Abstract
In heart failure (HF) patients with reduced ejection fraction, LIPCAR, a long noncoding RNA is elevated and is associated with left ventricular remodeling and poor prognosis. We studied the role of LIPCAR in patients with HF post-acute myocardial infarction (AMI) to find biomarkers for early detection of HF. We conducted a study of 127 patients with AMI, of which 59 were patients with HF post-AMI. LIPCAR levels were higher in HF patients post-AMI than patients without HF, and LIPCAR had a high predictive value for diagnosis of HF, which was estimated by receiver operating characteristic curves (AUC: 0.985). The results indicate that LIPCAR may be a marker of early HF after AMI.
Keywords: LIPCAR, biomarker, acute myocardial infarction, heart failure
Impact statement
In the present study, our aim was to determine the utility of LIPCAR as a heart failure (HF) biomarker after acute myocardial infarction (AMI). New biomarkers of HF after AMI could lead to more proactive and preventative treatments for early stage patients, thereby advancing the field. This is the first report of the diagnostic value of LIPCAR for HF after AMI. Our results may have wide-reaching implications in terms of the early identification of HF after AMI.
Introduction
Acute myocardial infarction (AMI) is one of the most serious coronary artery diseases (CADs), characterized by multiple complications, high morbidity, and high mortality. 1 AMI is often followed by heart failure (HF), which is one of the leading causes of death and disability in the world.2–4 Drug therapy is the most valid treatment for HF, but the lack of early symptoms leads to a poor prognosis. Thence, finding effective biomarkers of early HF is of great significance to improve the prognosis of HF after AMI.
Long noncoding RNAs (lncRNAs), in length of over 200 nucleotides, are located in the nucleus or cytoplasm. They lack protein-coding capability but can epigenetically regulate gene expression and may have multiple functions inside the cell. 5 , 6 Recent studies have shown that lncRNAs are participated in many complicated processes of cardiovascular disease.7–9 For example, it has been reported that lncRNA uc022bqs.1, LIPCAR, located in mitochondria, is upregulated in the plasma of patients with HF. 10 , 11 It has also been shown that high levels of plasma LIPCAR predict left ventricular (LV) remodeling and are independently associated with a higher risk of cardiovascular death 10 and CAD. 11 But the value of LIPCAR in the diagnosis of HF post-AMI remains unknown. Here, we evaluated the expression of LIPCAR in AMI patients and determined the diagnostic value of LIPCAR in AMI patients with HF.
Materials and methods
Participants
This cross-sectional study was conducted in the Department of Cardiology at Shanghai Gong Hospital from June 2018 to January 2020. All subjects involved in the study did not have acute HF caused by acute valvular insufficiency, severe arrhythmias, or other reasons, had no history of coronary atherosclerotic heart disease with percutaneous coronary interventions (PCIs) or coronary artery bypass graft, no renal failure, liver failure, hepatitis, hemorrhagic disease, previous chest radiotherapy, pregnancy, history of cardiomyopathy, congenital heart disease, or malignant disease. A total of 157 participants were enrolled in the study. Following diagnosis of AMI, all patients underwent PCI at an early time. According to the diagnostic criteria, the participants were divided into two groups: non-HF post-AMI (n = 68) and HF post-AMI (n = 59), and 30 controls matched with basic clinical data. HF was diagnosed according to the 2016 European Society of Cardiology (ESC) “Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure.” 12 HF patients have at least one of the following manifestations: N-terminal pro-brain natriuretic peptide (NT-proBNP; age >75 y: >1800 ng/L, age 50–75 y: >900 ng/L, age <50 y: >450 ng/L), specific signs (hepatic jugular vein reflux, increased jugular venous pressure, peripheral edema, pulmonary crack), and typical symptoms (fatigue, dyspnoea), left ventricular ejection fraction (LVEF) <50% 12 ; no patients present with serious cardiogenic shock. The diagnosis of AMI was based on the 2017 ESC “Guidelines for the management of AMI in patients presenting with ST segment elevation.” 13 Patients with AMI had at least one of the following conditions: myocardial signs of ischemia (12-lead ECG: ST segment elevation> 0.2 mm), continuous pectoralgia> 30 min, and at minimum a twofold increase in troponin I (TnI). 13 Finally, 59 HF post-AMI patients were incorporated into the study. All participants offered written informed consent prior to registration. The study design is described in detail in Figure S1. All procedures conducted in the study implicating human participants were in compliance with the Declaration of Helsinki. The protocol was registered with the Chinese Clinical Trial Registration Center and sanctified by the Medicine Ethics Committee of Shanghai Gong Li Hospital (KY2018-168).
Anthropometric measurements
Anthropometric measurements were performed on the day of admission. Participants’ weight and height measurement was taken barefoot and in casual indoor attire, using inflexible height measuring bars and calibrated electronic scales. The calculation of BMI used the standard BMI formula: weight (kg) divided by the height (m) square. Nonsmokers refer to patients who had stopped smoking ≥1 y or had never smoked before enrolment in this study, and the rest were classified as smokers, smoking more than one cigarette per day.
Blood sample preparation
Clinical variables included high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglyceride (TG), total cholesterol (TC), serum creatinine (SCr), estimated glomerular filtration rate (eGFR), cystatin C (CysC), urea nitrogen (UN), TnI, myoglobin, and creatine kinase-MB (CK-Mb). Ortho-3160 biochemical analyzer (Pencoed, Bridgend, UK) was used to determine serum HDL-C, LDL-C, TG, TC, TnI, CK-Mb, and other routine serum biochemical indicators. 14 NT-proBNP and LIPCAR levels were detected at the diagnosis of AMI (before PCI), intraprocedural and 4 h, 12 h, 24 h, and 72 h postoperatively. 14 Serum NT-proBNP determination was used the nanoscale Checker 710 biochemical analyzer (Nano-Ditech, Cranbury, NJ, USA). 14 Every blood samples were collected after the participants fasting overnight. All measured values were acquired from blinded quality control specimens in the Biochemical Laboratory of Shanghai Gong Li Hospital. All blood samples used for LIPCAR analysis were placed in the collecting vessels containing EDTA immediately and stored at –80°C prior to RNA extraction. 14 LIPCAR expression was detected by reverse transcription polymerase chain reaction (RT-PCR) analysis.
RNA extraction and real-time PCR analysis
Trizol reagent (Invitrogen, Carlsbad, USA) was used to isolate total RNA from venous samples and Nanodrop (Thermo Scientific, Rockford, IL, USA) was used for RNA quantification. cDNA synthesis used the Impromptu II reverse transcription kit (Promega, Madison, WI, USA) after the instructions of manufacturer. 14 In order to detect circulating levels of LIPCAR, RT-PCR was executed using GAPDH as an internal reference and SYBR Green to detect LIPCAR levels; the 2–ΔΔCT method was used to calculate the relative expression level of LIPCAR. The primers of the target genes were acquired from GenePharma (Shanghai, China). 14
Statistical analysis
The continuous variable is represented as the mean ± standard difference or median (quartile range), and the categorical variable is expressed as a number (percentage). Differences between continuous variables were assessed for normally distributed data using independent student’s t tests and Wilcoxon’s rank sum tests for asymmetrically distributed data. Categorical variables were analyzed using Chi-squared tests and Fisher’s exact tests. The Pearson correlation coefficient was used to determine the association between NT-proBNP and LIPCAR. Diagnostic test of value of LIPCAR was generated by receiver operating characteristic (ROC) web-tool. A P value <0.05 was considered statistically significant.
Results
The basic clinical characteristics of the research group
The study involved 68 patients without HF post-PCI and 59 patients with HF after PCI. The clinical features of the study group are exhibited in Table 1. There was no significant difference between the two groups in terms of gender (P = 0.876), age (P = 0.567), BMI (P = 0.945), history of smoking (P = 0.192), hypertension (P = 0.751), or diabetes (P = 0.447). In comparison to the group of non-HF, the group of HF had observably higher CysC (P < 0.001) and lower eGFR (P < 0.01), but no significant difference was noted between the two groups in terms of SCr (P = 0.313) and UN (P = 0.062). The levels of LIPCAR were significantly increased in the group of HF compared to the group of non-HF at AMI diagnosis (before PCI), intraprocedural and postoperatively at 4 h, 24 h, and 72 h (P < 0.001) and postoperatively at 12 h (P < 0.01; Table 1).
Table 1.
Anthropometric and biochemical characteristics of the subjects.
| HF post-AMI (n = 59) | Non-HF post-AMI (n = 68) | P value | |
|---|---|---|---|
| Male (n, %) | 33 (56) | 39 (57) | 0.872 |
| Age (years) | 63.85 ± 7.19 | 62.94 ± 10.47 | 0.567 |
| BMI (kg/m2) | 23.82 ± 2.91 | 23.86 ± 3.87 | 0.945 |
| Smoker (n, %) | 32 (54) | 29 (43) | 0.192 |
| Diabetic (n, %) | 22 (37) | 21 (31) | 0.447 |
| Hypertensive (n, %) | 41 (69) | 49 (72) | 0.751 |
| TG (mmol/L) | 1.36 ± 1.23 | 1.55 ± 0.72 | 0.295 |
| TC (mmol/L) | 4.26 ± 1.10 | 4.47 ± 1.06 | 0.276 |
| LDL-C (mmol/L) | 2.79 ± 0.96 | 2.95 ± 0.90 | 0.349 |
| HDL-C (mmol/L) | 1.01 ± 0.28 | 0.99 ± 0.42 | 0.774 |
| SCr (µmol/L) | 73 (60, 91) | 66 (50, 80) | 0.313 |
| UN (µmol/L) | 5.66 (3.98, 7.44) | 4.72 (4.05, 5.99) | 0.062 |
| CysC (mg/L) | 1.11 (0.94, 1.41) | 0.89 (0.74, 1.12) | <0.001*** |
| eGFR (mL/min/1.73m2) | 88 (67, 95) | 94 (85, 102) | <0.01** |
| LVEF (%) | 54.50 ± 9.22 | 61.22 ± 5.39 | <0.001** |
| LVFS (%) | 28.75 ± 5.88 | 32.83 ± 3.83 | <0.001** |
| LVEDD (mm) | 48.24 ± 6.20 | 47.38 ± 4.58 | 0.385 |
| NT-proBNP at AMI diagnosis (ng/L) | 2597 (1945, 4124) | 303 (100, 494) | <0.001** |
| Intraprocedural NT-proBNP (ng/L) | 589 (290, 950) | 202 (21, 438) | <0.001** |
| TnI | 18.41 ± 26.85 | 7.55 ± 18.26 | <0.01** |
| TIMI | 25.4 ± 0.95 | 2.66 ± 0.73 | 0.434 |
| Number of branch lesions | 0.85 ± 1.32 | 1.19 ± 1.40 | 0.159 |
| LIPCAR at AMI diagnosis | 5.45 (4.84, 15.33) | 0.0004 (0.0003, 0.44) | <0.001*** |
| Intraprocedural LIPCAR | 5.72 (2.66, 10.04) | 2.68 (2.46, 2.79) | <0.001*** |
| LIPCAR 4 h postoperative | 3.36 (2.91, 5.36) | 3.34 (1.85, 3.48) | <0.001** |
| LIPCAR 12 h postoperative | 2.94 (2.38, 4.30) | 2.99 (2.02, 3.11) | <0.01*** |
| LIPCAR 24 h postoperative | 3.24 (1.48, 3.95) | 2.16 (2.09, 2.29) | <0.001*** |
| LIPCAR 72 h postoperative | 3.68 (3.07, 4.23) | 0.58 (0.56, 0.61) | <0.001*** |
Results are expressed as mean ± standard deviation, median (interquartile range), or n (%).
BMI: body mass index; TG: triglycerides; TC: total cholesterol; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; SCr: serum creatinine; CysC: cystatin; UN: urea nitrogen; eGFR: estimated glomerular filtration rate; NT-proBNP: N-terminal pro-brain natriuretic peptide; LVEF: left ventricular ejection fraction; LVFS: left ventricular fractional shortening; LVEDD: left ventricular end-diastolic dimension; TnI: troponin I; TIMI: thrombolysis in myocardial infarction.
**P < 0.01, ***P < 0.001.
RT-PCR analyzes of LIPCAR expression
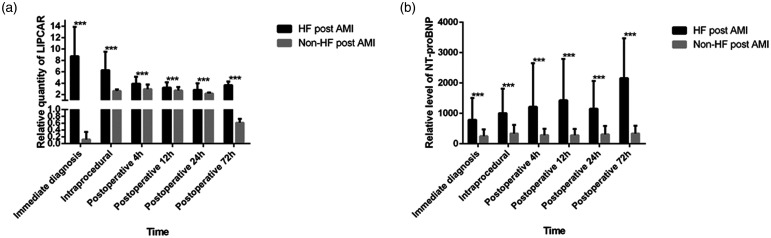
The peripheral blood LIPCAR levels of each group were measured by the RT-PCR method. The results showed that circulating LIPCAR levels at AMI diagnosis were observably higher in patients that developed HF compared to non-HF patients (Figure 1a, P < 0.001). This high level of circulating LIPCAR in HF patients gradually decreased following PCI but was still increased compared with non-HF patients 72 h after PCI (P < 0.001). Following PCI, circulating LIPCAR increased in patients without HF but decreased to normal levels by 72 h post-PCI. Levels of venous NT-proBNP were higher HF patients after AMI compared to patients with non-HF at 72 h after PCI (Figure 1b, P < 0.001).
Figure 1.
The levels of circulating LIPCAR in HF and non-HF patients after AMI (a) and the levels of NT-proBNP in HF and non-HF patients after AMI (b); ***P < 0.001.
HF: heart failure; AMI: acute myocardial infarction; NT-proBNP: N-terminal pro-brain natriuretic peptide.
Correlation between LIPCAR and clinicopathological characteristics
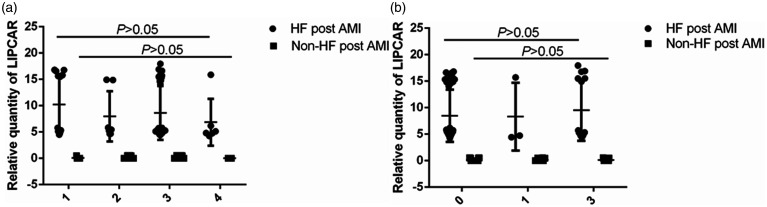
The Pearson correlation coefficient was used to assess the correlation between circulating LIPCAR and clinical characteristics. As shown in Table 2, at the time of diagnosis, the levels of LIPCAR were positively correlated with NT-proBNP levels (r2 = 0.643, P < 0.001) and negatively correlated with LVEF (r2 = 0.259, P < 0.001). No associations between LIPCAR and TnI (r2 = 0.018, P = 0.135) or CK-Mb (r2 = 0.038, P = 0.053) were observed. In addition, we found that the levels of circulating LIPCAR were not correlated with the burden of coronary disease or thrombolysis in myocardial infarction (TIMI) flow (Figure 2, Table 1, P > 0.05) in either of the two groups.
Table 2.
Pearson correlation coefficient of correlation between LIPCAR and clinicopathological characteristics.
| NT-proBNP |
LVEF |
TnI |
CK-Mb |
|||||
|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| LIPCAR | r2 = 0.643 | <0.001*** | r2 = 0.259 | <0.001*** | r2 = 0.018 | 0.135 | r2 = 0.038 | 0.053 |
NT-proBNP: N-terminal pro-brain natriuretic peptide; LVEF: left ventricular ejection fraction; TnI: troponin I; CK-Mb: creatine kinase-Mb. ***P < 0.001.
Figure 2.
Relationship between the level of intraoperative LIPCAR and the burden of coronary disease. The expression levels of LIPCAR in the patients of AMI with different coronary artery disease (a) and the expression levels of LIPCAR in the patients of AMI with different TIMI blood flow (b) HF: heart failure; AMI: acute myocardial infarction.
Diagnostic value of LIPCAR in HF post-AMI
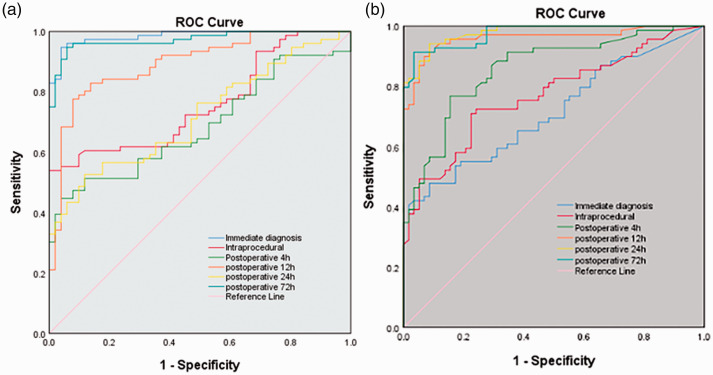
Having determined that LIPCAR is expressed in the peripheral and the levels of LIPCAR are increased in patients who develop HF after AMI, we attempted to ascertain the potential purpose of LIPCAR as a diagnostic marker for HF post-AMI. For this reason, the AUC calculation of ROC analysis proves the predictive ability of LIPCAR in diagnosis (AUC: 0.985), intraprocedural (AUC: 0.759) and postoperatively at 4 h (AUC: 0.686), 12 h (AUC: 0.894), 24 h (AUC: 0.719), and 72 h (AUC: 0.973) for HF post-AMI (Figure 3a). The ROC curve of NT-proBNP was calculated at the time of diagnosis (AUC: 0.714), at the intraprocedural timepoint (AUC: 0.769) and postoperatively at 4 h (AUC: 0.858), 12 h (AUC: 0.960), 24 h (AUC: 0.979), and 72 h (AUC: 0.975) (Figure 3b).
Figure 3.
ROC curve for HF patients post-AMI based on LIPCAR expression (a) and NT-proBNP expression (b). HF: heart failure; AMI: acute myocardial infarction. (A color version of this figure is available in the online journal.)
Discussion
In this study, we demonstrate that circulating LIPCAR is a standalone predictor of HF following AMI during hospitalization. Our results show that circulating LIPCAR levels are significantly elevated in patients with HF after AMI. Pearson correlation coefficient revealed that the circulating levels of LIPCAR are positively associated with NT-proBNP but negatively related to LVEF. The levels of circulating LIPCAR were positively related to the severity of HF. Our results indicate that circulating LIPCAR might be a promising early biomarker for HF post-AMI.
Increasing reports indicate that many noncoding RNAs originate from the mitochondria and that the contribution of mitochondrial lncRNAs to total lncRNA in the left ventricle is greater than 70%. This suggests that most of the circulating mitochondrial lncRNAs may be from the heart.15–17 Although it is still controversial whether LIPCAR is derived from the nucleus or mitochondria, it can be readily detected in the blood. 10 In patients with coronary heart disease (CAD), the correlation between elevated LIPCAR levels and the risk of infaust clinical outcomes reported that LIPCAR is independently related to the risk of CAD. 11 Another study has reported that the levels of circulating LIPCAR are increased in HF patients irrespectively of the pathogenesis, and higher levels of LIPCAR are significantly associated with cardiovascular mortality in patients with HF. 10 Our results show that circulating LIPCAR levels are remarkably higher in HF patients after AMI compared with patients who did not develop HF. While circulating levels of LIPCAR increase post-PCI in patients without HF, the levels return to normal by 72 h post-PCI. We also show that higher LIPCAR levels are independently correlated with increased risk of HF after AMI. In summary, the results imply that LIPCAR might be used to detect patients with undiagnosed or preclinical HF that is exacerbated by AMI, as well as to track events post-MI. Our results are in accord with recently published study which found that the levels of circulating LIPCAR is negatively associated with E/A peak flow, an index of mitral inflow that decreases with LV diastolic dysfunction, in nonelderly patients (mean age 57 y) without history of structural heart disease. 17
The mechanism potentially the correlation between LIPCAR and HF after AMI remains unclear. Be a mitochondrial-derived lncRNA, the underlying function of LIPCAR may be to regulate mitochondrial pathways, such as inflammasome activation and oxidative phosphorylation in the coronary arteries. 17 , 18 Moreover, Kumarswamy et al. indicated that LIPCAR is an autocephalous predictor of cardiac remodeling and predicts poor prognosis in patients with HF. 10 Further studies are requested to explore the relation between LIPCAR and HF post-AMI.
In this study, we also investigated the clinical significance of LIPCAR. Our findings indicate that abnormal LIPCAR expression is correlated with diagnosis of HF after AMI. The ROC curve of LIPCAR shows that its AUC is 0.985, which has a higher diagnostic value compared with NT-proBNP alone (AUC: 0.714).
Be careful to avoid bias in the present research. RT-PCR was blinded by a trained tester performed according to the manufacturer’s instructions. Furthermore, the propensity score matching was used to decrease the influence of result selection bias.
There are certain limitations in this study. First of all, it is a case-control study that it only present correlations, not causation. Second, all HF patients and some patients of non-HF were given drugs after surgery. In this study, the effects of drugs on LIPCAR levels have not been investigated. Third, the potential mechanism related to the upregulation of LIPCAR and the seriousness of HF after AMI requires further research; hence, the sample size should be expanded in the future to ulterior study the HF mechanism and prognosis of such patients. Fourth, since all the participants of the study were Chinese, the results of the study might not be related to other races and ought to be confirmed among other populations.
Conclusions
The levels of LIPCAR were significantly increased in HF patients after AMI compared to patients without HF after AMI. In addition, we confirmed that the levels of circulating LIPCAR were positively correlated with the seriousness of HF post-AMI. Nevertheless, the mechanism of action of LIPCAR on cardiovascular physiology and pathology still requires further study.
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_15353702211036055 for Circulating LIPCAR is a potential biomarker of heart failure in patients post-acute myocardial infarction by Li Yan, Yu Zhang, Mei Wang, Lu Wang, Wei Zhang and Zhi-Ru Ge in Experimental Biology and Medicine
Footnotes
AUTHORS’ CONTRIBUTIONS: Z-RG and LY designed the experiments and supervised the study. LY and YZ analyzed the data and wrote the manuscript. MW and LY performed the experiments. LW, WZ, and YZ collected samples and the clinical data. All authors have reviewed and approve the content manuscript.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ETHICAL APPROVAL: The study was approved by the Medicine Ethics Committee, Shanghai Gong Li Hospital, which is registered in the Chinese Clinical Trial Registry (KY2018-168).
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by Key Special Construction Project of Pudong Health and Family Planning Commission of Shanghai (grant number PWZzk2017-23), supported by Science and Technology Development Foundation of Pudong New Area (PKJ2020-Y26).
ORCID iD: Zhi-Ru Ge https://orcid.org/0000-0002-3733-4835
SupplementAL MATERIAL :Supplemental material for this article is available online.
References
- 1.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003; 361:13–20 [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Zhao T, Wei X, Lu H, Lin X. The prevalence of 30-day readmission after acute myocardial infarction: a systematic review and meta-analysis. Clin Cardiol 2019; 42:889–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bahit MC, Kochar A, Granger CB. Post-myocardial infarction heart failure. JACC Heart Failure 2018; 6:179–86 [DOI] [PubMed] [Google Scholar]
- 4.Hausenloy DJ. Cardiac innervation in acute myocardial ischaemia/reperfusion injury and cardioprotection. Cardiovasc Res 2019; 115:1167–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet 2016; 17:47–62 [DOI] [PubMed] [Google Scholar]
- 6.Derrien T, Guigó R. Long non-coding RNAs with enhancer-like function in human cells. Med Sci (Paris) 2011; 27:359–61 [DOI] [PubMed] [Google Scholar]
- 7.Jiang X, Ning Q. The emerging roles of long noncoding RNAs in common cardiovascular diseases. Hypertens Res 2015; 38:375–9 [DOI] [PubMed] [Google Scholar]
- 8.Huang Y. The novel regulatory role of lncRNA-miRNA-mRNA axis in cardiovascular diseases. J Cell Mol Med 2018; 22:5768–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu S, Kamato D, Little PJ, Nakagawa S, Pelisek J, Jin ZG. Targeting epigenetics and non-coding RNAs in atherosclerosis: from mechanisms to therapeutics. Pharmacol Ther 2019; 196:15–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumarswamy R. Circulating long noncoding RNA, LIPCAR, predicts survival in patients with heart failure. Circ Res 2014; 114:1569–75 [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z, Gao W, Long QQ, Zhang J, Li YF, Liu DC, Yan JJ, Yang ZJ, Wang LS. Increased plasma levels of lncRNA H19 and LIPCAR are associated with increased risk of coronary artery disease in a Chinese population. Sci Rep 2017; 7:7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Rev Esp Cardiol (Engl Ed) 2016; 69:1167. [DOI] [PubMed] [Google Scholar]
- 13.Ibanez B. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018; 39:119–77 [DOI] [PubMed] [Google Scholar]
- 14.Yan L, Zhang Y, Zhang W, Deng SQ, Ge ZR. lncRNA-NRF is a potential biomarker of heart failure after acute myocardial infarction. J Cardiovasc Transl Res 2020; 13:1008–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rackham O. Long noncoding RNAs are generated from the mitochondrial genome and regulated by nuclear-encoded proteins. RNA 2011; 17:2085–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ro S. The mitochondrial genome encodes abundant small noncoding RNAs. Cell Res 2013; 23:759–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Gonzalo-Calvo D. Circulating long-non coding RNAs as biomarkers of lef ventricular diastolic function and remodelling in patients with well-controlled type 2 diabetes. Sci Rep 2016; 6:37354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorn GW., 2nd. LIPCAR: a mitochondrial lnc in the noncoding RNA chain? Circ Res 2014; 114:1548–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_15353702211036055 for Circulating LIPCAR is a potential biomarker of heart failure in patients post-acute myocardial infarction by Li Yan, Yu Zhang, Mei Wang, Lu Wang, Wei Zhang and Zhi-Ru Ge in Experimental Biology and Medicine