Abstract
Toxocara canis is a helminth zoonosis that is estimated to infect more than 100 million dogs and 1 billion people, mostly in the tropics. Humans can be infected by accidentally ingesting embryonated T. canis eggs from the environment or occasionally after ingesting L3 larvae from paratenic hosts. This study investigated the importance of vertical transmission and the role of puppies in the epidemiology of T. canis through the examination of fecal samples from dogs less than one year of age in Grenada, West Indies, a small island tropical developing country. Samples were stored at 4 °C or in 10% formalin until microscopic examination for helminth eggs or using a rapid antigen test for the presence of protozoan species. A knowledge, attitudes and practices study was completed among dog owners, physicians and veterinary students.
Of 306 dogs less than one year of age, 147 (48%) were found to have T. canis eggs. Vertical transmission was indicated by the proportion of infected dogs increasing from 50% at two weeks of age (from in utero transmission) to 70% by 12 weeks (in utero and lactogenic transmission). After 12 weeks the positivity rate dropped rapidly with no dogs over 40 weeks of age being infected. As T. canis eggs were found in puppy feces at two weeks of age, initial treatment of puppies should begin earlier, at twelve days post-partum, than currently recommended to prevent shedding of eggs. Perhaps even more importantly, treatment of pregnant dogs, preventing vertical transmission, would have a major impact on the control of T. canis infection.
Knowledge of T. canis and other zoonotic helminths such as Ancylostoma caninum was found to be low among dog owners, physicians and veterinary students. None of the dog owners treated their dogs for helminths, all were unaware of the risk of zoonoses, and only 9% picked up dog feces. Efforts to prevent vertical transmission and to increase awareness and knowledge of these zoonoses could result in reducing their public health impact.
Keywords: Toxocara canis, Ancylostoma caninum, Puppies, Vertical transmission, Epidemiology, Control
1. Introduction
Toxocara canis is a helminth parasite with a worldwide distribution estimated to infect tens of millions of people annually (Fakhri et al., 2018; Ma et al., 2018; Dutra et al., 2014; Morgan et al., 2013; Macpherson, 2013). A recent meta-analysis suggests that over 100 million dogs are infected with T. canis, shedding billions of eggs into the environment annually (Rostami et al., 2020). The highest prevalence of T. canis was reported in young (< 1 year of age), stray, rural, male dogs, that live in tropical countries with a low Gross Domestic Product (Rostami et al., 2020; Hotez and Wilkins, 2009). Such conditions are ideal for the propagation of this helminth due to free roaming definitive hosts with unchecked dog reproduction ensuring a large percentage of young dogs that are untreated for the parasite while living in environmental conditions favorable to the parasite.
There are many routes of transmission of T. canis in dogs and the prepatent period (PPP) varies with the mode of transmission. Vertical transmission occurs when somatic larvae are reactivated during the third trimester of pregnancy, invading the gestational sac, and infecting the developing embryos (Roberts et al., 2013; Schnieder et al., 2011). Physiological changes during pregnancy alter the signaling pathways of the larvae leading to reactivation and migration of larvae (Schnieder et al., 2011; Ma et al., 2019). Puppies are born with prepatent worms and egg expulsion has been reported to start around day 16 postpartum (Lloyd et al., 1983). Vertical transmission continues postpartum as larvae can be passed lactogenically to the newborn pups (Ma et al., 2019; Burke and Roberson, 1985) with a PPP of 28 days (Stoye, 1976). Horizontal transmission is facilitated through ingestion of embryonated eggs with a PPP of 32–35 days (Dubey, 1978) or through ingestion of paratenic hosts with a PPP of 34–48 days (Manhardt and Stoye, 2010).
Transmission to humans is via ingestion of larvae from paratenic hosts or eggs from the environment. Once embryonated eggs are ingested, they travel down the digestive tract, hatch in the small intestine, penetrating its wall, enter the circulatory system and migrate to the liver, lungs, eyes or central nervous system, causing both mechanical and immunopathological damage (Hotez and Wilkins, 2009; Lee et al., 2014). Infection can result in any of the four clinical syndromes of toxocariasis: visceral larva migrans (VLM) or visceral toxocariasis (VT), ocular larva migrans (OLM) or ocular toxocariasis, covert toxocariais (CT), and neurotoxocariasis (NT). Toxocara larvae will eventually die within the human host, although the internal tissue damage may be irreparable, especially damage to the eye (Azira and Zeehaida, 2011). Many behavioral factors predispose children to infection such as closer contact with contaminated soil in public parks, playgrounds and beaches (Hotez and Wilkins, 2009) and naïve hygiene practices (Sowemimo et al., 2017).
After young dogs ingest embryonated eggs, L3 larvae are released from the egg, invade the gut wall, and undergo hepatic-tracheal migration ending up in the small intestine where they mature into adult worms (Roberts et al., 2013). With age, larvae tend to become developmentally arrested in tissues. T. canis adult worms, like other members of the Family Ascaroidea, have a life span of only a few months (Urquhart et al., 1987). Other zoonotic parasites investigated in this study were Ancylostoma spp., Strongyloides spp., and Trichuris vulpis.
There are four zoonotic species of Ancylostoma spp. (Stracke et al., 2020), with varying zoonotic importance and some are geographically restricted. All can result in cutaneous larva migrans (CLM), a serpiginous, pruritic, creeping dermal eruption, lasting for a few days to weeks but can be prolonged in A. braziliense infections. A. caninum has recently been shown to reach patency in humans (Ngcamphalala et al., 2020) and is a known cause of eosinophilic enteritis in humans. A. ceylanicum and Uncinaria stenocephala are geographically restricted to South-East Asia and the Pacific and temperate climates, respectively. A. ceylanicum is the only canine hookworm species which is fully adapted to humans, canids and felids and contributes to the burden of disease caused by the soil transmitted helminths. Transmission from dogs to humans is facilitated by percutaneous or ingestion of the L3 larvae from the environment (Stracke et al., 2020). Of the 6 widely recognized strongloides spp., S. stercoralis has a global distribution, particularly in rural areas, throughout the tropics and subtropics. In humans, strongyloidiasis can result in larva currens and non-pathognomonic clinical symptoms including abdominal pain, diarrhea and weight loss with autoinfection in some individuals resulting in life-threatening hyperinfection which can be fatal. Studies using nuclear and mitochondrial DNA sequence polymorphisms have demonstrated that humans and dogs can share genetically similar S. stercoralis populations providing evidence for zoonotic transmission (Sanpool et al., 2019; Jaleta et al., 2017). Strongloides spp. reproduces in the small intestine, but free-living stages can additionally reproduce in the environment and transmission is facilitated through skin penetration by L3 larvae. T. vulpis has been reported in up to 10% of dogs and has been found very rarely to occur in humans and thought to be of minor zoonotic relevance (Dunn et al., 2002).
Zoonotic protozoans include Cryptosporidium spp. and Giardia spp. Both protozoans can be transmitted to humans through ingestion and thought to be mostly waterborne and occasionally foodborne. Using a variety of detection methods, Cryptosporidium infection was found in 6–8% of approximately 18,000 dogs studied (Taghipour et al., 2020). Most human cases of Crytposporidium spp. are due to C. hominis and C. parvum, while the most common Cryptosporidium spp. found in dogs are C. canis (3.6%) and C. parvum (1.8%) (Taghipour et al., 2020). A meta-analysis provided a pooled prevalence of 15.2% Giardia spp. in dogs out of 4.3 million dogs (Bouzid et al., 2015).
This study was conducted to examine the prevalence of T. canis and the role of vertical transmission and the epidemiology and potential for control in the small-island developing-state of Grenada, West Indies. Other helminths and protozoan parasites in puppies were also recorded. This study also explored the knowledge, attitudes and practices (KAP) of dog owners, physicians, and Doctor of Veterinary Medicine (DVM) students who had completed courses in veterinary public health and veterinary parasitology. These groups represent stakeholders who would be responsible for implementing a helminth control program using a One Health approach in a sustainable, cost-effective way.
2. Material and methods
Fecal samples were collected between January and November 2017 from Pothounds less than one year of age. Pothounds are nondescript, medium sized dogs, weighing up to 50 pounds, usually brown or black in colour, and are the most common dog found throughout the Caribbean and tropics (Catan and Macpherson, 2007). They are often loosely owned within a community, and are allowed mostly to roam free. The sample size was determined based on the intent to use point biserial correlation to test for a relationship between parasite positivity and age with 95% confidence, 95% power, and an effect size of 20%.
Fecal samples were collected from puppies following consent from the owner and confirmation of the puppies' ages and lack of any prior treatment with anthelmentics from all communities throughout Grenada. Every effort was made to ascertain the exact age of the puppies sampled from the owner and from physical examination of the puppies. Despite this, some minor variation in recollection may have occurred, especially with puppies older than 1 month of age. Samples were located on each owner's property, usually from under the house or porch where the puppies were seen to congregate.
Collection was from the top of a fresh fecal deposit, and immediately split into two aliquots with one being submerged in 10% formalin and the other stored fresh, placed into a cold box for transportation and stored at 4 °C until examined. Two grams (g) (+/− 0.02 g) of each sample was removed from the formalin, weighed in a small weigh boat, transferred to a paper cup and mixed with 13 mL of zinc sulfate (specific gravity of 1.18). The mixture was poured through a fine mesh tea strainer, and then transferred to a pre-numbered conical tube to be centrifuged at 1200 rpm for 5 min. Additional zinc sulfate was pipetted into each tube to create a positive meniscus onto which a coverslip was placed. After 5 min, the coverslip was removed and placed on a pre-numbered slide for examination by light microscopy using 40× magnification. A sensitivity of almost 90% has been described using this method for detecting helminth eggs, when present, including Toxocara (Dryden et al., 2005). Eggs are not shed continuously and are not evenly distributed in feces. These factors should also be considered when estimating the true prevalence of a particular parasite species in cross-sectional point prevalence surveys. As a consequence, the T. canis prevalence found in this study's puppy population represents the minimum prevalence in this population. T. canis eggs were identified from all other helminth eggs as they have a unique appearance, being 80-85um, round, thick shelled and dense. These characteristics separate them from other helminths, in particular, Toxascaris leonina, which are smaller (55-75um), oval, translucent and are largely geographically restricted to Africa and the Eastern Mediterranean with rare reports from the Western Hemisphere (Rostami et al., 2020).
Fresh fecal aliquots were tested using the Quick Check assay (Thermo Fisher Scientific, USA) on a proportion of the samples to examine the presence of Cryptosporidium and Giardia antigens.
Approval for this study was obtained from the St George's University (SGU) Institutional Animal Care and Use Committee (IACUC) to examine the dogs. All puppies found to be positive for T. canis or other helminths were subsequently treated. Approval from the SGU Institutional Review Board (IRB) was obtained to complete oral and on-line knowledge, attitudes, and practices (KAP) questionnaires from a convenient sample of dog owners, physicians and veterinary students.
3. Results
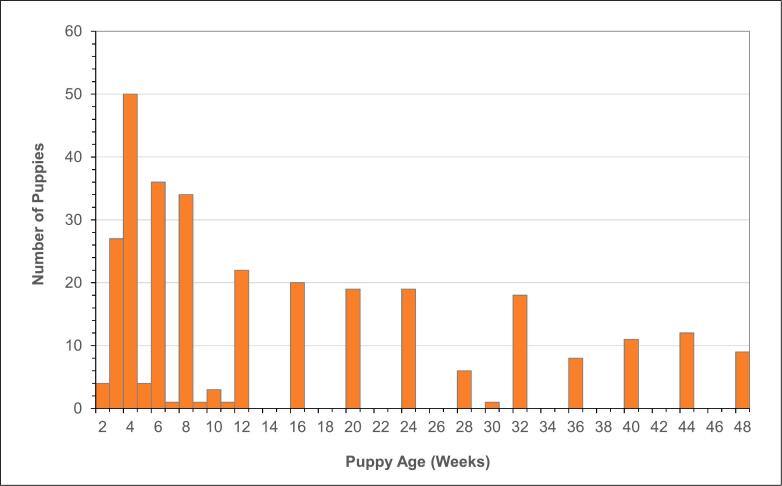
A total of 306 fecal samples were collected from dogs ranging in age from 2 weeks to 48 weeks with a median age of 8 weeks. There were 183 puppies (60%) 12 weeks of age or younger and 123 puppies (40%) over 12 weeks (Fig. 1).
Fig. 1.
Age profile of the sample of 306 puppies providing fecal samples examined for Toxocara canis.
Of the 306 samples, 147 (48%: 95% CI, 42.3% to 53.8%) were positive for T. canis. The youngest puppies found to be shedding T. canis eggs were 2 weeks old and the oldest 40 weeks. Further, 162 samples (53%: 95% CI, 47.5% to 59.0%) tested positive for Ancylostoma spp. with 76 samples (25%: 95% CI, 20.1% to 30.7%) testing positive for both parasites. The rates of positivity and polyparasitism are shown in Fig. 2. Other parasite species were found in 32 samples including Strongyloides spp., Trichuris vulpis, and Isospora spp. (13%: 95% CI, 9.2% to 17.0%) (Fig. 2).
Fig. 2.
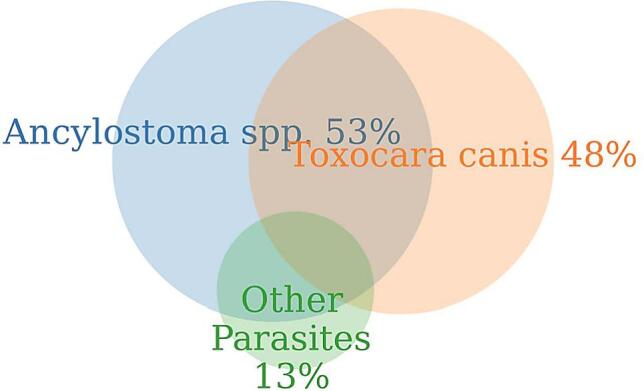
Venn diagram of the proportion of positive cases for Ancylostoma spp. (53%) T. canis (48%) and other parasites commonly found in puppy feces (13%) – Stronglyloides spp., Trichuris vulpis, and Isospora spp. The intersections indicate the occurrence of polyparasitism. Twenty five percent of the cases were positive for both Ancylostoma spp. and T. canis.
Point biserial correlation of positivity and age showed evidence of a moderately strong negative relationship between puppy age and testing positive for T. canis (rpb = −0.396, p < 0.001). In comparison, for Ancylostoma spp. there was evidence of a weak positive relationship (rpb = 0.156, p = 0.006). There was no evidence of a relationship between sex and positivity, since 47% of female and 48% of male puppies tested positive (rpb = 0.058, p = 0.310).
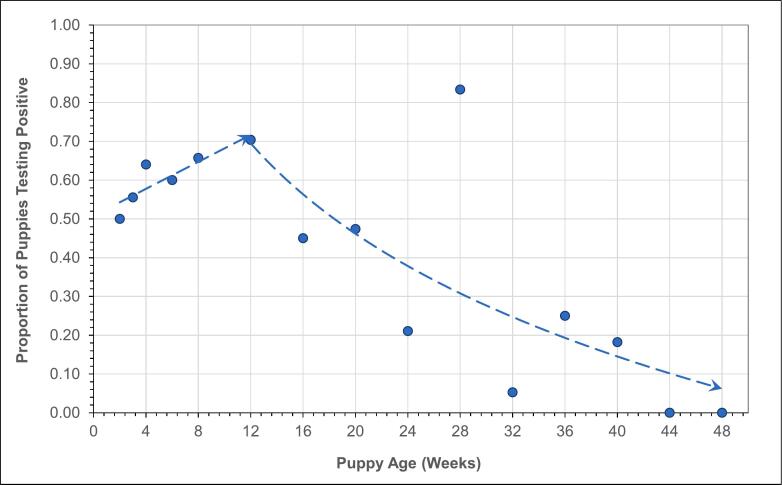
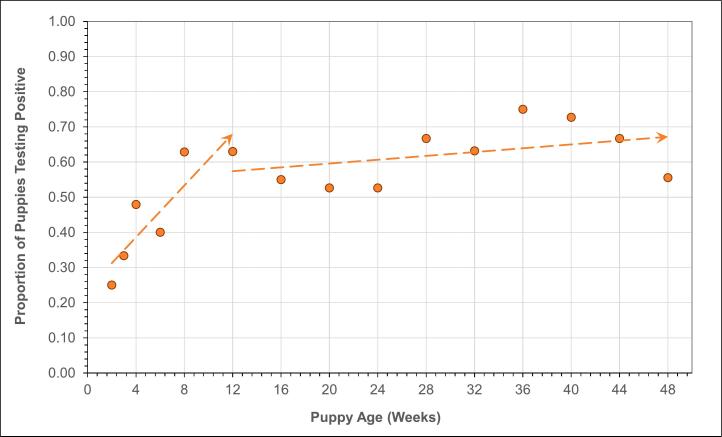
A plot of the proportion of samples positive for T. canis by age shown in Fig. 3 displays a clear trend from two weeks of age (50%) increasing to a peak at 12 weeks (70%), and then a logarithmic decrease to 0% by 48 weeks. In comparison, a similar plot for Ancylostoma spp. in Fig. 4 displays a possible upward trend in positivity from 2 to 12 weeks, but no strong evidence of a change in positivity on average after 12 weeks.
Fig. 3.
Proportion of cases positive for T. canis by puppy age showing a high rate of positivity at 2 weeks (50%) and increasing to 12 weeks of age (70%) followed by a logarithmic decline through 48 weeks to near zero.
Fig. 4.
Proportion of cases positive for Ancylostoma spp. by puppy age showing a low rate of positivity at 2 weeks (25%) and increasing to 12 weeks of age followed by a plateau at about 60% positivity through 48 weeks.
Puppies 12 weeks of age and younger were 4.8 times more likely to be positive for T. canis than puppies over 12 weeks (Odds Ratio = 4.8: 95% CI, 2.9 to 8.0, p < 0.001). In comparison, there was no evidence the proportion of positive cases was different below or above 12 weeks of age for puppies with Ancylostoma spp. (Odds Ratio = 0.6: 95% CI, 0.4 to 1.0, p = 0.977).
A KAP survey was conducted on 35 local puppy owners, 35 community physicians and 60 SGU veterinary students. Based on the small finite size of each target population, the sample sizes were determined with the intent of producing a margin of error of approximately ±10% for the survey results. The KAP survey showed that none of the 35 puppy owners interviewed had any knowledge of zoonoses, 34 (97%) had not spayed or neutered their dogs, 24 (68%) allowed their puppies to roam freely and 3 (9%) cleaned up after their puppies. None of the 35 physicians interviewed had previously diagnosed toxocariasis in children or adults, and consequently, 21 (60%) cited ‘no concern’ regarding the zoonotic potential of T. canis and 6 had ever discussed the potential of any zoonotic diseases with their patients. The majority 39 (65%) of 60 veterinary students surveyed were able to correctly identify the main routes of transmission of toxocariasis to humans, and 4 (7%) ranked T. canis as having significant zoonotic potential whilst 2 (3%) were aware of the clinical syndromes produced by T. canis in humans.
4. Discussion
Vertical transmission was indicated by the proportion of infected dogs increasing from 50% at two weeks of age (from in utero transmission) to 70% by 12 weeks (in utero and lactogenic transmission).
In this study, vertical transmission of T. canis accounted for up to ¾ of all the infections found in the puppies confirming the paramount importance of vertical transmission in the maintenance of this parasite. It is likely that many of the remaining infections were also as a result of vertical transmission but the role of horizontal transmission through the ingestion of embryonated eggs cannot be ruled out. Puppies, through the excretion of millions of eggs, with each female worm capable of contributing 200,000 eggs daily (Harvey et al., 1991) are important hosts for potential human exposure. Eggs expelled by puppies, although highly resistant, are susceptible to environmental conditions, and depend on the accidental ingestion by other definitive and accidental hosts for their successful transmission. Vertical transmission removes environmental constraints and its efficiency results in bitches being the most important reservoirs of T. canis infection and maintenance of the parasite for subsequent generations of the definitive host population (Morgan et al., 2013; Rostami et al., 2020; Kazacos, 1978).
Finding T. canis eggs in puppies two weeks of age strongly suggests that initial treatment of puppies, which for decades has been set at two weeks postpartum (Macpherson, 2013; Nijsse et al., 2015; Harvey et al., 1991; Kazacos, 1978) needs to be re-evaluated. The focus on the treatment of puppies does not prevent the development of T. canis adults in puppies nor their associated clinical manifestations and does not prevent environmental contamination with eggs. The prevention of vertical transmission should be prioritized in any control initiative. Such an approach would need to involve the treatment of pregnant bitches with anthelmintics administered under veterinary supervision as some anthelmintics are not recommended for treatment of a bitch during pregnancy due to negative side effects to unborn pups such as palatoschisis and low birth rates (Ziegler and Macpherson, 2019; Bosse et al., 1980; Overgaauw and Boersema, 1998). Anthelmintics that may be considered include ivermectin at 300 μg/kg, which when administered experimentally to greyhounds during gestation on days 0, 30 and 60, was shown to reduce vertical transmission by 90%. In another litter, where ivermectin was administered at days 0, 30, and 60 plus 10 days post-whelping, the worm burden was reduced by 100% (Payne and Ridley, 1999). These dosages are well above the level of ivermectin used in heartworm prophylaxis. Additionally, four experimentally infected beagles treated subcutaneously with moxidectin at 1 mg/kg on days 40 and 55 of pregnancy, completely prevented pre-natal and lactogenic infections in puppies (Krämer et al., 2006). In the same Kramer et al. study, one untreated bitch who served as a control, showed to have been infected with one adult and 26 somatic larvae of T. canis at necropsy.
Anthelmentics administered after 43 days of pregnancy are useful as this timing coincides with the activation of somatic larvae and their migration to the fetuses, which are under the influence of maternal hormonal levels (Ivanova and Georgiev, 2018). Programs implementing the use of ivermectin or moxidectin to prevent vertical transmission of T. canis will provide the additional benefit of preventing heartworm disease, treating ear mites, sarcoptic and demodectic mange as well as other intestinal, including zoonotic, nematodes (Sharun et al., 2019).
Implementation of a vertical transmission prevention program would require funding to support the veterinary services which would be required as treatment of pets is not usually performed in most low and middle income countries (LMICs). The high prevalence and favorable environmental conditions that occur in the tropics create a public health threat (Fakhri et al., 2018; Ma et al., 2019; Azam et al., 2012; Rocha et al., 2011). Such regions are thought to have the highest incidence of human infection which results in over ten million people, especially children, being infected with this parasite annually (Fakhri et al., 2018; Dutra et al., 2014; Morgan et al., 2013; Rostami et al., 2020; Cong et al., 2014).
In countries that largely lack both treatment and dog population control options, other appropriate affordable control measures should be implemented, such as education on the need to pick up and remove dog feces. This would require behavioral changes which have proven to be difficult for most parasitic disease control programs (Macpherson, 2005). In this study, only 9% of dog owners regularly picked up dog feces, and none were aware of the zoonotic threat of T. canis. Such lack of knowledge and proper behavior presents an intervention opportunity.
A lack of discussion about zoonoses by veterinarians with animal owners is a global problem (Harvey et al., 1991; Robertson et al., 2000; Alho et al., 2018; Kantarakia et al., 2020). This deficiency needs to be addressed not only to practitioners but also in the professional curriculum if any change is to occur. This study also revealed that veterinary students were largely unconcerned about the zoonotic nature of T. canis and only a small percentage of students could identify the main route of transmission to humans. This deficiency should be addressed in veterinary education, as veterinarians are the best target group for imparting accurate knowledge to pet owners about this zoonosis (Glickman and Schantz, 1981) and for implementing prevention protocols. Veterinarians would also be responsible for implementing dog population control programs that, if successful, could help reduce the number of young definitive hosts of T. canis in the communities.
The lack of recognition by physicians of the public health importance of T. canis may be explained by the fact that none of the physicians surveyed had ever diagnosed a case of toxocariasis in humans. The four clinical syndromes of toxocariasis do not exhibit pathognomonic signs and diagnostic tests are locally unavailable, expensive to import and require a cold chain for their importation. There have been no seroprevalence studies conducted in Grenada and the public health and economic importance, as in most other LMICs, of toxocariasis in humans, remains unknown. Enlisting physicians' assistance in an educational control initiative would require evidence of local clinical importance and an ability to make a diagnosis so that treatment can be provided.
Stressing the advantage of reducing the risk of contracting other, more well-known zoonoses such as Ancylostoma spp., Cryptosporidium spp. and Giardia spp., which were found in puppy fecal samples in this study, would be useful to include in any educational message. Molecular differentiation of the Ancylostoma spp. in the region to more fully appreciate their zoonotic importance could also be beneficial (Ngcamphalala et al., 2020). Improved hygiene practices, especially in children (Kroten et al., 2018; Moreira et al., 2014; Ahn et al., 2014), picking up pet feces (Vanhee et al., 2015) and increasing dog population control measures, all could lower T. canis and other parasitic zoonotic transmission, providing health benefits for both pets and people.
Declaration of Competing Interest
We the authors, Regan Schwartz (DVM, MPH), Satesh Bidaisee (DVM, MSPH, EdD), Paul Fields (PhD), Maxine Macpherson (BSc), and Calum Macpherson (PhD), have no conflicts of interest.
Acknowledgements
We thank the study participants for their time and participation in the study. This study was supported by a small research grant initiative grant from SGU.
References
- Ahn S.J., Ryoo N.-K., Woo S.J. Ocular toxocariasis: clinical features, diagnosis, treatment, and prevention. Asia Pac. Allergy. 2014;4:134. doi: 10.5415/apallergy.2014.4.3.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alho A.M., Lima C., Colella V., de Carvalho L. Madeira, Otranto D., Cardoso L. Awareness of zoonotic diseases and parasite control practices: a survey of dog and cat owners in Qatar. Parasites Vectors. 2018;11:133. doi: 10.1186/s13071-018-2720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam D., Ukpai O.M., Said A., Abd-Allah G.A., Morgan E.R. Temperature and the development and survival of infective Toxocara canis larvae. Parasitol. Res. 2012;110:649–656. doi: 10.1007/s00436-011-2536-8. [DOI] [PubMed] [Google Scholar]
- Azira N., Zeehaida M. A case report of ocular toxocariasis. Asian Pac. J. Trop. Biomed. 2011;1:164–165. doi: 10.1016/S2221-1691(11)60018-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosse M., Manhardt J., Stoye M. Epizootiology and control of neonatal helminth infection in dogs. Fortschritte der Veterinarmedizin. 1980;3:247–256. [Google Scholar]
- Bouzid M., Halai K., Jeffreys D., Hunter P.R. The prevalence of Giardia infection in dogs and cats, a systematic review and meta-analysis of prevalence studies from stool samples. Vet. Parasitol. 2015;207:181–202. doi: 10.1016/j.vetpar.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Burke T.M., Roberson E.L. Prenatal and lactational transmission of Toxocara canis and Ancylostoma caninum: experimental infection of the bitch before pregnancy. Int. J. Parasitol. 1985;15:71–75. doi: 10.1016/0020-7519(85)90104-3. [DOI] [PubMed] [Google Scholar]
- Catan P., Macpherson C.N.L. In: Encyclopedia of Human-Animal Relationships. Bekoff M., editor. Greenwood Press; Westport, Connecticut: 2007. The pothounds and pompeks of Grenada; pp. 576–579. [Google Scholar]
- Cong W., Zhang X.-X., Zhou N., Yu C.-Z., Chen J., Wang X.-Y., Li B., Qian A.-D., Zhu X.-Q. Toxocara seroprevalence among clinically healthy individuals, pregnant women and psychiatric patients and associated risk factors in Shandong Province, Eastern China. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryden M.W., Payne P.A., Ridley R., Smith V. Comparison of common fecal flotation techniques for the recovery of parasite eggs and oocysts. Vet. Ther. 2005;6:15–28. [PubMed] [Google Scholar]
- Dubey J.P. Patent Toxocara canis infection in ascarid-naive dogs. J. Parasitol. 1978;64:1021–1023. [PubMed] [Google Scholar]
- Dunn J.J., Columbus S.T., Aldeen W.E., Davis M., Carroll K.C. Trichuris vulpis recovered from a patient with chronic diarrhea and five dogs. J. Clin. Microbiol. 2002;40:2703–2704. doi: 10.1128/JCM.40.7.2703-2704.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra G.F., Pinto N.S.F., de Avila L.F.C., Dutra P.C., de Telmo P.L., Rodrigues L.H., Silva A.M.W.A., Scaini C.J. Risk of infection by the consumption of liver of chickens inoculated with low doses of Toxocara canis eggs. Vet. Parasitol. 2014;203:87–90. doi: 10.1016/j.vetpar.2014.03.025. [DOI] [PubMed] [Google Scholar]
- Fakhri Y., Gasser R.B., Rostami A., Fan C.K., Ghasemi S.M., Javanian M., Bayani M., Armoon B., Moradi B. Toxocara eggs in public places worldwide - a systematic review and meta-analysis. Environ. Pollut. 2018;242:1467–1475. doi: 10.1016/j.envpol.2018.07.087. [DOI] [PubMed] [Google Scholar]
- Glickman L.T., Schantz P.M. Epidemiology and pathogenesis of zoonotic toxocariasis. Epidemiol. Rev. 1981;3:230–250. doi: 10.1093/oxfordjournals.epirev.a036235. [DOI] [PubMed] [Google Scholar]
- Harvey J.B., Roberts J.M., Schantz P.M. Survey of veterinarians’ recommendations for treatment and control of intestinal parasites in dogs: public health implications. J. Am. Vet. Med. Assoc. 1991;199:702–707. [PubMed] [Google Scholar]
- Hotez P.J., Wilkins P.P. Toxocariasis: America’s most common neglected infection of poverty and a helminthiasis of global importance? PLoS Negl. Trop. Dis. 2009;3 doi: 10.1371/journal.pntd.0000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova C., Georgiev P. Pregnancy in the bitch- a physiological condition requiring specific care-review. Tradit. Modern. Vet. Med. 2018;3:77–82. [Google Scholar]
- Jaleta T.G., Zhou S., Bemm F.M., Schär F., Khieu V., Muth S., Odermatt P., Lok J.B., Streit A. Different but overlapping populations of Strongyloides stercoralis in dogs and humans-dogs as a possible source for zoonotic strongyloidiasis. PLoS Negl. Trop. Dis. 2017;11(8) doi: 10.1371/journal.pntd.0005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarakia C., Tsoumani M.E., Galanos A., Mathioudakis A.G., Giannoulaki E., Beloukas A., Voyiatzaki C. Comparison of the level of awareness about the transmission of Echinococcosis and Toxocariasis between pet owners and non-pet owners in Greece. IJERPH. 2020;17:5292. doi: 10.3390/ijerph17155292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazacos K.R. Gastrointestinal helminths in dogs from a humane shelter in Indiana. J. Am. Vet. Med. Assoc. 1978;173:995–997. [PubMed] [Google Scholar]
- Krämer F., Hammerstein R., Stoye M., Epe C. Investigations into the prevention of prenatal and lactogenic Toxocara canis infections in puppies by application of moxidectin to the pregnant dog. J. Vet. Med. B Infect. Dis Vet. Public Health. 2006;53:218–223. doi: 10.1111/j.1439-0450.2006.00948.x. [DOI] [PubMed] [Google Scholar]
- Kroten A., Toczylowski K., Oldak E., Sulik A. Toxocarosis in children: poor hygiene habits and contact with dogs is related to longer treatment. Parasitol. Res. 2018;117:1513–1519. doi: 10.1007/s00436-018-5833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R.M., Moore L.B., Bottazzi M.E., Hotez P.J. Toxocariasis in North America: a systematic review. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd S., Amerasinghe P.H., Soulsby E.J.L. Periparturient immunosuppression in the bitch and its influence on infection with Toxocara canis. J. Small Anim. Pract. 1983;24:237–247. doi: 10.1111/j.1748-5827.1983.tb00437.x. [DOI] [Google Scholar]
- Ma G., Holland C.V., Wang T., Hofmann A., Fan C.-K., Maizels R.M., Hotez P.J., Gasser R.B. Human toxocariasis. Lancet Infect. Dis. 2018;18:e14–e24. doi: 10.1016/S1473-3099(17)30331-6. [DOI] [PubMed] [Google Scholar]
- Ma G., Wang T., Korhonen P.K., Nie S., Reid G.E., Stroehlein A.J., Koehler A.V., Chang B.C.H., Hofmann A., Young N.D., Gasser R.B. Comparative bioinformatic analysis suggests that specific dauer-like signalling pathway components regulate Toxocara canis development and migration in the mammalian host. Parasites Vectors. 2019;12:32. doi: 10.1186/s13071-018-3265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson C.N.L. Human behaviour and the epidemiology of parasitic zoonoses. Int. J. Parasitol. 2005;35:1319–1331. doi: 10.1016/j.ijpara.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Macpherson C.N.L. The epidemiology and public health importance of toxocariasis: a zoonosis of global importance. Int. J. Parasitol. 2013;43:999–1008. doi: 10.1016/j.ijpara.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Manhardt J., Stoye M. Zum verhalten der larven von Toxocara canis WERNER 1782 (Anisakidae) während und nach der Lungenwanderung im definitiven Wirt (Beagle)1. Zentralbl. Veterinarmed. B. 2010;28:386–406. doi: 10.1111/j.1439-0450.1981.tb01928.x. [DOI] [PubMed] [Google Scholar]
- Moreira G.M.S.G., de Telmo P.L., Mendonça M., Moreira A.N., McBride A.J.A., Scaini C.J., Conceição F.R. Human toxocariasis: current advances in diagnostics, treatment, and interventions. Trends Parasitol. 2014;30:456–464. doi: 10.1016/j.pt.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Morgan E.R., Azam D., Pegler K. Quantifying sources of environmental contamination with Toxocara spp eggs. Vet. Parasitol. 2013;193:390–397. doi: 10.1016/j.vetpar.2012.12.034. [DOI] [PubMed] [Google Scholar]
- Ngcamphalala P.I., Lamb J., Mukaratirwa S. Molecular identification of hookworm isolates from stray dogs, humans and selected wildlife from South Africa. J. Helminthol. 2020;94 doi: 10.1017/S0022149X19000130. [DOI] [PubMed] [Google Scholar]
- Nijsse R., Mughini-Gras L., Wagenaar J.A., Franssen F., Ploeger H.W. Environmental contamination with Toxocara eggs: a quantitative approach to estimate the relative contributions of dogs, cats and foxes, and to assess the efficacy of advised interventions in dogs. Parasites Vectors. 2015;8:397. doi: 10.1186/s13071-015-1009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaauw P.A.M., Boersema J.H. Nematode infections in dog breeding kennels in the Netherlands, with special reference to Toxocara. Vet. Q. 1998;20:12–15. doi: 10.1080/01652176.1998.9694827. [DOI] [PubMed] [Google Scholar]
- Payne P.A., Ridley R.K. Strategic use of ivermectin during pregnancy to control Toxocara canis in greyhound puppies. Vet. Parasitol. 1999;85:305–312. doi: 10.1016/S0304-4017(99)00124-7. [DOI] [PubMed] [Google Scholar]
- Roberts L., Nadler S., Schmidt G., Janovy J. McGraw-Hill Higher Education; New York: 2013. Foundations of Parasitology. [Google Scholar]
- Robertson I.D., Irwin P.J., Lymbery A.J., Thompson R.C. The role of companion animals in the emergence of parasitic zoonoses. Int. J. Parasitol. 2000;30:1369–1377. doi: 10.1016/s0020-7519(00)00134-x. [DOI] [PubMed] [Google Scholar]
- Rocha S., Pinto R.M.F., Floriano A.P., Teixeira L.H., Bassili B., Martinez A., da Costa S.O.P., Caseiro M.M. Environmental analyses of the parasitic profile found in the sandy soil from the Santos municipality beaches, SP, Brazil. Rev. Inst. Med. Trop. S. Paulo. 2011;53:277–281. doi: 10.1590/S0036-46652011000500007. [DOI] [PubMed] [Google Scholar]
- Rostami A., Riahi S.M., Hofmann A., Ma G., Wang T., Behniafar H., Taghipour A., Fakhri Y., Spotin A., Chang B.C.H., Macpherson C.N.L., Hotez P.J., Gasser R.B. Global prevalence of Toxocara infection in dogs. Adv. Parasitol. 2020;109:561–583. doi: 10.1016/bs.apar.2020.01.017. [DOI] [PubMed] [Google Scholar]
- Sanpool O., Intapan P.M., Rodpai R., Laoraksawong P., Sadaow L., Tourtip S., Piratae S., Maleewong W., Thanchomnang T. Dogs are reservoir hosts for possible transmission of human strongyloidiasis in Thailand: molecular identification and genetic diversity of causative parasite species. J. Helminthol. 2019;94 doi: 10.1017/S0022149X1900107X. [DOI] [PubMed] [Google Scholar]
- Schnieder T., Laabs E.-M., Welz C. Larval development of Toxocara canis in dogs. Vet. Parasitol. 2011;175:193–206. doi: 10.1016/j.vetpar.2010.10.027. [DOI] [PubMed] [Google Scholar]
- Sharun K., Shyamkumar T.S., Aneesha V.A., Dhama K., Pawde A.M., Pal A. Current therapeutic applications and pharmacokinetic modulations of ivermectin. Vet. World. 2019;12:1204–1211. doi: 10.14202/vetworld.2019.1204-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowemimo O.A., Lee Y.-L., Asaolu S.O., Chuang T.-W., Akinwale O.P., Badejoko B.O., Gyang V.P., Nwafor T., Henry E., Fan C.-K. Seroepidemiological study and associated risk factors of Toxocara canis infection among preschool children in Osun State, Nigeria. Acta Trop. 2017;173:85–89. doi: 10.1016/j.actatropica.2017.05.030. [DOI] [PubMed] [Google Scholar]
- Stoye M. Galaktogene und pränatale infektionen mit Toxocara canis beim hund (Beagle), brief communication. Dtsch. Tierarztl. Wochenschr. 1976;83:107–108. [PubMed] [Google Scholar]
- Stracke K., Jex A.R., Traub R.J. Zoonotic ancylostomiasis: an update of a continually neglected zoonosis. Am. J. Trop. Med. Hygiene. 2020;103:64–68. doi: 10.4269/ajtmh.20-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghipour A., Olfatifar M., Bahadory S., Godfrey S.S., Abdoli A., Khatami A., Javanmard E., Shahrivar F. The global prevalence of Cryptosporidium infection in dogs: a systematic review and meta-analysis. Vet. Parasitol. 2020;281 doi: 10.1016/j.vetpar.2020.109093. [DOI] [PubMed] [Google Scholar]
- Urquhart G.M., Armour J., Duncan J.L., Dunn A.M., Jennings F.W. Longman Group; UK: 1987. Veterinary Parasitology. [Google Scholar]
- Vanhee M., Dalemans A.-C., Viaene J., Depuydt L., Claerebout E. Toxocara in sandpits of public playgrounds and kindergartens in Flanders (Belgium) Vet. Parasitol. 2015;1–2:51–54. doi: 10.1016/j.vprsr.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Ziegler M.A., Macpherson C.N.L. Toxocara and its species. CAB Rev. 2019;14 doi: 10.1079/PAVSNNR201914053. [DOI] [Google Scholar]