, there was a significant increase in the number of cells that were in the pre-G1 phase (P < .05) when compared to cells in the control group or the blank micelle group. The increased numbers of cancerous cells in the pre-G1 phase could be also seen in the existing literature (Lee et al, 2008). The effect on the G0-G1 and S phase of the cell cycle by the free drug and drug-loaded micelle is not significant. Interestingly, a characteristic sign of an apoptotic potential is the increased percentage of cells in the pre-G1 phase of the cell cycle (Anwar et al, 2019; McKenzie and Kyprianou, 2006; Md et al, 2020; Wang and Lin, 2008). Thus, the induction of apoptosis and the alterations observed in the cell cycle stages could indicate increased cytotoxicity of the micellar formulation of 2-ME. This increased cytotoxicity could be also reflected by changes in the expressions of cellular markers involved in apoptosis and cell cycle. The upregulation of the tumor suppressor gene, caspase-3, p53, within the cancerous cells was found to be significant when the cells were incubated with the treatments, in which the maximum response was obtained from the 2ME-loaded micelle. This significant alteration in the expression of these markers could be critical in the context of breast cancer management (McKenzie and Kyprianou, 2006; Wang and Lin, 2008)
Acknowledgment
The authors, therefore, acknowledge with thanks DSR for technical and financial support.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. (RG-16-166-41).
ORCID iDs
Samah Alshehri https://orcid.org/0000-0002-3668-4905
Sabna Kotta https://orcid.org/0000-0001-6350-5733
References
- Akrawi SH, Gorain B, Nair AB, et al. Development and optimization of naringenin-loaded chitosan-coated nanoemulsion for topical therapy in wound healing. Pharmaceutics 2020;12:893. doi: 10.3390/pharmaceutics12090893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhakamy NA, Fahmy UA, Badr-Eldin SM, et al. Optimized icariin phytosomes exhibit enhanced cytotoxicity and apoptosis-inducing activities in ovarian cancer cells. Pharmaceutics. 2020;12:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhakamy NA, Ahmed OA, Fahmy UA, et al. Development, optimization and evaluation of 2-methoxy-estradiol loaded nanocarrier for prostate cancer. Front Pharmacol. 2021. a;1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhakamy NA, Ahmed OAA, Fahmy UA, Md S. Development and in vitro evaluation of 2-methoxyestradiol loaded polymeric micelles for enhancing anticancer activities in prostate cancer. Polymers. 2021. b;13:884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhakamy NA, Md S. Repurposing itraconazole loaded PLGA nanoparticles for improved antitumor efficacy in non-small cell lung cancers. Pharmaceutics. 2019;11:685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar MM, Abd El-Karim SS, Mahmoud AH, Amr AE-GE, Al-Omar MA. A comparative study of the anticancer activity and PARP-1 inhibiting effect of benzofuran-pyrazole scaffold and its nano-sized particles in human breast cancer cells. Molecules. 2019;24:2413. DOI: 10.3390/molecules24132413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attalla H, Mäkelä TP, Adlercreutz H, Andersson LC. 2-methoxyestradiol arrests cells in mitosis without depolymerizing tubulin. Biochem Biophys Res Commun. 1996;228:467-473. DOI: 10.1006/bbrc.1996.1683. [DOI] [PubMed] [Google Scholar]
- Balachandran C, Sangeetha B, Duraipandiyan V, et al. A flavonoid isolated from streptomyces sp. (ERINLG-4) induces apoptosis in human lung cancer A549 cells through p53 and cytochrome c release caspase dependant pathway. Chem Biol Interact. 2014;224:24-35. DOI: 10.1016/j.cbi.2014.09.019. [DOI] [PubMed] [Google Scholar]
- Chin LY, Tan JYP, Choudhury H, Pandey M, Sisinthy SP, Gorain B. Development and optimization of chitosan coated nanoemulgel of telmisartan for intranasal delivery: a comparative study. J Drug Deliv Sci Technol. 2021;62:102341. DOI: 10.1016/j.jddst.2021.102341. [DOI] [Google Scholar]
- Choudhury H, Gorain B, Pandey M, Khurana RK, Kesharwani P. Strategizing biodegradable polymeric nanoparticles to cross the biological barriers for cancer targeting. Int J Pharm. 2019;565:509-522. DOI: 10.1016/J.IJPHARM.2019.05.042. [DOI] [PubMed] [Google Scholar]
- Du B, Li Y, Li X, A Y Chengqun C, Zhang Z. Preparation, characterization and in vivo evaluation of 2-methoxyestradiol-loaded liposomes. Int J Pharm. 2010;384:140-147. DOI: 10.1016/j.ijpharm.2009.09.045. [DOI] [PubMed] [Google Scholar]
- Faramarzi L, Dadashpour M, Sadeghzadeh H, Mahdavi M, Zarghami N. Enhanced anti-proliferative and pro-apoptotic effects of metformin encapsulated PLGA-PEG nanoparticles on SKOV3 human ovarian carcinoma cells. Artificial Cells, Nanomedicine, and Biotechnology. 2019;47:737-746. DOI: 10.1080/21691401.2019.1573737. [DOI] [PubMed] [Google Scholar]
- Gorain B, Choudhury H, Pandey M, Kesharwani P. Paclitaxel loaded vitamin E-TPGS nanoparticles for cancer therapy. Mater Sci Eng C. 2018;91:868-880. DOI: 10.1016/j.msec.2018.05.054. [DOI] [PubMed] [Google Scholar]
- Harrison MR, Hahn NM, Pili R, et al. A phase II study of 2-methoxyestradiol (2ME2) nanocrystal dispersion (NCD) in patients with taxane-refractory, metastatic castrate-resistant prostate cancer (CRPC). Invest N Drugs. 2011;29:1465-1474. DOI: 10.1007/s10637-010-9455-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhani NJ, Sarkar MA, Venitz J, Figg WD. 2-Methoxyestradiol, a promising anticancer agent. Pharmacotherapy. 2003;23:165-172. [DOI] [PubMed] [Google Scholar]
- Lee Y-M, Ting C-M, Cheng Y-K, et al. Mechanisms of 2-methoxyestradiol-induced apoptosis and G2/M cell-cycle arrest of nasopharyngeal carcinoma cells. Cancer Lett. 2008;268:295-307. DOI: 10.1016/j.canlet.2008.04.010. [DOI] [PubMed] [Google Scholar]
- McKenzie S, Kyprianou N. Apoptosis evasion: the role of survival pathways in prostate cancer progression and therapeutic resistance. J Cell Biochem. 2006;97:18-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Md S, Alhakamy NA, Aldawsari HM, et al. Formulation design, statistical optimization, and in vitro evaluation of a naringenin nanoemulsion to enhance apoptotic activity in a549 lung cancer cells. Pharmaceuticals. 2020;13:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed ME, Abduldaium YS, Younis NS. Ameliorative effect of linalool in cisplatin-induced nephrotoxicity: the role of HMGB1/TLR4/NF-κB and Nrf2/HO1 pathways. Biomolecules. 2020;10:1-19. DOI: 10.3390/biom10111488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neophytou CM, Constantinou AI. Drug delivery innovations for enhancing the anticancer potential of vitamin e isoforms and their derivatives. BioMed Res Int. 2015. DOI: 10.1155/2015/584862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai GJ, Paul-Prasanth B, Nair SV, Menon D. Influence of surface passivation of 2-methoxyestradiol loaded PLGA nanoparticles on cellular interactions, pharmacokinetics and tumour accumulation. Colloids Surf B Biointerfaces. 2017;150:242-249. [DOI] [PubMed] [Google Scholar]
- Sweeney C, Liu G, Yiannoutsos C, et al. A phase II multicenter, randomized, double-blind, safety trial assessing the pharmacokinetics, pharmacodynamics, and efficacy of oral 2-methoxyestradiol capsules in hormone-refractory prostate cancer. Clin Cancer Res. 2005;11:6625-6633. DOI: 10.1158/1078-0432.CCR-05-0440. [DOI] [PubMed] [Google Scholar]
- Tevaarwerk AJ, Holen KD, Alberti DB, et al. Phase I trial of 2-methoxyestradiol nanocrystal dispersion in advanced solid malignancies. Clin Cancer Res. 2009;15:1460-1465. DOI: 10.1158/1078-0432.CCR-08-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lin Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol Sin. 2008;29:1275-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Guo R, Cao X, Shen M, Shi X. Encapsulation of 2-methoxyestradiol within multifunctional poly(amidoamine) dendrimers for targeted cancer therapy. Biomaterials. 2011;32:3322-3329. DOI: 10.1016/j.biomaterials.2010.12.060. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Patel SB, King MR. Micelle-in-liposomes for sustained delivery of anticancer agents that promote potent TRAIL-induced cancer cell apoptosis. Molecules. 2020;26:157. DOI: 10.3390/molecules26010157. [DOI] [PMC free article] [PubMed] [Google Scholar]