Abstract
This study aims to compare the meat quality of Shaziling and Yorkshire pigs and to find the potential indicator in serum for superior meat quality. Six Shaziling and Yorkshire pigs at 30, 60, 90, 150, 210, and 300 d of age were selected to examine carcass traits, meat quality, and serum metabolome. The results showed that the body weight, carcass length, and loin eye area of Shaziling pigs at 150, 210, and 300 d of age were significantly lower than those of Yorkshire pigs (P < 0.05). Shaziling pigs at 150 and 300 d of age had significantly lower backfat thickness than Yorkshire pigs (P < 0.05). Compared with Yorkshire pigs, Shaziling pigs at all 6 ages had a lower lean percentage and a higher fat percentage (P < 0.05). At 60, 90, and 150 d of age, the post-mortem pH-decline, b∗ value (yellowness), and drip loss of Shaziling pigs were significantly lower than those of Yorkshire pigs (P < 0.05). Moreover, at 150 d of age, Shaziling pigs had significantly higher a∗ value (redness) and intramuscular fat (IMF) content than Yorkshire pigs (P < 0.05). Correlation analysis between the top 40 metabolites and phenotypes indicated that L-carnitine had positive correlations with fat percentage, pH24h, and IMF content, but had negative correlations with lean percentage, L∗ value (lightness), and b∗ value (P < 0.05). Serum L-carnitine content, fat percentage, pH24h, and IMF content all decreased first and then increased as the pigs grew, which verified the positive correlations between L-carnitine and these phenotypes. In conclusion, Shaziling pigs have a slower growth rate but a better meat quality than Yorkshire pigs. The meat quality of Shaziling pigs is the best from 150 to 210 d of age. This study suggests that a higher serum L-carnitine content is a promising indicator for better meat quality.
Keywords: Shaziling pig, Yorkshire pig, Carcass trait, Meat quality, Serum metabolome
1. Introduction
China owns the most abundant genetic resources in pig breeds. One hundred and twenty-eight Chinese indigenous breeds, almost one-third of all in the world, were documented in the domestic animal diversity information system of the Food and Agriculture Organization (Yang et al., 2003). The Shaziling pig is a lard-type breed living in the Hunan Province of China, which have superior meat quality, strong resistance to diseases, and slow growth rates (Yang et al., 2016). Previous studies have investigated the mitochondrial genome of Shaziling pigs and compared the transcriptome and proteome of skeletal muscles between Shaziling and Yorkshire pigs (Xu et al., 2015; Yang et al., 2016). However, among the 15 results searched in PubMed (https://pubmed.ncbi.nlm.nih.gov/) with “Shaziling pig” as the keyword, no one has investigated the carcass traits and meat quality of Shaziling pigs. Due to the lack of relevant studies, the excellent meat quality of Shaziling pigs is still limited to sensory awareness and lacks solid evidence and specific phenotypes. Furthermore, how the meat quality alters as the pig grows is also unclear. Investigating this issue may provide references for the determination of the best slaughtering time of Shaziling pigs.
The interaction between genetic, breeding, feeding, slaughtering, and processing factors results in the final quality of pork (Berri et al., 2019). The complex determinism of meat quality makes it hard to predict ante-mortem or quickly after slaughter. Metabolomics is an emerging tool to identify signature substances for some phenotypes of livestock. For example, through metabolomics, former studies identified 15 high-confidence biomarkers that could be applied to predict the quality of chicken breast (Beauclercq et al., 2016). Based on the untargeted metabolomics, the other study demonstrated that there was a molecular signature for feed efficiency of beef cattle (Novais et al., 2019). Therefore, there is great potential to exploit the biomarkers for pork quality with the help of metabolomics. Moreover, by detecting serum metabolites, a new method may be created to predict pork quality before slaughtering.
The present study investigated the carcass traits, meat quality, and serum metabolome of Shaziling pigs and Yorkshire pigs at 6 different ages. There were 3 objectives in this study. Firstly, by comparing the meat quality of Shaziling and Yorkshire pigs, we expect to confirm the superiority of Shaziling pork and find out the specific superior phenotypes. Secondly, based on the horizontal and vertical comparisons, we intend to clarify when the meat quality of Shaziling pig is at its best. Finally, through metabolomics and correlation analysis, we hope to identify potential serum biomarkers for pork quality predicting.
2. Materials and methods
2.1. Animals and sample collection
The experiment was approved by the Animal Care Committee of the Institute of Subtropical Agriculture, the Chinese Academy of Sciences under ethic approval number ISA-2020-023.
2.2. Animals and sample collection
A total of 72 healthy male pigs (36 Shaziling pigs and 36 Yorkshire pigs) were selected and kept on the same diets and under the same environmental conditions. After overnight fasting, blood samples and the longissimus dorsi muscle (LM) were obtained from purebred castrated male Shaziling and Yorkshire pigs at 30, 60, 90, 150, 210, and 300 d of age, with 6 pigs per age. Blood samples were centrifuged at 3,000 × g at 4 °C for 15 min to obtain serum. The serum was stored at −80 °C until analysis. LM samples were processed immediately or preserved at 4 °C and −80 °C, according to the requirements of specific measurements.
2.3. Carcass traits
The body weight (kg) of each pig was recorded before slaughtering. The weight of the left half of the carcass was recorded as carcass weight (kg). The distance from the anterior margin of the symphysis pubis to the fovea of the first cervical spine on the left half of the carcass was measured as carcass length (cm). Loin eye area (height × width, 0.7 cm2) and backfat thickness (mm) were measured between the 6th and 7th ribs by vernier caliper. Then, the left half of the carcass was dissected by a professional meatman according to the normal commercial requirements to separate leans, fats, bones, and skins. The lean/fat percentage was calculated as the percentage of lean/fat weight to carcass weight, respectively.
2.4. Meat quality
Post-mortem pH at 45 min and 24 h was measured by a portable pH meter (Matthaus pH Star, Germany). The difference between pH45min and pH24h was recorded as post-mortem pH-decline.
Meat color parameters (L∗, lightness; a∗, redness, and b∗, yellowness) were determined in duplicate at 45 min post-mortem, using a CR-410 hand-held colorimeter (Kinica Minolta Sensing Inc., Osaka, Japan). The mean value of the 2 measurements was used as the final result.
For drip loss measurements, meat samples were cut into pieces (1 cm × 1 cm × 2 cm) and weighed. Then, the trimmed pieces were hung in plastic cups for 24 h at 4 °C. The percentage of weight loss to initial weight was recorded as the drip loss (%).
The shear force values were measured by a Warner-Bratzler shear force device (TA. XT Plus, Stable Micro Systems, Godalming, UK) according to our previous study (Li et al., 2018).
The IMF content was determined with petroleum ether using a Soxtec Extraction method.
All meat quality traits were measured at the same anatomical location on the LM sample from the left carcass.
2.5. Metabolite extraction, separation, and UHPLC-MS/MS analysis
A volume of 100 μL serum sample was mixed with 400 μL methanol–water (4:1, vol:vol) solution and then settled at −20 °C. The mixture then went through vortex for 30 s and ultrasound at 40 kHz for 30 min at 5 °C. Next, the samples were placed at −20 °C for 30 min to precipitate proteins. After being centrifuged at 13,000 × g and 4 °C for 15 min, the supernatant was transferred to a specialized vial for UHPLC-MS/MS analysis. A Thermo UHPLC system equipped with an ACQUITY BEH C18 column (length: 100 mm; inner diameter: 2.1 mm; grain diameter: 1.7 μm; Waters, Milford, USA) was used to perform chromatographic separation of the metabolites. The elution gradient of the mobile phase are shown in Table 1. The samples were injected at a volume of 2 μL. The column temperature was maintained at 40 °C. All samples were stored at 4 °C during the analysis period.
Table 1.
Elution gradient of mobile phase.
| Time | Flow rate, mL/min | Solution A1, % | Solution B2, % |
|---|---|---|---|
| 0 min | 0.4 | 95 | 5 |
| 3 min | 0.4 | 80 | 20 |
| 9 min | 0.4 | 5 | 95 |
| 13 min | 0.4 | 5 | 95 |
| 13 min 10 s | 0.4 | 95 | 5 |
| 16 min | 0.4 | 95 | 5 |
Solution A is 95% water + 5% acetonitrile (containing 0.1% formic acid).
Solution B is 47.5% acetonitrile + 47.5% isopropanol + 5% water (containing 0.1% formic acid).
The mass spectrometric data were collected using a Thermo UHPLC-Q Exactive Mass Spectrometer equipped with an electrospray ionization (ESI) source operating in either positive or negative ion mode. The optimal conditions were set as followed: heater temperature, 425 °C; Sheath gas flow rate 50 arb (arbitrary unit); Aux gas flow rate 13 arb; ion-spray voltage floating (ISVF), −3,500 V in negative mode and 3,500 V in positive mode, respectively; Normalized collision energy, 20 to 40 to 60 V rolling for MS/MS. Data acquisition was performed with the Data Dependent Acquisition (DDA) mode. The detection range was 70 to 1,050 m/z.
2.6. Data processing and annotation
Peak detection and alignment of raw data were performed in Progenesis QI 2.3 (Nonlinear Dynamics, Waters, USA). Metabolic features that were detected in less than 80% in any of the set of samples were eliminated. For specific samples in which the metabolite levels fell below the lower limit of quantitation, minimum metabolite values were imputed. Each metabolic feature was normalized by the sum. Metabolic features with a relative standard deviation (RSD) higher than 30% were discarded. After normalization and imputation procedures, statistical analysis was performed on log10 transformed data to identify significant differences in metabolite levels between comparable groups. Mass spectra of these metabolic features were identified by searching in reliable biochemical databases like the Human metabolome database (HMDB) (http://www.hmdb.ca/) and Kyoto Encyclopedia of Genes and Genomes (KEGG) database (http://www.genome.jp/kegg/).
2.7. Statistical analysis
Carcass traits and meat quality in the same breed at different ages were expressed as mean ± SEM and analyzed by one-way ANOVA in SAS 8.2. Different lowercases/uppercases denote significant differences among Shaziling/Yorkshire pigs at different ages (P < 0.05). The unpaired t-test in SAS 8.2 was applied to compare the above results in the 2 breeds at the same age. Significant differences from the t-test were marked as ∗ for P < 0.05, ∗∗ for P < 0.01, and ∗∗∗ for P < 0.001.
Principle component analysis (PCA) and orthogonal partial least squares discriminate analysis (OPLS-DA) were performed using ropls (Version1.6.2, http://bioconductor.org/packages/release/bioc/html/ropls.html) R package on Majorbio Cloud Platform (https://cloud.majorbio.com). Variable importance in the projection (VIP) was calculated in the OPLS-DA model. Differential metabolites were identified according to the standard of VIP >1, P < 0.05, and FC < 0.5 or >2. Then the differential metabolites were summarized and mapped into biochemical pathways through KEGG enrichment analysis. The significantly enriched pathways were identified by Fisher's exact test. The correlational heatmaps were generated according to the result of Pearson correlation analysis. These 2 processes are both conducted in the environment of Python package named Scipy.stats (https://docs.scipy.org/doc/scipy/).
3. Results
3.1. Carcass traits of Shaziling and Yorkshire pigs
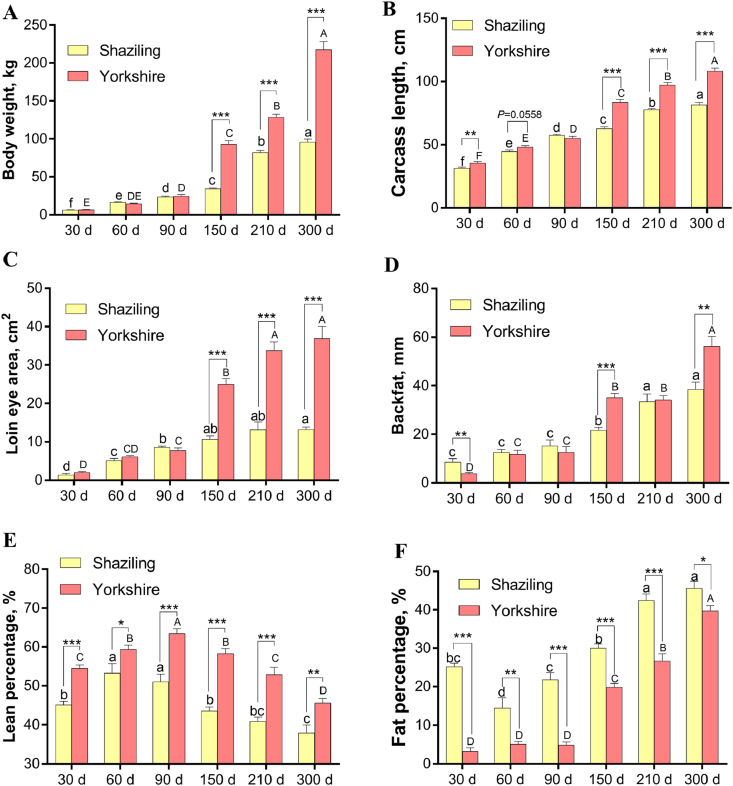
The body weight, carcass length, loin eye area, and backfat thickness of Shaziling and Yorkshire pigs elevated with the increase of age (P < 0.05, Fig. 1A–D). At 150, 210, and 300 d of age, the body weight, carcass length, and loin eye area of Shaziling pigs were significantly lower than those of Yorkshire pigs (P < 0.05, Fig. 1A–C). At 150 and 300 d of age, the backfat thickness of Shaziling pigs was significantly lower than those of Yorkshire pigs (P < 0.05, Fig. 1D). With the increase of age, the lean percentage of Shaziling and Yorkshire pigs first increased and then decreased (P < 0.05, Fig. 1E), the fat percentage of Shaziling pigs first decreased and then increased (P < 0.05, Fig. 1F), and the fat percentage of Yorkshire pigs augmented (P < 0.05, Fig. 1F). At 150, 210, and 300 d of age, the fat percentage of Yorkshire pigs was significantly higher than those at 30, 60, and 90 d of age (P < 0.05, Fig. 1F). During the whole experiment, the lean percentage of Shaziling pigs was significantly lower, and the fat percentage significantly higher, than those of Yorkshire pigs (P < 0.05, Fig. 1E to F).
Fig. 1.
Carcass traits of Shaziling and Yorkshire pigs (A) Body weight (B) carcass length (C) loin eye area (D) backfat (E) lean percentage, and (F) fat percentage. Data are presented as means ± SEM (n = 6). ato e Different letters denotes differences among Shaziling pigs (P < 0.05). A to E Different letters denotes differences amongYorkshire pigs (P < 0.01). Significant differences in comparison between the 2 breeds at the same age are marked as ∗ for P < 0.05, ∗∗ for P < 0.01, and ∗∗∗ for P < 0.001.
3.2. Meat quality of shaziling pigs and Yorkshire pigs
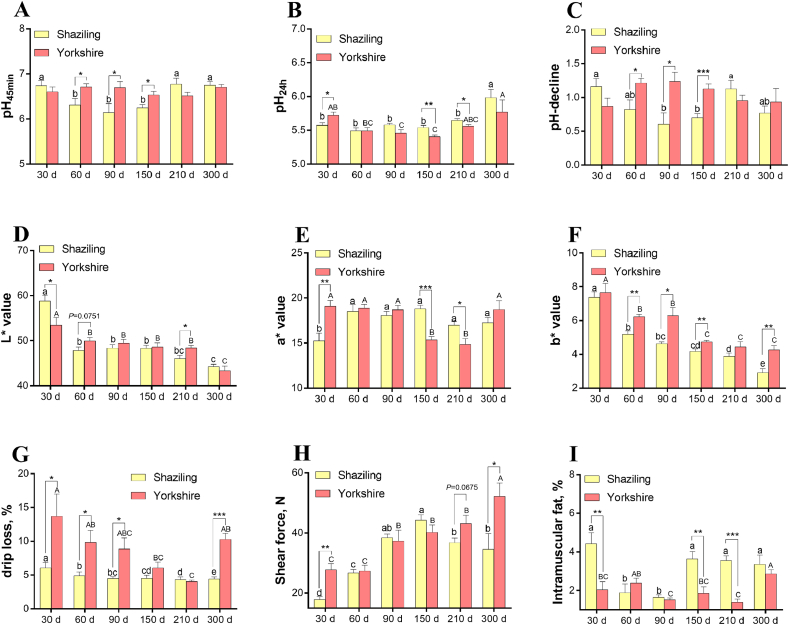
The pH45min values of Shaziling pigs at 30, 210, and 300 d of age were significantly higher than those at 60, 90, and 150 d of age (P < 0.05, Fig. 2A). The pH24h values of Shaziling pigs at 300 d of age were significantly higher than those at other ages (P < 0.05, Fig. 2B). The post mortem pH-decline of Shaziling pigs at 90 and 150 d of age was significantly lower than that at 30 and 210 d of age (P < 0.05, Fig. 2C). There was no significant difference for the pH45min and post mortem pH-decline of Yorkshire pigs at all ages (Fig. 2A and C). The pH24h of Yorkshire pigs at 90 and 150 d of age was significantly lower than that at 30 and 300 d of age (P < 0.05, Fig. 2B). The pH45min and post mortem pH-decline of Shaziling pigs at 60, 90, and 150 d of age were significantly lower than those of Yorkshire pigs (P < 0.05, Fig. 2A and C). Compared with Yorkshire pigs, Shaziling pigs at the 150 and 210 d of age had significantly higher pH24h (P < 0.05, Fig. 2B). L∗/a∗ values of Shaziling pigs at 30 d of age were significantly higher/lower than those at other ages (P < 0.05, Fig. 2D to E). The b∗ value of Shaziling pigs decreased with the growth of age (P < 0.05, Fig. 2F). The L∗ value of Yorkshire pigs at 30 d of age was significantly higher than that at other ages (P < 0.05, Fig. 2D). The a∗ value of Yorkshire pigs at 150 and 210 d of age was significantly lower than that at other ages (P < 0.05, Fig. 2E). The b∗ value of Yorkshire pigs at 150, 210, and 300 d of age was significantly lower than that at other ages (P < 0.05, Fig. 2F). The L∗ value of Shaziling pigs at 210 d of age was significantly lower than that of Yorkshire pigs (P < 0.05, Fig. 2D). The a∗ value of Shaziling pigs at 150 and 210 d of age was significantly higher than that of Yorkshire pigs (P < 0.05, Fig. 2E). Compared with Yorkshire pigs, Shaziling pigs at 60, 90, 150, and 300 d of age had significantly lower b∗ values (P < 0.05, Fig. 2F). The drip loss of Shaziling pigs decreased with the growth of age (P < 0.05, Fig. 2G). The drip loss of Yorkshire pigs first decreased and then elevated with the increase of age and was the lowest at 210 d of age (P < 0.05, Fig. 2G). At 30, 60, 90, and 300 d of age, drip loss of Shaziling pigs was significantly lower than that of Yorkshire pigs (P < 0.05, Fig. 2G). The shear force of Shaziling pigs first elevated and then decreased with the increase of age and reached the peak at 150 d of age (P < 0.05, Fig. 2H). The shear force of Yorkshire pigs at 30 and 60 d of age was significantly lower than that at 300 d of age (P < 0.05, Fig. 2H). The shear force of Shaziling pigs at 30 and 300 d of age was significantly lower than that of Yorkshire pigs (P < 0.05, Fig. 2H). The IMF content of Shaziling pigs at 30, 150, 210, and 300 d of age was significantly higher than that at 60 and 90 d of age (P < 0.05, Fig. 2I). The IMF content of Yorkshire pigs at 90 and 210 d of age was significantly lower than that at 300 d of age (P < 0.05, Fig. 2I). The IMF content of Shaziling pigs at 30, 150, and 210 d of age was significantly higher than that of Yorkshire pigs (P < 0.05, Fig. 2I).
Fig. 2.
Meat quality of Shaziling and Yorkshire (A) pH45min, post-mortem pH at 45 min (B) pH24h, post-mortem pH at 24 h (C) pH-decline, the difference between pH45min and pH24h (D) L∗ value (lightness) (E) a∗ value (redness) (F) b∗ value (yellowness) (G) drip loss (H) shear force, and (I) intramuscular fat. Data are presented as means ± SEM (n = 6). atoc Different letters denotes significant differences among Shaziling pigs (P < 0.05). A to E Different letters denotes significant differences among Yorkshire pigs (P < 0.01). Significant differences between 2 breeds at the same age were marked as ∗ for P < 0.05, ∗∗ for P < 0.01, and ∗∗∗ for P < 0.001.
3.3. Serum metabolome of shaziling pigs and Yorkshire pigs
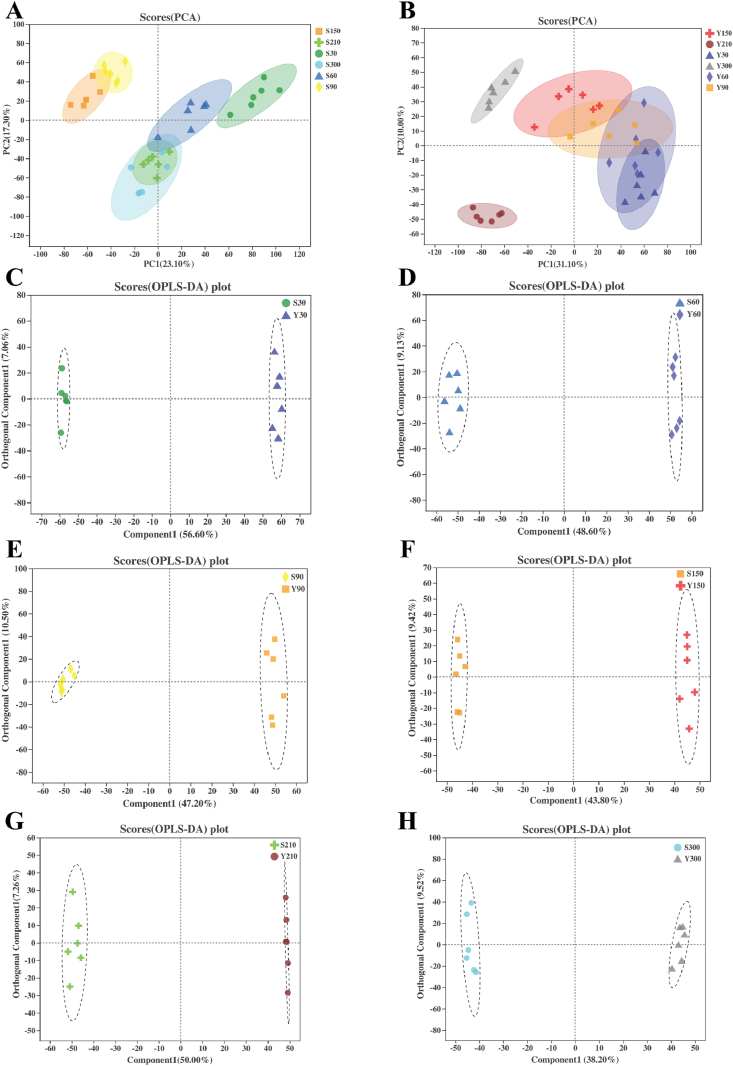
A total of 20,090 peaks were detected by UHPLC-MS/MS method in the serum of Shaziling and Yorkshire pigs. A total of 987 metabolites were identified by comparison with reliable databases. The total ion current intensity is obtained by summing up all the ionic intensities in each mass spectrum. As shown in Appendix Fig. 1A and B, the shape of peaks is good and the distribution is relatively uniform. In the following analysis of serum metabolome, pigs of different ages were abbreviated as (breed + age), such as S30. The PCA analysis showed that the development of serum metabolite patterns of Shaziling and Yorkshire pigs had distinct stages (Fig. 3A and B). Serum metabolite patterns of S90 and S150 were significantly different from those of other Shaziling pigs (Fig. 3A). Serum metabolite patterns of Y210 and Y300 distributed separately from each other and other Yorkshire pigs (Fig. 3B). OPLS-DA analyses suggested that there were also significant differences in serum metabolite patterns between different breeds at the same age (Fig. 3C–H). Differential metabolites between 2 breeds at the same age were screened out by the standard of VIP >1, P-value of t-test < 0.05, and fold change (FC) > 2 or < 0.5 (Appendix Fig. 2). A total of 16 differential metabolites were identified, such as arginyl-glycine, gamma-glutamyl-L-putrescine, and tubaic acid. KEGG enrichment analysis was applied to identify specific pathways in which these differential metabolites participated. It turned out that only one of these metabolites, gamma-glutamyl-L-putrescine, was mapped into the arginine and proline metabolism pathway (Table 2). Interestingly, the relative content of gamma-glutamyl-L-putrescine in Y30 pigs was 2-fold higher than that of S30 pigs (P < 0.05, Table 2). However, after 90 d of age, it was significantly lower in Yorkshire pigs, especially at 150 d of age (P < 0.05, Table 2).
Fig. 3.
PCA and OPLS-DA scatter plots of Shaziling and Yorkshire pigs (A) and (B) PCA of pigs (C to H) OPLS-DA scatter plots of the 2 breeds (n = 6). PCA = principal component analysis; OPLS-DA = orthogonal partial least squares discrimination analysis. Pigs of different ages were abbreviated as (breed + age), such as S30 means Shaziling pigs at 30 d of age, Y30 means Yorkshire pigs at 30 d of age.
Table 2.
KEGG enrichment analysis of differential metabolite between 2 breeds at the same ages.
| Metabolite | Comparison1 | VIP2 | P-value 3 | FC4 | KEGG pathway5 |
|---|---|---|---|---|---|
| Gamma-glutamyl-L-putrescine | Y30 vs. S30 | 2.30 | <0.01 | 2.02 | Arginine and proline metabolism |
| Y60 vs. S60 | 0.04 | 0.92 | 0.99 | ||
| Y90 vs. S90 | 1.61 | <0.01 | 0.85 | ||
| Y150 vs. S150 | 3.87 | <0.01 | 0.30 | ||
| Y210 vs. S210 | 1.32 | 0.01 | 0.87 | ||
| Y300 vs. S300 | 1.78 | 0.01 | 0.80 |
KEGG = Kyoto Encyclopedia of Genes and Genomes; VIP = variable importance in the projection; FC = fold change.
The way of comparison. Pigs of different ages were abbreviated as (breed + age), such as S30 means Shaziling pigs at 30 d of age, Y30 means Yorkshire pigs at 30 d of age.
VIP value of OPLS-DA (Orthogonal Partial Least Squares Discrimination Analysis).
P-value of t-test. P < 0.05 means a significant difference.
Fold change (Y to S) of relative contents between different breeds.
The KEGG pathway that differential metabolite participates in.
3.4. Correlation analyses and potential indicators for meat quality
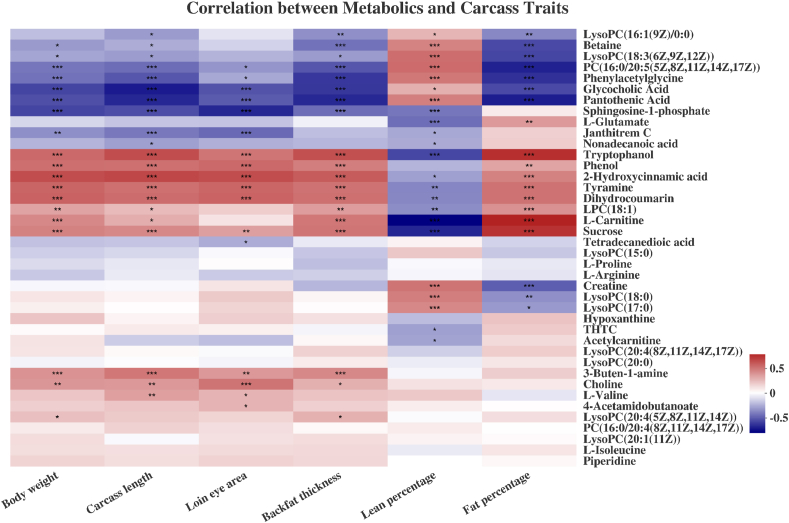
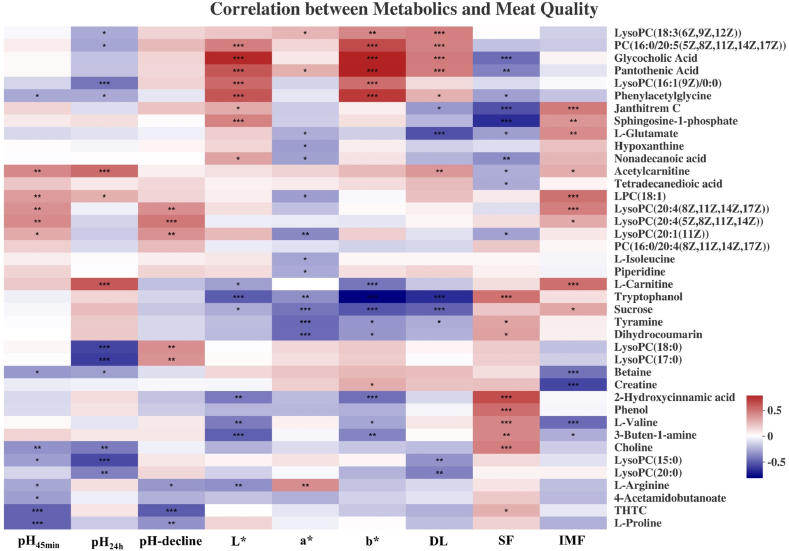
To identify potential indicators of pig development and meat quality, correlation analyses were conducted between the top 40 metabolites and these phenotypes. As shown in Fig. 4, the relative contents of serum carnitine, tryptophanol, 2-hydroxycinnamic acid, tyramine, and dihydrocoumarin were positively correlated with body weight, carcass length, backfat thickness, and fat percentage, but negatively correlated with lean percentage (P < 0.05, Fig. 4). On the contrary, the relative contents of betaine, pantothenic acid, and glycocholic acid were negatively correlated with body weight, carcass length, backfat thickness, and fat percentage, but positively correlated with lean percentage (P < 0.05, Fig. 4). Negative correlations were observed between sphingosine-1-phosphate and body weight, carcass length, loin eye area, backfat thickness, and lean percentage (P < 0.05, Fig. 4). Creatine had a positive correlation with lean percentage, but a negative correlation with fat percentage (P < 0.05, Fig. 4). L-glutamate had a positive correlation with fat percentage and a negative correlation with lean percentage (P < 0.05, Fig. 4).
Fig. 4.
Correlation analysis between the top 40 serum metabolites and carcass traits of Shaziling and Yorkshire pigs at 30, 60, 90, 150, 210, and 300 d of age. Significant correlations between the 2 variables were marked as ∗ for P < 0.05, ∗∗ for P < 0.01, and ∗∗∗ for P < 0.001. Different color of blocks denotes different correlation coefficients. LysoPC or LPC = lysophosphatidylcholine; THTC = tetrahydrothiophene carboxylic acid; PC = phosphatidylcholine.
Nine metabolites, including sphingosine-1-phosphate, L-glutamate, acetylcarnitine, lysophosphatidylcholine (18:1), and L-carnitine, were positively correlated with IMF content (P < 0.05, Fig. 5). Among these nine metabolites, only acetylcarnitine, lysophosphatidylcholine (18:1), and L-carnitine were positively correlated with pH24h (P < 0.05, Fig. 5). However, acetylcarnitine was positively correlated with drip loss, and lysophosphatidylcholine (18:1) was negatively correlated with a∗ value (P < 0.05, Fig. 5). Moreover, L-carnitine was negatively correlated with L∗ and b∗ values (P < 0.05, Fig. 5). These results suggest that L-carnitine might be the most suitable indicator for superior meat quality.
Fig. 5.
Correlation analysis between top 40 serum metabolites and meat quality of Shaziling and Yorkshire pigs at 30, 60, 90, 150, 210, and 300 d of age. Different color of blocks denotes different correlation coefficients. Significant correlations between the 2 variables were marked as ∗ for P < 0.05, ∗∗ for P < 0.01, and ∗∗∗ for P < 0.001. pH-decline, the difference between pH45min and pH24h. L∗, lightness; a∗, redness; b∗, yellowness. DL = drip loss; SF = shear force; IMF = intramuscular fat. LysoPC or LPC = lysophosphatidylcholine; THTC = tetrahydrothiophene carboxylic acid; PC = phosphatidylcholine.
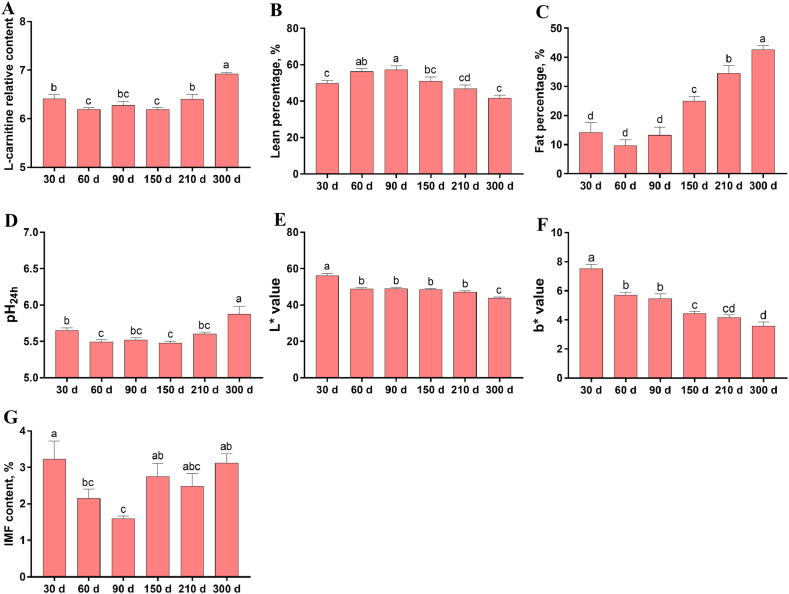
To confirm this suggestion, we further investigated the alterations of serum L-carnitine content and related phenotypes by merging the results of Shaziling and Yorkshire pigs (Fig. 6). Serum L-carnitine content, fat percentage, pH24h, and IMF content all first decreased and then increased with the increase of age (P < 0.05, Fig. 6A, C, D, and G). Lean percentage first increased and then decreased with the increase of age (P < 0.05, Fig. 6B). L∗ and b∗ values both decreased with the increase of age (P < 0.05, Fig. 6E and F).
Fig. 6.
Merged results of highly correlated variables in Shaziling and Yorkshire pigs at 30, 60, 90, 150, 210, and 300 d of age (A) Serum relative content of L-carnitine (B) lean percentage (C) fat percentage (D) post-mortem pH at 24 h (E) lightness (L∗) (F) yellowness (b∗) (G) intramuscular fat (IMF) content. Data are presented as means ± SEM (n = 12). a to d Different letters denote significant differences (P < 0.05).
4. Discussion
It is well-known that indigenous pigs have much lower growth rates and lean percentages than commercial lean pigs (Yang et al., 2003). The present study found that carcass traits, except for lean and fat percentage, were almost the same for the 2 breeds before 150 d of age. From 150 d of age, the body size of Shaziling pig was much smaller than that of Yorkshire pig, evidenced by significantly lower body weight and carcass length. As a fat-type breed, Shaziling pigs had a higher fat percentage and a lower lean percentage than Yorkshire pigs at all ages. These results are consistent with other studies that compared the growth performance or carcass traits of indigenous pigs with those of commercial breeds (Wang et al., 2021; Touma and Oyadomari, 2020; Miao et al., 2009). It is hence reasonable to speculate that Yorkshire pigs grow rapidly after 90 d of age, far outpacing Shaziling pigs. Whether differences in gene expression contribute to differences in growth performance between the 2 breeds is an issue worthy of further investigation. A thought-provoking result is that the backfat thickness of Yorkshire pigs at 150 and 300 d of age was significantly higher than that of Shaziling pigs. We propose that there are 2 possible explanations for this. Firstly, the body weight of Shaziling pigs at 150 and 300 d of age was merely half of Yorkshire pigs. Therefore, the effect of body size might have covered up the effect of breed on fat percentage. Supporting evidence is found in a comparison of carcass traits between Mashen and Large White pigs, where Mashen pigs that weigh approximately 50 kg lighter than Large White pigs had lower lean meat rates and backfat thickness (Guo et al., 2019). Secondly, Shaziling pigs might have a higher proportion of visceral fat than Yorkshire pigs. Although we did not weigh the visceral fat separately, nor did we find relevant evidence in other research, the abdominous appearance of Shaziling pigs makes the conjecture somewhat possible and worth testing.
Despite the slower growth rate and lower lean percentage, superior meat quality and strong resistance to diseases still make Shaziling pigs economically and genetically valuable (Yang et al., 2016). The present study found that, compared with Yorkshire pigs, Shaziling pigs had lower post-mortem pH-decline and b∗ values, but higher a∗ values and IMF content at 150 d of age. At 210 d of age, Shaziling pigs had a higher a∗ value and IMF content than Yorkshire pigs. At 300 d of age, Shaziling pigs had a lower b∗ value, drip loss, and shear force than Yorkshire pigs. Post-mortem pH variation is an important indicator of metabolic changes in meat. Specifically, increased glycolysis in meat can create a high rate of post-mortem pH-decline and a low ultimate pH, resulting in protein denaturation and diminished quality parameters (Ryu and Kim, 2005). The IMF content can not only greatly improve the flavor of meat, but also has a positive correlation with the juiciness of meat (Hocquette et al., 2010; Joo et al., 2013). Therefore, our results confirmed that the superior meat quality of Shaziling pigs was embodied in better color, water-holding capacity, tenderness, juiciness, and flavor. Similar results can be found in comparisons between Mashen and Large White pigs (Guo et al., 2019), Jinhua and Landrace pigs (Guo et al., 2011; Miao et al., 2009), Tibetan and Duroc × Landrace × Yorkshire (DLY) pigs (Shen et al., 2014), and Liang-Shan and DLY pigs (Shen et al., 2014). At 60, 90, and 300 d of age, the a∗ value and IMF content, which indicate the meat redness, marbling, and flavor, had no significant difference between Shaziling and Yorkshire pigs. Therefore, the meat quality of Shaziling pigs might be the best at 150 to 210 d of age.
Serum metabolome is the so-called intermediate phenotype that lies in the middle between the genomic and the final phenotypes, such as growth rate and fat deposition. It could be an advanced approach to improve phenotype description before formulating novel breeding strategies (Fontanesi, 2016). In this study, a comparative analysis of serum metabolome identified 16 metabolites that contribute to the metabolic differences between Shaziling and Yorkshire pigs at the same ages. Given the same age and nutritional conditions, the most likely reason for these differences is breed. Among these metabolites, only gamma-glutamyl-L-putrescine was enriched in a KEGG pathway, namely arginine and proline metabolism. Gamma-glutamyl-L-putrescine is an intermediate metabolite in the process of arginine producing gamma-aminobutyric acid (GABA) (https://www.kegg.jp/kegg-bin/show_pathway?Map00330+C15699). However, in this study, we did not detect the presence of GABA in the serum of Shaziling and Yorkshire pigs. Therefore, the meaning of the difference between breeds warrants further investigation. A previous study compared the serum metabolome between Ningxiang and DLY pigs at 4 months of age and detected a total of 34 metabolites and 14 differential metabolites that participate in lipogenesis, lipid oxidation, protein and amino acid metabolism, energy utilization and partition, and fermentation of gastrointestinal microbes (He et al., 2012). Discrepancies between the present and previous study might be due to the differences in detection methods of metabolites and pig breeds.
To find out the potential indicators for meat quality, we analyzed the correlations between the top 40 metabolites and those phenotypes. It was revealed that L-carnitine had positive correlations with fat percentage, pH24h, and IMF content, and negative correlations with the lean percentage, L∗ value, and b∗ value. Moreover, the alteration of L-carnitine content with age is consistent with that of fat percentage, pH24h, and intramuscular fat, which verified the positive correlation between these parameters. The alteration of lean percentage is opposite to that of L-carnitine content, which is consistent with the negative correlations between them. From 60 d of age, the decreasing alteration of L∗ and b∗ values is opposite to the uptrend of L-carnitine content, which fits in with the negative correlations among L-carnitine and L∗ and b∗ values. Except for its best-known function of importing long-chain fatty acid into the mitochondrion, L-carnitine can also regulate the activity of the pyruvate dehydrogenase complex to promote energy production from glucose (Ringseis et al., 2018). Combined with our results of higher serum L-carnitine content and IMF content in Shaziling pigs, we speculated that the carnitine distribution in the Shaziling pigs was different from that in the Yorkshire pigs, with more carnitine distributed in the serum and less in the muscle. Therefore, the fat decomposition in the muscle of Shaziling pigs may be less than that in Yorkshire pigs, which can indirectly lead to more fat deposition in the muscle of Shaziling pigs. The reasons for the difference in L-carnitine distribution may be ascribed to the differences between the 2 breeds at the gene level. Inspired by the function of L-carnitine, some studies have investigated the effect of L-carnitine as a potential feed additive in pigs. In finishing pigs fed ractopamine hydrochloride, supplementation of L-carnitine decreased the L∗ value and increased the ratio of the a∗ value to b∗ value (James et al., 2013). Moreover, without ractopamine hydrochloride supplementation, L-carnitine decreased the drip loss and tended to increase pH24h independently (James et al., 2013). Another study showed that dietary supplementation of L-carnitine increased the mRNA expression of fatty acid synthase in the longissimus thoracis muscle of growing-finishing pigs when supplemented with lecithin (Meng et al., 2018). In pigs fed 50 mg/kg dietary L-carnitine, the carcass yields and fat depths were greater than pigs fed no L-carnitine (Ying et al., 2013). When supplemented together with distillers dried grains with solubles, 50 mg/kg dietary L-carnitine decreased the shear force in LM (Ying et al., 2013). However, some effects of L-carnitine in the above studies were dependent on other additives. Besides, it should be emphasized that the results of correlation analysis in the present study only described associations and did not prove causation. From another point of view, serum L-carnitine might just be an indicator, namely the result, of great meat quality. In a comparison of metabolome between high and low-marbling beef, the relative amount of L-carnitine was significantly higher in high-marbling beef (Jeong et al., 2020). Therefore, serum L-carnitine is more likely an indicator of great meat quality. Specifically, higher content of carnitine in serum may suggest better meat quality.
In conclusion, our study confirmed the superior meat quality of Shaziling pigs compared with the commercial lean breed, as evidenced by better color, water-holding capacity, tenderness, and IMF content. The comparison between Shaziling and Yorkshire pigs indicated that the meat quality of Shaziling pigs was the best at 150 to 210 d of age. Due to its highly positive correlation with pH24h and IMF content, serum L-carnitine is suggested to be the indicator of great pork quality. This study expanded our understanding of pork quality and provided references for the exploration of the economic and genetic resources offered by Shaziling pigs.
Author contributions
Bo Song: Investigation, Formal analysis, Data curation, Writing – original draft; Changbing Zheng: Investigation, Formal analysis; Jie Zheng: Investigation, Formal analysis; Shiyu Zhang: Formal analysis; Yinzhao Zhong: Formal analysis; Qiuping Guo: Data curation; Fengna Li: Data curation; Cimin Long: Resources; Kang Xu: Resources; Yehui Duan: Validation, Resources, Writing – review & editing; Yulong Yin: Funding acquisition, Conceptualization, Project administration.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This study was jointly supported by the National Natural Science Foundation of China (U19A2037, 31802077), the Changsha Natural Science Funds for Distinguished Young Scholar (kq2009020), the Natural Science Foundation of Guangxi Province (2020JJA130102, 2018JJB130239), Young Elite Scientists Sponsorship Program by CAST (2020QNRC001), Special funds for the construction of innovative provinces in Hunan Project (2019NK2193, 2019RS3022), China Agriculture Research System of MOF and MARA (CARS-35), the ‘Strategic Priority Research Program’ of the Chinese Academy of Sciences (XDA24030204), Open Fund of Key Laboratory of Agro-ecological Processes in Subtropical Region, Chinese Academy of Sciences (ISA2020203), and Taishan industry leading talent project special funds.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
The appendix to this article can be found online at https://doi.org/10.1016/j.aninu.2021.06.011.
Contributor Information
Yehui Duan, Email: duanyehui@isa.ac.cn.
Yulong Yin, Email: yinyulong@isa.ac.cn.
Appendix.
The following is the Supplementary data to this article:
References
- Beauclercq S., Nadal-Desbarats L., Hennequet-Antier C., Collin A., Tesseraud S., Bourin M., et al. Serum and muscle metabolomics for the prediction of ultimate pH, a Key factor for chicken-meat quality. J Proteome Res. 2016;15(4):1168–1178. doi: 10.1021/acs.jproteome.5b01050. [DOI] [PubMed] [Google Scholar]
- Berri C., Picard B., Lebret B., Andueza D., Lefevre F., Le Bihan-Duval E., et al. Predicting the quality of meat: myth or reality? Foods. 2019;8(10) doi: 10.3390/foods8100436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanesi L. Metabolomics and livestock genomics: insights into a phenotyping frontier and its applications in animal breeding. Animal Frontiers. 2016;6(1):73–79. [Google Scholar]
- Guo J., Shan T., Wu T., Zhu L.N., Ren Y., An S., et al. Comparisons of different muscle metabolic enzymes and muscle fiber types in Jinhua and Landrace pigs. J Anim Sci. 2011;89(1):185–191. doi: 10.2527/jas.2010-2983. [DOI] [PubMed] [Google Scholar]
- Guo X., Qin B., Yang X., Jia J., Niu J., Li M., et al. Comparison of carcass traits, meat quality and expressions of MyHCs in muscles between Mashen and Large White pigs. Ital J Anim Sci. 2019;18(1):1410–1418. [Google Scholar]
- He Q., Ren P., Kong X., Wu Y., Wu G., Li P., et al. Comparison of serum metabolite compositions between obese and lean growing pigs using an NMR-based metabonomic approach. J Nutr Biochem. 2012;23(2):133–139. doi: 10.1016/j.jnutbio.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Hocquette J.F., Gondret F., Baeza E., Medale F., Jurie C., Pethick D.W. Intramuscular fat content in meat-producing animals: development, genetic and nutritional control, and identification of putative markers. Animal. 2010;4(2):303–319. doi: 10.1017/S1751731109991091. [DOI] [PubMed] [Google Scholar]
- James B.W., Tokach M.D., Goodband R.D., Nelssen J.L., Dritz S.S., Owen K.Q., et al. Interactive effects of dietary ractopamine HCl and L-carnitine on finishing pigs: II. Carcass characteristics and meat quality. J Anim Sci. 2013;91(7):3272–3282. doi: 10.2527/jas.2011-4287. [DOI] [PubMed] [Google Scholar]
- Jeong J.Y., Kim M., Ji S.Y., Baek Y.C., Lee S., Oh Y.K., et al. Metabolomics analysis of the beef samples with different meat qualities and tastes. Food Sci Anim Resour. 2020;40(6):924–937. doi: 10.5851/kosfa.2020.e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo S.T., Kim G.D., Hwang Y.H., Ryu Y.C. Control of fresh meat quality through manipulation of muscle fiber characteristics. Meat Sci. 2013;95(4):828–836. doi: 10.1016/j.meatsci.2013.04.044. [DOI] [PubMed] [Google Scholar]
- Li Y.H., Li F.N., Duan Y.H., Guo Q.P., Wen C.Y., Wang W.L., et al. Low-protein diet improves meat quality of growing and finishing pigs through changing lipid metabolism, fiber characteristics, and free amino acid profile of the muscle. J Anim Sci. 2018;96(8):3221–3232. doi: 10.1093/jas/sky116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q., Sun S., Sun Y., Li J., Wu D., Shan A., et al. Effects of dietary lecithin and l-carnitine on fatty acid composition and lipid-metabolic genes expression in subcutaneous fat and longissimus thoracis of growing-finishing pigs. Meat Sci. 2018;136:68–78. doi: 10.1016/j.meatsci.2017.10.012. [DOI] [PubMed] [Google Scholar]
- Miao Z.G., Wang L.J., Xu Z.R., Huang J.F., Wang Y.R. Developmental changes of carcass composition, meat quality and organs in the Jinhua pig and Landrace. Animal. 2009;3(3):468–473. doi: 10.1017/S1751731108003613. [DOI] [PubMed] [Google Scholar]
- Novais F.J., Pires P.R.L., Alexandre P.A., Dromms R.A., Iglesias A.H., Ferraz J.B.S., et al. Identification of a metabolomic signature associated with feed efficiency in beef cattle. BMC Genom. 2019;20(1):8. doi: 10.1186/s12864-018-5406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringseis R., Keller J., Eder K. Basic mechanisms of the regulation of L-carnitine status in monogastrics and efficacy of L-carnitine as a feed additive in pigs and poultry. J Anim Physiol Anim Nutr (Berl) 2018;102(6):1686–1719. doi: 10.1111/jpn.12959. [DOI] [PubMed] [Google Scholar]
- Ryu Y.C., Kim B.C. The relationship between muscle fiber characteristics, postmortem metabolic rate, and meat quality of pig longissimus dorsi muscle. Meat Sci. 2005;71(2):351–357. doi: 10.1016/j.meatsci.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Shen L., Lei H., Zhang S., Li X., Li M., Jiang X., et al. Comparison of energy metabolism and meat quality among three pig breeds. Anim Sci J. 2014;85(7):770–779. doi: 10.1111/asj.12207. [DOI] [PubMed] [Google Scholar]
- Touma S., Oyadomari M. Comparison of growth performances, carcass characteristics, and meat qualities of Okinawan indigenous Agu pigs and crossbred pigs sired by Agu or Duroc boar. Anim Sci J. 2020;91(1) doi: 10.1111/asj.13362. [DOI] [PubMed] [Google Scholar]
- Wang Y., Thakali K., Morse P., Shelby S., Chen J., Apple J., et al. Comparison of growth performance and meat quality traits of commercial cross-bred pigs versus the Large black pig breed. Animals (Basel) 2021;11(1) doi: 10.3390/ani11010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Li Q.H., He C.Q., Wang L.Y., Ma H.M. The complete mitochondrial genome of the Shaziling pig. Mitochondrial DNA. 2015;26(4):619–620. doi: 10.3109/19401736.2013.834431. [DOI] [PubMed] [Google Scholar]
- Yang H., Xu X.L., Ma H.M., Jiang J. Integrative analysis of transcriptomics and proteomics of skeletal muscles of the Chinese indigenous Shaziling pig compared with the Yorkshire breed. BMC Genet. 2016;17(1):80. doi: 10.1186/s12863-016-0389-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.-L., Wang Z.-G., Liu B., Zhang G.-X., Zhao S.-H., Yu M., et al. Genetic variation and relationships of eighteen Chinese indigenous pig breeds. Genet Sel Evol. 2003;35(6):657–671. doi: 10.1186/1297-9686-35-7-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying W., Tokach M.D., DeRouchey J.M., Houser T.E., Dritz S.S., Goodband R.D., et al. Effects of dietary L-carnitine and dried distillers grains with solubles on growth, carcass characteristics, and loin and fat quality of growing-finishing pigs. J Anim Sci. 2013;91(7):3211–3219. doi: 10.2527/jas.2012-5606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.