Abstract
The discs large (Dlg) protein, or synapse-associated protein 97 (SAP97), is a member of the membrane-associated guanylate kinase family of multidomain scaffolding proteins which recruits transmembrane and signaling molecules to localized plasma membrane sites. Murine dlg is the homologue of the Drosophila dlg tumor suppressor gene. The loss of dlg function in Drosophila disrupts cellular growth control, apicobasal polarity, and cell adhesion of imaginal disc epithelial cells, resulting in embryonic lethality. In this study, we isolated a mutational insertion in the murine dlg locus by gene trapping in totipotent embryonic stem cells. This insertion results in a truncated protein product that contains the N-terminal three PSD-95/DLG/ZO-1 domains of Dlg fused to the LacZ reporter and subsequently lacks the src homology 3 (SH3), protein 4.1 binding, and guanylate kinase (GUK)-like domains. The Dlg-LacZ fusion protein is expressed in epithelial, mesenchymal, neuronal, endothelial, and hematopoietic cells during embryogenesis. Mice homozygous for the dlg mutation exhibit growth retardation in utero, have hypoplasia of the premaxilla and mandible, have a cleft secondary palate, and die perinatally. Consistent with this phenotype, Dlg-LacZ is expressed in mesenchymal and epithelial cells throughout palatal development. Our genetic and phenotypic analysis of dlg mutant mice suggests that protein-protein interactions involving the SH3, protein 4.1 binding, and/or GUK-like domains are essential to the normal function of murine Dlg within craniofacial and palatal morphogenesis.
The product of the murine discs large (dlg) gene (25) belongs to the family of membrane-associated guanylate kinase (MAGUK) scaffolding proteins (3, 5). The first member of the MAGUK family was identified in Drosophila as recessive lethal mutations in dlg associated with neoplastic overgrowth of imaginal disc epithelial cells. In addition to loss of cellular growth control, epithelial cells in dlg mutants also demonstrated abnormalities in septate junction formation, cell polarity, and cell adhesion (40, 45, 46). Two recent reports have also presented evidence that mammalian Dlg is involved in regulation of cell growth (17, 41), raising the possibility that mammalian Dlg is involved in oncogenesis, consistent with the loss of cellular growth control in loss-of-function Drosophila dlg mutants.
Members of the MAGUK family (the Dlg-like, p55-like, lin2-like, and ZO-1-like proteins) (5) have a protein domain structure in common that includes one to three PDZ (PSD-95/DLG/ZO-1) domains, a src homology 3 (SH3) domain, and a guanylate kinase (GUK)-like domain (Fig. 1A). These domains mediate interactions with a variety of transmembrane, ion channel, signaling, and cytoskeletal proteins, thereby localizing these protein complexes to specialized membrane sites, such as regions of cell-cell contact in epithelial cells, the plasma membrane of red blood cells, and synaptic junctions (5). The PDZ domains of human Dlg (hDlg) and the rat homologue, synapse-associated protein 97 (SAP97), interact with the C-terminal Ser/Thr-XXX-Val/Ile consensus sequence found in a wide variety of cellular proteins (38). In particular, the second PDZ domain of Dlg interacts with the C terminus of the N-methyl-d-aspartate- and α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid-type glutamate receptors (24), the Shaker-type K+ channel (19), the adenomatous polyposis coli (APC) and PTEN tumor suppressors (1, 27), PDZ-binding kinase (9), and several viral oncoproteins (20, 23, 32, 41). In addition to the common MAGUK domains, isoforms of hDlg possess a proline-rich region that mediates interactions with lck, a member of the src family of tyrosine kinases (11), and regions that bind the Band 4.1 protein family members, which in turn associate with the cytoskeleton (26). The GUK-like domain of hDlg does not possess enzymatic activity but instead is involved in the mediation of interactions with a family of proteins termed SAPAP/GKAP in the brain (18, 35, 42) and GAKIN in T lymphocytes (12). Thus, the MAGUK family proteins appear to function as scaffolding proteins that mediate multiple protein-protein interactions in different cell types.
FIG. 1.
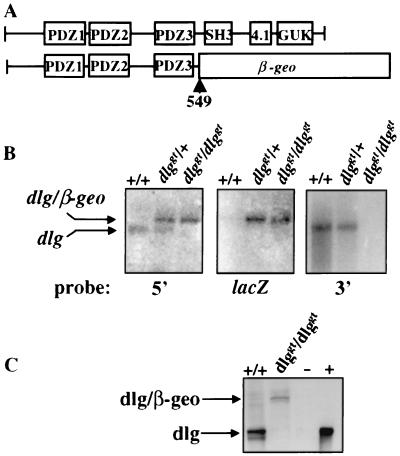
Characterization of the gene trap vector insertion in the dlg locus. (A) Domain structures of wild-type Dlg protein and mutant Dlg protein with the fusion occurring at position 549. Wild-type Dlg contains three PDZ domains and SH3, protein 4.1 binding, and GUK-like domains. (B) Northern blot analysis of brain mRNA extracted from +/+, dlggt/+, and dlggt/dlggt E17.5 embryos for the presence of endogenous dlg and/or dlg-β-geo fusion transcripts. Probes 5′ and 3′ to the vector insertion site were used, as well as a lacZ probe. (C) Western blot analysis of lung lysate from wild-type and dlggt/dlggt mutant newborn pups using antiserum raised against the N terminus of Dlg. A doublet around 120 to 140 kDa was detected in wild-type lysates which was absent in dlggt/dlggt mutant mice; however, the expected Dlg–β-Geo fusion protein of 190 kDa was detected. −, untransfected HEK 293 cells; +, HEK 293 cells transfected with full-length murine dlg cDNA.
The specific biological functions of the different protein modular domains of mammalian Dlg are still unclear. To address the biological functions of some of these domains in vivo, we analyzed the development of mice containing a gene trap insertion within the dlg locus (25). The resulting mutation generates a truncated Dlg protein which retains the first three PDZ domains fused to the Escherichia coli β-galactosidase (LacZ) reporter, thus enabling us to monitor the in vivo expression pattern of Dlg. This analysis revealed that Dlg is expressed in epithelial, mesenchymal, neuronal, endothelial, and hematopoietic cells during embryogenesis. In addition, mice homozygous for the gene trap insertion displayed growth retardation in utero, craniofacial abnormalities with a cleft secondary palate, and perinatal lethality. These results demonstrate that murine Dlg is required for normal craniofacial morphogenesis and strongly suggest that loss of one or more of the C-terminal SH3, protein 4.1, or GUK-like domains disrupts one or more of the protein-protein interactions that are essential for normal Dlg function.
MATERIALS AND METHODS
Generation of gene trap ES cells.
R1 embryonic stem (ES) cells were electroporated with the GT1.8geo vector, which contains the En-2 splice acceptor sequence upstream of the lacZ-neomycin (β-geo) fusion gene and a polyadenylation signal (37), as previously described (30). Using an expression-based gene trap screen (39), we identified an ES cell clone containing a gene trap insertion in the dlg locus as determined by 5′ rapid amplification of cDNA ends (RACE) and cDNA sequencing (39).
Generation of chimeras and transgenic mice.
Dlggt/+ ES cells were aggregated with ICR morulae (33) and transferred into pseudopregnant ICR females to generate several strong male chimeras. Chimeric males were bred with ICR females to transmit the transgene through the germ line. Tail DNAs of F1 and F2 offspring were prepared, digested with EcoRI, and analyzed by Southern blotting and hybridization with an En-2–LacZ probe, which identified the presence of the gene trap vector, and with the RACE fragment probe. The latter detected a polymorphism between the mutant and wild-type dlg alleles. Mice were maintained on a 12-h light-dark cycle. Noon on the plug date was designated day 0.5. Experimental procedures were performed on a mixed 129-ICR background.
Northern blot analysis.
Northern blot analysis was performed using 2 μg of poly(A) mRNA extracted from embryonic day 17.5 (E17.5) brain as previously described (10). The probes used were the 5′ RACE product, a PCR fragment 3′ to the gene trap vector insertion, and a lacZ probe.
Western blot analysis.
Lungs from wild-type and dlggt/dlggt mutant late-stage embryos were dissected, homogenized, and lysed in modified radioimmunoprecipitation assay buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% [wt/vol] Triton X-100, 1% [wt/vol] sodium deoxycholate, 0.1% [wt/vol] sodium dodecyl sulfate) containing a cocktail of protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 100 μg of leupeptin per ml, 10 μg of aprotinin per ml). Total protein (100 μg) was resolved on sodium dodecyl sulfate–7.5% agarose gels, transferred onto nitrocellulose filters (Amersham), and probed with anti-Dlg antibody (1:500 dilution; Transduction Laboratories, Lexington, Ky.). Dlg protein was visualized by incubation of the filters with horseradish peroxidase-conjugated goat anti-mouse antibody (Bio-Rad), followed by enhanced chemiluminescence assay in accordance with the manufacturer's (Amersham) instructions.
Whole-mount 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining and immunohistochemistry.
β-Galactosidase activity in embryos was detected as previously described (39). For whole-mount immunohistochemistry, embryos were stained with anti-Dlg antibody (5 μg/ml; Transduction Laboratories) essentially as previously described (14), except that the 2% skim milk was replaced with 1% Blocking Reagent (Boehringer Mannheim). For immunohistochemistry of paraffin-embedded, 4% paraformaldehyde-fixed gut sections, sections were deparaffinized in xylene and then rehydrated in a graded series of ethanol–phosphate-buffered saline. Endogenous peroxidase was inhibited by bleaching in H2O2-methanol (1:5). Antigen unmasking was performed by immersing the sections in 10 mM sodium citrate (pH 6) at 95°C for 5 min and allowing them to cool in the solution for 20 min prior to washing in H2O. Sections were blocked in 10% normal goat serum (NGS) in phosphate-buffered saline containing 0.1% NP-40 (PBSN) for 1 h at room temperature. The sections were incubated with anti-Dlg antibody (5 μg/ml) in 10% NGS–PBSN overnight at 4°C. The sections were washed three times for 5 min each time in PBSN prior to incubation with Texas red-conjugated goat anti-mouse immunoglobulin G (1/50; Molecular Probes, Eugene, Oreg.) in 10% NGS–PBSN for 1 h in the dark. The sections were washed three times as described above and mounted with coverslips. Digital images were captured using a Leica Leitz DMRD microscope and the Northern Eclipse program.
Skeletal staining and SEM.
Newborns or late gestational stage embryos were prepared for staining with alcian blue and alizarin red as previously described (15). Scanning electron microscopy (SEM) was performed as previously described (13).
RESULTS
Mutagenesis of the dlg locus.
Using an expression-based gene trap screen (39), we isolated ES cell clones expressing LacZ in hematopoietic and/or endothelial cell lineages during in vitro differentiation. One of these ES cell clones contained an integration of the gene trap vector in the murine homologue of the Drosophila dlg gene (25). This insertion occurred at position 1653 of the dlg cDNA sequence (GenBank accession no. U93309), generating a truncated dlg RNA transcript fused to the β-geo reporter gene (dlggt) (Fig. 1B). The deduced fusion protein retains the first 549 amino acids, which includes the three PDZ domains, but lacks the SH3, protein 4.1, and GUK-like domains (Fig. 1A). ES cells carrying the dlggt allele were aggregated with diploid embryos, and the trapped allele was transmitted through the germ line to produce F1 heterozygotes (dlggt/+). Heterozygous animals were viable and fertile and did not exhibit any obvious abnormalities, indicating that the truncated dlggt fusion protein did not exert any dominant-negative effects on the wild-type Dlg protein. Newborns of all genotypes were obtained from heterozygous matings at the expected Mendelian frequency (23% +/+, 50% +/−, 27% −/−; n = 117), indicating that truncation of the Dlg protein does not result in embryonic lethality. The presence of the fusion protein in embryos homozygous for dlggt was confirmed by Western blot analysis (Fig. 1C).
Expression of Dlg-LacZ in vivo.
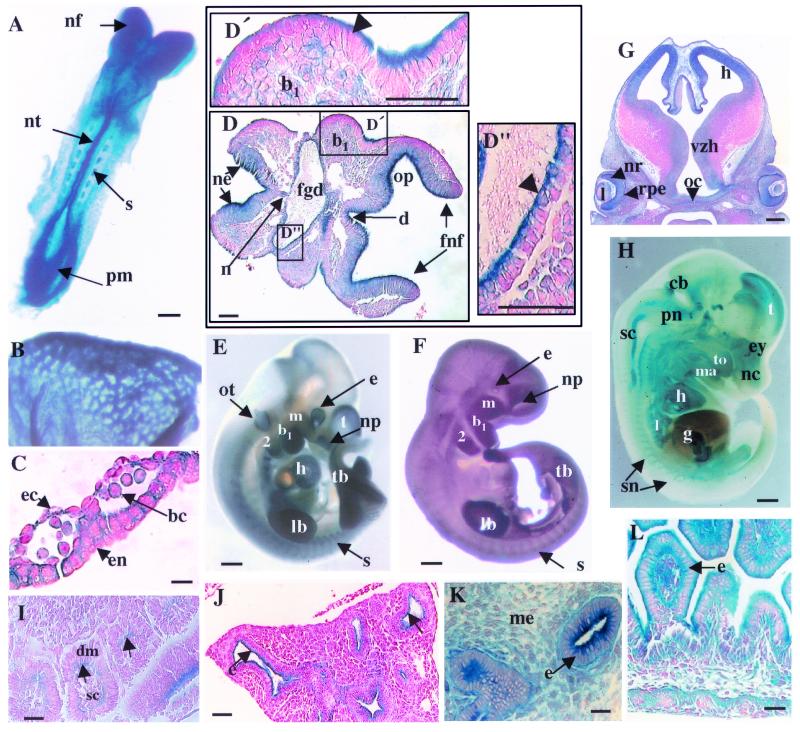
To determine the pattern of expression of the murine dlg gene in vivo, we took advantage of the insertion of the promoterless lacZ gene within dlg, thereby placing it under the transcriptional control of the dlg promoter. In heterozygous E8.5 embryos, Dlg-LacZ was expressed in the presomitic mesoderm, the mesenchyme and neural epithelium of the cephalic head folds, the rhombomeres, the neural tube, the notochord, the apical membrane of the epithelium of the foregut diverticulum, the branchial arch, the dermomyotome epithelial component of the somites at the face of the adjacent scleratome, the myocardium, and the yolk sac (Fig. 2A, B, C, D, and I). Expression in the yolk sac was associated with blood cells, endothelial cells, and endodermal cells (Fig. 2C). The hematopoietic and endothelial pattern of Dlg-LacZ expression observed in the yolk sac recapitulated the in vitro expression pattern used to isolate the dlggt ES clone in the primary gene trap screen (39). Later in embryogenesis, Dlg-LacZ was expressed in various hematopoietic compartments, including the developing liver, spleen, and thymus, in adult bone marrow cells, and in the meninges and vasculature of the head (data not shown).
FIG. 2.
Expression of Dlg-LacZ during embryonic development in heterozygous dlggt embryos. (A) Expression in E8.5 embryos (dorsal view) was detected in the cephalic neural folds (nf), neural tube (nt), somites (s), and presomitic mesoderm (pm). Bar, 100 μm. Whole-mount LacZ staining in the vasculature of the yolk sac (E8.5) (B) and histological sections (C) demonstrated that the fusion protein was expressed in blood cells (bc), at regions of cell contact in endothelial cells (ec), and at the basal and lateral membranes of the epithelial cells of the endoderm (en). Magnification, ×100; bar, 12 μm. (D) At E8.5, transverse histological sections demonstrated that LacZ was expressed in the neural epithelium of the forebrain neural head folds (fnf), diencephelon (d), and otic pit (op), with the strongest expression detected at the apical surface. In the epithelium of the branchial arch (b1) (D′), foregut diverticulum (fgd) (D"), and surface ectoderm, expression was seen only at the apical face of the cells (arrowheads). Magnifications: panel D, ×20; panels D′ and D", ×40. Bars, 50 μm. (E) Whole-mount Dlg-LacZ expression in E10.5 embryos. (F) Endogenous Dlg expression in E10.5 wild-type embryos detected using Dlg antisera. Bars, 500 μm. (G) Dlg-LacZ expression in the brain (E12.5) was seen in the ventricular zone of the hypothalmus (vzh) and hippocampus (h). In the eye, Dlg-LacZ was expressed in the neural retina (nr), lens (l), retinal pigmented epithelium (rpe), and optic chiasm (oc). Magnification, ×5; bar, 200 μm. (H) Whole-mount Dlg-LacZ expression at E14.5 after only 4 h of X-Gal staining. Bar, 1 mm. (I) In the somites (E9.5), expression was detected in the epithelial dermomyotome (dm) component at the face adjacent to the scleratome (sc) (arrows). Magnification, ×40; bar, 25 μm. (J) Expression was seen predominantly at the apical membrane of epithelial cells (e) (arrows) within the lung (E14.5) but also at all membranes of epithelial cells of the (K) kidney (E14.5) and (L) gut (E14.5). Magnification in panel J, ×20; bar, 50 μm. Magnification in panel K, ×40; bar, 8 μm. Magnification in panel L, ×40; bar, 50 μm. b1 and b2, branchial arches 1 and 2, respectively; cb, cerebellum; ey, eye; g, gut; h, heart; lb, limb bud; l, lung; ma, mandible; m, maxillary component of b1; me, mesenchyme; nc, nasal cavity; np, nasal pit; ne, neural epithelium; n, notochord; ot, otic vesicle; pn, pons; sc, spinal cord; sn, spinal nerve; tb, tailbud; to, tongue; t, telencephalon.
At E10.5, expression was observed in the telecephalon, eye, otic vesicle, nasal pit, branchial arches, limb buds, apical ectodermal ridge, and tailbud (Fig. 2E). These patterns of Dlg-LacZ expression were verified by examining expression of the endogenous dlg gene using antisera to the Dlg protein (Fig. 2F). Dlg-LacZ was expressed throughout the brain in the ventricular zone of the hypothalmus, the ganglionic eminence, the hippocampus, the pons, the cerebellum, the choroid plexus, and the spinal cord (Fig. 2G and H), mirroring the expression pattern reported for SAP97 in the rat brain (29). Expression was also detected in the peripheral nervous system, for example, in the trigeminal ganglia (VG), accessory (X1) nerve, and dorsal root ganglia (data not shown). In the developing eye, expression was associated with the neural retina, the retinal pigmented epithelium, the lens, and the optic chiasma and nerve (Fig. 2G).
At E14.5, Dlg-LacZ expression continued in the mesenchyme and epithelium of the nasal cavity and in the oral cavity (maxillary and mandibular). Expression was also observed in the epithelium of the developing gut, lung, kidney, and pancreas (Fig. 2J, K, and L; data not shown). Dlg-LacZ was expressed at all membranes in the epithelium of the gut and kidney (E14.5; Fig. 2L and K) but was restricted to the apical membrane in the epithelium of the lung (E14.5; Fig. 2J). Dlg-LacZ was also expressed in the surface ectoderm, developing epidermis, and hair follicles (data not shown). The expression pattern of Dlg-LacZ was also monitored throughout embryogenesis in homozygous dlggt embryos and was identical to that observed in dlggt/+ embryos (data not shown).
Truncation of Dlg affects its subcellular localization.
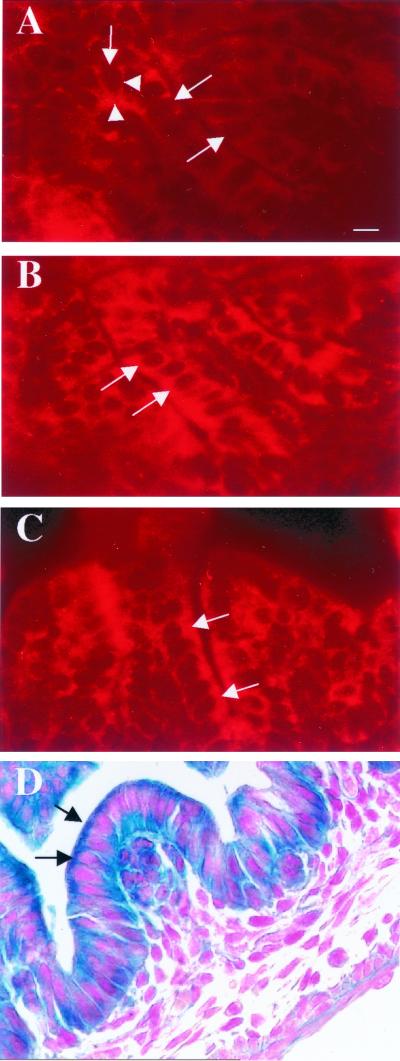
Recent deletion studies of Dlg have demonstrated that various domains are important in the localization of the protein (16, 48). In mammals, the first 65 amino acids of Dlg are essential for its localization to the inner lateral cell membrane at regions of cell-cell contact in epithelial cells (48). The regions which bind protein 4.1, which in turn associate with the cytoskeleton, also contribute to the localization of Dlg. The SH3 and GUK domains do not appear to be necessary for Dlg localization in epithelial cells (48). Because integration of the gene trap vector into Dlg results in the loss of one of the protein 4.1 binding domains, we next investigated whether localization of the protein was affected using an antibody directed against the N terminus of Dlg. As shown in Fig. 3A, the wild-type Dlg protein was localized predominantly to the basal and lateral membranes of colon epithelial cells, as previously reported (27, 29). In both dlggt/+ and dlggt/dlggt colon epithelial cells, the overall expression levels appeared higher and strong expression was also observed at the apical membrane, in addition to the expression seen at the basal and lateral membranes (Fig. 3B and C). The basal, lateral, and apical expression of Dlg-LacZ determined by immunofluorescence (Fig. 3B and C) correlates with the LacZ expression observed in colon epithelial cells of dlggt mutant embryos (Fig. 3D).
FIG. 3.
Localization of Dlg in colon epithelial cells. Sections of E17.5 colons were stained with an anti-Dlg antibody. (A) Dlg was localized predominantly to the lateral and basal (arrowheads) membranes in wild-type epithelial cells. In dlggt/+ (B) and dlggt/dlggt (C) colon epithelial cells, Dlg-LacZ was expressed at the basal and lateral membranes and also highly expressed at the apical membrane. Arrows depict the apical membrane of epithelial cells. Magnification, ×100; bar, 12.5 μm.
Disruption of murine dlg results in craniofacial abnormalities.
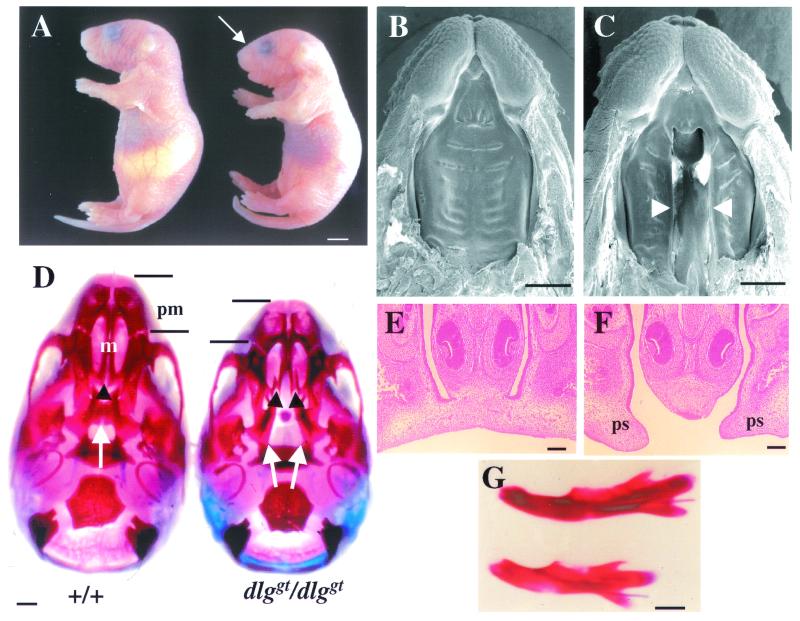
Newborn pups homozygous for the dlggt mutation lacked milk in their stomachs, became cyanotic, and developed distended abdomens as a result of a buildup of air in their stomachs and intestines. All dlggt/dlggt pups were smaller than their wild-type and heterozygous littermates and exhibited shortened snouts and dome-shaped skulls (Fig. 4A). Approximately 50% of the dlggt/dlggt pups displayed kinked or ventrally curled tails, and all dlggt/dlggt pups died within 24 h of parturition. Growth retardation was observed in utero from around E14.5.
FIG. 4.
Phenotype of dlggt/dlggt embryos and mice. (A) Newborn dlggt/dlggt pups are smaller than their wild-type littermates, lack milk in their stomachs, and have dome-shaped skulls (arrow). Bar, 2.5 mm. SEM of the upper jaws of wild-type (B) and dlggt/dlggt (C) embryos at E16.5 is shown. Arrowheads depict the cleft secondary palate. Bar, 1 mm. (D) Whole-mount alizarin red- and alcian blue-stained skeletal preparations of the skulls of wild-type and dlggt/dlggt mutant E18.5 embryos. In wild-type embryos, the palatine (arrows) and maxillary (m) (arrowheads) shelves are fused. In dlggt/dlggt mutant embryos, both shelves are open. Shortening of the premaxilla (pm) is also indicated. Bar, 1 mm. Coronal sections of E15.5 wild-type (E) and dlggt/dlggt mutant (F) embryos demonstrate partial elevation and lack of contact and fusion of the palatal shelves (ps). Magnification, ×10; bar, 100 μm. (G) Whole-mount alizarin red and alcian blue skeletal staining of the mandibles of wild-type (top) and dlggt/dlggt mutant embryos (bottom). Bar, 1 mm.
The inability of dlggt/dlggt pups to feed and the change in the shape of the skull suggested that these animals had skeletal abnormalities. The most obvious defect was a cleft secondary palate, allowing a direct view into the nasal cavity (Fig. 4B, C, E, and F). Skeletal staining demonstrated that both the maxillary and palatine shelves were cleft (Fig. 4D). In addition, dlggt/dlggt mice demonstrated premaxilla and mandible hypoplasia (Fig. 4D and G). The width of the skull was not altered (Fig. 4D).
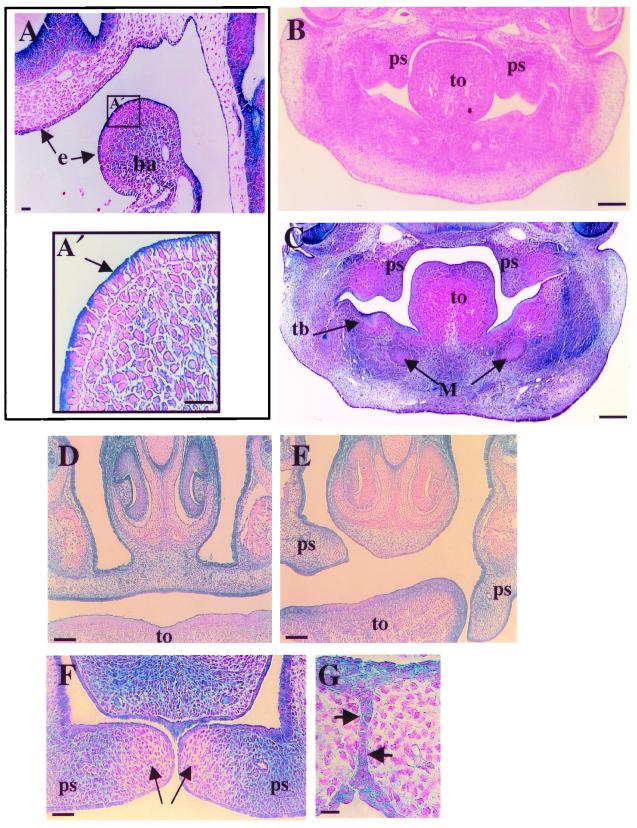
Normal palate formation relies on a number of timed developmental processes (7). Therefore, we histologically analyzed mutant embryos at relevant time points to monitor the progression of palatal development and the expression of the Dlg-LacZ fusion protein. The Dlg-LacZ protein was expressed in the ectomesenchyme, mesoderm core, and apical surface of epithelial cells of the first branchial arch, which gives rise to the maxillary and mandibular components (Fig. 2D and 5A). At E12.5, the palatal shelves of dlggt/dlggt embryos were normal in size and grew down the side of the tongue (Fig. 5B and C). Dlg-LacZ was expressed in both the mesenchyme and epithelial cells of the palatal shelves (Fig. 5C). Expression was also seen in the epithelium of the developing tooth bud (Fig. 5C); however, tooth development was not affected in dlggt/dlggt mice. At E16.5, when palatal-shelf elevation and fusion should normally be complete (Fig. 5D), dlggt/dlggt embryos displayed only partial elevation of both palatal shelves, total elevation of one shelf but not the other, or elevation of both palatal shelves but lack of fusion at the midline (Fig. 4F and 5E). When one palatal shelf was elevated, we observed that the tongue was trapped between the palatal shelf and nasal septum, perhaps inhibiting palatal elevation (Fig. 5E). Although there appeared to be variations in palatal-shelf elevation, the resulting cleft palate in dlggt homozygotes occurred with complete penetrance and was observed on the 129/ICR, C57BL/6 (six backcrosses), and BALB/c (six backcrosses) backgrounds. Prior to palatal-shelf fusion in dlggt/+ embryos (Fig. 5F) and in unfused palatal shelves in dlggt/dlggt embryos (Fig. 5E), we noted low levels of LacZ expression in the mesenchyme at the tips of the palatal shelves, with stronger expression adjacent to this region (Fig. 5F). Dlg-LacZ expression was also seen in the medial-edge epithelial cells prior to and at the time of palatal fusion (Fig. 5G). Strong Dlg-LacZ expression was also seen in the pseudostratified ciliated columnar and stratified squamous epithelia of the nasal and oral cavities, respectively (Fig. 5D to F).
FIG. 5.
Histology of palate development and Dlg-LacZ expression in mutant dlggt embryos. (A) Dlg-LacZ was expressed at the apical membrane of the epithelium of the first branchial arch (ba) and the epithelium (e) associated with the olfactory placode in dlggt/+ embryos (E9.5) (arrows). Magnification, ×20; bar, 50 μm. (A′) Higher magnification (×40) of the boxed area in panel A demonstrating the apical expression of Dlg-LacZ in the epithelium of the branchial arch. Bar, 25 μm. Coronal sections of E12.5 wild-type (B) and dlggt/dlggt (C) heads, the latter demonstrating Dlg-LacZ expression in the palatal shelves (ps), toothbud (tb), and facial mesenchyme. Magnification, ×5; bar, 200 μm. (D) Palatal shelves are elevated and fused in dlggt/+ E15.5 embryos. (E) Only one palatal shelf is elevated in dlggt/dlggt embryos. Dlg-LacZ is expressed within the mesenchyme and epithelial cells of the fused palatal shelves (D) and unfused palatal shelves (E). Magnification, ×10; bar, 100 μm. (F) Dlg-LacZ expression in the palatal shelves of dlggt/+ prior to fusion demonstrates low levels of expression in the tips of the shelves (arrows) with higher expression adjacent to this region. Magnification, ×20. Bar, 200 μm. (G) Dlg-LacZ expression was observed in the medial-edge epithelial cells at the time of palatal-shelf fusion (arrows). Magnification, ×100; bar, 100 μm. M, Meckel's cartilage; to, tongue.
DISCUSSION
In this study, we demonstrated that disruption of the murine dlg gene results in craniofacial dysmorphogenesis. The craniofacial structures affected are derived from cephalic neural crest cells, cells that migrate during development from the posterior midbrain-hindbrain region into the first branchial arch. Here, the neural crest-derived ectomesenchyme undergoes inductive changes, resulting in the development of craniofacial bones and cartilage (21). Dlg-LacZ was expressed in structures containing neural crest cells or their derivatives, such as the cephalic head folds, rhombomeres, branchial arches, peripheral nerves of the head, and mesenchymal and epithelial cells throughout craniofacial and palatal morphogenesis. Together, these data suggest that there is a requirement for Dlg in craniofacial development, possibly in a subset of neural crest cells or their derivatives.
The palatal shelves arise as bilateral outgrowths of the maxillary process of the first branchial arch. The shelves grow vertically down the sides of the tongue and then elevate into a horizontal position above the tongue, adhere at the midline, and fuse (7). Analysis of a number of mutant mice has demonstrated that development of a cleft palate can result from the disruption of any one of these processes or as a consequence of disruption of the development of other craniofacial structures (7, 8). In dlggt/dlggt embryos, palatal-shelf elevation occurred in some instances; however, contact and subsequent fusion were never observed. That elevation did occur in some embryos suggests that mechanical hindrance, possibly due to shortening of the lower jaw and mispositioning of the tongue, may have prevented or delayed palatal-shelf elevation (7). Interestingly, Dlg-LacZ expression was continuous throughout the mesenchyme of the palatal shelves at E12.5 (Fig. 5C). However, upon palatal-shelf elevation and just prior to fusion, Dlg-LacZ was not uniformly expressed but was present at very low levels at the tips of the shelves with elevated levels of expression adjacent to this region (Fig. 5F). These data suggest that Dlg expression is regulated spatially during palate formation. Strong Dlg-LacZ expression was also observed in the medial-edge epithelial cells, suggesting that Dlg plays a role in cell adhesion between the two opposing palatal shelves and/or in the transformation of epithelial cells to mesenchymal cells during the fusion process.
Interestingly, the phenotype exhibited by the dlggt/dlggt mutants is similar to that resulting from a transgene insertion in CASK, another MAGUK family member (22, 44). The CASK gene is X linked, and insertional mutagenesis results in shortening of the mandible and a cleft secondary palate in male mice. Unlike the CASK mutants, the snouts of the dlggt/dlggt mutants were not pointed. This difference may reflect the fact that, in addition to the smaller mandible, the premaxilla was also shorter in dlggt/dlggt mutants, the likely cause of the overall dome-shaped appearance of the skull and a likely contributing factor in the formation of the cleft palate in these mice. The lack of contact and fusion of the palatal shelves that did elevate may be due to a reduction in the size of the palatal shelves or a delay in palatal-shelf elevation. In addition, both CASK and dlggt/dlggt mutant mice displayed kinks in their tails. The similarity in the phenotypes of mice carrying mutations in these MAGUK family members may be explained by recent findings showing that the SH3 domain of Dlg interacts with the GUK domain of CASK (31). In dlggt/dlggt mutants, this intermolecular interaction is lost, thus raising the intriguing possibility that the Dlg-CASK scaffolding complex is involved in craniofacial morphogenesis.
In addition, the SH3 and GUK domains of MAGUKs have also been shown to be involved in intramolecular interactions (28, 31, 36, 47). This association has been shown to regulate the intermolecular binding of MAGUKs (31), the binding of the GUK-interacting GKAP (47), and the clustering of PDZ binding proteins (36). Together, these data suggest that the loss of the SH3 and GUK domains in the dlggt/dlggt mutants may disrupt any one of these functions of Dlg which may be critical for normal craniofacial development.
The loss of cell growth control in Drosophila dlg mutants suggests that the Dlg protein possesses tumor suppressor activity. Recent studies have demonstrated that in association with the tumor suppressor APC, Dlg negatively regulates cell proliferation by blocking cell cycle progression from the G0/G1 phase to the S phase. Although APC interacts with the second PDZ domain of Dlg, mutation of the SH3 or GUK-like domain abolished the inhibition of cell cycle progression (17). Thus, it is possible that the SH3-GUK interactions described above also regulate the control of cell proliferation in conjunction with APC. Interestingly, we did not observe any obvious tumor phenotype in either heterozygous (>18 months) or homozygous dlggt mutant mice. Thus, any possible tumor suppressor function of murine Dlg may be compensated by other members of the MAGUK family or it may be that tumor formation can only be detected in homozygous dlggt mice if they survive to adulthood.
Dlg is also involved in protein localization to specialized membrane sites. In epithelial cells, the first 65 amino acids of Dlg (SAP97) (which are absent in other MAGUKs, including the Drosophila homologue), in conjunction with the protein 4.1 binding domains, are involved in localization of the protein to regions of cell contact (48). The Dlg-LacZ fusion protein retains the N-terminal 65 amino acids and the protein 4.1 binding region but lacks the C-terminal protein 4.1 binding domain. The subcellular expression pattern of Dlg-LacZ in mesenchymal cells during craniofacial development appeared throughout the cytoplasm. During craniofacial development, Dlg-LacZ was first expressed at the apical face of the epithelial cells lining the branchial arches (Fig. 2D and 5A), which we confirmed to be consistent with the localization of the endogenous Dlg protein (data not shown). Subsequently, Dlg was expressed at all membranes once the palatal shelves had started to develop (Fig. 5). To date, the localization of Dlg has only been analyzed in polarized epithelial cells, such as those of the imaginal discs in Drosophila and colonic epithelial cells in the rat (27, 29, 45). In the latter, Dlg has been reported to localize to the basolateral membranes of epithelial cells (27, 29). In this study, we observed Dlg-LacZ in epithelial cells throughout gut development, with expression initially being localized to the apical surface in early gut epithelial cells (E8.5) (Fig 2D) and then at all membranes of gut epithelial cells later in development (E14.5 to E18.5) (Fig. 2L and 3.). This observation suggests that the localization of Dlg is developmentally regulated within epithelial cells during the development of various tissue structures. As expected, the expression pattern of endogenous Dlg in wild-type colon epithelial cells (E18.5) was absent from the apical membrane (Fig 3A; reference 29). These results therefore suggest that loss of the protein 4.1, SH3, and/or GUK-like domains prevents downregulation of Dlg-LacZ expression from the apical surface. Thus, the sustained apical expression of the Dlg-LacZ fusion protein may reflect overexpression of Dlg-LacZ, possibly due to increased stability of the fusion protein (43). This raises the question of whether the gene trap insertion that created the Dlg-LacZ fusion protein is a gain-of-function event leading to the expression of a novel protein which can localize proteins which interact with the retained PDZ domains to the apical surface and/or are able to interact with new binding partners found only at the apical surface. However, even though additional expression was observed in the gut epithelial cells, no overt phenotype was observed in the gut epithelium of newborn mutant mice. The possible scenarios described above may contribute to the disruption of craniofacial development seen in dlggt/dlggt mutants; however, arguing against this possibility is the lack of any phenotype in the dlggt/+ heterozygotes.
Although Dlg-LacZ was expressed in a number of tissues, the phenotype of the dlggt/dlggt mice demonstrated that the SH3, protein 4.1, and/or GUK-like domains were essential to the function of Dlg within a subset of cells, possibly of neural crest origin, involved in craniofacial development. Therefore, in other tissues that express Dlg but where we observed no defects, other MAGUK family members may compensate for the loss of Dlg function. The identification of additional Dlg binding partners and the generation of new mutant dlg alleles will further clarify the biological functions of the various protein-protein interacting domains of Dlg and the molecular mechanisms involved in craniofacial and palatal morphogenesis. Interestingly, human Dlg is localized to chromosome 3q29 (2, 4), a region that is duplicated in partial trisomy 3q syndromes which commonly display craniofacial defects (6, 34). Thus, the phenotype exhibited by the dlggt/dlggt mutant mice makes dlg a candidate gene for the craniofacial defects associated with these human disorders.
ACKNOWLEDGMENTS
We thank S. Tondat for ES cell aggregations; K. Harpal for sectioning of embryos; D. Holmyard for SEM; W. Skarnes for providing the GT1.8geo vector; and D. Donoviel, A. Hart, M. Puri, and L. Velazquez for helpful discussions and critical reading of the manuscript.
This work was supported by a Terry Fox Program Project Grant from the National Cancer Institute of Canada, the Canadian Institutes of Health Research, and Bristol-Myers Squibb, Inc. G.C. is a recipient of an Ontario Research and Development Challenge Funds Multidisciplinary Program in Biomedical Research for the 21st Century fellowship.
REFERENCES
- 1.Adey N B, Huang L, Ormonde P A, Baumgard M L, Pero R, Byreddy D V, Tatigian S V. Threonine phosphorylation of the MMAC/PTEN PDZ binding domain both inhibits and stimulates PDZ binding. Cancer Res. 2000;60:35–37. [PubMed] [Google Scholar]
- 2.Alexander C, Stathakis D G, Lin L, Rahman S, Bryant P J, Auburger G, Chishti A H. Fine scale mapping places DLG1, the gene encoding hDlg, telomeric to the OPA1 candidate region. Mamm Genome. 1997;8:795–796. doi: 10.1007/s003359900578. [DOI] [PubMed] [Google Scholar]
- 3.Anderson J M. Cell signalling: MAGUK magic. Curr Biol. 1996;6:382–384. doi: 10.1016/s0960-9822(02)00501-8. [DOI] [PubMed] [Google Scholar]
- 4.Azim A C, Knoll J H, Marfatia S M, Peel D J, Bryant P J, Chishti A H. DLG1: chromosome location of the closest human homologue of the Drosophila discs large tumor suppressor gene. Genomics. 1995;30:613–616. doi: 10.1006/geno.1995.1286. [DOI] [PubMed] [Google Scholar]
- 5.Dimitratos S D, Woods D F, Stathakis D G, Bryant P J. Signaling pathways are focussed at specialized regions of the plasma membrane by scaffolding proteins of the MAGUK family. Bioessays. 1999;21:912–921. doi: 10.1002/(SICI)1521-1878(199911)21:11<912::AID-BIES3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 6.Fear C, Brigg A. Familial partial trisomy of the long arm of chromosome 3 (3q) Arch Dis Child. 1979;54:135–138. doi: 10.1136/adc.54.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson M W. Craniofacial malformations: towards a molecular understanding. Development. 1988;103(Suppl.):41–60. [Google Scholar]
- 8.Francis-West P, Ladher R, Barlow A, Graveson A. Signalling interactions during facial development. Mech Dev. 1998;75:3–28. doi: 10.1016/s0925-4773(98)00082-3. [DOI] [PubMed] [Google Scholar]
- 9.Gaudet S, Branton D, Lue R A. Characterization of PDZ-binding kinase, a mitotic kinase. Proc Natl Acad Sci USA. 2000;97:5167–5172. doi: 10.1073/pnas.090102397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonda T J, Sheiness D K, Bishop J M. Transcripts from the cellular homologs of retroviral oncogenes: distribution among chicken tissues. Mol Cell Biol. 1982;2:617–624. doi: 10.1128/mcb.2.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanada T, Lin L, Chandy K G, Oh S S, Chishti A H. Human homologue of the Drosophila Discs large tumor suppressor binds to p56lck tyrosine kinase and Shaker Type Kv1.3 potassium channel in T lymphocytes. J Biol Chem. 1997;272:26899–26904. doi: 10.1074/jbc.272.43.26899. [DOI] [PubMed] [Google Scholar]
- 12.Hanada T, Lin L, Tibaldi E V, Reinherz E L, Chishti A H. GAKIN: a novel kinesin-like protein associates with the human homologue of the Drosophila Discs Large tumor suppressor in T lymphocytes. J Biol Chem. 2000;275:28774–28784. doi: 10.1074/jbc.M000715200. [DOI] [PubMed] [Google Scholar]
- 13.Hayat M. Principles and techniques of scanning electron microscopy: biological applications. New York, N.Y.: Van Nostrand Rienhold; 1974. [Google Scholar]
- 14.Henkemeyer M, Marengere L E M, McGlade J, Olivier J P, Conlon R A, Holmyard D P, Letwin K, Pawson T. Immunolocalization of the Nuk receptor tyrosine kinase suggests roles in segmental patterning of the brain and axonogenesis. Oncogene. 1994;9:1001–1014. [PubMed] [Google Scholar]
- 15.Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the mouse embryo: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 16.Hough C D, Woods D F, Park S, Bryant P J. Organizing a functional junctional complex requires specific domains of the Drosophila MAGUK Discs large. Genes Dev. 1997;11:3242–3253. doi: 10.1101/gad.11.23.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishidate T, Matsumine A, Toyoshima K, Akiyama T. The APC-hDLG complex negatively regulates cell cycle progression from the G0/G1 to S phase. Oncogene. 2000;19:365–372. doi: 10.1038/sj.onc.1203309. [DOI] [PubMed] [Google Scholar]
- 18.Kim E, Naisbitt S, Hseuh Y P, Rao A, Rothschild A, Craig A M, Sheng M. GKAP, a novel synaptic protein that interacts with the guanylate kinase-like domain of the PSD-95/SAP90 family of channel clustering molecules. J Biol Chem. 1997;136:669–678. doi: 10.1083/jcb.136.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim E, Niethammer M, Rothschild A, Jan Y N, Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- 20.Kiyono T, Hiraiwa A, Fujita M, Hayashi Y, Akiyama T, Ishibashi M. Binding of high-risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophila discs large tumor suppressor protein. Proc Natl Acad Sci USA. 1997;94:11612–11616. doi: 10.1073/pnas.94.21.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kontges G, Lumsden A. Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development. 1996;122:3299–3242. doi: 10.1242/dev.122.10.3229. [DOI] [PubMed] [Google Scholar]
- 22.Laverty H G, Wilson J B. Murine CASK is disrupted in a sex-linked cleft palate mouse mutant. Genomics. 1998;53:29–41. doi: 10.1006/geno.1998.5479. [DOI] [PubMed] [Google Scholar]
- 23.Lee S S, Weiss R S, Javier R T. Binding of human virus oncoproteins to hDlg/SAP97, a mammalian homolog of the Drosophila discs large tumor suppressor protein. Proc Natl Acad Sci USA. 1997;94:6670–6675. doi: 10.1073/pnas.94.13.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leonard A S, Davare M A, Horne M C, Garner C C, Hell J W. SAP97 is associated with the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit. J Biol Chem. 1998;273:19518–19524. doi: 10.1074/jbc.273.31.19518. [DOI] [PubMed] [Google Scholar]
- 25.Lin L, Sahr K E, Chishti A H. Identification of the mouse homologue of human discs large and rat SAP97 genes. Biochim Biophys Acta. 1997;1362:1–5. doi: 10.1016/s0925-4439(97)00059-8. [DOI] [PubMed] [Google Scholar]
- 26.Lue R A, Marfatia S M, Branton D, Chishti A H. Cloning and characterization of hdlg: the human homologue of the Drosophila discs large tumor suppressor binds to protein 4.1. Proc Natl Acad Sci USA. 1994;91:9818–9822. doi: 10.1073/pnas.91.21.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsumine A, Ogai A, Senda T, Okumure T, Kobayashi S, Okada M, Toyoshima K, Akiyama T. Binding of APC to the human homolog of the Drosophila discs large tumor suppressor protein. Science. 1996;272:1020–1023. doi: 10.1126/science.272.5264.1020. [DOI] [PubMed] [Google Scholar]
- 28.McGee A W, Bredt D S. Identification of an intramolecular interaction between the SH3 and guanylate kinase domains of PSD-95. J Biol Chem. 1999;274:17431–17436. doi: 10.1074/jbc.274.25.17431. [DOI] [PubMed] [Google Scholar]
- 29.Müller B M, Kistner U, Veh R W, Cases-Langhoff C, Becker B, Gundelfinger E D, Garner C C. Molecular characterization and spatial distribution of SAP97, a novel presynaptic protein homologous to SAP90 and the Drosophila discs-large tumor suppressor protein. J Neurosci. 1995;15:2354–2366. doi: 10.1523/JNEUROSCI.15-03-02354.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder J C. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci USA. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nix, S. L., A. H. Chishti, J. M. Anderson, and Z. Walther. hCASK and hDlg associate in epithelia, and their SH3 and guanylate kinase domains participate in both intramolecular and intermolecular interactions. J. Biol. Chem., in press. [DOI] [PubMed]
- 32.Pim D, Thomas M, Javier R, Gardiol D, Banks L. HPV E6 targeted degradation of the discs large protein: evidence for the involvement of a novel ubiquitin ligase. Oncogene. 2000;19:719–725. doi: 10.1038/sj.onc.1203374. [DOI] [PubMed] [Google Scholar]
- 33.Pirity M, Hadjantonakis A K, Nagy A. Embryonic stem cells, creating transgenic animals. Methods Cell Biol. 1998;57:279–293. doi: 10.1016/s0091-679x(08)61585-x. [DOI] [PubMed] [Google Scholar]
- 34.Salazar D, Rosenfeld W, Verma R S, Jhaveri R C, Dosik H. Partial trisomy of chromosome 3 (3q12-qter) owing to 3q/18p translocation. Am J Dis Child. 1979;133:1006–1008. doi: 10.1001/archpedi.1979.02130100030005. [DOI] [PubMed] [Google Scholar]
- 35.Satoh K, Yanai H, Senda T, Kohu K, Nakamura T, Okamura N, Matsumine A, Koyabashi S, Toyoshima K, Akiyama T. DAP-1, a novel protein that interacts with the guanylate kinase-like domains of hDLG and PSD-95. Genes Cells. 1997;2:415–424. doi: 10.1046/j.1365-2443.1997.1310329.x. [DOI] [PubMed] [Google Scholar]
- 36.Shin H, Hsueh Y-P, Yang F-C, Kim E, Sheng M. An intramolecular interaction between Src homology 3 domain and guanylate kinase-like domain required for channel clustering by postsynaptic density-95/SAP90. J Neurosci. 2000;20:3580–3587. doi: 10.1523/JNEUROSCI.20-10-03580.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skarnes W C, Moss J E, Hurtley S M, Beddington R S P. Capturing genes encoding membrane and secreted proteins important for mouse development. Proc Natl Acad Sci USA. 1995;92:6592–6596. doi: 10.1073/pnas.92.14.6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sonyang Z, Fanning A S, Fu C, Xu J, Marfatia S M, Chishti A H, Crompton A, Chan A C, Anderson J M, Cantley L C. Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science. 1997;265:73–77. doi: 10.1126/science.275.5296.73. [DOI] [PubMed] [Google Scholar]
- 39.Stanford W L, Caruana G, Vallis K A, Inamdar M, Hidaka M, Bernstein A. Expression trapping: identification of novel genes expressed in hematopoietic and endothelial lineages by gene trapping in ES cells. Blood. 1998;92:4622–4631. [PubMed] [Google Scholar]
- 40.Stewart M, Murphy C, Fristrom J. The recovery and preliminary characterization of X chromosome mutants affecting imaginal discs of Drosophila melanogaster. Dev Biol. 1972;27:71–83. doi: 10.1016/0012-1606(72)90113-3. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki T, Ohsugi Y, Uchida-Toita M, Akiyama T, Yoshida M. Tax oncoprotein of HTLV-1 binds to the human homologue of the Drosophila discs large tumor suppressor protein, hDlg, and perturbs its function in cell growth control. Oncogene. 1999;18:5967–5972. doi: 10.1038/sj.onc.1203008. [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi M, Hata Y, Hirao K, Toyoda A, Irie M, Takai Y. SAPAPs: a family of PSD-95/SAP90-associated proteins localized at postsynaptic density. J Biol Chem. 1997;272:11943–11951. doi: 10.1074/jbc.272.18.11943. [DOI] [PubMed] [Google Scholar]
- 43.Thomas T, Voss A K, Gruss P. Distribution of a murine protein tyrosine phosphatase BL-beta-galactosidase fusion protein suggests a role in neurite outgrowth. Dev Dyn. 1998;212:250–257. doi: 10.1002/(SICI)1097-0177(199806)212:2<250::AID-AJA9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 44.Wilson J B, Ferguson M W J, Jenkins N A, Lock L F, Copeland N G, Levine A J. Transgenic mouse model of X-linked cleft palate. Cell Growth Differ. 1993;4:67–76. [PubMed] [Google Scholar]
- 45.Woods D F, Bryant P J. The Discs-Large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- 46.Woods D F, Hough C, Peel D, Callaini G, Bryant P J. Dlg protein is required for junction structure, cell polarity, and proliferation control in Drosophila epithelia. J Cell Biol. 1996;134:1469–1482. doi: 10.1083/jcb.134.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu H, Reissner C, Kuhlendahl S, Coblentz B, Reuver S, Kindler S, Gundelfinger E D, Garner C C. Intramolecular interactions regulate SAP97 binding to GKAP. EMBO J. 2000;19:5740–5751. doi: 10.1093/emboj/19.21.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu H, Reuver S M, Kuhlendahl S, Chung W J, Garner C C. Subcellular targeting and cytoskeletal attachment of SAP97 to the epithelial lateral membrane. J Cell Sci. 1998;111:2365–2376. doi: 10.1242/jcs.111.16.2365. [DOI] [PubMed] [Google Scholar]