Abstract
Pyogenic liver abscess (PLA) remains a significant challenge for modern clinicians. Serum albumin/globulin ratio (AGR) can reflect the progress of many diseases. However, the clinical significance of AGR in PLA has not been evaluated. The aim of this study was to explore the effect of AGR on the clinical characteristic and prognosis in PLA patients. This retrospective study included 392 PLA patients who admitted to the First Affiliated Hospital of Xi'an Jiaotong University from January, 2007 to December, 2016. The medical records on admission were collected. Compared with the healthy controls and the patients with extraperitoneal infection or non-infectious liver disease, PLA patients had lower levels of AGR. The mean level of AGR in PLA patients was 1.02 ± 0.25. There were 179 (45.4%) patients with AGR > 1.02 and 213 (54.6%) patients with AGR ≤ 1.02. The baseline data and treatment plans of PLA patients with high or low AGR were comparative. However, PLA patients with a low AGR had higher body temperature, leukocytes and neutrophils, lower hemoglobin, poorer liver and coagulation function, larger abscess diameter, higher positive rate of pus culture and proportion of Escherichia coli, and were more susceptible to multiple bacteria. Moreover, PLA patients with a low AGR had more complications, including systemic inflammatory response syndrome (SIRS), peritoneal effusion and pleural effusion. And it also needs longer time for temperature normalization and hospital stay. In conclusion, PLA patients have lower AGR and lower AGR is associated with worse clinical manifestations, more complications and poorer prognosis. Thus, monitoring of AGR is of great clinical significance for evaluating the progress of PLA patients.
Keywords: pyogenic liver abscess (PLA), albumin/globulin ratio, AGR, clinical characteristics, prognosis
Introduction
Pyogenic liver abscess (PLA) is a type of pyogenic infection in the liver, which can be life-threatening without appropriate treatment. PLA had a high morbidity and mortality in the past (1). The advent of modern antibiotics and intensive care techniques have dramatically reduced the mortality of PLA patients. However, the PLA incidence is still rising due to the aging of the population, the use of immunosuppressive agents, and the increase of diabetes (2). Many factors, such as malnutrition (3), diabetes (4, 5), hepatobiliary diseases (6), or accompanied by shock, low hemoglobin, high urea nitrogen, and multiple lesions in the liver (7) can influence the prognosis of PLA. Therefore, PLA remains an important challenge for today's clinicians.
The serum protein is composed by albumin (ALB) and globulin (GLO). Albumin is mainly synthesized in the liver and its level can reflect liver function. Albumin has a variety of physiological functions, such as maintaining osmotic pressure, transporting nutrient contents (fatty acids, bile acids, and cholesterol), scavenging free oxygen radicals (8–11). In addition, albumin also plays an important role in a variety of diseases (12, 13). Globulin, known as immune globulin, is mainly used to evaluate the overall status of immune and inflammatory responses (14–16).
Several recent studies have found that serum albumin/globulin ratio (AGR) can predict the prognosis of various diseases, including cancers, chronic kidney disease, heart failure, and peritoneal dialysis (17–21). As a serious infectious disease of the liver, PLA was associated with the decreased albumin and increased globulin, however, the clinical significance of AGR in PLA patients remains largely unknown. The aim of this study is to explore the effect of AGR on the clinical characteristics and prognosis of PLA patients.
Materials and Methods
Patients
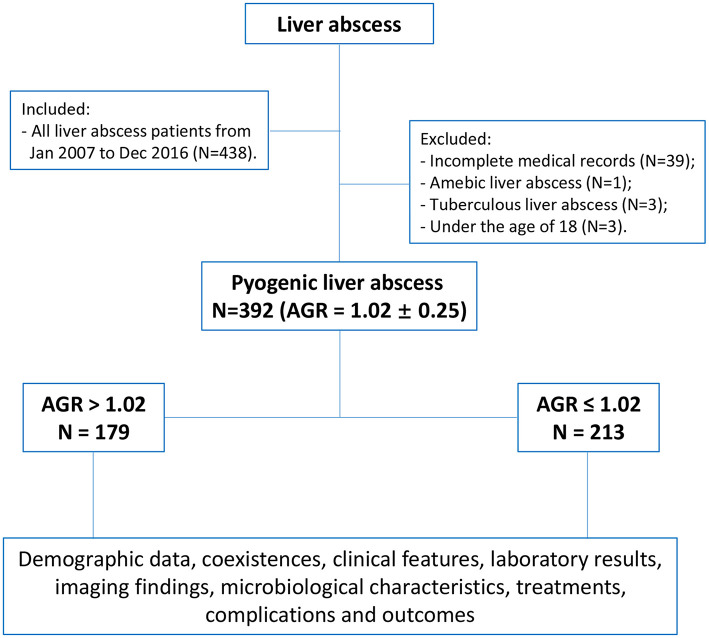
There were 438 patients were collected who diagnosed with liver abscess in the First Affiliated Hospital of Xi'an Jiaotong University during the 10 years from January 2007 to December 2016. The diagnosis of PLA was based on the clinical features, imaging and laboratory results, blood and pus cultures. The inclusion and exclusion criteria, and therapeutic criteria were described previously (22–24). Finally, 392 patients with pyogenic liver abscess were included in this study (Figure 1). This study was authorized by the ethic committee of the First Affiliated Hospital of Xi'an Jiaotong University (No. XJTU1AF2015LSL-057). Due to the nature of retrospective study, the patient informed consent was waived.
Figure 1.
Flow chart of the study.
Data Collection
The medical records on admission were collected, including baseline information (age, gender, height, weight, coexistences), clinical features (symptoms and signs), laboratory examinations (blood routine, liver function, kidney function, and coagulation function), imagological examination (the number, size, site of abscesses), microbiological characteristics (pus culture and blood culture), treatments (antibiotics alone, antibiotics combined with percutaneous drainage or surgical drainage), complications (systemic inflammatory response syndrome, pleural effusion, peritoneal effusion, acute respiratory distress syndrome, septic shock, spontaneous rupture of abscess, portal venous thrombosis, acute kidney injury), and outcomes (time for temperature normalization, length of hospital stay, and mortality in hospital). In addition, the AGR levels on day 1, 3, and 7 after admission and on discharge were collected.
Statistical Analysis
Categorical variables were presented in absolute values (%) and compared by the Chi-square test or the Fisher exact test. Continuous variables were presented as mean ± standard deviation (SD) or standard error (SE) and compared by the Students t-test. All statistical analyses were conducted in SPSS 22.0, and the two-tailed P < 0.05 was considered statistically significant.
Results
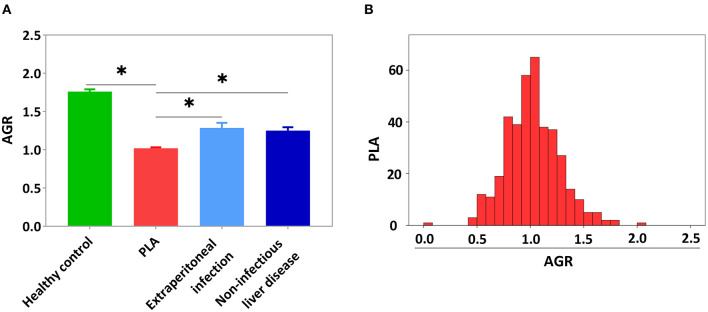
To explore the effect of PLA on serum AGR, the AGR levels of healthy controls (n = 50) and the patients with PLA (n = 392), extraperitoneal infection (n = 38) and non-infectious liver disease (n = 28) were collected and the three controls were matched with the PLA patients by age and gender (Supplementary Table 1, P > 0.05). However, other factors were not matched in this study. As showed in Supplementary Table 2, the most included extraperitoneal infection was pulmonary infection (26, 68.4%), and the most included non-infectious liver disease was hepatic hemangioma (16, 57.1%). Compared with healthy controls and patients with extraperitoneal infection or non-infectious liver disease, PLA patients had lower level of AGR (Figure 2A, P < 0.05,). There were 69.9% of PLA patients with an AGR between 0.8 and 1.3 (Figure 2B). The mean level of AGR in PLA patients was 1.02 ± 0.25. PLA patients were divided into low-AGR group (AGR ≤ 1.02) and high-AGR group (AGR > 1.02). There were 213 (54.3%) PLA patients in the low-AGR group, and 179 (45.7%) PLA patients in the high-AGR group. This cut-off value was similar to previous studies (25–27).
Figure 2.
The PLA patients had lower AGR level compared with the healthy controls and the patients with extraperitoneal infection or non-infectious liver disease. (A) The AGR levels in healthy controls and the patients with PLA, extraperitoneal infection and non-infectious liver disease. (B) The AGR distribution of PLA patients. Data were presented as the mean ± SE. Differences between the groups were compared by the One-way ANOVA. *P < 0.05.
Baseline Data
Demographic Data
As shown in the Table 1, the mean age of the patients was 57.8 ± 13.0 in the low-AGR group and 55.4 ± 13.5 in the high-AGR group. The ratio of males to females in the two groups was 108/71 and 115/98, respectively. The body mass index (BMI) of PLA patients was 22.6 ± 2.7 kg/m2. There was no statistically significance in age, gender, and BMI between the two groups (P > 0.05).
Table 1.
Baseline data of demographic data and coexistences.
| Total N = 392 | AGR > 1.02 N = 179 | AGR ≤ 1.02 N = 213 | P-value | |
|---|---|---|---|---|
| Age (years) | 56.8 ± 13.4 | 55.4 ± 13.5 | 57.8 ± 13.0 | 0.068 |
| Gender (Male/Female) | 223/169 | 108/71 | 115/98 | 0.206 |
| BMI (kg/m2) | 22.6 ± 2.7 | 22.7 ± 2.9 | 22.5 ± 2.6 | 0.731 |
| Coexistences ( n , %) | ||||
| History of smoking | 106 (27.0%) | 53 (29.6%) | 53 (24.9%) | 0.294 |
| History of alcohol | 65 (16.6%) | 36 (20.1%) | 29 (13.6%) | 0.085 |
| Hypertension | 77 (19.6%) | 37 (20.7%) | 40 (18.8%) | 0.639 |
| Diabetes mellitus | 124 (31.6%) | 49 (27.4%) | 75 (35.2%) | 0.097 |
| Type 2 diabetes | 109 (27.8%) | 40 (22.3%) | 69 (32.4%) | 0.080 |
| Type 1 diabetes | 2 (0.5%) | 1 (0.6%) | 1 (0.5%) | |
| Undefined | 13 (3.3%) | 8 (4.5%) | 5 (2.3%) | |
| Cirrhosis | 16 (4.1%) | 8 (4.5%) | 8 (3.8%) | 0.722 |
| Viral hepatitis | 29 (7.4%) | 15 (8.4%) | 14 (6.6%) | 0.496 |
| HBV | 21 (5.4%) | 14 (3.6%) | 7 (3.3%) | 0.055 |
| HCV | 6 (1.5%) | 0 (0) | 6 (2.8%) | |
| HBV/HCV | 2 (0.5%) | 1 (0.6%) | 1 (0.5%) | |
| Hepatobiliary malignant diseases | 44 (11.2%) | 15 (8.4%) | 29 (13.6%) | 0.102 |
| Primary hepatic carcinoma | 15 (3.8%) | 7 (3.9%) | 8 (3.8%) | 0.472 |
| Cholangiocarcinoma | 13 (3.3%) | 3 (1.7%) | 10 (4.7%) | |
| Gallbladder carcinoma | 10 (2.6%) | 3 (1.7%) | 7 (3.3%) | |
| Liver metastatic carcinoma | 6 (1.5%) | 2 (1.1%) | 4 (1.9%) | |
| Lithiasis | 150 (38.3%) | 68 (38.0%) | 82 (38.5%) | 0.918 |
| Cholecystolithiasis | 104 (26.5%) | 51 (28.5%) | 53 (24.9%) | 0.476 |
| Intrahepatic biliary stone | 17 (4.3%) | 5 (2.8%) | 12 (5.6%) | |
| Extrahepatic biliary stone | 12 (3.1%) | 5 (2.8%) | 7 (3.3%) | |
| Polylithiasis | 17 (4.3%) | 7 (3.9%) | 10 (4.7%) | |
| Abdominal surgery history | 177 (45.2%) | 79 (44.1%) | 98 (46.0%) | 0.710 |
| PLA history | 42 (10.7%) | 18 (10.1%) | 24 (11.3%) | 0.699 |
PLA, pyogenic liver abscess; AGR, Albumin/Globulin Ratio; HBV, hepatitis B virus; HCV, hepatitis C virus; BMI, body mass index.
Coexistences
There were no significant differences between the high-AGR group and the low-AGR group in terms of the coexistences, such as history of smoking and alcohol, hypertension, diabetes mellitus, cirrhosis, viral hepatitis, hepatobiliary malignant diseases, lithiasis, abdominal surgery history, and PLA history (Table 1, P > 0.05).
Clinical Features, Laboratory Results, and Imaging Findings
Symptoms and Signs
As shown in Table 2, in terms of clinical symptoms, the two groups had similar proportions of patients with fever, chills, abdominal pain, nausea, vomiting, fatigue (P > 0.05). In terms of the clinical signs, respiratory rate, heart rate, and mean arterial pressure were comparable between the two groups (P > 0.05). However, the low-AGR group had higher temperature at admission than the high-AGR group (P < 0.05).
Table 2.
Clinical features, laboratory results, and imaging findings.
| Total N = 392 | AGR > 1.02 N = 179 | AGR ≤ 1.02 N = 213 | P-value | |
|---|---|---|---|---|
| Symptoms and signs (n, % or mean ± SD) | ||||
| Fever | 340 (87.2%) | 150 (83.8%) | 190 (89.2%) | 0.116 |
| Chill | 197 (50.3%) | 91 (50.8%) | 106 (49.8%) | 0.832 |
| Abdominal pain | 172 (43.9%) | 75 (41.9%) | 97 (45.5%) | 0.469 |
| Nausea | 91 (23.2%) | 38 (21.2%) | 53 (24.9%) | 0.393 |
| Vomit | 60 (16.3%) | 25 (14.0%) | 35 (16.4%) | 0.499 |
| Fatigue | 69 (17.6%) | 25 (14.0%) | 44 (20.7%) | 0.083 |
| Temperature (°C) | 37.3 ± 1.1 | 37.2 ± 1.1 | 37.4 ± 1.0 | 0.048 |
| Respiratory rate (/min) | 19.8 ± 1.8 | 19.9 ± 1.9 | 19.8 ± 1.7 | 0.854 |
| Heart rate (/min) | 85.1 ± 12.9 | 84.5 ± 13.5 | 85.6 ± 12.5 | 0.387 |
| Mean arterial pressure (mmHg) | 90.5 ± 25.3 | 92.2 ± 27.6 | 89.2 ± 23.2 | 0.247 |
| Laboratory results (mean ± SD) | ||||
| Leucocytes ( × 109/L) | 11.2 ± 5.7 | 10.2 ± 5.3 | 12.0 ± 6.0 | 0.002 |
| Neutrophils ( × 109/L) | 9.1 ± 5.5 | 8.3 ± 5.1 | 9.8 ± 5.7 | 0.007 |
| Lymphocytes ( × 109/L) | 1.3 ± 0.6 | 1.3 ± 0.7 | 1.2 ± 0.6 | 0.351 |
| Hemoglobin (g/L) | 111.6 ± 19.3 | 117.3 ± 17.7 | 106.9 ± 19.4 | <0.001 |
| Platelet count ( × 109/L) | 223.7 ± 125.3 | 221.1 ± 125.2 | 225.9 ± 125.7 | 0.705 |
| ALT (U/L) | 63.9 ± 98.7 | 64.5 ± 71.5 | 63.4 ± 117.1 | 0.912 |
| AST (U/L) | 54.9 ± 129.1 | 49.1 ± 48.8 | 59.8 ± 169.6 | 0.415 |
| ALP (U/L) | 195.0 ± 134.5 | 158.4 ± 103.1 | 225.9 ± 149.6 | <0.001 |
| GGT (U/L) | 168.7 ± 156.5 | 143.8 ± 138.6 | 189.7 ± 167.6 | 0.003 |
| TBIL (μmol/L) | 20.9 ± 24.7 | 21.7 ± 30.5 | 20.3 ± 18.6 | 0.587 |
| DBIL (μmol/L) | 11.2 ± 17.4 | 11.3 ± 20.9 | 11.1 ± 13.7 | 0.902 |
| Cr (umol/L) | 67.1 ± 47.4 | 69.6 ± 50.4 | 65.0 ± 44.7 | 0.345 |
| BUN (mmol/L) | 5.2 ± 3.1 | 5.0 ± 2.7 | 5.3 ± 3.3 | 0.337 |
| PT (s) | 14.5 ± 1.8 | 14.1 ± 1.4 | 14.8 ± 2.1 | <0.001 |
| APTT (s) | 39.0 ± 5.9 | 38.0 ± 4.9 | 39.7 ± 6.6 | 0.004 |
| INR | 1.16 ± 0.20 | 1.12 ± 0.12 | 1.19 ± 0.21 | <0.001 |
| FIB (g/L) | 5.9 ± 1.9 | 5.8 ± 1.8 | 6.0 ± 1.9 | 0.226 |
| Imaging findings (n, % or mean ± SD) | ||||
| Gas formation | 66 (16.8%) | 23 (12.8%) | 43 (20.2%) | 0.053 |
| Abscess number | ||||
| Single lesion | 291 (74.2%) | 136 (76.0%) | 155 (72.8%) | 0.469 |
| Multiple lesions | 101 (25.8%) | 43 (24.0%) | 58 (27.2%) | |
| Maximal diameter of abscess (cm) | 6.7 ± 2.8 | 6.0 ± 2.5 | 7.3 ± 3.0 | <0.001 |
| Abscess site | N = 345 | N = 154 | N = 191 | |
| Left lobe | 46 (13.3%) | 23 (14.9%) | 23 (12.0%) | 0.555 |
| Right lobe | 255 (73.9%) | 114 (74.0%) | 141 (73.8%) | |
| BOTH-LOBES | 44 (12.8%) | 17 (11.0%) | 27 (14.1%) | |
PLA, pyogenic liver abscess; AGR, Albumin/Globulin Ratio; ALT, alanine transaminase; AST, aspartate transaminase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transpeptidase; TBIL, total bilirubin; DBIL, direct bilirubin; Cr, creatinine; BUN, blood urea nitrogen; PT, prothrombin time; APTT, activated partial thromboplastin time; INR, international normalized ratio; FIB, fibrinogen. Bold values: P < 0.05.
Laboratory Results
The blood routine, liver function, kidney function, and coagulation function on admission were collected and showed in Table 2. Compared with the high-AGR group, the low-AGR group had higher leukocytes, neutrophils, alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT), international standardized ratio (INR), longer prothrombin time (PT), activated partial thromboplastin time (APTT), and lower hemoglobin (P < 0.05), which means that the PLA patients in low-AGR group had more severe infection, poorer liver function, and coagulation function.
Imaging Finding
Abdominal computed tomography (CT)/ultrasound (US) was collected to determine the gas formation, number, size and site of abscesses and the results were showed in Table 2. Gas formation of abscess was comparative between the two groups (23, 12.8%/43, 20.2%, P > 0.05). In terms of the abscess number, single lesion was common in both groups and there was no statistical difference in the abscess number (136, 76.0%/155, 72.8%, P > 0.05). Abscesses in the right lobe were common and did not differ between the two groups (114, 74.0%/141, 73.8%, P > 0.05). However, the maximal diameter of abscess in low-AGR group was larger than that in high-AGR group (7.3 ± 3.0/6.0 ± 2.5, cm, P < 0.05).
Microbiological Characteristics
Pus Culture
The results of pus culture from 231 PLA patients were presented in Table 3. Klebsiella pneumoniae was the most common pathogen in both groups (40, 33.6%/39, 34.8%, P > 0.05). However, the positive rate of pus culture (87, 77.7%/67, 56.3%) and the proportion of Escherichia coli (21, 18.8%/3, 2.5%) were higher in low-AGR group (P < 0.05), and patients in low-AGR group were more susceptible to the infection of multiple bacteria (15, 13.4%/5, 4.2%, P < 0.05).
Table 3.
Microbiological characteristics.
| Total | AGR > 1.02 | AGR ≤ 1.02 | P-value | |
|---|---|---|---|---|
| Pus culture ( n , %) | N = 231 | N = 119 | N = 112 | |
| Klebsiella pneumoniae | 79 (34.2%) | 40 (33.6%) | 39 (34.8%) | 0.956 |
| Escherichia coli | 24 (10.4%) | 3 (2.5%) | 21 (18.8%) | <0.001 |
| Enterococcus | 7 (3.0%) | 4 (3.4%) | 3 (2.7%) | 1 |
| Streptococcus | 8 (3.5%) | 5 (4.2%) | 3 (2.7%) | 0.723 |
| Staphylococcus | 4 (1.7%) | 2 (1.7%) | 2 (1.8%) | 1 |
| Aerogen (Enteroaerogen) | 1 (0.4%) | 1 (0.8%) | 0 (0) | 1 |
| Other | 11 (4.8%) | 7 (5.9%) | 4 (3.6%) | 0.410 |
| Multiple bacteria | 20 (8.7%) | 5 (4.2%) | 15 (13.4%) | 0.013 |
| No growth | 77 (33.3%) | 52 (43.7%) | 25 (22.3%) | 0.001 |
| Blood culture ( n , %) | N = 163 | N = 74 | N = 89 | |
| Klebsiella pneumoniae | 14 (8.6%) | 7 (9.5%) | 7 (7.9%) | 0.718 |
| Escherichia coli | 8 (4.9%) | 1 (1.4%) | 7 (7.9%) | 0.073 |
| Enterococcus | 2 (1.2%) | 1 (1.4%) | 1 (1.1%) | 1 |
| Streptococcus | 4 (2.5%) | 2 (2.7%) | 2 (2.2%) | 1 |
| Staphylococcus | 4 (2.5%) | 1 (1.4%) | 3 (3.4%) | 0.627 |
| Aerogen (Clostridium perfringens) | 1 (0.6%) | 1 (1.4%) | 0 (0) | 0.454 |
| Other | 5 (3.1%) | 3 (4.1%) | 2 (2.2%) | 0.660 |
| Multiple bacteria | 6 (3.7%) | 1 (1.4%) | 5 (5.6%) | 0.222 |
| No growth | 119 (73.0%) | 57 (77.0%) | 62 (69.7%) | 0.292 |
AGR, Albumin/Globulin Ratio. Bold values: P < 0.05.
Blood Culture
According to the analysis of 163 PLA patients whose samples were sent for blood culture, Klebsiella pneumoniae was the most common pathogens in both groups (7, 9.5%/7, 7.9%, Table 3, P > 0.05), which was consistent with the result of pus culture. In addition, the two groups had similar positive rate and composition of positive bacteria in blood culture (Table 3, P > 0.05).
Treatments, Complications, and Outcomes
Treatments
Patients in both groups were mainly treated with antibiotics combined with percutaneous drainage (85, 47.5%/119, 55.9%), followed by antibiotics alone (51, 28.5%/56, 26.3%), and antibiotics combined with surgical drainage was used in the least number of PLA patients (43, 24.0%/38, 17.8%). There was no statistical difference in the choice of treatments (Table 4, P > 0.05).
Table 4.
Treatments, complications, and outcomes.
| Total N = 392 | AGR > 1.02 N = 179 | AGR ≤ 1.02 N = 213 | P-value | |
|---|---|---|---|---|
| Treatments ( n , %) | ||||
| Antibiotics alone | 107 (27.3%) | 51 (28.5%) | 56 (26.3%) | 0.194 |
| Antibiotics combined with percutaneous drainage | 204 (52.0%) | 85 (47.5%) | 119 (55.9%) | |
| Antibiotics combined with surgical drainage | 81 (20.7%) | 43 (24%) | 38 (17.8%) | |
| Complications ( n , %) | ||||
| SIRS | 140 (35.7%) | 51 (28.5%) | 89 (41.8%) | 0.006 |
| Pleural effusion | 138 (35.2%) | 48 (26.8%) | 90 (42.3%) | 0.001 |
| Peritoneal effusion | 51 (13.0%) | 15 (8.4%) | 36 (16.9%) | 0.012 |
| ARDS | 4 (1.0%) | 2 (1.1%) | 2 (0.9%) | 1 |
| Septic shock | 3 (0.8%) | 1 (0.6%) | 2 (0.9%) | 1 |
| Spontaneous rupture of abscess | 3 (0.8%) | 3 (1.7%) | 0 (0) | 0.094 |
| Portal venous thrombosis | 3 (0.8%) | 1 (0.6%) | 2 (0.9%) | 1 |
| AKI | 1 (0.3%) | 0 (0) | 1 (0.5%) | 1 |
| Outcomes (% or mean ± SD) | ||||
| Time for temperature normalization (days) | 7.4 ± 6.3 | 6.7 ± 6.2 | 8.0 ± 6.1 | 0.045 |
| Length of hospital stay (days) | 16.8 ± 9.4 | 15.5 ± 8.6 | 17.9 ± 9.9 | 0.009 |
| Mortality in hospital | 0 | 0 | 0 | 1 |
PLA, pyogenic liver abscess; AGR, Albumin/Globulin Ratio; SIRS, systemic inflammatory response syndrome; ARDS, acute respiratory distress syndrome; AKI, acute kidney injury. Bold values: P < 0.05.
Complications
The common complications are listed in Table 4, systemic inflammatory response syndrome (SIRS) (51, 28.5%/89, 41.8%), pleural effusion (48, 26.8%/90, 42.3%), peritoneal effusion (15, 8.4%/36, 16.9%) were the most common complications in two groups. However, the number of patients with SIRS, pleural effusion, peritoneal effusion was higher in low-AGR group than that in high-AGR group (P < 0.05). There was no statistically difference between the two groups in other less common complications, such as acute respiratory distress syndrome (ARDS), septic shock, spontaneous rupture of abscess, portal venous thrombosis, and acute kidney injury (AKI) (P > 0.05).
Outcomes
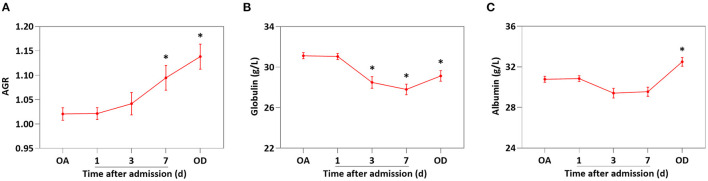
Table 4 showed the analysis of patients' outcomes. Specifically speaking, the low-AGR group needed longer to temperature normalization and longer hospital stay than that in high-AGR group (P < 0.05). There were no in-hospital deaths in either group. In additon, the AGR level of PLA patients was increased gradually after treatment (Figure 3A, P < 0.05 vs. AGR level on admission). However, the decrease of globulin (day 3 after admission, Figure 3B) was significantly earlier than the increase of albumin (on discharge, Figure 3C).
Figure 3.
The AGR level was increased gradually after treatment in PLA patients. The levels of AGR (A), globulin (B), and albumin (C) on day 1, 3, and 7 after admission and on discharge. Data were presented as the mean ± SE. Differences between the groups were compared by the One-way ANOVA. *P < 0.05. OA, on admission; OD, on discharge.
Discussion
In this study, the PLA patients had lower AGR level, compared with the healthy controls and the patients with extraperitoneal infection and non-infectious liver disease. The 392 PLA patients were divided into high-AGR group and low-AGR group according to the mean of AGR 1.02. There were 179 (45.4%) patients in the high-AGR group and 213 (54.6%) patients in the low-AGR group. The baseline data were consistent between the two groups. However, the low-AGR group had more serious infections, lower hemoglobin, worse liver function and coagulation function than high-AGR group. In addition, the maximal diameter of abscess in the low-AGR group was larger than that in the high-AGR group. On the other hand, the low-AGR group had higher positive rate, proportion of Escherichia coli and proportion of multiple bacteria. The treatments of the two groups were comparable, but the patients in low-AGR group were more likely to have complications such as SIRS, pleural effusion and peritoneal effusion. The low-AGR group also needed longer to temperature normalization and longer hospital stay than that in the high-AGR group.
PLA is one of the largest solid abscesses in the human body, it has multiple etiologies, which have been changed recently (1). Biliary diseases, including biliary stones, biliary tumors and biliary strictures, have replaced appendicitis as the most common cause of PLA (28, 29). Meanwhile, PLA with cryptogenic infection are also increasing gradually (30). Albumin/globulin ratio (AGR) is often used to guide treatment and predict prognosis in a variety of diseases. In cancers, AGR is a potential prognostic biomarker for several cancers, such as oral cavity cancer, lung cancers, nasopharyngeal cancers, renal cell carcinoma, and gastric cancers (31–35). In addition, AGR also plays a role in non-neoplastic diseases. Serum levels of ALB and GLB can be used in combination to predict the survival of patients with heart failure (18). The low AGR is associated with the significantly increased risk for subsequent vascular events in stroke patients and in subjects with clinical risk factors for stroke (17). In chronic kidney disease, AGR was suggested a simple and inexpensive measurement for detecting the risk of mortality (20). AGR has the potential to predict all-cause and cardiovascular mortality in patients on peritoneal dialysis (21). However, the effect of AGR on the clinical characteristic and prognosis in PLA patients remains unknown.
Liver is the body's albumin synthesis organ and globulin is associated with inflammation. PLA could affect the production of both albumin and globulin. In contrast, extra-abdominal infection mainly caused the increase of globulin and non-infectious liver disease mainly caused the decrease of albumin. Our results showed that AGR in both groups was lower than healthy controls, but AGR in extra-peritoneal infection and non-infectious liver disease was higher than PLA patients. This demonstrated the importance of AGR in PLA.
In the study, PLA patients was divided into two groups based on the levels of AGR. PLA patients in low-AGR group had higher body temperature, leukocytes and neutrophils on admission, which were the indication of poor prognosis (36). Moreover, the patients in low-AGR group had higher ALP, GGT, INR, longer PT, APTT, which indicating that the patients in the low-AGR group had more severe liver injury and inflammation, and worse liver function. This is not surprisingly that albumin is synthesized and secreted by hepatocytes and globulin is associated with inflammation. Patients with low AGR often have lower albumin and higher globulin, which predicts more severe liver injury and inflammation, and worse liver function. On the other hand, patients in low-AGR group had lower hemoglobin. Research has shown that hemoglobin was one of the independent prognostic factors of PLA (37). Furthermore, several studies have explored the effect of gas formation on PLA (38, 39) and gas formation of abscess was comparative between the two groups in our study. In addition, we found that the abscess diameter of the patients in the low-AGR group was significantly larger than that in the high-AGR group. Our previous study found that the larger abscess always accompanies with higher leukocytes, lower albumin, and longer time for temperature normalization (40).
With regard to microbiological characteristics, Klebsiella pneumoniae and Escherichia coli were still the most common pathogenic bacteria and Klebsiella pneumoniae has gradually take the place of Escherichia coli as the main pathogenic bacteria of PLA (41). In this study, Klebsiella pneumoniae was the main pathogenic bacteria, which was consistently with a previous study (42). A higher positive rate, proportion of Escherichia coli and susceptibility to multiple bacteria in pus culture were observed in the low-AGR group. In general, the factors, such as positive rate of bacterial culture and infections of Escherichia coli or multiple bacteria, always predicted a worse prognosis for PLA patients (1, 43). In addition, PLA with Aerogen infection may cause acute cholecystitis (44), however, there was no difference between the two groups.
In terms of treatments, sensitive antibiotics should be used as the preferred treatment, and combined with percutaneous drainage or surgical drainage if necessary (45). Percutaneous drainage is regarded as the preferred drainage due to its advantages of fewer complications, lower mortality, anesthetic risk, cost and shorter hospital stay (1, 46–48). Surgical drainage can be selected in the case of failure of percutaneous drainage, abscess diameter > 5 cm or multiple lesions (45, 49). In this study, antibiotics combined with percutaneous drainage was the most common treatment and the treatments were similar in both groups. Eighty-One (20.7%) PLA patients were treated with surgical drainage, which was a high percentage compared with a previous study (50). A possible reason is that the different standard (e.g., smaller abscess size) for surgical drainage was used in our study.
Many researches have shown that the low AGR is often associated with the poorer prognosis in many diseases (51–53). In the present study, although there were no in-hospital deaths in either group, patients in the low-AGR group had more complications and needed longer time for temperature normalization and hospital stay. This indicated that patients in the low-AGR group had a worse prognosis than those in the high-AGR group, which was consistent with the above results. Meanwhile, SIRS is a prevalent feature of patients with sepsis and SIRS has high sensitivity in predicting intensive care unit (ICU) admission and morbidity (54, 55). Thus, more attention needs to be paid to the low-AGR group of PLA patients. In addition, the AGR level of PLA patients was increased gradually after treatment. Therefore, AGR may be a prognostic factor for PLA patients. However, due to the retrospective nature and its inherent biases of this study, we cannot prove a cause-and-effect relationship of AGR on PLA patients, further prospective studies and larger multicenter studies are still needed to verify it. Specifically, it would be interesting to investigate if a patient, with more severe disease (lower AGR) would benefit from a more aggressive treatment in a prospective study.
In conclusion, PLA patients in low-AGR group are associated with worse clinical manifestations, more complications and poorer prognosis. AGR level may be used to measure severity and predict prognosis in PLA patients. Monitoring of AGR is of great clinical significance for the treatment and prognosis of PLA patients.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This study was authorized by the ethic committee of the First Affiliated Hospital of Xi'an Jiaotong University (No. XJTU1AF2015LSL-057). Due to the nature of retrospective study, the patient informed consent was waived.
Author Contributions
RW and JZhang designed the research. JZhang and TW wrote the manuscript. JZhang, TW, YF, MW, WL, and JZhao collected the data. JZhang, TW, and RW analyzed the data. BW, ZW, and YL interpreted the data. RW supervised the study and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Innovation Capacity Support Plan of Shaanxi Province (Grant No. 2020TD-040).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer JZ declared a shared affiliation, with no collaboration, with the authors to the handling editor at the time of the review.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
Abbreviations
- PLA
pyogenic liver abscess
- ALB
albumin
- GLO
globulin
- AGR
Albumin/Globulin Ratio
- CT
computed tomography
- US
ultrasound
- SD
standard deviation
- ALT
alanine transaminase
- AST
aspartate transaminase
- ALP
alkaline phosphatase
- GGT
gamma-glutamyl transpeptidase
- TBIL
total bilirubin
- DBIL
direct bilirubin
- Cr
creatinine
- BUN
blood urea nitrogen
- PT
prothrombin time
- APTT
activated partial thromboplastin time
- INR
international normalized ratio
- FIB
fibrinogen
- SIRS
systemic inflammatory response syndrome
- ARDS
acute respiratory distress syndrome
- AKI
acute kidney injury
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- BMI
body mass index
- ICU
intensive care unit.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2021.677799/full#supplementary-material
References
- 1.Chattopadhyay B. Pyogenic liver abscess. J Infect. (1983) 6:5–12. 10.1016/S0163-4453(83)95356-2 [DOI] [PubMed] [Google Scholar]
- 2.Chen YC, Lin CH, Chang SN, Shi ZY. Epidemiology and clinical outcome of pyogenic liver abscess: an analysis from the national health insurance research database of Taiwan, 2000-2011. J Microbiol Immunol Infect. (2016) 49:646–53. 10.1016/j.jmii.2014.08.028 [DOI] [PubMed] [Google Scholar]
- 3.Xu J, Zhou X, Zheng C. The geriatric nutritional risk index independently predicts adverse outcomes in patients with pyogenic liver abscess. BMC Geriatr. (2019) 19:14. 10.1186/s12877-019-1030-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomsen RW, Jepsen P, Sorensen HT. Diabetes mellitus and pyogenic liver abscess: risk and prognosis. Clin Infect Dis. (2007) 44:1194–201. 10.1086/513201 [DOI] [PubMed] [Google Scholar]
- 5.Du Z, Zhou X, Zhao J, Bi J, Ren Y, Zhang J, et al. Effect of diabetes mellitus on short-term prognosis of 227 pyogenic liver abscess patients after hospitalization. BMC Infect Dis. (2020) 20:145. 10.1186/s12879-020-4855-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heneghan HM, Healy NA, Martin ST, Ryan RS, Nolan N, Traynor O, et al. Modern management of pyogenic hepatic abscess: a case series and review of the literature. BMC Res Notes. (2011) 4:80. 10.1186/1756-0500-4-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alvarez Perez JA, Gonzalez JJ, Baldonedo RF, Sanz L, Carreno G, Junco A, et al. Clinical course, treatment, and multivariate analysis of risk factors for pyogenic liver abscess. Am J Surg. (2001) 181:177–86. 10.1016/S0002-9610(00)00564-X [DOI] [PubMed] [Google Scholar]
- 8.Friedman AN, Fadem SZ. Reassessment of albumin as a nutritional marker in kidney disease. J Am Soc Nephrol. (2010) 21:223–30. 10.1681/ASN.2009020213 [DOI] [PubMed] [Google Scholar]
- 9.Ferrie S, Allman-Farinelli M. Commonly used “nutrition” indicators do not predict outcome in the critically ill: a systematic review. Nutr Clin Pract. (2013) 28:463–84. 10.1177/0884533613486297 [DOI] [PubMed] [Google Scholar]
- 10.Kim S, Mcclave SA, Martindale RG, Miller KR, Hurt RT. Hypoalbuminemia and clinical outcomes: what is the mechanism behind the relationship? Am Surg. (2017) 83:1220–7. 10.1177/000313481708301123 [DOI] [PubMed] [Google Scholar]
- 11.Wada Y, Takeda Y, Kuwahata M. Potential role of amino acid/protein nutrition and exercise in serum albumin redox state. Nutrients. (2017) 10:17. 10.3390/nu10010017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Stijn MF, Korkic-Halilovic I, Bakker MS, Van Der Ploeg T, Van Leeuwen PA, Houdijk AP. Preoperative nutrition status and postoperative outcome in elderly general surgery patients: a systematic review. JPEN J Parenter Enteral Nutr. (2013) 37:37–43. 10.1177/0148607112445900 [DOI] [PubMed] [Google Scholar]
- 13.Li S, Zhang J, Zheng H, Wang X, Liu Z, Sun T. Prognostic role of serum albumin, total lymphocyte count, and mini nutritional assessment on outcomes after geriatric hip fracture surgery: a meta-analysis and systematic review. J Arthroplasty. (2019) 34:1287–96. 10.1016/j.arth.2019.02.003 [DOI] [PubMed] [Google Scholar]
- 14.Liao CH, Li HY, Yu HJ, Chiang HS, Lin MS, Hua CH, et al. Low serum sex hormone-binding globulin: marker of inflammation? Clin Chim Acta. (2012) 413:803–7. 10.1016/j.cca.2012.01.021 [DOI] [PubMed] [Google Scholar]
- 15.Hill LA, Bodnar TS, Weinberg J, Hammond GL. Corticosteroid-binding globulin is a biomarker of inflammation onset and severity in female rats. J Endocrinol. (2016) 230:215–25. 10.1530/JOE-16-0047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer EJ, Nenke MA, Rankin W, Lewis JG, Torpy DJ. Corticosteroid-Binding globulin: a review of basic and clinical advances. Horm Metab Res. (2016) 48:359–71. 10.1055/s-0042-108071 [DOI] [PubMed] [Google Scholar]
- 17.Beamer N, Coull BM, Sexton G, De Garmo P, Knox R, Seaman G. Fibrinogen and the albumin-globulin ratio in recurrent stroke. Stroke. (1993) 24:1133–9. 10.1161/01.STR.24.8.1133 [DOI] [PubMed] [Google Scholar]
- 18.Li K, Fu W, Bo Y, Zhu Y. Effect of albumin-globulin score and albumin to globulin ratio on survival in patients with heart failure: a retrospective cohort study in China. BMJ Open. (2018) 8:e022960. 10.1136/bmjopen-2018-022960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lv GY, An L, Sun XD, Hu YL, Sun DW. Pretreatment albumin to globulin ratio can serve as a prognostic marker in human cancers: a meta-analysis. Clin Chim Acta. (2018) 476:81–91. 10.1016/j.cca.2017.11.019 [DOI] [PubMed] [Google Scholar]
- 20.Wu PP, Hsieh YP, Kor CT, Chiu PF. Association between albumin-globulin ratio and mortality in patients with chronic kidney disease. J Clin Med. (2019) 8:1991. 10.3390/jcm8111991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng F, Sun L, Chen T, Zhu Y, Zhou W, Li P, et al. Albumin-globulin ratio and mortality in patients on peritoneal dialysis: a retrospective study. BMC Nephrol. (2020) 21:51. 10.1186/s12882-020-1707-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Du Z, Bi J, Wu Z, Lv Y, Zhang X, et al. The impact of previous abdominal surgery on clinical characteristics and prognosis of pyogenic liver abscess: a 10-year retrospective study of 392 patients. Medicine. (2018) 97:e12290. 10.1097/MD.0000000000012290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Du Z, Bi J, Wu Z, Lv Y, Zhang X, et al. Comparison of clinical characteristics and outcomes of pyogenic liver abscess patients <65 years of age versus >/= 65 years of age. BMC Infect Dis. (2019) 19:233. 10.1186/s12879-019-3837-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Gao Y, Du Z, Ren Y, Bi J, Wu Z, et al. Clinical features and prognosis of gas-forming and non-gas-forming pyogenic liver abscess: a comparative study. Surg Infect. (2021) 22:427–33. 10.1089/sur.2020.245 [DOI] [PubMed] [Google Scholar]
- 25.Swarup D, Upadhyay DS, Pachauri SP. Some biochemical indices in naturally occurring fascioliasis in goats. Res Vet Sci. (1986) 40:276–7. 10.1016/S0034-5288(18)30528-9 [DOI] [PubMed] [Google Scholar]
- 26.Xue F, Lin F, Yin M, Feng N, Zhang X, Cui YG, et al. Preoperative albumin/globulin ratio is a potential prognosis predicting biomarker in patients with resectable gastric cancer. Turk J Gastroenterol. (2017) 28:439–45. 10.5152/tjg.2017.17167 [DOI] [PubMed] [Google Scholar]
- 27.Lu J, Xun Y, Yu X, Liu Z, Cui L, Zhang J, et al. Albumin-globulin ratio: a novel predictor of sepsis after flexible ureteroscopy in patients with solitary proximal ureteral stones. Transl Androl Urol. (2020) 9:1980–9. 10.21037/tau-20-823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Longworth S, Han J. Pyogenic liver abscess. Clin Liver Dis. (2015) 6:51–4. 10.1002/cld.487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qian Y, Wong CC, Lai S, Chen H, He X, Sun L, et al. A retrospective study of pyogenic liver abscess focusing on Klebsiella pneumoniae as a primary pathogen in China from 1994 to 2015. Sci Rep. (2016) 6:38587. 10.1038/srep38587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu X, Wang S, Jacob R, Fan Z, Zhang F, Ji G. A 10-year retrospective analysis of clinical profiles, laboratory characteristics and management of pyogenic liver abscesses in a chinese hospital. Gut Liver. (2011) 5:221–7. 10.5009/gnl.2011.5.2.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gundog M, Basaran H. Pretreatment low prognostic nutritional index and low albumin-globulin ratio are predictive for overall survival in nasopharyngeal cancer. Eur Arch Otorhinolaryngol. (2019) 276:3221–30. 10.1007/s00405-019-05595-2 [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Li S, Hu X, Wang Y, Wu Y, Li P, et al. The prognostic value of serum albumin-globulin ratio in early-stage non-small cell lung cancer: a retrospective study. Cancer Manag Res. (2019) 11:3545–54. 10.2147/CMAR.S191288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung JW, Park DJ, Chun SY, Choi SH, Lee JN, Kim BS, et al. The prognostic role of preoperative serum albumin/globulin ratio in patients with non-metastatic renal cell carcinoma undergoing partial or radical nephrectomy. Sci Rep. (2020) 10:11999. 10.1038/s41598-020-68975-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang YT, Fang KH, Hsu CM, Chang GH, Lai CH, Lee YC, et al. Retrospective study on the potential of albumin/globulin ratio as a prognostic biomarker for oral cavity cancer patients. Eur Arch Otorhinolaryngol. (2020) 278:227–38. 10.1007/s00405-020-06145-x [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Zhu JY, Zhou LN, Tang M, Chen MB, Tao M. Predicting the prognosis of gastric cancer by albumin/globulin ratio and the prognostic nutritional index. Nutr Cancer. (2020) 72:635–44. 10.1080/01635581.2019.1651347 [DOI] [PubMed] [Google Scholar]
- 36.Park KS, Lee SH, Yun SJ, Ryu S, Kim K. Neutrophil-to-lymphocyte ratio as a feasible prognostic marker for pyogenic liver abscess in the emergency department. Eur J Trauma Emerg Surg. (2019) 45:343–51. 10.1007/s00068-018-0925-8 [DOI] [PubMed] [Google Scholar]
- 37.Mischinger HJ, Hauser H, Rabl H, Quehenberger F, Werkgartner G, Rubin R, et al. Pyogenic liver abscess: studies of therapy and analysis of risk factors. World J Surg. (1994) 18:852–7; discussion 858. 10.1007/BF00299085 [DOI] [PubMed] [Google Scholar]
- 38.Chan KS, Thng CB, Chan YH, Shelat VG. Outcomes of gas-forming pyogenic liver abscess are comparable to non-gas-forming pyogenic liver abscess in the era of multi-modal care: a propensity score matched study. Surg Infect. (2020) 21:884–90. 10.1089/sur.2019.278 [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Gao Y, Du Z, Ren Y, Bi J, Wu Z, et al. Clinical features and prognosis of gas-forming and non-gas-forming pyogenic liver abscess: a comparative study. Surg Infect. (2021) 22:427–33. 10.21203/rs.2.24160/v1 [DOI] [PubMed] [Google Scholar]
- 40.Du ZQ, Zhang LN, Lu Q, Ren YF, Lv Y, Liu XM, et al. Clinical charateristics and outcome of pyogenic liver abscess with different size: 15-year experience from a single center. Sci Rep. (2016) 6:35890. 10.1038/srep35890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rahimian J, Wilson T, Oram V, Holzman RS. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis. (2004) 39:1654–9. 10.1086/425616 [DOI] [PubMed] [Google Scholar]
- 42.Shelat VG, Wang Q, Chia CL, Wang Z, Low JK, Woon WW. Patients with culture negative pyogenic liver abscess have the same outcomes compared to those with Klebsiella pneumoniae pyogenic liver abscess. Hepatobiliary Pancreat Dis Int. (2016) 15:504–11. 10.1016/S1499-3872(16)60127-3 [DOI] [PubMed] [Google Scholar]
- 43.Chen SC, Yen CH, Lai KC, Tsao SM, Cheng KS, Chen CC, et al. Pyogenic liver abscesses with Escherichia coli: etiology, clinical course, outcome, prognostic factors. Wien Klin Wochenschr. (2005) 117:809–15. 10.1007/s00508-005-0481-1 [DOI] [PubMed] [Google Scholar]
- 44.Teng TZJ, Shelat VG. Aeromonas caviae and Aeromonas veronii causing acute cholecystitis. Surg Infect. (2021) 22:873–4. 10.1089/sur.2020.474 [DOI] [PubMed] [Google Scholar]
- 45.Lardiere-Deguelte S, Ragot E, Amroun K, Piardi T, Dokmak S, Bruno O, et al. Hepatic abscess: diagnosis and management. J Visc Surg. (2015) 152:231–43. 10.1016/j.jviscsurg.2015.01.013 [DOI] [PubMed] [Google Scholar]
- 46.Ch Yu S, Hg Lo R, Kan PS, Metreweli C. Pyogenic liver abscess: treatment with needle aspiration. Clin Radiol. (1997) 52:912–6. 10.1016/S0009-9260(97)80223-1 [DOI] [PubMed] [Google Scholar]
- 47.Vogl TJ, Estifan F. [Pyogenic liver abscess: interventional versus surgical therapy: technique, results and indications]. Rofo. (2001) 173:663–7. 10.1055/s-2001-15845 [DOI] [PubMed] [Google Scholar]
- 48.Yu SC, Ho SS, Lau WY, Yeung DT, Yuen EH, Lee PS, et al. Treatment of pyogenic liver abscess: prospective randomized comparison of catheter drainage and needle aspiration. Hepatology. (2004) 39:932–8. 10.1002/hep.20133 [DOI] [PubMed] [Google Scholar]
- 49.Shelat VG, Chia CL, Yeo CS, Qiao W, Woon W, Junnarkar SP. Pyogenic liver abscess: does Escherichia coli cause more adverse outcomes than klebsiella pneumoniae? World J Surg. (2015) 39:2535–42. 10.1007/s00268-015-3126-1 [DOI] [PubMed] [Google Scholar]
- 50.Ahmed S, Chia CL, Junnarkar SP, Woon W, Shelat VG. Percutaneous drainage for giant pyogenic liver abscess–is it safe and sufficient? Am J Surg. (2016) 211:95–101. 10.1016/j.amjsurg.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 51.Kumar P. Grading of severity of the condition in burn patients by serum protein and albumin/globulin studies. Ann Plast Surg. (2010) 65:74–9. 10.1097/SAP.0b013e3181c47d71 [DOI] [PubMed] [Google Scholar]
- 52.Chi J, Xie Q, Jia J, Liu X, Sun J, Chen J, et al. prognostic value of albumin/globulin ratio in survival and lymph node metastasis in patients with cancer: a systematic review and meta-analysis. J Cancer. (2018) 9:2341–8. 10.7150/jca.24889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qin J, Qin Y, Wu Y, Wei A, Luo M, Liao L, et al. Application of albumin/globulin ratio in elderly patients with acute exacerbation of chronic obstructive pulmonary disease. J Thorac Dis. (2018) 10:4923–30. 10.21037/jtd.2018.07.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simpson SQ. SIRS in the time of sepsis-3. Chest. (2018) 153:34–8. 10.1016/j.chest.2017.10.006 [DOI] [PubMed] [Google Scholar]
- 55.Mak MHW, Low JK, Junnarkar SP, Huey TCW, Shelat VG. A prospective validation of sepsis-3 guidelines in acute hepatobiliary sepsis: qSOFA lacks sensitivity and SIRS criteria lacks specificity (cohort study). Int J Surg. (2019) 72:71–7. 10.1016/j.ijsu.2019.10.022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.