Abstract
Background and aim
To investigate whether a striped occipital cortex and intragyral hemorrhage, two markers recently detected on ultra-high-field 7-tesla-magnetic resonance imaging in hereditary cerebral amyloid angiopathy (CAA), also occur in sporadic CAA (sCAA) or non-sCAA intracerebral hemorrhage (ICH).
Methods
We performed 7-tesla-magnetic resonance imaging in patients with probable sCAA and patients with non-sCAA-ICH. Striped occipital cortex (linear hypointense stripes perpendicular to the cortex) and intragyral hemorrhage (hemorrhage restricted to the juxtacortical white matter of one gyrus) were scored on T2*-weighted magnetic resonance imaging. We assessed the association between the markers, other CAA-magnetic resonance imaging markers and clinical features.
Results
We included 33 patients with sCAA (median age 70 years) and 29 patients with non-sCAA-ICH (median age 58 years). Striped occipital cortex was detected in one (3%) patient with severe sCAA. Five intragyral hemorrhages were found in four (12%) sCAA patients. The markers were absent in the non-sCAA-ICH group. Patients with intragyral hemorrhages had more lobar ICHs (median count 6.5 vs. 1.0), lobar microbleeds (median count >50 vs. 15), and lower median cognitive scores (Mini Mental State Exam: 20 vs. 28, Montreal Cognitive Assessment: 18 vs. 24) compared with patients with sCAA without intragyral hemorrhage. In 12 (36%) patients, sCAA diagnosis was changed to mixed-type small vessel disease due to deep bleeds previously unobserved on lower field-magnetic resonance imaging.
Conclusion
Whereas a striped occipital cortex is rare in sCAA, 12% of patients with sCAA have intragyral hemorrhages. Intragyral hemorrhages seem to be related to advanced disease and their absence in patients with non-sCAA-ICH could suggest specificity for CAA.
Keywords: Striped occipital cortex, intragyral hemorrhage, magnetic resonance imaging, intracerebral hemorrhage, cerebral amyloid angiopathy
Introduction
Sporadic cerebral amyloid angiopathy (sCAA) is a frequent cause of intracerebral hemorrhage (ICH) in the elderly, characterized by the deposition of the protein amyloid-β in the cerebrovasculature. 1 The Boston criteria enable clinicians to diagnose possible or probable CAA during life based on clinical symptoms and imaging markers. 2 Since the development of the criteria, new CAA-related magnetic resonance imaging (MRI) markers have improved their sensitivity and specificity. 2
Recently, we detected two novel MRI markers on 7-tesla (7 T) MRI in patients with Hereditary Dutch-type CAA (D-CAA), an hereditary form of CAA: a pattern of the occipital cortex and intragyral hemorrhages. 3 A striped occipital cortex, defined as a pattern of separate, hypointense linear stripes at T2*-weighted MRI perpendicular to the pial surface of the cortex, occurred in 40% of patients with symptomatic D-CAA. Intragyral hemorrhage, defined as parenchymal hemorrhage restricted to the juxtacortical white matter of an individual gyrus, occurred in 47% of patients with symptomatic D-CAA. 3 The prevalence of these markers in sCAA and their specificity for CAA pathology is yet unknown.
Aims
Our aim was to investigate the prevalence and specificity of the striped occipital cortex and intragyral hemorrhages in patients with sCAA and in patients with non-sCAA-related ICH and to assess their association with clinical features and CAA-related MRI markers.
Methods
Study participants
We included patients diagnosed with sCAA who participated in our ongoing studies on sCAA disease progression: FOCAS (Follow-up in sporadic CAA Study) and STRIP (The striped occipital cortex sign, a new MRI marker for sCAA). We included patients with non-sCAA-related ICH from the FETCH (Finding the ETiology in spontaneous Cerebral Hemorrhage) study, a collaborative study between the University Medical Centers of Utrecht, Nijmegen and Leiden. Details on the inclusion process of the studies can be found in the Supplementary Methods.
For all participants data on demographics, medical history (hypertension, diabetes mellitus, and hypercholesterolemia) and clinical symptoms (symptomatic ICH and cognition) were obtained by questionnaires. All patients with sCAA underwent cognitive screening in the form of a Mini Mental State Exam (MMSE) and Montreal Cognitive Assessment (MOCA).4,5 The ethics committees of the University Medical Centers of Leiden, Utrecht and Nijmegen approved the studies. Written informed consent was obtained from all participants.
Magnetic resonance imaging
Details on image acquisition and scan parameters can be found in the Supplementary Methods.
Image analysis
We scored the striped occipital cortex and intragyral hemorrhages as previously described by our group on T2*-weighted images: the striped occipital cortex was defined as linear hypointense stripes perpendicular to the pial surface of the cortex, intragyral hemorrhage was defined as an hemorrhage restricted to the juxtacortical white matter of the brain following the contours of the cortical gray matter and was scored according to number and location. The following MRI markers associated with small vessel disease (SVD) were scored according to the Standards for Reporting Vascular changes on neuroimaging (STRIVE) criteria: microbleeds, macrobleeds, cortical superficial siderosis (cSS), white matter hyperintensities, and enlarged perivascular spaces (EPVS) in the centrum semiovale. 6 Two independent observers (EAK and SV) scored the two novel MRI markers. The other markers were scored by one observer (EAK). Because the observers had to review the MRI scans for the study they could not be blinded for ICH location or CAA status. However, both observers were blinded for the demographics, medical history, and clinical symptoms of the participants. Non-concordant findings were discussed with a third observer with >15 years of experience in the field (MAAvW) to obtain consensus. Definitions of the markers can be found in the Supplementary Methods.
Data analysis
Descriptive statistics were performed for baseline characteristics. For both novel MRI markers, the interobserver variation (kappa) and the grading of interobserver agreement was assessed. 7 We calculated the proportion of patients with sCAA (all and in patients with sCAA patients and previous symptomatic ICH) and patients with non-sCAA-ICH with a striped occipital cortex and/or intragyral hemorrhages as well as the difference between these proportions including 95% confidence intervals (95% CIs) with continuity correction. We calculated proportions and medians of MRI markers and cognitive scores in patients with sCAA and compared these in patients with sCAA with and without intragyral hemorrhages. Because our study was exploratory and the number of participants with the novel markers was expected to be relatively low, we decided not to calculate formal p-values but to perform only descriptive statistics for the association between the markers and the MRI and cognitive outcomes.
Results
We included 33 patients with probable sCAA and 29 patients with non-sCAA-ICH. The median age of the 33 patients with sCAA was 70 years (range 55–83), 13 (39%) were women, and 19 (58%) had a history of symptomatic lobar ICH. The median age of the 29 patients with non-sCAA-ICH was 58 years (range 18–84) and 7 (24%) of them were women. Of the 29 non-sCAA-ICH participants, 23 (79%) had a deep and 6 (21%) an infratentorial ICH (Table 1).
Table 1.
Clinical characteristics and presence of the novel MRI markers
| sCAA (n = 33) | Non-sCAA-ICH (n = 29) | |
|---|---|---|
| Median age in years (range) | 70 (55–83) | 58 (18–84) |
| Women (%) | 13 (39) | 7 (24) |
| Symptomatic ICH (%) | 19 (58) | 29 (100) |
| Lobar (%) | 19 (58) | 0 (0) |
| Deep (%) | 0 | 23 (79) |
| Infratentorial (%) a | 0 | 6 (21) |
| Hypertension (%) | 16 (49) b | 19 (67) |
| Hypercholesterolemia (%) | 9 (27) b | 8 (28) |
| Diabetes mellitus type 2 (%) | 1 (3) b | 0 |
| Novel markers | ||
| Striped occipital cortex on MRI (%) | 1 (3) | 0 |
| Intragyral hemorrhage on MRI (%) | 4 (12) | 0 |
Note: MRI: magnetic resonance imaging; ICH: intracerebral hemorrhage; sCAA: sporadic cerebral amyloid angiopathy.
ICH located in the cerebellum or brainstem.
n = 32, information was not available for one CAA participant.
We found a striped occipital cortex in one sCAA patient (3%) and in none of the patients with non-sCAA-ICH (difference in proportions 0.03, 95% CI −0.12 to 0.18). We found five intragyral hemorrhages in four patients with sCAA (12%) and none of the patients with non-sCAA-ICH had intragyral hemorrhage (difference in proportions 0.12, 95% CI −0.05 to 0.29). If we looked at the presence of the novel markers in patients with sCAA and previous symptomatic ICH only, we found that 1 of 19 (5%) had a striped occipital cortex compared to none of the patients with non-sCAA-ICH (difference in proportions 0.05, 95% CI −0.10 to 0.28), and 4/19 (21%) of patients with sCAA and previous symptomatic ICH had intragyral hemorrhages compared to none of the patients with non-sCAA-ICH (difference in proportions 0.21, 95% CI 0.01 to 0.46). The interobserver variation (Kappa statistic) for striped occipital cortex was perfect (1.00) and for intragyral hemorrhage almost perfect (0.90).
We compared the patients with sCAA and the novel MRI markers to the patients with sCAA without. The participant with the striped occipital cortex was 64 years old and had an advanced stage of sCAA; he had 8 lobar ICH on MRI of which at least 1 had been documented to be symptomatic, over 50 lobar Microbleeds (MB) and focal cSS. Clinically, he had vascular dementia with an MOCA score of 13/25 (MMSE score was not available for this participant). The characteristics of the patients with sCAA and intragyral hemorrhage and the patients with sCAA without intragyral hemorrhage are shown in Table 2. Two participants with intragyral hemorrhage had EPVS visible in the same gyrus as the intragyral hemorrhage (Figure 2).
Table 2.
Characteristics of sCAA patients with intragyral hemorrhage compared to sCAA patients without intragyral hemorrhage.
| sCAA with intragyral hemorrhage (n = 4) | sCAA without intragyral hemorrhage (n = 29) | |
|---|---|---|
| Median age in years (range) | 64.5 (62–71) | 71 (55–83) |
| Symptomatic ICH (%) | 4 (100) | 15 (52) |
| Median number of symptomatic lobar ICH (range) | 1.5 (1–2) | 0.5 (0–2) |
| ICH on MRI (%) | 4 (100) | 17 (59) |
| Lobar ICH (%) | 4 (100) | 17 (59) |
| Deep ICH (%) | 1 (25) | 2 (7) |
| Median lobar ICH count (range) | 6.5 (4–10) | 1.0 (0–9) |
| Median deep ICH count (range) | 0.0 (0–2) | 0.0 (0–1) |
| Median lobar microbleed count (range) | >50 (42 – >50) | 15 (0 – >50) |
| Median deep microbleed count (range) | 9.5 (0–36) | 0 (0–11) |
| Median Fazekas score periventricular (range) a | 3 (2–3) | 3 (1–3) |
| Median Fazekas score deep (range) a | 3 (2–3) | 2 (1–3) |
| Enlarged CSO-EPVS (%) b | 3 (100) | 12 (100) |
| 11–20 CSO-EPVS (%) | 0 (0) | 2 (17) |
| 21–40 CSO-EPVS (%) | 0 (0) | 2 (17) |
| >40 CSO-EPVS (%) | 3 (100) | 8 (67) |
| Median MMSE score (range) c | 20 (17–24) | 28 (18–30) |
| Median MOCA score (range) d | 18 (13–21) | 24 (13–29) |
| cSS (%) | 2 (50) | 19 (66) |
| Focal (%) | 1 (25) | 2 (7) |
| Disseminated (%) | 1 (25) | 17 (59) |
| Median hemisphere score (range) | 0.5 (0–4) | 2.0 (0–4) |
Note: MRI: magnetic resonance imaging; ICH: intracerebral hemorrhage; sCAA: sporadic cerebral amyloid angiopathy; CSO-EPVS: enlarged perivascular spaces in the centrum semiovale; MMSE: Mini Mental State Exam; MOCA: Montreal Cognitive Assessment; cSS: cortical superficial siderosis.
Intragyral hemorrhage participants n = 3, CAA participants without intragyral hemorrhage n = 28, due to missing MRI sequences.
Intragyral hemorrhage participants n = 3, CAA participants without intragyral hemorrhage n = 12, due to missing MRI sequences.
Intragyral hemorrhage participants n = 3, CAA participants without intragyral hemorrhage n = 27.
Intragyral hemorrhage participants n = 3, CAA participants without intragyral hemorrhage n = 28.
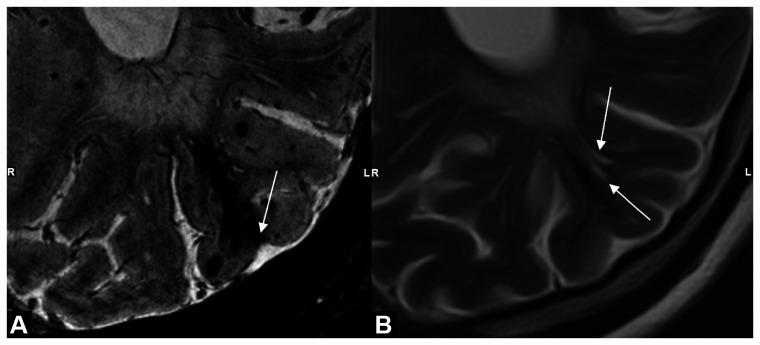
Figure 2.
Example of characteristic shape of intragyral hemorrhage and extended perivascular spaces. MRI scans of a patient with sCAA showing: (a) intragyral hemorrhage on T2*-weighted 7 T-MRI with cortical involvement (white arrow) and (b) presence of enlarged perivascular spaces (EPVS) (white arrows) in the same gyrus as the intragyral hemorrhage on T2-weighted 3 T-MRI; note the similar shape of the EPVS and the susceptibility artifact of the intragyral hemorrhage on T2-weighted MRI, possibly suggesting that intragyral hemorrhage is caused by blood leakage from a cortical microbleed into an EPVS. LR: right and left.
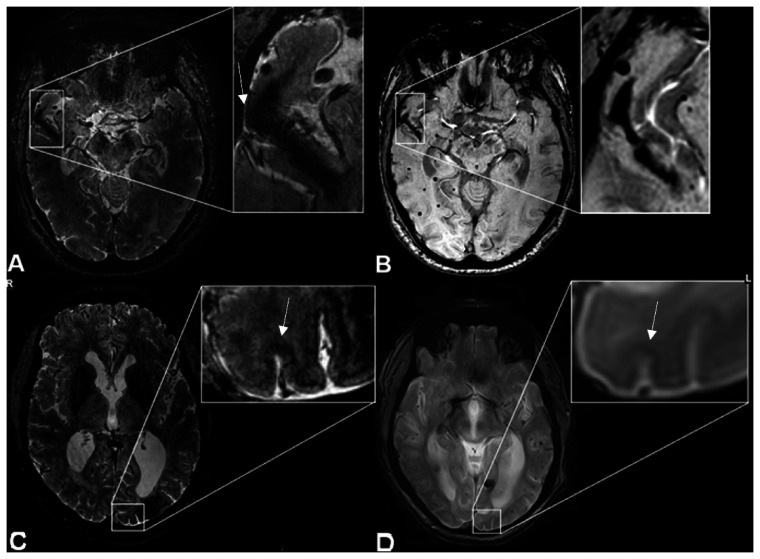
Two of the intragyral hemorrhages were located in the temporal lobe and three in the occipital lobe. The participant with the striped occipital cortex had an occipitally located intragyral hemorrhage. When examining the intragyral hemorrhages closely, we noticed that the susceptibility artifact on T2*-weighted MRIs—although largely confined to the juxtacortical white matter—also showed some extension in the overlying cortex. This cortical involvement was always of limited size but was present in all intragyral hemorrhages (Figure 1(b)). Three of the four patients with intragyral hemorrhages also underwent 3 T-MRI on the same day. We screened the corresponding susceptibility-weighted 3 T-MRI scans and were able to distinguish the intragyral hemorrhages retrospectively in all three patients (Figure 1(c)). The patient with a striped occipital cortex and an intragyral hemorrhage had a T2*-weighted 3 T-MRI of the brain six months after the 7 T-MRI was performed. We were not able to distinguish the characteristic features of a striped occipital cortex at 3 T, although we did see an intracortical hypointense signal at the location of the striped occipital cortex (Figure 1(d)).
Figure 1.
Intragyral hemorrhage and striped occipital cortex on 7 T- and 3 T-MRI. MRI scans of a patient with sCAA showing: (a) intragyral hemorrhage on T2*-weighted 7 T-MRI, showing cortical involvement (arrow), (b) the same intragyral hemorrhage on susceptibility weighted (SWI) 3 T-MRI, (c) striped occipital cortex on T2*-weighted 7 T-MRI (arrow), and (d) on SWI 3 T-MRI (arrow).
We found that in 12 (36%) of the patients with sCAA, including two of the patients with intragyral hemorrhages, the 7 T-MRI showed signs of hypertensive SVD (Table 2 and Figure 1 of the Supplementary Results). One of the patients with intragyral hemorrhages had two deep ICHs on MRI as well as several deep MBs, the other had deep MBs, changing their diagnosis to a mixed form of CAA and hypertension related SVD. However, all 12 patients had more extensive CAA-related SVD than hypertensive SVD: 9 (75%) had a symptomatic lobar ICH, 7 (58%) had cSS, and all had multiple lobar microbleeds.
Discussion
We found that two recently described new MRI markers, a striped occipital cortex and intragyral hemorrhage, also occur in sCAA although less frequently than in hereditary D-CAA and only in patients with previous symptomatic ICH. Neither marker was observed in patients with non-sCAA-ICH.
A striped occipital cortex on 7 T-MRI has been described previously in a small histopathological study from our group, which identified iron depositions and calcification of the penetrating arteries of the cerebral occipital cortex as the histopathological substrate of the striping in one patient with sCAA and two patients with D-CAA. 8 As both iron and calcium co-localize with amyloid-β and represent an advanced stage of amyloid-β accumulation resulting in calcified arterioles, a possible explanation for the presence of the stripes is that they are amyloid-filled arterioles penetrating the occipital cortex. 9 Unfortunately, the striped occipital cortex is only visible on high-field MRI, limiting its use in clinical practice, although some darkening of the cortex could be seen in retrospect on 3 T-MRI (Figure 1).
The pathophysiology underlying intragyral hemorrhages is unclear. In CAA, the cortical and leptomeningeal arterioles are predominantly affected and not the vessels in the white matter. Therefore, it is striking that intragyral hemorrhages would be restricted to the juxtacortical white matter. However, all intragyral hemorrhages in this study showed cortical involvement and, therefore, we speculate that intragyral hemorrhages may have their origin in a cortical (micro) hemorrhage from which blood leaks into an enlarged perivascular space in the juxtacortical white matter, subsequently creating the characteristic shape of an intragyral hemorrhage. By looking in retrospect at the seven intragyral hemorrhages originally described in patients with D-CAA, we found that they, too, show cortical involvement. 3 Two patients with intragyral hemorrhages had EPVS in the same gyrus as the intragyral hemorrhage, and the shapes of the intragyral hemorrhages and EPVS seem to correspond (Figure 2). Future prospective follow-up studies are necessary to investigate presence of EPVS at the location of future intragyral hemorrhages and to investigate their prognostic meaning in CAA.
To our surprise, we found that 36% of the patients previously diagnosed with probable CAA according to the modified Boston criteria based on 1.5 or 3 T-MRI had deep hemorrhages on ultra-high-field 7 T-MRI. The explanation for this finding might be that diagnosis of CAA had been made several months prior, based on lower field strength MR images which have a lower sensitivity to hemorrhagic markers compared to 7 T-MRI.10,11 The Boston criteria have not been validated for 7 T-MRI. By using this type of high-quality MRI scans in this study, we might have unearthed deep bleeds which could not be seen on lower field MRI, which might be reason to consider changing the diagnosis from “pure” CAA to a mixed form of CAA and possibly hypertension related changes. 12 This finding supports recent theories state that SVD is a spectrum with pure CAA (lobar) and non-CAA, often hypertension related (deep) cerebral disease on opposing ends.12–15 Many patients with sCAA will have signs of non-CAA-related SVD or will develop these changes over time, due to the age-related comorbidity of (hypertensive) arteriopathy, which might be undetected in studies using conventional MRI techniques. The findings in this study highlight the difficulty of diagnosing CAA with accuracy during life and the fact that pure CAA might be less common than previously thought. The patients with mixed pathology in our study still had predominantly CAA pathology. If we would exclude these patients from our analysis, the proportion of striped occipital cortex would increase to 1/21 (5%), and the proportion of intragyral hemorrhages would decrease to 2/21 (10%).
A limitation of this study is the small number of included patients. In addition, patients with very severe clinical symptoms due to CAA who were not able to undergo 7 T-MRI because of their clinical condition could not be included in this study. We did not have pathological material to confirm the diagnosis CAA, and observers could not be blinded to the initial diagnosis of either sCAA or non-CAA-ICH which could have caused a bias. Furthermore, there were differences in the 7 T scan protocols for the CAA participants (FOCAS and STRIP) and the non-sCAA-ICH participants (FETCH) although both studies used the same type of MRI scanner. The in-plane resolution of the FETCH T2*-weighted scans was 0.5 × 0.5 mm, versus 0.24 × 0.24 mm of the FOCAS and STRIP T2*-weighted scans. As a striped pattern of the occipital cortex can be very subtle, it is possible that this pattern was missed in the non-CAA-ICH group. The patients with non-sCAA were younger compared to the patients with sCAA and we did not have access to healthy, age-matched controls. Our results suggest that the two markers could be specific for sCAA, however, as they were only found in patients with sCAA and previous ICH the markers might be specific for the CAA-ICH phenotype. Therefore, replication of our findings is warranted in additional (preferably larger) cohorts of CAA patients with different phenotypes, in other amyloid related diseases such as Alzheimer’s disease and in age-matched healthy individuals
Diagnosing CAA during life remains difficult, especially in the early stage of the disease. Although the two new MRI markers are only seen in advanced disease stages, they could help diagnose CAA in cases of doubt. Further research is necessary to investigate the prognostic value of these two new MRI markers, to further investigate the specificity of the markers for sCAA and to assess their underlying pathophysiology.
Supplemental Material
Supplemental material, sj-pdf-1-wso-10.1177_1747493021991961 for Striped occipital cortex and intragyral hemorrhage: Novel magnetic resonance imaging markers for cerebral amyloid angiopathy by EA Koemans, S Voigt, I Rasing, WMT Jolink, TW van Harten, J van der Grond, S van Rooden, FHBM Schreuder, WM Freeze, MA van Buchem, EW van Zwet, SJ van Veluw, GM Terwindt, MJP van Osch, CJM Klijn, MAA van Walderveen and MJH Wermer in International Journal of Stroke
Supplemental material, sj-pdf-2-wso-10.1177_1747493021991961 for Striped occipital cortex and intragyral hemorrhage: Novel magnetic resonance imaging markers for cerebral amyloid angiopathy by EA Koemans, S Voigt, I Rasing, WMT Jolink, TW van Harten, J van der Grond, S van Rooden, FHBM Schreuder, WM Freeze, MA van Buchem, EW van Zwet, SJ van Veluw, GM Terwindt, MJP van Osch, CJM Klijn, MAA van Walderveen and MJH Wermer in International Journal of Stroke
Declaration of conflicting interests: EA Koemans, S Voigt, I Rasing, WMT Jolink, TW van Harten, J van der Grond, S van Rooden, WM Freeze, MA van Buchem, EW van Zwet, and MAA van Walderveen report no disclosures. FHBM Schreuder reports support by the Dutch Heart Foundation (clinical established investigator grant 2012T077). SJ van Veluw reports support from Nederlandse organisatie voor Wetenschappelijk Onderzoek (NWO) (VENI grant 91619021). GM Terwindt reports independent support from NWO, European Community, the Dutch Heart Foundation, the Dutch Brain Foundation, and the Dutch CAA foundation. MJP van Osch reports independent support from NWO (VICI grant 016.160.351), European Community (CDS–QUAMRI, Grant/Award Number 634541), the Netherlands Heart Foundation, and NWO (Brain@Risk) and research support from Philips. CJM Klijn reports support from the Dutch Heart Foundation (clinical established investigator grant 2012T077) and from the Netherlands Organization for Health Research and Development (Aspasia grant ZonMw no. 015.008.048). MJH Wermer reports independent support from NWO ZonMw (VIDI grant 91717337), the Netherlands Heart Foundation, and the Dutch CAA foundation. The authors declare that there is no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by an Clinical Established Investigator grant of the Netherlands Heart Foundation 2016T086 to MJH Wermer, and by the Dutch CAA foundation. The funding agency had no role in the design or conduct of the study.
Supplemental material: Supplemental material for this article is available online.
ORCID iDs
EA Koemans https://orcid.org/0000-0003-0560-8077
S Voigt https://orcid.org/0000-0002-5182-6676
TW van Harten https://orcid.org/0000-0002-3408-9379
References
- 1.Viswanathan A, Greenberg SM. Cerebral amyloid angiopathy in the elderly. Ann Neurol 2011; 70: 871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linn J, Halpin A, Demaerel P, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology 2010; 74: 1346–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koemans EA, van Etten ES, van Opstal AM, et al. Innovative magnetic resonance imaging markers of hereditary cerebral amyloid angiopathy at 7 tesla. Stroke 2018; 49: 1518–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 5.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–699. [DOI] [PubMed] [Google Scholar]
- 6.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–174. [PubMed] [Google Scholar]
- 8.Bulk M, Moursel LG, van der Graaf LM, et al. Cerebral amyloid angiopathy with vascular iron accumulation and calcification: a high-resolution magnetic resonance imaging histopathology study. Stroke 2018; 49: 2081–2087. [DOI] [PubMed] [Google Scholar]
- 9.van Rooden S, Maat-Schieman ML, Nabuurs RJ, et al. Cerebral amyloidosis: postmortem detection with human 7.0-T MR imaging system. Radiology 2009; 253: 788–796. [DOI] [PubMed] [Google Scholar]
- 10.Theysohn JM, Kraff O, Maderwald S, et al. 7 tesla MRI of microbleeds and white matter lesions as seen in vascular dementia. J Magn Reson Imaging 2011; 33: 782–791. [DOI] [PubMed] [Google Scholar]
- 11.Ni J, Auriel E, Martinez-Ramirez S, et al. Cortical localization of microbleeds in cerebral amyloid angiopathy: an ultra high-field 7T MRI study. J Alzheimers Dis 2015; 43: 1325–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasi M, Charidimou A, Boulouis G, et al. Mixed-location cerebral hemorrhage/microbleeds: underlying microangiopathy and recurrence risk. Neurology 2018; 90: e119–e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mustapha M, Nassir C, Aminuddin N, Safri A, Ghazali M. Cerebral small vessel disease (CSVD) – lessons from the animal models. Front Physiol 2019; 10: 1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schreiber S, Wilisch-Neumann A, Schreiber F, et al. Invited review: the spectrum of age-related small vessel diseases: potential overlap and interactions of amyloid and nonamyloid vasculopathies. Neuropathol Appl Neurobiol 2020; 46: 219–239. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigues MA, Samarasekera N, Lerpiniere C, et al. The Edinburgh CT and genetic diagnostic criteria for lobar intracerebral haemorrhage associated with cerebral amyloid angiopathy: model development and diagnostic test accuracy study. Lancet Neurol 2018; 17: 232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-wso-10.1177_1747493021991961 for Striped occipital cortex and intragyral hemorrhage: Novel magnetic resonance imaging markers for cerebral amyloid angiopathy by EA Koemans, S Voigt, I Rasing, WMT Jolink, TW van Harten, J van der Grond, S van Rooden, FHBM Schreuder, WM Freeze, MA van Buchem, EW van Zwet, SJ van Veluw, GM Terwindt, MJP van Osch, CJM Klijn, MAA van Walderveen and MJH Wermer in International Journal of Stroke
Supplemental material, sj-pdf-2-wso-10.1177_1747493021991961 for Striped occipital cortex and intragyral hemorrhage: Novel magnetic resonance imaging markers for cerebral amyloid angiopathy by EA Koemans, S Voigt, I Rasing, WMT Jolink, TW van Harten, J van der Grond, S van Rooden, FHBM Schreuder, WM Freeze, MA van Buchem, EW van Zwet, SJ van Veluw, GM Terwindt, MJP van Osch, CJM Klijn, MAA van Walderveen and MJH Wermer in International Journal of Stroke