Abstract
Methionine and its hydroxy analogue (MHA) have been shown to benefit mouse intestinal regeneration. The intestinal organoid is a good model that directly reflects the impact of certain nutrients or chemicals on intestinal development. Here, we aimed to establish a chicken intestinal organoid culture method first and then use the model to explore the influence of methionine deficiency and MHA on intestinal organoid development. The results showed that 125-μm cell strainer exhibited the highest efficiency for chicken embryo crypt harvesting. We found that transforming growth factor-β inhibitor (A8301) supplementation promoted enterocyte differentiation at the expense of the proliferation of intestinal stem cells (ISC). The mitogen-activated protein kinase p38 inhibitor (SB202190) promoted intestinal organoid formation and enterocyte differentiation but suppressed the differentiation of enteroendocrine cells, goblet cells and Paneth cells. However, the suppression of enteroendocrine cell and Paneth cell differentiation by SB202190 was alleviated at the presence of A8301. The glycogen synthase kinase 3 inhibitor (CHIR99021), valproic acid (VPA) alone and their combination promoted chicken intestinal organoid formation and enterocyte differentiation at the expense of the expression of Paneth cells and goblet cells. Chicken serum significantly improved organoid formation, especially in the presence of A8301, SB202190, CHIR99021, and VPA, but inhibited the differentiation of Paneth cells and enteroendocrine cells. Chicken serum at a concentration of 0.25% meets the requirement of chicken intestinal organoid development, and the beneficial effect of chicken serum on chicken intestinal organoid culture could not be replaced by fetal bovine serum and insulin-like growth factor-1. Moreover, commercial mouse organoid culture medium supplemented with A8301, SB202190, CHIR99021, VPA, and chicken serum promotes chicken organoid budding. Based on the chicken intestinal organoid model, we found that methionine deficiency mimicked by cycloleucine suppressed organoid formation and organoid size, and this effect was reinforced with increased cycloleucine concentrations. Methionine hydroxy analogue promoted regeneration of ISC but decreased cell differentiation compared with the results obtained with L-methionine. In conclusion, our results provide a potentially excellent guideline for chicken intestinal organoid culture and insights into methionine function in crypt development.
Keywords: Chicken intestinal organoid, Chicken serum, Methionine
1. Introduction
Methionine is an essential sulfur-containing amino acid that cannot be synthesized by animals. Dietary methionine supplementation benefits intestinal villus development and growth performance (Su et al., 2018; Bin et al., 2018), and its deficiency causes increased apoptosis of intestinal epithelial cells (Tang et al., 2015). Besides, methionine also regulates cell differentiation by methylation induced by its metabolite S-adenosylmethionine (SAM) (Shiraki et al., 2014; Castellano et al., 2017; He et al., 2018). Methionine hydroxy analogue (MHA) is a methionine substitute that is increasingly being used in livestock (Wang et al., 2019a, Wang et al., 2019b). Our previous work showed that a diet supplemented with MHA promotes the SAM synthesis in broilers (Wang et al., 2019b). However, how the mechanisms through which methionine deficiency and MHA alternatives influence chicken intestinal stem cell (ISC) development remains unknown.
Nowadays, mouse/human intestinal organoids are becoming an increasingly useful protocol for investigating the intestine because the intestinal organoid partially reconstructs the typical dynamics of the intestine, and these organoids thus provide researchers with a more physiological context for their experiments. A cocktail (denoted by ENR) of 3 basal growth factors (epidermal growth factor [EGF], Noggin, and R-spondin 1) needs to be present in the intestinal organoid culture medium for the culture of murine and human intestinal organoids (Sato et al., 2011a). EGF, Noggin, and R-spondin 1 separately benefit organoid growth by suppressing cell shedding (Miguel et al., 2017), promoting epithelial cell self-renewal (Chung et al., 2018; Hoffmann et al., 2020), and maintaining stem cell renewal, and cell proliferation and differentiation (Steinhart and Angers, 2018). Besides, the transforming growth factor-β (TGF-β) inhibitor (A8301), the mitogen-activated protein kinase p38 inhibitor (SB202190), valproic acid (VPA), and a highly specific glycogen synthase kinase 3 (GSK3) inhibitor (CHIR99021) have also been shown to benefit the intestinal organoid development (Gottlicher et al., 2001; Ring et al., 2003; Sato et al., 2011a). Recently, Ji et al. (2018) reported that serum supplementation increases cell proliferation via the insulin-like growth factor (IGF)-related signaling pathway. It has been determined that the concentrations of EGF, Noggin, R-spondin 1 in chicken intestinal crypt culture are the same as those in mouse crypts (Li et al., 2018). However, whether chemicals such as A8301, SB202190, VPA, CHIR99021, IGF-1 and serum can further improve chicken organoid growth is still unknown.
In the present study, based on the ENR medium, we aimed to explore the impact of A8301, SB202190, CHIR99021, VPA (together denoted as ASCV), IGF-1, and serum alone or in combination on chicken intestinal crypt development. Based on this culture system, we studied the influence of methionine deficiency and its hydroxy analogue on chicken intestinal crypt development.
2. Materials and methods
2.1. Animal ethics
All the procedures in this experiment were approved by the China Agricultural University Laboratory Animal Welfare and Animal Experimental Ethical Inspection Committee and conducted following the Guidelines for Experimental Animals.
2.2. Culture medium
Penicillin-streptomycin (Thermo Fisher Scientific, Waltham, USA), HEPES (a zwitterionic sulfonic acid buffering agent, 10 mmol/L, Thermo Fisher Scientific, Waltham, USA), B27 (1:50, Thermo Fisher Scientific, Waltham, USA), N2 (1:100, Thermo Fisher Scientific, Waltham, USA), and Glutamax (2 mmol/L, Thermo Fisher Scientific, Waltham, USA) were added to advanced DMEM/F12 (Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12, Thermo Fisher Scientific, Waltham, USA) to form a basal culture medium. On the basis of basal culture medium, different growth factors such as EGF (50 ng/mL, Thermo Fisher Scientific, Waltham, USA), Noggin (100 ng/mL, R&D, Minneapolis, USA), R-spondin 1 (500 ng/mL, R&D, Minneapolis, USA), A8301 (500 nmol/L, ABS, Shanghai, China), SB202190 (10 μmol/L, Sigma, Hunterdon County, USA), CHIR99021(3 μmol/L, Sigma, Hunterdon County, USA), VPA (1 mmol/L, Sigma, Hunterdon County, USA), IGF-1 (PeproTech, Rocky Hill, USA) and chicken serum (Thermo Fisher Scientific, Waltham, USA) or fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, USA) were added to make an assembled medium. Besides, Y27632 (a selective Rho-associated kinase inhibitor, ABS, Shanghai, China) was added only on the first 3 d of the cell culture. A commercial medium (IntestiCult Organoid Growth Medium [IOGM]) was bought from STEMCELL Technologies (CAS 06005, Vancouver, Canada).
2.3. Crypt isolating and seeding
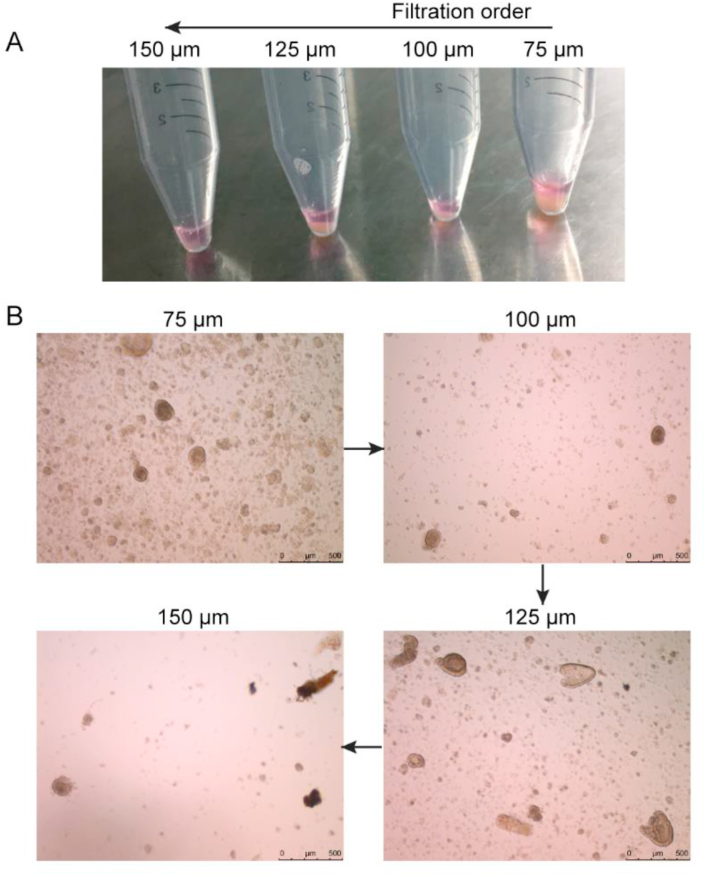
Based on the mouse crypt isolation method (Sato et al., 2011a; Fujii et al., 2015), we simply modified the procedure. Briefly, 18- to 20-d-old chicken embryos (Arbor Acre plus) were sterilized with 70% ethanol. The shell was cracked, and the embryo was taken out and decapitated. The embryo was placed in a cold Petri dish on ice and the small intestine was isolated. The connective tissue was then removed, and the lumen was longitudinally opened and washed with cold PBS (HyClone, Logan, USA) to remove the luminal content. The tissue was then cut into pieces and further washed with cold PBS. The tissue fragments were incubated with 2.5 mmol/L ethylenediaminetetraacetic acid (EDTA, Invitrogen, Waltham, USA) in cold Dulbecco's phosphate buffered saline (DPBS, without calcium and magnesium ions, Solarbio, Beijing, China) for 40 min on an orbital shaker at 0 °C. After the removal of EDTA, the tissue fragments were resuspended in PBS and shaken vigorously to release crypts. Supernatant fractions enriched in crypts were passed through a 70-μm cell strainer first, the filtrate was saved, the residue on the strainer was rinsed, and the flushing fluid was collected. The flushing fluid from the 70-μm cell strainer was filtrated through a 100-μm strainer, the filtrate was saved, the residue on the strainer was rinsed, and the flushing fluid was collected. Subsequently, 125- and 150-μm cell strainers were used sequentially for filtration of crypts (Fig. 1A). The filtrates from strainers with different pore sizes were centrifuged at 150 × g for 3 min and the crypt pellets were suspended with the same volume of advanced DMEM/F12. Then 10 μL of suspension was dropped on a glass slide, and the crypts were observed under a microscope.
Fig. 1.
Chicken crypt isolation. (A) Sequence of the filtration of the digested intestine debris. (B) Photographs of the filtered solution obtained with cell sieves of different diameters (scale bar, 500 μm).
The purified crypt pellet was resuspended in complete culture medium and then mixed with the same volume of Matrigel (Life Science, NY, USA). Then, 50 μL of the mixture suspension that containing at least 1,000 crypts/fragment was dispensed into the center of each well of a preheated 24-well plate and allowed to solidify for 10 to 20 min in a 37 °C incubator immediately. Finally, 500 μL of complete culture medium was overlaid onto the Matrigel and changed every 3 d, and the plate was maintained at 37 °C in an incubator containing 5% CO2.
2.4. Methionine deficiency and its hydroxy analogue alternative in organoid culture
Crypts were isolated as described above, seeded in dishes and cultured with different concentrations of methionine analogue cycloleucine (Shanghai Yingxin Laboratory, Shanghai, China). Besides, the effects of MHA (Adisseo, Commentry, France, purity ≥88%, density 1.23 g/mL) in the methionine-reduced medium were also assayed. Methionine hydroxy analogue was added at a concentration of 1.15 μL/mL, which was equal to 100 μmol/L-methionine. Organoid growth was viewed by an inverted microscope (Leica DMI8, Wetzlar, Germany), and the organoid numbers after 0, 3, and 5 d of culture were counted. Organoids cultured for 5 d were used for RNA analysis.
2.5. 5-Ethynyl-2′-deoxyuridine (EdU) staining
After 5 d of culture, EdU (30 μmol/L) was administrated to the culture medium. After 2 h of incubation, the culture medium was discarded, and the Matrigel was washed 3 times with PBS. The organoids were then fixed in 4% paraformaldehyde for 30 min at room temperature and then glycine was used to neutralize the aldehyde group (2 mg/mL) for 5 min. The organoids were washed with PBS, permeabilized with 0.5% Triton X-100 in PBS for 10 min, and stained using a Cell-Light EdU Apollo 643 In Vivo Kit (Ribobio, Guangzhou, China). Mounting medium with DAPI was used for cell nucleus staining (Solarbio Life Science, Beijing, China). The organoids were then visualized under a laser-scanning confocal microscope (A1 HD25, Nikon, Tokyo, Japan) and the EdU-positive cells appeared in red.
2.6. Quantitative RT-qPCR analysis
The organoids cultured for 5 d were harvested for RNA detection. Organoids mixed with TRIzol reagent (TAKARA, Kyoto, Japan) were blown with a pipette until the organoid pellet disappeared. The purity and concentration of the total RNA were measured with a nucleic acid analyzer (Nano-drop 2000, Thermo Fisher Scientific, Waltham, USA) using the 260:280 nm absorbance ratio. An amount of 900 ng RNA was reverse-transcribed using a PrimeScript RT reagent kit (TAKARA, Kyoto, Japan), and 5 ng of cDNA was used as a template for amplification using an SYBR Premix ExTaq kit (TAKARA, Kyoto, Japan). Two-step RT-qPCR was carried out in a 20-μL reaction volume on a 7500-fluorescence detection system (Applied Biosystems, Carlsbad, USA). Primer sequences are listed in Table 1. The threshold cycle method of comparative PCR was used to analyze the results. All the values were normalized to the level of the housekeeping gene β-actin.
Table 1.
Primers sequences for quantitative RT-qPCR.
| Gene | Accession number | Forward sequence (5′→3′) | Reverse sequence (5′→3′) |
|---|---|---|---|
| LGR5 | XM_425441 | TCAATACCTGAGCGTGCGTT | TGTGAGTGTCAAACTCTCCAGAC |
| MUC2 | XM_001234581.3 | CCCTGGAAGTAGAGGTGACTG | TGACAAGCCATTGAAGGACA |
| CHGA | XM_421330 | GCTATCTCCCTTCCTGTGACAAATG | TGAGTTCTCTCATTGGCACCTTG |
| LYZ | NM_205281 | TACAGCCTGGGAAACTGGGT | CTCCCATCGGTGTTACGGTT |
| SI | XM_015291762 | GTACGCTACGCTTGGAGGTT | TGAAGAGTCACATCCATCGCAT |
| MAT2A | XM_025142868.1 | GGGTGTTGGTGCAGGTTTCCTA | TTCGCTCTTCTGCGACGTTC |
| β-actin | XM_027015741.1 | CAACACAGTGCTGTCTGGTGGTAC | CTCCTGCTTGCTGATCCACATCTG |
LGR5 = leucine-rich-repeat-containing G-protein-coupled receptor 5; MUC2 = mucin 2; CHGA = chromogranin A; LYZ = lysozyme; SI = sucrase-isomaltase; MAT2A = methionine adenosyltransferase 2A.
2.7. Statistical analysis
The data were analyzed by two-way ANOVA with the organoid culture periods and chemical supplementation as main effects using the General Linear Models procedures and followed by post-hoc Duncan's test using the statistical software SPSS 20.0 (SPSS Inc., Chicago, US). A polynomial regression analysis was used to predict the effects of the inclusion of various levels of chicken serum on organoid growth. A Student’s t-test was used for comparison between 2 groups. The data are presented as the means ± SEM. Treatments with different letters were significantly different (P ≤ 0.05).
3. Results
3.1. Isolation of chicken embryo intestinal crypt
As shown in Fig. 1B, most crypts were isolated using a 70-μm strainer, but most of the resulting crypts were broken. However, the most intact crypts were observed in the filtrate obtained after passed through a 125-μm cell strainer. Some unknown big tissue debris was observed in the filtrate obtained after passage through a 150-μm strainer. In summary, a cell strainer with a 125-μm pore size is the most efficient for the isolation of chick embryo intestinal crypt.
3.2. Effect of A8301 and SB202190 on chicken intestinal organoid culture
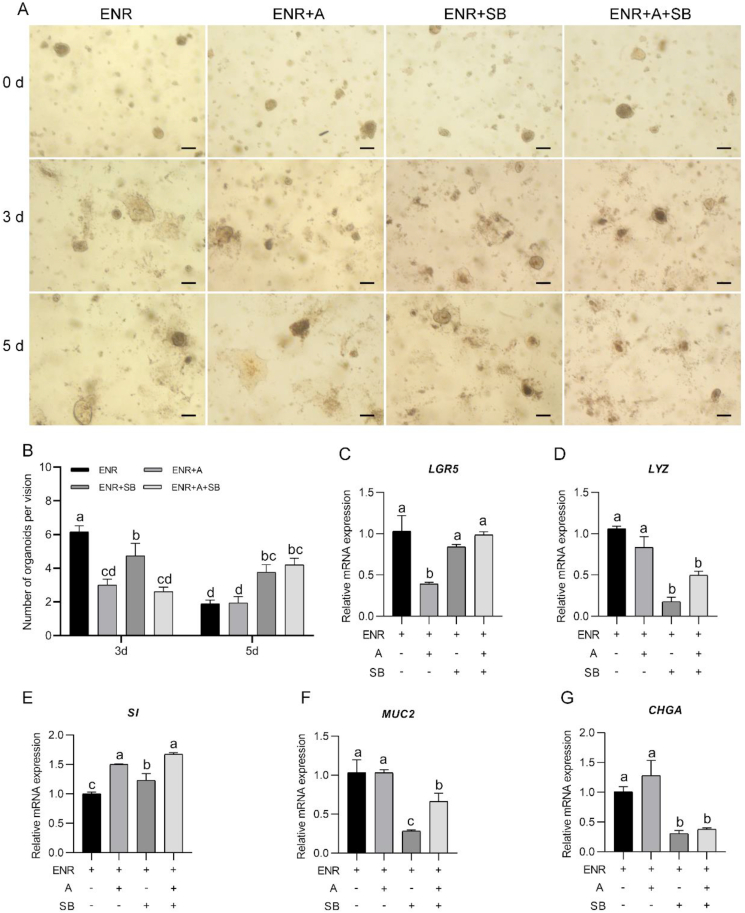
The culture period and additive (A8301 and SB202190) had an interaction effect on the number of formed organoids (P < 0.05). The A8301 treatment decreased the organoid number at 3 d of culture (P < 0.05). However, after 5 d of culture, the number of organoids obtained with the A8301 treatment was comparable to that found with the ENR-only treatment (P > 0.05). SB202190 alone or combined with A8301 decreased the organoid number after 3 d of culture (P < 0.05). However, after 5 d of culture, the organoid numbers obtained with SB202190 alone or combined with A8301 were higher than those obtained with the ENR-only treatment (P < 0.05).
The quantitative RT-PCR analysis showed that treatment with SB202190 alone increased the mRNA expression level of sucrase-isomaltase (SI) (P = 0.021, Fig. 2E), and decreased expression of lysozyme (LYZ), mucin 2 (MUC2) and chromogranin A (CHGA) (P < 0.001, P = 0.001, and P = 0.006, respectively, Fig. 2D, F and G) compared with the expression levels in the crypts cultured under ENR-only conditions. Treatment with A8301 alone increased the mRNA expression of SI (P < 0.001, Fig. 2E), but decreased the expression of leucine-rich-repeat-containing G-protein-coupled receptor 5 (LGR5) (P = 0.002) compared with the levels found in the crypts cultured in ENR-only medium (Fig. 2C). Compared with the expression levels found in organoids cultured in ENR medium, the combination of SB202190 and A8301 upregulated the expression of SI (P < 0.001, Fig. 2E), but downregulated the expression of LYZ, MUC2 and CHGA (P = 0.001, P = 0.031, and P = 0.011, respectively, Fig. 2D, F and G).
Fig. 2.
Influence of A8301 and SB202190 alone or in combination on chicken crypt development. (A) Growth of chicken crypts with different treatments (scale bar, 200 μm). (B) Number of live intestinal organoids obtained with different treatments. At least 15 fields were used for counting the organoids obtained with each treatment. (C to G) Relative mRNA expression of mature intestinal epithelial markers of organoids after 5 d of culture with different treatments. Experiments were performed at least 3 times and a representative one was exhibited. Error bars, SEM (n = 3 wells). a to d Different letters on bars mean a significant difference (P ≤ 0.05). ENR, the mixture of epidermal growth factor, Noggin and R-spondin 1; A, A8301, an inhibitor of transforming growth factor β (TGF-β); SB, SB202190, an inhibitor of mitogen-activated protein kinase p38. LGR5 = leucine-rich-repeat-containing G-protein-coupled receptor 5; LYZ = lysozyme; SI = sucrase-isomaltase; MUC2 = mucin 2; CHGA = chromogranin A.
3.3. Effect of CHIR99021 and VPA on chicken intestinal organoid culture
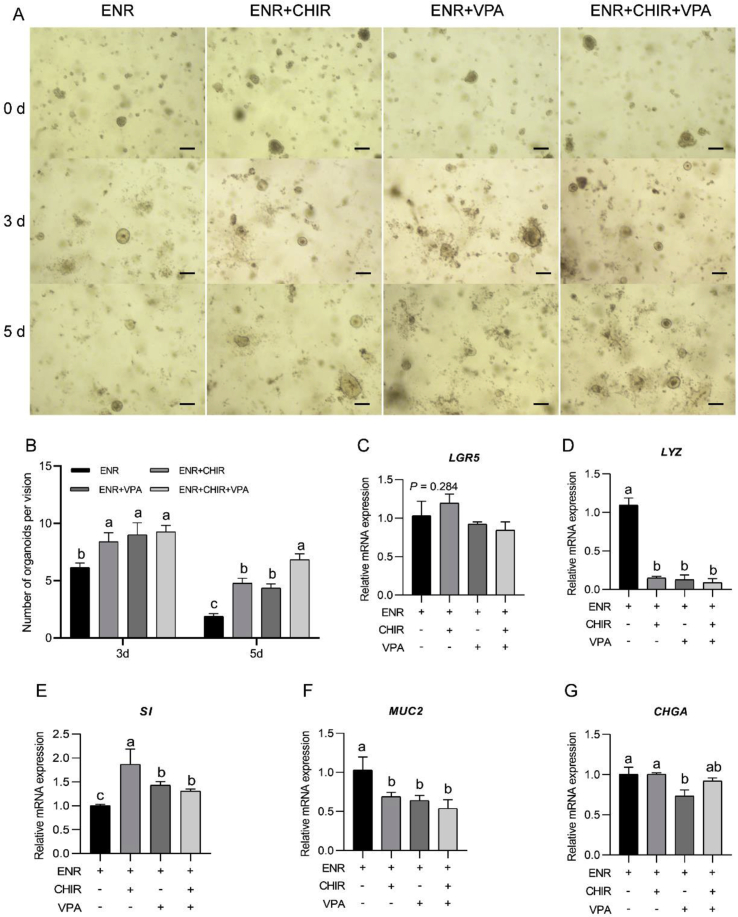
As shown in Fig. 3A and B, the culture period exerted a major effect on the number of formed organoids (P < 0.001). We found that the number of surviving organoids decreased with extensions in the culture period (P < 0.001). Furthermore, the additive exerted the main effect on the number of formed organoids (P < 0.001). Supplementation with CHIR99021, VPA or their combination decreased the number of formed organoids compared with that found in the ENR-only group (P < 0.001). The culture period and additive (CHIR99021 and VPA) exerted no interaction effect on the organoid formation (P > 0.05).
Fig. 3.
Influence of CHIR99021 and valproic acid (VPA) alone or in combination on chicken crypt development. (A) Growth of chicken crypts with different treatments (scale bar, 200 μm). (B) Number of live intestinal organoids obtained with different treatments. At least 15 fields were used for counting the organoids obtained with each treatment. (C to G) Relative mRNA expression of mature intestinal epithelial markers of organoids after 5 d of culture with different treatments. Experiments were performed at least 3 times and a representative one was exhibited. Error bars, SEM (n = 3 wells). a, b, c Different letters on bars mean a significant difference (P ≤ 0.05). ENR, the mixture of epidermal growth factor, Noggin and R-spondin 1; CHIR, CHIR99021, an inhibitor of glycogen synthase kinase 3. LGR5 = leucine-rich-repeat-containing G-protein-coupled receptor 5; LYZ = lysozyme; SI = sucrase-isomaltase; MUC2 = mucin 2; CHGA = chromogranin A.
Compared with the organoids cultured under ENR conditions, we found that treatment with CHIR99021 alone increased the expression of SI (P < 0.001, Fig. 3E), but decreased the expression of LYZ and MUC2 (both P < 0.001, Fig. 3D and F), and did not influence the mRNA levels of LGR5 and CHGA (P = 0.383, P = 0.986, respectively, Fig. 3C and G). Treatment with VPA alone decreased the mRNA levels of LYZ, MUC2 and CHGA (P < 0.001, P = 0.035, and P = 0.012, respectively, Fig. 3D and F to G), but increased the expression of SI (P < 0.001). The combination of CHIR99021 and VPA decreased the expression of LYZ and MUC2 (P < 0.001 and P = 0.013, respectively, Fig. 3D and F), whereas increased the expression of SI (P = 0.002, Fig. 3E).
3.4. Chicken serum supplementation substantially improved the intestinal organoid plating efficiency
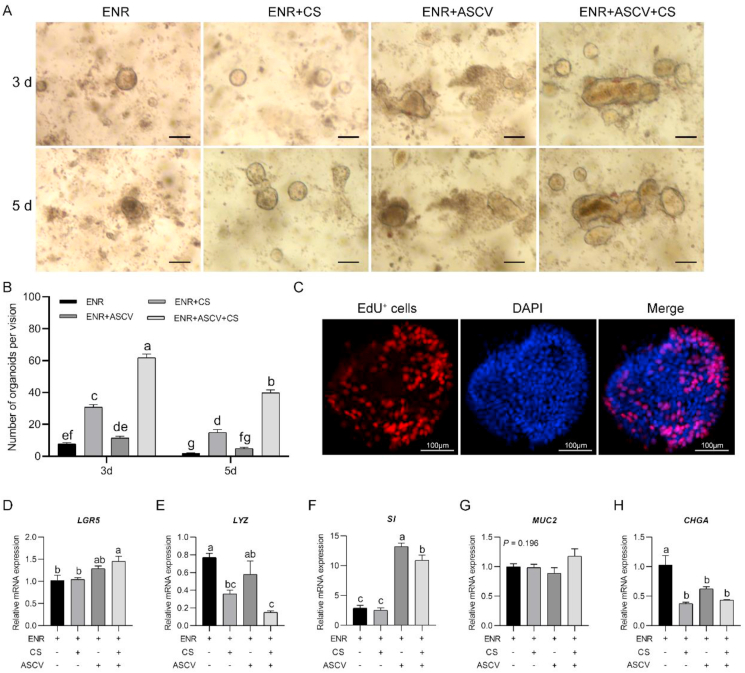
An interaction effect between the culture period and additive was observed (P < 0.05). The organoid number obtained with the chicken serum treatment was greater than that found with the ENR-only medium (P < 0.001), and this finding was particularly notable in the presence of ASCV was present (P < 0.001, Fig. 4A and B). Crypts cultured with ASCV for 3 or 5 d did not improve the organoid formation compared with those cultured in ENR-only medium (both P > 0.05, Fig. 4B). The organoid numbers obtained with all the treatments were decreased with extensions in the culture period (all P < 0.05, Fig. 4B). 5-Ethynyl-2′-deoxyuridine positive cells were distributed around the organoids (Fig. 4C), which implies that ISC are located in spheroids and proliferate and differentiate into epithelial cells.
Fig. 4.
Influence of chicken serum supplementation on chicken crypt development. (A) Growth of chicken crypts with different treatments (scale bar, 200 μm). (B) Number of live intestinal organoids obtained with different treatments. At least 15 fields were used for counting the organoids obtained with each treatment. (C) 5-Ethynyl-2′-deoxyuridine (EdU) positive cells in organoids cultured for 5 d in medium supplemented with chicken serum and A8301, SB202190, CHIR99021 and valproic acid (scale bar, 100 μm). (D to G) Relative mRNA expression of mature intestinal epithelial markers of organoids after 5 d of culture with different treatments. Experiments were performed at least 3 times and a representative one was exhibited. Error bars, SEM (n = 3 wells). a, b, c Different letters on bars mean a significant difference (P ≤ 0.05). ENR, the mixture of epidermal growth factor, Noggin and R-spondin 1; ASCV, the mixture of A8301, SB202190, CHIR99021 and valproic acid; CS = chicken serum; DAPI = 4′,6-diamidino-2-phenylindole; LGR5 = leucine-rich-repeat-containing G-protein-coupled receptor 5; LYZ = lysozyme; SI = sucrase-isomaltase; MUC2 = mucin 2; CHGA = chromogranin A.
Organoids cultured with chicken serum and ASCV were used for RT-PCR detection. We found that chicken serum supplementation decreased the mRNA levels of LYZ and CHGA (P = 0.008 and P = 0.001, respectively, Fig. 4E and H) but did not influence the mRNA levels of LGR5, SI or MUC2 (all P > 0.05, Fig. 4D, F and G). Surprisingly, the mixture of ASCV significantly promoted the expression of SI (P < 0.001, Fig. 4F), decreased the expression of CHGA (P = 0.010, Fig. 4H) but did not influence the expression of LGR5, LYZ, or MUC2 (all P > 0.05, Fig. 4D, E, and G). Chicken serum in combination with ASCV increased the mRNA levels of LGR5 and SI (P = 0.010, P < 0.001, respectively, Fig. 4D and F), but decreased the mRNA levels of LYZ and CHGA (both P = 0.001, Fig. 4E and H).
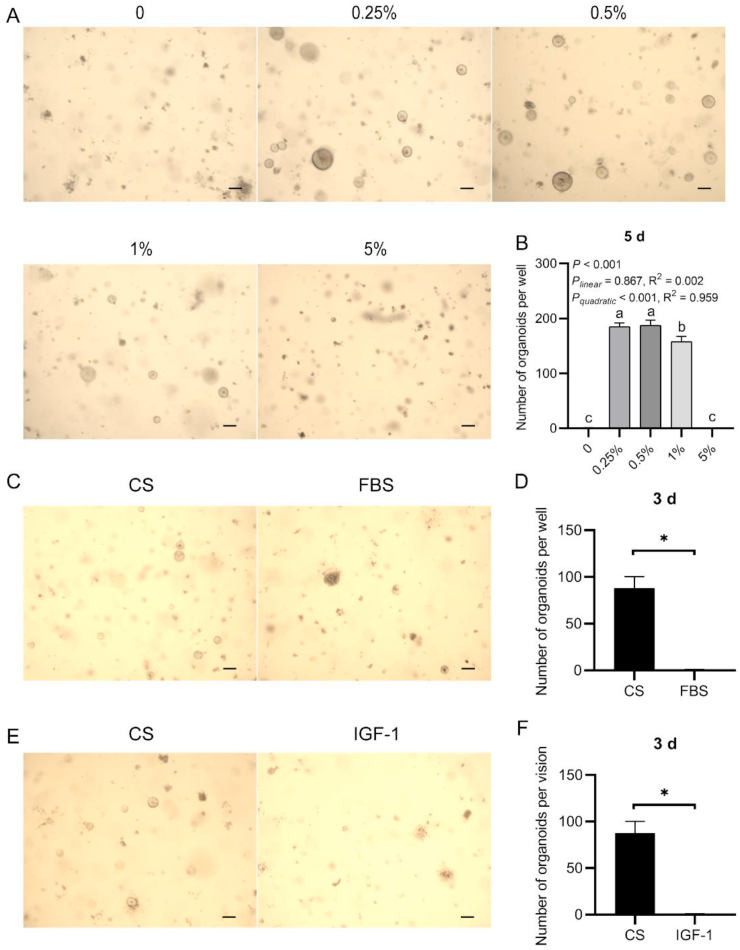
We then studied the influence of the concentration of chicken serum on chicken intestinal organoid growth, and we found that the organoid plating efficiency was quadratically related to the chicken serum concentration (PLinear = 0.867, R2 = 0.002; PQuadratical < 0.001, R2 = 959, Fig. 5A and B). Chicken serum at 5% had no benefit on crypt growth (P = 0.784). Besides, FBS showed no improvement in chicken intestinal organoid growth (Fig. 5C and D).
Fig. 5.
Influence of the (A and B) serum concentration, (C and D) serum source, and (E and F) insulin-like growth factor 1 (IGF-1) on the chicken intestinal organoid growth (scale bar, 200 μm). Error bars, SEM (n = 3 wells). Experiments were performed at least 3 times and a representative one was exhibited. Error bars, SEM (n = 3 wells). ∗, P ≤ 0.05. a, b Different letters on bars mean a significant difference (P ≤ 0.05). CS = chicken serum; FBS = fetal bovine serum.
It has been reported that serum supplementation benefits cell growth and proliferation via IGF-1 (Ji et al., 2018). We here hypothesized that IGF-1 was the compound responsible for the observed improvement in chicken intestinal organoid growth. However, nearly no live organoids were observed in the IGF-1 replacement group after 3 d of culture (Fig. 5E and F). This finding indicates that IGF-1 is not the key chemical in chicken serum that improves chicken intestinal organoid growth.
3.5. Comparison between commercial medium and the assembled medium
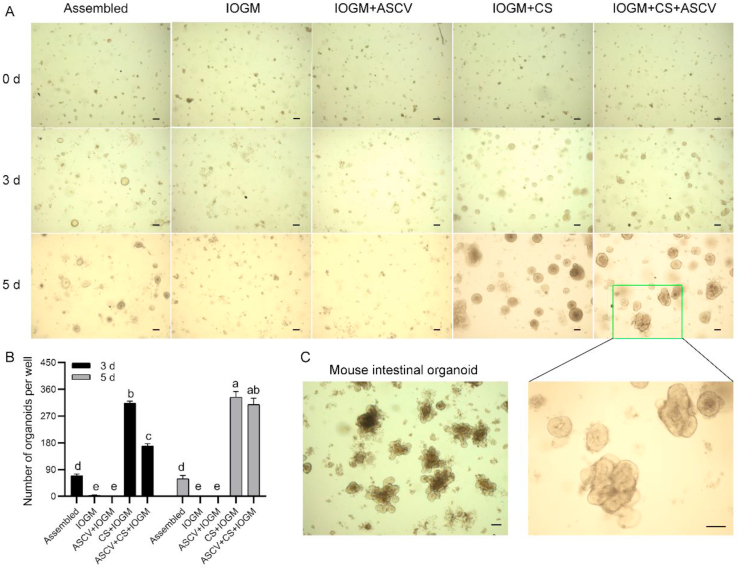
A commercial mouse intestinal organoid medium named IOGM was compared with the assembled culture medium. An interaction effect between the culture period and additive was observed on the number of formed organoids (P < 0.05). Nearly no living organoids were observed in the IOGM alone or in combination with ASCV (Fig. 6A and B). However, we found that IOGM supplemented with chicken serum yielded a higher number of formed organoids after 3 or 5 d of culture than those obtained with the culture of crypts in the assembled medium (both P < 0.05). In the presence of chicken serum, the number of formed intestinal organoids obtained after 3 d of culture with the ASCV treatment was lower than that obtained with the medium without ASCV (P < 0.001). However, after 5 d of culture, the organoid number obtained in the culture with ASCV was comparable to that found in the medium without ASCV. Surprisingly, we found that supplementation with ASCV contributed to budding organoid formation when cocultured with IOGM (Fig. 6A), even though the budding number could not be compared to the mouse intestinal organoid number (Fig. 6C).
Fig. 6.
Comparison between commercial medium and the assembled medium in culturing chicken crypts. (A) Growth of chicken crypts under different treatments. (B) Number of live organoids obtained with different treatments. a to e Different letters on bars mean a significant difference (P ≤ 0.05). (C) Mouse crypts cultured for 5 d in the assembled medium but without chicken serum (scale bar, 200 μm). Experiments were performed at least 3 times and a representative one was exhibited. Error bars, SEM (n = 3 wells). The assembled medium is composed of advanced DMEM/F12 with supplemented with epidermal growth factor, Noggin, R-spondin 1, A8301, SB202190, CHIR99021 and valproic acid. IOGM, a commercial medium named IntestinCult Organoid Growth Medium. ASCV, the mixture of A8301, SB202190, CHIR99021, and valproic acid; CS, chicken serum.
3.6. Influence of methionine deficiency on intestinal organoid growth and differentiation
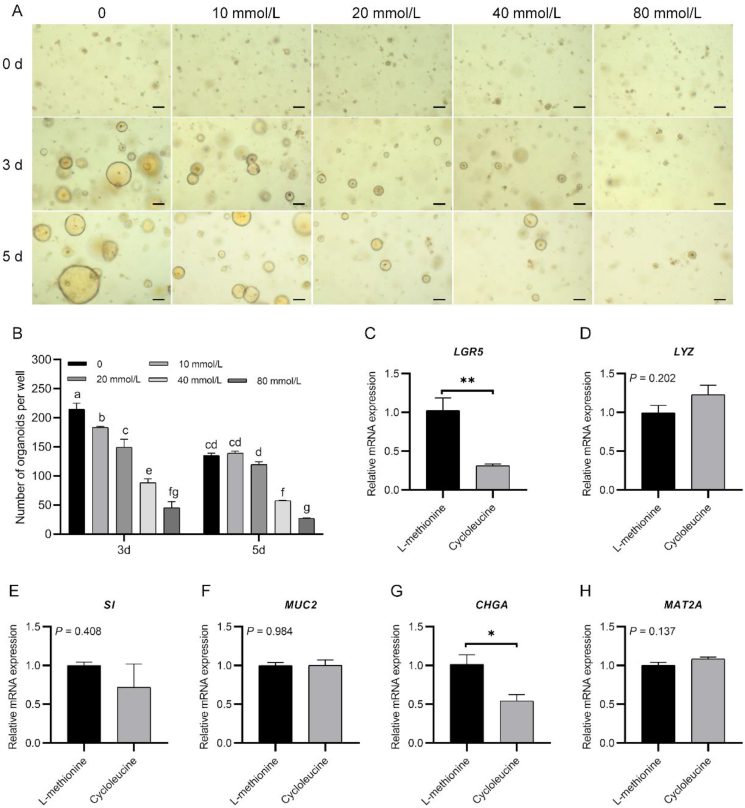
Different concentrations of cycloleucine were used to mimic varying degrees of methionine deficiency (Jani et al., 2009). An interaction effect of the cycloleucine concentration and culture period on the number of formed organoids was observed in this study (P < 0.05). The results showed that intestinal organoid plating efficiency and size gradually decreased with increases in the cycloleucine concentration after 3 and 5 d of culture (Fig. 7A and B). With the different treatments, a lower intestinal organoid number was detected after 5 d of culture than after 3 d of culture. Furthermore, methionine deficiency decreased the expression of LGR5 and CHGA (both P = 0.05, Fig. 7C and G), but could not influence the expression of LYZ, SI or MUC2 (all P > 0.05, Fig. 7D to F). Methionine deficiency did not influence the methionine adenosyltransferase 2A (MAT2A) expression (P = 0.137, Fig. 7H). In summary, methionine deficiency suppresses organoid growth.
Fig. 7.
Chicken crypt growth and development under different degrees of methionine deficiency. (A) Growth of chicken crypts under different treatments (scale bar, 200 μm). (B) Number of live intestinal organoids obtained with different treatments. (C to G) Comparison of relative mRNA expression of mature intestinal epithelial markers in crypts cultured for 5 d in medium with methionine or in medium additionally supplemented with 10 mmol/L cycloleucine. (H) Comparison of relative mRNA expression of MAT2A in crypts cultured for 5 d in medium with methionine or in medium additionally supplemented with 10 mmol/L cycloleucine. Experiments were performed at least 3 times and a representative one was exhibited. Error bars, SEM (n = 3 wells). a to g Different letters on bars mean a significant difference (P ≤ 0.05). ∗P < 0.05; ∗∗P < 0.01. LGR5 = leucine-rich-repeat-containing G-protein-coupled receptor 5; LYZ = lysozyme; SI = sucrase-isomaltase; MUC2 = mucin 2; CHGA = chromogranin A; MAT2A = methionine adenosyltransferase 2A.
3.7. Influence of L-methionine hydroxy analogue on organoid growth and differentiation
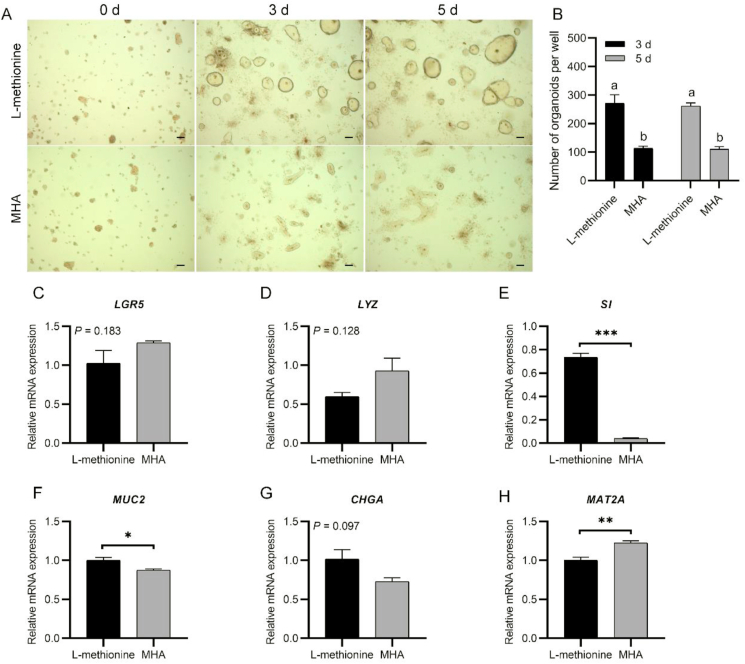
No interaction effect between the culture period and methionine form was observed on the number of formed organoids (P > 0.05). The methionine form exerted a main effect on the number of formed organoids (P < 0.05). The intestinal organoid plating efficiency obtained with MHA supplementation was significantly lower than that obtained with L-methionine supplementation (P < 0.05, Fig. 8A and B). We found that MHA supplementation significantly decreased the gene expression of SI and MUC2 (P < 0.001 and P < 0.05, respectively, Fig. 8E and F) and slightly decreased the mRNA level of CHGA (P = 0.097). However, organoids cultured with MHA seemed to show improved mRNA levels of LGR5 and LYZ, although the difference was not statistically significant (P = 0.183, P = 0.128, respectively, Fig. 8C and D). Besides, we found that MHA increased the expression of MAT2A (P < 0.01, Fig. 8H).
Fig. 8.
Influence of methionine hydroxy analogue (MHA) alternatives on chicken crypt growth and development. (A) Growth of chicken crypts under different treatments (scale bar, 200 μm). (B) Number of live intestinal organoids obtained with different treatments. (C to H) Relative mRNA expression of mature intestinal epithelial markers and MAT2A in crypts cultured for 5 d. Experiments were performed at least 3 times and a representative one was exhibited. Error bars, SEM (n = 3 wells). a, b Different letters on bars mean a significant difference (P ≤ 0.05). ∗, P ≤ 0.05; ∗∗, P ≤ 0.01; ∗∗∗, P ≤ 0.001. LGR5 = leucine-rich-repeat-containing G-protein-coupled receptor 5; LYZ = lysozyme; SI = sucrase-isomaltase; MUC2 = mucin 2; CHGA = chromogranin A; MAT2A = methionine adenosyltransferase 2A.
4. Discussion
Intestinal crypts sourced from mice and humans have been successfully cultured in vitro and are becoming increasingly popular in the exploration of intestinal development. Most studies have attempted to identify an ideal method for the culture of chicken intestinal organoid (Pierzchalska et al., 2012; Powell and Behnke, 2017; Li et al., 2018; Acharya et al., 2020; Nash et al., 2021). Here, we successfully applied chicken serum and A8301, SB202190, VPA and CHIR99021 to promote chicken intestinal organoid formation.
Cell strainers with a diameter of 70 μm are usually used for the isolation of mice crypts (Yin et al., 2014; Hou et al., 2018). For chicken crypts, cell strainers with different pore sizes, including 40 μm (Acharya et al., 2020), 70 μm (Li et al., 2018), and 100 μm (Pierzchalska et al., 2012) have been used. However, reliable pore diameters are lacking. The chicken crypt has an irregular shape. Determining the filter size suitable for achieving improved filtering according to the chicken crypt length and width is challenging. In this study, we gradually increased the pore size of cell strainers for filtration of the crypts and found that a 125-μm pore diameter was beneficial for the harvesting of intact crypts and thus improves the chicken crypt isolation efficiency and decreases the utilization of experimental animals.
Transforming growth factor-β expressed in the mucosa throughout the gastrointestinal tract plays important roles in promoting the proliferation of intestinal epithelial cells and in stimulating gut growth, maturation, and repair (Xian et al., 2002). However, in vitro cultures have shown that TGF-β induces apoptosis in human tubular adenoma organoid culture (Fessler et al., 2016) and inhibits intestinal organoid proliferation (Sato et al., 2011a). Interestingly, in the present study, we found that TGF-β inhibitor A8301 decreased the organoid plating efficiency early during the culture period, but protected cells from death later during the culture period. Previous work has shown that decreased TGF-β signaling in the crypt and increased signaling in differentiated cells (Mishra et al., 2005; Cammareri et al., 2017). Reasonably, newly seeded crypts have a low level of TGF-β signaling that may not require TGF-β inhibition. These results imply that A8301 may be more beneficial in chicken intestinal organoid formation when it is added after the first few days of culture. We found that A8301 increased the expression of the marker gene of enterocytes but decreased that of the marker gene of ISC, which is opposite to the finding that TGF-β promotes proliferation of intestinal epithelial cell (Xian et al., 2002). This difference implies that TGF-β may have different functions in vitro and in vivo.
Organoids sourced from the human colon disintegrated with the extended culture period (Sato et al., 2011a), which may be attributed to the cell apoptosis processes involved in MAPK (JNK, ERK, and p38)-related signaling pathways (Li et al., 2012). SB202190 is a MAPK signaling inhibitor that improves the organoid plating efficiency by inducing autophagy and upregulating the cytoprotective enzyme heme oxygenase-1 (Sato et al., 2011a; Schwartz et al., 2018). Consistently, the comparable organoid plating efficiency 5 and 3 d of culture observed in the present study implies that SB202190 supplementation prevents organoid death. Previous studies have shown that p38 inhibitor treatment suppresses goblet cell and enteroendocrine cell differentiation (Otsuka et al., 2010; Sato et al., 2011a). Similarly, the present study revealed that SB202190 decreased the expression of marker genes of goblet cells and enteroendocrine cells. Besides, increased expression of SI and inhibited expression of LYZ imply that SB202190 supplementation promotes enterocyte differentiation but suppresses Paneth cell differentiation. In summary, SB202190 supplementation efficiently prevents organoid from death at the expense of Paneth cell, goblet cell and enteroendocrine cell differentiation.
We then tried to maintain ISC renewal. The Wnt/β-catenin signaling pathway is an evolutionarily conserved system that is vital for stem cell renewal, cell proliferation and cell differentiation (Steinhart and Angers, 2018). However, β-catenin can be phosphorylated by GSK3 and then degraded (Cselenyi et al., 2008) and ultimately induce cell apoptosis (Wang et al., 2010). CHIR99021 is a highly specific GSK3 inhibitor that suppresses GSK3β-mediated β-catenin degradation (Kazi et al., 2018). And it has been reported that CHIR99021 boosts the induction of pluripotent stem cell generation from murine embryonic fibroblasts (Bar-Nur et al., 2014). In line with previous studies, a significant improvement in the chicken intestinal organoid plating efficiency was found in CHIR99021 supplementation treatments. Additionally, experiments with mouse intestinal organoids have shown that CHIR99021 supplementation reduces enterocyte differentiation but increases Paneth cell differentiation (Yin et al., 2014). Inversely, the present study revealed that treatment with CHIR99021 increases enterocyte differentiation but decreases the differentiation of Paneth cell and goblet cell. Why CHIR99021 exerted an inverse effect on the intestinal organoid development of chickens and mice remains unclear.
Interestingly, Yin et al. (2014) found that CHIR99021 and VPA not only synergistically maintain the self-renewal of mouse ISC, but also significantly improve the intestinal organoid plating efficiency at least 100-fold greater than that obtained with medium without CHIR99021 and VPA. Valproic acid is a histone deacetylation inhibitor (Gottlicher et al., 2001) and abnormal HDAC leads to cell metabolism disorders (Li and Seto, 2016). In this study, organoid culture with VPA promoted chicken intestinal organoid formation, particularly in the presence of CHIR99021, which is similar to the results in a published paper (Yin et al., 2014). Besides, it has been reported that VPA suppresses the expression of the neuroendocrine tumor marker chromogranin A by activating Notch-1 signaling (Stockhausen et al., 2005; Greenblatt et al., 2007). Notch-1 signaling blocks the differentiation of stem cells to secretory lineages such as goblet cells, Paneth cells and enteroendocrine cells (Gehart and Clevers, 2019). Consistently, in the present study, we found that VPA supplementation suppressed the gene expression of LYZ, MUC2 and CHGA but increased the gene expression of SI.
Fetal bovine serum is widely used in cell culture. We then explored the impact of chicken serum and FBS on the culture of chicken crypt. Here, we found that organoids cultured with chicken serum showed better growth than organoids cultured in medium without chicken serum. However, excessive chicken serum supplementation was dangerous to chicken intestinal organoid growth, and this negative effect may be caused by the TNFα in the chicken serum (Grabinger et al., 2017). Besides, FBS treatment suppressed the chicken intestinal organoid formation. A previous report showed that serum supplementation benefits cell growth and proliferation by its IGF-1 (Ji et al., 2018). We then explored the effect of IGF-1 on chicken intestinal organoid culture. However, further study indicated that IGF-1 is not the key factor in chicken serum that promotes chicken intestinal organoid formation. In the present study, we found that chicken serum treatments had lower expression of LYZ and CHGA, which means that chicken serum inhibits Paneth cell and enteroendocrine cell differentiation. Given that chicken serum has unknown components that may affect our research on some mechanisms, further studies are needed to explore the types of growth factors in chicken serum that play such an important role in chicken intestinal organoid culture.
We then compared the culture efficiency of our assembled medium and commercial mouse culture medium and found that chicken intestinal crypts could not grow in the IOGM. However, in the presence of chicken serum, the IOGM improved the formation of chicken intestinal organoids compared with that obtained with the assembled medium, which provides further evidence showing that chicken serum is important for chicken intestinal crypt culture. Besides, ASCV supplementation improved budding organoid formation in the presence of IOGM, which means that certain compounds in the commercial mouse intestinal organoid culture medium cooperate with the ASCV to promote organoid budding. Considering that approximately 80% of human genes have a direct 1:1 ortholog in the mouse genome (Waterston et al., 2002), and that the medium for mouse intestinal organoid culture is different from that for the culture of human intestinal organoid (Sato et al., 2011a), whether commercial medium for the culture of human intestinal organoids is suitable for the culture of chicken crypts needs further study.
We studied the mechanism underlying the effects of methionine deprivation and its hydroxy analogue on chicken intestinal development based on the assembled culture medium for chicken intestinal organoids. Dietary methionine supplementation benefits intestinal villus development (Su et al., 2018). Cycloleucine is a cyclic analogue of methionine, which acts as a competitive inhibitor of MAT (Lombardini and Sufrin, 1983). Cycloleucine blocks MAT2A activity and decreases SAM (Jani et al., 2009), and decreased SAM suppresses ISC proliferation (Obata et al., 2018). Here, we found that cycloleucine at 10 mmol/L suppressed organoid formation and LGR5 expression, which agrees with the results of previous study on murine intestinal organoids (Saito et al., 2017). Our results also showed that cycloleucine did not influence the expression of MAT2A. In summary, this study indicates that methionine is important for the development of ISC.
Methionine hydroxy analogue is usually used in livestock as a methionine substitution (Kluge et al., 2016; Wang et al., 2019a, Wang et al., 2019b). In this study, we found that MHA promoted MAT2A expression, which means that more methionine will be converted into SAM. This result agrees with previous work showing that dietary MHA promotes SAM synthesis in broilers (Wang et al., 2019b). S-adenosylmethionine is an important methyl donor (Chiang et al., 1996). The increased SAM catalyzed by upregulated MAT2A during MHA supplementation explains why MHA supplementation significantly decreased SI expression. Because genes essential to enterocyte differentiation are de-methylated (Huang et al., 2015). Besides, it has been reported that the depletion of MAT2A decreases SAM synthesis, the H3K4me3 levels and the self-renewal of stem cells (Ang et al., 2011; Shyh-Chang et al., 2013). Similarly, we found that MHA treatment appeared to improve the expression of a marker gene of ISC. Moreover, the slightly upregulated expression of LYZ obtained with MHA treatment conducive to maintaining the function of ISC (Sato et al., 2011b). Therefore, these results demonstrate that MHA promotes ISC regeneration but not for differentiation.
5. Conclusion
This study notes that a cell strainer with a diameter of 125 μm exhibits the highest efficiency for the isolation of chicken embryo crypts. Chicken serum is essential for chicken intestinal organoid culture. Based on the chicken intestinal organoid model, we successfully examined the impact of methionine deficit and its hydroxy analogue on organoid growth and development.
Author contributions
Youli Wang: Writing – original draft; Qihang Hou: Methodology; Yuqin Wu: Data curation, Software; Yanwei Xu: Data curation, Software; Yan Liu: Visualization, Investigation; Jing Chen: Visualization, Investigation; Lingling Xu: Visualization, Investigation; Yuming Guo: Writing - Reviewing and Editing; Shuai Gao: Validation, Conceptualization, Supervision; Jianmin Yuan: Validation, Conceptualization, Supervision.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This work was supported by the founding of the National Natural Science Foundation of China (No. 32072752, No. 31772620, No. 31970814), the System for Poultry Production Technology, Beijing Agriculture Innovation Consortium (Project Number: BAIC04-2020), and Natural Key R&D Project of China (2020YFA0113200).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Shuai Gao, Email: gaoshuai1959@163.com.
Jianmin Yuan, Email: yuanjm@cau.edu.cn.
References
- Acharya M., Arsi K., Donoghue A.M., Liyanage R., Rath N.C. Production and characterization of avian crypt-villus enteroids and the effect of chemicals. BMC Vet Res. 2020;16(1) doi: 10.1186/s12917-020-02397-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang Y.S., Tsai S.Y., Lee D.F., Monk J., Su J., Ratnakumar K., et al. Wdr5 mediates self-renewal and reprogramming via the embryonic stem cell core transcriptional network. Cell. 2011;145(2):183–197. doi: 10.1016/j.cell.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Nur O., Brumbaugh J., Verheul C., Apostolou E., Pruteanu-Malinici I., Walsh R.M., et al. Small molecules facilitate rapid and synchronous iPSC generation. Nat Methods. 2014;11(11):1170–1176. doi: 10.1038/nmeth.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin P., Azad M.A., Liu G., Zhu D., Kim S.W., Yin Y.L. Effects of different levels of methionine on sow health and plasma metabolomics during late gestation. Food Funct. 2018;9(9):4979–4988. doi: 10.1039/c8fo01477a. [DOI] [PubMed] [Google Scholar]
- Cammareri P., Vincent D.F., Hodder M.C., Ridgway R.A., Murgia C., Nobis M., et al. TGFbeta pathway limits dedifferentiation following WNT and MAPK pathway activation to suppress intestinal tumourigenesis. Cell Death Differ. 2017;24(10):1681–1693. doi: 10.1038/cdd.2017.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano R., Perruchot M., Tesseraud S., Métayer-Coustard S., Baeza E., Mercier Y., et al. Methionine and cysteine deficiencies altered proliferation rate and time-course differentiation of porcine preadipose cells. Amino Acids. 2017;49(2):355–366. doi: 10.1007/s00726-016-2369-y. [DOI] [PubMed] [Google Scholar]
- Chiang P.K., Gordon R.K., Tal J., Zeng G.C., Doctor B.P., Pardhasaradhi K., et al. S-Adenosylmethionine and methylation. Faseb J. 1996;10(4):471–480. [PubMed] [Google Scholar]
- Chung M., Bujnis M., Barkauskas C.E., Kobayashi Y., Hogan B.L.M. Niche-mediated BMP/SMAD signaling regulates lung alveolar stem cell proliferation and differentiation. Development. 2018;145(9):v163014. doi: 10.1242/dev.163014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cselenyi C.S., Jernigan K.K., Tahinci E., Thorne C.A., Lee L.A., Lee E. LRP6 transduces a canonical Wnt signal independently of axin degradation by inhibiting GSK3's phosphorylation of beta-catenin. Proc Natl Acad Sci USA. 2008;105(23):8032–8037. doi: 10.1073/pnas.0803025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler E., Drost J., van Hooff S.R., Linnekamp J.F., Wang X., Jansen M., et al. TGFbeta signaling directs serrated adenomas to the mesenchymal colorectal cancer subtype. EMBO Mol Med. 2016;8(7):745–760. doi: 10.15252/emmm.201606184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii M., Matano M., Nanki K., Sato T. Efficient genetic engineering of human intestinal organoids using electroporation. Nat Protoc. 2015;10(10):1474–1485. doi: 10.1038/nprot.2015.088. [DOI] [PubMed] [Google Scholar]
- Gehart H., Clevers H. Tales from the crypt: new insights into intestinal stem cells. Nat Rev Gastroenterol Hepatol. 2019;16(1):19–34. doi: 10.1038/s41575-018-0081-y. [DOI] [PubMed] [Google Scholar]
- Gottlicher M., Minucci S., Zhu P., Kramer O.H., Schimpf A., Giavara S., et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20(24):6969–6978. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabinger T., Bode K.J., Demgenski J., Seitz C., Delgado M.E., Kostadinova F., et al. Inhibitor of apoptosis protein-1 regulates tumor necrosis factor-mediated destruction of intestinal epithelial cells. Gastroenterology. 2017;152(4):867–879. doi: 10.1053/j.gastro.2016.11.019. [DOI] [PubMed] [Google Scholar]
- Greenblatt D.Y., Vaccaro A.M., Jaskula Sztul R., Ning L., Haymart M., Kunnimalaiyaan M., et al. Valproic acid activates notch-1 signaling and regulates the neuroendocrine phenotype in carcinoid cancer cells. Oncol. 2007;12(8):942–951. doi: 10.1634/theoncologist.12-8-942. [DOI] [PubMed] [Google Scholar]
- He Y., Zuo Q., Edwards J., Zhao K., Lei J., Cai W., et al. DNA methylation and regulatory elements during chicken germline stem cell differentiation. Stem Cell Rep. 2018;10(6):1793–1806. doi: 10.1016/j.stemcr.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann K., Berger H., Kulbe H., Thillainadarasan S., Mollenkopf H.J., Zemojtel T., et al. Stable expansion of high-grade serous ovarian cancer organoids requires a low-Wnt environment. EMBO J. 2020;39(6) doi: 10.15252/embj.2019104013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q., Ye L., Liu H., Huang L., Yang Q., Turner J.R., et al. Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22. Cell Death Differ. 2018;25(9):1657–1670. doi: 10.1038/s41418-018-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Yu T., Chen Q. DNA methylation dynamics during differentiation, proliferation, and tumorigenesis in the intestinal tract. Stem Cell Dev. 2015;24(23):2733–2739. doi: 10.1089/scd.2015.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jani T.S., Gobejishvili L., Hote P.T., Barve A.S., Joshi-Barve S., Kharebava G., et al. Inhibition of methionine adenosyltransferase II induces FasL expression, Fas-DISC formation and caspase-8-dependent apoptotic death in T leukemic cells. Cell Res. 2009;19(3):358–369. doi: 10.1038/cr.2008.314. [DOI] [PubMed] [Google Scholar]
- Ji Y., Wang Z., Chen H., Zhang L., Zhuo F., Yang Q. Serum from chronic hepatitis B patients promotes growth and proliferation via the IGF-II/IGF-IR/MEK/ERK signaling pathway in hepatocellular carcinoma cells. Cell Physiol Biochem. 2018;47(1):39–53. doi: 10.1159/000489744. [DOI] [PubMed] [Google Scholar]
- Kazi A., Xiang S., Yang H., Delitto D., Trevino J., Jiang R.H.Y., et al. GSK3 suppression upregulates β-catenin and c-Myc to abrogate KRas-dependent tumors. Nat Commun. 2018;9(1) doi: 10.1038/s41467-018-07644-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluge H., Gessner D.K., Herzog E., Eder K. Efficacy of DL-methionine hydroxy analogue-free acid in comparison to DL-methionine in growing male white Pekin ducks. Poultry Sci. 2016;95(3):590–594. doi: 10.3382/ps/pev355. [DOI] [PubMed] [Google Scholar]
- Li Y., Seto E. HDACs and HDAC inhibitors in cancer development and therapy. Cold Spring Harb Perspect Med. 2016;6(10):a26831. doi: 10.1101/cshperspect.a026831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Liu Y., Fu Y., Wei T., Le Guyader L., et al. The triggering of apoptosis in macrophages by pristine graphene through the MAPK and TGF-beta signaling pathways. Biomaterials. 2012;33(2):402–411. doi: 10.1016/j.biomaterials.2011.09.091. [DOI] [PubMed] [Google Scholar]
- Li J., Li J., Zhang S.Y., Li R.X., Lin X., Mi Y.L., et al. Culture and characterization of chicken small intestinal crypts. Poultry Sci. 2018;97(5):1536–1543. doi: 10.3382/ps/pey010. [DOI] [PubMed] [Google Scholar]
- Lombardini J.B., Sufrin J.R. Chemotherapeutic potential of methionine analogue inhibitors of tumor-derived methionine adenosyltransferases. Biochem Pharmacol. 1983;32(3):489–495. doi: 10.1016/0006-2952(83)90528-2. [DOI] [PubMed] [Google Scholar]
- Miguel J.C., Maxwell A.A., Hsieh J.J., Harnisch L.C., Al Alam D., Polk D.B., et al. Epidermal growth factor suppresses intestinal epithelial cell shedding through a MAPK-dependent pathway. J Cell Sci. 2017;130(1):90–96. doi: 10.1242/jcs.182584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra L., Shetty K., Tang Y., Stuart A., Byers S.W. The role of TGF-beta and Wnt signaling in gastrointestinal stem cells and cancer. Oncogene. 2005;24(37):5775–5789. doi: 10.1038/sj.onc.1208924. [DOI] [PubMed] [Google Scholar]
- Nash T.J., Morris K.M., Mabbott N.A., Vervelde L. Inside-out chicken enteroids with leukocyte component as a model to study host–pathogen interactions. Commun Biol. 2021;4(1) doi: 10.1038/s42003-021-01901-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata F., Tsuda-Sakurai K., Yamazaki T., Nishio R., Nishimura K., Kimura M., et al. Nutritional control of stem cell division through S-adenosylmethionine in Drosophila intestine. Dev Cell. 2018;44(6):741–751. doi: 10.1016/j.devcel.2018.02.017. [DOI] [PubMed] [Google Scholar]
- Otsuka M., Kang Y.J., Ren J., Jiang H., Wang Y., Omata M., et al. Distinct effects of p38α deletion in myeloid lineage and gut epithelia in mouse models of inflammatory bowel disease. Gastroenterology. 2010;138(4):1255–1265. doi: 10.1053/j.gastro.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierzchalska M., Grabacka M., Michalik M., Zyla K., Pierzchalski P. Prostaglandin E2 supports growth of chicken embryo intestinal organoids in matrigel matrix. Biotechniques. 2012;52(5):307–315. doi: 10.2144/0000113851. [DOI] [PubMed] [Google Scholar]
- Powell R.H., Behnke M.S. WRN conditioned media is sufficient for in vitro propagation of intestinal organoids from large farm and small companion animals. Biol Open. 2017;6(5):698–705. doi: 10.1242/bio.021717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring D.B., Johnson K.W., Henriksen E.J., Nuss J.M., Goff D., Kinnick T.R., et al. Selective glycogen synthase kinase 3 inhibitors potentiate insulin activation of glucose transport and utilization in vitro and in vivo. Diabetes. 2003;52(3):588–595. doi: 10.2337/diabetes.52.3.588. [DOI] [PubMed] [Google Scholar]
- Saito Y., Iwatsuki K., Hanyu H., Maruyama N., Aihara E., Tadaishi M., et al. Effect of essential amino acids on enteroids: methionine deprivation suppresses proliferation and affects differentiation in enteroid stem cells. Biochem Biophys Res Commun. 2017;488(1):171–176. doi: 10.1016/j.bbrc.2017.05.029. [DOI] [PubMed] [Google Scholar]
- Sato T., Stange D.E., Ferrante M., Vries R.G.J., van Es J.H., van den Brink S., et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and barrett's epithelium. Gastroenterology. 2011;141(5):1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- Sato T., van Es J.H., Snippert H.J., Stange D.E., Vries R.G., van den Born M., et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469(7330):415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M., Böckmann S., Borchert P., Hinz B. SB202190 inhibits endothelial cell apoptosis via induction of autophagy and heme oxygenase-1. Oncotarget. 2018;9(33):23149–23163. doi: 10.18632/oncotarget.25234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraki N., Shiraki Y., Tsuyama T., Obata F., Miura M., Nagae G., et al. Methionine metabolism regulates maintenance and differentiation of human pluripotent stem cells. Cell Metabol. 2014;19(5):780–794. doi: 10.1016/j.cmet.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Shyh-Chang N., Locasale J.W., Lyssiotis C.A., Zheng Y., Teo R.Y., Ratanasirintrawoot S., et al. Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science. 2013;339(6116):222–226. doi: 10.1126/science.1226603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhart Z., Angers S. Wnt signaling in development and tissue homeostasis. Development. 2018;145(11):v146589. doi: 10.1242/dev.146589. [DOI] [PubMed] [Google Scholar]
- Stockhausen M., Sjölund J., Manetopoulos C., Axelson H. Effects of the histone deacetylase inhibitor valproic acid on Notch signalling in human neuroblastoma cells. Br J Cancer. 2005;92(4):751–759. doi: 10.1038/sj.bjc.6602309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W., Zhang H., Ying Z., Li Y., Zhou L., Wang F., et al. Effects of dietary L-methionine supplementation on intestinal integrity and oxidative status in intrauterine growth-retarded weanling piglets. Eur J Nutr. 2018;57(8):2735–2745. doi: 10.1007/s00394-017-1539-3. [DOI] [PubMed] [Google Scholar]
- Tang Y., Tan B., Xiong X., Li F., Ren W., Kong X., et al. Methionine deficiency reduces autophagy and accelerates death in intestinal epithelial cells infected with enterotoxigenic Escherichia coli. Amino Acids. 2015;47(10):2199–2204. doi: 10.1007/s00726-014-1781-4. [DOI] [PubMed] [Google Scholar]
- Wang L., Yang H., Xia Y., Feng Z. Insulin-like growth factor 1 protects human neuroblastoma cells SH-EP1 against MPP+-induced apoptosis by AKT/GSK-3β/JNK signaling. Apoptosis. 2010;15(12):1470–1479. doi: 10.1007/s10495-010-0547-z. [DOI] [PubMed] [Google Scholar]
- Wang J., Yang G., Zhang K., Ding X., Bai S., Zeng Q. Effects of dietary supplementation of DL-2-hydroxy-4(methylthio) butanoic acid on antioxidant capacity and its related gene expression in lung and liver of broilers exposed to low temperature. Poultry Sci. 2019;98(1):341–349. doi: 10.3382/ps/pey371. [DOI] [PubMed] [Google Scholar]
- Wang Y., Yin X., Yin D., Lei Z., Mahmood T., Yuan J. Antioxidant response and bioavailability of methionine hydroxy analog relative to DL-methionine in broiler chickens. Anim Nutr. 2019;5(3):241–247. doi: 10.1016/j.aninu.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterston R.H., Birney E., Rogers J., Abril J.F., Agarwala R., Ainscough R., et al. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420(6915):520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- Xian C.J., Cool J.C., Howarth G.S., Read L.C. Effects of TGF-alpha gene knockout on epithelial cell kinetics and repair of methotrexate-induced damage in mouse small intestine. J Cell Physiol. 2002;191(1):105–115. doi: 10.1002/jcp.10079. [DOI] [PubMed] [Google Scholar]
- Yin X., Farin H.F., van Es J.H., Clevers H., Langer R., Karp J.M. Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat Methods. 2014;11(1):106–112. doi: 10.1038/nmeth.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]