Abstract
In breast cancer, tumor-associated macrophages with activated phenotypes promote tumor invasion and metastasis. The more aggressive mesenchymal-like breast cancer cells have a selective advantage, skewing macrophages toward the more immunosuppressive subtype. However, the mechanism underlying this shift is poorly understood. Cyclin D1b is a highly oncogenic variant of cyclin D1. Our previous study showed that non-metastatic epithelial-like breast cancer cells were highly metastatic in vivo when cyclin D1b was overexpressed. The present study determined whether cyclin D1b contributed to the interaction between breast cancer cells and macrophages. The results showed that cyclin D1b promoted the invasion of breast cancer cells in vitro. Specifically, through overexpression of cyclin D1b, breast cancer cells regulated the differentiation of macrophages into a more immunosuppressive M2 phenotype. Notably, tumor cells overexpressing cyclin D1b activated macrophages and induced migration of breast cancer cells. Further investigations indicated that SDF-1 mediated macrophage activation through breast cancer cells overexpressing cyclin D1b. These results revealed a previously unknown link between aggressive breast cancer cells and Tumor-associated macrophages, and highlighted the importance of cyclin D1b activity in the breast cancer microenvironment.
Keywords: Cyclin D1b, breast cancer, tumor-associated macrophage, tumor metastasis, tumor invasion, SDF-1
Impact statement
Cyclin D1b is a highly carcinogenic variant of cyclin D1. Non-metastatic epithelial-like breast cancer cells have been shown to be highly metastatic in vivo when cyclin D1b was overexpressed. Tumor-associated macrophages are important components of leukocyte infiltration into tumor microenvironment. Tumor-associated macrophages in breast cancer microenvironment usually exhibit an M2 phenotype, and their primary function is to promote tumor growth, remodel tissue, promote angiogenesis, and inhibit adaptive immunity. Using overexpression of cyclin D1b in non-metastatic epithelial-like breast cancer cells, we show that breast cancer cells modulate the differentiation of macrophages into a more immunosuppressive M2 phenotype. The interaction between tumor-associated macrophages and tumor cells further revealed that high cyclin D1b-expressing breast cancer cells promote macrophage M2-like polarization, and macrophages activated by high cyclin D1b-expressing breast cancer cell induce migration of breast cancer cells in return. Interestingly, SDF-1 mediated the activation of macrophages induced by high cyclin D1b-expressing breast cancer cells.
Introduction
Recent studies have shown that the development of inflammatory environment in tumors is crucial for tumor progression. 1 Tumor microenvironment is composed of many kinds of cells, and their interaction with tumor cells is the key factor determining tumor progression. 2 Tumor-associated macrophages (TAMs) are an important component of leukocyte infiltration into tumor microenvironment. 3 Macrophage polarization is a continuum, spanning two extremes from classic activated M1 macrophages to alternatively activated M2 macrophages. 4 TAMs in breast cancer (BRC) microenvironment usually exhibit an M2 phenotype, whose primary function is to promote tumor growth, remodel tissue, promote angiogenesis, and inhibit adaptive immunity. 5 , 6 However, the interaction between TAMs and tumor cells requires further study.
Wyckoff et al. 7 identified the existence of a paracrine loop between TAMs and cancer cells in breast tumor. Tumor cell-derived factors, such as chemokines, may perform an indispensable role in macrophage recruitment and differentiation. 8 Interestingly, the more aggressive mesenchymal-like BRC cells showed a selective advantage, skewing macrophages toward the more immunosuppressive subtype. 9 Epithelial-mesenchymal transition (EMT) has been shown to be associated with the induction of immunosuppression. 10 In addition, TAMs and invasive cancer cells appear at the same time in the invasive front of advanced tumors.11–13 These data suggest that aggressive cancer cells may have a selective advantage in modulating TAM polarization. However, the underlying mechanisms of these findings are still poorly understood.
Cyclin D1 is a highly expressed oncogene in several solid tumors, including BRC. 14 It exists as two isoforms: conventional cyclin D1 (referred to cyclin D1a) and cyclin D1b. Cyclin D1b, derived from alternative splicing of CCND1 transcript, is a highly carcinogenic variant of cyclin D1. 15 Our previous studies showed that cyclin D1b may perform an aggressive role in BRC as a metastasis-inducing factor through transcriptional regulation. 16 , 17 These data indicate the presence of communication between aggressive BRC cells and promonocytes. In the present study, we assessed whether cyclin D1b contributes to the interaction between BRC cells and macrophages. Specifically, we focused on the ex vivo differentiation of human macrophages in the presence of BRC conditioned media (CM) in order to explore the role of cyclin D1b in promoting tumor metastasis by activating macrophages and pushing them toward a TAM-like phenotype. Overexpression of cyclin D1b in non-metastatic epithelial-like BRC cells was used to show that BRC cells modulated the differentiation of macrophages into a more immunosuppressive M2 phenotype. The potential mechanism underlying crosstalk between BRC cells and macrophages induced by cyclin D1b through transcriptional regulation of secretory factors of BRC cells was further evaluated to highlight the importance of cyclin D1b activity in the BRC microenvironment.
Materials and methods
Animals and cells
BALB/c athymic nude mice (nu/nu) aged 6–8 weeks, approximately 20 g, were purchased from Shanghai SLAC Laboratory Animal Co. Ltd, and raised in an accredited animal facility. Human BRC cell lines MCF-7 and MDA-MB-231 were purchased from China Center for Type Culture Collection (CCTCC No. GDC0055 and GDC0297, respectively) and cultured as described by CCTCC.
Plasmid and cell transfection
As described in our previous study, 17 pcDNA3.1-cyclin D1b plasmid and cyclin D1b-expressing MCF-7 cells were established. MCF-7 cell expressing exogenous cyclin D1b was termed MCF-7-D1b. The sequence of small interfering (si)RNA against cyclin D1b was 5′-UCUUCCACUGCUCCUAGAAdTdT-3′. 18 According to the manufacturer’s protocol (Invitrogen; Thermo Fisher Scientific, Inc.), transfection of the plasmid and small interfering (si)RNA was performed using Lipofectamine PLUS reagent and Lipofectamine 3000, respectively. Puromycin (0.2–5 µg/mL) or G418 (100–200 µg/mL) was used to screen cells for further experiments.
Macrophage differentiation and co-culture experiments
Human myeloid leukemia mononuclear cells (THP-1) cells treated with 100 nM phorbol-12-myristate-13-acetate (PMA) for three days were differentiated into macrophages. The macrophages were further cultured in RPMI 1640 medium supplemented with 15% fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.) in the presence or absence of 50% CM from different BRC cells for six days. AMD3100 (Plerixafor) (10 μg/mL; Sigma-Aldrich; Merck KGaA), a specific CXCR4 antagonist, was added along with the BRC cell CM.
For the co-culture experiments, both BRC cells and differentiated macrophages were respectively added to the lower and upper chambers of a 12- or 6-well Transwell apparatus with a 0.4-μm pore size (Corning, Inc.).
Matrigel invasion and Boyden chamber assay
Boyden chambers (Transwell; Corning, Inc.) were used for Matrigel invasion assays. Briefly, Transwell inserts were coated with the Matrigel. RPMI 1640 medium containing 10% FBS was added to the lower chamber. In total, ∼5 × 104 differentiated macrophages were placed in the upper chamber. The non-invading cells were removed after 24 h of incubation at 37°C with 5% CO2. To quantify invasion, five fields of each membrane were randomly selected for examination, and the invasive cells attached to the lower surface of the membrane insert were fixed, stained, and counted. The number of cells per field was calculated and averaged for each insert. The differences among groups were analyzed.
For Boyden chamber assay, differentiated macrophages were isolated by addition of a cell eluant (0.02% versene) and collected. Similar to the protocol described above, ∼5 × 104 macrophages were added to the upper well of Boyden chamber. The lower well contained supplemented BRC-conditioned medium (RPMI 1640 medium with 50% CM from different BRC cells). The two wells were separated by an 8.0-μm polycarbonate polyvinylidene fluoride (PVDF) membrane. After incubation at 37°C for 4 h, the non-migrated cells were removed, and the membrane with migrated cells was fixed and stained, as described above.
Analysis of gene expression
Based on the manufacturer’s protocol, total RNA of cells was extracted by TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Reverse transcription (RT)-quantitative polymerase chain reaction (PCR) was used to assess gene expression. In brief, 100 ng RNA was used for RT by Superscript II RNase H (Invitrogen; Thermo Fisher Scientific, Inc.) in a final volume of 25 μL. After that, 2 μL cDNA was amplified using SYBR Green Universal PCR MasterMix (Bio-Rad Laboratories, Inc.) in duplicate with three repetitions. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the loading control. The target gene expression was relatively evaluated using the 2–△△Cq method. 19 The sequences of the primers used were as follows: IL-8 (CXCL8) forward, 5′-GAATTCTCAGCCCTCTTCAAAAAC-3′ and reverse, 5′-GCCAAGGAGTGCTAAAGA ACTTAG-3′; hIL-4 forward, 5′-CCACGGACACAAGTGCGATA-3′ and reverse, 5′-CCCTG CAGAAGGTTTCCTTCT-3′; hIL-10 forward, 5′-GTGATGCCCCAAGCTGAGA-3′ and reverse, 5′-CACGGCCTTGCTCTTGTTTT-3′; hIFN-γ forward 5′-TCAGCTCTGCATCGTT TTGG-3′ and reverse, 5′-GTTCCATTATCCGCTACATCTGAA-3′; hTNF-α forward, 5′-TCT TCTCGAACCCCGAGTGA-3′ and reverse, 5′-CCTCTGATGGCACCACCAG-3′; and GAPDH forward, 5′-TCATTGACCTCAACTACATGGTTT-3′ and reverse 5′-GAAGATGGT GATGGGATTTC-3′. PCR was performed with the following conditions: 4 min at 95°C, followed by 30 cycles of 95°C (60 s), 52°C (55 s), and 72°C (50 s).
Flow cytometry
According to the manufacturer’s instructions, non-specific antibodies to Fc receptors on harvested cells were blocked by BD Fc Block™ (BD Bioscience, San Jose, CA, USA), and the cells were stained using mouse anti-human antibodies for examining the expression of M2 cell markers such as CD206 or co-regulatory molecules such as CD68. All antibodies were directly bound to allophycocyanin or phycoerythrin. The corresponding IgG homotype matched control was used as negative control. In phosphate-buffered saline (PBS) containing 1% heat inactivated fetal bovine serum (FBS; Life Technologies, Carlsbad, CA, USA) and 0.1% sodium azide (Sigma-Aldrich, St. Louis, MO, USA) at 4°C and dark, the antibody was stained for 30 min. Antibodies came from eBioscience (San Diego, CA, USA) and BD Bioscience, BioLegend (San Diego, CA, USA). Intracellular staining for CD68 was performed by Cytofix/Cytoperm™Kit (BD Bioscience). Samples after washing were immediately tested by flow cytometry using an Attune® Acoustic Focusing Cytometer (Life Technologies). The acquired data were assessed by FlowJo software (TreeStar, Ashland, OR, USA).
Matrix metalloproteinase assay using gelatin zymography
For assaying matrix metalloproteinase (MMP) activity using gelatin zymography, tumor cells or macrophages were cultured for 24 h in a culture medium containing 1% FBS in plates pre-coated with Matrigel, and the supernatants were collected. MMP-2 and MMP-9 expression in supernatants was detected as described previously. 20 The band gray values of different groups were analyzed by Iamge J software.
Breast tumor metastasis in mice
MCF-7 cells transfected by pcDNA3.1-cyclin D1b were co-cultured with macrophages using the 6-well Transwell co-culture system for six days. For the sake of assaying tumor cell arrest in lungs during blood flow, the collected tumor cells were labeled using CFSE (carboxyfluorescein succinimidyl ester) 21 and then injected into mice via the tail vein (1 × 106 cells/mouse, n = 8 for each group) after mice were anesthetized. Mice were anesthetized by intraperitoneal injection of sodium pentobarbital saline (6.7 mg/mL) based on their body weight (50 mg/kg). All mice were euthanized by the anesthesia method (100 mg/kg pentobarbital) to obtain the lungs. For alleviating their pain, mice before sacrifice were prevented from observing the execution process. In order to test for CFSE-labeled fluorescence, the lungs of a group of mice were obtained 24 h after tumor cell injection. According to the manufacturer’s instructions, 21 the lungs were embedded with a cryoembedding agent at –30°C for 15 min. Subsequently, 5-µm sections were obtained at –15°C and analyzed using fluorescence microscopy (200×; Zeiss Axioscope and AxioVision software; Carl Zeiss Microscopy GmbH, Jena, Germany). To examine the formation of tumor nodules in mice lung, the lungs of another group of sacrificed mice were harvested 28 days after tumor cell injection and fixed in Bouin’s solution for 16 h at 25°C. Similar to routine, pathological examinations were performed. Mice lungs’ paraffin-embedded blocks were continuously sliced into 4-µm-thick sections and stained using hematoxylin and eosin (HE). Magnified lung pathological images were captured by an Axiophot microscope (Zeiss, Austin, TX, USA) and a digital camera (Leaf Systems Lumina, Southborough, MA, USA). The images were assayed with Optima Imaging Analysis Systems (Version 6.5, Media Cybernetics, Silver Springs, MD, USA). Tumor nodules on mice lung surface were counted in each group. The fluorescence and HE positive spots of different groups were analyzed by Iamge J software.
Western blotting analysis 16 , 17
In 0.5 mL of cold lysis buffer (50 mM NaCl, 10 mM Tris, 1 mM ethylenediaminetetraacetic acid, 1 mM phenylmethylsulfonyl fluoride, 0.5 mM Na3VO4·12H2O, 50 mM NaF, and 1 mM benzamidine), the cells in every group were homogenized and centrifuged at 12,500 × g for 15 min at 4°C. The protein concentration was tested by bicinchoninic acid (BCA) reagent kit. Twenty micrograms of equal amounts of lysate protein for each sample were loaded on 7.5% sodium dodecyl sulfate-polyacrylamide gels for electrophoresis and then blot transmembrane. At room temperature (RT), 3% bovine serum albumin was used to block the nonspecific binding with the membrane in Tris-buffered saline for 1 h. The membranes were then respectively incubated with different primary antibodies overnight at 4°C, as follows: anti-E-cadherin rabbit polyclonal antibodies (1:1000, no. SAB4503751, Sigma-Aldrich, Shanghai, China), anti-Vimentin rabbit polyclonal antibodies (1:1000, no. SAB4503081, Sigma-Aldrich, Shanghai, China), and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:1000, no. sc-25778, Santa Cruz, CA, USA). Horseradish peroxidase-conjugated secondary goat anti-rabbit IgG2a antibodies (1:1000, no. sc-2061, Santa Cruz) were used as the secondary antibodies. Based on the manufacturer’s instructions, Western blots were colored by an enhanced chemiluminescence kit (Sigma, St. Louis, MO, USA). Using Bio-Rad GelDoc XR and Chemi Doc XRS systems (Bio-Rad, Hercules, CA, USA), Quantification of protein bands was performed and analyzed with Quantity One 1-D Analysis Software Version 4.6.3 (Bio-Rad).
Detection of cell-derived cytokines using antibody arrays (human cytokine antibody array G-Series 5 cat# AAH-CYT-G5-4)
BRC cells and different BRC cell-activated macrophages were cultured in medium with 1% FBS for 24 h. The CM was collected and used for Human Cytokine Antibody Array. Human Cytokine Antibody Array 5 (G-Series; RayBio, Inc.) was used according to the manufacturer’s protocol. Briefly, the arrays were blocked at RT by incubating them with 100 µL of CM overnight, and then incubated with biotin-conjugated antibodies (1/250) for 2 h and with Streptavidin-Fluor reagent while avoiding exposure to light. After allowing the glass chip to dry in a laminar flow hood, a laser scanner (Innopsys, InnoScan®) of Cy3 fluorophore or the “green” channel (excitation frequency, 532 nm) was used to scan the glass chip.
Statistical analysis
SPSS 19.0 software (SPSS, Chicago, IL, USA) was used for statistical analysis. All data are presented as the mean ± standard deviation of three independent repeats, and the mean results of different groups were compared by LSD and Tukey’s tests following ANOVA to determine statistical significance. P < 0.05 was considered statistically significant difference.
Results
Cyclin D1b promote invasiveness of BRC cell
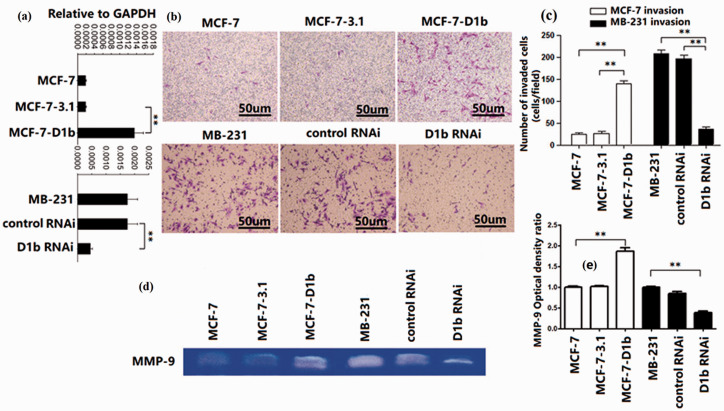
To investigate the effect of cyclin D1b on the invasiveness of human BRC cell, pcDNA3.1-cyclin D1b was transfected into non-metastatic MCF-7 cells with low cyclin D1b transcript expression. In another experiment, cyclin D1b expression in the metastatic MDA-MB-231 cells was downregulated by transfection of siRNA (Figure 1(a)). To quantitatively evaluate the invasive ability of these cancer cell lines, Transwell assay was used, and the results showed that ectopic cyclin D1b increased the invasive ability of MCF-7 cells in vitro. Matrigel invasion assay also showed that MDA-MB-231 cell invasiveness was significantly reduced in the cells transfected with cyclin D1b siRNA, but not in the siRNA control group (Figure 1(b) and (c)). MMP-9 is related to higher invasiveness and metastatic potential of BRC cells. 20 The production of MMP-9 was increased by cyclin D1b overexpression in MCF-7 cells and decreased by the cyclin D1b siRNA in MB-231 cells (Figure 1(d) and (e)). These data suggest that cyclin D1b is related to increased tumor invasion of BRC cells.
Figure 1.
The cyclin D1b variant promotes invasion of BRC cells. (a) Cyclin D1b expression was assessed using reverse-transcription PCR analysis. MCF-7 cells were transfected with plasmid DNA containing the cyclin D1b sequence. MDA-MB-231 cells were transfected with siRNA against cyclin D1b. (b, c) Invasion of cells was assessed using a Matrigel invasion assay. Magnification, ×100. (d, e) MMP-9 expression was assessed in the indicated BRC cells. MMP-9 production was evaluated using a zymography assay (48-h culture). The MMP-9 optical density ratio of the left three groups is relative to MCF-7. The MMP-9 optical density ratio of the right three groups is relative to MB-231. BRC: breast cancer; siRNA: small interfering; RNAi: RNA interference (siRNA); MMP: matrix metalloproteinase. The differences between the two groups were analyzed by ANOVA. **P < 0.01. (A color version of this figure is available in the online journal.)
High cyclin D1b-expressing tumor cell promote macrophage M2 polarization
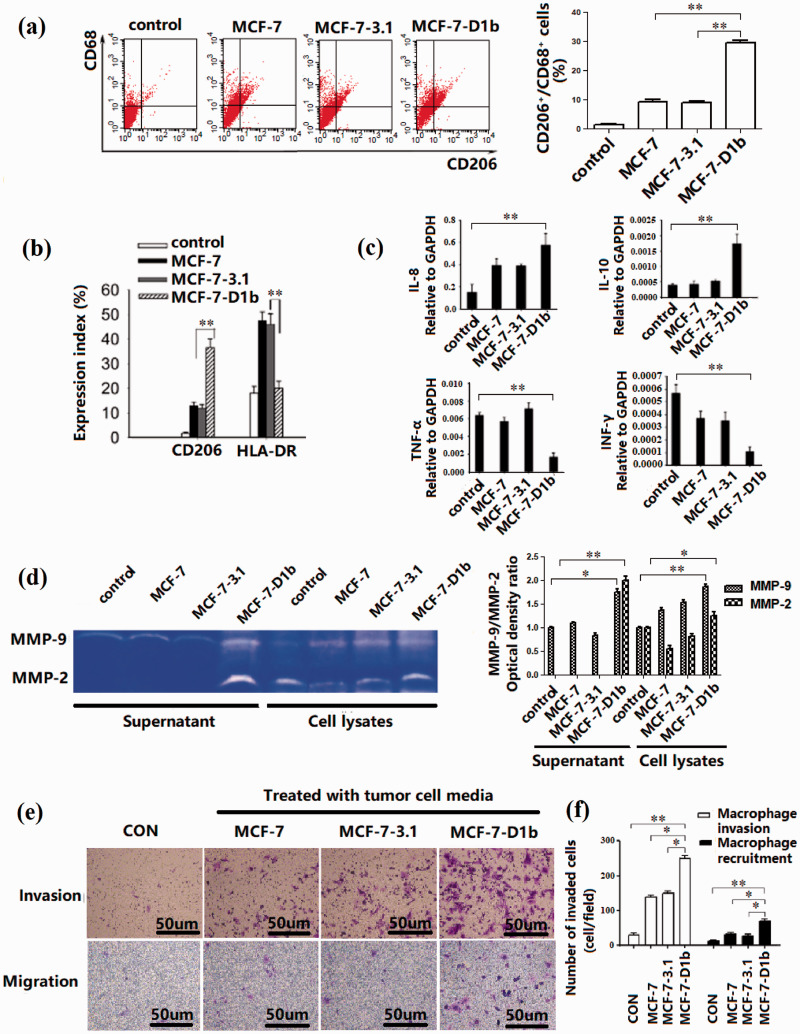
In order to establish a macrophage differentiation model in vitro, the human monocyte cell line THP-1 was differentiated into macrophages by administration of PMA following culture filtrates of different tumor cells. In the process of differentiation, THP-1 cells gradually changed from suspended growth to adherent diffusion, and the increase in CD68 expression indicated that the cells had differentiated into macrophage-like cells. In flow cytometric analyses, the CD206 expression of M2 marker was significantly higher in MCF-7-D1b cells than in other three groups (Figure 2(a)). Expression of HLA-DR, M1 macrophage marker, was lower in MCF-7-D1b cells (Figure 2(b)). Macrophages treated with CM from MCF-7-D1b cells exhibited a CD206high/HLA-DRlow phenotype, similar to primary TAMs, suggesting that CM from MCF-7-D1b cells may be a potent activator of macrophage differentiation. To confirm the expression of characteristic cytokines in these macrophages, the mRNA levels of the macrophage cytokines (IL-8, IL-10, TNF-α, IFN-γ) were investigated. The mRNA levels of these cytokines showed a similar expression pattern as that of macrophage marker expression (Figure 2(c)). Secretion of MMPs by TAMs has been well documented. 22 To determine whether MCF-7-D1b cells induced macrophage M2 polarization, MMP activity of the indicated macrophages treated with different CMs was assessed using the zymography assay. CM from MCF-7-D1b cells resulted in an increase in both MMP-9 and MMP-2 activity (Figure 2(d)). Accordingly, Transwell invasion assay was used to evaluate the degradation of extracellular matrix (ECM) and basement membrane by seeding macrophages into the upper well, and a similar tendency was observed, as shown in Figure 2(e) (upper panel) and Figure 2(f) (left). Tumor cells are known to be able to recruit macrophages. 23 In the present study, Boyden chamber assay showed that more macrophages migrated towards the MCF-7-D1b cancer cell CM, in comparison with other tumor cell groups (Figure 2(e), lower panel and Figure 2(f), right).
Figure 2.
Differentiation of M2 macrophages derived from the human monocyte cell line (THP-1). (a) Expression of CD206/CD68 in macrophages. THP-1 cells were differentiated using different conditional culture media. The percentage of CD206+/CD68+ positive cells was detected using flow cytometry. (b) Expression of CD206 or HLA-DR on the cell surface of macrophages was detected and calculated using flow cytometry. (c) mRNA expression of the cytokine profiles of THP-1-derived macrophages. (d) MMP production in the THP-1-derived macrophages. THP-1-derived macrophages were incubated in the presence of different BRC culture media for six days and then cultured for 24 h in DMEM supplemented with 1% FBS. Conditioned media (supernatant) and indicated macrophages (cell lysates) were collected, and gelatinase production was assessed using a zymography assay. The MMP-9 optical density ratio of different groups is relative to the control group, respectively. The MMP-2 optical density ratio of different groups is relative to the cell lysates control group. (e, f) Invasion and migration of macrophages. Invasion of macrophages induced by different BRC culture media was assessed using a Transwell invasion assay (upper panel). The Transwell migration assay demonstrated that the number of macrophages migrating toward the MCF-7D1b-conditioned medium (lower panel) was highest. BRC: breast cancer; MMP: matrix metalloproteinase. The differences between the two groups were analyzed by ANOVA. *P < 0.05, **P < 0.01. (A color version of this figure is available in the online journal.)
Macrophage activated by high cyclin D1b-expressing tumor cell and induce migration of BRC cell in return
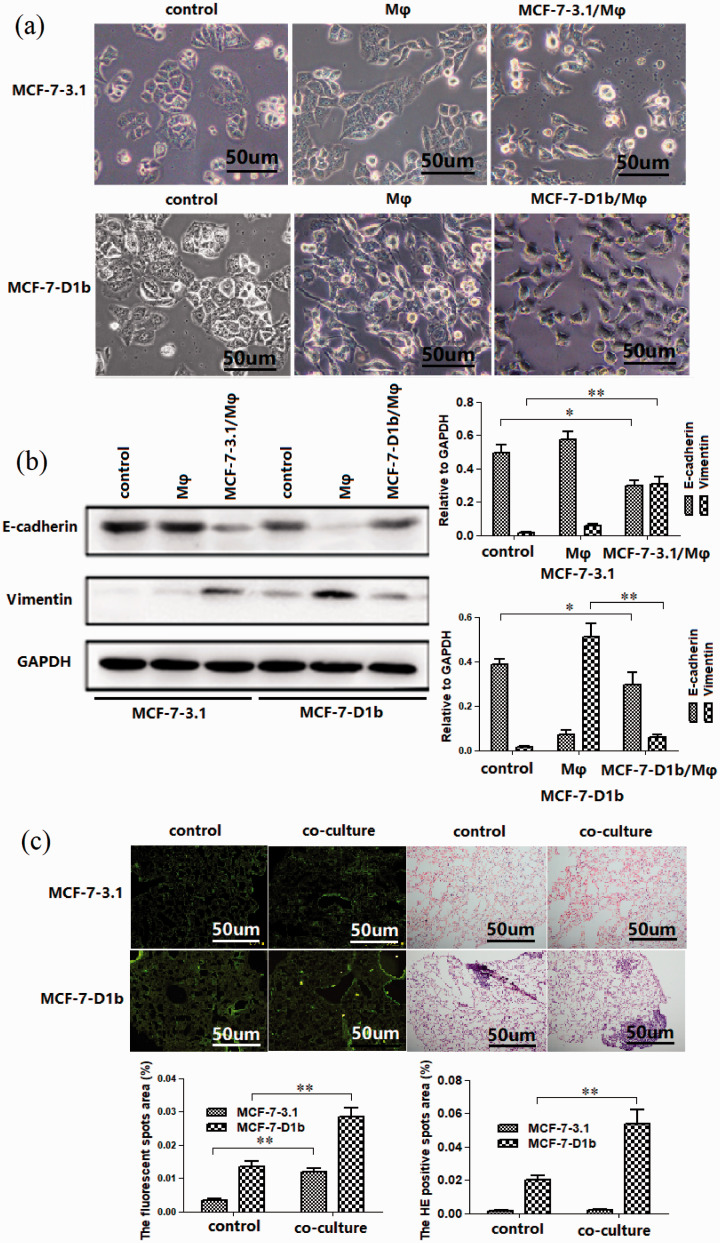
TAMs have been previously reported to enhance metastasis of BRC cells, which is often associated with EMT of BRC cells. 24 Thus, we observed whether macrophage activated by high cyclin D1b-expressing tumor cell induced migration of BRC cell in return. MCF-7-D1b cell co-cultured with THP-1 derived macrophage for six days changed from a rounded shape to an elongated one (Figure 3(a)) and showed reduced E-cadherin expression as well as increased Vimentin expression (Figure 3(b)). Conversely, MCF-7 cells co-cultured with macrophages which have been co-cultured with MCF-7-D1b cells for another 24 h changed from a rounded shape to an elongated one, too. The protein levels of E-cadherin and Vimentin showed a similar expression pattern as that of co-cultured MCF-7-D1b cell (Figure 3(b)), revealing that MCF-7-D1b cell activated macrophage-mediated induction of EMT in BRC cell in return. To assess the effect of the activated macrophages on tumor cell retention in lungs under dynamic flow conditions, CFSE-labeled tumor cells were injected into nude mice via the tail vein. A total of 24 h after injection, cell extravasation in the lungs was assessed by counting the fluorescent spots in lung tissues. As shown in Figure 3(c), 24 h after injection, the retention of MCF-7-D1b cells co-cultured with macrophages in lung tissue was increased, and this may have contributed to BRC metastasis in vivo. Similarly, the number of metastatic nodules in the lungs was also increased 28 days after inoculation.
Figure 3.
Macrophages activated high cyclin D1b expression in tumor cells, which, in turn, induced migration of BRC cells. (a) Co-culture with macrophages increased the fibroblastic morphology of MCF-7-D1b cells. MCF-7-D1b cell lines and control MCF-7-3.1 cell lines were indirectly co-cultured with THP-1-derived macrophages using a Transwell system. MCF-7-D1b cells co-cultured with THP-1 derived macrophages for six days changed from a rounded shape to an elongated one ((a) second line middle panel), but MCF-7-3.1 cells have not morphological change ((a) first line middle panel). MCF-7-3.1 cells co-cultured with macrophages which have been co-cultured with MCF-7-D1b cells for another 24 h changed from a rounded shape to an elongated one ((a) first line right panel), and MCF-7-D1b cells co-cultured with macrophages which have been co-cultured with MCF-7-3.1 cells for another 24 h changed from a rounded shape to an elongated one ((a) second line right panel), too. (b) Protein expression of E-cadherin and Vimentin in the tumor cells pretreated above was analyzed by Western blotting. For MCF-7-3.1 and MCF-7-D1b cells, the control group, Mφ group, and D1b/Mφ group were set up for co-culturing respectively. The protein expression of E-cadherin and Vimentin was investigated. (c) Tumor cell retention in lungs after co-culture with macrophages. MCF-7-D1b cells co-cultured with macrophages were collected, labeled with CFSE, and injected into mice via the tail vein. Mice were sacrificed after 24 h. Scale bar, 50 μm. After 30 days, lung tissues were dissected and used for histological examination. BRC: breast cancer. The differences between the two groups were analyzed by ANOVA. *P < 0.05, **P < 0.01. (A color version of this figure is available in the online journal.)
Cyclin D1b promotes SDF-1 secretion from BRC cells
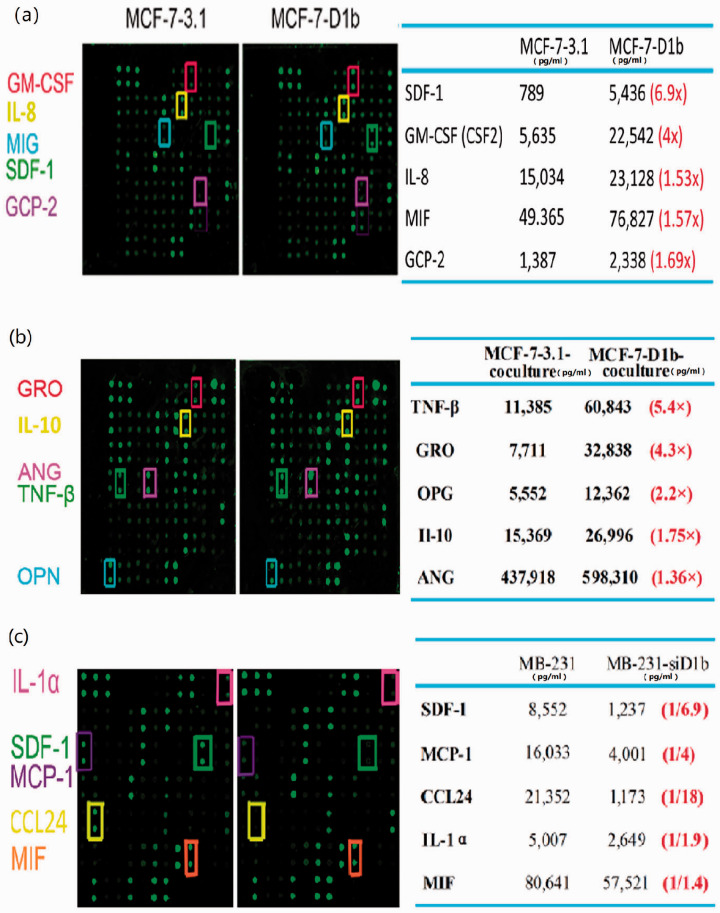
To identify the mechanism underlying activation of macrophage by critical cytokines secreted by high cyclin D1b-expressing BRC cell, the cytokine profiles of CM from MCF-7-D1b and MCF-7-3.1 cell were analyzed using RayBio Human Cytokine Antibody Array. The levels of three cytokines, SDF-1, GM-CSF, and GCP-2, were significantly increased in the CM of MCF-7-D1b cell in comparison with the CM of MCF-7-3.1 cell (Figure 4(a)). The cytokine profiles of CM from MCF-7-D1b cell-activated macrophage showed M2 subtype cytokine TNF-βhigh GROhigh IL-10high characteristics (Figure 4(b)). Cyclin D1b expression in the MDA-MB-231 cell was reduced by the siRNA, and the cytokine profile of CM from MDA-MB-231-siD1b cell was assessed. The levels of four cytokines (SDF-1, MCP-1, CCL24, and IL-α) in the CM of MDA-MB-231-siD1b cell were significantly decreased compared with those in the control cell (Figure 4(c)), of which SDF-1, MCP-1, and CCL24 were associated with monocyte accumulation or M2-macrophage polarization. 8 , 25 Collectively, SDF-1 secreted by high cyclin D1b-expressing BRC cell may be a critical factor mediating the activation of macrophage.
Figure 4.
Cytokine array of the CM of different BRC cells and co-cultured macrophages. (a) Cytokine array of the CM of the MCF-7-D1b and control MCF-7-3.1 cells. (b) Cytokine array of CM of MCF-7-D1b and control MCF-7-3.1 cells were respectively co-cultured with activated macrophages. (c) Cytokine array of the CM of MDA-MB-231-siD1b and control MDA-MB-231 cells. CM: conditioned media; BRC: breast cancer; si: small interfering; GM-CSF: granulocyte-macrophage colony stimulating factor; IL-8: interleukin 8; MIG: monokine induce by INF-γ; SDF-1: stromal cell-derived factor-1; GCP-2: granulocyte chemotactic protein 2; MIF: macrophage migration inhibitory factor; GRO: growth regulated protein; IL-10: interleukin 10; ANG: angiopoietin; TNF-β: tumor necrosis factor-β; OPG: osteoprotegerin; IL-1α: interleukin 1α; MCP-1: monocyte chemoattractant protein-1; CCL24: C-C motif chemokine 24. (A color version of this figure is available in the online journal.)
SDF-1 mediates the activation of macrophage induced by high cyclin D1b-expressing BRC cell
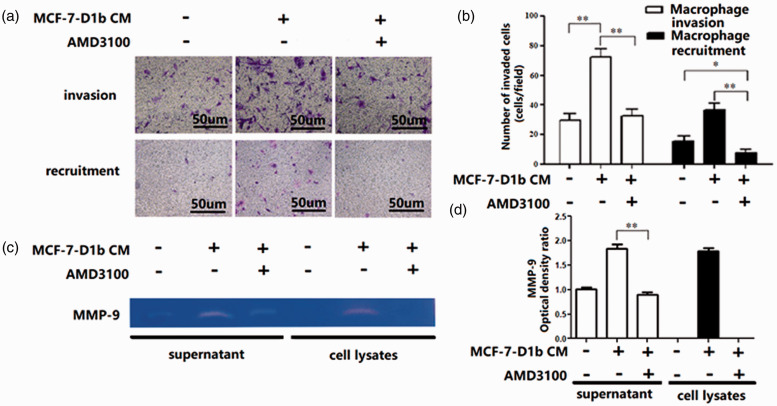
To investigate the role of SDF-1 in augmenting crosstalk between BRC cell and macrophage, AMD3100, a specific antagonist of SDF-1 receptor CXCR4, was used in the co-culture system. AMD3100 (10 μg/mL) was added to the CM from BRC cell, and this abrogated the enhanced ability of macrophage to degrade the ECM. In Boyden chamber assay, when AMD3100 was added to the lower well, the number of migrating macrophages was significantly lower than that with the untreated controls (Figure 5(a) and (b)). Consistent with this result, the increased release of MMP-9 from macrophage was abrogated as well (Figure 5(c) and (d)). These results show that SDF-1 may serve a critical role in the process of macrophage polarization induced by cyclin D1b-expressing BRC cell.
Figure 5.
Functional alteration of macrophages treated with AMD3100. (a, b) Invasion assay and Boyden chamber assays. Macrophages were incubated with CM from MCF-7-D1b treated with or without AMD3100. Invasion of macrophages was measured using a Transwell invasion assay (upper panel). Boyden chamber assays were used to measure macrophage migration toward MCF-7-D1b-CM which had been treated with or without AMD3100 (lower panel). (c, d) MMP-9 production in THP-1-derived macrophages incubated with CM from MCF-7-D1b with or without AMD3100 administration. CM: conditioned media; MMP: matrix metalloproteinase. The differences between the two groups were analyzed by ANOVA. *P < 0.05, **P < 0.01. (A color version of this figure is available in the online journal.)
Discussion
The present study showed that the cyclin D1b splice variant facilitated tumor metastasis by promoting SDF-1-mediated macrophage polarization.
In tumor microenvironment, cancer cells actively modulate nonmalignant stromal cells, such as macrophages, to enhance tumor development and metastasis. 26 Interestingly, distinct types of cancer cells exhibit differing abilities to modulate the tumor microenvironment. In BRC, the highly aggressive cancer cells may have a selective advantage in modulating the tumor microenvironment. 27 The relationship between cancer cell and nonmalignant stromal cell is the focus of clinical attention. Cyclin D1b has been reported to be associated with worse disease outcomes, and its influence is not dependent on the relative levels of cyclin D1a. In our previous study, the endogenous and ectopic expression of cyclin D1b in human BRC cell lines was shown to be correlated with an aggressive phenotype via transcriptional regulation of functions other than that of classical cyclin D1a. The present study is the first to show that cyclin D1b expression in BRC made non-metastatic MCF-7 cells metastatic, and its silencing made metastatic MDA-MB-231 cells less metastatic. Thus, is cyclin D1b expression in BRC associated with macrophage polarization-mediated metastasis?
Macrophages have been previously reported to constitute the majority of tumor-infiltrating immune cells in tumor microenvironment. 28 Several different types of cancer tissues possess numerous M2 macrophage-like TAMs, 29 which express specific markers, such as CD206 and CD163, and CD83, CD80, and HLA-DR for M1 macrophages. CD68 is expressed on both M1 and M2 macrophages.30–32 The in vitro model system was successfully established to examine the interaction between macrophage and tumor cell. Macrophage could undergo differentiation into M2-like macrophage (CD206high HLA-DRlow) in response to CM from high cyclin D1b-expressing tumor cell. The secretion of MMP-9 by TAMs has been well documented. 25 Increased MMP-9 expression in cancer tissues via degradation of the ECM and basement membrane, liberation of matrix-bound growth factors, and activation of tumor-promoting agents is considered to be an important step in promoting invasiveness and angiogenesis. 33 The present study revealed that THP-1-derived macrophages treated with MCF-7-D1b CM showed an altered proteolytic profile, including increased MMP-9 production and enhanced ECM degradation ability. Furthermore, the CM of MCF-7-D1b cancer cell attracted more macrophages, suggesting that BRC cell with high expression levels of cyclin D1b may both recruit tumor-supportive macrophages and promote their polarization toward the M2 phenotype.
With the colocalization of TAMs and cancer cells that had undergone EMT at the invasive front of tumors, both BRC cells and macrophages co-migrated and were dependent upon each other to be invasive in vivo. 34 When co-cultured with macrophages, MCF-7-D1b cells transformed from a rounded to a more elongated morphology and exhibited decreased E-cadherin and increased Vimentin expression, resulting in increased migration and invasiveness. Furthermore, metastasis was also increased following intravenous injection transplantation. These results suggest that tumor metastasis is augmented by crosstalk between aggressive BRC cell and TAM, and this may be promoted by the cyclin D1b splice variant.
In tumor microenvironment, the cytokine communication exists between tumor cells and pro-monocytes, e.g. CCL2-mediated recruitment of monocytes 35 and the role of GROs in neutrophil recruitment 36 and IL-8 in angiogenesis, 37 and this was an important factor increasing the expression of genes associated with tumor malignancy. Another report recently showed that tumors specifically alter tumor cells to interact with virtually all host cells present in the microenvironment to promote malignancy via functional communication through cytokines. 38 This study showed that the interactions between macrophage and cancer cell with high cyclin D1b expression resulted in upregulation of numerous cytokines, particularly SDF-1. Expression of SDF-1 (CXCL12) by tumor cell has been shown to result in an increase in macrophage and microvessel density and in vivo invasiveness. 8 Furthermore, CXCR4, the only receptor for CXCL12, was essential for M2-skewed TAM differentiation. 39 Slug transcriptional induction is essential for cyclin D1b-mediated invasive properties, 40 and Snail family members (Snail and Slug) promote EMT and mediate myeloid cell recruitment by increasing the release of soluble factors, including SDF-1. 41 These data suggest that cyclin D1b induces pro-tumorigenic phenotypes and a unique gene expression program associated with metastasis. Cyclin D1 has been confirmed to have transcriptional function in vivo. 42 However, studies have found that the main function of cyclin D1b is to induce nuclear receptor dependent transcription events related to tumor cell migration, invasion, or differentiation. 40 In fact, gene expression profiles show that cyclin D1b alters some cellular processes, including transcriptional and translational regulation. 43 It is speculated that cyclind1b may cause changes in cytokines through transcriptional regulation.
The previous studies on cyclin D1b were mainly limited to the tumor cells themselves. 42 , 43 This study further evaluated the role and mechanism of cyclin D1b in remodeling the tumor microenvironment by changing the biological characteristics of tumor cell. A large number of studies have shown that stromal tumor cells play an important role in remodeling the tumor microenvironment. 44 Because of the heterogeneity of tumor itself, our study further explored the specific molecular targets of this cell population, thereby placing more emphasis on studying the tumor microenvironment and tumor cell dialogue mechanisms. However, there are some limitations associated with the in vivo experiments in this study. In order to replicate the experimental model in vivo and further study the role of cyclin D1b tumor cells in the process of interaction with macrophages, an immune reconstitution model must be used for in vivo experiments, which has some limitations. Studies have shown that different subpopulations of BRC cells have different induction effects on macrophages. 45 The highly invasive BRC cells with stromal-like characteristics show a stronger induction effect. Aggressive cancer cells may have a selective advantage in modulating the tumor microenvironment. 9 When macrophages polarize into tumor-promoting subtypes, this vicious circle promotes tumor development. Thus, clarification of the characteristics of the dominant BRC cell subpopulation induced by macrophage polarization and identification of the specific molecular targets of the subtype are of great significance for BRC treatment.
Previous studies have shown that the positive feedback loop between aggressive cancer cells and macrophages is critical for metastasis of BRC. 9 , 24 The present study showed that cyclin D1b in aggressive BRC cell promotes the release of soluble factors from cancer cell to recruit and “train” macrophage. These results provide insight into the extent of interactions between cancer cell and macrophage, highlighting the convergence networks of cyclin D1b expression in cancer cells and TAMs and promoting phenotypes implicated in progression of metastasis. This unexpected pathway highlights a potentially novel means of therapeutic intervention for treatment of metastatic cancer.
ACKNOWLEDGMENTS
We thank Associate Professor Xiao Chen for technical assistance and Dr Yujie Sun for providing the BRC cells.
AUTHORS’ CONTRIBUTIONS: All authors participated in the design and interpretation of the study and analysis of the data and review of the manuscript: YXW, YL, LX, LTH, and XWY conducted the experiments and wrote the manuscript; YXW, YBH, and FHW reviewed the manuscript; and YXW and FHW designed and interpreted the data and reviewed the manuscript.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Science Foundation of China (grant nos. 81702920 and 81702948).
ORCID iD: Yuxue Wang https://orcid.org/0000-0003-2756-9494
References
- 1.Korbecki J, Bajdak-Rusinek K, Kupnicka P, Kapczuk P, Simińska D, Chlubek D, Baranowska-Bosiacka I. The role of CXCL16 in the pathogenesis of cancer and other diseases. Int J Mol Sci 2021; 22:3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spaw M, Anant S, Thomas SM. Stromal contributions to the carcinogenic process. Mol Carcinog 2017; 56:1199–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol 2013; 33:S79–84 [DOI] [PubMed] [Google Scholar]
- 4.Albini A, Bruno A, Noonan DM, Mortara L. Contribution to tumor angiogenesis from innate immune cells within the tumor microenvironment: Implications for immunotherapy. Front Immunol 2018; 9:527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Yao Y, Gong C, Yu F, Su S, Chen J, Liu B, Deng H, Wang F, Lin L, Yao H, Su F, Anderson KS, Liu Q, Ewen ME, Yao X, Song E. CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell 2011; 19:541–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamilton MJ, Bosiljcic M, Lepard NE, Halvorsen EC, Ho VW, Banáth JP, Krystal G, Bennewith KL. Macrophages are more potent immune suppressors ex vivo than immature myeloid-derived suppressor cells induced by metastatic murine mammary carcinomas. J Immunol 2014; 192:512–22 [DOI] [PubMed] [Google Scholar]
- 7.Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, Graf T, Pollard JW, Segall J, Condeelis J. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res 2004; 64:7022–9 [DOI] [PubMed] [Google Scholar]
- 8.Wani N, Nasser MW, Ahirwar DK, Zhao H, Miao Z, Shilo K, Ganju RK. C-X-C motif chemokine 12/C-X-C chemokine receptor type 7 signaling regulates breast cancer growth and metastasis by modulating the tumor microenvironment. Breast Cancer Res 2014; 16:R54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi J, Gyamfi J, Jang H, Koo JS. The role of tumor-associated macrophage in breast cancer biology. Histol Histopathol 2018; 33:133–45 [DOI] [PubMed] [Google Scholar]
- 10.Kudo-Saito C, Shirako H, Ohike M, Tsukamoto N, Kawakami Y. CCL2 is critical for immunosuppression to promote cancer metastasis. Clin Exp Metastasis 2013; 30:393–405 [DOI] [PubMed] [Google Scholar]
- 11.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev 2009; 28:15–33 [DOI] [PubMed] [Google Scholar]
- 12.Francí C, Takkunen M, Dave N, Alameda F, Gómez S, Rodríguez R, Escrivà M, Montserrat-Sentís B, Baró T, Garrido M, Bonilla F, Virtanen I, García de Herreros A. Expression of snail protein in tumor-stroma interface. Oncogene 2006; 25:5134–44 [DOI] [PubMed] [Google Scholar]
- 13.Laoui D, Movahedi K, Van Overmeire E, Van den Bossche J, Schouppe E, Mommer C, Nikolaou A, Morias Y, De Baetselier P, Van Ginderachter JA. Tumor-associated macrophages in breast cancer: distinct subsets, distinct functions. Int J Dev Biol 2011; 55:861–7 [DOI] [PubMed] [Google Scholar]
- 14.Casimiro MC, Velasco-Velázquez M, Aguirre-Alvarado C, Pestell RG. Overview of cyclins D1 function in cancer and the CDK inhibitor landscape: past and present. Expert Opin Investig Drugs 2014; 23:295–304 [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Dean JL, Millar EK, Tran TH, McNeil CM, Burd CJ, Henshall SM, Utama FE, Witkiewicz A, Rui H, Sutherland RL, Knudsen KE, Knudsen ES. Cyclin D1b is aberrantly regulated in response to therapeutic challenge and promotes resistance to estrogen antagonists. Cancer Res 2008; 68:5628–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo BP, Luo J, Hu YB, Yao XW, Wu FH. Cyclin D1b splice variant promotes αvβ3-mediated EMT induced by LPS in breast cancer cells. Curr Med Sci 2018; 38:467–72 [DOI] [PubMed] [Google Scholar]
- 17.Wu FH, Luo LQ, Liu Y, Zhan QX, Luo C, Luo J, Zhang GM, Feng ZH. Cyclin D1b splice variant promotes αvβ3-mediated adhesion and invasive migration of breast cancer cells. Cancer Lett 2014; 355:159–67 [DOI] [PubMed] [Google Scholar]
- 18.Kovar H. Blocking the road, stopping the engine or killing the driver? Advances in targeting EWS/FLI-1 fusion in Ewing sarcoma as novel therapy. Expert Opin Ther Targets 2014; 18:1315–28 [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCq method. Methods 2001; 25:402–8 [DOI] [PubMed] [Google Scholar]
- 20.Rolli M, Fransvea E, Pilch J, Saven A, Felding-Habermann B. Activated integrin alphavbeta3 cooperates with metalloproteinase MMP-9 in regulating migration of metastatic breast cancer cells. Proc Natl Acad Sci U S A 2003; 100:9482–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakakura K, Takahashi H, Kaira K, Toyoda M, Murata T, Ohnishi H, Oyama T, Chikamatsu K. Relationship between tumor-associated macrophage subsets and CD47 expression in squamous cell carcinoma of the head and neck in the tumor microenvironment. Lab Invest 2016; 96:994–1003 [DOI] [PubMed] [Google Scholar]
- 22.Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology 2013; 218:1402–10 [DOI] [PubMed] [Google Scholar]
- 23.Qin M, Wang L, Li F, Yang M, Song L, Tian F, Yukht A, Shah PK, Rothenberg ME, Sharifi BG. Oxidized LDL activated eosinophil polarize macrophage phenotype from M2 to M1 through activation of CD36 scavenger receptor. Atherosclerosis 2017; 263:82–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su S, Liu Q, Chen J, Chen J, Chen F, He C, Huang D, Wu W, Lin L, Huang W, Zhang J, Cui X, Zheng F, Li H, Yao H, Su F, Song E. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell 2014; 25:605–20 [DOI] [PubMed] [Google Scholar]
- 25.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013; 19:1423–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith HA, Kang Y. The metastasis-promoting roles of tumor-associated immune cells. J Mol Med (Berl) 2013; 91:411–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Pul KM, Vuylsteke R, de Beijer MTA, van de Ven R, van den Tol MP, Stockmann HBAC, de Gruijl TD. Breast cancer-induced immune suppression in the sentinel lymph node is effectively countered by CpG-B in conjunction with inhibition of the JAK2/STAT3 pathway. J Immunother Cancer 2020; 8:e000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sica A, Allavena P, Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett 2008; 267:204–15 [DOI] [PubMed] [Google Scholar]
- 29.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer 2006; 42:717–27 [DOI] [PubMed] [Google Scholar]
- 30.Nicod LP, Joudrier S, Isler P, Spiliopoulos A, Pache JC. Upregulation of CD40, CD80, CD83 or CD86 on alveolar macrophages after lung transplantation. J Heart Lung Transplant 2005; 24:1067–75 [DOI] [PubMed] [Google Scholar]
- 31.Costa A, Naranjo JD, Londono R, Badylak SF. Biologic scaffolds. Cold Spring Harb Perspect Med 2017; 7:a025676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarado-Vazquez PA, Bernal L, Paige CA, Grosick RL, Moracho Vilrriales C, Ferreira DW, Ulecia-Morón C, Romero-Sandoval EA. Macrophage-specific nanotechnology-driven CD163 overexpression in human macrophages results in an M2 phenotype under inflammatory conditions. Immunobiology 2017; 222:900–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vandooren J, Van den Steen PE, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): the next decade. Crit Rev Biochem Mol Biol 2013; 48:222–72 [DOI] [PubMed] [Google Scholar]
- 34.Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol 2020; 15:123–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen H, Liu D, Guo L, Cheng X, Guo N, Shi M. Chronic psychological stress promotes lung metastatic colonization of circulating breast cancer cells by decorating a pre-metastatic niche through activating β-adrenergic signaling. J Pathol 2018; 244:49–60 [DOI] [PubMed] [Google Scholar]
- 36.Jinquan T, Frydenberg J, Mukaida N, Bonde J, Larsen CG, Matsushima K, Thestrup-Pedersen K. Recombinant human growth-regulated oncogene-alpha induces T lymphocyte chemotaxis. A process regulated via IL-8 receptors by IFN-gamma, TNF-alpha, IL-4, IL-10, and IL-13. J Immunol 1995; 155:5359–68 [PubMed] [Google Scholar]
- 37.Todorović-Raković N, Milovanović J. Interleukin-8 in breast cancer progression. J Interferon Cytokine Res 2013; 33:563–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt T, Ben-Batalla I, Schultze A, Loges S. Macrophage–tumor crosstalk: role of TAMR tyrosine kinase receptors and of their ligands. Cell Mol Life Sci 2012; 69:1391–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown JM. Radiation damage to tumor vasculature initiates a program that promotes tumor recurrences. Int J Radiat Oncol Biol Phys 2020; 108:734–44 [DOI] [PubMed] [Google Scholar]
- 40.Augello MA, Burd CJ, Birbe R, McNair C, Ertel A, Magee MS, Frigo DE, Wilder-Romans K, Shilkrut M, Han S, Jernigan DL, Dean JL, Fatatis A, McDonnell DP, Visakorpi T, Feng FY, Knudsen KE. Convergence of oncogenic and hormone receptor pathways promotes metastatic phenotypes. J Clin Invest 2013; 123:493–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suarez-Carmona M, Bourcy M, Lesage J, Leroi N, Syne L, Blacher S, Hubert P, Erpicum C, Foidart JM, Delvenne P, Birembaut P, Noël A, Polette M, Gilles C. Soluble factors regulated by epithelial-mesenchymal transition mediate tumour angiogenesis and myeloid cell recruitment. J Pathol 2015; 236:491–504 [DOI] [PubMed] [Google Scholar]
- 42.Bienvenu F, Jirawatnotai S, Elias JE, Meyer CA, Mizeracka K, Marson A, Frampton GM, Cole MF, Odom DT, Odajima J, Geng Y, Zagozdzon A, Jecrois M, Young RA, Liu XS, Cepko CL, Gygi SP, Sicinski P. Transcriptional role of cyclin D1 in development revealed by a genetic-proteomic screen. Nature 2010; 463:374–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pestell RG. New roles of cyclin D1. Am J Pathol 2013; 183:3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen X, Zhou J, Li X, Wang X, Lin Y, Wang X. Exosomes derived from hypoxic epithelial ovarian cancer cells deliver microRNAs to macrophages and elicit a tumor-promoted phenotype. Cancer Lett 2018; 435:80–91 [DOI] [PubMed] [Google Scholar]
- 45.Guo L, Cheng X, Chen H, Chen C, Xie S, Zhao M, Liu D, Deng Q, Liu Y, Wang X, Chen X, Wang J, Yin Z, Qi S, Gao J, Ma Y, Guo N, Shi M. Induction of breast cancer stem cells by M1 macrophages through lin-28B-let-7-HMGA2 axis. Cancer Lett 2019; 452:213–25 [DOI] [PubMed] [Google Scholar]