Introduction
Dupilumab is a fully human monoclonal antibody that functions as an interleukin (IL) 4α receptor antagonist, inhibiting the activity of both IL-4 and IL-13, drivers of T helper 2 mediated inflammation. Although injection-site reactions are the most reported side effect, there have been several reported cases of adverse dermatologic reactions, including facial and neck erythema.1,2 We present a case of a patient with severe eosinophilic asthma and chronic sinusitis with nasal polyps in whom drug-induced erythema nodosum (EN) developed after 8 weeks of dupilumab therapy.
Case report
A 74-year-old woman presented to the dermatology clinic with a 3-week history of pruritic nodules on the bilateral lower extremities. Her past medical history was remarkable for severe eosinophilic asthma and chronic sinusitis with nasal polyps. Her diagnosis of severe eosinophilic asthma was based on a clinical history of symptoms ongoing for the past year and obstructive spirometry with mild peripheral blood eosinophilia (600 cells/μL), as well as eosinophilic inflammation on endobronchial biopsy. She had undergone a negative workup for underlying vasculitis and other secondary causes of eosinophilia. A 600-mg subcutaneous injection of dupilumab, followed by 300 mg every 2 weeks thereafter, had been initiated 8 weeks before her presentation to the dermatology clinic with associated significant improvement in both upper and lower respiratory tract symptoms.
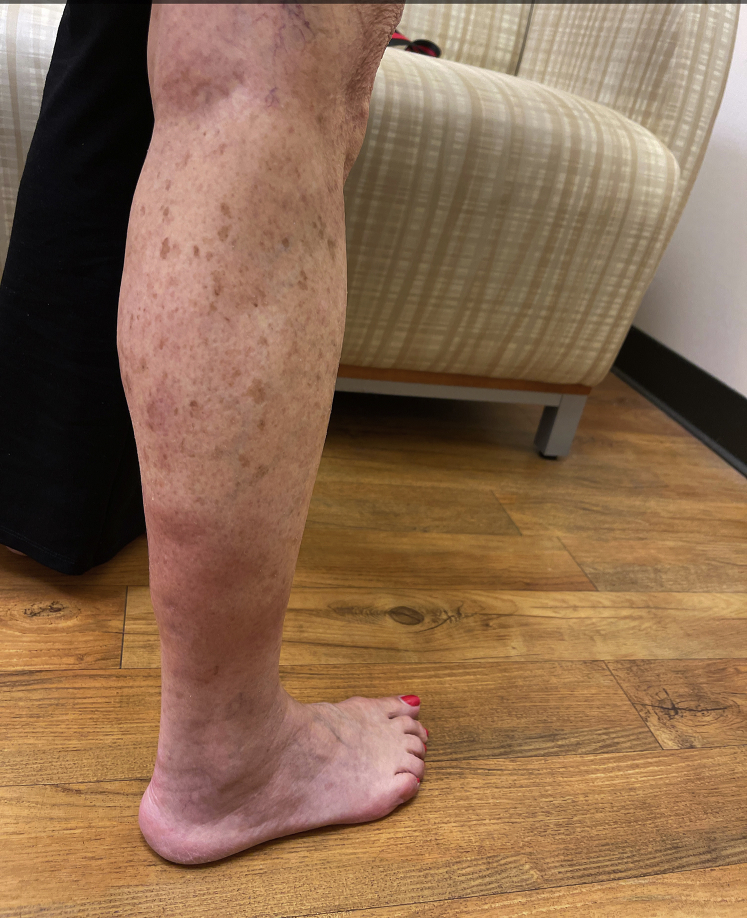
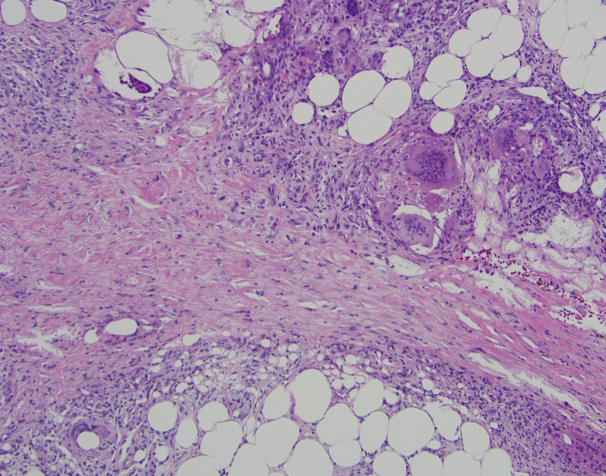
Physical examination revealed multiple erythematous subcutaneous nodules on the bilateral lower extremities, extending from the thighs to the posterior calves (Fig 1). The peripheral blood eosinophil count increased from baseline to >2500 cells/μL. Excisional biopsy of a nodule on the right calf was performed, which showed a predominantly septal panniculitis characterized by septal fibrosis and a mixed granulomatous and lymphohistiocytic inflammatory infiltrate with scattered admixed eosinophils (Fig 2). There was minor involvement of the fat lobules and no evidence of vasculitis. Grocott's methenamine silver and acid-fast histochemical stains were negative for infectious panniculitis. No exogenous material was identified under examination with polarized light. A diagnosis of EN was made. Because the rash appeared at the same time as initiation of dupilumab, dupilumab was discontinued. She was given an intramuscular injection of corticosteroid and started on a prednisone taper, resulting in improvement in the lower extremity nodules in 4 weeks. The eosinophil count decreased to 610 cells/μL after 3 days of steroid treatment.
Fig 1.
Clinical presentation. Erythematous nodules and plaques on the right lower extremity.
Fig 2.
Punch biopsy from the lower leg. Septal fibrosis with a mixed granulomatous and lymphohistiocytic inflammatory infiltrate with scattered admixed eosinophils. (Original magnification: ×100.)
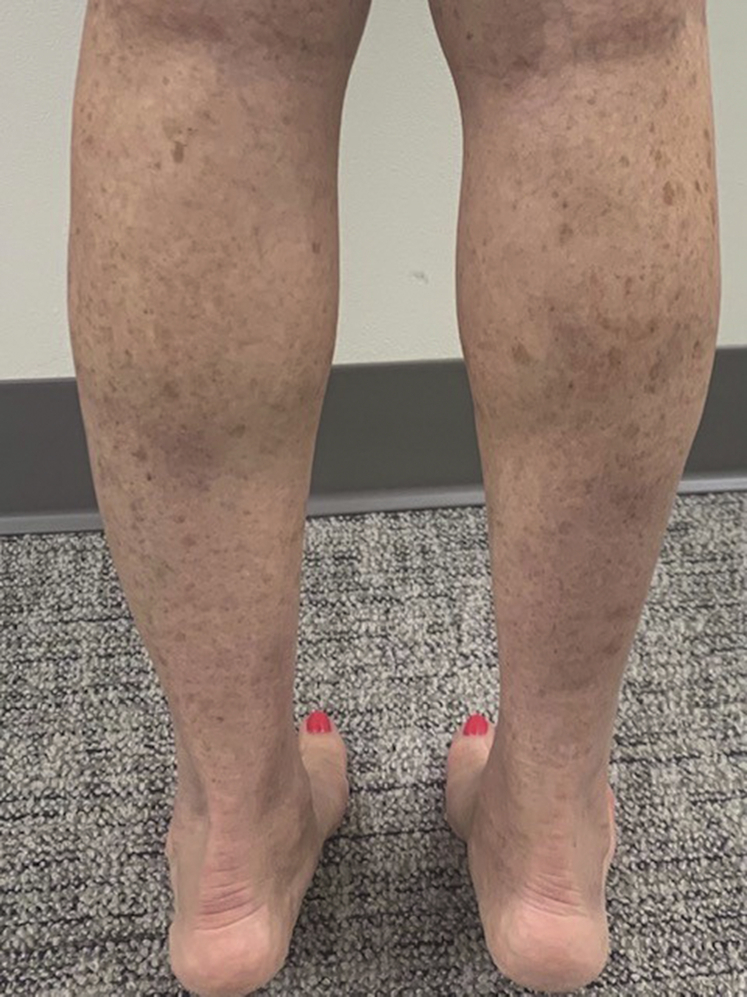
Because of the improvement in respiratory symptoms, the patient restarted dupilumab, with subsequent recurrence of lower extremity nodules within 1 week. She again discontinued dupilumab and started ibuprofen 400 mg 3 times daily along with the use of compression stockings and leg elevation. She switched to omalizumab for management of her eosinophilic asthma, and after 4 weeks, complete resolution of the lower extremity nodules occurred (Fig 3). The blood eosinophil counts returned to baseline. The patient has not experienced any upper or lower airway symptoms or recurrence of skin nodules after 3 doses of omalizumab.
Fig 3.
Clinical resolution. Resolution of nodules and plaques on the lower extremities 6 weeks after discontinuation of dupilumab therapy.
Discussion
Dupilumab was first approved for the treatment of moderate-to-severe atopic dermatitis (AD) and, more recently, for moderate-to-severe asthma with an eosinophilic phenotype or oral corticosteroid-dependent asthma.1 Clinical trials to date have shown that dupilumab therapy significantly improves lung function, reduces severe exacerbations of asthma, and improves quality of life in patients with moderate-to-severe asthma.3 Dupilumab was relatively well tolerated in asthma trials; the commonly observed adverse events were injection-site reactions, nasopharyngitis, and eosinophilia, without increased incidence of conjunctivitis, as seen in AD clinical trials.1,3,4 A similar safety profile has been observed in post–Food and Drug Administration approval observation studies.5,6 The dupilumab package insert warns that EN, among other hypersensitivity reactions, has occurred.1 We have identified a single reported case of EN in a cohort study of dupilumab therapy for AD, although the authors did not elaborate on the diagnosis or clinical course of the EN.6 Reports of EN in patients receiving dupilumab for the treatment of asthma are absent in the existing medical literature.
Although EN is the most common type of panniculitis, its pathophysiology is not fully understood. It is a hypersensitivity reaction precipitated by numerous antigens. The most commonly identified triggers include streptococcal pharyngitis, sarcoidosis, drugs, pregnancy, and enteropathies, none of which were present in this case.7 Up to 55% of cases are idiopathic.7 Histopathologic examination of early lesions of EN reveals a neutrophil-predominant infiltrate in the subcutaneous septa, whereas later lesions are characterized by fibrosis and chronic inflammation, including multinucleated histiocytic giant cells. Elevated levels of proinflammatory cytokines, such as tumor necrosis factor α and IL-8, involved in neutrophil recruitment and activation have been found in patients with EN.8 The pathogenesis of this adverse event in our patient while receiving dupilumab therapy is unclear. Blockage of IL-4 and IL-13 may lead to a dysregulated immune response and a shift toward mediators of neutrophilic inflammation. Alternatively, the EN eruption in the setting of peripheral eosinophilia along with scattered eosinophils noted on histopathologic examination may suggest the involvement of eosinophil sequestration in the subcutis in triggering EN. Eosinophilia and hypereosinophilia occurred in both trials and in post–Food and Drug Administration approval studies of patients with asthma receiving dupilumab.3,5 This was not observed in clinical trials of AD but was recently demonstrated in a retrospective cohort.9 In post–Food and Drug Administration approval studies of asthma, hypereosinophilia was considered asymptomatic, although there have been case reports of hypereosinophilia leading to adverse events.3,10 In a recently reported case of eosinophilic vasculitis that developed in a patient with asthma treated with dupilumab, the investigators concluded that the prominent perivascular eosinophilic infiltrate suggested the pathologic role of eosinophils and not the biologic itself.10 In our patient, although there were eosinophils scattered among the septal infiltrate, the overall histologic findings were not consistent with eosinophilic panniculitis or vasculitis.
Some investigators have suggested that the occurrence of EN is a contraindication to further use of dupilumab, but a correlation between the drug and the adverse event cannot be confirmed without re-exposure.11 In consultation with the Allergy Department, it was decided to restart dupilumab, given the remarkable improvement in asthma symptoms. The temporal relationship between the initiation of dupilumab treatment and onset of the EN eruption, improvement after discontinuation, and recurrence after rechallenge, suggests a potential correlation between dupilumab and EN.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Regeneron Pharmaceuticals DUPIXENT (dupilumab) prescribing information. 2021. https://www.regeneron.com/downloads/dupixent_fpi.pdf
- 2.Jo C.E., Finstad A., Georgakopoulos J.R., Piguet V., Yeung J., Drucker A.M. Facial and neck erythema associated with dupilumab treatment: a systematic review. J Am Acad Dermatol. 2021;84(5):1339–1347. doi: 10.1016/j.jaad.2021.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Xiong X.F., Zhu M., Wu H.X., Fan L.L., Cheng D.Y. Efficacy and safety of dupilumab for the treatment of uncontrolled asthma: a meta-analysis of randomized clinical trials. Respir Res. 2019;20(1):108. doi: 10.1186/s12931-019-1065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y., Jorizzo J. Difference in the rate of ocular adverse events with dupilumab between asthma and atopic dermatitis patients. Int J Dermatol. 2021;60(9):e382. doi: 10.1111/ijd.15524. [DOI] [PubMed] [Google Scholar]
- 5.Dupin C., Belhadi D., Guilleminault L., et al. Effectiveness and safety of dupilumab for the treatment of severe asthma in a real-life French multi-centre adult cohort. Clin Exp Allergy. 2020;50(7):789–798. doi: 10.1111/cea.13614. [DOI] [PubMed] [Google Scholar]
- 6.Faiz S., Giovannelli J., Podevin C., et al. Effectiveness and safety of dupilumab for the treatment of atopic dermatitis in a real-life French multicenter adult cohort. J Am Acad Dermatol. 2019;81(1):143–151. doi: 10.1016/j.jaad.2019.02.053. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz R.A., Nervi S.J. Erythema nodosum: a sign of systemic disease. Am Fam Physician. 2007;75(5):695–700. [PubMed] [Google Scholar]
- 8.De Simone C., Caldarola G., Scaldaferri F., et al. Clinical, histopathological, and immunological evaluation of a series of patients with erythema nodosum. Int J Dermatol. 2016;55(5):e289–e294. doi: 10.1111/ijd.13212. [DOI] [PubMed] [Google Scholar]
- 9.Marcant P., Balayé P., Merhi R., et al. Dupilumab-associated hypereosinophilia in patients treated for moderate-to-severe atopic dermatitis. J Eur Acad Dermatol Venereol. 2021;35(6):e394–e396. doi: 10.1111/jdv.17177. [DOI] [PubMed] [Google Scholar]
- 10.Descamps V., Deschamps L., El Khalifa J., et al. Eosinophilic vasculitis associated with persistent dupilumab-induced hypereosinophilia in severe asthma. Respir Med Res. 2021;79:100821. doi: 10.1016/j.resmer.2021.100821. [DOI] [PubMed] [Google Scholar]
- 11.Brooks G.D. Updated evaluation of dupilumab in the treatment of asthma: patient selection and reported outcomes. Ther Clin Risk Manag. 2020;16:181–187. doi: 10.2147/TCRM.S192392. [DOI] [PMC free article] [PubMed] [Google Scholar]