Abstract
Brain arteriovenous malformations (bAVM) are an important cause of intracranial hemorrhage (ICH), especially in younger patients. The pathogenesis of bAVM are largely unknown. Current understanding of bAVM etiology is based on studying genetic syndromes, animal models, and surgically resected specimens from patients. The identification of activating somatic mutations in the Kirsten rat sarcoma viral oncogene homologue (KRAS) gene and other mitogen-activated protein kinase (MAPK) pathway genes has opened up new avenues for bAVM study, leading to a paradigm shift to search for somatic, de novo mutations in sporadic bAVMs instead of focusing on inherited genetic mutations. Through the development of new models and understanding of pathways involved in maintaining normal vascular structure and functions, promising therapeutic targets have been identified and safety and efficacy studies are underway in animal models and in patients. The goal of this paper is to provide a thorough review or current diagnostic and treatment tools, known genes and key pathways involved in bAVM pathogenesis to summarize current treatment options and potential therapeutic targets uncovered by recent discoveries.
Keywords: Brain arteriovenous malformation, mouse models, somatic mutations, signaling pathways, therapeutic targets
Introduction
Brain arteriovenous malformations (bAVMs) represent a relatively rare but important cause of intracranial hemorrhage (ICH) and neurological morbidity, especially in children and young adults. The population prevalence of bAVM is 10-18 per 100,000 adults, with a new detection rate of ∼1.3 per 100,000 person-years. BAVMs are comprised of a complex tangle of blood vessels called the nidus, in which there are direct arterial-venous connections without a normal intervening capillary bed. These high-flow, arteriovenous shunts are prone to rupture with an overall annual ICH rate of 1-3% per year. 1
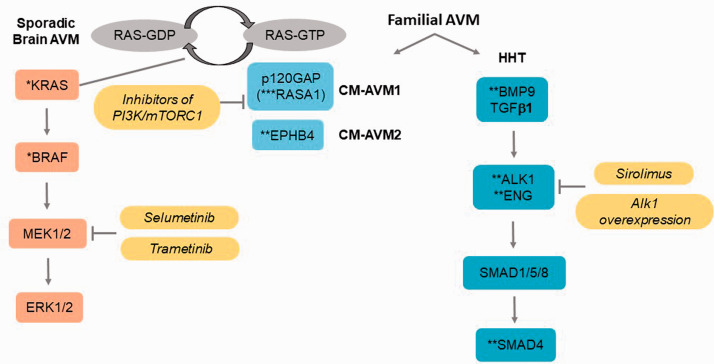
The vast majority of bAVMs present as a solitary lesion without known family history (sporadic), while about 5% of bAVMs occur in patients with genetic syndromes, primarily Hereditary Hemorrhagic Telangiectasia (HHT; also called Osler-Weber-Rendu syndrome) and capillary malformation-arteriovenous malformation (CM-AVM, Figure 1). Many early animal models were established by knocking out HHT causative genes. Similarly, many signaling pathways involved in bAVM pathogenesis and therapeutic targets have been identified through establishing and analyzing these models as well as studying HHT and sporadic bAVM patients. However, there are still no specific medical therapies available for the treatment of bAVMs. Treatment modalities are limited to interventional approaches, such as surgery, radiosurgery, or endovascular embolization, and many complex lesions require multi-modal therapy. However, these procedures are associated with risks, including transient or permanent neurological morbidity or death, 2 and not all patients can be offered treatment. There is a compelling need for novel therapies to prevent and/or reduce bAVM bleeding or rupture. In this review, we have summarized current and potential therapeutic targets identified based on clinical and experimental findings highlighting relevant signaling pathways (Figure 2).
Figure 1.
Major pathways for familial vascular diseases with brain AVMs (Hereditary Hemorrhagic Telangiectasis (HHT) and Capillary Malformation-Ateriovenous Malformation (CM-AVM)), sporadic brain AVM, and therapeutic targets. *Somatic mutations, and **germline mutations, and ***germline and somatic mutations identified in vascular malformations.
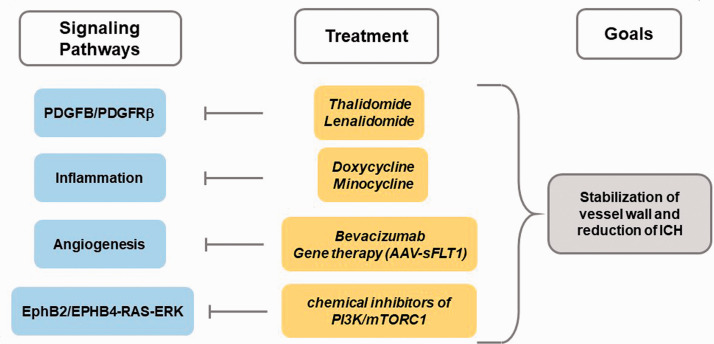
Figure 2.
Other pathways dysregulated in brain AVM pathogenesis and implicated therapeutic targets.
Current diagnosis and treatment options for brain arteriovenous malformation
Diagnosis
BAVMs are most commonly diagnosed as part of a work up for a new neurological deficit typically related to a spontaneous ICH. Overall approximately half of all bAVM patients will present initially with ICH, though this fraction is higher for younger (< 20 years) patients given the reduced use of screening imaging for issues like headache or other neurological issues. 3 The annual rate of ICH after diagnosis but before treatment (i.e., natural history) is estimated to be 2.3% (95% CI: 2.0% - 2.7%), and is higher for ruptured (4.8%, 95% CI: 3.9%-5.9%) than unruptured (1.3%, 95% CI: 1.0%-1.7%) cases at presentation. 3
Computerized tomography (CT) scan is the primary means to screen patients with new focal neurological deficits demonstrating a high degree of sensitivity for hemorrhage. CT angiography also provides excellent accuracy in the detection of bAVMs. 4 In the event of a spontaneous ICH in a patient <40 years or ≥40 years without hypertensive or coagulopathic risk factors, magnetic resonance imaging (MRI) is often performed given its more sensitive soft tissue differentiation. 5 High-resolution post-contrast enhanced imaging is also essential in the identification of small or micro (< 1 cm) bAVMs typical of HHT syndrome. In addition to these more standard MR series, there is growing evidence that quantitative flow methods (e.g., 4D flow) may be used to grade pre- and post-treatment effects on fluid dynamics to and surrounding an AVM. 6
In the event CT and/or MR reveal findings suggestive of a bAVM or the clinical scenario is concerning for a potential secondary vascular malformation in the setting of ICH regardless the cross-sectional angiographic findings, digital subtraction angiography (DSA) is required. DSA better delineates arterial afferent and venous efferent components, the presence of flow-related aneurysms, venous stenoses, and physiological proliferative angiopathy, all high-risk features implicated in natural history and/or surgical risk assessment of bAVM. Additionally, DSA may be used to quantify flow characteristics – information that may prove helpful in determining management risks. 7 Molecular and mechanical imaging may also prove helpful in grading bAVM characteristics. 8 Such methods are experimental at this time, though have proven useful in other vascular and other hyper-vascular neurological pathologies.
Treatment
Decisions to treat a bAVM should carefully weigh the risks of neurologic morbidity from eventual ICH versus those associated with interventional treatment. With high-risk features, hemorrhage rates of bAVM may be up to 34%. 9 Randomized controlled trials and clinical studies have suggested risks of treatment may outweigh risks of rupture for unruptured bAVMs, and treatment remains controversial given the lower risk of ICH in these lesions.1,10 Like most neurovascular disorders, the timing and modality of bAVM treatment is largely dictated by the acuity and severity of ICH. For those cases with rapid clinical deterioration, surgical decompression with or without hematoma and/or nidal resection based on CT imaging alone is often needed. For the majority of cases, however, treatment considerations are multidisciplinary with input from neurological surgery, neurology, and neurointerventional radiology teams. There is no established natural history scoring system, though a number of series have noted increased ICH risks for deep seated lesions, those with a single or deep draining vein, the presence of nidal or peri-nidal aneurysm, and infratentorial location. 11 For patients with a prior history of hemorrhage, treatment is often recommended given the elevated observed rate of secondary hemorrhage, particularly within the first year of ictus. 12 As a general rule, smaller (< 3 cm), well-circumscribed lesions with fewer arterial afferents and venous efferents centered in non-eloquent locations respond more favorably to any intervention. For larger, more angiographically complex, and/or eloquently located lesions, decisions are more nuanced and a treatment team will often discuss combination strategies designed to address discrete high-risk bAVM features (e.g. flow-related, nidal, or pseudo-aneurysms), deep or perforating arterial afferents more difficult to surgically access, and/or high-flow direct AV shunts through embolization.
Higher rates of obliteration are reported with surgical resection and patient outcome heavily driven by patient selection. Patient selection for surgical resection is aided by surgical grading scales which estimate risks of outcomes after surgery – such as the Spetzler-Martin and Lawton-Young grading scales.13,14 Factors including size, patterns of venous drainage, eloquence of the brain region containing the bAVM, patient age, rupture status, and configuration of the nidus (diffuse vs. compact) have been shown to impact outcomes following surgery. Due to neuroplasticity, surgical outcomes are better in younger patients. 15 With appropriate patient selection, surgery is also a safe, viable treatment option in the elderly. 16
The intent of bAVM surgery is the complete removal of the nidus and elimination of AV shunting. When these are not possible, surgery can be a useful adjuvant to eliminate blood flow from high risk features – such as intranidal or flow-related aneurysms. 17 Successful surgical resection is defined as no residual arteriovenous shunting on postoperative angiography. However, in certain high risk populations, such as ruptured bAVMs in children, higher rates of recurrence (20%) have been reported and delayed angiography is often recommended between 1 and 5 years after complete resection.18,19
For bAVMs in deep, eloquent or surgically in accessible locations, stereotactic radiosurgery (SRS) may be an attractive treatment option. Radiation damages the endothelial cell lining and induces proliferation of smooth-muscle cells which leads to progressive stenosis of bAVM feeding arteries. Eventual occlusion occurs over several years. 20 Radiation induced changes may be seen in the surrounding brain and limits radiation dosage which may be safely applied. 20 With large bAVMs, volume staged radiosurgery may be required. 21 Even if complete obliteration is not achieved, volume staged radiosurgery may decrease the size of the nidus to make it acceptable for surgery.
Embolization has largely been used as an adjunctive aimed to make microsurgical resection safer and more complete. Embolization carries 1-10% peri-procedural stroke risk depending on the series and is often a function of the number of pedicles treated and embolic agent used. 22 Complications may occur when embolizing neighboring physiological arteries, and obstructing venous outflows causing subarachnoid hemorrhage, ischemia, and ICH, respectively. There is limited evidence as to the impact of embolization on surgical performance, 23 as well as evidence of better clinical outcomes from such a tandem approach. 24 Embolization as an adjunct to radiosurgery has also evolved over time, with evidence suggesting that such practice may lessen the effect of radiation therapy while adding embolic risk. 25 As such, this practice, unless embolization is used to target a high-risk feature (e.g. pseudoaneurysm), is less favorable.
A number of case series describe embolization as a curative approach. 26 There is less evidence in support of this practice, though some have proved successful with follow up intervals up to 3 years without evidence of angiographic recurrence. 26 As with the other modalities, smaller, angiographically simpler lesions prove safer and more amenable to definitive treatment. 27 Within this curative cohort is a group using a combination of transarterial and transvenous techniques. This approach has proven effective in a select number of cases,28–30 though may carry significantly higher rates of peri-procedural stroke relative to adjunctive embolization when applied to larger, more complex lesions.
Palliative embolization may be used in certain cases where conventional therapies cannot safely and effectively treat a bAVM and a patient has progressive signs or symptoms refractory to medical interventions. In the setting of pain or bothersome pulsatile tinnitus, targeted embolization of dural arterial supply to a bAVM may reduce such symptoms. In rare instances where progressive venous stenosis occurs, venous hypertension can cause headaches, seizures, and/or other focal neurological issues. In these instances targeted embolization of arterial afferents to reduce the shunt volume may be effective.
These interventional options focus on removing the bAVM nidus or high-risk features to reduce flow through the lesion. However, not all bAVM lesions can be safely treated with available options, and the ARUBA randomized controlled trial results suggest that unruptured bAVMs (roughly half of all cases) should not be treated. 1 Therefore, many groups have been focused on identifying medical therapies to slow or stabilize lesion progression based on genetic discoveries and signaling pathways identified to date and described below.
Genetic discoveries in bAVM
The genetic contributions to bAVMs are multi-factorial, including germline and somatic mutations, epigenetic alterations, and genetic modifiers which can alter the expressivity of disease-causing genes. Different approaches have been used to inform on the key genes and biological pathways that contribute to bAVM development and disease progression. Genetic and epigenetic factors may also have clinical utility as bAVM diagnostic or prognostic markers, or as potential therapeutic targets. To identify new therapeutic targets, we highlight human studies that have identified key genetic discoveries that have resulted in a paradigm shift and suggest areas for future bAVM research strategies.
Germline mutation in bAVM
There are two main subgroups of bAVM patients with known underlying genetic disorders: HHT and CM-AVM (Figure 1). HHT is an autosomal dominant disease characterized by systemic vascular fragility, telangiectasias and AVMs in various organs, including the brain. The majority of HHT is caused by heterozygous, loss-of-function mutations in the following genes: (1) endoglin (ENG, HHT1), encoding an ancillary TGFβ receptor; (2) activin receptor-like kinases 1 (ALK1, also named ACVRL1, HHT2), encoding a type I TGFβ receptor; and (3) mothers against decapentaplegic homology 4 (SMAD4, juvenile polyposis-HHT), encoding a mediator of TGFβ signaling. Mutations in bone morphogenetic protein 9 (BMP9, also named GDF2, HHT5), encoding a secreted ligand of the TGFβ superfamily, have also been reported to cause an HHT-like syndrome. 31 ENG and ALK1 are primarily expressed in endothelial cells to regulate the development of AV network through TGFβ and BMP signaling pathways.
CM-AVM is another autosomal dominant disorder caused by germline, heterozygous loss of function mutations in RASA1 (CM-AVM1) or EPHB4 (CM-AVM2), and characterized by multiple cutaneous capillary malformations co-occurring with fast-flow vascular anomalies, such as AVM or AV fistula. RASA1 encodes RAS p21 protein activator 1, which is a negative regulator of the Ras pathway through its GTPase activating protein. RASA1 interacts with receptor tyrosine kinases including EPH family receptors, amongst which the EPHB4 receptor is involved in regulating AV morphology. 32
Interestingly, de novo damaging heterozygous germline mutations in EPHB4 have been identified in Vein of Galen malformations (VOGM), another rare congenital AVM sometimes present in newborns.33,34 The Ephrin receptor signaling pathway was over-represented in VOGM cases with the following genes in an interactome: EPHB4, RASA1, EPHA4, EPHA6, ITGB1, ITNS1, and NGEF. 34 In addition, one RASA1 mutation was identified in 55 VOGM probands, suggesting a potentially shared molecular mechanism between VOGM and CM-AVM. 34 Therefore, the EphrinB2-EphB4-RASA1 signaling axis has a role in human cerebrovascular development and disease, which may provide diagnostic and therapeutic targets for patients with cerebrovascular disorders including bAVM. 33
Findings in sequencing studies
Whole exome sequencing studies in bAVM have identified several rare germline mutations. For example, a stop-gain mutation (c.C739T:p.R247X) in SMAD9 was discovered, with reduced levels of vascular SMAD9 protein and phosphorylated SMAD4, a downstream effector of the BMP signaling pathway, in AVM peri-nidal blood vessels. 35 Whole exome sequencing of 5 bAVM patients identified germline mutations enriched in pathways controlling endothelial homeostasis and 2 novel pathways: cilia morphogenesis and ion homeostasis. 36 In addition, Scimone et al. 37 performed whole exome sequencing to evaluate a young boy with a sporadic bAVM and detected 20 likely gene-disrupting variants affecting many genetic loci, including a de novo nonsense variant in the STK4 gene.
Whole exome sequencing of 100 unrelated bAVM trios led to the discovery of four pathogenic heterozygous variants in four bAVM patients. 38 One variant was in ENG, and three others were damaging variants in novel candidate genes: PITPNM3, SARS and LEMD3. These whole exome sequencing data were included in a larger follow-up study of a total of 112 bAVM trios which investigated rare and deleterious compound heterozygous mutations associated with bAVM. 39 A total of 16 genes had compound heterozygous variants that were recurrent in more than one trio. Two genes, LRP2 and MUC5B, were recurrently mutated in three trios. LRP2 is a receptor for lipocalin 2 (LCN2), which is involved in inflammation and may have a role in brain andothelial cell angiogenesis. 40 The LRP2 mutations identified in bAVM trios were all novel and predicted to be harmful, and all three bAVMs were located in the left parietal lobe. The mutations in the MUC5B gene were all missense mutations. However, MUC5B is a large gene and sequencing studies often reveal many unexplained variations in this gene; thus MUC5B is not considered a strong candidate gene for bAVM. The following genes were recurrently mutated in two trios: DNAH14, DNAH5, FCGBP, HERC2, HMCN1, MYH1, NHSL1, PLEC, RP1L1 and five genes known to have a role in vascular disease or angiogenesis including MYLK, HSPG2, PEAK1, PIEZO1, and PRUNE2. This study supports a role for rare recessive compound heterozygous variants in bAVM and future functional studies will be required to assess the impact on bAVM pathology. 39
Somatic mutation in bAVM
Somatic mutations arise during development or in disease pathogenesis in a somatic cell and are subsequently found only in a subset of cells in each affected individual. Activating somatic mutations in the Kirsten rat sarcoma viral oncogene homologue (KRAS) gene and other mitogen-activated protein kinase (MAPK) pathway genes have been detected in bAVM tissue using high-throughput or targeted sequencing technology,41–44 suggesting a role for the RAS/RAF pathway and MAPK-ERK signaling pathway. Using whole exome sequencing of DNA from sporadic bAVM tissue, Nikolaev et al. 41 identified the presence of 3 recurrent, somatic activating mutations in KRAS (c.35G>A [G12D], c.35G>T [G12V], c.183A>T [Q61H]), with low-allelic representation (<5%) and not present in DNA from paired blood samples. Since that report, several groups have confirmed the presence of these rare but recurrent KRAS mutations, identified additional KRAS and BRAF mutations, 42 and found that these mutations are also present in spinal cord AVM. 45 Peripheral AVM lesions also harbor somatic mutations in other members of the RAS/MAPK pathway, including MAP2K1 and BRAF, a proto-oncogene,46,47 which suggests there may be a potential common signaling pathway for treatment of AVMs located both within or outside the central nervous system (CNS). A recent meta-analysis of 6 studies including 1726 patients with bAVM estimated the frequency of KRAS somatic mutations is 55%, while the prevalence of BRAF somatic mutation is 7.5%. 44 Gao et al. 48 performed whole exome sequencing in 14 paired bAVM tissue and blood DNA samples, and validated KRAS mutations in 56 patients. A total of 24 candidate somatic variants in 11 MAPK pathway genes were identified, including KRAS G12V in 15% and KRAS G12D in 32% of bAVM lesions, 48 and novel mutations in PDGFRB. Only KRAS mutations have been reported in multiple patients (recurrent), whereas remaining somatic mutations identified to date are private mutations (e.g., specific and isolated to a single individual or family).
It is unknown whether somatic mutation burden is an inciting event in bAVM formation or appears later in the disease course, as part of endothelial repair, progression to hemorrhage (e.g., as a result of high intra-nidal blood flow and chronic inflammation triggering changes in vascular genes such as flow-sensitive genes), or development of high-risk features (e.g., associated aneurysms).42,47 BAVMs are rarely observed in utero, VOGM being the exception, hence the somatic mutations are likely to occur later in post-natal development. Genotype-phenotype studies have not identified associations with age at presentation, sex, presenting symptom, AVM size, or location, when comparing patients with and without somatic mutations or with mutation burden.43,48 These findings highlight the need to characterize bAVM tissue for somatic KRAS and other RAS/MAPK gene mutations to better understand the relevance in bAVM pathogenesis and phenotypes. Future studies will need to elucidate the precise timing of when these somatic mutations arise in the endothelial cells or other cell types.
Somatic mutations have also been observed in vascular malformations from patients with HHT and CM-AVM. Two somatic mosaic RASA1 mutations (c.2035C>T and c.1507C>T) were identified in a facial AVM of a patient with CM-AVM who has the germline RASA1 c.2035C>T mutation. 49 Somatic mutations identified in telangiectasia from HHT patients resulted in bi-allelic loss of ENG or ALK1. 50 These studies suggest that the focal nature of vascular malformations in these familial diseases follow a two-hit genetic mechanism, where patients inherit a germline mutation followed by a second somatic mutation in the same gene to seed lesion formation, rather than haploinsufficiency of the protein. 50 The mechanism for sporadic lesions likely follows a two-hit mechanism, although the second hit may be genetic or environmental, as also suggested by animal studies. Additional studies are needed to further define the impact of these mutations in bAVM development or progression.
A current limitation of somatic mutation studies is that they rely on availability of AVM tissue. For peripheral AVMs, conventional biopsy methods may be used. However for CNS AVMs, not all can be safely treated by microsurgical resection and open surgical biopsy is not possible due the risks of stroke. As such, our group has demonstrated a method to safely and accurately collect cells using endovascular means for bAVM specific genetic diagnosis. 51 This technique may prove instrumental in determining which cases will most favorably respond to certain therapies, medical or otherwise, in addition to more generally expanding our understanding of the molecular genetics of secondary vascular disorders. Additionally, next generation sequencing liquid biopsy using cell-free DNA may be a useful noninvasive approach to investigate KRAS mutations in bAVM patients. 52 Future studies are needed to determine whether blood-based markers can inform on KRAS somatic mutation burden and the relevance to bAVM hemorrhage.
Differential microRNA and mRNA expression in bAVM
Expression studies have demonstrated a role for gene regulation in bAVM, including microRNAs, which are non-coding RNA that regulate the expression of target genes. 53 Specific microRNAs (miR-18a, miR-137, and miR-195 all downregulated in bAVM) have been shown to inhibit vasculogenesis or improve endothelial cell function in bAVM. 54 A recent study of patient-derived bAVM endothelial cells demonstrated that miR-18a increases TSP-1 and decreases VEGF by reducing plasminogen activator inhibitor-1/SERPINE1 (PAI-1) levels. 55 In addition, miR-18a decreased the expression of bone morphogenetic protein 4 (BMP4) and hypoxia-inducible factor 1α (HIF-1α), and blocked the BMP4/ALK2/ALK1/ALK5 and Notch signaling pathways. 55 miR-199a-5p, miR-7-5p and miR-200b-3b are upregulated in peripheral blood of bAVM patients, involved in VEGF signaling, and may be useful biomarkers for bAVMs. 56 Chen et al. also observed upregulated let-7b-3p in the blood of bAVM patients, although the function of this miRNA in bAVM is unknown. 56 It remains unknown if miRNAs are involved in AVM development, However in mice, mutations in Drosha, a core nuclease that executes the initiation step of miRNA processing in the nucleus, caused vascular abnormalities similar to HHT telangiectasia in mice. 57
Next-generation RNA sequencing has identified differential expression on a transcriptome-wide level comparing tissue samples of 12 bAVMs to 16 intracranial control arteries. 58 A total of 736 upregulated genes in bAVM are implicated in cytoskeletal machinery, cell-migration, neutrophils and macrophages, and inflammatory cytokines, which is consistent with older transcriptome studies in bAVM tissue.59–61 In addition, 498 genes are downregulated, including genes involved in the angiopoietin-TIE system and TGF-β signaling. In line with previous studies, ANGPT1 and its receptor (TEK) were downregulated. 60 The study points to involvement of loss of cerebrovascular quiescence, and impaired integrity of the vascular wall in the pathophysiology of bAVMs, and supports a potential role for therapeutics promoting vessel maturation.
Whole blood transcriptome (mRNA) profiling in 40 bAVM patients (20 ruptured vs. 20 unruptured) identified molecular signatures of ICH, including increased levels of FAS, TLR10, TNFAIP6, IL1R1, and IL18R1, and suggests involvement of the MAPK, VEGF, Wnt and several inflammatory pathways. 53 Future studies will be needed to further define the role of microRNAs and mRNAs in bAVM and determine whether they can be useful noninvasive clinical biomarkers for bAVM.
Epigenetic factors in cerebrovascular disease and bAVM
Epigenetic mechanisms provide tight control at the transcriptional level that differentially modifies gene expression and protein activity. Targeted candidate gene methylation studies in bAVM and intracranial aneurysm patients suggest that methylation of CDKN2A is associated with bAVM and methylation of PDGFD is associated with increased risk of both bAVM and intracranial aneurysm. 62 Genetic variants in these or related genes have been associated with bAVM or bAVM-associated aneurysms.48,63 In addition, key components of the m6A methyltransferase complex, Wilms’ tumour 1-associating protein (WTAP) and Methyltransferase-like 3 (METTL3), which is an important epigenetic regulator, are down-regulated in bAVM lesions and inhibits angiogenesis, 64 with METTL3 downregulation leading to continuous activation of the Notch signaling pathway. 64 DNA methylation of key candidate genes involved in pathways that contribute to bAVM progression, such as flow-sensitive genes, may disrupt the regulation of transcription in immune cells or supporting vascular cells that stabilize the bAVM lesion. Future studies are needed to investigate the role of gene regulation, e.g., through mechanisms such as DNA methylation, in bAVM pathogenesis. These new avenues of bAVM research may also lead to the identification of new therapeutic targets for bAVM, as epigenetic regulators may be targeted to correct gene expression perturbations in disease.
Other genetic factors associated with bAVM and bAVM hemorrhage
Common genetic variants may influence bAVM disease course and increase risk of ICH. We have identified inflammatory genes associated with risk of bAVM hemorrhage in three settings: presentation with ICH, 65 new ICH after diagnosis, 66 and ICH after treatment. 67 In particular, our group and others have identified several pro-inflammatory cytokine variants that increase risk of ICH by 2-4 fold in bAVM patients, including interleukins and tumor necrosis factor alpha (TNFA). 68 In addition, two EPHB4 variants (rs314313 and rs314308) were found to be associated with risk of ICH in Caucasian subjects with sporadic bAVM. 65 EPHB4, which is expressed by venous endothelial cells, is involved in kinase dependent forward signaling, which regulates diverse endothelial cell functions and angiogenesis along with concomitant activation of ERK1/2. 69 Thus, it is plausible that common RASA1 and EPHB4 variants could influence disease severity in vascular diseases with involvement in the complex RAS-ERK and EPHRINB2-EPHB4-RASA1 signaling pathways.
In summary, we do not yet know the cause of sporadic bAVM, however there are several genetic factors that may influence sporadic bAVM disease including, e.g., gain or loss of function genetic mutations, epigenetic changes, or genetic modifiers. Figure 1 illustrates several major pathways (and key genes) in both hereditary diseases with bAVM and sporadic bAVM. Although these are not the same genes, the pathways interact, suggesting a spectrum of vascular diseases displaying vascular malformations as part of the phenotype. Genetic associations are not necessarily causal for bAVM. However, mechanistic/functional studies in in vitro and in vivo animal models as described in detail in the next section below reveal a strong role for these genes in bAVM development and/or hemorrhage.
Signaling pathways, current AVM animal models, and potential therapeutic targets
Tgf-β signaling and HHT animal models
Mutations in the TGF-β pathway impairs vascular morphogenesis and angiogenesis. Mice deficient in the components of the TGF-β pathway exhibited embryonic lethality due to vascular defects. Five type I receptors and seven type II receptors have been identified thus far. Accessory receptors have also been identified to be involved in the formation of receptor complexes, including ENG, beta-glycan, BMP, and activin membrane-bound inhibitor homology (BAMBI). In the canonical Smad pathway, TGFβ/BMP dimers induce the heteromeric complex formation of TβRI/TβRII. The TβRII then phosphorylates and activates the TβRI, which in turn propagates the signal to the nucleus through the Smad family of co-activators. Alternatively, the phosphorylated TβRI receptor can activate non-Smad pathways.
Mutations of TGF-β signaling pathway genes, including ENG, ALK1, SMAD4 and BMP9 cause HHT. The endothelial cell TGF-β signaling is characterized by the balanced signaling through the TGF-β type I receptors: the endothelial cell dominant ALK1 and the uniquitously expressed ALK5. TGF-β fine tunes the intricate equilibrium between ALK1 and ALK5. Low doses of TGF-β stimulates endothelial cell proliferation and migration via ALK1 to activate angiogenesis, while high doses of TGF-β increases the production of extracellular matrix (ECM) components, leading to a quiescent endothelium. ALK5 is required for efficient ALK1 signaling and the ratio of those two receptors as well as accessory receptor ENG determines the relative response to TGF-β. 70 Interestingly, ENG can be shed off from the endothelial cell membrane as a soluble form (solENG) affecting the delicate balance of TGF-β signaling required for angiogenesis by scavenging TGF-β ligands. Overexpression of solENG caused bAVMs in mice. 71 solENG was also found to specifically bind to BMP9 and BMP 10, leading to the inhibition of blood vessel formation. 72 BMPs have also been implicated in endothelial cell function and angiogenesis. Blocking both BMP9 and BMP10 induced AVM development in the retinal vasculature, but it remains elusive whether BMP9 and BMP10 are both required for ENG-ALK1 signaling.
Mice carrying mutations on both alleles of Eng or Alk1 genes were embryonic lethal and showed obvious defects in angiogenesis and cardiac development.73,74 However, mice with heterozygous mutations in either of these genes can survive to adult stage and recapitulate relatively mild phenotypes seen in HHT patients,74,75 suggesting that additional factors, such as mutation on the other allele (second hit) and environmental mediators are required for bAVM development. Moreover, morpholino-induced knockdown of Eng or Alk1 in zebrafish models recapitulate the morphologic, functional, and molecular defects seen in human AVMs, allowing visualization of precise spatiotemporal patterns during vascular development. 76
Tamoxifen-inducible conditional knockout (iKO) mouse models have been developed to allow temporal control of Eng or Alk1 deletion in specific cells and at specific developmental stages. Brain focal angiogenic stimulation (VEGF administration) with either Eng or Alk1 iKO globally or specifically in endothelial cells induced a robust and reproducible bAVM phenotype in adult mice, including vascular dysplasia, arteriovenous shunt, and microhemorrhage.77–79 In the skin, arteriovenous shunts only developed around skin wounds in Alk1 or Eng iKO mice.80,81 In addition, bAVM can develop spontaneously in mice that have Alk1 or Eng deleted at the perinatal stage.79,82 These data indicate that in addition to Eng or Alk1 mutation, response to injury/angiogenic stimulation is necessary to cause AVM development in the brain and other organs.
Recently, Kim et. al. demonstrated that overexpression of Alk1 can rescue the AVM phenotypes in both Alk1- and Eng-iKO mice through normalizing the expression of Smad and Notch target genes and restoring the effect of Bmp9 on suppression of pAkt in Eng-deficient endothelial cells. 81 However, overexpression of Eng failed to inhibit the AVM manifestations in Alk1-iKO mice. Therefore, Eng is signaling upstream of Alk1. Increasing Alk1 expression could be a therapeutic option.
KRAS-MAPK signaling and sporadic bAVM models
Emerging evidence suggests the RAS-MAPK signaling cascade is important in sporadic bAVMs and non-CNS AVMs.41,45–47 The somatic, de novo activation mutations in KRAS/BRAF and MAP2K1/MEK were shown to activate the MAPK-ERK signaling pathway in AVM endothelial cells, leading to increased angiogenesis, cell migration and proliferation.41,45–47 Robust MAPK/ERK activity were detected in all bAVM tissues, including those without detectable KRAS mutations, suggesting that this pathway plays a key role in AVM pathogenesis. 41
Endothelial KRAS activating mutations cause conformational changes in KRAS and render it constitutively active by preventing GTP hydrolysis. 83 BRAF is the downstream effector of KRAS, which is recruited to the cell membrane following KRAS activation. As a serine/threonine kinase, RAF activation phosphorylates MAPK (a.k.a. MEK, mitogen-activated protein kinase kinase 1), which phosphorylates and activates downstream ERK1 and ERK2. ERK1 and ERK2 further activate and phosphorylate a variety of nuclear transcription factors and kinases, resulting in a large number of KRAS-induced cellular responses. Expression of KRASG12V in endothelial cells in vitro stimulated ERK activity, and activated specific genes involved in angiogenesis and Notch signaling. These effects of KRASG12V were reversed by inhibition of MAPK-ERK signaling using MEK inhibitor (U0126). 41
Mouse and zebrafish models that mimic sporadic bAVM features have been recently generated through somatic endothelial cell-specific gain of function mutation in KRAS.84,85 Using both postnatal and adult mice, Fish et al. demonstrated that endothelial cell-specific gain of function mutations in KRAS (G12D or G12V) are sufficient to induce bAVMs, 84 even in the setting of uninjured adult vasculature. Using a brain endothelial cell-specific AAV vector, AAV-BR1, mediated brain endothelial cell gene transfer, Park et al. confirmed that KRAS mutations promote bAVM development via the MEK/ERK pathway. 85 In addition, using the embryonic zebrafish model, Fish et al. demonstrated that activation of MEK but not PI3K signaling is required for KRAS-mediated AVM progression. 84 Similarly, Park et al showed that inhibition of MEK/ERK by trametinib treatment attenuated KRASG12V-induced bAVM growth in mice. 85
There are two case reports of off-label use of the MEK-inhibitor trametinib in patients with KRAS-positive chest wall AVMs, one of which demonstrated significant reduction in the cardiac output fraction to the lesion after 6 months of treatment.86,87 Together, these animal and human studies indicate that MEK inhibition is a promising therapy for the treatment of bAVMs and should be evaluated in future studies.
Notch signaling and bAVM models
Aberrant activation of Notch signaling is involved in the etiology of bAVMs.88,89 Canonical Notch signaling controls cell fate decisions in various developmental processes. This pathway is an intercellular signaling pathway, where both receptor and ligand are membrane-bound on adjacent juxtaposed cells. There are four transmembrane Notch receptors (1-4) and five membrane-bound ligands (Jagged 1, 2, D-like ligand 1, 3, and 4). Notch ligand-receptor interaction is followed by proteolytic cleavage to release the intracellular domain of the receptor, which is subsequently trafficked to the nucleus to mediate the transcription of Notch target genes.
Endothelial cell expression of a constitutively active Notch-4 allele in adult mice caused vascular defects in the liver, uterus, and skin, but not in brain. 88 The defective vessels were reversed upon repression of Notch-4 expression. Similarly, endothelial cell expression of a constitutively active Notch-1 resulted in similar hepatic vascular lesions. These findings provide the first evidence that Notch signaling in adult endothelium is sufficient to render the development of AVMs. 88 Expression of constitutive Notch-4 or Notch-1 in neonatal mice recapitulated the phenotypes of human bAVMs. 88 Blockage of Notch signaling through deletion of Rbpj in endothelial cells of postnatal mice also caused features of bAVMs. 90 Arteriovenous shunts showed decreased Efnb2 (arterial marker) and increased Ephb4 (venous marker) expression.
Previous studies have shown that arteriovenous shunts were observed in both mouse and zebrafish models carrying mutants of genes in the Notch pathway, which prompted the investigation of the Notch pathway in AVM pathogenesis.81,91 In Alk1 KO mouse models, decreased Notch signaling was found in AVMs. 91 Alk1 signaling inhibits angiogenesis by cooperating with the Notch pathway. In addition, combined blockade of Alk1 and Notch signaling exacerbated hypervascularization, and activation of Alk1 by its high-affinity ligand Bmp9 rescued the hyper-sprouting induced by Notch inhibition. 91 These findings demonstrate a direct crosstalk between ALK1 and NOTCH pathways during vascular morphogenesis that may be relevant to the pathogenesis of HHT.
Notch signaling also plays a very important role in regulating mural cell differentiation and function. There are 2 types of mural cells: pericytes and vascular smooth muscle cells (vSMCs). Pericytes and vSMCs both express Notch 1, 2, and 3. Notch signaling has been found to modulate vSMC differentiation, survival, and vasculature. In vitro studies demonstrated that Notch signaling is essential for pericyte survival and adhesion to endothelial cells. In vivo studies using mouse and zebrafish models found that Notch-3 signaling promoted pericyte proliferation and limited vascular permeability. 92 Deletion of Rbpj in pericytes resulted in reduced pericyte coverage and induced AVM development. 92 Moreover, the loss of Notch signaling in pericytes downregulated Pdgfrb levels and increased pericyte apoptosis, indicating a critical role for Notch in pericyte survival. 93
Proteins involved in Notch signaling, including the receptor, its ligands, and downstream signals, are expressed in bAVM tissue.89,94 Therefore, the role of Notch in bAVM pathogenesis merits further exploration.
Other pathways and AVM models
Matrix GLA protein (MGP)
MGP, an antagonist of BMPs, is expressed in endothelial cells and plays an essential role in endothelial cell function by affecting BMP, TGFβ and VEGF signaling. 95 BMP-SMAD signaling increases the expression of ALK1, which in turn induces the expression of MGP and further sequesters BMP, thereby forming a negative feedback loop. 96 Deletion of Mgp (Mgp−/−) in mice induces Notch signaling by enhancing expression of Notch ligands, Jagged 1 and Jagged 2, dysregulates endothelial cell differentiation, and causes bAVM development. 97 Crossing Mgp−/− mice with Jagged deficient mice diminished Notch activity, normalized endothelial cell differentiation, and prevented bAVMs, but not pulmonary or renal AVMs. 97 These findings suggest that endothelial cell-Rbpj is required at postnatal stage for maintaining of vascular integrity and preventing arteriovenous shunt and AVM development.
PDGFB/PDGFRβ signaling
Accumulating data demonstrate that the abnormal vascular remodeling and vascular instability are associated with bAVM development and progression, including dilated perinidal capillaries, 98 intranidal or feeding artery aneurysms, 99 and microhemorrhage and rupture. 100 However, the exact mechanisms underlying bAVM hemorrhage remain unclear. Abnormal expression of PDGFB and PDGFRβ has been described in bAVMs in humans and rodents.101–103 Pdgfrβ expression was reduced in the bAVM lesions of Alk1 iKO mice, which was associated with a reduction of mural cell coverage, suggesting a possible crosstalk between ALK1 and PDGFB/PDGFRβ signaling pathways. 103 Both pericyte number and coverage are reduced in resected tissue from sporadic bAVM patients. 101 Importantly, pericyte reductions are greatest in bAVMs with clinical hemorrhage and are associated with a higher microhemorrhage burden in unruptured cases, suggesting that reduction of pericytes contributes to bAVM hemorrhage. 101
PDGFRβ is expressed in multiple cell types, including pericytes, vSMCs, and neurons. 104 Its ligand, PDGFB is secreted from the endothelial cells of angiogenic sprouts where it works as an attractant for recruiting pericytes. PDGFB and PDGFRβ are key elements in regulating pericyte recruitment and maintaining vascular integrity and stabilization. 105
Thalidomide treatment was shown to increase PDGFB expression in endothelial cells and induce vessel maturation by increasing mural cell coverage. 106 Thalidomide belongs to a group of drugs known as immunomodulatory drugs (IMIDs), which works to modulate the immune system. Neurotoxic adverse effects of thalidomide promoted the discovery of newer derivatives, e.g. lenalidomide, which demonstrates effectiveness in treating multiple myeloma and myelodysplastic syndrome. 107 The IMIDs have been shown to inhibit endothelial cell proliferation and migration. 108 The anti-angiogenic mechanism of thalidomide remains elusive, but it has demonstrated clinical benefits in treating gastrointestinal hemorrhage and epistaxis in HHT patients.106,109 Thalidomide or lenalidomide treatment also reduced hemorrhage, attenuated dysplastic vessel formation, and improved vascular smooth muscle cell coverage in mouse bAVM lesions. 110 In addition, lentiviral vector mediated overexpression of Pdgfb in mouse brain has also reduced bAVM severity in Alk1 iKO bAVM mouse model. 110 These data demonstrate that PDGFB/PDGFRβ pathway can be a target for developing new therapies to reduce AVM hemorrhage.
Ephrinb2/EphB4 signaling
Elevated expression of EphB4 and ephrinB2 were detected in patients with AVMs.111,112 Among the 14 Eph receptors and 8 Ephrin, EphrinB2/EphB4 are the first ones discovered to be differentially expressed in arterial and venous endothelial cells. EphrinB2/EphB4 signaling has been implicated in the regulation of multiple vascular events, including sprouting angiogenesis, vascular morphogenesis, arteriovenous differentiation and vascular homeostasis. 113
EphrinB2 is expressed in endothelial cells and their surrounding mesenchymal and mural cells, while EphB4 is specifically expressed in endothelial cells. 114 EphrinB2 and EphB4 have been deemed as the primary molecular markers for endothelial cell arteriovenous specification. Accumulating evidence suggest that EphrinB2-EphB4 signaling plays an very important role in AVMs and other cerebrovascular disorders. 111 Embryos harboring homozygous mutations in Efnb2 and Ephb4 exhibited vascular defects and AVMs. 115 An in vitro model of HHT2 showed that loss of Alk1 gene blocked Bmp9 signaling, resulting in reduced EphrinB2 expression, enhanced Vegfr2 expression, and dysregulated endothelial cell sprouting and anastomosis. 116 In addition, EphrinB2 is a crucial regulator of Pdgfrβ expression in vSMCs, and thereby acts as a molecular switch controlling the downstream signaling activity induced by PDGFB/PDGFRβ. mTORC1 overactivition was observed in both morpholino-treated zebrafish and cultured HEK293T cells with EphB4 knocked in. 117 The zebrafish phenotype could be rescued by inhibiting mTOR or RAS-MAPK signaling. EphrinB2 ablation enhanced Pdgfb-induced Mapk and Jnk activation and diminished Tiam1/Rac1 signaling, a pathway critical for cell migration, proliferation, and spreading. 118
RASA1 is a direct downstream effector of EPHB4. Knockdown of Ephb4 and Rasa1 in zebrafish shared a similar phenotype of vascular deformities and caudal vascular plexus malformation. 32 Rasa1 KO mice are embryonic lethal and exhibited several blood vessel abnormalities, suggesting that RASA1 is essential in vasculogenesis. 119 Knock-in Rasa1 lacking the arginine finger, which is required for its interaction with Ras, resulted in embryonic lethality and several vascular abnormalities similar to Rasa1 KO mice. These findings suggest that dysfunction of Ras-Mapk and EphrinB2/EphB4 pathways work synergistically in the context of vascular development. Phenotypes induced by knockdown of Ephb4a or Rasa1 can be rescued by chemical inhibitors of PI3K/mTORC1.
Altogether, these data demonstrate that the ephrinB2-ephB4-RASA1 signaling axis is essential for development of the vascular system. Inhibition of PI3K/mTORC1 could be a therapeutic target for the treatment of vascular malformation induced by RASA1 or EPHB4 mutation.
Additional therapeutic targets not related to specific genetic alteration
Anti-angiogenesis
Excessive expression of VEGF was detected in both HHT and sporadic bAVMs, and angiogenesis is necessary to induce bAVM development in adult mice.77,120 Compelling evidence supports that inhibitors of VEGF signaling can block angiogenesis and reduce AVM severity in HHT mouse models. Intraperitoneal bevacizumab injection reduced the number of malformed vessels in bAVM model of an Alk1 iKO mouse. 121 Several VEGF inhibitors have demonstrated clinical efficacy in patients with cancer or ocular vascular disease.122,123 Of these, a humanized anti-VEGF monoclonal antibody (bevacizumab; Avastin), approved by the FDA for treatment of several cancers, showed promise in treating HHT patients. It normalized cardiac output and improved anemia in HHT patients with severe liver failure and/or refractory anemia. 124 It also demonstrated clinical efficacy and safety in the treatment of severe epistaxis caused by hemorrhage from small mucosal AVMs (telangiectasias). 125 There have been several case reports of off label use of bevacizumab in treating sporadic bAVMs.126,127 No serious adverse events have been noted, though bAVMs did not change in size during the study interval.126,127 However, two reports using bevacizumab to treat adverse radiation effects demonstrated a reduction in perilesional edema by imaging and marked improvement in clinical symptoms.126,127
Despite the clinical benefits of bevacizumab, this antibody-based therapy has several drawbacks, including hemorrhage 128 and frequent dosing over an extended period of time. The use of AAV-mediated expression of soluble FMS-related tyrosine kinase 1 (sFLT1), the extracellular domain of VEGFR-1, is a promising alternative to the bevacizumab for the treatment of bAVM. sFLT1 is capable of binding VEGF in tissues, preventing its binding to VEGFRs and thus inhibits VEGF-mediated angiogenesis. Intravenous injection of AAV9-sFlt1 reduced bAVM development and bAVM severity in two Eng iKO mouse models. 129
Anti-inflammation
AVMs in humans and animal models are associated with increased inflammation and overexpressed inflammatory markers, e.g., MPO, IL-6, and MMP-9.103,130 Tetracyclines are clinically available non-specific MMP inhibitors. They can potentially increase vascular stability and reduce the risk of spontanous hemorrhage in a variety of human diseases, including ICH 131 and traumatic brain injury. 132 Doxycycline has been shown to reduce MMP level in bAVMs. 133 Animal studies show that doxycycline is also effective in reducing bleeding risk in bAVMs133,134 via MMP-9 inhibition. 135 Doxycycline or minocycline has been used in a small pilot study of bAVM patients, with no serious adverse effects noted for up to 2 years. 136 However, there is also no significant evidence of clinical efficacy or hemorrhagic risk reduction in patients.
Bone marrow (BM)/monocyte transfusion
BM-derived cells participate in VEGF-stimulated brain angiogenesis. 137 BM-derived MMP-9 plays an important role in bone marrow cell mobilization and VEGF-induced brain angiogenesis. 137 Transplatation of Eng+/− mouse bone marrow to WT mice resulted in similar degree of capillary dysplasia in the brain angiogenesis in the brain angiogenic region of Eng+/− mice. Transplatation of WT bone marrow to Eng+/− mice reduced the severity of vascular dysplasia. 138 Another study found that injection of normal human monocytes can rescue the defective vessel formation and heart function in Eng+/− mice. 139 Together, these data suggest that bone marrow has a crucial role in blood cell-mediated vascular repair and bone marrow/monocyte transfusion could be used as an potential therapy to reduce bAVM severity.
In summary, the most devastating symptom of bAVM patients is ICH. The treatment for bAVM should aim to stabilize vascular tissue thereby decreasing the risk of spontaneous ICH. Risk factors for hemorrhage of bAVM include elevation of VEGF, loss of vessel wall integrity, and alteration in hemodynamics. 105 Several therapeutic options identified through pathway studies can be explored to strengthen vessel wall and reduce the possibility of ICH, such as increase PDGFB level through thalidomide or lenalidomide treatment or overexpression of PDGFB and anti-angiogenesis through Bevacizumab treatment or overexpression of sFLT1. In addition, anti-inflammation can also stabilize vessel wall and reduce ICH of bAVM patients (Figure 2).
Future prospects
In summary, much progress has been made in understanding sporadic bAVM pathogenesis. The recent discovery of somatic gene mutations in the RAS/MAPK/ERK signaling pathway in bAVMs suggest a common pathway with peripheral AVMs, and have added to the growing number of relevant signaling pathways involved in bAVM. Novel animal models have been developed to elucidate the molecular mechanisms involved and have identified several potential therapeutic targets. Due to the size limitations, AVM models in rodents cannot be used for many preclinical tests, such as for development of novel endovascular treatment. Large animal AVM models generated by creating carotid-jugular fistula, using species exhibiting rete mirabile or autologous implants feature various conceptual advantages in translational research. 140 However, the carotid-jugular fistula, rete mirabile or autologous implants are not true brain AVM. With the advantage of molecular tools, it is possible to induce AVM development in the brain parenchymal in large animals in the future.
Current therapeutic strategies for bAVM are to remove or reduce the risk of hemorrhage with interventional treatment. Likewise potential medical therapies targeting relevant signaling pathways highlighted in this review also aim to stabilize vascular tissue thereby decreasing the risk of spontaneous ICH. Future studies should focus on validating existing targets in animal models, and moving towards clinical trials in AVM patients.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the National Institutes of Health to H.S. (R01 HL122774, NS027713 and NS112819), to H.K. (R01 NS034949, NS099268), and from the Michael Ryan Zodda Foundation and the Leslie Munzer Neurovascular Research Fund.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: HK and HS: participated in conception and design of the review; PP, SW, DC, AA and EW participated in writing, HK and HS finalized the manuscript.
ORCID iD: Hua Su https://orcid.org/0000-0003-1566-9877
References
- 1.Mohr JP, Parides MK, Stapf C, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (Aruba): a multicentre, non-blinded, randomised trial. Lancet 2014; 383: 614–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Beijnum J, van der Worp HB, Buis DR, et al. Treatment of brain arteriovenous malformations: a systematic review and meta-analysis. Jama 2011; 306: 2011–2019. [DOI] [PubMed] [Google Scholar]
- 3.Kim H, Al-Shahi Salman R, McCulloch CE, et al. Untreated brain arteriovenous malformation: patient-level meta-analysis of hemorrhage predictors. Neurology 2014; 83: 590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, Ye X, Gao X, et al. The diagnosis of arteriovenous malformations by 4D-CTA: a clinical study. J Neuroradiol 2014; 41: 117–123. [DOI] [PubMed] [Google Scholar]
- 5.Hung YC, Mohammed N, Eluvathingal Muttikkal TJ, et al. The impact of preradiosurgery embolization on intracranial arteriovenous malformations: a matched cohort analysis based on de novo lesion volume. J Neurosurg 2019; 30; 1–12. [DOI] [PubMed]
- 6.Li CQ, Hsiao A, Hattangadi-Gluth J, et al. Early hemodynamic response assessment of stereotactic radiosurgery for a cerebral arteriovenous malformation using 4D flow MRI. AJNR Am J Neuroradiol 2018; 39: 678–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narsinh KH, Mueller K, Nelson J, et al. Interrater reliability in the measurement of flow characteristics on color-coded quantitative DSA of brain AVMs. AJNR Am J Neuroradiol 2020; 41: 2303–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong H, Jin N, Kannengiesser S, et al. Magnetic resonance elastography for estimating in vivo stiffness of the abdominal aorta using cardiac-gated spin-echo echo-planar imaging: a feasibility study. NMR Biomed 2021; 34: e4420. [DOI] [PubMed] [Google Scholar]
- 9.Stapf C, Mast H, Sciacca RR, et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology 2006; 66: 1350–1355. [DOI] [PubMed] [Google Scholar]
- 10.Volovici V, Schouten JW, Vajkoczy P, et al. Unruptured arteriovenous malformations: do we have an answer after the final follow-up of Aruba? A Bayesian viewpoint. Stroke 2021; 52: 1143–1146. [DOI] [PubMed] [Google Scholar]
- 11.Feghali J, Yang WY, Xu RS, et al. R(2)eD AVM score a novel predictive tool for arteriovenous malformation presentation with hemorrhage. Stroke 2019; 50: 1703–1710.31167618 [Google Scholar]
- 12.Garzelli L, Shotar E, Blauwblomme T, et al. Risk factors for early brain AVM rupture: cohort study of pediatric and adult patients. AJNR Am J Neuroradiol 2020; 41: 2358–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg 1986; 65: 476–483. [DOI] [PubMed] [Google Scholar]
- 14.Lawton MT, Kim H, McCulloch CE, et al. A supplementary grading scale for selecting patients with brain arteriovenous malformations for surgery. Neurosurgery 2010; 66: 702–713; discussion 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winkler EA, Lu A, Morshed RA, et al. Bringing high-grade arteriovenous malformations under control: clinical outcomes following multimodality treatment in children. J Neurosurg Pediatr 2020; : 1–10. 2020/04/11. DOI: 10.3171/2020.1.PEDS19487. [DOI] [PubMed] [Google Scholar]
- 16.Burkhardt JK, Lasker GF, Winkler EA, et al. Microsurgical resection of brain arteriovenous malformations in the elderly: outcomes analysis and risk stratification. J Neurosurg 2018; 129: 1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raper DMS, Winkler EA, Rutledge WC, et al. An update on medications for brain arteriovenous malformations. Neurosurgery 2020; 87: 871–878. [DOI] [PubMed] [Google Scholar]
- 18.Copelan A, Drocton G, Caton MT, et al. Brain arteriovenous malformation recurrence after apparent microsurgical cure: increased risk in children who present with arteriovenous malformation rupture. Stroke 2020; 51: 2990–2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorenson TJ, Brinjikji W, Bortolotti C, et al. Recurrent brain arteriovenous malformations (AVMs): a systematic review. World Neurosurg 2018; 116: e856–e866. [DOI] [PubMed] [Google Scholar]
- 20.Ilyas A, Chen CJ, Ding D, et al. Radiation-Induced changes after stereotactic radiosurgery for brain arteriovenous malformations: a systematic review and meta-analysis. Neurosurgery 2018; 83: 365–376. [DOI] [PubMed] [Google Scholar]
- 21.Seymour ZA, Sneed PK, Gupta N, et al. Volume-staged radiosurgery for large arteriovenous malformations: an evolving paradigm. J Neurosurg 2016; 124: 163–174. [DOI] [PubMed] [Google Scholar]
- 22.See AP, Mohammaden MH, Rizko M, et al. Morbidity and mortality associated with sequential flow reduction embolization technique of cerebral arteriovenous malformations using n-butyl cyanoacrylate. J NeuroIntervent Surg 2021; 13: 237–241. [DOI] [PubMed] [Google Scholar]
- 23.Donzelli GF, Nelson J, McCoy D, et al. The effect of preoperative embolization and flow dynamics on resection of brain arteriovenous malformations. J Neurosurg 2019; 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Catapano JS, Frisoli FA, Nguyen CL, et al. Spetzler-Martin grade III arteriovenous malformations: a multicenter propensity-adjusted analysis of the effects of preoperative embolization. Neurosurgery 2021; 88(5): 996–1002. DOI: 10.1093/neuros/nyaa551. [DOI] [PMC free article] [PubMed]
- 25.Chen CJ, Ding D, Lee CC, et al. Stereotactic radiosurgery with versus without prior onyx embolization for brain arteriovenous malformations. J Neurosurg 2020; 1–9. [DOI] [PMC free article] [PubMed]
- 26.Wu EM, El Ahmadieh TY, McDougall CM, et al. Embolization of brain arteriovenous malformations with intent to cure: a systematic review. J Neurosurg 2019; 1–12. [DOI] [PubMed]
- 27.Baharvahdat H, Blanc R, Fahed R, et al. Endovascular treatment for low-grade (Spetzler-Martin I-II) brain arteriovenous malformations. AJNR Am J Neuroradiol 2019; 40: 668–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Sousa JMB, Iosif C, Sganzerla LZ, et al. Selection of patients for treatment of brain arteriovenous malformations by the transvenous approach: relationship with venous anatomy and risk of hemorrhagic complications. AJNR Am J Neuroradiol 2020; 41: 2311–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koyanagi M, Mosimann PJ, Nordmeyer H, et al. The transvenous retrograde pressure cooker technique for the curative embolization of high-grade brain arteriovenous malformations. J Neurointerv Surg 2020. [DOI] [PubMed] [Google Scholar]
- 30.Fang YB, Byun JS, Liu JM, et al. Transvenous embolization of brain arteriovenous malformations: a systematic review and meta-analysis. J Neurosurg Sci 2019; 63: 468–472. [DOI] [PubMed] [Google Scholar]
- 31.Wooderchak-Donahue WL, McDonald J, O'Fallon B, et al. BMP9 mutations cause a vascular-anomaly syndrome with phenotypic overlap with hereditary hemorrhagic telangiectasia. Am J Hum Genet 2013; 93: 530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawasaki J, Aegerter S, Fevurly RD, et al. RASA1 functions in EPHB4 signaling pathway to suppress endothelial mTORC1 activity. J Clin Invest 2014; 124: 2774–2784. 2014/05/20. DOI: 10.1172/JCI67084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng X, Hunt A, Jin SC, et al. EphrinB2-EphB4-RASA1 signaling in human cerebrovascular development and disease. Trends Mol Med 2019; 25: 265–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duran D, Zeng X, Jin SC, et al. Mutations in chromatin modifier and ephrin signaling genes in vein of galen malformation. Neuron 2019; 101: 429–443 e424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walcott BP, Winkler EA, Zhou S, et al. Identification of a rare BMP pathway mutation in a non-syndromic human brain arteriovenous malformation via exome sequencing. Hum Genome Var 2018; 5: 18001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scimone C, Granata F, Longo M, et al. Germline mutation enrichment in pathways controlling endothelial cell homeostasis in patients with brain arteriovenous malformation: Implication for molecular diagnosis. Int J Mol Sci 2020; 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scimone C, Donato L, Alafaci C, et al. High-throughput sequencing to detect novel likely gene-disrupting variants in pathogenesis of sporadic brain arteriovenous malformations. Front Genet 2020; 11: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang K, Zhao S, Liu B, et al. Perturbations of BMP/TGF-beta and VEGF/VEGFR signalling pathways in non-syndromic sporadic brain arteriovenous malformations (BAVM). J Med Genet 2018; 55: 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang M, Ding X, Zhang Q, et al. Exome sequencing of 112 trios identifies recessive genetic variants in brain arteriovenous malformations. J Neurointerv Surg 2020. [DOI] [PubMed] [Google Scholar]
- 40.Wu L, Du Y, Lok J, et al. Lipocalin-2 enhances angiogenesis in rat brain endothelial cells via reactive oxygen species and iron-dependent mechanisms. J Neurochem 2015; 132: 622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nikolaev SI, Vetiska S, Bonilla X, et al. Somatic activating KRAS mutations in arteriovenous malformations of the brain. N Engl J Med 2018; 378: 250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Priemer DS, Vortmeyer AO, Zhang S, et al. Activating KRAS mutations in arteriovenous malformations of the brain: frequency and clinicopathologic correlation. Hum Pathol 2019; 89: 33–39. [DOI] [PubMed] [Google Scholar]
- 43.Goss JA, Huang AY, Smith E, et al. Somatic mutations in intracranial arteriovenous malformations. PLoS One 2019; 14: e0226852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bameri O, Salarzaei M, Parooie F. KRAS/BRAF mutations in brain arteriovenous malformations: a systematic review and meta-analysis. Interv Neuroradiol. Epub ahead of print 7. January 2021. DOI: 10.1177/1591019920982810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hong T, Yan Y, Li J, et al. High prevalence of KRAS/BRAF somatic mutations in brain and spinal cord arteriovenous malformations. Brain 2019; 142: 23–34. [DOI] [PubMed] [Google Scholar]
- 46.Couto JA, Huang AY, Konczyk DJ, et al. Somatic MAP2K1 mutations are associated with extracranial arteriovenous malformation. Am J Hum Genet 2017; 100: 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al-Olabi L, Polubothu S, Dowsett K, et al. Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy. J Clin Invest 2018; 128: 1496–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao S, Nj Weinsheimer S, Winkler EA, Rutledge C, et al. Somatic mosaicism in the mapk pathway in sporadic brain arteriovenous malformation and association with phenotype. J Neurosurg 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Revencu N, Fastre E, Ravoet M, et al. RASA1 mosaic mutations in patients with capillary malformation-arteriovenous malformation. J Med Genet. Epub ahead of print 14 July 2019. DOI: 10.1136/jmedgenet-2019-106024. [DOI] [PubMed]
- 50.Snellings DA, Gallione CJ, Clark DS, et al. Somatic mutations in vascular malformations of hereditary hemorrhagic telangiectasia result in bi-allelic loss of ENG or ACVRL1. Am J Hum Genet 2019; 105: 894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cooke DL, McCoy DB, Halbach VV, et al. Endovascular biopsy: in vivo cerebral aneurysm endothelial cell sampling and gene expression analysis. Transl Stroke Res 2018; 9: 20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maria Palmieri AC, Tommasi A, Sarno LD, et al. Cell-free DNA next-generation sequencing liquid biopsy as a new revolutionary approach for arteriovenous malformation. JVS: Vascular Science 2020; 1: 176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weinsheimer SM, Xu H, Achrol AS, et al. Gene expression profiling of blood in brain arteriovenous malformation patients. Transl Stroke Res 2011; 2: 575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang J, Song J, Qu M, et al. MicroRNA-137 and microRNA-195* inhibit vasculogenesis in brain arteriovenous malformations. Ann Neurol 2017; 82: 371–384. [DOI] [PubMed] [Google Scholar]
- 55.Marin-Ramos NI, Thein TZ, Ghaghada KB, et al. miR-18a inhibits BMP4 and HIF-1alpha normalizing brain arteriovenous malformations. Circ Res 2020; 127: e210–e231. [DOI] [PubMed] [Google Scholar]
- 56.Chen Y, Li Z, Shi Y, et al. Deep sequencing of small RNAs in blood of patients with brain arteriovenous malformations. World Neurosurg 2018; 115: e570–e579. [DOI] [PubMed] [Google Scholar]
- 57.Jiang X, Wooderchak-Donahue WL, McDonald J, et al. Inactivating mutations in Drosha mediate vascular abnormalities similar to hereditary hemorrhagic telangiectasia. Sci Signal 2018; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hauer AJ, Kleinloog R, Giuliani F, et al. RNA-sequencing highlights inflammation and impaired integrity of the vascular wall in brain arteriovenous malformations. Stroke 2020; 51: 268–274. [DOI] [PubMed] [Google Scholar]
- 59.Hashimoto T, Lawton MT, Wen G, et al. Gene microarray analysis of human brain arteriovenous malformations. Neurosurgery 2004; 54: 410–423; discussion 415–423. [DOI] [PubMed] [Google Scholar]
- 60.Shenkar R, Elliott JP, Diener K, et al. Differential gene expression in human cerebrovascular malformations. Neurosurgery 2003; 52: 465–477; discussion 468–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takagi Y, Aoki T, Takahashi JC, et al. Differential gene expression in relation to the clinical characteristics of human brain arteriovenous malformations. Neurol Med Chir (Tokyo) 2014; 54: 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen X, Liu Y, Zhou S, et al. Methylation of the CDKN2A gene increases the risk of brain arteriovenous malformations. J Mol Neurosci 2019; 69: 316–323. [DOI] [PubMed] [Google Scholar]
- 63.Bendjilali N, Nelson J, Weinsheimer S, et al. Common variants on 9p21.3 are associated with brain arteriovenous malformations with accompanying arterial aneurysms. J Neurol Neurosurg Psychiatry 2014; 85: 1280–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang LJ, Xue Y, Huo R, et al. N6-methyladenosine methyltransferase METTL3 affects the phenotype of cerebral arteriovenous malformation via modulating notch signaling pathway. J Biomed Sci 2020; 27: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weinsheimer S, Kim H, Pawlikowska L, et al. EPHB4 gene polymorphisms and risk of intracranial hemorrhage in patients with brain arteriovenous malformations. Circ Cardiovasc Genet 2009; 2: 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim H, Hysi PG, Pawlikowska L, et al. Common variants in interleukin-1-beta gene are associated with intracranial hemorrhage and susceptibility to brain arteriovenous malformation. Cerebrovasc Dis 2009; 27: 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Achrol AS, Kim H, Pawlikowska L, et al. Association of tumor necrosis factor-alpha-238G>a and apolipoprotein E2 polymorphisms with intracranial hemorrhage after brain arteriovenous malformation treatment. Neurosurgery 2007; 61: 731–739; discussion 740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mouchtouris N, Jabbour PM, Starke RM, et al. Biology of cerebral arteriovenous malformations with a focus on inflammation. J Cereb Blood Flow Metab 2015; 35: 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.You C, Zhao K, Dammann P, et al. EphB4 forward signalling mediates angiogenesis caused by CCM3/PDCD10-ablation. J Cell Mol Med 2017; 21: 1848–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lebrin F, Deckers M, Bertolino P, et al. TGF-beta receptor function in the endothelium. Cardiovasc Res 2005; 65: 599–608. [DOI] [PubMed] [Google Scholar]
- 71.Chen Y, Hao Q, Kim H, et al. Soluble endoglin modulates aberrant cerebral vascular remodeling. Ann Neurol 2009; 66: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Castonguay R, Werner ED, Matthews RG, et al. Soluble endoglin specifically binds bone morphogenetic proteins 9 and 10 via its orphan domain, inhibits blood vessel formation, and suppresses tumor growth. J Biol Chem 2011; 286: 30034–30046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arthur HM, Ure J, Smith AJ, et al. Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev Biol 2000; 217: 42–53. [DOI] [PubMed] [Google Scholar]
- 74.Bourdeau A, Dumont DJ, Letarte M. A murine model of hereditary hemorrhagic telangiectasia. J Clin Invest 1999; 104: 1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Srinivasan S, Hanes MA, Dickens T, et al. A mouse model for hereditary hemorrhagic telangiectasia (HHT) type 2. Hum Mol Genet 2003; 12: 473–482. [DOI] [PubMed] [Google Scholar]
- 76.Walcott BP. BMP signaling modulation attenuates cerebral arteriovenous malformation formation in a vertebrate model. J Cereb Blood Flow Metab 2014; 34: 1688–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Walker EJ, Su H, Shen F, et al. Arteriovenous malformation in the adult mouse brain resembling the human disease. Ann Neurol 2011; 69: 954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen W, Sun Z, Han Z, et al. De novo cerebrovascular malformation in the adult mouse after endothelial Alk1 deletion and angiogenic stimulation. Stroke 2014; 45: 900–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Choi EJ, Chen W, Jun K, et al. Novel brain arteriovenous malformation mouse models for type 1 hereditary hemorrhagic telangiectasia. PLoS One 2014; 9: e88511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park SO, Wankhede M, Lee YJ, et al. Real-time imaging of de novo arteriovenous malformation in a mouse model of hereditary hemorrhagic telangiectasia. J Clin Invest 2009; 119: 3487–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim YH, Phuong NV, Choe SW, et al. Overexpression of activin receptor-like kinase 1 in endothelial cells suppresses development of arteriovenous malformations in mouse models of hereditary hemorrhagic telangiectasia. Circ Res. Epub ahead of print 9 August 2020. DOI: 10.1161/CIRCRESAHA.119.316267. [DOI] [PMC free article] [PubMed]
- 82.Milton I, Ouyang D, Allen CJ, et al. Age-dependent lethality in novel transgenic mouse models of central nervous system arteriovenous malformations. Stroke 2012; 43: 1432–1435. [DOI] [PubMed] [Google Scholar]
- 83.Serebriiskii IG, Connelly C, Frampton G, et al. Comprehensive characterization of RAS mutations in colon and rectal cancers in old and young patients. Nat Commun 2019; 10: 3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fish JE, Flores Suarez CP, Boudreau E, et al. Somatic gain of KRAS function in the endothelium is sufficient to cause vascular malformations that require MEK but not PI3K signaling. Circ Res 2020; 127: 727–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park ES, Kim S, Huang S, et al. Selective endothelial hyperactivation of oncogenic KRAS induces brain arteriovenous malformations in mice. Ann Neurol 2021; 89: 926–941. [DOI] [PubMed] [Google Scholar]
- 86.Edwards EA, Phelps AS, Cooke D, et al. Monitoring arteriovenous malformation response to genotype-targeted therapy. Pediatrics 2020; 146: e20193206. [DOI] [PubMed] [Google Scholar]
- 87.Lekwuttikarn R, Lim YH, Admani S, et al. Genotype-Guided medical treatment of an arteriovenous malformation in a child. JAMA Dermatol 2019; 155: 256–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carlson TR, Yan Y, Wu X, et al. Endothelial expression of constitutively active Notch4 elicits reversible arteriovenous malformations in adult mice. Proc Natl Acad Sci U S A 2005; 102: 9884–9889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.ZhuGe Q, Zhong M, Zheng W, et al. Notch-1 signalling is activated in brain arteriovenous malformations in humans. Brain 2009; 132: 3231–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nielsen CM, Cuervo H, Ding VW, et al. Deletion of Rbpj from postnatal endothelium leads to abnormal arteriovenous shunting in mice. Development 2014; 141: 3782–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Larrivee B, Prahst C, Gordon E, et al. ALK1 signaling inhibits angiogenesis by cooperating with the notch pathway. Dev Cell 2012; 22: 489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nadeem T, Bogue W, Bigit B, et al. Deficiency of notch signaling in pericytes results in arteriovenous malformations. JCI Insight 2020; 5(21): e125940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang Y, Pan L, Moens CB, et al. Notch3 establishes brain vascular integrity by regulating pericyte number. Development 2014; 141: 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murphy PA, Lu G, Shiah S, et al. Endothelial notch signaling is upregulated in human brain arteriovenous malformations and a mouse model of the disease. Lab Invest 2009; 89: 971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bostrom K, Zebboudj AF, Yao Y, et al. Matrix GLA protein stimulates VEGF expression through increased transforming growth factor-beta1 activity in endothelial cells. J Biol Chem 2004; 279: 52904–52913. [DOI] [PubMed] [Google Scholar]
- 96.Yao Y, Jumabay M, Wang A, et al. Matrix GLA protein deficiency causes arteriovenous malformations in mice. J Clin Invest 2011; 121: 2993–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yao Y, Yao J, Radparvar M, et al. Reducing jagged 1 and 2 levels prevents cerebral arteriovenous malformations in matrix GLA protein deficiency. Proc Natl Acad Sci U S A 2013; 110: 19071–19076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tu J, Stoodley MA, Morgan MK, et al. Ultrastructure of perinidal capillaries in cerebral arteriovenous malformations. Neurosurgery 2006; 58: 961–970; discussion 961–970. [DOI] [PubMed] [Google Scholar]
- 99.Gross BA, Du R. Natural history of cerebral arteriovenous malformations: a meta-analysis. J Neurosurg 2013; 118: 437–443. [DOI] [PubMed] [Google Scholar]
- 100.Pekmezci M, Nelson J, Su H, et al. Morphometric characterization of brain arteriovenous malformations for clinical and radiological studies to identify silent intralesional microhemorrhages. Clin Neuropathol 2016; 35: 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Winkler EA, Birk H, Burkhardt JK, et al. Reductions in brain pericytes are associated with arteriovenous malformation vascular instability. J Neurosurg 2018; 129: 1464–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barbosa Do Prado L, Han C, Oh SP, et al. Recent advances in basic research for brain arteriovenous malformation. Int J Mol Sci 2019; 20: 5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen W, Guo Y, Walker EJ, et al. Reduced mural cell coverage and impaired vessel integrity after angiogenic stimulation in the Alk1-deficient brain. Arterioscler Thromb Vasc Biol 2013; 33: 305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ishii Y, Oya T, Zheng L, et al. Mouse brains deficient in neuronal PDGF receptor-beta develop normally but are vulnerable to injury. J Neurochem 2006; 98: 588–600. [DOI] [PubMed] [Google Scholar]
- 105.Shaligram SS, Winkler E, Cooke D, et al. Risk factors for hemorrhage of brain arteriovenous malformation. CNS Neurosci Ther 2019; 25: 1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lebrin F, Srun S, Raymond K, et al. Thalidomide stimulates vessel maturation and reduces epistaxis in individuals with hereditary hemorrhagic telangiectasia. Nat Med 2010; 16: 420–428. [DOI] [PubMed] [Google Scholar]
- 107.Quach H, Kalff A, Spencer A. Lenalidomide in multiple myeloma: current status and future potential. Am J Hematol 2012; 87: 1089–1095. [DOI] [PubMed] [Google Scholar]
- 108.Dredge K, Horsfall R, Robinson SP, et al. Orally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and Akt phosphorylation in vitro. Microvasc Res 2005; 69: 56–63. [DOI] [PubMed] [Google Scholar]
- 109.Faughnan ME, Gossage JR, Chakinala MM, et al. Pazopanib may reduce bleeding in hereditary hemorrhagic telangiectasia. Angiogenesis 2019; 22: 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhu W, Chen W, Zou D, et al. Thalidomide reduces hemorrhage of brain arteriovenous malformations in a mouse model. Stroke 2018; 49: 1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bai J, Wang YJ, Liu L, et al. Ephrin B2 and EphB4 selectively mark arterial and venous vessels in cerebral arteriovenous malformation. J Int Med Res 2014; 42: 405–415. [DOI] [PubMed] [Google Scholar]
- 112.Fehnel KP, Penn DL, Duggins-Warf M, et al. Dysregulation of the EphrinB2-EphB4 ratio in pediatric cerebral arteriovenous malformations is associated with endothelial cell dysfunction in vitro and functions as a novel noninvasive biomarker in patients. Exp Mol Med 2020; 52: 658–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Luxan G, Stewen J, Diaz N, et al. Endothelial EphB4 maintains vascular integrity and transport function in adult heart. Elife 2019; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gerety SS, Anderson DJ. Cardiovascular ephrinB2 function is essential for embryonic angiogenesis. Development 2002; 129: 1397–1410. [DOI] [PubMed] [Google Scholar]
- 115.Krebs LT, Starling C, Chervonsky AV, et al. Notch 1 activation in mice causes arteriovenous malformations phenocopied by EphrinB2 and EphB4 mutants. Genesis 2010; 48: 146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim JH, Peacock MR, George SC, et al. BMP9 induces EphrinB2 expression in endothelial cells through an Alk1-BMPRII/ActRII-ID1/ID3-dependent pathway: implications for hereditary hemorrhagic telangiectasia type II. Angiogenesis 2012; 15: 497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li D, Wenger TL, Seiler C, et al. Pathogenic variant in EPHB4 results in central conducting lymphatic anomaly. Hum Mol Genet 2018; 27: 3233–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nakayama A, Nakayama M, Turner CJ, et al. Ephrin-B2 controls PDGFRbeta internalization and signaling. Genes Dev 2013; 27: 2576–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Henkemeyer M, Rossi DJ, Holmyard DP, et al. Vascular system defects and neuronal apoptosis in mice lacking ras GTPase-activating protein. Nature 1995; 377: 695–701. [DOI] [PubMed] [Google Scholar]
- 120.Kim H, Su H, Weinsheimer S, et al. Brain arteriovenous malformation pathogenesis: a response-to-injury paradigm. Acta Neurochir Suppl 2011; 111: 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Walker EJ, Su H, Shen F, et al. Bevacizumab attenuates VEGF-induced angiogenesis and vascular malformations in the adult mouse brain. Stroke 2012; 43: 1925–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ferrara N, Damico L, Shams N, et al. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 2006; 26: 859–870. [DOI] [PubMed] [Google Scholar]
- 123.Ng EW, Shima DT, Calias P, et al. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov 2006; 5: 123–132. [DOI] [PubMed] [Google Scholar]
- 124.Vazquez C, Gonzalez ML, Ferraris A, et al. Bevacizumab for treating hereditary hemorrhagic telangiectasia patients with severe hepatic involvement or refractory anemia. PLoS One 2020; 15: e0228486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Al-Samkari H, Albitar HA, Olitsky SE, et al. Systemic bevacizumab for high-output cardiac failure in hereditary hemorrhagic telangiectasia: an international survey of HHT centers. Orphanet J Rare Dis 2019; 14: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Muster Nk R, Smith W, Su H, et al. Proof-of-concept one-armed open label trial of bevacizumab therapy for brain arteriovenous malformation. BMJ Neurol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Deibert CP, Ahluwalia MS, Sheehan JP, et al. Bevacizumab for refractory adverse radiation effects after stereotactic radiosurgery. J Neurooncol 2013; 115: 217–223. [DOI] [PubMed] [Google Scholar]
- 128.Tanvetyanon T, Murtagh R, Bepler G. Rupture of a cerebral arteriovenous malformation in a patient treated with bevacizumab. J Thorac Oncol 2009; 4: 268–269. [DOI] [PubMed] [Google Scholar]
- 129.Zhu W, Shen F, Mao L, et al. Soluble FLT1 gene therapy alleviates brain arteriovenous malformation severity. Stroke 2017; 48: 1420–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guo Y, Saunders T, Su H, et al. Silent intralesional microhemorrhage as a risk factor for brain arteriovenous malformation rupture. Stroke 2012; 43: 1240–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Power C, Henry S, Del Bigio MR, et al. Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Ann Neurol 2003; 53: 731–742. [DOI] [PubMed] [Google Scholar]
- 132.Sanchez-Mejia RO, Ona VO, Li M, et al. Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery 2001; 48: 1393–1399; discussion 1399–1401. [DOI] [PubMed] [Google Scholar]
- 133.Hashimoto T, Matsumoto MM, Li JF, et al. Suppression of MMP-9 by doxycycline in brain arteriovenous malformations. BMC Neurol 2005; 5: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee BC, Ahn SY, Doo HK, et al. Susceptibility for ischemic stroke in Korean population is associated with polymorphisms of the interleukin-1 receptor antagonist and tumor necrosis factor-alpha genes, but not the interleukin-1beta gene. Neurosci Lett 2004; 357: 33–36. [DOI] [PubMed] [Google Scholar]
- 135.Lee CZ, Xu B, Hashimoto T, et al. Doxycycline suppresses cerebral matrix metalloproteinase-9 and angiogenesis induced by focal hyperstimulation of vascular endothelial growth factor in a mouse model. Stroke 2004; 35: 1715–1719. [DOI] [PubMed] [Google Scholar]
- 136.Frenzel T, Lee CZ, Kim H, et al. Feasibility of minocycline and doxycycline use as potential vasculostatic therapy for brain vascular malformations: pilot study of adverse events and tolerance. Cerebrovasc Dis 2008; 25: 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hao Q, Su H, Palmer D, et al. Bone marrow-derived cells contribute to vascular endothelial growth factor-induced angiogenesis in the adult mouse brain by supplying matrix metalloproteinase-9. Stroke 2011; 42: 453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Choi EJ, Walker EJ, Degos V, et al. Endoglin deficiency in bone marrow is sufficient to cause cerebrovascular dysplasia in the adult mouse after vascular endothelial growth factor stimulation. Stroke 2013; 44: 795–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.van Laake LW, van den Driesche S, Post S, et al. Endoglin has a crucial role in blood cell-mediated vascular repair. Circulation 2006; 114: 2288–2297. [DOI] [PubMed] [Google Scholar]
- 140.Herrmann AM, Meckel S, Gounis MJ, et al. Large animals in neurointerventional research: a systematic review on models, techniques and their application in endovascular procedures for stroke, aneurysms and vascular malformations. J Cereb Blood Flow Metab 2019; 39: 375–394. [DOI] [PMC free article] [PubMed] [Google Scholar]