Abstract
The use of medical imaging as a non-invasive or minimally invasive method to assess disease or treatment response continues to grow. A similar trend is observed in pre-clinical research, in general, and more specifically in macaques, enabling longitudinal assessment of disease in individual animals. Computed tomography (CT) is such an imaging technique used to obtain clinically applicable data. To acquire a chest CT using a cone beam tomography system, some kind of respiration control is needed. A commonly used technique for this is endotracheal intubation and mechanical ventilation. However, although routinely performed this can increase the risk of impact on welfare in comparison with non-invasive imaging. Therefore, we studied the option of retrospectively gated CTs: acquiring high resolution chest CTs in freely breathing macaques. For this, we compared 748 CTs obtained during free breathing with 881 CTs obtained with mechanical ventilation in combination with a breath-hold procedure predominantly on the appearance of misregistration artifacts. The scans were obtained during different stages of multiple experimentally induced respiratory diseases. The comparison shows that although there are still streaking artifacts present in the retrospective gated scans, the amount of shading artifacts is reduced to such a level that it possibly dominates underlying lesions, causing misdiagnosis. Our data reveal that the use of retrospective gating in high resolution CTs for macaques can be successfully applied. With the use of this technique, artifacts due to free breathing are reduced to a diagnostically appropriate level. Most importantly, this technique makes chest CTs with this instrumentation a non-invasive modality.
Keywords: non-human primate, chest CT, refinement, respiratory management
Acquisition affinée d’images de TDM thoracique haute résolution par respiration libre chez les macaques Résumé
L'utilisation de l'imagerie médicale comme méthode non invasive ou invasive minimale pour évaluer la réponse à la maladie ou au traitement continue de croître. Une tendance similaire est observée dans la recherche préclinique, en général et plus spécifiquement chez les macaques, pour permettre une évaluation longitudinale de la maladie chez des animaux individuels. La tomodensitométrie (TDM) est une technique d'imagerie utilisée pour obtenir des données cliniquement applicables. Pour acquérir des images de TDM thoracique à l’aide d’un système de tomographie à faisceau conique, le contrôle de la respiration est nécessaire. Bien que l’intubation endotrachéale et la ventilation mécanique effectuées régulièrement facilitent le contrôle respiratoire, elles peuvent aussi augmenter le risque d'impact sur le bien-être par rapport à l'imagerie non invasive. Par conséquent, nous avons étudié l’option de TDM rétrospectivement synchronisées: acquisition d’images de TDM thoracique haute résolution chez des macaques respirant librement. Pour ce faire, nous avons comparé 748 TDM obtenues pendant la respiration libre avec 881 TDM obtenues avec une ventilation mécanique en combinaison avec une procédure d'apnée principalement sur l'apparition d'artefacts d'erreur d'enregistrement. Les images ont été obtenues à différents stades de multiples maladies respiratoires induites expérimentalement. La comparaison montre qu’il existe toujours des artefacts striés dans les acquisitions d’images synchronisées rétrospectives. Cependant, la quantité d’artefacts d’ombre est réduite à un niveau susceptible de masquer des lésions sous-jacentes causant des erreurs de diagnostic. Nos données montrent que l’utilisation de la synchronisation rétrospective dans les TDM haute résolution est possible chez les macaques. Plus important encore, cette technique fait des TDM thoraciques effectuées avec cet instrument une modalité non invasive.
Verbesserte Akquisition von hochauflösenden Thorax-CT bei Makaken durch freie Atmung Abstract
Medizinische Bildgebung als nicht- oder minimal-invasive Methode zur Beurteilung von Krankheiten oder des Ansprechens auf eine Behandlung findet zunehmend Verwendung. Ein ähnlicher Trend ist in der präklinischen Forschung zu beobachten, wo diese Verfahren generell und speziell bei Makaken eine longitudinale Beurteilung von Krankheiten bei einzelnen Tieren ermöglichen. Die Computertomographie (CT) ist ein solches bildgebendes Verfahren, mit dem klinisch anwendbare Daten gewonnen werden können. Um eine Thorax-CT mit einem Kegelstrahltomographen aufzunehmen, ist eine Art der Atemkontrolle erforderlich. Die routinemäßig durchgeführte endotracheale Intubation und mechanische Beatmung, die der Atemkontrolle dient, kann im Vergleich zur nicht-invasiven Bildgebung das Risiko einer Beeinträchtigung des Wohlbefindens erhöhen. Daher untersuchten wir die Option des retrospektiven CT-Gatings: die Aufnahme von hochauflösenden Thorax-CT bei frei atmenden Makaken. Dazu verglichen wir 748 CT, die während der freien Atmung gewonnen wurden, mit 881 CT, die unter mechanischer Beatmung in Kombination mit einer Atemanhalteprozedur erfolgten, wobei das Auftreten von Fehlregistrierungsartefakten im Vordergrund stand. Die Scans erfolgten während verschiedener Stadien von mehreren experimentell induzierten Atemwegserkrankungen. Der Vergleich zeigt, dass in den retrospektiven Scan-Gatings weiterhin Streifenartefakte vorhanden sind. Die Menge der Schattenartefakte ist jedoch auf ein solches Maß reduziert, dass sie möglicherweise die darunter liegenden Läsionen dominieren und eine Fehlbefundung verursachen. Unsere Daten zeigen, dass die Verwendung von retrospektivem Gating in hochauflösenden CT bei Makaken möglich ist. Am wichtigsten ist, dass diese Methode Thorax-CT mit dieser Instrumentierung zu einer nicht-invasiven Modalität macht.
Adquisición refinada de TC pectoral de alta resolución en macacos mediante respiración libre Resumen
El uso de radiografías medicas como un método no invasivo o de invasión mínima para identificar enfermedades o respuestas a tratamientos continúa creciendo. Se ha observado una tendencia parecida en investigaciones preclínicas, en general y más concretamente en macacos, lo que permite una evaluación longitudinal de enfermedades en animales individuales. La tomografía computarizada (TC) es una técnica de radiografía utilizada para obtener datos clínicamente aplicables. Para realizar una TC pectoral utilizando un sistema de tomografía de haz cónico, se necesita algún tipo de control respiratorio. Aunque se utilicen habitualmente, la intubación endotraqueal y la ventilación mecánica que ayuda en el control respiratorio pueden aumentar el riesgo de impacto en el bienestar del animal en comparación a las radiografías no invasivas. Por tanto, hemos estudiado la opción de una TC retrospectivamente controlada: adquiriendo una TC pectoral de alta resolución con respiración libre en macacos. Para ello comparamos 748 TC obtenidas con respiración libre con 881 TC obtenidas con ventilación mecánica en combinación con un procedimiento de retención de la respiración predominantemente con la aparición de artefactos de falta de registro. Los escáneres se obtuvieron durante distintas fases de enfermedades respiratorias inducidas de forma experimental. La comparación muestra que todavía existen artefactos de retransmisión presentes en los escáneres controlados retrospectivamente. Sin embargo, la cantidad de artefactos de tonalidades se ha visto reducida a un nivel que posiblemente domina las lesiones subyacentes que provocan un diagnóstico erróneo. Nuestros datos muestran que el uso de control retrospectivo en TC de alta resolución para macacos es posible. Y lo que es más importante, esta técnica hace que las TC pectorales sean no invasivas con este instrumento.
Introduction
Respiratory diseases are the leading causes of morbidity and mortality in the world, affecting all age categories of the population, 1 highlighting the importance of both basic and translational research. For translational research, it is essential to use a model that closely mimics the human respiratory system. Macaques are most suitable for this purpose, based on their structure, physiology and the immune mechanisms of their lungs. 2 Another key point in translational research is the use of methodologies similar, or at least close to, those used in the clinic. Imaging is such a method; in this regard, computed tomography (CT) is used, allowing serial assessment with high detail.3,4
With fan beam technology, a whole-body CT is performed in only a few seconds. More importantly, in order to reduce motion artefacts due to breathing, human subjects are instructed to hold their breath during image capture. As this strategy is not possible with anaesthetised macaques, obtaining high-resolution CT images using cone beam technology requires respiratory control during the 30-s capture period. Respiratory control can be achieved by a breath-hold or via a high frequency jet ventilation technique. 5 Both techniques require the insertion of an endotracheal tube and the need to perform respiratory control with mechanical ventilation. Invasive handlings, although performed routinely, increase the chance of animal handling complications and possible cross-contamination with highly contagious diseases. Non-invasive imaging by measuring the breathing pattern and applying a respiratory-synchronized technique can avoid these potential problems. With retrospective respiratory gating, the same region is scanned several times and data from only certain intervals of the breathing cycle are used. This report describes retrospective respiratory gating for macaques and validates its effectiveness for respiratory control during free breathing CTs.
Material and methods
Animals and procedure
A longitudinal cohort study was conducted to calculate the effectiveness of retrospective respiratory gating for macaques. The protocol was conducted entirely at the Biomedical Primate Research Centre (BPRC, Rijswijk, Netherlands). No animals were used directly for this study. These data were obtained as part of multiple studies, e.g. tuberculosis (AVD5020020172645), influenza (AVD502002016704) and COVID-19 (AVD5020020209404) as longitudinal follow up after infection. Thus, separate ethical approval was not required. Animals on which the CTs were based originated from and were housed socially in pairs at the BPRC. All procedures, husbandry and housing performed in this study were in accordance with the Dutch laws on animal experimentation, with the regulations for animal handling as described in the EU Directive 63/2010 and with the Weatherall report (2006). BPRC is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International.
Animals included male and female rhesus monkeys (RM, Macaca mulatta) (4.8–13.8 kg, 4–21 years old) and cynomolgus macaques (CM, Macaca fascicularis) (3.1–11.8 kg, 4–16 years old). All animals were classified healthy according to physical examination and the evaluation of complete blood count and serum chemistry before the start of each study. The current protocol involved 565 RM and 183 CM free breathing scans and 756 RM and 125 CM breath-hold scans. The scans were acquired in a dedicated scanner room away from the home cages. The scans were performed from January 2018 until May 2021 in the morning. All macaques were fasted before the scans but water was freely available.
Macaques were anaesthetized, primarily to reduce motion artifacts 6 . Induction was performed, for both scan protocols, with an intramuscular (IM) injection of ketamine 10 mg/kg (ketamine hydrochloride, Ketamine 10%; Alfasan Nederland BV, Woerden, Netherlands, 100 mg/ml) in combination with medetomidine hydrochloride (medetomidine hydrochloride, Sedastart; AST Farma BV, Oudewater, Netherlands, 1 mg/ml 0.05 mg/kg).7–9 For the free breathing protocol, no further anaesthetics were applied. For mechanical ventilation after induction, anaesthesia was maintained with inhalant anaesthetics. The breath-hold was induced at maximum inspiration by blocking the airflow manually for 35 s. For both procedures, when the macaques returned to their home cage, atipamezole (atipamezole hydrochloride, sedastop; ASTFarma B.V., Oudewater, Netherlands, 5 mg/ml) was provided IM (0.25 mg/kg).
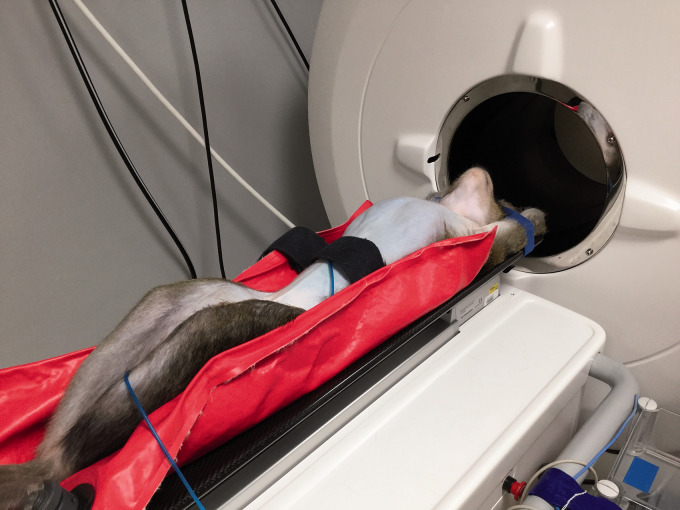
In both scan protocols, the macaques were placed head-first-supine in the scanner, lying on their back with their arms placed above their head (Figure 1). The macaque was positioned using a vacuum pillow with an additional band around the umbilical area. The band is to fixate the pressure cushion and the vacuum pillow (Mecan VHM BV, Nijmegen, Netherlands) is to restrain the position but, more importantly, to limit the breathing movement of the abdomen in the ventro-dorsal direction only. In this way the signals detected by the pressure cushion are more clearly distinguishable from other internal movements like the heartbeat, and the breaths per minute (BPM) of respiration can be monitored accurately. The arms are up to avoid truncation artifacts around the thorax.
Figure 1.
A sedated free breathing cynomolgus macaque (Macaca fascicularis) in front of the CT showing the position of the vacuum pillow, band around the umbilical area and pressure cushion.
Scanner
The current protocol was performed on a compact dedicated positron emission tomography (PET)-CT for non-human primate research: MultiScan LFER150 PET-CT (Mediso Medical Imaging Systems Ltd., Budapest, Hungary). 10 To achieve compact size and high spatial resolution, the system utilizes cone-beam CT technology. Cone-beam technology relies on large area detectors, decreased collimation, at the cost of scattered radiation affecting the data.
The scanner covers a 150 × 200 × 200 mm3 Field of View (FoV) in a single 32.4 s rotation. It features a complementary metal oxide semiconductor (CMOS)-based flat panel detector with 75 µm native pixel spacing and an 80 kVp/80W microfocus X-ray tube supporting isotropic high-resolution imaging, exceeding 2.8 lp/mm at 10% modulation transfer function (MTF). The scatter correction algorithm of the scanner effectively reduces both qualitative and quantitative distortions. A CT scan with 80 kVp voltage leads to a CT dose index (CTDI) of around 24.53 mGy for each 32.4 sec long rotation, estimating a 16 cm diameter CTDI phantom.
Procedures
In this manuscript three breathing techniques are included; breath-hold, free breathing with retrospective gating and free breathing without retrospective gating. For this, two scan protocols are used:
A breath-hold technique: 80 kVp; 360 raw projections per rotation, an exposure time of 90 ms and 360° rotation (n =881 scans). This technique is also used for the free breathing scans without retrospective gating.
Retrospective gating: 80 kVp; 480 projections, an exposure time of 90 ms and three or four rotations of 360° are used as parameters (n =748 scans). The increased projection number is suitable for a finer differentiation between moving and resting periods.
With the breath-hold technique, CT image reconstruction is performed automatically after the scan is finished in contrast to the retrospective gating protocol. For this a workflow consisting of three consecutive steps is done: scanning, gating post-processing and image reconstruction.
Gating post-processing is a semi-automatic classification of the raw projection data into two phases: silent and motion. The silent phase takes from the end-exhalation to start-inhalation in one respiratory cycle. The motion phase is the remaining part of the cycle. The robust post-processing algorithm is written in Matlab. 11 The program calculates a new signal from the motion of the diaphragm within a user selected region of interest. The signal with some editable parameters transforms into a binary series, meaning a labelling of the projection with silent or motion phase that is used during the image reconstruction as a filter of input data. Executing the gating post-processing and reconstruction takes around 15–20 min.
CT image reconstructions were carried out using filtered back projection (FBP) with a RamLak filter, voxel size and slice thickness are 321 µm. Additionally, an adaptive bilateral post filter and a strong unsharp mask are applied to enhance the sharpness and remove noise.
Data analysis
The software package used for data analysis was GraphPad Prism software (8.4.2, PRISM, San Diego, CA, USA). We used a Spearman r correlate analysis for BPM versus silent rate per respiratory cycle. All tests were two-tailed, and results with p < 0.05 were considered to be statistically significant.
Image quality was inspected visually by three experienced persons and based subjectively on the number of artifacts visible. In addition, quantification of visibility of the lungs was measured with two objective metrics: quantification of the contrast between the brighter object and the darker background in CT Hounsfield units (HU), and the second parameter was the contrast to noise ratio (CNR), which is more correlative with the human visibility of the objects.
Results
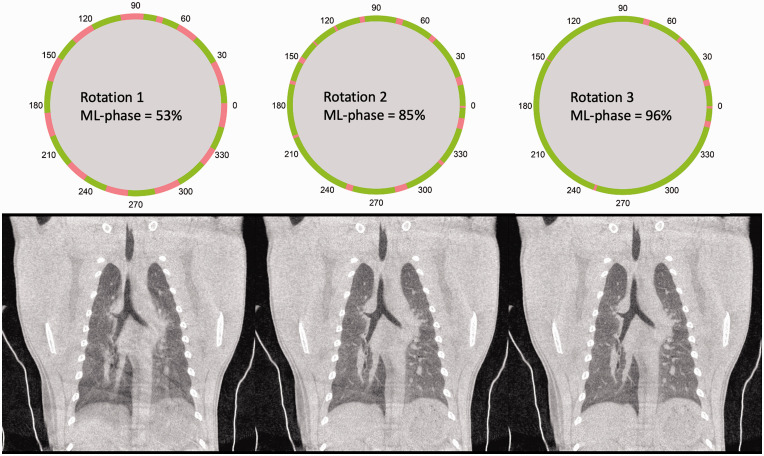
In general, all procedures were uneventful. The projection set from a single rotation with a silent phase of only 53% is inadequate for a faithful reconstruction (Figure 2). The gaps in the angular distribution of the data cause streak artifacts and blurring of the image, most prominently around the trachea and diaphragm. To avoid such distortions, the gaps in the data have to be closed, primarily by repeating the scan over the same trajectory and filtering the silent phase projections, to avoid piling, and filling any residual gaps with motion phase projections. After the second rotation, the silent phase in the projection set increased to 85%, significantly decreasing blurring; nevertheless, there were still a number of streak artifacts present. After the third rotation, the silent phase was further increased to 96% and hardly any streak artifact was detectable. The number of repetitions needed depends on the breathing pattern and the delay time between consecutive scans and can be different for each animal.
Figure 2.
Visualisation of the results of the retrospective respiratory gating in combination with the increase in percentage of silent phase (green) in the final reconstruction of 53% after the first rotation adding up until 85% and 96% after the second and third rotation, respectively.
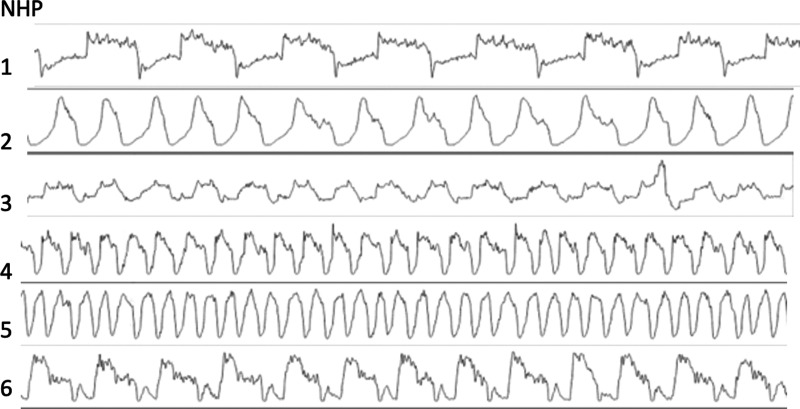
The respiratory cycle of 20 scans derived from ten freely breathing animals was examined and the breathing pattern of a selection is visualized in Figure 3. These scans are all representations of non-infected healthy animals. The average respiration period is 2440 ms [standard deviation (SD) 760 ms, range 1120–3800 ms], equalling a respiration rate of 28 BPM (SD 10 BPM, range 16–54 BPM) with quite stable frequency during the scans. On average, the silent part is 42.3% of the cycle (SD 7.6%, range 28.4–54.1%). This means that, in theory, three rotations are sufficient to achieve a complete raw data set without motion. However, as the silent parts can overlap during the repetitions, the gaps in the sorted projection set can still be considerable, impacting image quality as visualized in Figure 2. In practise, in about 95% of cases, three rotations suffice when a delay of 2 s between rotations is applied.
Figure 3.
Examples of breathing patterns of six anesthetized free breathing healthy rhesus macaques (Macaca mulatta) of 5–6 years of age, 7.10–9.19 kg and a weight-to-height index (WHI) of 56.8–-62.7 kg/m3. 12 The upper three rows represent males and the bottom three rows females.
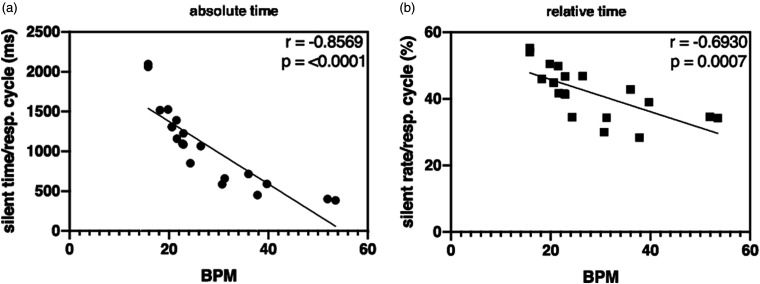
Next, the correlation between the BPM and silent time/silent rate was determined (Figure 4). Silent rate is the percentage of the silent time per respiratory cycle. A useful statistic in the beginning of the study was the clear negative correlation for both the absolute silent time and relative silent rate of r=–0.8569 and r=–0.6930 (P < 0.0001 and P = 0.0007), respectively. The higher the BPM, the lower the silent rate, and the more likely it is that three rotations are not sufficient for an optimal image. As the physiology of the animal cannot be adjusted, the optimized scan count (three or four rotations) in our studies became dependent on a subjective evaluation of the respiration rate upfront.
Figure 4.
Correlation graphs of the absolute (a) and relative (b) time in silent phase of the respiration cycles as a function of the respiratory frequency. (BPM= breathings per minute)
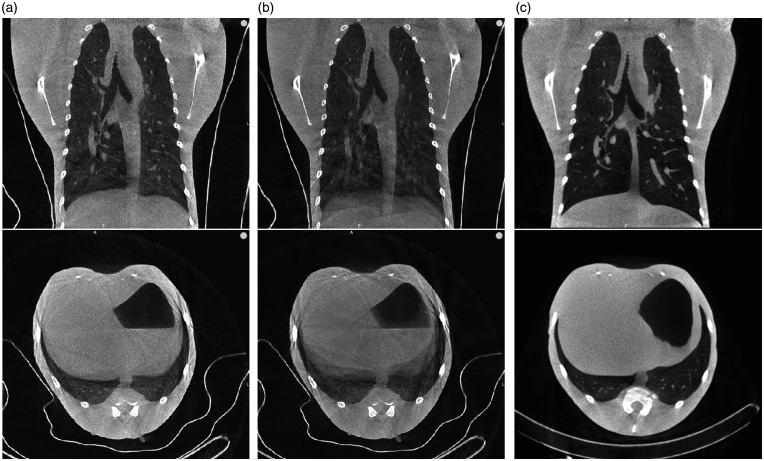
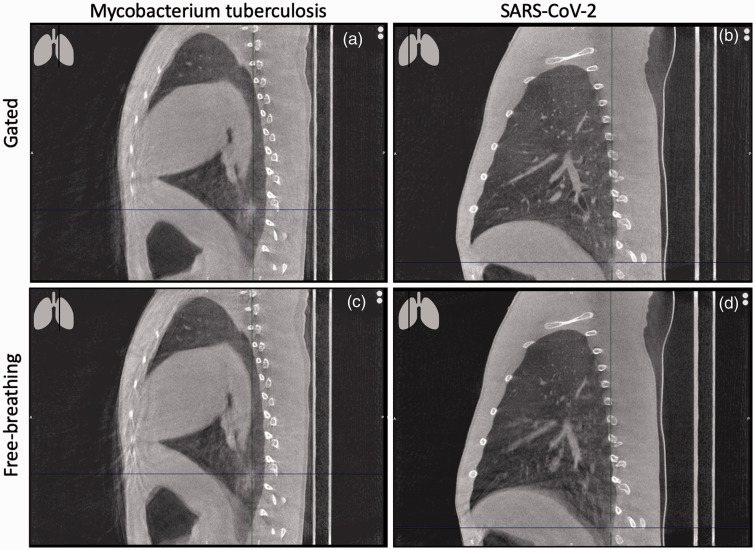
Lastly, the image quality of the optimized retrospective gating reconstruction was visually compared with a free breathing and a breath-hold procedure in Figure 5a–c. Images were obtained from the same animal within 2 days of each other. A free breathing image was reconstructed as part of the gated scan. Besides the comparison of the three imaging techniques with healthy macaques, two examples of infected animals are also shown (Figure 6). Figure 6a,c shows an example of a rhesus macaque 6 weeks after the first infection with Mycobacterium tuberculosis following a repeated low dose scheme of 8 weeks 1 colony forming unit (CFU) with two consolidations in the lower left part of the lung. Figure 6b,d shows an example of a rhesus macaque 7 days after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection with a ground glass opacity (GGO) in the lower dorsal part of the right lung.
Figure 5.
(a–c) Free breathing with retrospective gating (a), free breathing without retrospective gating (b) and breath-hold (c) coronal and transversal representative CT slices of one rhesus macaque (Macaca mulatta).
Figure 6.
(a–d) Free breathing with retrospective gating (a and b) and free breathing without retrospective gating (c and d) sagittal CT slices of two rhesus macaques (Macaca mulatta) after infection with mycobacterium tuberculosis (a and c) or SARS-CoV-2 (b and d). The cross hairs in the image mark the lesion.
The quantitative image quality indexes of the CT images of Figures 2, 5 and 6 are presented in Table 1 with the CT dose index of the source scans. The numerical evaluation shows evidence of the superiority in average contrast-to-noise ratio (CNR) of the gated images (average 4.6) over the free breathing, non-gated image (average 3.0). Although the comparison between breath-hold and gated technique is less clear since the animal scans are not the same, the breath-hold images are more satisfactory. An explanation of this may be the difference in air-density inside the expanded lung during the breath-hold, plus natural evaporation is paused, resulting in less water content in the air. The smaller the density the smaller the HU in air and the higher the contrast of lesions/bronchioles.
Table 1.
| Image label | a | b | c | d | |
|---|---|---|---|---|---|
| Figure 2 (gated (c), coronal) | CTDI (mGy) | 32.7 | 65.4 | 98.1 | |
| Average contrast (HU) | 157.8 | 218.7 | 241.7 | ||
| Average CNR | 2.3 | 3.3 | 3.7 | ||
| Figure 5 (gated (a), free breathing (b), breath-hold (c), coronal) | CTDI (mGy) | 98.1 | 32.7 | 32.7 | |
| Average contrast (HU) | 398. | 244.1 | 672.7 | ||
| Average CNR | 5.8 | 2.8 | 8.3 | ||
| Figure 6 (gated (a+b) and free breathing (c+d), sagittal) | CTDI (mGy) | 98.1 | 98.1 | 32.7 | 33.7 |
| Average contrast (HU) | 332.0 | 371.9 | 273.4 | 311.7 | |
| Average CNR | 4.2 | 4.8 | 3.0 | 3.3 |
HU, Hounsfield units; CNR, contrast to noise ratio; CTDI, computed tomography dose index.
Discussion
This report demonstrates the effectiveness of retrospective respiratory gating in improving high resolution CT images from free breathing macaques. To evaluate the effectiveness of the gating procedure, one has to compare it with the other two options: free breathing, and some type of respiratory control, in our case a breath hold procedure.
Provided that the captured data is of sufficiently high quality, imaging can address the 3Rs by providing refinement as longitudinal information is obtained in a minimal or non-invasive way that reduces procedure severity. 12 Nevertheless, this refinement can be shattered by reduced image quality. Image quality of a CT is, for the most part, determined by the number of artifacts present. Respiratory motion artifacts, so called misregistration artifacts, cause blur, shading and streaking on the reconstructed image. 13 Streaking appear as straight lines, bright and dark, across the image. Closer to the bones (vertebrae, ribs) the streaks intensify. In general, these artifacts are unlikely to cause misdiagnosis, since pathology rarely appears like this but they reduce the reliability of the results. 14 Streaks are present in both the free breathing and gated reconstruction though their intensity is reduced with gating. Shading artifacts show often in regions of high contrast (e.g. between soft-tissue and bones or soft tissue and air), causing shifts in HU representations. This artifact is less predictable compared with streaks, and the boundaries are less easy to identify since natural anatomy also presents gradual changes in HUs. As a result, shading can mimic pathology on it is own or dominate underlying lesions. This last point can lead to misdiagnosis; for instance, in COVID-19 research, the most common lesion types found after SARS-CoV-2 infection on CT are GGOs, which are characterized by only a slight increase in lung opacity.15,16 The risk of missing these types of lesions is visualised in Figure 6, disturbing the image quality in a clinical relevant way. 14 When comparing the three breathing techniques, shading is totally absent in the breath-hold image and almost absent in the gated image compared with the free breathing image when focussing on the diaphragm and trachea. The findings of this study are likely to be translated to other animal species, including other non-human primate species, when respiratory control is necessary. In humans, for certain CT protocols a similar type of retrospective gating is applied. 17
Our data show that the use of retrospective respiratory gating in high resolution CTs for macaques is successful. With the use of this technique, artifacts due to free breathing are reduced to a diagnostically appropriate level. Most importantly, this technique makes chest CTs with this instrumentation a non-invasive modality.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Botond Tölgyesi and Kálmán Nagy are employees of Mediso Medical Imaging Systems Ltd., without any financial interest in the current publication.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Botond Tölgyesi https://orcid.org/0000-0003-1882-4289
Jaco Bakker https://orcid.org/0000-0001-6670-1887
Kálmán Nagy https://orcid.org/0000-0001-9731-4599
Marieke A. Stammes https://orcid.org/0000-0003-4261-6535
References
- 1.Forum of International Respiratory Societies, The Global Impact of Respiratory Disease. 2017. Sheffield.
- 2.Miller LA, Royer CM, Pinkerton KE, et al. Nonhuman primate models of respiratory disease: past, present, and future. ILAR J 2017; 58: 269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Berk IAH, Kanglie M, van Engelen TSR, et al. OPTimal IMAging strategy in patients suspected of non-traumatic pulmonary disease at the emergency department: chest X-ray or ultra-low-dose CT (OPTIMACT) – a randomised controlled trial chest X-ray or ultra-low-dose CT at the ED: design and rationale. Diagn Progn Res 2018; 2: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin PL, Coleman T, Carney JP, et al. Radiologic responses in cynomolgus macaques for assessing tuberculosis chemotherapy regimens. Antimicrob Agents Chemother 2013; 57: 4237–4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharpe SA, Scott S, Taylor I, et al. Use of high frequency jet ventilation as a refinement for imaging macaques with respiratory disease. Lab Anim 2020: 54: 386–390. [DOI] [PubMed] [Google Scholar]
- 6.Winkelmann C. Imaging in research using nonhuman primates. In: Winkelmann C, Krause SM, McCracken P, et al. (eds) Nonhuman primates in biomedical research. New York: Academic Press, 2012, pp 795–815.
- 7.Cullen LK. Medetomidine sedation in dogs and cats: a review of its pharmacology, antagonism and dose. Br Vet J 1996; 152: 519–535. [DOI] [PubMed] [Google Scholar]
- 8.Sinclair MD. A review of the physiological effects of alpha2-agonists related to the clinical use of medetomidine in small animal practice. Can Vet J 2003; 44: 885–897. [PMC free article] [PubMed] [Google Scholar]
- 9.Verstegen J, Fargetton X, Ectors F. Medetomidine/ketamine anaesthesia in cats. Acta Vet Scand Suppl 1989; 85: 117–123. [PubMed] [Google Scholar]
- 10.Stammes MA, Bakker J, Vervenne RAW, et al. Recommendations for standardizing thorax PET-CT in non-human primates by recent experience from macaque studies. Animals (Basel ) 2021; 11: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartling SH, Dinkel J, Stiller W, et al. Intrinsic respiratory gating in small-animal CT. Eur Radiol 2008; 18: 1375–1384. [DOI] [PubMed] [Google Scholar]
- 12.Tannenbaum J, Bennett BT. Russell and Burch's 3Rs then and now: the need for clarity in definition and purpose. J Am Assoc Lab Anim Sci 2015; 54: 120–132. [PMC free article] [PubMed] [Google Scholar]
- 13.Barrett JF, Keat N. Artifacts in CT: recognition and avoidance. Radiographics 2004; 24: 1679–1691. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh J. Computed tomography: principles, design, artifacts, and recent advances. Bellingham WA: SPIE, 2015. [Google Scholar]
- 15.Finch CL, Crozier I, Lee JH, et al. Characteristic and quantifiable COVID-19-like abnormalities in CT- and PET/CT-imaged lungs of SARS-CoV-2-infected crab-eating macaques (Macaca fascicularis). Preprint at bioRxiv 14 May 2020.
- 16.Warman A, Warman P, Sharma A, et al. Interpretable artificial intelligence for COVID-19 diagnosis from chest CT reveals specificity of ground-glass opacities. Preprint at medRxiv 18 May 2020.
- 17.Pan CH, Shiau AC, Li KC, et al. The irregular breathing effect on target volume and coverage for lung stereotactic body radiotherapy. J Appl Clin Med Phys 2019; 20: 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterck EHM, Zijlmans DGM, de Vries H, et al. Determining overweight and underweight with a new weight-for-height index in captive group-housed macaques. Am J Primatol 2019; 81: e22996. doi:10.1002/ajp.22996. Epub 2019 Jun 13. PMID: 31192494; PMCID: PMC6772146. [DOI] [PMC free article] [PubMed] [Google Scholar]