Abstract
High levels of blood glucose and lipids are well-known risk factors for heart diseases. Bee venom is a natural product that has a potent hypoglycemic, hypolipidemic, anti-inflammatory, and antioxidant effects. The current study aimed to determine the bee venom effects on cardiac dysfunction compared to combined therapy of metformin and atorvastatin in diabetic hyperlipidemic rats. The median lethal dose of bee venom was estimated, and then 50 adult male albino rats were categorized into five groups. One group was fed a standard diet and served as a negative control, while the other groups were given nicotinamide and streptozotocin injections to induce type 2 diabetes. After confirming diabetes, the rats were fed a high-fat diet for four weeks. The four groups were divided as follows: one group served as a positive control, whereas the other three groups were treated with bee venom (0.5 mg/kg), bee venom (1.23 mg/kg), and combined therapy of metformin (60 mg/kg) and atorvastatin (10 mg/kg), respectively, for four weeks. Upon termination of the experiment, blood samples and heart tissue were obtained. Administration of bee venom using both doses (0.5 and 1.23 mg/kg) and combined therapy of metformin and atorvastatin revealed a significant decrease in the concentrations of glucose, total cholesterol, triacylglycerol, low-density lipoprotein cholesterol, very low-density lipoprotein cholesterol, troponin I, creatine kinase, and lactate dehydrogenase activities. Moreover, a significant decrease had been detedcted in malondialdehyde, nuclear factor-kappa-β levels, and relative mRNA expression of vascular cell adhesion molecule-1 and galectin-3 in heart tissue compared to the positive control (P < 0.0001). Furthermore, there was a significant increase in bodyweight levels of insulin, high-density lipoprotein cholesterol, and total antioxidant capacity in heart tissue compared to the positive control (P < 0.0001). The results indicate that bee venom can ameliorate cardiac dysfunction through attenuating oxidative stress and downregulating the NF-κβ signaling pathway.
Keywords: Heart, bee venom, diabetes, hyperlipidemia, oxidative stress, NF-κβ, VCAM-1, galectin-3
Impact statement
Diabetes and hyperlipidemia are associated with a significantly higher risk of cardiovascular diseases. The present study describes the cardiovascular complications in diabetic hyperlipidemic rats with an emphasis on the importance of using natural products such as bee venom to treat cardiovascular diseases. Bee venom can attenuate cardiac dysfunction in diabetic hyperlipidemic rats in a dose-dependent manner compared to the combined therapy of metformin and atorvastatin. Instead of synthetic drugs, bee venom can be used as an alternative therapy for treating diabetes associated with hyperlipidemia.
Introduction
Type 2 diabetes mellitus (T2DM) is a metabolic disorder defined by persistent hyperglycemia, deficiency in insulin secretion and insulin response defects. 1
Diabetes mellitus contributes to quality-of-life impairments, including cardiovascular diseases (CVDs), retinopathy, which can lead to blindness, a renal failure because of diabetic nephropathy (DN), autonomic neuropathy, that can result in bowel and bladder dysfunction risk, and foot ulcers risk and difficulty walking.2–5
Hyperlipidemia is a metabolic disease described by elevated levels of triacylglycerol (TAG), total cholesterol (TC), and low-density lipoprotein-cholesterol (LDL-C), and reduced levels of high-density lipoprotein-cholesterol (HDL-C). 6 The presence of hyperlipidemia, along with diabetes mellitus, increases the chance of developing cardiovascular disease. 7
The association between diabetes mellitus, hyperlipidemia, and cardiovascular diseases can be attributed to oxidative stress and inflammation. 8 Excess reactive oxygen species induce nuclear factor kappa (NF-κβ) activation, promoting the transcription of inflammatory mediators and triggers myocardial inflammation. 9
In the development of alternative therapies for many illnesses, much attention has been given to natural products, including certain species’ venom. 10 Bee venom consists of low molecular ingredients, enzymes, and peptides. These peptides include adolapin, apamin, melittin, and mast cell degranulating peptide. Hyaluronidase, phosphatase, ᾳ-glucosidase, phospholipaseA2 (PLA2), and phospholipase B enzymes are included. In addition to some carbohydrates, non-peptides like histamine, dopamine, and norepinephrine are also present in bee venom components. 11
The use of bee venom in the treatment of a variety of diseases has shown to be effective. 12 Several studies have shown bee venom is utilized as a hypoglycemic, hypolipidemic, anti-inflammatory, and antioxidant agent.13–16
Melittin, apamin, and phospholipase A2 are the key components of bee venom. Individual components, as well as the whole bee venom, have been shown to exhibit myocardium protective effects through regulation of inflammation, magnesium ions, calcium ions, and activation of peroxisome proliferator-activated receptor-α and -γ. Phospholipase A2 inhibits lipid accumulation in the aortic valve and aorta, and protects against atherosclerosis by suppressing the transition of macrophages into foam cells. 17
This research aims to determine the bee venom effects on cardiac dysfunction compared to the combined therapy of metformin and atorvastatin in diabetic hyperlipidemic rats.
Materials and methods
Bee venom
The Carniolan bee venom (Apis mellifera carnica) specimen was obtained from the Beekeeping Research Department of the Plant Protection Research Institute of the Agriculture Research Centre in Dokki, Giza governorate, Egypt.
Chemicals and drugs
Streptozotocin and nicotinamide were purchased from sigma chemicals Co. (St. Louis, Mo. USA). Metformin hydrochloride and atorvastatin calcium were obtained as a gift from Amoun pharmaceutical company, Egypt. Cholesterol, cholic acid, and tert. Butylhydroquinone were obtained from Alpha chemical company, India. Vitamins mixture, minerals mixture, and L-cystine were obtained from Acros-Organics Chemical Co. (USA).
Animals
Adult male albino rats weighing 150–170 g were housed in the animal house of the Faculty of Science, Zagazig University. Rats were kept under laboratory conditions of temperature (20–25°C), humidity (60–65%), and 12-h light/dark cycle. Rats had free access to tap water and a regular chow diet ad libitum. The Ethical Committee of Zagazig University approved the experimental design and animal handling (Approval number ZU-IAUUC/1/F/32/2019).
Determination of the median lethal dose of carniolan bee venom
The median lethal dose (LD50) of carniolan bee venom was calculated by arranging ascending concentrations of five bee venom doses in regular progression, starting with a dose that kills approximately 0–20% of the animals and ending with a dose that kills approximately 80–100% of the injected animals. Each dose level was tested in five rats, and all injections were given intraperitoneally. Mortality and survival rates of injected animals were recorded after 24 h from the time of injection. The following equations were used to measure LD50 using a previous method: 18
Log LD50 = log dose next below 50% + [log increasing factor × proportional distance]. Proportional distance = [50% – mortality next below]/[mortality next above – mortality next below].
Preparation of experimental diets
The American Institute of Nutrition designed the standard diet, 19 and the high-fat diet (HFD) had the same components as the standard diet with the addition of 23% fats, 1% cholesterol, and 0.2% cholic acid, 20 as shown in Table 1.
Table 1.
Standard diet and high-fat diet composition.
| Ingredients(g/100g diet) | Standard diet | High-fat diet |
|---|---|---|
| Corn starch | 57.0692 | 32.8692 |
| Casein | 14 | 14 |
| Sucrose | 10 | 10 |
| Soyabean oil | 4 | 4 |
| Cellulose | 5 | 5 |
| Mixture of vitaminsa | 3.5 | 3.5 |
| Mixture of mineralsa | 1 | 1.0 |
| L-cystine | 0.18 | 0.18 |
| choline bitartrate | 0.25 | 0.25 |
| Tert. Butylhydroquinone | 0.0008 | 0.0008 |
| Fats (tallow) | 5 | 28 |
| Cholesterol | 0 | 1 |
| Cholic acid | 0 | 0.2 |
aMixtures of minerals and vitamins that contributed to the diets were in line with the guidelines of American Institute of Nutrition. 19 .
Type 2 diabetes induction in rats
Type 2 diabetes was induced in 18-h fasted rats (without food but allowed free access to water) with a single intraperitoneal streptozotocin (60 mg/kg) injection dissolved in 0.1 M citrate buffer (pH 4.5) after 15 min of intraperitoneal injection of nicotinamide (120 mg/kg) dissolved in saline. 21 After 1 h of streptozotocin and nicotinamide administration, rats were given 5% glucose solution overnight to prevent hypoglycemia. Fasting blood glucose was determined using a portable glucometer after 72 h and on the seventh day of injection. Rats had a fasting blood glucose above 250 mg/dL were considered diabetic. 22
Hyperlipidemia induction
Diabetic rats were given a high-fat diet for four weeks to induce hyperlipidemia, while the negative control animals were fed a standard diet. The composition of experimental diets is presented in Table 1. At the end of the fourth week, about 1 mL of blood has been obtained from one of the lateral tail vein of rat using a 23-G needle. 23 Blood was collected in a free anti-coagulant tube to obtain serum in order to measure lipid profile to verify hyperlipidemia induction. 24 Subsequently, the high-fat diet was given along with treatment administration for four weeks.
Experimental design
After one week of acclimatization, 50 adult male albino rats were categorized into five groups (10/group) as listed below:
Group I: Negative control: Normal rats received saline solution by intraperitoneal injections daily for four weeks.
Group II: positive control: Diabetic hyperlipidemic rats.
Group III: Bee venom-treated group (0.5 mg/kg): Diabetic hyperlipidemic rats received bee venom (0.5 mg/kg) dissolved in distilled water by intraperitoneal injections daily for four weeks. 25
Group IV: Bee venom-treated group (1.23 mg/kg): Diabetic hyperlipidemic rats received bee venom (1.23 mg/kg) dissolved in distilled water by intraperitoneal injections daily for four weeks. This dose was determined according to its LD50, and 1/10th of LD50 (1.23 mg/kg) was taken as a therapeutic dosage. 26
Group V: Metformin and atorvastatin-treated group: Diabetic hyperlipidemic rats received metformin (60 mg/kg) and atorvastatin (10 mg/kg) dissolved in distilled water orally administrated daily for four weeks. 27
Monitoring of bodyweight and blood glucose throughout the treatment period
The bodyweights and fasting blood glucose rates were recorded weekly throughout the treatment period. The doses of bee venom, metformin, and atorvastatin were adjusted weekly to maintain the same dosage during the treatment period.
Specimen collection
Upon termination of the experiment, rats were fasted for 10 h, and all rats were euthanized by urethane. A drop of blood obtained from each rat tail-tip was utilized for measuring fasting blood glucose. Ten blood specimens from each group were obtained from the retro-orbital venous plexus in two tubes: one containing EDTA to get plasma via centrifugation at 4000 r/min for 20 min to estimate the insulin concentration, and the other is a free anti-coagulant tube to get serum via centrifugation at 4000 r/min for 20 min to evaluate the lipid profile and heart function tests. All these specimens were kept at −20°C until biochemical analysis. 28 Heart tissue was excised from each rat, rinsed in saline solution, cleared off blood, and cut into three parts. The first part (0.2 g) from each rat's heart tissue was homogenized in 2 mL phosphate buffer saline (pH 7.4) using a Teflon homogenizer, and then the homogenate was centrifuged at 3500 r/min for 15 min at 4°C. 29 The supernatants were used to determine the concentration of total antioxidant capacity, malondialdehyde, and nuclear factor-kappa-β for histopathological examination. The second portion of the heart tissue was immersed in 10% formalin solution, whereas the third part of heart tissue was kept at −80°C until RNA isolation. 30
Biochemical parameters
Fasting blood glucose
Fasting blood glucose (FBG) was measured using a portable glucometer (Right Tango, TD-4235, Taiwan) according to the method in literature. 31
Plasma insulin
Plasma insulin was estimated according to a previously described method 32 using an ELISA kit derived from Cloud-Clone Crop, USA.
Lipid profile
Serum triacylglycerol (TAG), total cholesterol (TC), and HDL-C were measured according to previous methods,33–35 using commercial kits purchased from Spectrum Diagnostics Company, Egypt. LDL-C was calculated by Friedewald’s equation: LDL-C = TC- (TAG/5+ HDL-C). 36 VLDL-C was calculated by Nobert’s equation: VLDL-C = TAG/5. 37
Serum creatine kinase, lactate dehydrogenase, and troponin I
Serum creatine kinase (CK-MB) was estimated according to a previous method 38 using commercial kits derived from Spectrum Diagnostics company, Egypt. Serum lactate dehydrogenase (LDH) was evaluated according to the method 39 using commercial kits purchased from Spinreact, Spain. Serum troponin I (TnI) was estimated according to a previous method 40 using ELISA kits derived from Eagle Biosciences company, USA.
Total antioxidant capacity, malondialdehyde, and nuclear factor-kappa-β
Total antioxidant capacity (TAC) and malondialdehyde (MDA) were estimated in heart tissue homogenate according to previous methods,41,42 using a commercial kit purchased from Biodiagnostic Company, Egypt. Nuclear factor-kappa-β (NF-κβ) was assessed in heart tissue homogenate based on an earlier method 43 using an ELISA kit derived from Cloud-Clone Crop, USA.
Quantification real-time polymerase chain reaction
RNA was extracted from six heart tissue samples in each group using TRIzol™ Reagent (Invitrogen, USA) according to the manufacturer’s protocol. The concentration and purity of the extracted RNA were estimated by measuring the absorbance at 260/280 nm using Beckman dual spectrophotometer, USA. RNA specimens with A260/A280 ratio between 1.9 and 2.1 were utilized for further analysis. A high-capacity cDNA synthesis kit (Qiagen, USA) was used to reverse transcribe 2 µg of extracted RNA into cDNA. Relative mRNA expression of vascular cell adhesion molecule-1(VCAM-1) and Galectin-3 was estimated by quantitative real-time PCR using SYBR Green Master Mix kit (Qiagen, Germany) using a real-time PCR System (Biometra, Germany). The real-time PCR thermal cycler was programmed as follows: 95°C for 5 min, 40 cycles (95°C for 10 sec, 55°C for 10 s, and 72°C for 30 s), and finally 72°C for 5 min. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was utilized as a housekeeping gene. The primer sequence of studied genes is listed in Table 2. The 2−ΔΔct formula determined the relative expression of the amplified products in the target genes. 44
Table 2.
Primer sequences and gene bank accession number of studied genes.
| Genes | Primer sequences | Gene bank accession number |
|---|---|---|
| VCAM-1 | Forward 5′-TAAGTTACACAGCAGTCAAATGGA-3′ | NM_012889.1 |
| Reverse 5′-CACATACATAAATGCCGGAATCTT-3′ | ||
| Galectin-3 | Forward 5′-ATGGCAGACGGCTTCTCACT-3′ | KF639958.1 |
| Reverse 5′-CGCGAAGGGTGCGGTACTAG-3′ | ||
| GAPDH | Forward 5′-ACCACAGTCCATGCCATCAC-3′ | XM_032909104.1 |
| Reverse 5′-TCCACCACCCTGTTGCTGTA -3′ |
Histopathological examination
Six samples of heart tissue from each group were used for histopathological examination. A piece of heart tissue was immersed for 24 h in 10% formalin for tissue fixation. The fixed tissues have been sectionalized, deparaffinized, rinsed, and stained with H&E for histopathological examination. 45
Quantification analysis of area percentage (area %) of abnormal myocytes
Area percentage (Area %) of abnormal myocytes (Cell Number/Specific area) was evaluated in six non-overlapping fields from different heart sections of each group. Cell numbers were calculated as the number of cells per cross-sectional area using some features (cell counter/color deconvolution/color threshold) of the Image J program (version 1.6.0_20, National Institutes of Health, Bethesda, MD). 46 They were used for the evaluation of area percentage (Area %) of abnormal myocytes in different heart sections.
Statistical analysis
The statistical analysis was done by the Social Science Package version 25 (SPSS). 47 Normality of the data was first tested using Shapiro–Wilk test. The data were shown as mean ± SEM. The statistical significance was determined using two-way ANOVA test followed by Tukey’s post hoc test. A value of P < 0.05 is statistically significant.
Results
The median lethal dose (LD50) of carniolan bee venom
The median lethal dose (LD50) of carniolan bee venom was 12.3 mg/kg by calculating the results in Table 3.
Table 3.
Carniolan bee venom toxicity in rats.
| Dosemg/kg(a) | Dead (b) | Alive (c) | Total(d) |
Percent of mortality(e) (%) | |
|---|---|---|---|---|---|
| Dead(d1) | Alive (d2) | ||||
| 10.9 | 1 | 4 | 1 | 10 | 9.09 |
| 11.9 | 2 | 3 | 3 | 6 | 33.33 |
| 12.9 | 3 | 2 | 6 | 3 | 66.66 |
| 14.1 | 4 | 1 | 10 | 1 | 90.9 |
| 15.4 | 5 | 0 | 15 | 0 | 100 |
Note: Column (a) represents tested doses. The numbers of dead and alive rats after 24 h of doses injection are entered in column (b) and (c). Column (d) represents the accumulated number of dead and alive rats at given doses. Column (b) is therefore added from the top, and the subtotal for each dose is entered in column (d1) as the accumulated number of dead rats at given doses plus those lower doses. Column (c) is therefore added from the bottom, and the subtotal for each dose is entered in column (d2) as the accumulated number of alive rats at given doses plus higher doses. Percent of mortality is calculated from column (d1 and d2) and is entered in column (e).
Serum lipid profile levels after hyperlipidemia induction
Table 4 summarizes the results of the lipid profile after consumption of standard and high-fat diets for four weeks. Triacylglycerol, total cholesterol, LDL-C, and VLDL-C showed a significant elevation, while HDL-C levels showed a significant reduction in the positive control group compared to the negative control group (P < 0.0001).
Table 4.
Serum lipid profile levels after hyperlipidemia induction.
| Groups | Negative control | Positive control | P value |
|---|---|---|---|
| Triacylglycerol (mg/dL) | 70.5 ± 1.29c | 181.9 ± 1.8 | <0.0001 |
| % change | ----- | 158% | |
| Total cholesterol (mg/dL) | 71.7 ± 1.36c | 189.3 ± 1.58 | |
| % change | ----- | 164% | |
| LDL-C (mg/dL) | 29.3 ± 1.07c | 135.12 ± 2.3 | |
| % change | ----- | 361.2% | |
| VLDL-C (mg/dL) | 14.1 ± 0.25c | 36.38 ± 0.36 | |
| % change | ----- | 158% | |
| HDL-C (mg/dL) | 28.3 ± 0.66c | 19.1 ± 0.43 | |
| % change | ----- | −32.5% |
Note: Values are expressed in mean ± SEM, n = 10. Statistical analysis was performed by two-way ANOVA test followed by Tukey's post hoc test. Values with different superscript letters represent significant differences (P < 0.05). aP < 0.05, bP < 0.01, cP < 0.001 compared to positive control group. % change of positive control was calculated according to negative control.
Effect of bee venom using both doses (0.5 and 1.23 mg/kg) and combined therapy (metformin and atorvastatin) on bodyweight and blood glucose
Table 5 summarizes the results of bodyweight monitoring during the experimental period for all studied groups. Bodyweight was significantly reduced (P < 0.0001) in the positive control group compared to the negative control group during the experimental period. Meanwhile, the bodyweight was gradually increased till the end of the treatment period in bee venom-treated groups (0.5 and 1.23 mg/kg), and metformin and atorvastatin-treated group compared to the positive control group (P < 0.0001).
Table 5.
Effect of bee venom using both doses (0.5 and 1.23 mg/kg) and combined therapy (metformin and atorvastatin) on body weight.
| Groups | Negative control | Positive control | Bee venom treated group(0.5 mg/kg) | Bee venom treated group (1.23 mg/kg) | Metformin- and atorvastatin- treated group | P value |
|---|---|---|---|---|---|---|
| Initial | 268.7 ± 5.8c | 134.5 ± 1.55 | 147.5 ± 1.88 | 157.4 ± 2.58c | 149.5 ± 2.31a | < 0.0001 |
| % change | ----- | −49.94% | 9.66% | 17% | 11.15% | |
| 1st week | 281.9 ± 7.65c | 132.1 ± 1.38 | 151.5 ± 1.91a | 163.9 ± 4.2c | 156.2 ± 2.59b | |
| % change | ----- | −53.14% | 14.68% | 24.07% | 18.24% | |
| 2nd week | 291.7 ± 8.76c | 127 ± 1.67 | 154.2 ± 2.17b | 166 ± 4.3c | 158.3 ± 2.49c | |
| % change | ----- | −56.46% | 21.41% | 30.7% | 24.64% | |
| 3rd week | 303.4 ± 9.77c | 119.8 ± 1.31 | 160.1 ± 2.92c | 169 ± 4.54c | 160.7 ± 2.49c | |
| % change | ----- | −60.5% | 33.63% | 41.06% | 34.14% | |
| 4th week | 314.9 ± 9.28c | 107.2 ± 2.07 | 165.5 ± 3.15c | 176 ± 4.9c | 170.3 ± 2.42c | |
| % change | ----- | −65.95% | 54.3% | 64.17% | 58.86% |
Initial means one day before administration of different treatments. Values are expressed in mean ± SEM, n= 10. Statistical analysis was performed by two-way ANOVA test followed by Tukey's post hoc test. Values with different superscript letters represent significant differences (P < 0.05). aP < 0.05, bP < 0.01, cP < 0.001 compared to positive control group. % change of positive control was calculated according to negative control. % change of all treated groups was calculated according to positive control.
Table 6 presents the results of fasting blood glucose monitoring during the experimental period of all studied groups. The fasting blood glucose levels were significantly elevated in the positive control group than the negative control group during the experimental period. Meanwhile, the levels of fasting blood glucose were gradually diminished till the end of the treatment period in bee venom-treated groups (0.5 and 1.23 mg/kg) and metformin and atorvastatin-treated group compared to the positive control group (P < 0.0001).
Table 6.
Effect of bee venom using both doses (0.5 and 1.23 mg/kg) and combined therapy (metformin and atorvastatin) on fasting blood glucose levels.
| Groups | Negative control | Positive Control | Bee venom- treated group (0.5 mg/kg) | Bee venom- treated group (1.23 mg/kg) | Metformin- and atorvastatin-treated group | P value |
|---|---|---|---|---|---|---|
| Initial | 97.4 ± 2.04c | 479.3 ± 16.6 | 501.9 ± 14.36 | 484.7 ± 6.49 | 482.8 ± 10.6 | < 0.0001 |
| % change | ----- | 392% | 4.7% | 1.13% | 0.7% | |
| 1st week | 98.9 ± 2.12c | 486.9 ± 21.6 | 450.8 ± 22.4a | 368.3 ± 11.9c | 355.5 ± 12.5c | |
| % change | ----- | 392.3% | −7.41% | −24.35% | −26.98% | |
| 2nd week | 95.6 ± 3.01c | 499.5 ± 23.7 | 336.4 ± 14.2c | 288.4 ± 7.5c | 266.6 ± 17.6c | |
| % change | ----- | 422.48% | −32.65% | −42.26% | −46.6% | |
| 3rd week | 101 ± 3.3c | 514.7 ± 16.9 | 236.7 ± 11.35c | 181 ± 7.06c | 174.2 ± 6.7c | |
| % change | ----- | 365.34% | −54% | −64.83% | −66.15% | |
| 4th week | 100.4 ± 3.5c | 520.6 ± 19.9 | 157.9 ± 5.12c | 118.9 ± 2.78c | 109 ± 3.23c | |
| % change | ----- | 418.5% | −69.6% | −77.16% | −79.06% |
Note: Initial means one day before administration of different treatments. Values are expressed in mean ± SEM, n= 10. Statistical analysis was performed by two-way ANOVA test followed by Tukey's post hoc test. Values with different superscript letters represent significant differences (P < 0.05). aP < 0.05, bP < 0.01, cP < 0.001 compared to positive control group. % change of positive control was calculated according to negative control. % change of all treated groups was calculated according to positive control.
Effect of bee venom using two doses (0.5 and 1.23 mg/kg) and combined therapy (metformin and atorvastatin) on levels of insulin and lipid profile
Table 7 displays the results of plasma insulin and lipid profile levels of all studied groups. For diabetic hyperlipidemic rats, a significant elevation in triacylglycerol, total cholesterol, LDL-C, and VLDL-C and a significant reduction (P < 0.0001) in HDL-C and plasma insulin levels were observed compared to the negative control. Treatment with bee venom using two doses (0.5 and 1.23 mg/kg) and a combination of metformin and atorvastatin significantly ameliorated (P < 0.0001) the undesirable changes in insulin and lipid profile compared to the positive control group.
Table 7.
Effect of bee venom using both doses (0.5 and 1.23 mg/kg) and combined therapy (metformin and atorvastatin) on levels of insulin and lipid profile.
| Groups | Negative control | Positive control | Bee venom-treated group (0.5 mg/kg) | Bee venom- treated group (1.23 mg/kg) | Metformin- and atorvastatin- treated group | P value |
|---|---|---|---|---|---|---|
| Insulin (pg/mL) | 126 ± 1.16c | 57.9 ± 1.32 | 82.7 ± 2.03c | 121.6 ± 0.96c | 123.1 ± 1.14c | <0.0001 |
| % change | --------- | −54.05% | 42.83% | 110.01% | 112.61% | |
| Triacylglycerol (mg/dL) | 82.8 ± 1.75c | 227.7 ± 4.3 | 158.5 ± 2.3c | 120.7 ± 1.7c | 108 ± 1.43c | |
| % change | --------- | 175% | −30.25% | −47% | −52.56% | |
| Total cholesterol (mg/dL) | 84.3 ± 1.57c | 235.6 ± 2.8 | 166.9 ± 2.25c | 104.6 ± 1.52c | 96.5 ± 1.15c | |
| % change | --------- | 179.47% | −29.15% | −55.6% | −59% | |
| LDL-C (mg/dL) | 40.14 ± 1.32c | 178.24 ± 2.5 | 113.6 ± 2.32c | 54.96 ± 1.63c | 46.1 ± 1.13c | |
| % change | --------- | 344.04% | −36.26% | −69.16% | −74.13% | |
| VLDL-C (mg/dL) | 16.56 ± 0.35c | 45.66 ± 0.91 | 31.7 ± 0.46c | 24.14 ± 0.34c | 21.6 ± 0.28c | |
| % change | --------- | 175.72% | −30.57% | −47.13% | −52.69% | |
| HDL-C (mg/dL) | 27.6 ± 0.47c | 11.7 ± 0.34 | 21.6 ± 0.45c | 25.5 ± 0.75c | 28.8 ± 0.62c | |
| % change | --------- | -57.6% | 84.6% | 117.9% | 146.15% |
Note: Values are expressed in mean ± SEM, n= 10. Statistical analysis was performed by two-way ANOVA test followed by Tukey's post hoc test. Values with different superscript letters represent significant differences (P < 0.05). aP < 0.05, bP < 0.01, cP < 0.001 compared to positive control group. % change of positive control was calculated according to negative control. % change of all treated groups was calculated according to positive control.
Effect of bee venom using both doses (0.5 and 1.23 mg/kg) and combined therapy (metformin and atorvastatin) on serum CK-MB, LDH, and TnI
Table 8 shows the results of serum creatine kinase-MB (CK-MB), lactate dehydrogenase (LDH) activities and troponin I (TnI) for all studied groups. CK-MB and LDH activities were significantly elevated in the positive control as compared to the negative control group (P < 0.0001), while their activity was significantly reduced in bee venom-treated groups (0.5 and 1.23 mg/kg) and metformin and atorvastatin-treated group when compared to the positive control group (P < 0.0001). Concentration of serum troponin I (TnI) was significantly increased in the positive control when compared to the negative control group (P < 0.0001), whereas its level was significantly reduced (P < 0.0001) in bee venom-treated groups (0.5 and 1.23 mg/kg) and metformin and atorvastatin-treated group when compared to the positive control group.
Table 8.
Effect of bee venom using both doses (0.5 and 1.23 mg/kg) and combined therapy (metformin and atorvastatin) on serum CK-MB, LDH, and TnI.
| Groups | Negative control | Positive control | Bee venom- treated group(0.5 mg/kg) | Bee venom- treated group(1.23 mg/kg) | Metformin- and atorvastatin -treated group | P value |
|---|---|---|---|---|---|---|
| CK-MB(U/L) | 210.8 ± 2.48c | 527.4 ± 28.39 | 324.9 ± 15.17c | 252.3 ± 4.95c | 240.8 ± 6.75c | <0.0001 |
| % change | ----- | 150.2% | −38.39% | −52.16% | −54.34% | |
| LDH (U/L) | 498.4 ± 2.65c | 1188.5 ± 42.57 | 711 ± 39.9c | 570.7 ± 10.45c | 567.2 ± 22.7c | |
| % change | ----- | 138.46% | −40.17% | −51.95% | −52.27% | |
| TnI (ng/mL) | 0.081 ± 0.004c | 0.167 ± 0.006 | 0.11 ± 0.005c | 0.089 ± 0.007c | 0.092 ± 0.003c | |
| % change | ----- | 106.17% | −34.13% | −46.7% | −44.9% |
Note: Values are expressed in Mean ± SEM, n= 10. Statistical analysis was performed by two-way ANOVA test followed by Tukey's post hoc test. Values with different superscript letters represent significant differences (P < 0.05). aP < 0.05, bP < 0.01, cP < 0.001 compared to positive control group. % change of positive control was calculated according to negative control. % change of all treated groups was calculated according to positive control.
Effect of bee venom using both doses (0.5 and 1.23 mg/kg) and combined therapy (metformin and atorvastatin) on TAC, MDA, and NF-κβ levels in heart tissue
Total antioxidant capacity (TAC), malondialdehyde (MDA), and nuclear factor-kappa-β (NF-κβ) levels in cardiac tissue of all studied groups are summarized in Table 9. TAC was significantly decreased, whereas MDA and NF-κβ were markedly increased in the positive control group when compared to the negative control (P < 0.0001). Compared to the positive control, the undesired elevations in MDA, NF-κβ, and decrease in TAC were substantially improved after the administration of bee venom (either 0.5 or 1.23 mg/kg) and the combined therapy of metformin and atorvastatin (P < 0.0001).
Table 9.
Effect of bee venom using both doses (0.5 and 1.23 mg/kg) and combined therapy (metformin and atorvastatin) on levels of TAC, MDA, and NF-κβ in heart tissue.
| Groups | Negative control | Positive control | Bee venom-treated group (0.5 mg/kg) | Bee venom- treated group (1.23 mg/kg) | Metformin- and atorvastatin -treated group | P value |
|---|---|---|---|---|---|---|
| TAC (mM/L) | 0.95 ± 0.03c | 0.62 ± 0.03 | 0.75 ± 0.014b | 0.81 ± 0.01c | 0.89 ± 0.02c | <0.0001 |
| % change | ------ | −34.7% | 20.96% | 30.6% | 43.54% | |
| MDA (nmol/g tissue) | 79.8 ± 1.03c | 117.9 ± 2.7 | 101.4 ± 1.29c | 94.5 ± 1.44c | 92 ± 1.1c | |
| % change | ------ | 47.74% | −13.99% | −19.84% | −21.96% | |
| NF-κβ (pg/mL) | 90.1 ± 1.47c | 238.3 ± 3.3 | 174.4 ± 3.08c | 158.3 ± 1.9c | 133.5 ± 1.4c | |
| % change | ----- | 164.5% | −26.81% | −33.57% | −43.97% |
Note: Values are expressed in mean ± SEM, n= 10. Statistical analysis was performed by two-way ANOVA test followed by Tukey's post hoc test. Values with different superscript letters represent significant differences (P < 0.05). aP < 0.05, bP < 0.01, cP < 0.001 compared to positive control group. % change of positive control was calculated according to negative control. % change of all treated groups was calculated according to positive control.
Effect of bee venom using two doses (0.5 and 1.23 mg/kg) and combined therapy (metformin and atorvastatin) on mRNA relative expression of vascular cell adhesion molecule-1 (VCAM-1) and galectin-3 in heart tissue
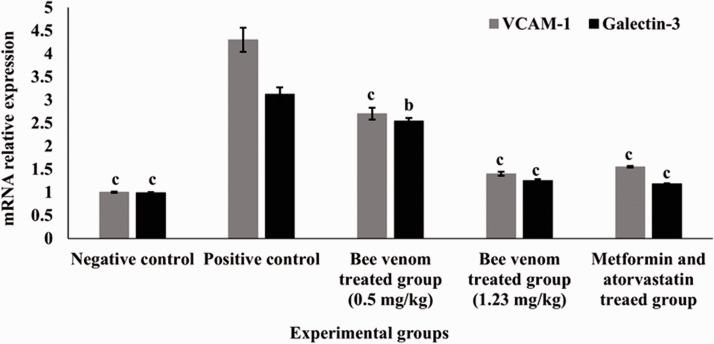
mRNA relative expression VCAM-1 and galectin-3 in heart tissue in all studied groups are demonstrated in Figure 1. The cardiac expression of VCAM-1 and galectin-3 genes of the positive control group was significantly higher than that of the negative control group (P < 0.0001). Overexpression of both VCAM-1 and galectin-3 was markedly declined after administration of bee venom (either 0.5 or 1.23 mg/kg) and the combined therapy of metformin and atorvastatin when compared to the positive control (P < 0.0001).
Figure 1.
Effect of bee venom using two doses (0.5 and 1.23 mg/kg) and combined therapy (metformin and atorvastatin) on mRNA relative expression of VCAM-1 and galectin-3 in heart tissue of all studied groups. Values are expressed in (mean ± SEM, n = 6).Statistical analysis was performed by two-way ANOVA test followed by Tukey's post hoc test. Values with different superscript letters represent significant differences (P < 0.05). aP < 0.05, bP < 0.01, cP < 0.001 compared to positive control group.
Histopathological examination
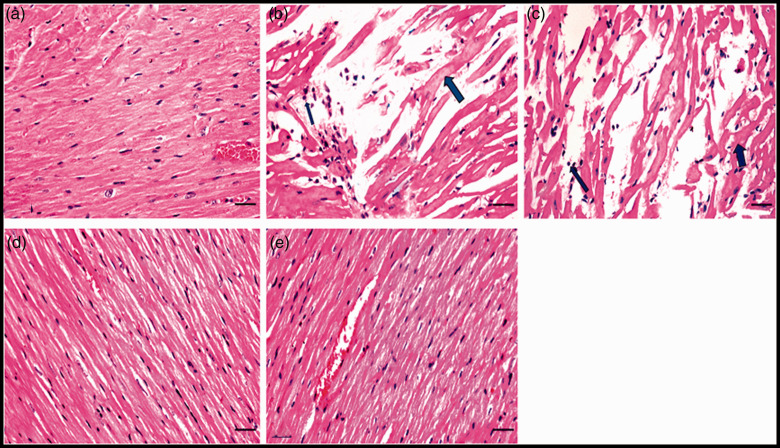
Histopathological results regarding cardiomyocytes in heart sections revealed normal myocardial muscles striation and nucleation in the negative control group (Figure 2(a)). Conversely, the positive group experienced myocarditis, hyalinization of myocytes and leucocytic cells infiltration (Figure 2(b)). Heart from bee venom-treated group (0.5 mg/kg) displayed a picture nearly resembling positive one as they existed with inflammation and hyalinization (Figure 2(c)). Unlike bee-venom-treated group (1.23 mg/kg) (Figure 2(d)), metformin- and atorvastatin-treated group (Figure 2(e)) exhibited a normal myocardial muscles striation and nucleation identical to negative control group.
Figure 2.
Photomicrographs of cardiomyocytes in heart sections of all studied groups: (a) Heart section from negative control group showed normal myocardial muscles striation and nucleation. (b) Heart section from positive group presented myocarditis, the hyalinized myocytes (thick arrow) and leucocytic cells infiltration (thin arrow). (c) Heart section from bee venom-treated group (0.5 mg/kg) displayed hyalinized myocytes (thick arrow) and leucocytic cells infiltration (thin arrow). Histological heart section from (d) bee venom-treated group (1.23 mg/kg) and (e) metformin- and atorvastatin-treated group exhibited the normal structure of myocardial muscles (H&E staining, scale bar = 100 μM). (A color version of this figure is available in the online journal.).
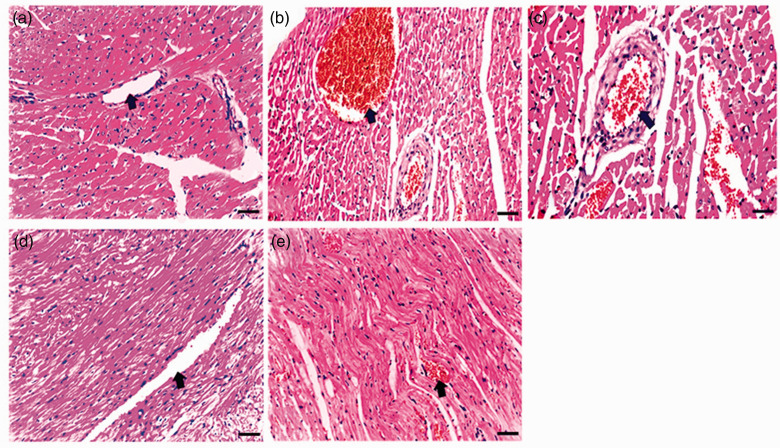
Regarding the blood vasculature in heart sections, outcomes revealed a normal histological structure of blood vessels in negative control group (Figure 3(a)) and bee venom-treated group (1.23 mg/kg) (Figure 3(d)). On the other hand, results from positive group (Figure 3(b)) and bee venom-treated group (0.5 mg/kg) (Figure 3(c)) confirmed a prominent congestion to vessels distended with blood and also to myocardial muscles near it. Metformin- and atorvastatin-treated group revealed blood congestion in some vessels and others seemed normally (Figure 3(e)).
Figure 3.
Photomicrographs of blood vasculature in heart sections of all studied groups: (a) Heart section from negative control group displayed normal blood vessels (arrow). Sections from (b) positive group and (c) bee venom-treated group (0.5 mg/kg) exhibited congested vessels distended with blood (arrows). (d) Blood vessels of heart section from bee venom-treated group (1.23 mg/kg) existed in a normal structure (arrow). (e) Heart section from metformin- and atorvastatin-treated group showed blood congestion in some vessels (arrow). (H&E staining, scale bar = 100 μM). (A color version of this figure is available in the online journal.).
Effect of bee venom using both doses (0.5 and 1.23 mg/kg) and combined therapy (metformin and atorvastatin) on area percentage (area %) of abnormal myocytes
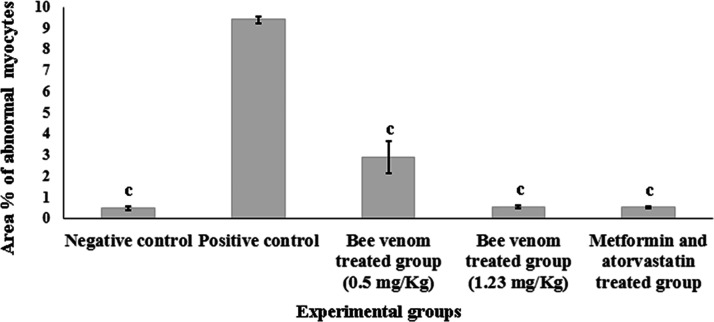
Quantitative analysis of area percentage (Area %) of abnormal myocytes confirmed the photographic sections and resulted in a significant variation between the positive control group and all studied groups (P < 0.0001), while a non-significant difference was recorded between the negative control group and bee venom-treated group (1.23 mg/kg), as well as for the group treated with metformin and atorvastatin as shown in Figure 4.
Figure 4.
Effect of bee venom using two doses (0.5 and 1.23 mg/kg) and combined therapy (metformin and atorvastatin) on area % of abnormal myocytes in H&E stained heart sections of all studied groups. Values are expressed in (mean ± SEM, n = 6). Statistical analysis was performed by two-way ANOVA test followed by Tukey's post hoc test. Values with different superscript letters represent significant differences (P < 0.05). aP < 0.05, bP < 0.01, cP < 0.001 compared to positive control group.
Discussion
Hyperglycemia and increased oxidative stress are associated with a higher risk of cardiovascular disease in diabetics. 48 Hyperlipidemia is a widespread metabolic disorder characterized by high levels of LDL-C and triacylglycerol, which play a critical role in the pathogenesis of cardiovascular disease. 49
Recently, great attention has been devoted to the treatment of different diseases using natural products. Bee venom is a natural substance secreted by honeybee queens and workers to defend themselves against invaders. 50 Venoms are toxic substances; however, they are characterized by therapeutic benefits. 51 The hypoglycemic activity of bee venom has been demonstrated in animal studies.13,25,52 In obese experimental animals, bee venom reduces lipid deposition and adipose tissue inflammation is induced by a high-fat diet. 14
This research aims to investigate the bee venom effects on cardiac dysfunction compared to the combined therapy of metformin and atorvastatin in diabetic hyperlipidemic rats.
The toxicity was estimated by intraperitoneal administration of Carniolan bee venom (Apis mellifera carnica) in rats to determine the median lethal dose (LD50). According to the results, LD50 was 12.3 mg/kg. These findings were near to those obtained in a previous study, 53 reporting the LD50 value of Carniolan bee venom (8.47 mg/kg) when injected intravenously into mice. In an earlier study, the LD50 of Sweet Bee Venom in rats was over 30 mg/kg through single-dose toxicity tests. 54 Melittin and phospholipase A2 resulted in the toxicity of bee venoms that act synergistically. 55 The venom of all Apis species is similar in quality and composition. However, the differences in LD50 values can be attributed to the geographical distribution of different Apis mellifera species 56 and variations in their production and toxicity depending on their size and physiological differences.57,58
Streptozotocin-induced diabetes mellitus causes a decline in the bodyweight due to structural protein degradation and carbohydrate metabolism disturbance related to increased blood glucose and deficiency or no insulin secretion. 59
Our data illustrated a significant decline in the bodyweight of the positive control group when compared to the negative control group. These findings support a previous study that found that insulin deficiency inhibits the body's ability to transfer glucose from the bloodstream to cells where it can be utilized. When this occurs, the body starts to consume fat and muscles for energy, resulting in decreased total bodyweight. 60 At the fourth week of the experimental trial, groups treated with bee venom using two doses (0.5 and 1.23 mg/kg) and combined therapy (metformin and atorvastatin) showed a significant elevation in bodyweight compared to the positive control group. Administration of bee venom improved the bodyweight, which may be due to improved glycemic control and its ability to elevate insulin secretion. 25 In streptozotocin-diabetic rats, the decline in blood glucose levels can contribute weight gain. 61
Streptozotocin (STZ) causes selective islet β-cell damage correlated with decreased β-cell function and viability, which eventually leads to an elevation in blood glucose and a reduction in insulin secretion. 62
The findings indicated that the positive control group had a significant increase in fasting blood glucose levels and a significant decrease in plasma insulin levels when compared to the negative control group. The current findings are in agreement with those of an earlier study, 63 which found that the high levels of glucose and the decreased levels of insulin in diabetic rats with hyperlipidemia are because of STZ's cytotoxic influence, causing the degradation of β cells and a decline in insulin secretion in the positive control group compared to the negative control group. Meanwhile, bee venom-treated groups using two doses (0.5 and 1.23 mg/kg) and combined therapy (metformin and atorvastatin) exhibited a significant decline in the levels of fasting blood glucose and a substantial increase in plasma insulin levels compared to the positive control group. These results are in line with other studies13,25,52 that confirmed the hypoglycemic activity of bee venom and its ability to raise insulin secretion. These glucose and insulin level improvements may be because of melittin and phospholipase-A2, which are active compounds in bee venom. They reduce the inflammation of the Langerhans islets and induce them to secrete insulin.64,65 Bee venom also blocks pro-inflammatory cytokines and free radicals production, leading to beta-cell apoptosis. 66 Furthermore, melittin can depolarize β-cells plasma membranes that results in opening Ca2+ channels, allowing a significant amount of Ca2+ to enter β-cells and stimulating insulin secretion. 67
Hyperglycemia and insulin action defects may contribute to lipid metabolism abnormalities and dyslipidemia in diabetics. 68 HFD consumption leads to increased serum total cholesterol triacylglycerol and LDL-C and causes hyperlipidemia. 69 Increased fatty acid oxidation and/or hepatic synthesis of VLDL-C, as well as the release of stored fatty acids from adipose tissues without a corresponding increase in the rate of clearance from the blood using lipoprotein lipase, may result in hyperlipidemia. 70
The results demonstrated a significant rise in total cholesterol, triacylglycerol l, LDL-C, and VLDL-C levels and a marked decline in HDL-C levels in the positive control group compared to the negative control group at the end of the experimental period. These findings are consistent with those in other studies,71,72 which reported that the levels of triacylglycerol, total cholesterol, LDL-C, and VLDL-C were significantly increased, and the HDL-C levels were declined considerably in diabetic hyperlipidemic rats because of the damaging effect of streptozotocin on β-cells and HFD consumption that results in reduced insulin secretion, hyperglycemia, and hyperlipidemia, while the groups treated with bee venom using both doses (0.5 and 1.23 mg/kg) and the combined therapy (metformin and atorvastatin) showed a significant decline in total cholesterol, triacylglycerol, LDL-C, VLDL-C levels, and a significant elevation in HDL-C levels in comparison to the positive control group at the end of the experimental period. The results are supported by other studies,73,74 which revealed the ability of bee venom to alleviate lipid profile abnormalities through increasing insulin secretion and sensitivity in experimental animal models. Phospholipase A2 activity in bee venom is essential for lowering total cholesterol, triacylglycerol (TAG) and LDL-C, and regulating the lipid profile. Phospholipase A2 partially lyses the cell membrane of fatty tissues, leading to an increase in lipid uptake and glucose transport into adipocytes and raises the affinity of insulin molecules. 75
Hyperglycemia, hyperlipidemia, and insulin resistance may result in raised cellular oxidative stress in the diabetic state. Reactive oxygen species (ROS) cause structural degradation of the cell membrane and lipid peroxidation, resulting in myocardial enzyme leakage. It is necessary to inhibit ROS generation to relieve myocardial reperfusion injury. 76
Our results illustrated a significant elevation (P < 0.0001) in the activities of CK-MB and LDH of the positive control when compared to the negative control. In addition, they are compatible with other studies,77,78 which found that the activities of CK-MB and LDH were raised in STZ-diabetic rats, likely due to disruption of myocardial structure induced by reactive oxygen species, while groups treated with bee venom using both doses (0.5 and 1.23 mg/kg) and the combined therapy (metformin and atorvastatin) showed a significant reduction (P < 0.0001) in CK-MB and LDH activities when compared to the positive control. These results are in accordance with Shaaban and Hamza, 79 who stated that treatment with bee venom resulted in a reduction in CK-MB and LDH activities compared to the positive control. The ameliorating effect of bee venom on CK-MB and LDH activities could be because of its antioxidant effect by suppressing the formation of free radicals, scavenging free radicals, preventing lipid peroxidation of membranes, and maintaining membrane integrity, thus limiting the outflow of these enzymes. 80
The results showed a significant increase in serum troponin I in the positive control group compared to the negative control group. These results are in line with those in other studies,81,82 which reported that the levels of troponin I were significantly increased in diabetic rats due to prolonged hyperglycemic condition, oxidative stress, and therefore the cardiomyocytes are damaged and troponin I is highly expressed which can be detected by various measures to indicate the myocardial injury. While groups treated with bee venom using both doses (0.5 and 1.23 mg/kg) and the combined therapy (metformin and atorvastatin) showed a significant reduction in the concentration of troponin I when compared to the positive control. These results are in accordance with Shaaban and Hamz,a 79 who stated that treatment with bee venom resulted in a reduction in troponin I levels compared to the positive control group, demonstrating the myocardial protection of bee venom because of the antioxidant effect of bee venom.
Numerous multiple causes have been proposed to understand why oxidative stress is raised, and these causes fall into two basic categories: raised reactive oxygen species (ROS) production and reduced antioxidant defense mechanisms. 83
The results reported a significant decline in total antioxidant capacity (TAC) in heart tissue of the positive control compared to the negative control. These findings are consistent with those of a previous study, 84 which stated that the levels of total antioxidant capacity were declined in the hearts of diabetic rats because of adverse consequences of diabetes mellitus. Groups treated with bee venom using two doses (0.5 and 1.23 mg/kg) and the combined therapy (metformin and atorvastatin) showed a significant elevation in levels of total antioxidant capacity in comparison to the positive control group. These findings are in line with those of a previous study, 13 which reported that total antioxidant capacity levels in in groups treated with bee venom using two doses low-dose (1 mg/kg) and high-dose (2 mg/kg) were increased compared to the positive control because of the antioxidant effect bee venom and its ability to suppress the production of free radicals and scavenge them.
Malondialdehyde is one of the end products of lipid peroxidation process and can be utilized as a biomarker to assess oxidative stress levels. It is elevated in diseases-induced oxidative stress, including diabetes mellitus. 85
Our results stated that the levels of malondialdehyde (MDA) in heart tissue of the positive control were significantly increased compared to the negative control. These data are in line with an earlier study, 86 which reported that the levels of malondialdehyde were increased in the heart of diabetic rats because of damage to the antioxidant defense system and a boost in lipid peroxidation. While groups treated with bee venom using both doses (0.5 and 1.23 mg/kg) and the combined therapy (metformin and atorvastatin) showed a significant decline in malondialdehyde levels in comparison to the positive control. These results follow a previous study, 79 which reported that the treatment with bee venom leads to a reduction in the malondialdehyde levels in heart tissue compared to positive control because of the anti-oxidative activity of bee venom.
Nuclear factor-kappa β (NF-κβ) is a critical transcriptional factor that is prone to oxidative stress induced by changes in glucose levels within the cell. 87 Activated NF-κβ acts as an inducer of many inflammatory-related genes, vascular cell adhesion molecules (VCAM-1), tumor necrosis factor-alpha (TNF-α), and cytokines like interleukin-1 beta (IL-1β), IL-6, aggravating the vascular endothelial dysfunction and tissue injury. 88
Our results reported that the levels of nuclear factor-kappa β (NF-κβ) in heart tissue in the positive control are significantly elevated compared to the negative control. These findings are supported by other studies,89,90 which stated that the levels of nuclear factor-kappa β were elevated in the positive control in comparison to the negative control because hyperglycemia and oxidative stress are associated with the activation of NF-κβ in the heart tissue of diabetic rats. Furthermore, activated NF-κβ contributes to an elevated inflammatory response to cardiac tissue injury in diabetic rats. Groups treated with bee venom using two doses (0.5 and 1.23 mg/kg) and combined therapy (metformin and atorvastatin) showed a significant decline in NF-κβ levels in heart tissue compared to the positive control. These results are compatible with those obtained in an earlier study, 91 demonstrating that bee venom exhibits an anti-inflammatory activity that is attributed to direct inhibition of the inflammatory response by inhibiting NF-κβ activation.
Vascular cell adhesion molecule-1 (VCAM-1) is one of the cell-surface proteins family that helps cells adhere to each other and the extracellular matrix. Several factors induce VCAM-1 expression on the cell surface, including hyperglycemia, LDL-C, pro-inflammatory cytokines, and oxidative stress, leading to initiation of several pathways that contributed to VCAM-1 activation, including interferon regulatory factor-1, NF-κβ, and activating protein-1 (AP-1). 92 Once VCAM-1 expressed on the endothelial cell membrane, it binds to multiple ligands on leucocytes, including α4β1 integrin, α4β7 integrin, and galectin-3,93,94 causing cytoskeletal remodeling and weakening of endothelial cell intercellular junctions, facilitating leukocyte transendothelial migration, which is an important step in the development of atherosclerosis. 95
Our results revealed that the relative mRNA expression of VCAM-1 was significantly increased in the positive control when compared to the negative control. These findings are in agreement with a previous study, 96 indicating that after STZ injection, severe inflammation was observed in diabetic rats' cardiac tissue, which was initiated by the activation of the NF-κβ signaling pathway, leading to an extreme expression of VCAM-1 identified by its mRNA level compared to the negative control group. Groups treated with bee venom using two doses (0.5 and 1.23 mg/kg) and the combined therapy (metformin and atorvastatin) showed a significant decline in relative mRNA expression of VCAM-1 in the heart tissue compared to positive control. These data are consistent with those of a previous research, 97 which demonstrated that treatment with bee venom resulted in a reduction in vascular inflammation and downregulation of VCAM-1 expression in the heart of atherosclerotic mice, implying that bee venom has anti-inflammatory and anti-atherogenic effects by suppressing the activity of pro-inflammatory cytokines like TNF-α and endothelial adhesion molecules such as VCAM-1 that contribute to atherosclerotic lesion formation.
Galectin-3 belongs to the β-galactoside-binding lectin family and is widely distributed in the heart, lung, brain, blood vessels, and visceral adipose tissue. Galectin-3 levels are higher in diabetes patients and obesity, which have been linked to problems with glucose homeostasis. 98 Overexpression of cardiac galectin-3 leads to interstitial fibrosis, myocardial macrophage infiltration, and heart dysfunction, and these effects are mediated by the activation of extracellular signal-regulated kinase 1/2 (ERK1/2), one of the best-characterized members of the mitogen-activated protein kinase (MAPK) family. 99 The activation of MAPK/ERK1/2 signaling pathway by pro-inflammatory mediators leads to activator protein-1 (AP-1) and transcription factor NF-κB, resulting in upregulation of galectin-3 expression. 100
The results indicate a significant rise in relative galectin-3's mRNA expression in the positive control group compared to the negative control group. These results are in accordance with an earlier study, 101 reporting that the galectin-3 levels were increased in the left ventricular (LV) tissue of diabetic mice. Furthermore, it has been demonstrated that the relative mRNA expression of galectin-3 expression was upregulated in the hearts of rats fed a high-fat diet compared to those fed a standard diet. 102 The observed elevation in relative gene expression of galectin-3 may be related to heart impairment, suggesting that diabetes mellitus and a high-fat diet cause vigorous cardiomyocyte damage. However, groups treated with bee venom using two doses (0.5 and 1.23 mg/kg) and combined therapy (metformin and atorvastatin) showed a significant decline in relative galectin-3's mRNA expression in the heart tissue compared to the positive control. These findings are compatible with a previous study 103 , stating that bee venom has anti-inflammatory activity and can inhibit the ERK1/2 pathway, leading to negative regulation of AP-1 and NF-κB that are two transcriptional factors involved in the regulation of galectin-3 expression.
Histopathological studies of cadiomyocytes and blood vasculature in heart sections revealed that there was myocarditis, hyalinized myocytes, leucocytic cell infiltration, and congested vessels distended with blood in the positive control rats, while the negative control rats showed normal myocardial muscle striation, nucleation and blood vessels. These results are in line with a previous study, 104 which reported an obvious inflammation, disarranged cardiac myocytes, congestion and intracytoplasmic vacuoles in the heart sections of the diabetic group. So, these results had illustrated the increased levels of CK-MB, LDH, TnI, and NF-κB. I. Bee venom-treated group (0.5 mg/kg) displayed a picture nearly resembling positive control as they existed with inflammation, hyalinization, and congested blood vessels, but treatment with bee venom (1.23 mg/kg) and combined therapy (metformin and atorvastatin) restored normal heart muscles, normal myocardial muscles striation, and nucleation in contrast to the positive control, but bee venom-treated group (1.23 mg/kg) showed a normal histological structure of blood vessels. Meanwhile metformin- and atorvastatin-treated group revealed blood congestion in some vessels and others seemed normally. These findings are consistent with those of a previous study, 105 which demonstrated the mitochondrial ultrastructural integrity and preserved myofilament in the heart tissue of the bee venom-treated group. Also in another study, 106 it was reported that bee venom treatment successfully improved the abnormalities observed in heart sections compared to positive control group. The low-dose bee venom-treated group exhibited slight capillary telangiectasia and congestion, arranged myocardial fibers and a few small round vacuoles were observed in the myocardium, but medium and high dose of bee venom performed better than the low dose; myocardial fibers were abundant, and the arrangement of the fibers was close without other obvious abnormalities. The cardioprotective effects of bee venom can be ascribed to the reduction of oxidative stress and inflammation through inhibition production of reactive oxygen spices and inflammatory mediators due to its antioxidant and anti-inflammatory activities.15,16,106
A quantification analysis of the area % abnormal cardiomyocytes was also performed, revealing a significant difference between the positive control and all treated groups and a non-significant difference between the negative control and all treated groups. These results demonstrate the potential effect of bee venom-treated group (1.23 mg/kg) plus metformin and atorvastatin treated group. These results are in accordance with a previous study, 106 which confirmed the cardioprotctive effects of bee venom.
Conclusions
Based on the results obtained, the bee venom (BV) administration has a therapeutic effect on biochemical, mRNA expression, and histopathological abnormalities in diabetic hyperlipidemic rats by promoting insulin secretion, glucose, and lipid uptake in adipose tissue through its hypoglycemic and hypolipidemic activities. It can suppress upregulation of VCAM-1 and galactin-3 genes expression in the heart, which is associated with cardiac disease improvement through attenuating oxidative stress and concentrations of nuclear factor-kappa-β (NF-κβ) due to its antioxidantand anti-inflammatory effects. Bee venom can attenuate cardiac dysfunction in diabetic hyperlipidemic rats in a dose-dependent manner. Therapeutic dose (1.23 mg/kg) of bee venom and combined therapy (metformin and atorvastatin) roughly correct biochemical, mRNA expression, and histopathological abnormalities in comparison to the therapeutic dose (0.5 mg/kg) of bee venom.
Footnotes
AUTHORS’ CONTRIBUTIONS: All authors participated in the design, the interpretation of the studies, data analysis and review of the manuscript.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Nabila Zein https://orcid.org/0000-0003-0921-6919
References
- 1.. Kerner W, Brückel J. Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes 2014; 122:384–6 [DOI] [PubMed] [Google Scholar]
- 2.Rawshani A, Rawshani A, Franzén S, Sattar N, Eliasson B, Svensson AM, Gudbjörnsdottir S. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2018; 379:633–44 [DOI] [PubMed] [Google Scholar]
- 3.Solomon SD, Chew E, Duh EJ, Sobrin L, Sun JK, VanderBeek BL, Gardner TW. Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care 2017; 40:412–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al Hroob AM, Abukhalil MH, Alghonmeen RD, Mahmoud AM. Ginger alleviates hyperglycemia-induced oxidative stress, inflammation and apoptosis and protects rats against diabetic nephropathy. Biomed Pharmacother 2018; 106:381–9 [DOI] [PubMed] [Google Scholar]
- 5.Pop-Busui R, Boulton AJM, Feldman EL, Bril V, Freeman R, Malik RA, Ziegler D. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care 2017; 40:136–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han Q, Yeung SC, Ip MSM, Mak JC. Dysregulation of cardiac lipid parameters in the high-fat high-cholesterol diet-induced rat model. Lipids Health Dis 2018; 17:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tadic M, Cuspidi C. Type 2 diabetes mellitus and atrial fibrillation: from mechanisms to clinical practice. Arch Cardiovasc Dis 2015; 108:269–76 [DOI] [PubMed] [Google Scholar]
- 8.Khanra R, Dewanjee S, Dua TK, Sahu R, Gangopadhyay M, De Feo V, Zia-Ul-Haq M. Abroma augusta L.(malvaceae) leaf extract attenuates diabetes-induced nephropathy and cardiomyopathy via inhibition of oxidative stress and inflammatory response. J Transl Med 2015; 13:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res 2016; 118:1808–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roudbari L, Imani S. The effects of androctonus crassicauda scorpion venom in the treatment of diabetes mellitus type 1 in animal models. Ann Biol Res 2012; 3:5782–5 [Google Scholar]
- 11.Ali M. Studies on bee venom and its medical uses. Int J Adv Res Technol 2012; 1:69–83 [Google Scholar]
- 12.Pucca MB, Cerni FA, Oliveira IS, Jenkins TP, Argemí L, Sørensen CV, Laustsen AH. Bee updated: current knowledge on bee venom and bee envenoming therapy. Front Immunol 2019; 10:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hassan AK, El-Kotby DA, Tawfik MM, Badr RE, Bahgat IM. Antidiabetic effect of the egyptian honey bee (Apis mellifera) venom in alloxan-induced diabetic rats. J Basic Appl Zool 2019; 80:1–9 [Google Scholar]
- 14.Cheon SY, Chung KS, Roh SS, Cha YY, An HJ. Bee venom suppresses the differentiation of preadipocytes and high fat diet-induced obesity by inhibiting adipogenesis. Toxins 2018; 10:9–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim DH, Song HS. Atopic dermatitis-related inflammation in macrophages and keratinocytes: the inhibitory effects of bee venom. J Acupunct Res 2019; 36:80–7 [Google Scholar]
- 16.Park HG, Lee KS, Kim BY, Yoon HJ, Choi YS, Lee KY, Jin BR. Honeybee (Apis cerana) vitellogenin acts as an antimicrobial and antioxidant agent in the body and venom. Dev Comp Immunol 2018; 85:51–60 [DOI] [PubMed] [Google Scholar]
- 17.Kang GH, Lee S, Choi DB, Shin D, Kim J, Yang H, Bae H. Bee venom phospholipase A2 ameliorates atherosclerosis by modulating regulatory T cells. Toxins 2020; 12:609–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reed LJ, Muench H. A simple method of estimating 50 per cent end points. Am J Hyg 1938; 27:493–7 [Google Scholar]
- 19.Reeves PG, Nielsen FH, Fahey Jr GC. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 1993; 123:1939–51 [DOI] [PubMed] [Google Scholar]
- 20.Yuan Y, Liu Q, Zhao F, Cao J, Shen X, Li C. Holothuria leucospilota polysaccharides ameliorate hyperlipidemia in high-fat diet-induced rats via short-chain fatty acids production and lipid metabolism regulation. Int J Mol Sci 2019; 20:4738–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Punitha IR, Rajendran K, Shirwaikar A, Shirwaikar A. Alcoholic stem extract of coscinium fenestratum regulates carbohydrate metabolism and improves antioxidant status in streptozotocin–nicotinamide induced diabetic rats. Evid Based Complement Alternat Med 2005; 2:375–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deeds MC, Anderson JM, Armstrong AS, Gastineau DA, Hiddinga HJ, Jahangir A, Kudva YC. Single dose streptozotocin-induced diabetes: considerations for study design in islet transplantation models. Lab Anim 2011; 45:131–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar M, Dandapat S, Sinha MP, Kumar A, Raipat BS. Different blood collection methods from rats: a review. Balneo Res J 2017; 8:46–50 [Google Scholar]
- 24.Sunder AS, Reddy ARN, Kiran G, Thirumurugu S. Antihyperlipidemic and antioxidant activity of methanolic extract of trianthema portulacastrum in rats fed a high-fat diet. J Herbs Spices Med Plants 2010; 16:193–202 [Google Scholar]
- 25.Mousavi SM, Imani S, Haghighi S, Mousavi SE, Karimi A. Effect of iranian honey bee (Apis mellifera) venom on blood glucose and insulin in diabetic rats. J Arthropod Borne Dis 2012; 6:136–43 [PMC free article] [PubMed] [Google Scholar]
- 26.Phatak RS, Khanwelkar CC, Matule SM, Datkhile K, Hendre A. Antihyperglycemic activity of murraya koenigii leaves extract on blood sugar level in streptozotocin-nicotinamide induced diabetes in rats. Biomed Pharmacol J 2019; 12:597–602 [Google Scholar]
- 27.Matafome P, Louro T, Rodrigues L, Crisostomo J, Nunes E, Amaral C, Seica R. Metformin and atorvastatin combination further protect the liver in type 2 diabetes with hyperlipidaemia. Diabetes Metab Res Rev 2011; 27:54–62 [DOI] [PubMed] [Google Scholar]
- 28.Thavasu PW, Longhurst S, Joel SP, Slevin ML, Balkwill FR. Measuring cytokine levels in blood. Importance of anti-coagulants, processing, and storage conditions. J Immunol Methods 1992; 153:115–24 [DOI] [PubMed] [Google Scholar]
- 29.Badawy SM, Hammad SA, Amine SA, El-Seidy AM, Slima SRA. Biochemical and histopathological changes in the brain of albino rats treated with profenofos and the possible protective effect of vitamins C and E. Menoufia Med J 2017; 30:278–85 [Google Scholar]
- 30.Vaught JB, Henderson MK. Biological sample collection, processing, storage and information management. IARC Sci Publ 2011; 163:23–42 [PubMed] [Google Scholar]
- 31.Panigrahy SK, Kumar A, Bhatt R. In vitro and in vivo anti-diabetic activity of fractions obtained from the unexplored hedychium coronarium rhizome. Proc Natl Acad Sci India Sect B 2019; 90:605–14 [Google Scholar]
- 32.Morgan CR, Lazarow A. Immunoassay of insulin: two antibody system: plasma insulin levels of normal, subdiabetic and diabetic rats. Diabetes 1963; 12:115–26 [Google Scholar]
- 33.Stein EA, Myers GL. Lipids, lipoproteins and apolipoproteins. Fundam Clin Chem 1987; 3:478–9 [Google Scholar]
- 34.Young DS, Friedman RB, Ng V. Effects of disease on clinical laboratory tests, vol. 1 and 2. Clin Chem 2002; 48:682–3 [Google Scholar]
- 35.Warnick GR, Wood PD. National cholesterol education program recommendations for measurement of high-density lipoprotein cholesterol: executive summary. The national cholesterol education program working group on lipoprotein measurement. Clin Chem 1995; 41:1427–33 [PubMed] [Google Scholar]
- 36.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18:499–502 [PubMed] [Google Scholar]
- 37.Norbert WT. Clinical guide to laboratory tests. 3rd ed. Philadelphia: Saunders W.B. Company, 1995, pp. 268–73. [Google Scholar]
- 38.Wu AH, Bowers Jr GN. Evaluation and comparison of immunoinhibition and immunoprecipitation methods for differentiating MB and BB from macro forms of creatine kinase isoenzymes in patients and healthy individuals. Clin Chem 1982; 28:2017–21 [PubMed] [Google Scholar]
- 39.Bergmeyer HU. Methods of enzymatic analysis. Vol. 42. New York: Academic, 1974, pp.321–7. [Google Scholar]
- 40.Kiyasu Y. Current status of detecting Troponin-I for patient management. Am Clin Prod Rev 1985; 4:29–31 [Google Scholar]
- 41.Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. Method for the measurement of antioxidant activity in human fluids. J Clin Pathol 2001; 54:356–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta 1978; 90:37–43 [DOI] [PubMed] [Google Scholar]
- 43.Heppner C, Bilimoria KY, Agarwal SK, Kester M, Whitty LJ, Guru SC, Burns AL. The tumor suppressor protein menin interacts with NF-κB proteins and inhibits NF-κB-mediated transactivation. Oncogene 2001; 20:4917–25 [DOI] [PubMed] [Google Scholar]
- 44.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001; 25:402–8 [DOI] [PubMed] [Google Scholar]
- 45.Bancroft JD, Gamble M. Theory and practice of histological techniques. 6th ed. London: Elsevier health Sciences, 2008, pp.83–134. [Google Scholar]
- 46.Valentin J, Frobert A, Ajalbert G, Cook S, Giraud MN. Histological quantification of chronic myocardial infarct in rats. J Visual Exp 2016; 118:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abu-Bader SH. Using statistical methods in social science research: with a complete SPSS guide. 3rd ed. Oxford: Oxford University Press, 2021, pp.297–302. [Google Scholar]
- 48.Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative Meta-analysis of 102 prospective studies. Lancet 2010; 375:2215–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boren J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, Ginsberg HN. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2020; 41:2313–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wehbe R, Frangieh J, Rima M, El Obeid D, Sabatier JM, Fajloun Z. Bee venom: overview of main compounds and bioactivities for therapeutic interests. Molecules 2019; 24:2997–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Souza JM, Goncalves BDC, Gomez MV, Vieira LB, Ribeiro FM. Animal toxins as therapeutic tools to treat neurodegenerative diseases. Front Pharmacol 2018; 9:1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raafat M, Hamam GG. The possible role of bee venom on gastric fundic mucosa in streptozotocin induced diabetes mellitus in rats. A histological study. Egypt J Histol 2020; 42:1029–43 [Google Scholar]
- 53.Zidan H-G, Mostafa ZK, Ibrahim MA, Haggag SI, Darwish DA, Elfiky AA. Venom composition of Egyptian and Carniolan honeybee, Apis mellifera L. Affected by collection methods. Egypt Acad J Biol Sci A Entomol 2018; 11:59–71 [Google Scholar]
- 54.Kim Y-J, Lim C-S, Kwon K-R. Study of single dose test of sweet bee venom in rats. J Pharmacopuncture 2009; 12:5–32 [Google Scholar]
- 55.Quistad GB, Skinner WS, Schooley DA. Venoms of social hymenoptera – toxicity to the lepidopteran, manduca sexta. Insect Biochem 1988; 18:511–4 [Google Scholar]
- 56.Habermann E. Bee and wasp venoms. Science 1972; 177:314–22 [DOI] [PubMed] [Google Scholar]
- 57.Devi A, Sangeeta NR, Kaur J. Honey bee venom and its composition: focusing on different apis species – a review. World 2016; 68:51–71 [Google Scholar]
- 58.Farshineh Adl MB, Gençer HV, Firatli Ç, Bahreini R. Morphometric characterization of iranian (Apis mellifera meda), Central anatolian (Apis mellifera anatoliaca) and caucasian (Apis mellifera caucasica) honey bee populations. J Apic Res 2007; 46:225–31 [Google Scholar]
- 59.Rocha DS, Casagrande L, Model JFA, dos Santos JT, Hoefel AL, Kucharski LC. Effect of yerba mate (ilex paraguariensis) extract on the metabolism of diabetic rats. Biomed Pharmacother 2018; 105:370–6 [DOI] [PubMed] [Google Scholar]
- 60.Al-Awaida WJ, Sharab AS, Al-Ameer HJ, Ayoub NY. Effect of simulated microgravity on the antidiabetic properties of wheatgrass (Triticum aestivum) in streptozotocin-induced diabetic rats. npj Microgravity 2020; 6:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Al-Enazi MM. Combined therapy of rutin and silymarin has more protective effects on streptozotocin-induced oxidative stress in rats. J Appl Pharm Sci 2014; 4:21–8 [Google Scholar]
- 62.Kumar R, Arora V, Ram V, Bhandari A, Vyas P. Hypoglycemic and hypolipidemic effect of allopolyherbal formulations in streptozotocin induced diabetes mellitus in rats. Int J Diabetes Mellit 2015; 3:45–50 [Google Scholar]
- 63.Asokan SM, Wang R-Y, Hung T-H, Lin WT. Hepato-protective effects of glossogyne tenuifolia in streptozotocin-nicotinamide-induced diabetic rats on high fat diet. BMC Complement Altern Med 2019; 19:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park HJ, Lee HJ, Choi MS, Son DJ, Song HS, Song MJ, Hong JT. JNK pathway is involved in the inhibition of inflammatory target gene expression and NF-kappaB activation by melittin. J Inflamm 2008; 5:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simonsson E, Karlsson S, Ahrén B. Islet phospholipase A2 activation is potentiated in insulin resistant mice. Biochem Biophys Res Commun 2000; 272:539–43 [DOI] [PubMed] [Google Scholar]
- 66.Badr G, Hozzein WN, Badr BM, Al Ghamdi A, Saad Eldien HM, Garraud O. Bee venom accelerates wound healing in diabetic mice by suppressing activating transcription factor‐3 (ATF‐3) and inducible nitric oxide synthase (iNOS)‐mediated oxidative stress and recruiting bone marrow‐derived endothelial progenitor cells. J Cell Physiol 2016; 231:2159–71 [DOI] [PubMed] [Google Scholar]
- 67.Morgan NG, Montague W. Stimulation of insulin secretion from isolated rat islets of langerhans by melittin. Biosci Rep 1984; 4:665–71 [DOI] [PubMed] [Google Scholar]
- 68.Elberry AA, Harraz FM, Ghareib SA, Gabr SA, Nagy AA, Abdel-Sattar E. Methanolic extract of marrubium vulgare ameliorates hyperglycemia and dyslipidemia in streptozotocin-induced diabetic rats. Int J Diabetes Mellit 2015; 3:37–44 [Google Scholar]
- 69.Adekunle AS, Adedeji AL, Oyewo EO, Adedosu OT, Omotoso AT. Hyperlipidemia induced by atherogenic diet enhanced oxidative stress in the kidney and inflammatory responses: an in-vivo study. Asian J Nat Appl Sci 2013; 2:82–93 [Google Scholar]
- 70.Wang X, Gao L, Lin H, Song J, Wang J, Yin Y, Li L. Mangiferin prevents diabetic nephropathy progression and protects podocyte function via autophagy in diabetic rat glomeruli. Eur J Pharmacol 2018; 824:170–8 [DOI] [PubMed] [Google Scholar]
- 71.Vengala N. Antihyperlipidemic activity of flax lignan concentrate in nicotinamide-streptozotocin induced diabetic hyperlipidemia rats. Res Rev Biosci 2017; 12:120–33 [Google Scholar]
- 72.Elseweidy MM, Aly SI, Hammad SK, Shershir NI. Early myocardial injury biomarkers in diabetic hyperlipidemic rats: impact of 10-dehydrogingerdione and vitamin D3. Exp Biol Med 2020; 245:1326–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khulan TS, Ambaga M, Chimedragcha CH. Effect of honey bee venom (Apis mellifera) on hyperglycemia and hyperlipidemia in alloxan induced diabetic rabbits. J Diabetes Metab 2015; 6:3–6 [Google Scholar]
- 74.Hanafi MY, Zaher ELM, El-Adely SEM, Sakr A, Ghobashi AH, Hemly MH, Kamel MA. The therapeutic effects of bee venom on some metabolic and antioxidant parameters associated with HFD-induced non-alcoholic fatty liver in rats. Exp Ther Med 2018; 15:5091–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singh J, Ranganathan R. Quantitation of lysolipids, fatty acids, and phospholipase A2 activity and correlation with membrane polarity. J Lipid Res 2012; 53:1993–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.González-Montero J, Brito R, Gajardo AIJ, Rodrigo R. Myocardial reperfusion injury and oxidative stress: therapeutic opportunities. World J Cardiol 2018; 10:74–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pranav Nayak B, Ganesha KR, Minaz N, Razdan R, Goswami SK. Phloroglucinol, a nutraceutical for IR‐induced cardiac damage in diabetic rats. Animal Model Exp Med 2019; 2:210–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qiu Z, Ming H, Zhang Y, Yu Y, Lei S, Xia ZY. The protective role of Bmal1-Regulated autophagy mediated by HDAC3/SIRT1 pathway in myocardial ischemia/reperfusion injury of diabetic rats. Cardiovasc Drugs Ther 2021; 35:1–15 [DOI] [PubMed] [Google Scholar]
- 79.Shaaban AMM, Hamza RG. Studying the ameliorative effect of bee venom against damage and inflammation induced in gamma-irradiated rats. Arab J Nucl Sci Appl 2019; 52:178–84 [Google Scholar]
- 80.Sobral F, Sampaio A, Falcão S, Queiroz MJR, Calhelha RC, Vilas-Boas M, Ferreira IC. Chemical characterization, antioxidant, anti-inflammatory and cytotoxic properties of bee venom collected in northeast Portugal. Food Chem Toxicol 2016; 94:172–7 [DOI] [PubMed] [Google Scholar]
- 81.Safhi MM, Anwer T, Khan G, Siddiqui R, Sivakumar SM, Alam MF. The combination of canagliflozin and omega-3 fatty acid ameliorates insulin resistance and cardiac biomarkers via modulation of inflammatory cytokines in type 2 diabetic rats. Korean J Physiol Pharmacol 2018; 22:493–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thakur V, Alcoreza N, Delgado M, Joddar B, Chattopadhyay M. Cardioprotective effect of glycyrrhizin on myocardial remodeling in diabetic rats. Biomolecules 2021; 11:569–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hisalkar PJ, Patne AB, Fawade MM. Assessment of plasma antioxidant levels in type 2 diabetes patients. Int J Biol Med Res 2012; 3:1796–800 [Google Scholar]
- 84.Rahimi R, Karimi J, Khodadadi I, Tayebinia H, Kheiripour N, Hashemnia M, Goli F. Silymarin ameliorates expression of urotensin II (U-II) and its receptor (UTR) and attenuates toxic oxidative stress in the heart of rats with type 2 diabetes. Biomed Pharmacother 2018; 101:244–50 [DOI] [PubMed] [Google Scholar]
- 85.Tripathi P, Verma MK, Tripathi SS, Singh SP. Comparative study of malondialdehyde and vitamin C in type 2 diabetes mellitus and non diabetic individuals. Int J Life Sci Sci Res 2016; 2:9–14 [Google Scholar]
- 86.Pektaş A, Pektaş MB, Koca HB, Tosun M, Aslan E, Koca S, Sadi G. Effects of resveratrol on diabetes-induced vascular tissue damage and inflammation in male rats. Turkish J Biochem 2017; 42:451–8 [Google Scholar]
- 87.Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res 2018; 122:624–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yuan T, Yang T, Chen H, Fu D, Hu Y, Wang J, Xie X. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol 2019; 20:247–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kumar S, Prasad S, Sitasawad SL. Multiple antioxidants improve cardiac complications and inhibit cardiac cell death in streptozotocin-induced diabetic rats. PLoS One 2013; 8:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.ALTamimi JZ, BinMowyna MN, AlFaris NA, Alagal RI, El-Kott AF, Al-Farga AM. Fisetin protects against streptozotocin-induced diabetic cardiomyopathy in rats by suppressing fatty acid oxidation and inhibiting protein kinase R. Saudi Pharm J 2021; 29:27–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim W-H, An H-J, Kim J-Y, Gwon MG, Gu H, Park JB, Park KK. Bee venom inhibits porphyromonas gingivalis lipopolysaccharides-induced pro-inflammatory cytokines through suppression of NF-κB and AP-1 signaling pathways. Molecules 2016; 21:1508–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thayse K, Kindt N, Laurent S, Carlier S. VCAM-1 target in non-invasive imaging for the detection of atherosclerotic plaques. Biology 2020; 9:368–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grayson MH, Van der Vieren M, Sterbinsky SA, Michael Gallatin W, Hoffman PA, Staunton DE, Bochner BS. αdβ2 integrin is expressed on human eosinophils and functions as an alternative ligand for vascular cell adhesion molecule 1 (VCAM-1). J Exp Med 1998; 188:2187–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rao SP, Wang Z, Zuberi RI, Sikora L, Bahaie NS, Zuraw BL, Sriramarao P. Galectin-3 functions as an adhesion molecule to support eosinophil rolling and adhesion under conditions of flow. J Immunol 2007; 179:7800–7 [DOI] [PubMed] [Google Scholar]
- 95.Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Milstone DS. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest 2001; 107:1255–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yin Y, Xu Y, Ma H, Tian X. Hesperetin ameliorates cardiac inflammation and cardiac fibrosis in streptozotocin-induced diabetic rats by inhibiting NF-kB signaling pathway. Biomed Res 2017;28:223–9
- 97.Lee WR, Kim SJ, Park JH, Kim KH, Chang YC, Park YY, Park KK. Bee venom reduces atherosclerotic lesion formation via anti-inflammatory mechanism. Am J Chin Med 2010; 38:1077–92 [DOI] [PubMed] [Google Scholar]
- 98.Weigert J, Neumeier M, Wanninger J, Bauer S, Farkas S, Scherer MN, Buechler C. Serum galectin-3 is elevated in obesity and negatively correlates with glycosylated hemoglobin in type 2 diabetes. J Clin Endocrinol Metab 2010; 95:1404–11 [DOI] [PubMed] [Google Scholar]
- 99.Sonkawade SD, Suzuki G, Xu S, Kc K, Pokharel S, Sharma UC. Cardioselective galectin-3 overexpression in mice activates MAPK/ERK1/2 signaling and induces myocardial fibrosis. Circulation 2019; 140:A12548 [Google Scholar]
- 100.Salem AM, Mahdy KA, Hassan NS, El-Saeed GS, Farrag ARH, Monem MAA. Nigella sativa seed reduced galectin-3 level and liver fibrosis in thioacetamide-induced liver injury in rats. J Arab Soc Med Res 2017; 12:46–55 [Google Scholar]
- 101.Thandavarayan RA, Watanabe K, Ma M, Veeraveedu PT, Gurusamy N, Palaniyandi SS, Aizawa Y. 14-3-3 Protein regulates Ask1 signaling and protects against diabetic cardiomyopathy. Biochem Pharmacol 2008; 75:1797–806 [DOI] [PubMed] [Google Scholar]
- 102.Marín-Royo G, Gallardo I, Martínez-Martínez E, Gutiérrez B, Jurado-López R, López-Andrés N, Cachofeiro V. Inhibition of galectin-3 ameliorates the consequences of cardiac lipotoxicity in a rat model of diet-induced obesity. Dis Model Mech 2018; 11:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jeong CH, Cheng WN, Bae H, Lee KW, Han SM, Petriello MC, Han SG. Bee venom decreases LPS-induced inflammatory responses in bovine mammary epithelial cells. J Microbiol Biotechnol 2017; 27:1827–36 [DOI] [PubMed] [Google Scholar]
- 104.Alshabi AM, Alkahtani SA, Shaikh I, Habeeb MS. Caffeine modulates pharmacokinetic and pharmacodynamic profiles of pioglitazone in diabetic rats: impact on therapeutics. Saudi Med J 2021; 42:151–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Saleh NK, Saleh HA. Cardiac effects of bee venom in rats. Saudi Med J 2011; 32:563–70 [PubMed] [Google Scholar]
- 106.Sun Y, Han M, Shen Z, Huang H, Miao X. Anti-hypertensive and cardioprotective effects of a novel apitherapy formulation via upregulation of peroxisome proliferator-activated receptor-α and-γ in spontaneous hypertensive rats. Saudi J Biol Sci 2018; 25:213–9 [DOI] [PMC free article] [PubMed] [Google Scholar]