Abstract
Endoplasmic reticulum (ER) stress has been associated with the dysfunction of intestinal barrier in humans and animals. We have previously shown that oral administration of glycine to suckling-piglets improves ER stress-related intestinal mucosal barrier impairment and jejunal epithelial apoptosis. However, the underlying mechanism remains unknown. In this study, the protective effect and the mechanism of glycine on apoptosis and dysfunction in intestinal barrier induced by brefeldin A (BFA), an ER stress inducer, was explored in porcine intestinal epithelial cells (IPEC-1). The results showed that BFA treatment led to enhanced apoptosis and upregulation of proteins involved in ER stress signaling, including inositol-requiring enzyme 1α (IRE1α), activating transcription factor 6α (ATF6α), c-Jun N-terminal kinase (JNK), and C/EBP-homologous protein (CHOP). In addition, BFA induced a dysfunction in intestinal epithelial barrier, as evidenced by the increased paracellular permeability, decreased transepithelial electrical resistance (TEER), and reduced abundance of tight junction proteins (occludin, claudin-1, zonula occludens [ZO]-1, and ZO-2). These alterations triggered by BFA were significantly abolished by glycine treatment (P < 0.05), indicating a protective effect of glycine on barrier function impaired by ER stress. Importantly, we found that the regulatory effect of glycine on intestinal permeability, proteins implicated in ER stress and apoptosis, as well as the morphological alterations of the ER were reversed by rapamycin. In summary, our results indicated that glycine alleviates ER stress-induced apoptosis and intestinal barrier dysfunction in IPEC-1 cells in a mammalian target of rapamycin complex 1 (mTORC1)-dependent manner. The data provides in vitro evidence and a mechanism for the protective effect of glycine against the disruption of intestinal barrier integrity induced by ER stress.
Keywords: Glycine, ER stress, Apoptosis, mTORC1, Intestinal barrier
1. Introduction
Enterocytes are the predominant cell type in the small intestine and play a critical role for the integrity of intestinal barrier (Vancamelbeke and Vermeire, 2017). It has been reported that the unfolded protein response (UPR) plays an important role in the maintenance of the intracellular homeostasis and a normal physiological function of enterocytes (Grootjans et al., 2016). In response to endoplasmic reticulum (ER) stress, ER-resident chaperone binding immunoglobulin protein (BiP) dissociates from the luminal domains of the three protein sensors, leading to activation of protein kinase R-like ER kinase (PERK), inositol-requiring enzyme 1α (IRE1α), and activating transcription factor 6α (ATF6α) to restore intracellular homeostasis by inhibiting protein synthesis, and enhancing the capacity of protein folding and the degradation of misfolded proteins (Iurlaro and Munoz-Pinedo, 2016; Ma et al., 2017). ER stress in enterocytes has been associated with various intestinal diseases, including inflammatory bowel diseases and irritable bowel syndrome (Hosoi and Ozawa, 2009; McGuckin et al., 2010). In contrast, inactivation of the ER stress signaling is relevant to the maintenance of intestinal barrier function and a positive therapeutic outcome in clinical patients and experimental animals (Wlodkowic et al., 2007; Walter and Ron, 2011; Hetz, 2012; Luo and Cao, 2015). Of note, prolonged or severe ER stress may result in apoptosis via the intrinsic pathway of apoptotic cell death in which B-cell lymphoma 2 (Bcl-2) family proteins are implicated.
Glycine, a nutritionally nonessential amino acid, has been reported to exert anti-apoptotic activity in multiple cell types, including endothelial cells (Weinberg et al., 1992, 2016; Nishimura and Lemasters, 2001), hepatocytes (Dickson et al., 1992; Nyberg et al., 2000), and intestinal epithelial cells (Howard et al., 2010). In our recent study, suckling-piglets receiving glycine presented an improvement in intestinal mucosal barrier function and a repression of ER stress-related apoptosis in jejunal epithelial cells during the postweaning period (Fan et al., 2019). This finding is consistent with a previous report (Sim et al., 2016), indicating a regulatory effect of glycine on ER stress. However, the underlying mechanism responsible for the effect of glycine on ER stress has not been fully defined. Previously, glycine has been shown to active mammalian target of rapamycin complex 1 (mTORC1) signaling in enterocytes (Wang et al., 2014). Importantly, mTORC1 signaling is critical for cell survival and intestinal integrity in response to ER stress (Ji et al., 2018). Therefore, we propose the hypothesis that mTORC1 may be involved in the regulation of glycine on ER stress-induced apoptosis and barrier dysfunction in intestinal epithelium. To confirm our hypothesis, porcine intestinal epithelial cell line-1 (IPEC-1) was treated with brefeldin A (BFA), a fungal metabolite with an ability to inhibit protein trafficking between the ER and the Golgi apparatus (Chardin and McCormick, 1999) to induce ER stress in the presence or absence of glycine. Cell viability, trans-epithelial electrical resistance, cell apoptosis, the abundance of proteins related to ER stress signaling and intestinal integrity, and the morphological alterations of ER were determined. In addition, we tested the key signaling of ER stress in response to glycine treatment and whether mTORC1 is involved in the modulation of glycine in BFA-induced intestinal barrier dysfunction. Our results uncover a novel mechanism for glycine in the regulation of ER stress-associated cell death and barrier dysfunction in enterocytes of piglets.
2. Materials and methods
2.1. Materials
Fetal bovine serum (FBS), Dulbecco's modified Eagle medium/Nutrient Ham's Mixture F12 (DMEM/F12), glycine-free custom-made DMEM, and antibiotics (penicillin and streptomycin) were obtained from Gibco (Carlsbad, CA, USA). Glycine and fluorescein isothiocyanate (FITC)-dextran were purchased from Sigma (Saint Louis, MO, USA). Plastic culture plates were manufactured by Corning Inc (Corning, NY, USA). Cell Counting Kit (CCK-8) and Annexin V-FITC Apoptosis Detection Kit were purchased from Beyotime Biotechnology (Shanghai, China). Anisomycin and rapamycin were procured from MedChemExpress (NJ, USA). Anti-p-IRE1α (Ser724, ab48187) antibody was obtained from Abcam (Cambridge, MA, USA). The antibodies against protein kinase B (Akt) (9272), phosphorylated (p)-Akt (Ser473, 9271), mTORC1 (2972), p-mTORC1 (Ser2448, 5536), p70S6 kinase (p70S6K) (9202), p-p70S6K (Thr389, 9205), p-c-Jun N-terminal kinase (JNK) (Thr183/Tyr185, 9251), JNK (9252), eukaryotic initiation factor 2α (eIF2α) (2103), p-eIF2α (Ser51, 3398), cleaved-caspase3 (9661), and p53 (2524) were purchased from Cell Signaling Technology (Massachusetts, USA). Anti-ATF6α (sc-22799), anti-Bcl-2 (sc-492), anti-Bcl-xL (sc-634), anti-Bax (sc-493), anti-C/EBP-homologous protein (CHOP) (sc-575), and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (sc-59540) antibodies were obtained from Santa Cruz (California, USA). Anti-occludin (40-4700), anti-claudin-1 (51-9000), anti-claudin-3 (34-1700), anti-zonula occludens (ZO)-1 (61-7300), anti-ZO-2 (38-9100), and anti-ZO-3 (36-4100) antibodies were obtained from Invitrogen (Grand Island, NY, USA).
2.2. Cell culture
IPEC-1 cells, isolated from the jejunum of an un-suckling newborn piglet immediately after birth, were cultured in DMEM/F12 media supplemented with 10% FBS and 1% penicillin-streptomycin. The cells were maintained at 37 °C in a 5% CO2 incubator (Sanyo, Japan).
2.3. Cell treatment
Cells were seeded in culture plates in DMEM/F12 media containing 10% FBS to permit cell adherence. The adhered cells were pretreated with serum-free custom-made DMEM containing 0 or 0.5 mmol/L glycine for 6 h. The basal DMEM medium containing 5 mmol/L of D-glucose, no glycine, and the physiologic concentrations of other amino acids was prepared as previously described (Wang et al., 2014). Cells were then treated with or without BFA (0.1 μmol/L) for 12 h.
2.4. Cell viability
Cells were seeded in a 96-well plate (10,000 cells per well). Cell viability was evaluated using a cell counting kit-8 following the protocol provided by the manufacturer. The absorbance was measured at 450 nm by a microplate reader (SpectraMax M3, Molecular Devices, Sunnyvale, CA, USA). Results are expressed as a percentage relative to the controls.
2.5. Transepithelial electrical resistance (TEER) measurements
Cells were seeded at a density of 50,000 cells per well into the transwells (membrane area, 0.33 cm2; pore size, 0.4 μm) equipped in 24-well culture plates. An EVOM epithelial volt-ohmmeter with STX2 electrode (World Precision Instruments, USA) was employed for TEER determination. The final values of TEER were obtained by deducting the resistance value of the filter and culture medium. All values were converted to the percentages of the controls.
2.6. Determination of monolayer paracellular permeability
To determine the paracellular permeability, FITC-dextran/mL (20 kDa) was applied to the apical side of monolayer at a final concentration of 1 mg/mL. By sampling the basolateral compartment at specified times, the flux of FITC-dextran was measured. The content of FITC-dextran was measured under the excitation and emission wavelengths of 490 and 520 nm respectively using a SpectraMax M3 multi-mode microplate reader (Molecular Devices, Sunnyvale, CA, USA). The permeability of monolayers was evaluated by the amount of FITC-dextran delivered from the apical side to the basolateral side. The concentration of FITC-dextran was calculated by a subduction of the fluorescence value obtained from FITC-free medium.
2.7. Detection of apoptosis by flow cytometry
The harvested cell pellets were washed twice with pre-cooled PBS, and then were resuspended in 0.5 mL of 1 × binding buffer. Afterwards, cells were incubated with FITC-labeled Annexin V for 15 min and then were stained with propidium iodide (50 μg/mL) for 5 min before detection. Samples were analyzed by a flow cytometer (Beckman, USA). Data were assessed using the CytExpert software.
2.8. Transmission electronic microscope (TEM)
IPEC-1 cells were fixed in 2.5% glutaraldehyde for 4 h and then in 1% osmium tetroxide for 1.5 h at 4 °C, after which they were dehydrated in gradient ethanol solutions and propylene oxide. Subsequently, the cells were embedded in Epon 812. Ultrathin sections (1 μm) were cut with the Ultramicrotome Leica EM UC7 (Wetzlar, Germany) and then stained with uranyl acetate and lead citrate. The sections were examined under a TEM (JEM-1400, JEOL, Japan).
2.9. Western blot analysis
The total protein from harvested cells was isolated as previously described (Ji et al., 2016). Samples were separated by SDS-PAGE gels and transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). The membranes were incubated with primary antibodies (1:2,000) at 4 °C overnight, followed by an incubation with corresponding horseradish peroxidase (HRP)-conjugated secondary antibody (1:2,000) for 1 h at room temperature. The blots were visualized with an ImageQuant LAS 4000 mini system (GE Healthcare BioSciences, USA) after reaction with ECL Plus detection reagents (Huaxingbio, Beijing, China). The gray value for each band was quantified by One Quantity software (Bio-Rad Laboratories, USA). All results were normalized to GAPDH and expressed as the relative values to the control group.
2.10. Immunofluorescence imaging
Cells fixed with 4% paraformaldehyde for 20 min were permeabilized with 0.1% Triton X-100 in PBS for 15 min at room temperature. After being blocked for 1 h at room temperature with 3% goat serum, samples were incubated with specific primary antibodies (1:100) against occludin, claudin-1, claudin-3, ZO-1, ZO-2, or ZO-3 overnight at 4 °C. Following washing with PBS for 3 times, cells were incubated with an appropriate secondary antibody (1:100) at 25 °C for 1 h. The nuclei were stained by Hoechst 33342 (1 μg/mL) for 1 min at room temperature. The distribution of tight junction proteins was visualized under a fluorescence microscope (Axio Vert. A1, Zeiss, Germany).
2.11. Statistical analysis
All of the experimental data are expressed as the means ± SEM. Data were analyzed statistically by one-way ANOVA followed by the Duncan's multiple range tests using the SAS 9.1 software (SAS Institute Inc., Cary, NC, USA). Probability values < 0.05 were considered statistically significant.
3. Results
3.1. Glycine blocked BFA-induced apoptosis in IPEC-1 cells
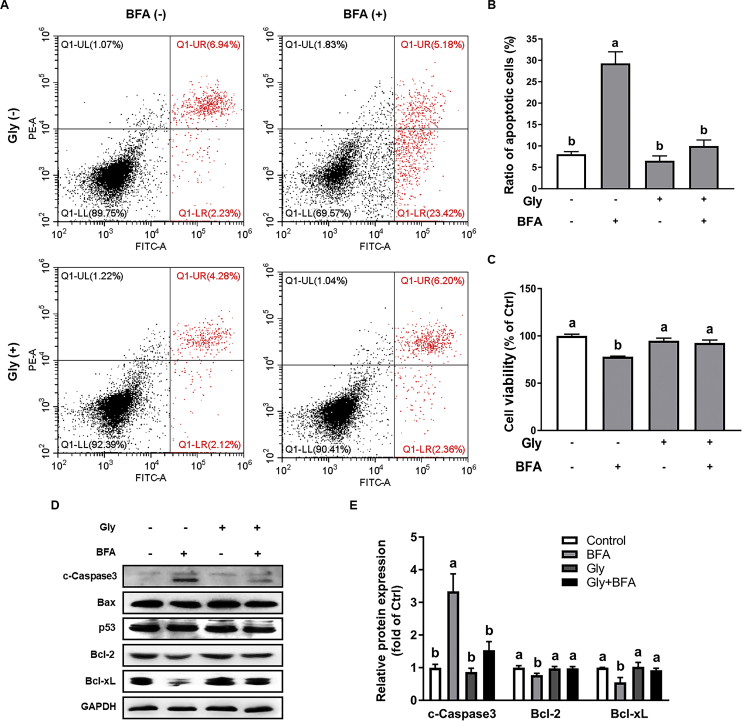
Compared with the control, brefeldin A (BFA, 0.1 μmol/L) treatment led to increased cell apoptosis (P < 0.05), which was significantly reduced (P < 0.05) by 0.5 mmol/L glycine pretreatment (Fig. 1A and B). Consistently, BFA-induced decrease in cell viability was reversed by glycine administration. Western blot analysis indicated that BFA exposure resulted in down-regulation (P < 0.05) of anti-apoptotic proteins (Bcl-2 and Bcl-xL) and up-regulation (P < 0.05) of pro-apoptotic protein (cleaved-Caspase-3) in IPEC-1 cells, which were abrogated by glycine (Fig. 1D and E).
Fig. 1.
Glycine (Gly) inhibited brefeldin A (BFA)-induced apoptosis in intestinal epithelial (IPEC-1) cells. Apoptosis were determined by flow cytometric analysis (A) and analyzed (B). Cell viability (C) was assessed by Cell Counting Kit-8 (CCK8) assay. Protein abundances of c-Caspase3, Bax, p53, Bcl-2, and Bcl-xL were analyzed by Western blot (D) and (E). Values are means ± SEM of 3 independent experiments. Means without a common letter differ, P < 0.05. FITC = fluorescein Isothiocyanate; c-caspase3 = cleaved-caspase3; Bcl-2 = B-cell lymphoma.
3.2. Glycine repressed IRE1α-JNK stress signaling in IPEC-1 cells
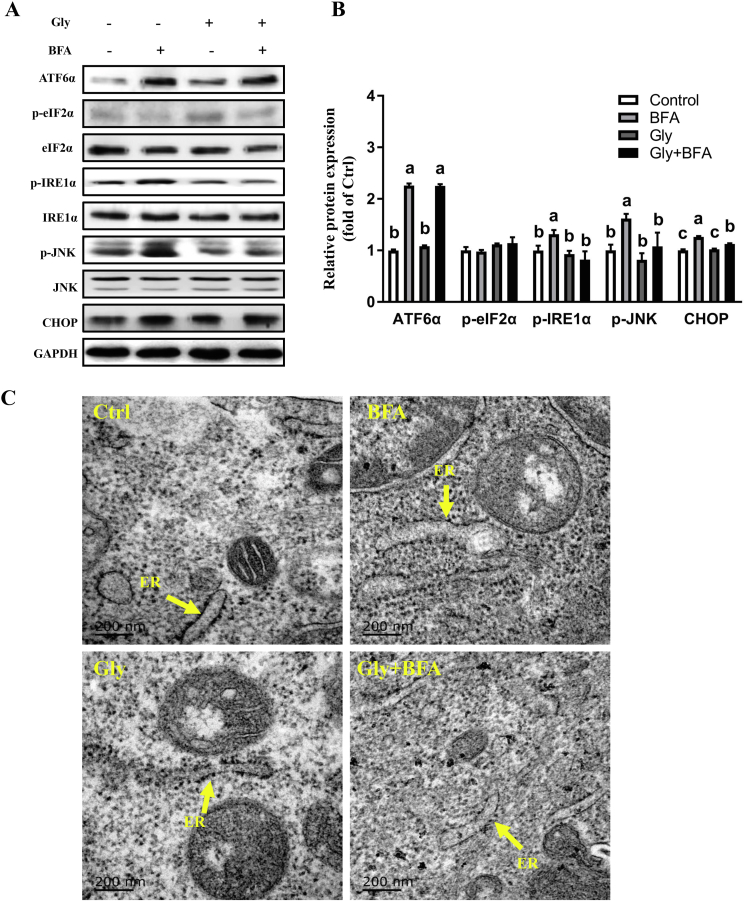
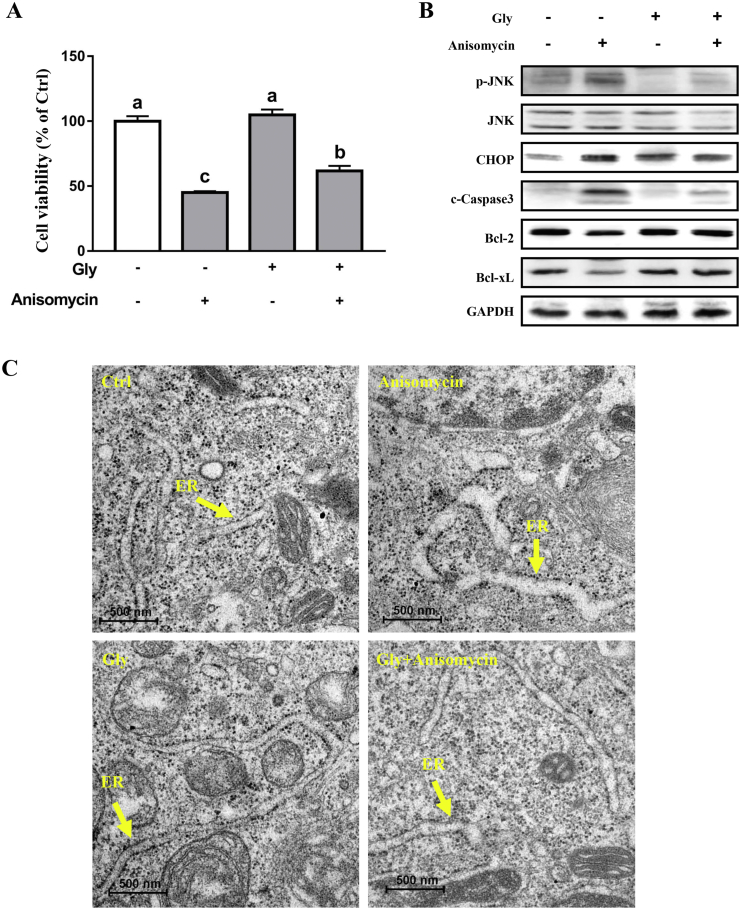
Western blot results showed that BFA treatment significantly increased (P < 0.05) the protein abundance of ATF6α, p-IRE1α, p-JNK, and CHOP, without affecting those of JNK and eIF2α (Fig. 2A and B). A single treatment with glycine showed no effect (P > 0.05) on the abundance of proteins implicated in ER stress signaling. However, BFA-triggered upregulation of p-IRE1α, p-JNK, and CHOP were abolished by glycine pretreatment. In addition, as shown in Fig. 2C, swelled ER and degranulation of the ribosome were observed in BFA-challenged cells, compared with the controls. These alterations were significantly prevented by glycine treatment (Fig. 2C) (P < 0.05). To validate the contribution of JNK to apoptosis as well as the inhibitory effect of glycine on JNK signaling, cells pretreated with glycine were subjected to anisomycin, a JNK agonist, or vehicle solution (Chen et al., 2017). As shown in Fig. 3A, cell viability was reduced (P < 0.05) following anisomycin treatment, as compared with the control. Western blot analysis showed that cells treated with anisomycin presented increased (P < 0.05) protein levels of p-JNK, CHOP, and cleaved-Caspase3, as well as decreased (P < 0.05) protein expression of Bcl-2 and Bcl-xL. These alterations were prevented by glycine treatment (Fig. 3B). Moreover, anisomycin triggered swell of the ER and degranulation of the ribosome were abrogated by glycine treatment (Fig. 3C). All these results indicated that a critical role of JNK activation is related to ER stress signaling in the induction of apoptosis in IPEC-1 cells. Glycine treatment alleviated JNK activation and thus the cell apoptosis induced by ER stress.
Fig. 2.
Glycine repressed brefeldin A (BFA) -triggered IRE1α-JNK signaling in intestinal epithelial (IPEC-1) cells. Protein abundances of ATF6α, p-eIF2α, eIF2α, p-IRE1α, IRE1α, p-JNK, JNK, and CHOP were analyzed by Western blot (A) and relative protein expression levels were normalized to GAPDH (B). Ultrastructure of rough endoplasmic reticulum under transmission electron microscope (C). Values are means ± SEM of 3 independent experiments. Relative expression of a protein without a common letter differ, P < 0.05. Scale bar: 200 nm. IRE1α = inositol-requiring enzyme 1α; JNK = c-Jun N-terminal kinase.
Fig. 3.
Glycine inhibited anisomycin-induced apoptosis. Cell viability (A) and protein abundance of p-JNK, JNK, CHOP, c-caspase3, Bcl-2, and Bcl-xL (B) were analyzed. Ultrastructure of the endoplasmic reticulum were examined under transmission electron microscope (C). Values are means ± SEM of 3 independent experiments. Means without a common letter differ, P < 0.05. Scale bar: 500 nm.
3.3. Glycine attenuated ER stress-related apoptosis in mTORC1-dependent manner
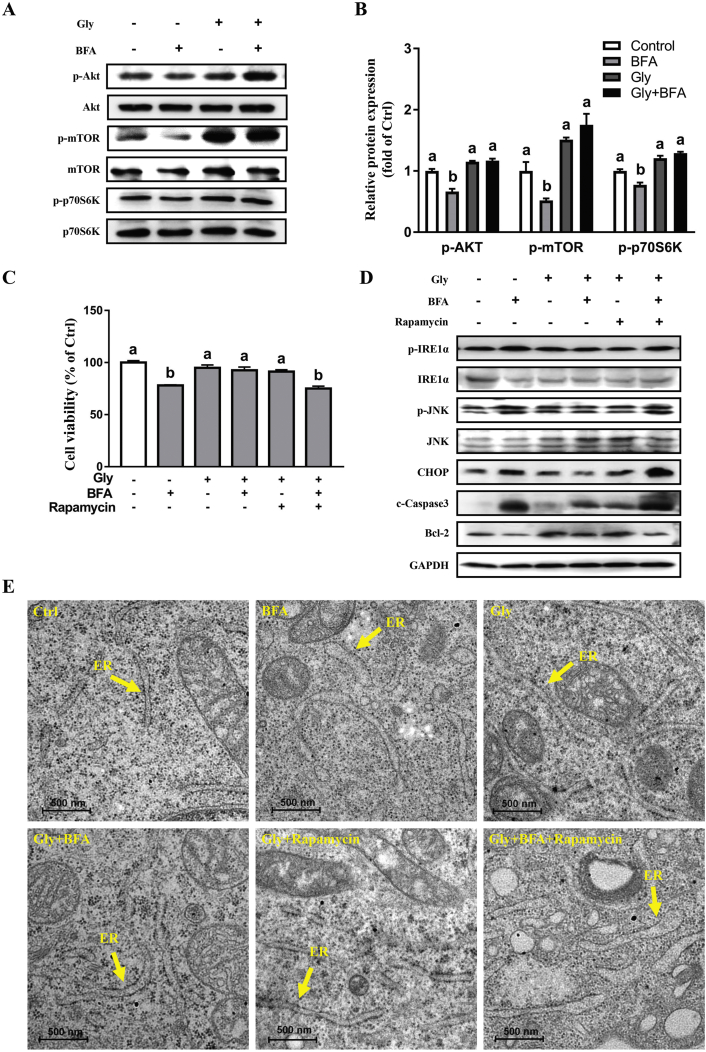
Western blot analysis was conducted to investigate an involvement of mTORC1 in cellular responses to ER stress. As shown, we observed downregulation (P < 0.05) of p-Akt, p-mTOR, and p-p70S6K in BFA-challenged cells, as compared with the control. Interestingly, these alterations were significantly prevented by glycine pretreatment (Fig. 4A and B) (P < 0.05). Rapamycin, an inhibitor of mTORC1, was used to further validate the functional role of mTORC1 in the effects of glycine. As shown, the protective effects of glycine on cell viability (Fig. 4C), activation of IRE1-JNK-CHOP (Fig. 4D), as well as morphological alteration of the ER (Fig. 4E) were abrogated by rapamycin. These results indicated that glycine attenuated ER stress-induced apoptosis of IPEC-1 in a mTORC1-dependent manner.
Fig. 4.
The repressive effect of glycine on the IRE1α-JNK endoplasmic reticulum (ER) stress signaling was dependent on mechanistic target of rapamycin complex 1 (mTORC1). Protein abundances of p-Akt, Akt, p-mTOR, mTOR, p-p70S6K, and p70S6K were determined by Western blot (A) and (B); Cell viability was measured by CCK-8 assay (C). Protein abundances of p-IRE1α, IRE1α, p-JNK, JNK, CHOP, and cleaved-Caspase3 (c-Caspase3), Bcl-2 were determined by Western blot (D). Ultrastructure of rough endoplasmic reticulum under transmission electron microscope (E). Values are means ± SEM of 3 independent experiments. Means without a common letter differ, P < 0.05. Scale bar: 500 nm. p = phosphate; Akt = protein kinase B; mTOR = mechanistic target of rapamycin; p70S6K = p70S6 kinase.
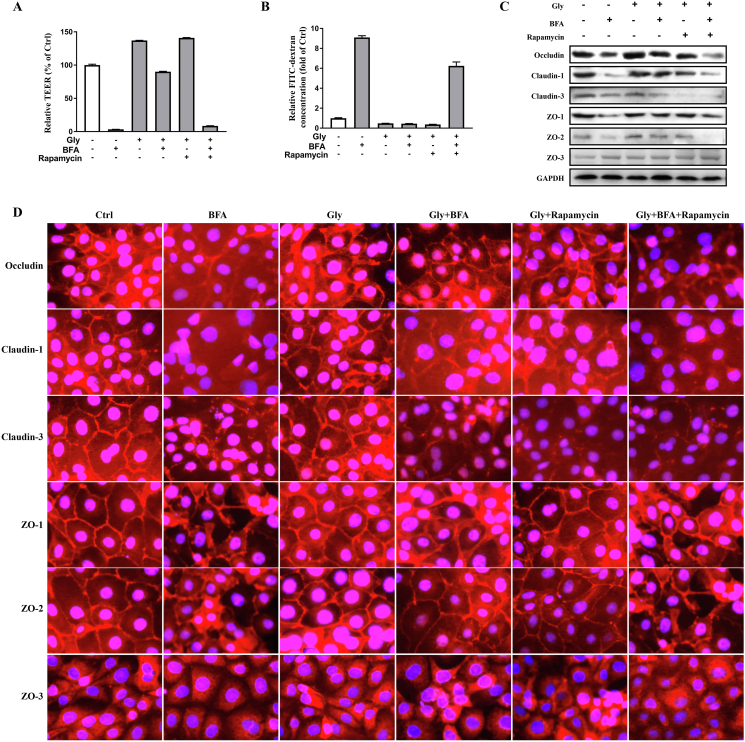
3.4. Glycine treatment attenuated BFA-induced impairment of intestinal integrity by regulating tight junction proteins in IPEC-1 cells
To investigate the effect of glycine on intestinal integrity in BFA-treated cells, monolayer TEER and permeability were determined. As shown in Fig. 5A and B, BFA treatment led to decreased (P < 0.05) TEER and increased intestinal permeability, which were significantly inhibited (P < 0.05) by glycine. Results from Western blot showed that BFA treatment decreased the abundance of tight junction proteins and induced a disruption in the distribution of occludin, claudin-1, claudin-3, ZO-1, ZO-2 and ZO-3 (Fig. 5C and D). Glycine markedly improved the abundance and localization of occludin, claudin-1, ZO-1, ZO-2, without affecting these of claudin-3 and ZO-3 (Fig. 5C and D). Importantly, the beneficial effect of glycine on TEER, intestinal permeability, abundance and distribution of the tight junction proteins in cells exposed to BFA were significantly abrogated by rapamycin (P < 0.05). These results indicated that glycine effectively protected intestinal epithelium from ER stress-induced intestinal barrier disruption, which was dependent on the activation of mTORC1.
Fig. 5.
Glycine alleviated brefeldin A (BFA)-induced intestinal barrier dysfunction in mTORC1-dependent manner. Transepithelial electrical resistance (TEER) (A) and paracellular permeability (B) were determined. Protein abundances (C) and localization of occludin, claudin-1, claudin-3, zonula occludens (ZO)-1, ZO-2 and ZO-3 (D) were analyzed by Western blot or immunofluorescence. Means without a common letter differ, P < 0.05. Scale bar: 50 μm.
4. Discussion
In the present study, we found that intestinal porcine epithelial cells (IPEC-1) exposed to BFA, a fungal metabolite with an ability to inhibit protein trafficking between the ER and the Golgi apparatus, exhibited activation of ER stress signaling and apoptosis, and impairment of the intestinal barrier function. These alterations were abolished by glycine treatment. Mechanically, the beneficial effect of glycine was mainly mediated by inactivation of IRE1α-JNK signaling pathway. Moreover, we demonstrated that the protective effect of glycine against ER stress-induced intestinal barrier dysfunction is dependent on mTORC1 activation.
Recent studies have highlighted a regulatory effect of glycine on ER stress-related cell death in jejunal epithelium of post-weaning piglets (Fan et al., 2019). In human endothelial cells, homocysteine-induced ER stress-associated apoptosis was lowered by glycine supplementation (Sim et al., 2016). Interestingly, a study on Caenorhabditis elegans model demonstrated that glycine modulates the sensitivity of cells to ER stress (Higuchi-Sanabria et al., 2020). Consistent with these reports, we found that ER stress-induced apoptosis was blocked by glycine treatment in IPEC-1 cells. A novel finding of our study is that glycine treatment abolished BFA-induced activation of IRE1α and downstream targets, including JNK and CHOP. The phosphorylated IRE1α activates apoptosis signal-regulating kinase 1 (ASK1), which phosphorylates and leads to activation of downstream target JNK, a stress kinase related to apoptosis (Chen et al., 2017). In agreement with our study, results from diet-induced ER stress in liver tissue were attenuated by glycine treatment, which was linked with the reduced level of CHOP and phosphorylated JNK (Zhou et al., 2016). Our results suggested that a suppression of IRE1α-JNK signaling is associated with glycine-mediated protective effect on BFA-induced apoptosis in IPEC-1 cells.
As a substrate for protein synthesis, glycine has been shown to activate the Akt-mTORC1 signaling in porcine intestine epithelial cells (Wang et al., 2014; Xu et al., 2018). In contrast, it has been reported that BFA suppresses the activation of Akt and mTORC1 in keratinocytes, which was accompanied by a reduction in cellular survival (Nam and Lee, 2016). Interestingly, we showed that the effect of glycine on BFA-induced reduction in cell viability, morphological alteration of the ER, as well as the abundance of proteins implicated in ER stress were abolished by rapamycin, indicating a dependence on mTORC1 signaling. A recent study demonstrated that Akt-mTORC1 signaling controlled the dynamics of IRE1α deactivation by regulating ER-mitochondria physical contacts and the autophosphorylation state of IRE1α, in order to limit prolonged IRE1α activity and related apoptosis (Sanchez-Alvarez et al., 2017). Inactivation of IRE1α contributes to a recovery from ER stress and a restoration of cellular homeostasis. AKT-mTORC1 signaling has been shown to regulate the dynamics of IRE1 RNAse activity (Tubbs et al., 2014; Sanchez-Alvarez et al., 2017). In agreement with these findings, an inverse correlation between IRE1α-JNK and Akt-mTORC1 was observed in our study. However, further studies are required to elucidate the crosstalk between these two critical signaling pathways regulated by glycine.
The ER stress-related apoptosis of enterocytes may lead to disruption of barrier integrity and intestinal dysfunction (Marchiando et al., 2010; Chotikatum et al., 2018). To explore the functional role of glycine on intestinal barrier in BFA-challenged cells, intestinal permeability and abundance of tight junction proteins were determined. BFA-induced increase in intestinal permeability was abolished by glycine, indicating a restoration of the intestinal epithelial integrity. The intestinal permeability is mainly dependent on the abundance and localization of the tight junction proteins of intestinal epithelium (Li et al., 2016; Fan et al., 2019). Claudin-1, one of the claudin family proteins, plays a vital role in tightening intercellular connection (Gunzel and Yu, 2013). Occludin is abundantly expressed at the contact sites of cells and is considered to be required for the assembling of tight junctions (Chiba et al., 2008). ZO-1 and ZO-2 independently determine the polymerized position of claudins protein in the formation of tight junctions (Umeda et al., 2006). In the present study, we showed that glycine attenuated BFA-induced downregulation of occludin, claudin-1, ZO-1, and ZO-2, as well as the aberrant distribution of TJPs. A study on rat intestinal epithelial cells proved that an inhibition of mTORC1 with rapamycin leads to increased cell apoptosis and intestinal permeability in response to ER stress (Ji et al., 2018). In our study, the regulatory effect of glycine on permeability, and tight junction proteins was abrogated by rapamycin. These data suggested that the enhancement of epithelial barrier function by glycine under ER stress relies, at least in part, on mTORC1 signaling.
5. Conclusion
In the present study, using IPEC-1 as an in vitro model, we demonstrated that glycine suppressed ER stress-triggered apoptosis and dysfunction of intestinal epithelial barrier. Further study showed that the beneficial effect of glycine was mainly mediated by the inactivation of IRE1α-JNK signaling in a mTORC1-dependent manner. Hence, glycine supplementation might be a therapeutic intervention in ER stress-related intestinal diseases in piglets.
Author contributions
Zhenlong Wu designed the research. Xiaoxiao Fan, Ju Li, performed the research. Zhaolai Dai, and Zhenlong Wu analyzed the data; Ying Yang, Yun Li, and Zhenlong Wu wrote the paper. Zhenlong Wu had primary responsibility for the final content. All authors read and approved the final manuscript.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31625025, 32172749, 31572410, 31272451), the Zhengzhou 1125 Talent Program, Jingxinnong Animal Science Development Foundation, and the “111” Project (B16044).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Chardin P., McCormick F. Brefeldin A: The advantage of being uncompetitive. Cell. 1999;97(2):153–155. doi: 10.1016/s0092-8674(00)80724-2. [DOI] [PubMed] [Google Scholar]
- Chen S., Wang Y., Yang Y., Xiang T., Liu J., Zhou H., Wu X. Psoralen inhibited apoptosis of osteoporotic osteoblasts by modulating IRE1-ASK1-JNK pathway. BioMed Res Int. 2017;2017:3524307. doi: 10.1155/2017/3524307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba H., Osanai M., Murata M., Kojima T., Sawada N. Transmembrane proteins of tight junctions. Biochim Biophys Acta. 2008;1778(3):588–600. doi: 10.1016/j.bbamem.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Chotikatum S., Naim H.Y., El-Najjar N. Inflammation induced ER stress affects absorptive intestinal epithelial cells function and integrity. Int Immunopharm. 2018;55:336–344. doi: 10.1016/j.intimp.2017.12.016. [DOI] [PubMed] [Google Scholar]
- Dickson R.C., Bronk S.F., Gores G.J. Glycine cytoprotection during lethal hepatocellular injury from adenosine triphosphate depletion. Gastroenterology. 1992;102(6):2098–2107. doi: 10.1016/0016-5085(92)90338-y. [DOI] [PubMed] [Google Scholar]
- Fan X., Li S., Wu Z., Dai Z., Li J., Wang X., Wu G. Glycine supplementation to breast-fed piglets attenuates post-weaning jejunal epithelial apoptosis: a functional role of CHOP signaling. Amino Acids. 2019;51(3):463–473. doi: 10.1007/s00726-018-2681-9. [DOI] [PubMed] [Google Scholar]
- Grootjans J., Kaser A., Kaufman R.J., Blumberg R.S. The unfolded protein response in immunity and inflammation. Nat Rev Immunol. 2016;16(8):469–484. doi: 10.1038/nri.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunzel D., Yu A.S. Claudins and the modulation of tight junction permeability. Physiol Rev. 2013;93(2):525–569. doi: 10.1152/physrev.00019.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13(2):89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- Higuchi-Sanabria R., Shen K., Kelet N., Frankino P.A., Durieux J., Bar-Ziv R., Sing C.N., Garcia E.J., Homentcovschi S., Sanchez M., Wu R., Tronnes S.U., Joe L., Webster B., Ahilon-Jeronimo A., Monshietehadi S., Dallarda S., Pender C., Pon L.A., Zoncu R., Dillin A. Lysosomal recycling of amino acids affects ER quality control. Sci Adv. 2020;6(26) doi: 10.1126/sciadv.aaz9805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi T., Ozawa K. Endoplasmic reticulum stress in disease: mechanisms and therapeutic opportunities. Clin Sci (Lond) 2009;118(1):19–29. doi: 10.1042/CS20080680. [DOI] [PubMed] [Google Scholar]
- Howard A., Tahir I., Javed S., Waring S.M., Ford D., Hirst B.H. Glycine transporter GLYT1 is essential for glycine-mediated protection of human intestinal epithelial cells against oxidative damage. Journal of Physiology-London. 2010;588(6):995–1009. doi: 10.1113/jphysiol.2009.186262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iurlaro R., Munoz-Pinedo C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016;283(14):2640–2652. doi: 10.1111/febs.13598. [DOI] [PubMed] [Google Scholar]
- Ji Y., Luo X., Yang Y., Dai Z., Wu G., Wu Z. Endoplasmic reticulum stress-induced apoptosis in intestinal epithelial cells: a feed-back regulation by mechanistic target of rapamycin complex 1 (mTORC1) J Anim Sci Biotechnol. 2018;9:38. doi: 10.1186/s40104-018-0253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y., Wu Z., Dai Z., Sun K., Zhang Q., Wu G. Excessive L-cysteine induces vacuole-like cell death by activating endoplasmic reticulum stress and mitogen-activated protein kinase signaling in intestinal porcine epithelial cells. Amino Acids. 2016;48(1):149–156. doi: 10.1007/s00726-015-2071-5. [DOI] [PubMed] [Google Scholar]
- Li W., Sun K., Ji Y., Wu Z., Wang W., Dai Z., Wu G. Glycine regulates expression and distribution of claudin-7 and ZO-3 proteins in intestinal porcine epithelial cells. J Nutr. 2016;146(5):964–969. doi: 10.3945/jn.115.228312. [DOI] [PubMed] [Google Scholar]
- Luo K., Cao S.S. Endoplasmic reticulum stress in intestinal epithelial cell function and inflammatory bowel disease. Gastroenterol Res Pract. 2015;2015:328791. doi: 10.1155/2015/328791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Dai Z., Sun K., Zhang Y., Chen J., Yang Y., Tso P., Wu G., Wu Z. Intestinal epithelial cell endoplasmic reticulum stress and inflammatory bowel disease pathogenesis: an update review. Front Immunol. 2017;8:1271. doi: 10.3389/fimmu.2017.01271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiando A.M., Graham W.V., Turner J.R. Epithelial barriers in homeostasis and disease. Annu Rev Pathol. 2010;5:119–144. doi: 10.1146/annurev.pathol.4.110807.092135. [DOI] [PubMed] [Google Scholar]
- McGuckin M.A., Eri R.D., Das I., Lourie R., Florin T.H. ER stress and the unfolded protein response in intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;298(6):G820–G832. doi: 10.1152/ajpgi.00063.2010. [DOI] [PubMed] [Google Scholar]
- Nam Y.J., Lee C.S. Brefeldin A reduces tumor necrosis factor-alpha-stimulated production of inflammatory mediators by suppressing the Akt, mTOR, and NF-kappaB pathways in human keratinocytes. Naunyn-Schmiedeberg’s Arch Pharmacol. 2016;389(9):951–960. doi: 10.1007/s00210-016-1242-6. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Lemasters J.J. Glycine blocks opening of a death channel in cultured hepatic sinusoidal endothelial cells during chemical hypoxia. Cell Death Differ. 2001;8(8):850–858. doi: 10.1038/sj.cdd.4400877. [DOI] [PubMed] [Google Scholar]
- Nyberg S.L., Hardin J.A., Matos L.E., Rivera D.J., Misra S.P., Gores G.J. Cytoprotective influence of ZVAD-fmk and glycine on gel-entrapped rat hepatocytes in a bioartificial liver. Surgery. 2000;127(4):447–455. doi: 10.1067/msy.2000.103162. [DOI] [PubMed] [Google Scholar]
- Sanchez-Alvarez M., Del Pozo M.A., Bakal C. AKT-mTOR signaling modulates the dynamics of IRE1 RNAse activity by regulating ER-mitochondria contacts. Sci Rep. 2017;7(1):16497. doi: 10.1038/s41598-017-16662-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim W.C., Han I., Lee W., Choi Y.J., Lee K.Y., Kim D.G., Jung S.H., Oh S.H., Lee B.H. Inhibition of homocysteine-induced endoplasmic reticulum stress and endothelial cell damage by l-serine and glycine. Toxicol Vitro. 2016;34:138–145. doi: 10.1016/j.tiv.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Tubbs E., Theurey P., Vial G., Bendridi N., Bravard A., Chauvin M.A., Ji-Cao J., Zoulim F., Bartosch B., Ovize M., Vidal H., Rieusset J. Mitochondria-associated endoplasmic reticulum membrane (MAM) integrity is required for insulin signaling and is implicated in hepatic insulin resistance. Diabetes. 2014;63(10):3279–3294. doi: 10.2337/db13-1751. [DOI] [PubMed] [Google Scholar]
- Umeda K., Ikenouchi J., Katahira-Tayama S., Furuse K., Sasaki H., Nakayama M., Matsui T., Tsukita S., Furuse M., Tsukita S. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126(4):741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- Vancamelbeke M., Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expet Rev Gastroenterol Hepatol. 2017;11(9):821–834. doi: 10.1080/17474124.2017.1343143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- Wang W., Wu Z., Lin G., Hu S., Wang B., Dai Z., Wu G. Glycine stimulates protein synthesis and inhibits oxidative stress in pig small intestinal epithelial cells. J Nutr. 2014;144(10):1540–1548. doi: 10.3945/jn.114.194001. [DOI] [PubMed] [Google Scholar]
- Weinberg J.M., Bienholz A., Venkatachalam M.A. The role of glycine in regulated cell death. Cell Mol Life Sci. 2016;73(11–12):2285–2308. doi: 10.1007/s00018-016-2201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J.M., Varani J., Johnson K.J., Roeser N.F., Dame M.K., Davis J.A., Venkatachalam M.A. Protection of human umbilical vein endothelial cells by glycine and structurally similar amino acids against calcium and hydrogen peroxide-induced lethal cell injury. Am J Pathol. 1992;140(2):457–471. [PMC free article] [PubMed] [Google Scholar]
- Wlodkowic D., Skommer J., Pelkonen J. Brefeldin A triggers apoptosis associated with mitochondrial breach and enhances HA14-1- and anti-Fas-mediated cell killing in follicular lymphoma cells. Leuk Res. 2007;31(12):1687–1700. doi: 10.1016/j.leukres.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Xu X., Wang X., Wu H., Zhu H., Liu C., Hou Y., Dai B., Liu X., Liu Y. Glycine relieves intestinal injury by maintaining mTOR signaling and suppressing AMPK, TLR4, and NOD signaling in weaned piglets after lipopolysaccharide challenge. Int J Mol Sci. 2018;19(7) doi: 10.3390/ijms19071980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Han D., Xu R., Wu H., Qu C., Wang F., Wang X., Zhao Y. Glycine protects against high sucrose and high fat-induced non-alcoholic steatohepatitis in rats. Oncotarget. 2016;7(49):80223–80237. doi: 10.18632/oncotarget.12831. [DOI] [PMC free article] [PubMed] [Google Scholar]