Abstract
Objective: Electroconvulsive therapy (ECT) is the most effective acute treatment for depression, but its use in younger patients is rare and heavily regulated in many U.S. states. It is unclear whether age modifies treatment response or tolerability in adolescents, transitional age youth, and young adults. We examined the effects of ECT on depression and cognition in patients aged 16–30 years.
Methods: A retrospective cohort study of patients aged 16–30 years receiving ECT between 2011 and 2020 who were evaluated with the Quick Inventory of Depressive Symptomatology (QIDS), the Behavior and Symptom Identification Scale-24 (BASIS-24), and the Montreal Cognitive Assessment (MoCA) at baseline and following treatment #10.
Results: Among the 424 patients who met the inclusion criteria, ECT was associated with a decrease in depression symptoms (ΔQIDS −6.7; Kruskal–Wallis rank sum test; χ2 = 293.37; df = 2; p < 0.0001) and improvement in overall self-reported mental health status (ΔBASIS-24 − 0.70; Kruskal–Wallis rank sum test; χ2 = 258.5; df = 2; p < 0.0001) during the first 10 treatments, with a slight reduction in cognition as measured by the MoCA (ΔMoCA −1.1; Kruskal–Wallis rank sum test; χ2 = 33.7; df = 1; p < 0.0001). Age was not a significant predictor of QIDS, BASIS-24, or MoCA changes.
Conclusions: Among 424 patients aged 16–30 years receiving acute course ECT, age was not a significant predictor of improvement in depression, change in overall self-reported mental health status, or change in cognition. These results support the utility of ECT in the treatment of adolescents and young adults.
Keywords: electroconvulsive therapy, depressive disorders, cohort studies, adolescent psychiatry
Introduction
Timely identification and intervention for adolescent depression is of extreme importance, given the morbidity associated with delays in diagnosis and treatment (Lewandowski et al. 2013). In particular, early-onset depression often extends into adulthood and is associated with high rates of disability, impairment, and suicidality (Zisook et al. 2007). Furthermore, despite the high prevalence of adolescent depression, currently available pharmacological interventions are limited to only two Food and Drug Administration (FDA)-approved selective serotonin reuptake inhibitor medications. Many adolescents with depression go through off-label trials of multiple antidepressant medications, but the treatment of resistant depression in adolescents randomized controlled trial of antidepressant switch plus cognitive behavioral therapy found that just 54.8% of patients responded to even combined medication and psychotherapy treatment at 12 weeks, leaving a sizable portion of the population with residual symptoms and dysfunctions (Brent et al. 2008). While there are no reports of suicides directly caused by antidepressant medications, the FDA Black Box warning contains statements of increased risk of suicidal thoughts and behavior in children, adolescents, and young adults treated with antidepressants, which often poses challenges in treatment decisions (Cousins and Goodyer 2015). In addition, adolescents with depression are prone to activation and manic switches associated with medication interventions compared with older patients (Baumer et al. 2006; Joseph et al. 2008). As such, there is a clear need for alternative methods of treatment for depression in young people.
Since its introduction in 1938, electroconvulsive therapy (ECT) has been the most effective treatment for affective disorders, achieving remission in more than 50% of patients with unipolar or bipolar illness (Dierckx et al. 2012; Bahji et al. 2019). ECT has a long history of use in young people, with publications dating back to 1941 for its use in a 3-year-old with epilepsy (Hemphill and Walter 1941). By 1943, the first case series of 40 pediatric patients aged 5–19 years was published in Paris demonstrating particular efficacy in melancholia and mania (Heuyer and Bour 1943), and a 1947 report of 98 children aged 12 years or younger receiving daily ECT for “childhood schizophrenia” demonstrated modest improvement in symptoms in two thirds of the patients, without evidence of cognitive dysfunction (Bender 1947).
Despite this early favorable literature, the use of ECT in young people has remained uncommon, and indeed, the 1947 report remains the largest case series of pediatric ECT (Rey and Walter 1997). A 1980 estimate suggested that only 500 patients aged 11–20 years were treated annually in the United States (Thompson and Blaine 1987), and a recent analysis of ECT treatments in the United States found that <1% of patients were younger than 18 years (Luccarelli et al. 2020a). Adolescent access to ECT is curtailed by laws in 21 U.S. states (Livingston et al. 2018): for instance, ECT is banned in all patients younger than 18 years in Ohio, and younger than 16 years in Colorado and Texas with no exemptions permitted. In Idaho, Illinois, Kansas, Missouri, and Utah, a court order is required for ECT in patients aged <18 years, even if the patient and family consent to treatment. Other states (California, Colorado, Illinois, Louisiana, Massachusetts, Michigan, Mississippi, New York, Tennessee, and Virginia) require approval of the State or of multiple independent physicians before treatment can be initiated in youth. In contrast, no state bans ECT for patients older than 18 years, and clinical treatment guidelines for patients older than 18 years do not generally differ in recommendations based on the age of the adult (Committ and Weiner 2001; Weiss et al. 2019).
Some authors have hypothesized that transitional age youth (TAY), generally defined as the those between the ages of 18 and 24–26 years, have social and neurobiological differences from adolescents and older adults (Giedd 2008; Wilens and Rosenbaum 2013). As a result, this population may also have a differential response to ECT. Given these regulatory and possible biological differences among younger patients, a comprehensive study of treatments among patients of different ages could guide treatment referrals and regulations. This study explores whether age modulates the effectiveness and cognitive effects of ECT among patients aged 16–30 years using the largest retrospective sample of these patients reported to date.
Methods
Population and setting
This was a single-center retrospective cohort study of patients aged 16–30 years who received ECT during the study period of May 2011 through June 2020. Patients were excluded if they lacked baseline symptom data. If a patient received more than one ECT course during the study period, only data from the earliest course were included. Patients were followed for the first 10 treatments, representing a typical acute course. This retrospective cohort study was approved by the Partners Healthcare Institutional Review Board with a waiver of informed consent.
Scales and measurements
As part of routine clinical care, patients receiving ECT are tracked using multiple self-reported scales. These include the Quick Inventory of Depressive Symptomatology–Self-Report 16 item scale (QIDS) (Rush et al. 2003), a measure of depressive symptoms; the Behavior and Symptom Identification Scale-24 (BASIS-24) (Eisen et al. 2006), a measure of overall self-reported mental health status; and the Montreal Cognitive Assessment (MoCA) (Nasreddine et al. 2005), a cognitive screening test. Measurements were obtained before the first treatment and repeated following the 5th and 10th treatments for the BASIS-24 and QIDS and following the 10th treatment for the MoCA. To reduce practice effects for the MoCA, alternative versions were given for the initial and follow-up assessment (Costa et al. 2012). Demographic information was extracted from the BASIS-24, and diagnosis at the time of first treatment was determined from the patient's records. Sample membership was not limited on the basis of diagnosis, and patients with major depressive disorder, bipolar disorder in any phase (manic, mixed, or depressed), or any other psychiatric diagnosis (including schizophrenia, schizoaffective disorder, and catatonia) were included.
Treatment procedure
All patients received ECT using a Mecta Spectrum 5000Q (Tualatin, OR), with individualized seizure threshold determination at the time of first treatment, as previously reported (Luccarelli et al. 2020b, 2021a). Subsequent supra-threshold treatments were given at a default frequency of three times weekly, with dose and electrode placement modified by the treating psychiatrist based on clinical judgment (Luccarelli et al. 2021b, 2021c). Methohexital was the default anesthetic agent, but etomidate, propofol, or ketamine could be used at the discretion of the treating psychiatrist or anesthesiologist. Succinylcholine was used as the muscle relaxant for all patients.
Statistical analysis
Patients were excluded from the primary analysis if they lacked follow-up QIDS results following treatment 10 ± 2. Comparisons between the included and excluded groups were made using two-sided t tests for continuous variables and chi-square tests for categorical variables. The change in QIDS, BASIS-24, or MoCA between baseline and treatment #10 was calculated using the Kruskal–Wallis rank sum test. For the primary analysis, the QIDS, BASIS-24, or MoCA following treatment #10 was analyzed using linear regression, with the baseline value for the scale, age, male sex, diagnosis (major depressive disorder, bipolar affective disorder, other), and initial treatment location (inpatient vs. outpatient) as descriptor variables. Analysis was completed using R (v 4; Vienna, Austria).
Results
A total of 662 patients aged 16–30 years were treated during the study period, of whom 424 met the inclusion criteria (Table 1); patients who were excluded were similar in baseline demographics and symptom severity with those who met the inclusion criteria (Supplementary Table S1). Among the 424 included patients, 23 (5.4%) were adolescents, 263 (62.0%) were TAY, and 138 (32.5%) were adults. Demographically, women made up 55.7% of the sample. The majority of patients (86.8%) self-identified as white, and 4.2% self-identified as Latino/Latina, although information about Hispanic ethnicity was not answered by 51.9% of patients. Diagnostically, the majority of patients (294; 63.9%) were diagnosed with major depressive disorder, whereas bipolar disorder (87; 20.5%) and other (43; 10.1%) made up the rest of the sample. Approximately two thirds (271; 63.9%) began ECT as inpatients, whereas the rest were outpatients at the time of the first treatment. Right unilateral electrode placement (411; 95.5%) and ultrabrief pulse width (405; 95.5%) were used for the majority of patients with the rest receiving bilateral or brief pulse treatments.
Table 1.
Baseline Characteristics of the Cohort
| Characteristic | n (%) |
|---|---|
| N | 424 |
| Age (mean ± SD), years | 23.4 ± 3.9 |
| Age distribution, years | |
| 16–17 | 23 (5.4) |
| 18–25 | 263 (62.0) |
| 26–30 | 138 (32.5) |
| Sex | |
| Female | 236 (55.7) |
| Male | 188 (44.3) |
| Race | |
| White | 368 (86.8) |
| Native American | 3 (0.7) |
| Asian | 28 (6.6) |
| Black | 12 (2.8) |
| Pacific Islander | 0 (0.0) |
| Other | 7 (1.7) |
| Unknown | 6 (1.4) |
| Ethnicity | |
| Latino/Latina | 18 (4.2) |
| Not Latino/Latina | 220 (51.9) |
| Unknown | 186 (43.9) |
| Employment in past 30 days | |
| Full time | 42 (9.9) |
| Part-time | 45 (10.6) |
| None | 269 (63.4) |
| Unknown | 68 (16.0) |
| Student (yes) | 203 (47.9) |
| On disability (yes) | 77 (18.2) |
| Education | |
| 8th Grade or less | 1 (0.2) |
| Some high school | 36 (8.5) |
| High school graduate or equivalent | 59 (13.9) |
| Some college | 202 (47.6) |
| 4 Year college graduate | 83 (19.6) |
| Postcollege education | 43 (10.1) |
| Number missing | 0 (0.0) |
| Subjective physical health | |
| Very poor | 4 (0.9) |
| Poor | 55 (13.0) |
| Good | 226 (53.3) |
| Very good | 106 (25.0) |
| Excellent | 33 (7.8) |
| Number missing | 0 (0.0) |
| Location where initially receiving ECT | |
| Inpatient | 271 (63.9) |
| Outpatient | 146 (34.4) |
| Number missing | 7 (1.7) |
| Clinical diagnosis | |
| MDD | 294 (69.3) |
| BPAD | 87 (20.5) |
| Other | 43 (10.1) |
| ECT electrode placement | |
| Unilateral | 411 (96.9) |
| Bilateral | 13 (3.1) |
| ECT pulse width | |
| Ultrabrief pulse (<0.5 ms) | 405 (95.5) |
| Brief pulse (0.5–1 ms) | 19 (4.5) |
| Baseline QIDS (mean ± SD) | 17.0 ± 4.9 |
| Baseline BASIS-24 (mean ± SD) | 2.00 ± 0.57 |
| Baseline MoCA (mean ± SD) | 26.7 ± 2.6 |
BASIS-24, Behavior and Symptom Identification Scale-24; BPAD, bipolar affective disorder; ECT, electroconvulsive therapy; MDD, major depressive disorder; MoCA, Montreal Cognitive Assessment; QIDS, Quick Inventory of Depressive Symptomatology; SD, standard deviation.
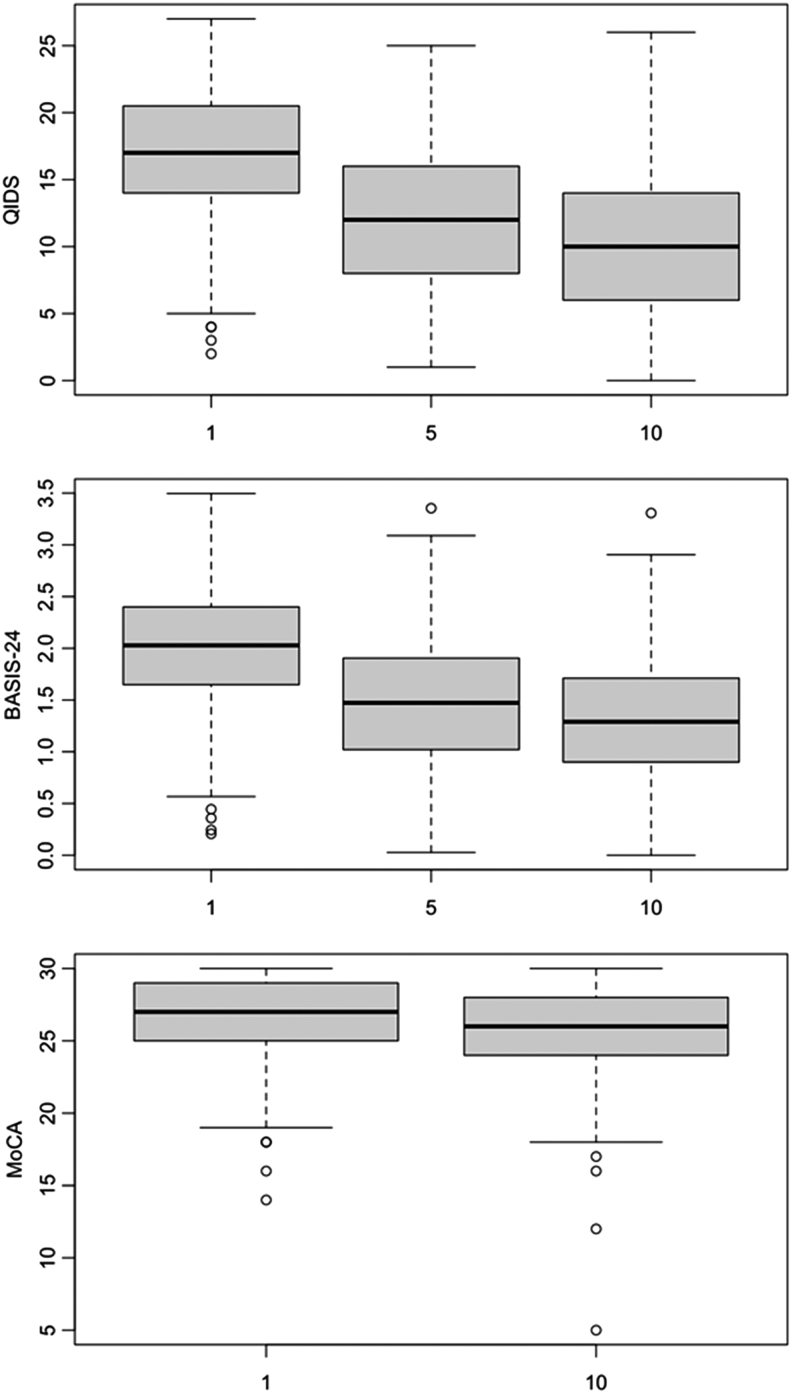
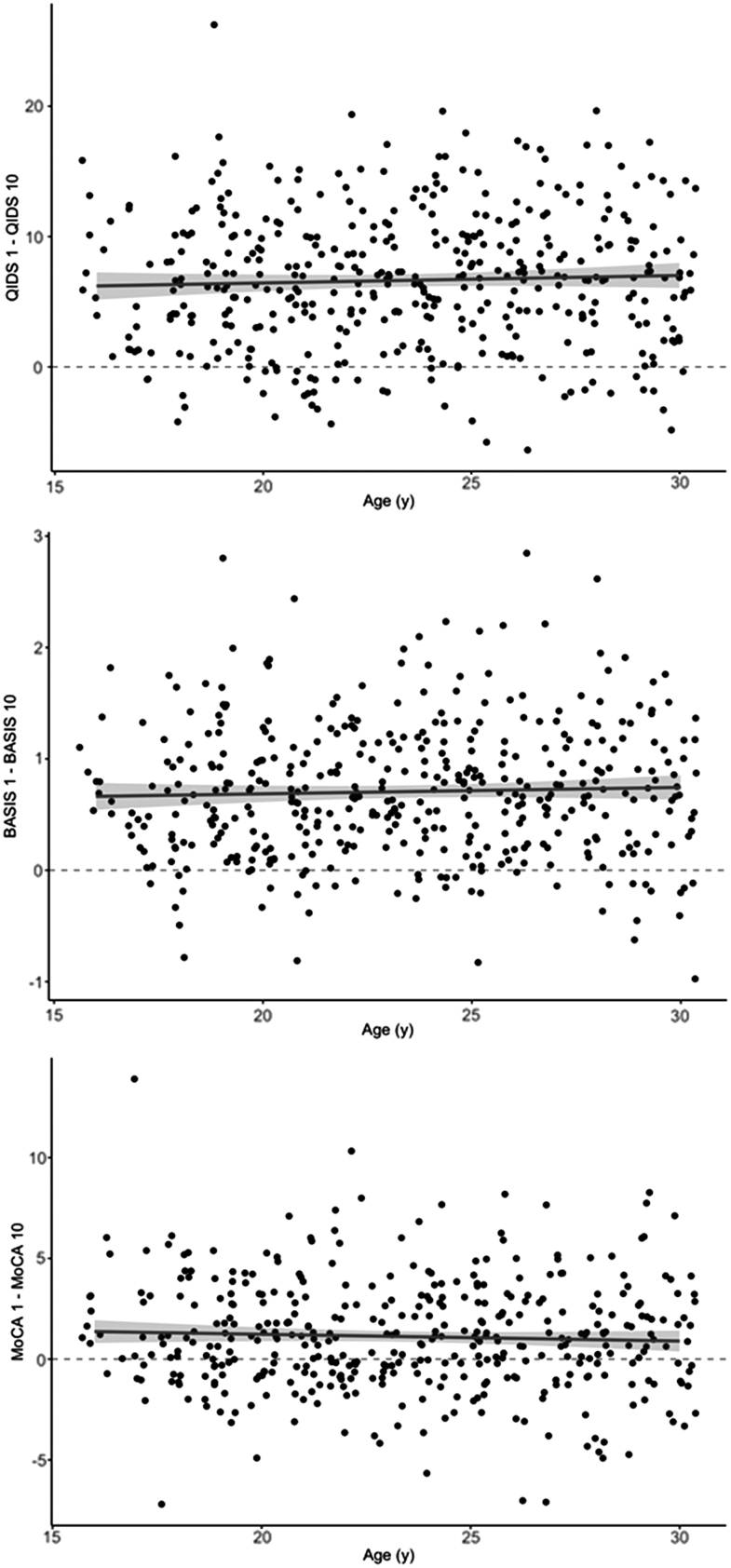
At baseline, the mean QIDS score was 17.0 ± 4.9, representing severe depressive symptom burden. Following treatment #5, this reduced to 11.8 ± 5.4, with a further reduction to 10.3 ± 5.3 after treatment #10 (Kruskal–Wallis rank sum test; χ2 = 293.37; df = 2; p < 0.0001), representing continued mild-to-moderate symptoms (Fig. 1, top). In a multivariable linear model of QIDS score at treatment #10, regressing on the baseline QIDS, age, sex, diagnosis, and initial treatment location, only baseline symptom severity was significantly associated with treatment #10 QIDS (estimate 0.48; 95% confidence interval [CI] 0.38–0.58; p < 0.001). No other variables were significantly associated (Table 2). A scatter plot of change in QIDS score between treatments #1 and #10 versus patient age illustrates the lack of univariate association between patient age and treatment response (Fig. 2, top).
FIG. 1.
Box plot of QIDS (top), BASIS-24 (middle), and MoCA (bottom) scores between treatment #1 and treatment #10. BASIS-24, Behavior and Symptom Identification Scale-24; MoCA, Montreal Cognitive Assessment; QIDS, Quick Inventory of Depressive Symptomatology.
Table 2.
Linear Regression of Quick Inventory of Depressive Symptomatology (QIDS) Score at Treatment #10, on the Baseline QIDS, Sex, Age, Diagnosis, and Initial Treatment Location
| Predictor | Estimate | CI | p |
|---|---|---|---|
| Baseline QIDS | 0.48 | 0.38 to 0.58 | <0.001 |
| Sex (male) | −0.77 | −1.73 to 0.19 | 0.113 |
| Age | 0.01 | −0.11 to 0.13 | 0.823 |
| Diagnosis | |||
| MDD | 0.55 | −1.03 to 2.13 | 0.494 |
| BPAD | −0.17 | −1.94 to 1.60 | 0.848 |
| Location (outpatient) | 0.64 | −0.33 to 1.60 | 0.194 |
Bold values are significant at a threshold of p < 0.05.
BPAD, bipolar affective disorder; CI, confidence interval; MDD, major depressive disorder; QIDS, Quick Inventory of Depressive Symptomatology.
FIG. 2.
Scatter plot of change in QIDS (top), BASIS-24 (middle), and MoCA (bottom) scores between treatment #1 and treatment #10 versus the age of the patient. BASIS-24, Behavior and Symptom Identification Scale-24; MoCA, Montreal Cognitive Assessment; QIDS, Quick Inventory of Depressive Symptomatology.
Baseline BASIS-24 for the sample was 2.00 ± 0.57, reducing to 1.48 ± 0.63 after treatment #5 and further reducing to 1.30 ± 0.60 after treatment #10 (Kruskal–Wallis rank sum test; χ2 = 258.5; df = 2; p < 0.0001; Fig. 1, middle). In a multivariable linear model of BASIS-24 score at treatment #10, regressing on the baseline BASIS-24, age, sex, diagnosis, and initial treatment location, only baseline BASIS-24 score was significantly associated with the follow-up score (estimate 0.48; 95% CI 0.39–0.57; p < 0.001). No other variables were significantly associated (Supplementary Table S2). A scatter plot of change in BASIS-24 score between treatments #1 and #10 versus patient age illustrates the lack of univariate association between patient age and treatment response (Fig. 2, middle).
Baseline MoCA for the sample was 26.7 ± 2.6, with a reduction to 25.6 ± 3.0 after treatment #10 (Kruskal–Wallis rank sum test; χ2 = 33.7; df = 1; p < 0.0001; Fig. 1, bottom). In a multivariable linear model of MoCA score at treatment #10, regressing on the baseline MoCA, age, sex, diagnosis, and initial treatment location, only baseline MoCA score was significantly associated with follow-up MoCA (estimate 0.6; 95% CI 0.5–0.71; p < 0.001); no other variables were significantly associated (Table 3). A scatter plot of change in MoCA score between treatments #1 and #10 versus patient age illustrates the lack of univariate association between patient age and treatment response (Fig. 2, bottom).
Table 3.
Linear Regression of Montreal Cognitive Assessment (MoCA) Score at Treatment #10, on the Baseline MoCA, Sex, Age, Diagnosis, and Initial Treatment Location
| Predictor | Estimate | CI | p |
|---|---|---|---|
| Baseline MoCA | 0.6 | 0.50 to 0.71 | <0.001 |
| Sex (male) | 0.07 | −0.46 to 0.60 | 0.79 |
| Age | 0.02 | −0.05 to 0.09 | 0.611 |
| Diagnosis | |||
| MDD | 0.75 | −0.17 to 1.67 | 0.11 |
| BPAD | 0.44 | −0.59 to 1.47 | 0.399 |
| Location (outpatient) | −0.17 | −0.72 to 0.38 | 0.544 |
Bold values are significant at a threshold of p < 0.05
BPAD, bipolar affective disorder; MDD, major depressive disorder; MoCA, Montreal Cognitive Assessment.
Discussion
In this large single-center sample of 424 adolescents, TAY, and young adults aged 16–30 years receiving 10 ECT treatments, patients had an improvement in depression symptoms from severe range to mild-to-moderate range. This was accompanied by an improvement in overall self-reported mental health on the BASIS-24, and a mean MoCA reduction of one point. Age was not a significant predictor of improvement in depression, change in overall self-reported mental health status, or change in cognition.
ECT has been demonstrated to be an effective treatment for depressive symptoms across the age range, with one study finding equal response for patients aged 18–45, 46–64, and 65+ years (Socci et al. 2018). In younger patients specifically, a prior analysis of treatment response on the BASIS-24 in 190 adolescents and TAY over the first 5 ECT treatments likewise found improvements over the first five treatments, an effect modulated by the presence or absence of baseline substance use disorder (Benson et al. 2019). This is consistent with prior smaller case series (Ghaziuddin et al. 2012; Puffer et al. 2016; Karayagmurlu et al. 2020) and present American practice guidelines (Ghaziuddin et al. 2004), which confirm the utility and overall tolerability of ECT in young people. Our study is the first to look specifically at the potential modulating effect of age among younger patients and finds no such effect on efficacy.
In addition to efficacy, our study tracks cognitive outcomes of acute course ECT and again found that age did not modulate changes in MoCA. The MoCA has been studied specifically in ECT and has been found to be more sensitive than the Mini-Mental State Examination for detecting subtle cognitive impairments (Moirand et al. 2018). Our results indicate an ∼1 point reduction in the mean MoCA score over the first 10 treatments of ECT. Of note, as the ECT in this study was given three times weekly, with the MoCA administered immediately before the 10th treatment, on average patients were tested 48, or at most 72 hours, after their previous treatment. Systematic study of ECT neurocognitive effects find that most deleterious effects last up to 3 days (Semkovska and McLoughlin 2010; Landry et al. 2020), so it is possible that these negative MoCA changes would be reduced or eliminated with a longer follow-up interval, and indeed, multiple case series of adolescent patients tested months to years after ECT have demonstrated no adverse cognitive changes (Cohen et al. 2000; Ghaziuddin et al. 2000; de la Serna et al. 2011). Moreover, the observed change of 1.1 points is below the minimal clinically important difference in MoCA that has been calculated in adult stroke patients of 1.22–2.15 (Tan et al. 2017; Wu et al. 2019), although the magnitude of clinically noticeable change has not been studied in younger patients. Notably, despite their young age patients at baseline had lower MoCA scores than the general population (Nasreddine et al. 2005), an effect may be related to cognitive impairment from depression (Vieira et al. 2021).
Limitations
As this is a retrospective observational study without control group, we are unable to explore the potential responsiveness of this sample to alternative treatment options or no treatment at all. Moreover, as ECT patients remained under the care of their primary psychopharmacologist, we are unable to assess the effects of possible concurrent medications or medication changes. Additionally, our study assessed diagnosis based on self-reported patient measures as well as primary clinical diagnosis, which may hinder comparisons to studies using structured clinical interviews but better represents ordinary clinical practices. Furthermore, patients were excluded from the cohort if baseline and follow-up survey responses were not complete, resulting in the exclusion of 36% of patients treated during the study period. While these excluded patients did not differ significantly from included patients in most baseline demographics, patients who were unable to complete these metric due to increased symptom burden or physical or cognitive limitations may have been excluded at a greater rate, potentially hindering analysis of those patients with the most severe illness. Reassuringly, in a large sample of 1793 patients of all ages initially receiving right unilateral ultrabrief pulse ECT at our study site, 33.1% discontinued ECT by treatment #10, a rate similar to observed in this sample (Luccarelli et al. 2021b). Moreover, our sample predominantly (96.9%) utilized right unilateral electrode placement at treatment #1, and we are unable to consistently track changes in electrode placement during treatment, so we are unable to assess for a possible difference in efficacy or side effects of unilateral or bilateral treatments, or to assess if these may differ based on age. This is likewise true for pulse width, as most initial treatments utilized ultrabrief pulse stimuli and we likewise are unable to assess for pulse width changes during the acute course.
Prior studies of ECT administration in the United States have highlighted disparities in ECT utilization (Williams et al. 2017; Luccarelli et al. 2020a), with white patients being treated at far higher rates than patients of other races. This disparity is likewise present in our predominantly white and non-Hispanic sample, and so generalizability of the findings to other sociodemographic populations is unclear. Furthermore, the QIDS is primarily a metric of depression symptoms, and its use in patients with alternative diagnoses (e.g., bipolar manic state or primary psychotic illness) is less studied, although this limitation is not present for the BASIS-24 results. Likewise as cognitive outcomes are assessed only following treatment #10, we are unable to assess the long-term cognitive impacts of ECT and whether these differ by age. As our sample was limited to patients completing a first course of ECT, we cannot assess for possible cumulative cognitive effects that could occur with repeated treatment courses.
Conclusions
In conclusion, among 424 adolescents, TAY, and young adults aged 16–30 years receiving acute course ECT, treatment is associated with improved depression symptoms and overall self-reported mental health with a slight negative effect on cognition that is likely below the minimally clinically important change as measured by the MoCA. Age did not modulate effectiveness or cognitive effects of treatment. These results do not support the notion that ECT in adolescents is less effective or less safe than in young adults, and call into question the different regulatory treatment of ECT among patients aged 16–30 years. Treatment of adolescent depression with ECT may provide an effective and alternative approach to mitigating the adverse consequences associated with early-onset depression.
Clinical Significance
This is the largest retrospective cohort of adolescents, TAY, and young adults receiving ECT yet reported. Among 424 patients aged 16–30 years, ECT treatment is associated with improved depression symptoms and overall self-reported mental health. Treatment was associated with a slight negative effect on cognition that is likely below the minimally clinically important change as measured by the MoCA. Age itself did not modulate any of these treatment effects. These results do not support the notion that ECT in adolescents is less effective or less safe than in young adults, and call into question the different regulatory treatment of ECT among patients aged 16–30 years.
Supplementary Material
Disclosures
T.H.M. receives research funding from the Stanley Center at the Broad Institute, the Brain and Behavior Research Foundation, the National Institute of Mental Health, the National Human Genome Research Institute Home, and Telefonica Alpha. The remaining authors have no disclosures to report.
Supplementary Material
References
- Bahji A, Hawken ER, Sepehry AA, Cabrera CA, Vazquez G: ECT beyond unipolar major depression: Systematic review and meta-analysis of electroconvulsive therapy in bipolar depression. Acta Psychiatr Scand 139:214–226, 2019. [DOI] [PubMed] [Google Scholar]
- Baumer FM, Howe M, Gallelli K, Simeonova DI, Hallmayer J, Chang KD: A pilot study of antidepressant-induced mania in pediatric bipolar disorder: Characteristics, risk factors, and the serotonin transporter gene. Biol Psychiatry 60:1005–1012, 2006. [DOI] [PubMed] [Google Scholar]
- Bender L: One hundred cases of childhood schizophrenia treated with electric shock. Trans Am Neurol Assoc 72:165–169, 1947. [Google Scholar]
- Benson NM, Seiner SJ, Bolton P, Fitzmaurice G, Meisner RC, Pierce C, Busch AB: Acute phase treatment outcomes of electroconvulsive therapy in adolescents and young adults. J ECT 35:178–183, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent D, Emslie G, Clarke G, Wagner KD, Asarnow JR, Keller M, Vitiello B, Ritz L, Iyengar S, Abebe K, Birmaher B, Ryan N, Kennard B, Hughes C, DeBar L, McCracken J, Strober M, Suddath R, Spirito A, Leonard H, Melhem N, Porta G, Onorato M, Zelazny J: Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: The TORDIA randomized controlled trial. JAMA 299:901–913, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D, Taieb O, Flament M, Benoit N, Chevret S, Corcos M, Fossati P, Jeammet P, Allilaire J-F, Basquin M: Absence of cognitive impairment at long-term follow-up in adolescents treated with ECT for severe mood disorder. Am J Psychiatry 157:460–462, 2000. [DOI] [PubMed] [Google Scholar]
- Committ APA, Weiner RD: Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Privileging. Washington, DC, American Psychiatric Publishing, Inc., 2001. [Google Scholar]
- Costa AS, Fimm B, Friesen P, Soundjock H, Rottschy C, Gross T, Eitner F, Reich A, Schulz JB, Nasreddine ZS, Reetz K: Alternate-form reliability of the Montreal cognitive assessment screening test in a clinical setting. Dement Geriatr Cogn Disord 33:379–384, 2012. [DOI] [PubMed] [Google Scholar]
- Cousins L, Goodyer IM: Antidepressants and the adolescent brain. J Psychopharmacol Oxf Engl 29:545–555, 2015. [DOI] [PubMed] [Google Scholar]
- de la Serna E, Flamarique I, Castro-Fornieles J, Pons A, Puig O, Andrés-Perpiña S, Lázaro L, Garrido JM, Bernardo M, Baeza I: Two-year follow-up of cognitive functions in schizophrenia spectrum disorders of adolescent patients treated with electroconvulsive therapy. J Child Adolesc Psychopharmacol 21:611–619, 2011. [DOI] [PubMed] [Google Scholar]
- Dierckx B, Heijnen WT, van den Broek WW, Birkenhäger TK: Efficacy of electroconvulsive therapy in bipolar versus unipolar major depression: A meta-analysis. Bipolar Disord 14:146–150, 2012. [DOI] [PubMed] [Google Scholar]
- Eisen SV, Gerena M, Ranganathan G, Esch D, Idiculla T: Reliability and validity of the BASIS-24 Mental Health Survey for Whites, African-Americans, and Latinos. J Behav Health Serv Res 33:304–323, 2006. [DOI] [PubMed] [Google Scholar]
- Ghaziuddin N, Dhossche D, Marcotte K: Retrospective chart review of catatonia in child and adolescent psychiatric patients. Acta Psychiatr Scand 125:33–38, 2012. [DOI] [PubMed] [Google Scholar]
- Ghaziuddin N, Kutcher SP, Knapp P: Practice parameter for use of electroconvulsive therapy with adolescents. J Am Acad Child Adolesc Psychiatry 43:1521–1539, 2004. [DOI] [PubMed] [Google Scholar]
- Ghaziuddin N, Laughrin D, Giordani B: Cognitive side effects of electroconvulsive therapy in adolescents. J Child Adolesc Psychopharmacol 10:269–276, 2000. [DOI] [PubMed] [Google Scholar]
- Giedd JN: The teen brain: Insights from neuroimaging. J Adolesc Health Off Publ Soc Adolesc Med 42:335–343, 2008. [DOI] [PubMed] [Google Scholar]
- Hemphill RE, Walter WG: The treatment of mental disorders by electrically induced convulsions. J Ment Sci 87:256–275, 1941. [Google Scholar]
- Heuyer G, Bour RL: L'électro-choc chez les enfants (Electroshock in children). Ann Méd-Psychol 101:402–407, 1943. [Google Scholar]
- Joseph MF, Youngstrom EA, Soares JC: Antidepressant-coincident mania in children and adolescents treated with selective serotonin reuptake inhibitors. Future Neurol 4:87–102, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayagmurlu A, Coskun M, Elboga G, Ghaziuddin N, Karayagmurlu E, Gökçen C, Altindag A: Efficacy and safety of electroconvulsive therapy in adolescents: A retrospective chart review study from Turkey. J ECT 36:54–59, 2020. [DOI] [PubMed] [Google Scholar]
- Landry M, Moreno A, Patry S, Potvin S, Lemasson M: Current practices of electroconvulsive therapy in mental disorders: A systematic review and meta-analysis of short and long-term cognitive effects. J ECT 37:119–127, 2021. [DOI] [PubMed] [Google Scholar]
- Lewandowski RE, Acri MC, Hoagwood KE, Olfson M, Clarke G, Gardner W, Scholle SH, Byron S, Kelleher K, Pincus HA, Frank S, Horwitz SM: Evidence for the management of adolescent depression. Pediatrics 132:e996–e1009, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston R, Wu C, Mu K, Coffey MJ: Regulation of electroconvulsive therapy: A systematic review of US state laws. J ECT 34:60, 2018. [DOI] [PubMed] [Google Scholar]
- Luccarelli J, Henry ME, McCoy TH: Demographics of patients receiving electroconvulsive therapy based on state-mandated reporting data. J ECT 36:229–233, 2020a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luccarelli J, McCoy TH, Seiner SJ, Henry ME: Charge required to induce a seizure during initial dose titration using right unilateral brief pulse electroconvulsive therapy. Brain Stimul Basic Transl Clin Res Neuromodulation 13:1504–1506, 2020b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luccarelli J, McCoy THJ, Seiner SJ, Henry ME: Total charge required to induce a seizure in a retrospective cohort of patients undergoing dose titration of right unilateral ultrabrief pulse electroconvulsive therapy. J ECT 37:40–45, 2021a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luccarelli J, McCoy TH, Shannon AP, Forester BP, Seiner SJ, Henry ME: Rate of continuing acute course treatment using right unilateral ultrabrief pulse electroconvulsive therapy at a large academic medical center. Eur Arch Psychiatry Clin Neurosci 271:191–197, 2021b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luccarelli J, McCoy THJ, Shannon AP, Forester BP, Seiner SJ, Henry ME: Duration of treatment in electroconvulsive therapy among patients beginning with acute course right unilateral brief pulse stimuli. J ECT 2021c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moirand R, Galvao F, Lecompte M, Poulet E, Haesebaert F, Brunelin J: Usefulness of the Montreal Cognitive Assessment (MoCA) to monitor cognitive impairments in depressed patients receiving electroconvulsive therapy. Psychiatry Res 259:476–481, 2018. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H: The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699, 2005. [DOI] [PubMed] [Google Scholar]
- Puffer CC, Wall CA, Huxsahl JE, Frye MA: A 20 year practice review of electroconvulsive therapy for adolescents. J Child Adolesc Psychopharmacol 26:632–636, 2016. [DOI] [PubMed] [Google Scholar]
- Rey JM, Walter G: Half a century of ECT use in young people. Am J Psychiatry 154:595–602, 1997. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB: The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biol Psychiatry 54:573–583, 2003. [DOI] [PubMed] [Google Scholar]
- Semkovska M, McLoughlin DM: Objective cognitive performance associated with electroconvulsive therapy for depression: A systematic review and meta-analysis. Biol Psychiatry 68:568–577, 2010. [DOI] [PubMed] [Google Scholar]
- Socci C, Medda P, Toni C, Lattanzi L, Tripodi B, Vannucchi G, Perugi G: Electroconvulsive therapy and age: Age-related clinical features and effectiveness in treatment resistant major depressive episode. J Affect Disord 227:627–632, 2018. [DOI] [PubMed] [Google Scholar]
- Tan HH, Xu J, Teoh HL, Chan BP-L, Seet RCS, Venketasubramanian N, Sharma VK, Chen CL-H, Dong Y: Decline in changing Montreal Cognitive Assessment (MoCA) scores is associated with post-stroke cognitive decline determined by a formal neuropsychological evaluation. PLoS One 12:e0173291, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JW, Blaine JD: Use of ECT in the United States in 1975 and 1980. Am J Psychiatry 144:557–562, 1987. [DOI] [PubMed] [Google Scholar]
- Vieira IS, Ferrugem SCR, Reyes AN, Branco JC, Mondin TC, Cardoso T de A, Kapczinski F, Souza LD de M, Jansen K, da Silva RA, Pedrotti Moreira F: Effects of depression and excess body weight on cognition and functioning in young adults: A population-based study. J Affect Disord 282:401–406, 2021. [DOI] [PubMed] [Google Scholar]
- Weiss A, Hussain S, Ng B, Sarma S, Tiller J, Waite S, Loo C: Royal Australian and New Zealand College of Psychiatrists professional practice guidelines for the administration of electroconvulsive therapy. Aust N Z J Psychiatry 53:609–623, 2019. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Rosenbaum JF: Transitional aged youth: A new frontier in child and adolescent psychiatry. J Am Acad Child Adolesc Psychiatry 52:887–890, 2013. [DOI] [PubMed] [Google Scholar]
- Williams J, Chiu L, Livingston R: Electroconvulsive therapy (ECT) and race: A report of ECT use and sociodemographic trends in Texas. J ECT 33:111–116, 2017. [DOI] [PubMed] [Google Scholar]
- Wu C-Y, Hung S-J, Lin K, Chen K-H, Chen P, Tsay P-K: Responsiveness, minimal clinically important difference, and validity of the MoCA in stroke rehabilitation. Occup Ther Int 2019:2517658, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisook S, Lesser I, Stewart JW, Wisniewski SR, Balasubramani G k., Fava M, Gilmer WS, Dresselhaus TR, Thase ME, Nierenberg AA, Trivedi MH, Rush AJ: Effect of age at onset on the course of major depressive disorder. Am J Psychiatry 164:1539–1546, 2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.