Abstract
Background
Ototoxicity has been reported after administration of aminoglycosides and glycopeptides.
Objectives
To identify available evidence for the occurrence and determinants of aminoglycoside- and glycopeptide-related ototoxicity in children.
Materials and methods
Systematic electronic literature searches that combined ototoxicity (hearing loss, tinnitus and/or vertigo) with intravenous aminoglycoside and/or glycopeptide administration in children were performed in PubMed, EMBASE and Cochrane Library databases. Studies with sample sizes of ≥50 children were included. The QUIPS tool and Cochrane criteria were used to assess the quality and risk of bias of included studies.
Results
Twenty-nine aminoglycoside-ototoxicity studies met the selection criteria (including 7 randomized controlled trials). Overall study quality was medium/low. The frequency of hearing loss within these studies ranged from 0%–57%, whereas the frequency of tinnitus and vertigo ranged between 0%–53% and 0%–79%, respectively. Two studies met the criteria on glycopeptide-induced ototoxicity and reported hearing loss frequencies of 54% and 55%. Hearing loss frequencies were higher in gentamicin-treated children compared to those treated with other aminoglycosides. In available studies aminoglycosides had most often been administered concomitantly with platinum agents, diuretics and other co-medication.
Conclusions
In children the reported occurrence of aminoglycoside/glycopeptide ototoxicity highly varies and seems to depend on the diagnosis, aminoglycoside subtype and use of co-administered medication. More research is needed to investigate the prevalence and determinants of aminoglycoside/glycopeptide ototoxicity. Our results indicate that age-dependent audiological examination may be considered for children frequently treated with aminoglycosides/glycopeptides especially if combined with other ototoxic medication.
Introduction
Aminoglycosides and glycopeptides are effective antibiotic families, frequently used for treatment of several bacterial infections in children. Aminoglycosides (such as amikacin, gentamicin, tobramycin and streptomycin) possess a concentration-dependent bactericidal activity against Gram-negative aerobic bacteria (including Pseudomonas, Acinetobacter, Klebsiella and Enterobacter species)1,2 and certain Gram-positive bacteria (including mycobacteria).3 Glycopeptides (such as vancomycin, teicoplanin and telavancin) inhibit the generation of peptidoglycan resulting in reduced synthesis of cell walls of Gram-positive bacteria including Enterococcus, Staphylococcus and Streptococcus species, and remain one of the first-line treatment options for MRSA and penicillin-resistant Streptococcus pneumoniae infections.4 Currently, paediatric patients receive aminoglycosides and glycopeptides for several types of infection including tuberculosis,5 pulmonary tract infections occurring in cystic fibrosis6 and cases of serious infections during treatment for (cancer-related) compromised hosts.7,8
From clinical experience and previous studies, it has been suggested that aminoglycosides and glycopeptide antibiotics may induce cochleotoxicity and/or vestibulotoxicity in a subset of paediatric patients, or may enhance the ototoxic effect of concomitantly administered drugs.9,10 Cochleotoxicity is caused by destruction of hair cells resulting in sensorineural hearing loss and/or tinnitus.11 Vestibulotoxicity is defined as damage to the vestibular system resulting in loss of balance and/or vertigo.9,12,13 The mechanism by which aminoglycosides and glycopeptides affect the vestibulocochlear structures is not fully understood yet. It seems that aminoglycosides interfere with mitochondrial ribosomes and thereby inhibit protein synthesis in mammalian cells and perturb cell respiration. This may lead to superoxide overproduction and accumulation of free ferrous iron caused by oxidative damage of mitochondrial aconitase, resulting in cell apoptosis via the Fenton reaction (Figure S1, available as Supplementary data at JAC-AMR Online), which causes destruction of inner ear cells.14,15 Inner ear cells possess a high amount of mitochondria and therefore these cells are highly sensitive to this aminoglycoside-induced toxicity.16 Vestibular hair cells are morphologically similar to cochlear hair cells. Peripheral vestibular cells rapidly take up systemically administered gentamicin via mechano-electric transduction channels at the tip of the stereocilia and apical endocytosis, eventually resulting in a decrease in the number of hair cells.17,18
Lanvers-Kaminsky et al. (2017)9 reported that the severity of ototoxicity varies among different types of aminoglycosides. Neomycin has been described as highly toxic, whereas gentamicin, kanamycin and tobramycin seem medium toxic, and amikacin and netilmicin are considered to be the least toxic aminoglycosides.19 Vancomycin-induced ototoxicity seems rare but discrepancies in the literature excist.20,21 Awareness of the ototoxic effect in growing and developing children is important as it may have a deleterious impact on speech recognition and vocabulary development, which in the long term may induce compromised communication abilities, school performance, psychosocial behaviour and (future) quality of life.22
To date, an overview of knowledge on the prevalence of aminoglycoside and glycopeptide-induced ototoxicity in children is lacking. Besides this, little is known about concomitant risk factors such as underlying disease, age, gender and use of other (potentially ototoxic) co-medication (chemotherapeutics, other antibiotics, diuretics and/or immunomodulatory agents). The aim of this review is to summarize all available literature on the ototoxic effect of aminoglycosides and glycopeptides in children, in order to obtain knowledge for future application in clinical settings.
Methods
Literature search strategy
Systematic electronic literature searches were performed in PubMed (Medline), EMBASE and Cochrane Library databases up to April 2021, by using Medical Subject Heading (MeSH) terms and Title/Abstract (TiAb) terms (Tables S1 and S2). Cross-reference checks were performed to detect additional articles that had not been identified by electronic searching.
Inclusion and exclusion criteria
Studies were selected based on the following inclusion criteria: (i) 75% of the study population was younger than 18 years of age at initiation of therapy; (ii) aminoglycosides and/or glycopeptides were administered intravenously (IV) for any disease or condition; (iii) audiological examinations (hearing function and/or vestibular function) were performed; (iv) the designs included original randomized controlled trial, controlled clinical trial, cross-sectional or cohort studies; (v) the sample sizes included ≥50 patients; and (vi) the articles were written in English. Studies were excluded if they were performed in neonates only; if aminoglycosides/glycopeptides were administered topically, via aerosols or intramuscularly; and if the designs of the studies were case reports or reviews. We decided to exclude studies with a completely neonatal study population as neonates often suffer from comorbidities including prematurity, dysmaturity and asphyxia, and differ in physiology compared with older children.23 Duplicate publications were removed.
Data extraction
Two independent reviewers (F.A.D. and A.J.M.M.) selected and screened all titles and abstracts of available publications. Abstracts selected for full-text inspection were checked based on inclusion criteria. Discrepancies were resolved by discussion with a third reviewer (M.M.v.d.H.-E.). Each identified paper was reviewed for the following data items: number and age of participants, type of disease or condition, aminoglycoside/glycopeptide type, dose and administration route, audiological and/or vestibular examination methods, ototoxicity classification, occurrence of hearing loss, tinnitus and/or vertigo (%) and possible risk factors for development of audiovestibular complications (e.g. dose, duration, concomitant administration of multiple drugs). The definition of relevant hearing loss was extracted from included articles as this influences the reported percentages of hearing loss. Due to heterogeneity among studies with regard to audiological examination methods, hearing loss definition and ototoxicity classification systems, a meta-analysis of the data was not performed.
Study quality assessment
The two independent reviewers assessed the quality and risk of bias within the included studies. For cohort studies and cross-sectional studies, the Quality In Prognosis Studies (QUIPS) tool was applied to assess the quality of the studies based on the different domains, i.e. study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding and statistical analysis and reporting.24 The risk of bias per domain was classified as ‘high’, ‘moderate’ or ‘low’. The risk of bias within randomized controlled trials (RCTs) was evaluated by the Cochrane criteria and included the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias.25,26 Here, the risk of bias per domain was classified as ‘low’, ‘high’ or ‘unclear’.
The evidence for a specific finding for each study question in each of the studies was critically appraised by the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) classification.27 An overall score was assigned to the summary of evidence, ranging from very high quality (A) to moderate (B), low (C) or very low quality evidence (D).
Results
Search results and quality of the included studies
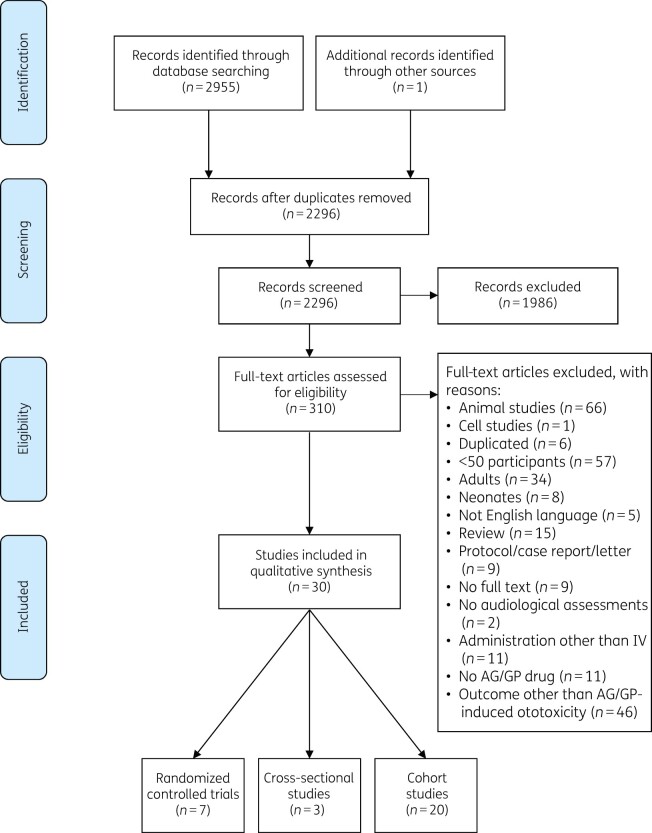
A total of 2956 articles were identified, of which 2296 remained after duplicates were removed (Figure 1). Thirty studies were selected based on the inclusion criteria after full-text screening. Seven were RCTs (Table S3),28–34 20 were cohort studies (Table S4)35–54 and 3 were cross-sectional studies (Table S5).55–57 Within the RCTs, one study33 had a low risk of bias, whereas the other six RCTs had a high or unclear risk of bias (Table S6).28–32,34 The risk of bias of all cohort and cross-sectional studies was moderate to high (Table S7). The total body of evidence for an association between aminoglycoside and/or glycopeptide administration and ototoxicity development in children was graded as low (level C) based on GRADE (Table S8).
Figure 1.
Flow diagram of literature search based on preferred reporting items for systematic reviews and meta-analyses (PRISMA) recommendations. AG, aminoglycoside; GP, glycopeptide.
Characteristics of included studies
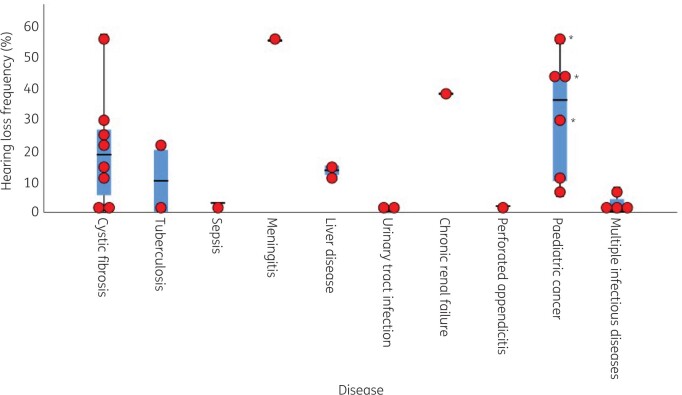
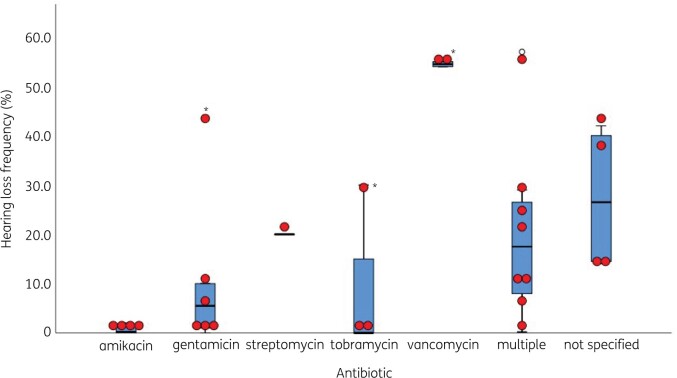
A summary of baseline characteristics of included studies is depicted in Tables S3–S5. Studies were carried out between 1975 and 2021.51,54 In total, 30 studies with more than 6000 children had been included with various diseases (Figure 2). Cross-sectional aetiology studies were performed in healthy children57 and children with hearing loss.55,56 Amikacin,32,35,42–46,48,50,53 gentamicin,28–30,34–36,41–43,48,50,52,55,56 streptomycin39,46,51,57 and tobramycin31,33,35,41–43,50 were the most frequently administered aminoglycosides, whereas vancomycin was the only administered glycopeptide antibiotic (Figure 3).38,41 Treatment dosages ranged between 4.5–8.0 mg/kg/day for gentamicin, 15–22.5 mg/kg/day for amikacin, 9.3–10.0 mg/kg/day for tobramycin, 8.0 mg/kg/day for netilmicin and 15 mg/kg/day for streptomycin. Treatment duration varied from 2 days to 23 days (Tables S3 and S4).45,52
Figure 2.
Boxplot with overlaid dot plot showing the hearing loss frequencies (%) per disease category. Cross-sectional aetiology studies and studies with a 100% hearing loss were not included in this figure. *Clemens et al. (2016)41 calculated hearing loss frequencies for gentamicin, tobramycin and vancomycin separately. The central lines represent the median frequency of hearing loss; the boxes represent the frequency of hearing loss within the 25th and 75th quartile; and the whiskers represent the frequency of hearing loss below the 25th quartile and above the 75th quartile.
Figure 3.
Boxplot with overlaid dot plot showing the hearing loss frequencies (%) per aminoglycoside/glycopeptide subtype. Cross-sectional aetiology studies and studies with a 100% hearing loss were not included in this figure. *Clemens et al. (2016)41 calculated hearing loss frequencies for gentamicin, tobramycin and vancomycin separately. The central lines represent the median frequency of hearing loss; the boxes represent the frequency of hearing loss within the 25th and 75th quartile; and the whiskers represent the frequency of hearing loss below the 25th quartile and above the 75th quartile. Outliers are defined as values 1.5 times the IQR above the upper quartile and below the lower quartile.
The median age of children at start of treatment within the included studies ranged from 2.5 months53 to 17.0 years.43 Hearing function was measured by various audiometric tests including extended-high frequency pure tone audiometry,28,31,35,54 conventional pure tone audiometry,29,33,34,36,37,39–41,43,47–49,51,52,55–57 otoacoustic emissions,30,32,36,37,43,48,49,53 auditory brainstem response,37,49,53 brainstem-evoked response audiometry,28–30 visual reinforcement audiometry,37,56 conditioned play audiometry32,37,56 and behavioural observational audiometry.32,44 The definition of hearing loss varied between 10–15 dB HL at ≥1 frequency32 and ≥50 dB HL in ≥1 ear38 (Tables S3 and S4, Figures S2 and S3). Middle ear status was tested by otoscopic examination,35,36,43,48,52–54 tympanometry35,36,43,44,47,48,54 and middle ear muscle reflexes.43,54 Information on tinnitus was obtained via questionnaires54,57 or self-reported.35,40,46,49,51,52 Tests for vertigo included Romberg, gait test, index finger point test, caloric stimulation, rotation acceleration test with electronystagmography,39 videonystagmography, sinusoidal and rotational step testing, dynamic visual acuity test43 and caloric examination of the vestibular labyrinth.51,53
Aminoglycoside-induced ototoxicity
Frequency of hearing loss
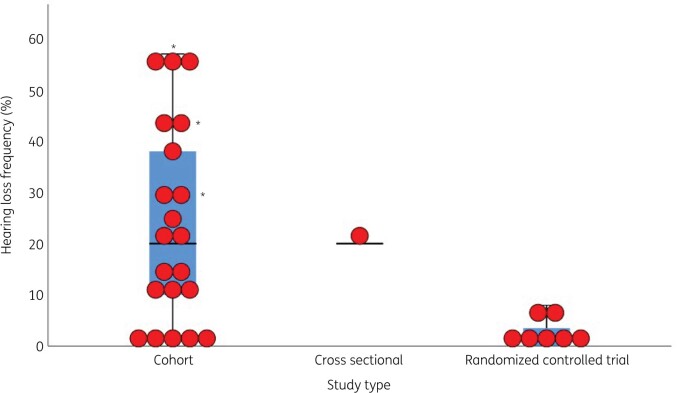
Twenty-nine studies evaluated the occurrence of hearing loss in children treated with aminoglycosides28–37,39–57 of which 7 were RCTs (Table S3),28–34 19 were cohort studies (Table S4)35–37,39–54 and three were cross-sectional studies (Figure 4, Table S5).55–57 In 24 of these studies, hearing loss was observed.28,29,32,34–37,39–44,46–52,54–57 When cross-sectional studies55–57 and one study with 100% hearing loss at time of inclusion were not taken into account,39 an overall frequency of hearing loss ranging from 0.0%30,31,33,45,53 to 57%54 was found within the included studies. Hearing loss frequencies greatly differed per disease (Figure 2) and aminoglycoside subtype (Figure 3).
Figure 4.
Boxplot with overlaid dot plot showing the hearing loss frequencies (%) per study type. Cross-sectional aetiology studies with a 100% hearing loss were not included in this figure. *Clemens et al. (2016)41 calculated hearing loss frequencies for gentamicin, tobramycin and vancomycin separately. The central lines represent the median frequency of hearing loss; the boxes represent the frequency of hearing loss within the 25th and 75th quartile; and the whiskers represent the frequency of hearing loss below the 25th quartile and above the 75th quartile.
Seven studies had an RCT study design.28–34 The primary aim of six of them was to compare a once-daily versus multiple times daily aminoglycoside administration regimen in terms of efficacy, safety and toxicities including hearing loss (Table S9).28–33 One RCT compared netilmicin and gentamicin treatment for life-threatening infections in febrile paediatric patients, and evaluated them for toxicities including hearing loss. In both arms two patients developed hearing loss (Tables S3 and S9).34 Hearing loss frequencies within these RCTs ranged from 0% to 8% (Figure 4).28,30,31,33
Three cross-sectional aetiology studies included aminoglycosides as a potential ototoxic causal factor for hearing loss/deafness (Table S5).55–57 In only one study was a statistically significant association found between aminoglycoside exposure and hearing loss (OR 2.28, 95% CI 0.96–6.28).56 The risk of bias was high in all cross-sectional studies.
Frequency of tinnitus
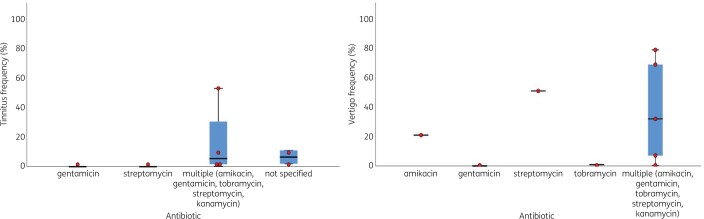
Eight cohort studies reported on tinnitus in children treated with aminoglycosides. The age of children at start of treatment varied from a median age of 5.7 years (range 0.5–16 years) to a mean age of 14.6 ± 3.6 years. All studies had a medium or high risk of bias.35,40,46,49,51,52,54,57 Children had received amikacin, tobramycin, gentamicin, streptomycin and/or kanamycin. The overall frequency of tinnitus ranged between 0% and 53% (Figure 5).35,40,46,49,51,52,54,57 In total, 54 children were reported with tinnitus; 39 of them were identified via questionnaires and in 15 children tinnitus was self-reported or the identification method was unclear. No multivariate analyses were performed to study the association between aminoglycosides and tinnitus.
Figure 5.
Boxplots with overlaid dot plots showing tinnitus and vertigo frequencies (%) per antibiotic subtype. The central lines represent the median frequency of hearing loss; the boxes represent the frequency of hearing loss within the 25th and 75th quartile; and the whiskers represent the frequency of hearing loss below the 25th quartile and above the 75th quartile.
Frequency of vertigo
Nine studies evaluated the occurrence of vertigo,33,39,43,46,51–54,57 of which one was an RCT, seven were cohort studies and one was a cross-sectional study. The risk of bias was low for the RCT33 and medium/high for the cohort and cross-sectional studies.39,43,46,51–54,57 Two children with vertigo in the RCT were withdrawn from the study.33 Children with vertigo had been treated with streptomycin, kanamycin, gentamicin, tobramycin and/or amikacin. The occurrence of vertigo ranged between 0% and 79% (Figure 5). Three studies—two cohort studies and one cross-sectional study—investigated vestibular impairment in combination with hearing loss. One cohort study showed a link between hearing loss and posterior labyrinth damage.39 Another cohort study showed that 18% of included cystic fibrosis patients developed hearing loss and vestibular impairment.43 In the cross-sectional study, 17% of the Chinese rural children with a decrease in hearing threshold levels at 4 kHz reported dizziness.57 No multivariate analyses were performed to study the association between aminoglycosides and vertigo.
Glycopeptide-induced ototoxicity
Frequency of hearing loss
Two cohort studies investigated the occurrence of hearing loss after glycopeptide treatment (Figure 3, Table S4).38,41 Both studies (n = 114 and n = 451) had a medium risk of bias (Table S7) and included children treated for pneumococcal meningitis and survivors of paediatric cancer. In both studies vancomycin was administered to 109 and 181 children, respectively. In the study that included patients with meningitis, hearing loss occurred in 54% of the patients. The multivariate logistic regression analysis revealed that early vancomycin administration, starting <2 h after the first dose of cefotaxime or ceftriaxone, was independently associated with an increased risk of hearing loss (OR 13.5, 95% CI 2.5–73.0).38 The other retrospective study in a selected platinum-treated cohort of childhood cancer survivors reported hearing loss in 55% of the patients. An association between vancomycin treatment and/or other predictors could not be shown, as co-medication data were not systematically collected.41
Concomitant administration of co-medication in included studies
In four studies that included paediatric cancer patients, cisplatin, carboplatin, vincristine and/or diuretics were concomitantly administered with aminoglycosides and/or glycopeptides.34,37,41,49 Here, the occurrence of hearing loss ranged from 5% to 54%. One study showed that treatment with a higher total cumulative dose of cisplatin (per 100 mg/m2 increase; OR 1.2, 95% CI 1.2–1.5) and co-treatment with furosemide (OR 2.3, 95% CI 1.4–3.9) were associated with ototoxicity.41 No significant correlation with aminoglycoside or vancomycin was found. In patients who suffered from chronic renal failure47 and in those who had received a liver transplant, diuretics were concomitantly applied with aminoglycosides.42 The frequency of hearing loss ranged from 12% to 38%. Other co-medication reported within studies included antibiotics such as cephalosporins,30,33,38,45,52 macrolides,30,31 penicillins,28,34,45,55 sulphonamides and trimethoprim,30,45 antiprotozoics,45 antifungals,52 inhaled or topical aminoglycosides,40,48 antimalarials55 and tuberculostatic agents (Tables S3–S5).46,51 One study in 70 cystic fibrosis patients reported that, beside aminoglycosides, IV vancomycin was administered in seven children. Four of these children developed hearing loss (P = 0.047, χ2 test).35
Discussion
Ototoxicity can occur after aminoglycosides and glycopeptide treatment for bacterial infections.9 The current review shows that hearing loss occurs in up to 57% of aminoglycoside-treated children and up to 55% of glycopeptide-treated children. Tinnitus and vertigo frequencies ranged between 0%–53% and 0%–79%, respectively. Ototoxicity occurred more often in children who were concomitantly treated with other potential ototoxic co-medication. This is relevant as ototoxicity can be a serious irreversible problem with implications on the psychosocial and developmental health of children.22,41,58
Aminoglycoside-related hearing loss frequencies found in this review seem to be in accordance with frequencies found in adults, in whom frequencies of 10%59 to 33%60 are reported. The heterogeneous frequency of hearing loss in studies that administered aminoglycosides may be explained by the various diagnoses, cohort selection biases, exposure to other various ototoxic (co-)medication and the variety of applied audiological assessment methods and classifications, as well as the variable cut-off points for hearing loss gradation that were used. In the RCTs, hearing loss occurred in 0%–8% of the patients. This small range might be explained by more homogeneity with regard to audiological assessment methods and hearing loss definition between these studies, the limited use of other ototoxic medication and better follow-up. The lack of consistency between non-RCT studies underscores the need for epidemiological studies on aminoglycoside- and glycopeptide-induced ototoxicity in paediatric patients with a set of standardized and methodologically consistent criteria. More prospective and unselected full cohort studies are needed in which complete data on aminoglycoside/glycopeptide dose and treatment duration as well as epidemiological data are accurately recorded, and audiological testing and grading is performed in a standardized manner at baseline and after cessation of treatment using age-appropriate tests [brainstem evoked response audiometry (0–6 months), visual reinforcement audiometry (6 months–3 years), conditioned play audiometry (3–5 years) and pure tone audiometry (>5 years)].61,62
Four studies in this review investigated aminoglycoside-induced ototoxicity in childhood cancer patients and survivors. In all of these studies, however, patients were co-treated with cisplatin, furosemide and/or vancomycin. Platinum agents including cisplatin and carboplatin are known to be ototoxic.63,64 Hearing loss induced by these agents starts in the high frequency ranges (8000 Hz and above), has an early and irreversible effect, and aggravates with increasing dose. Especially in young patients (≤5 years of age at start of platinum), awareness of this risk is important.7,41,64,65 Hence, it is likely that ototoxicity in platinum-treated childhood cancer patients or survivors is not only induced by aminoglycosides. Synergistic toxic effects of aminoglycosides and cisplatin have been reported in animal studies.66,67 Combined cisplatin and gentamicin administration in guinea pigs resulted in an augmented ototoxic effect when cisplatin was given early in a 14 day gentamicin course.66 Another study showed that a combination of antibiotics (vancomycin and gentamicin) led to a higher risk of ototoxicity.68
We identified only one study that investigated the role of chronic kidney disease (CKD) as an additional risk factor for ototoxicity, which showed that the occurrence of sensorineural hearing loss was significantly increased during the administration of aminoglycosides and furosemide in patients with CKD.47 A number of transporters and ion channels involved in K+ cycling and endolymphatic K+, Na+, Ca2+ and pH homeostasis are expressed in the kidney and inner ear.69 It has been suggested that fluid and/or electrolyte disturbances during chronic renal failure may be independently responsible for hearing loss development70 by changing electrolyte composition of the endolymph. Recently, a study in adults confirmed that reduced kidney function is independently associated with hearing loss development.71 However, aminoglycosides and glycopeptides are also known to be nephrotoxic,72,73 possibly leading to reduced kidney function. The exact mechanisms that may influence systemic and cochlear electrolyte homeostasis in children are still unexplored and unclear, which urges the need for further in vitro and in vivo studies. In addition to ototoxicity, nephrotoxicity may be a dose-limiting factor. Therefore, pharmacokinetics could be important, as high aminoglycoside/glycopeptide concentrations may lead to toxicities. This could be due to inappropriate or lacking therapeutic drug monitoring. Pharmacokinetic/pharmacodynamic modelling and simulation can be used to support therapeutic drug monitoring and consequently may reduce the occurrence of toxicities.74,75
Aminoglycosides are widely used in children with cystic fibrosis for antibiotic treatment during pulmonary exacerbations.35 In these children a prevalence between 0% and 57% has been described.31,33,54 The total cumulative number of IV courses of aminoglycosides (>10) and the total cumulative aminoglycoside dose induce a higher risk of ototoxicity.35,40,76,77 In addition to conductive, sensorineural and mixed hearing loss, substantially higher rates of multiple symptoms associated with audiological disorders (such as tinnitus, imbalance and dizziness) have been reported in cystic fibrosis children compared with healthy controls.54 Based on these findings, we suggest that routine hearing screening may be considered during and after aminoglycoside exposure.
Only two cohort studies investigated glycopeptide-induced ototoxicity.38,41 Their results might not be fully ascribed to possible independent ototoxic effect of vancomycin as patients in these studies were suffering from meningitis and childhood cancer. The latter group was treated with platinum. Meningitis is a clinical condition associated with hearing loss especially in case of pneumococcal infections.78,79 The mechanism underlying meningitis-associated hearing loss is not completely understood yet, but it has been shown that labyrinthitis ossificans and a decrease in spiral ganglion neuronal density are important factors.80,81 Spiral ganglion neuronal density is decreased 2 weeks after meningitis and correlated with the severity of permanent hearing loss as demonstrated in a rat model. The loss of neurons could be explained by meningitis-associated spiral ganglion neuronal necrosis rather than apoptosis.80
Historically, it has been described that vancomycin contained impurities that caused adverse reactions including ototoxicity.82 Currently, the occurrence of ototoxicity has decreased due to improvement in the manufacturing process. However, to our knowledge, no large prospective studies in older children have been performed since the discovery of vancomycin. This is in contrast to a study in neonatal patients who showed hearing loss at hospital discharge and 5 years after vancomycin treatment.83 Such studies would be highly relevant to determine the exact risk of vancomycin-induced ototoxicity in current practice.
Tinnitus is considered to be a symptom of various underlying somatic events, rather than a disease itself. Such events include middle ear pathology, vascular pathology including arteriovenous malformations, (iatrogenic) cochlear trauma or damaging lesions of the central nervous system.84 In a mouse model, electrophysiological assessments of hearing via auditory brain stem response were performed and showed an increased central activity in the auditory brainstem. This suggests that aminoglycosides induce central hyperactivity and possible manifestation of tinnitus.85 Tinnitus is most often subjective, indicating that it cannot be perceived by others. Thus, determination of the presence and severity of tinnitus during and after aminoglycoside/glycopeptide exposure is only possible through questioning. Even though the disorder is not life-threatening, patients may experience tinnitus as frustrating and annoying, leading to severe distress and reduced quality of life.84,86 Treatment for tinnitus can be effective in certain patients, and may include counselling and/or cognitive behavioural therapy.84 As the identification and quantification of tinnitus have remained difficult in young children, we expect that the actual tinnitus prevalence might be underestimated in this population.87 To the best of our knowledge, a validated tinnitus anamnesis/questionnaire in children younger than 8 years of age is currently not available. A detailed anamnestic screening for tinnitus as described by Savastano et al. (2007)87 may be considered in children aged 8 years and older and treated with potential ototoxic medication including aminoglycosides and glycopeptides.
In this review, the occurrence of vertigo in children was documented in eight studies. Even though we observed a broad frequency range within the studies, the prevalence of vertigo in children treated with aminoglycosides seems higher compared with the general paediatric population. In the USA, Brodsky et al. (2020)88 analysed the prevalence of dizziness and balance problems in healthy children and found an overall prevalence of 5.6%. Interestingly, a higher frequency of vertigo was described in patients treated with streptomycin.39,51 Streptomycin is known to be a more pronounced vestibulotoxic aminoglycoside that preferably affects the vestibular system rather than the cochlear structures.89,90 Another explanation for the broad range in vertigo prevalence could be the use of objective testing in some studies39,43,51,53 and self-reported vertigo in other studies.33,46,52,57 Objective testing (e.g. dynamic visual acuity, videonystagmography and sinusoidal and rotational step testing) could possibly lead to an earlier diagnosis of vertigo.43 As vertigo may lead to problems with posture, gait and coordination in children,91 early objective testing and treatment is suggested in children treated with vestibulotoxic medication.
Limitations
First, the overall quality of included studies was medium and low, and thus the risk of bias was high. Second, due to heterogeneity between studies it was not possible to conduct a meta-analysis. The studies had different inclusion and exclusion criteria, various types and doses of aminoglycosides were administered, therapeutic drugs monitoring was not always performed, and hearing loss testing and definitions highly varied. Although Clemens et al. (2019)92 conclude that there is good concordance between existing ototoxicity scales, diversity in definitions of functional outcomes still exist. In most cohort studies, baseline audiological assessments were not performed. Therefore, pre-existing hearing loss either congenital, induced by previous prescribed medication, or caused by the disease itself cannot be ruled out. Due to the large variance in administered co-medication, heterogeneity between included studies and overall study quality, the results should be interpreted with caution.
Conclusions
This systematic review reveals that the reported occurrence of hearing loss, tinnitus and vertigo related to aminoglycosides and glycopeptides in children varies highly and seems to depend on the diagnosis, type of medication, type of assessment and ototoxicity definition. Nevertheless, children treated with combinations of potential ototoxic medication seem more prone to develop hearing loss. As ototoxicity may have a considerable social and developmental impact on children, age-dependent audiological examinations should be considered for these children on a routine basis. The influence of glycopeptides on ototoxicity development has remained unclear so far. More research is needed on the pathophysiology basis of ototoxicity related to glycopeptides and aminoglycosides, as well as the potential synergistic interactions of ototoxic drugs in children. Future prospective studies are needed to gain insight into the independent as well as concomitant contribution of aminoglycosides and glycopeptides to ototoxicity development in children.
Funding
This study was carried out as part of our routine work.
Transparency declarations
None to declare.
Supplementary data
Tables S1 to S9 and Figures S1 to S3 are available as Supplementary data at JAC-AMR Online.
Supplementary Material
References
- 1. Jackson J, Chen C, Buising K.. Aminoglycosides: how should we use them in the 21st century? Curr Opin Infect Dis 2013; 26: 516–25. [DOI] [PubMed] [Google Scholar]
- 2. Poulikakos P, Falagas ME.. Aminoglycoside therapy in infectious diseases. Expert Opin Pharmacother 2013; 14: 1585–97. [DOI] [PubMed] [Google Scholar]
- 3. Farber SM, Eagle HR.. Streptomycin therapy of tuberculosis. Calif Med 1948; 69: 6–11. [PMC free article] [PubMed] [Google Scholar]
- 4. Butler MS, Hansford KA, Blaskovich MAT. et al. Glycopeptide antibiotics: back to the future. J Antibiot (Tokyo) 2014; 67: 631–44. [DOI] [PubMed] [Google Scholar]
- 5. de Jager P, van Altena R.. Hearing loss and nephrotoxicity in long-term aminoglycoside treatment in patients with tuberculosis. Int J Tuberc Lung Dis 2002; 6: 622–7. [PubMed] [Google Scholar]
- 6. Stavroulaki P, Vossinakis IC, Dinopoulou D. et al. Otoacoustic emissions for monitoring aminoglycoside-induced ototoxicity in children with cystic fibrosis. Arch Otolaryngol Head Neck Surg 2002; 128: 150–5. [DOI] [PubMed] [Google Scholar]
- 7. Landier W. Ototoxicity and cancer therapy. Cancer 2016; 122: 1647–58. [DOI] [PubMed] [Google Scholar]
- 8. Landier W, Freyer DR. et al. Adverse effects of cancer treatment on hearing. In: Schwartz C, Hobbie W, Constine L, eds. Survivors of Childhood and Adolescent Cancer. Springer, 2015; 131–49. [Google Scholar]
- 9. Lanvers-Kaminsky C, Zehnhoff-Dinnesen AG, Parfitt R. et al. Drug-induced ototoxicity: mechanisms, pharmacogenetics, and protective strategies. Clin Pharmacol Ther 2017; 101: 491–500. [DOI] [PubMed] [Google Scholar]
- 10. Kraus DM, Pai MP, Rodvold KA.. Efficacy and tolerability of extended-interval aminoglycoside administration in pediatric patients. Paediatr Drugs 2002; 4: 469–84. [DOI] [PubMed] [Google Scholar]
- 11. Jiang M, Karasawa T, Steyger PS.. Aminoglycoside-induced cochleotoxicity: a review. Front Cell Neurosci 2017; 11: 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Van Hecke R, Van Rompaey V, Wuyts FL. et al. Systemic aminoglycosides-induced vestibulotoxicity in humans. Ear Hear 2017; 38: 653–62. [DOI] [PubMed] [Google Scholar]
- 13. Rutka J. Aminoglycoside vestibulotoxicity. Adv Otorhinolaryngol 2019; 82: 101–10. [DOI] [PubMed] [Google Scholar]
- 14. Shulman E, Belakhov V, Wei G. et al. Designer aminoglycosides that selectively inhibit cytoplasmic rather than mitochondrial ribosomes show decreased ototoxicity: a strategy for the treatment of genetic diseases. J Biol Chem 2014; 289: 2318–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huth ME, Ricci AJ, Cheng AG.. Mechanisms of aminoglycoside ototoxicity and targets of hair cell protection. Int J Otolaryngol 2011; 2011: 937861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lesus J, Arias K, Kulaga J. et al. Why study inner ear hair cell mitochondria? HNO 2019; 67: 429–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu J, Kachelmeier A, Dai C. et al. Uptake of fluorescent gentamicin by peripheral vestibular cells after systemic administration. PLoS One 2015; 10: e0120612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Q, Steyger PS.. Trafficking of systemic fluorescent gentamicin into the cochlea and hair cells. J Assoc Res Otolaryngol 2009; 10: 205–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xie J, Talaska AE, Schacht J.. New developments in aminoglycoside therapy and ototoxicity. Hear Res 2011; 281: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bailie GR, Neal D.. Vancomycin ototoxicity and nephrotoxicity. A review. Med Toxicol Adverse Drug Exp 1988; 3: 376–86. [DOI] [PubMed] [Google Scholar]
- 21. Lestner JM, Hill LF, Heath PT. et al. Vancomycin toxicity in neonates: a review of the evidence. Curr Opin Infect Dis 2016; 29: 237–47. [DOI] [PubMed] [Google Scholar]
- 22. Knight KR, Kraemer DF, Neuwelt EA.. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol 2005; 23: 8588–96. [DOI] [PubMed] [Google Scholar]
- 23. Zimmerman KO, Benjamin DK, Becker ML.. Neonatal therapeutics: considerations for dosing. Am J Perinatol 2019; 36 Suppl 2: S18–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hayden JA, van der Windt DA, Cartwright JL. et al. Assessing bias in studies of prognostic factors. Ann Intern Med 2013; 158: 280–6. [DOI] [PubMed] [Google Scholar]
- 25. Cochrane. Risk of Bias Assessment Tool. http://handbook-5-1.cochrane.org/chapter_8/table_8_5_d_criteria_for_judging_risk_of_bias_in_the_risk_of.htm.
- 26. Higgins JPT, Altman DG, Gøtzsche PC. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aguayo-Albasini JL, Flores-Pastor B, Soria-Aledo V.. GRADE system: classification of quality of evidence and strength of recommendation. Cir Esp 2014; 92: 82–8. [DOI] [PubMed] [Google Scholar]
- 28. Elhanan K, Siplovich L, Raz R.. Gentamicin once-daily versus thrice-daily in children. J Antimicrob Chemother 1995; 35: 327–32. [DOI] [PubMed] [Google Scholar]
- 29. Carapetis JR, Jaquiery AL, Buttery JP. et al. Randomized, controlled trial comparing once daily and three times daily gentamicin in children with urinary tract infections. Pediatr Infect Dis J 2001; 20: 240–6. [DOI] [PubMed] [Google Scholar]
- 30. Chong CY, Tan AS, Ng W. et al. Treatment of urinary tract infection with gentamicin once or three times daily. Acta Paediatr 2003; 92: 291–6. [PubMed] [Google Scholar]
- 31. Mulheran M, Hyman-Taylor P, Tan KH. et al. Absence of cochleotoxicity measured by standard and high-frequency pure tone audiometry in a trial of once- versus three-times-daily tobramycin in cystic fibrosis patients. Antimicrob Agents Chemother 2006; 50: 2293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pérez V, Saénz D, Madriz J. et al. A double-blind study of the efficacy and safety of multiple daily doses of amikacin versus one daily dose for children with perforated appendicitis in Costa Rica. Int J Infect Dis 2011; 15: e569–75. [DOI] [PubMed] [Google Scholar]
- 33. Smyth A, Tan KH, Hyman-Taylor P. et al. Once versus three-times daily regimens of tobramycin treatment for pulmonary exacerbations of cystic fibrosis–the TOPIC study: a randomised controlled trial. Lancet 2005; 365: 573–8. [DOI] [PubMed] [Google Scholar]
- 34. Frazier JP, Kramer WG, Pickering LK. et al. Antimicrobial therapy of febrile children with malignancies and possible sepsis. Pediatr Infect Dis 1984; 3: 40–5. [DOI] [PubMed] [Google Scholar]
- 35. Al-Malky G, Dawson SJ, Sirimanna T. et al. High-frequency audiometry reveals high prevalence of aminoglycoside ototoxicity in children with cystic fibrosis. J Cyst Fibros 2015; 14: 248–54. [DOI] [PubMed] [Google Scholar]
- 36. Best EJ, Gazarian M, Cohn R. et al. Once-daily gentamicin in infants and children: a prospective cohort study evaluating safety and the role of therapeutic drug monitoring in minimizing toxicity. Pediatr Infect Dis J 2011; 30: 827–32. [DOI] [PubMed] [Google Scholar]
- 37. Bucuvalas JC, O'Connor A, Buschle K. et al. Risk of hearing impairment in pediatric liver transplant recipients: a single center study. Pediatr Transplant 2003; 7: 265–9. [DOI] [PubMed] [Google Scholar]
- 38. Buckingham SC, McCullers JA, Lujan-Zilbermann J. et al. Early vancomycin therapy and adverse outcomes in children with pneumococcal meningitis. Pediatrics 2006; 117: 1688–94. [DOI] [PubMed] [Google Scholar]
- 39. Camarda V, Moreno AM, Boschi V.. Vestibular ototoxicity in children: a retrospective study of 52 cases. Int J Pediatr Otorhinolaryngol 1981; 3: 195–8. [DOI] [PubMed] [Google Scholar]
- 40. Cheng AG, Johnston PR, Luz J. et al. Sensorineural hearing loss in patients with cystic fibrosis. Otolaryngol Head Neck Surg 2009; 141: 86–90. [DOI] [PubMed] [Google Scholar]
- 41. Clemens E, de Vries AC, Pluijm SF. et al. Determinants of ototoxicity in 451 platinum-treated Dutch survivors of childhood cancer: a DCOG late-effects study. Eur J Cancer 2016; 69: 77–85. [DOI] [PubMed] [Google Scholar]
- 42. Deutsch ES, Bartling V, Lawenda B. et al. Sensorineural hearing loss in children after liver transplantation. Arch Otolaryngol Head Neck Surg 1998; 124: 529–33. [DOI] [PubMed] [Google Scholar]
- 43. Handelsman JA, Nasr SZ, Pitts C. et al. Prevalence of hearing and vestibular loss in cystic fibrosis patients exposed to aminoglycosides. Pediatr Pulmonol 2017; 52: 1157–62. [DOI] [PubMed] [Google Scholar]
- 44. Hesseling PB, Mouton WL, Henning PA. et al. A prospective study of long-term use of amikacin in a paediatrics department. Indications, administration, side-effects, bacterial isolates and resistance. S Afr Med J 1990; 78: 192–5. [PubMed] [Google Scholar]
- 45. Kafetzis DA, Sianidou L, Vlachos E. et al. Clinical and pharmacokinetic study of a single daily dose of amikacin in paediatric patients with severe Gram-negative infections. J Antimicrob Chemother 1991; 27: 105–12. [DOI] [PubMed] [Google Scholar]
- 46. Li Y, Zhu Y, Zhong Q. et al. Serious adverse reactions from anti-tuberculosis drugs among 599 children hospitalized for tuberculosis. Pediatr Infect Dis J 2017; 36: 720–5. [DOI] [PubMed] [Google Scholar]
- 47. Mancini ML, Dello Strologo L, Bianchi PM. et al. Sensorineural hearing loss in patients reaching chronic renal failure in childhood. Pediatr Nephrol 1996; 10: 38–40. [DOI] [PubMed] [Google Scholar]
- 48. Martins LM, Camargos PA, Becker HM. et al. Hearing loss in cystic fibrosis. Int J Pediatr Otorhinolaryngol 2010; 74: 469–73. [DOI] [PubMed] [Google Scholar]
- 49. Olgun Y, Aktas S, Altun Z. et al. Analysis of genetic and non genetic risk factors for cisplatin ototoxicity in pediatric patients. Int J Pediatr Otorhinolaryngol 2016; 90: 64–9. [DOI] [PubMed] [Google Scholar]
- 50. Piltcher OB, Teixeira VN, De Oliveira MW. et al. The prevalence of neurosensorial hearing loss among cystic fibrosis patients from Hospital de Clínicas de Porto Alegre. Int J Pediatr Otorhinolaryngol 2003; 67: 939–41. [DOI] [PubMed] [Google Scholar]
- 51. Prazic M, Salaj B.. Ototoxicity with children caused by streptomycin. Audiology 1975; 14: 173–6. [DOI] [PubMed] [Google Scholar]
- 52. Tomlinson RJ, Ronghe M, Goodbourne C. et al. Once daily ceftriaxone and gentamicin for the treatment of febrile neutropenia. Arch Dis Child 1999; 80: 125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zagolski O. Vestibular system in infants after systemic aminoglycoside therapy. Int J Pediatr Otorhinolaryngol 2007; 71: 1797–802. [DOI] [PubMed] [Google Scholar]
- 54. Blankenship CM, Hunter LL, Feeney MP. et al. Functional impacts of aminoglycoside treatment on speech perception and extended high-frequency hearing loss in a pediatric cystic fibrosis cohort. Am J Audiol 2021; 30: 834–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Freeland A, Jones J, Mohammed NK.. Sensorineural deafness in Tanzanian children-is ototoxicity a significant cause? A pilot study. Int J Pediatr Otorhinolaryngol 2010; 74: 516–9. [DOI] [PubMed] [Google Scholar]
- 56. Judge PD, Jorgensen E, Lopez-Vazquez M. et al. Medical referral patterns and etiologies for children with mild-to-severe hearing loss. Ear Hear 2019; 40: 1001–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Morioka I, Luo WZ, Miyashita K. et al. Hearing impairment among young Chinese in a rural area. Public Health 1996; 110: 293–7. [DOI] [PubMed] [Google Scholar]
- 58. Rajput K, Edwards L, Brock P. et al. Ototoxicity-induced hearing loss and quality of life in survivors of paediatric cancer. Int J Pediatr Otorhinolaryngol 2020; 138: 110401. [DOI] [PubMed] [Google Scholar]
- 59. Smith CR, Lipsky JJ, Laskin OL. et al. Double-blind comparison of the nephrotoxicity and auditory toxicity of gentamicin and tobramycin. N Engl J Med 1980; 302: 1106–9. [DOI] [PubMed] [Google Scholar]
- 60. Brummett RE, Morrison RB.. The incidence of aminoglycoside antibiotic-induced hearing loss. Arch Otolaryngol Head Neck Surg 1990; 116: 406–10. [DOI] [PubMed] [Google Scholar]
- 61. Farinetti A, Raji A, Wu H. et al. International consensus (ICON) on audiological assessment of hearing loss in children. Eur Ann Otorhinolaryngol Head Neck Dis 2018; 135 Suppl 1: S41–8. [DOI] [PubMed] [Google Scholar]
- 62. Meijer AJM, van den Heuvel-Eibrink MM, Brooks B. et al. Recommendations for age-appropriate testing, timing, and frequency of audiologic monitoring during childhood cancer treatment: an International Society of Paediatric Oncology supportive care consensus report. JAMA Oncol 2021; 7: 1550–8. [DOI] [PubMed] [Google Scholar]
- 63. Paken J, Govender CD, Pillay M. et al. A review of cisplatin-associated ototoxicity. Semin Hear 2019; 40: 108–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Qaddoumi I, Bass JK, Wu J. et al. Carboplatin-associated ototoxicity in children with retinoblastoma. J Clin Oncol 2012; 30: 1034–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wei M, Yuan X.. Cisplatin-induced ototoxicity in children with solid tumor. J Pediatr Hematol Oncol 2019; 41: e97–e100. [DOI] [PubMed] [Google Scholar]
- 66. Riggs LC, Brummett RE, Guitjens SK. et al. Ototoxicity resulting from combined administration of cisplatin and gentamicin. Laryngoscope 1996; 106: 401–6. [DOI] [PubMed] [Google Scholar]
- 67. Kohn S, Fradis M, Podoshin L. et al. Toxic effects of cisplatin alone and in combination with gentamicin in stria vascularis of guinea pigs. Laryngoscope 1991; 101: 709–16. [DOI] [PubMed] [Google Scholar]
- 68. Brummett RE, Fox KE, Jacobs F. et al. Augmented gentamicin ototoxicity induced by vancomycin in guinea pigs. Arch Otolaryngol Head Neck Surg 1990; 116: 61–4. [DOI] [PubMed] [Google Scholar]
- 69. Lang F, Vallon V, Knipper M. et al. Functional significance of channels and transporters expressed in the inner ear and kidney. Am J Physiol Cell Physiol 2007; 293: C1187–208. [DOI] [PubMed] [Google Scholar]
- 70. Gatland D, Tucker B, Chalstrey S. et al. Hearing loss in chronic renal failure-hearing threshold changes following haemodialysis. J R Soc Med 1991; 84: 587–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liu W, Meng Q, Wang Y. et al. The association between reduced kidney function and hearing loss: a cross-sectional study. BMC Nephrol 2020; 21: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. McWilliam SJ, Antoine DJ, Smyth RL. et al. Aminoglycoside-induced nephrotoxicity in children. Pediatr Nephrol 2017; 32: 2015–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Feiten HDS, Okumura LM, Martinbiancho JK. et al. Vancomycin-associated nephrotoxicity and risk factors in critically ill children without preexisting renal injury. Pediatr Infect Dis J 2019; 38: 934–8. [DOI] [PubMed] [Google Scholar]
- 74. Germovsek E, Barker CI, Sharland M.. What do I need to know about aminoglycoside antibiotics? Arch Dis Child Educ Pract Ed 2017; 102: 89–93. [DOI] [PubMed] [Google Scholar]
- 75. Germovsek E, Kent A, Metsvaht T. et al. Development and evaluation of a gentamicin pharmacokinetic model that facilitates opportunistic gentamicin therapeutic drug monitoring in neonates and infants. Antimicrob Agents Chemother 2016; 60: 4869–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tarshish Y, Huang L, Jackson FI. et al. Risk factors for hearing loss in patients with cystic fibrosis. J Am Acad Audiol 2016; 27: 6–12. [DOI] [PubMed] [Google Scholar]
- 77. Chen KS, Bach A, Shoup A. et al. Hearing loss and vestibular dysfunction among children with cancer after receiving aminoglycosides. Pediatr Blood Cancer 2013; 60: 1772–7. [DOI] [PubMed] [Google Scholar]
- 78. Koomen I, Grobbee DE, Roord JJ. et al. Hearing loss at school age in survivors of bacterial meningitis: assessment, incidence, and prediction. Pediatrics 2003; 112: 1049–53. [DOI] [PubMed] [Google Scholar]
- 79. Kutz JW, Simon LM, Chennupati SK. et al. Clinical predictors for hearing loss in children with bacterial meningitis. Arch Otolaryngol Head Neck Surg 2006; 132: 941–5. [DOI] [PubMed] [Google Scholar]
- 80. Klein M, Koedel U, Pfister HW. et al. Morphological correlates of acute and permanent hearing loss during experimental pneumococcal meningitis. Brain Pathol 2003; 13: 123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bloch SL, McKenna MJ, Adams J. et al. Labyrinthitis ossificans: on the mechanism of perilabyrinthine bone remodeling. Ann Otol Rhinol Laryngol 2015; 124: 649–54. [DOI] [PubMed] [Google Scholar]
- 82. Tange RA, Kieviet HL, von Marle J. et al. An experimental study of vancomycin-induced cochlear damage. Arch Otorhinolaryngol 1989; 246: 67–70. [DOI] [PubMed] [Google Scholar]
- 83. Marissen J, Fortmann I, Humberg A. et al. Vancomycin-induced ototoxicity in very-low-birthweight infants. J Antimicrob Chemother 2020; 75: 2291–8. [DOI] [PubMed] [Google Scholar]
- 84. Langguth B, Kreuzer PM, Kleinjung T. et al. Tinnitus: causes and clinical management. Lancet Neurol 2013; 12: 920–30. [DOI] [PubMed] [Google Scholar]
- 85. Longenecker RJ, Gu R, Homan J. et al. A novel mouse model of aminoglycoside-induced hyperacusis and tinnitus. Front Neurosci 2020; 14: 561185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Han BI, Lee HW, Kim TY. et al. Tinnitus: characteristics, causes, mechanisms, and treatments. J Clin Neurol 2009; 5: 11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Savastano M. Characteristics of tinnitus in childhood. Eur J Pediatr 2007; 166: 797–801. [DOI] [PubMed] [Google Scholar]
- 88. Brodsky JR, Lipson S, Bhattacharyya N.. Prevalence of pediatric dizziness and imbalance in the United States. Otolaryngol Head Neck Surg 2020; 162: 241–7. [DOI] [PubMed] [Google Scholar]
- 89. Falbe-Hansen J, Rasmussen F, Worsoe-Petersen J.. Ototoxic side effects of the streptomycins, in particular dihydrostreptomycin. Scand J Respir Dis 1972; 53: 38–43. [PubMed] [Google Scholar]
- 90. Selimoğlu E, Kalkandelen S, Erdoğan F.. Comparative vestibulotoxicity of different aminoglycosides in the guinea pigs. Yonsei Med J 2003; 44: 517–22. [DOI] [PubMed] [Google Scholar]
- 91. Jahn K, Langhagen T, Heinen F.. Vertigo and dizziness in children. Curr Opin Neurol 2015; 28: 78–82. [DOI] [PubMed] [Google Scholar]
- 92. Clemens E, Brooks B, de Vries ACH. et al. A comparison of the Muenster, SIOP Boston, Brock, Chang and CTCAEv4.03 ototoxicity grading scales applied to 3,799 audiograms of childhood cancer patients treated with platinum-based chemotherapy. PLoS One 2019; 14: e0210646. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.