Highlights
-
•
Alzheimer’s disease biomarker tau improved by normalising to endogenous tau peptides.
-
•
Improved separation of Alzheimer patients and healthy controls in two cohorts.
-
•
Peptides measured by LC-MS were used to normalise protein sandwich ELISA results.
Abbreviations: AD, Alzheimer’s disease; AUC, Area under the ROC curve; CSF, cerebrospinal fluid; ELISA, enzyme-linked immunosorbent assay; GdnHCl, Guanidinium hydrochloride; PD, Parkinson’s disease; PSP, Progressive Supranuclear Palsy; P-tau, phospho-tau protein; SIL, peptide Synthetic isotope-labelled peptide; T-tau, total tau protein
Keywords: AD, Microtubule-associated protein tau, Endogenous peptides, Biomarker, Peptidomics, Mass spectrometry
Abstract
Cerebrospinal fluid (CSF) tau and phospho-tau are well established biomarkers of Alzheimer’s disease. While these measures are conventionally referred to as ‘total tau’ (T-tau) and ‘phospho-tau’ (P-tau), several truncated and modified tau forms exist that may relay additional diagnostic information. We evaluated the diagnostic performance of an endogenous tau peptide in CSF, tau 175–190, in the phosphorylated and non-phosphorylated state. A liquid chromatography-mass spectrometry (LC-MS) method was established to measure these peptides in CSF and was used to analyze two independent clinical cohorts; the first cohort included patients with Alzheimer’s disease (AD, n = 15), Parkinson’s disease (PD, n = 15), progressive supranuclear palsy (PSP, n = 15), and healthy controls (n = 15), the second cohort included AD patients (n = 16), and healthy controls (n = 24). In both cohorts T-tau and P-tau concentrations were determined by immunoassay. While tau 175–190 and P-tau 175–190 did not differentiate the study groups, the separation of AD and controls by T-tau (area under the ROC Curve (AUC) = 95%) and P-tau (AUC = 92%) was improved when normalizing the ELISA measurements to the concentrations of the endogenous peptides: T-tau/tau 175–190 (AUC = 100%), P-tau/P-tau 175–190 (AUC = 95%). The separation between patients and controls by T-tau (AUC = 88%) and P-tau (AUC = 82%) was similarly improved in the second cohort by taking the ratios of T-tau/tau 175–190 (AUC = 97%) and P-tau/P-tau 175–190 (AUC = 98%). In conclusion, our results suggest that the performance of the AD biomarkers T-tau and P-tau could be improved by normalizing their measurements to the endogenous peptides tau 175–190 and P-tau 175–190, possibly because these endogenous tau peptides serve to normalize for physiological, and disease-independent, secretion of tau from neurons to the extracellular space and the CSF. Finally, the observations made here add to the general applicability of mass spectrometry as a tool for rapid identification and accurate quantification of biomarker candidates.
1. Introduction
The core pathological hallmarks of Alzheimer’s Disease (AD) are cortical plaques, primarily composed of aggregated Aβ1-42, and neurofibrillary tangles, consisting of hyperphosphorylated fragments of microtubule-associated protein tau [1]. Current CSF biomarkers of AD reflect neurodegeneration (total tau; T-tau) and plaque and tangle pathologies (Aβ1-42, and phosphorylated tau; P-tau). Increased abundance of amyloid plaques directly correlates with a decrease in CSF Aβ1-42 concentration. While increased CSF T-tau is observed in other neurological conditions, such as stroke and Creutzfeldt-Jakob disease, as a consequence of neuronal loss, P-tau is specifically increased in AD [2].
Tau was identified by Weingarten et al. in 1975 and described as a heat-stable protein essential for microtubule assembly [3]. Subsequent studies have shown that its functions include stabilising and promoting microtubule polymerisation, regulating axonal transport, and affecting neurite growth [4], [5], [6], [7], [8], [9]. In 1986, tau was first associated with neurodegeneration and AD, being found to be the principal component of paired helical filaments [10]; a subunit or precursor of neurofibrillary tangles. Further, it was found that functional impairment of tau is dependent on the extent of protein phosphorylation, and that hyperphosphorylation – extensive phosphorylation of proline-rich motifs in the protein c-terminus – is a characteristic of pathological tau [10], [11].
Several forms of tau are present in humans as a result of alternative splicing and post-translational modification. A number of these forms have been detected in CSF, and there are indications that some of these may be altered in AD [12].
In a previous, explorative study, a large number of endogenous peptides in human CSF were identified [13]; this included seven peptides from tau, five of which spanned the diagnostically interesting site threonine-181, which is used to indicate degree of tau phosphorylation [14], [15], [16], [17], [18], [19].
In the present study, we evaluated the potential of a liquid-chromatography-mass spectrometry (LC-MS) based method for quantifying two forms (i.e., non-phosphorylated and phosphorylated) of an endogenous peptide corresponding to tau 175–190, as biomarkers of AD and other neurodegenerative disorders. The LC-MS method was established for peptide quantification and was initially used to analyse CSF from a cohort consisting of healthy controls and patients diagnosed with AD, Parkinson’s disease (PD) and Progressive Supranuclear Palsy (PSP). Findings were verified in an independent cohort of controls and patients diagnosed with AD.
2. Methods
2.1. CSF samples
The discovery cohort was sampled at the Memory and Neurology Clinics of Skåne University Hospital (Sweden). The included cases consisted of 60 individuals divided into four groups comprising healthy controls (n = 15), and patients clinically diagnosed with AD (n = 15), PD (n = 15) and PSP (n = 15). All study participants underwent cognitive, neurological and psychiatric assessments by a medical doctor, and brain imaging was performed. The control group consisted of cognitively healthy volunteers without history, symptoms or signs of significant neurological or psychiatric disorders. The AD group consisted of patients fulfilling the NINDS-ADRDA criteria for probable AD [20] and DSM-IV [21]. PD patients met the NINDS criteria [22] and PSP patients met the NINDS-SPSP criteria [23].
The validation cohort was sampled at the National Hospital for Neurology and Neurosurgery, Queen Square, London (England) and consisted of 40 individuals including AD patients (n = 16) and healthy controls (n = 24). The patients fulfilled the IWG-2 criteria for AD [24]. The controls had no subjective memory complaints or evidence of cognitive impairment on the Mattis dementia rating scale, and were followed up at one year to ensure they had not developed cognitive symptoms. Samples were collected according to a standard operating procedure in polypropylene tubes.
2.2. Stable isotope labelled peptide standards
Synthetic isotope-labelled (SIL) peptide standards of endogenous tau 175–190, 175-TPPAPKTPPSSGEPPK-190, and P-tau 175–190, 175-TPPAPK-[T(PO3H2)]-PPSSGEPPK-190 (the amino acid numeration corresponds to Isoform Tau-F), were acquired from Thermo Fisher Scientific. The heavy SIL peptides incorporated a C-terminal lysine residue labelled with 13C6: 175-TPPAPKTPPSSGEPP-[K(13C6]-190 and 175-TPPAPK-[T(PO3H2)]-PPSSGEPP-[K(13C6]-190. The peptide content of each standard was determined by quantitative amino acid analysis performed by the manufacturer.
2.3. Preparation of standard samples
Calibration curves were constructed by analysing samples containing 20 fmol/mL of each of the two SIL peptides, and non-labelled tau 175–190 in concentrations ranging from 4 to 40 fmol/mL (1–10 fmol on column). The samples were prepared in plasma diluted 1:200 with water, resulting in a total protein concentration of 60 µg/ml (by Bradford protein assay), and prepared in aliquots of 250 µL according to the protocol below. Samples for method precision evaluation were prepared by spiking five 250 µL CSF aliquots with 40 and 10 fmol/mL of SIL peptide tau 175–190 and P-tau 175–190, respectively.
2.4. Sample preparation
All study samples were blinded and randomly analysed. Aliquots of 250 µL of CSF were spiked with SIL peptides; 5 fmol/mL of tau 175–190 and 2.5 fmol/mL of P-tau 175–190. Samples were pH-stabilized by addition of 50 µL 0.5 M triethylammonium bicarbonate, pH 8.5 ± 0.1. Guanidinium hydrochloride (GdnHCl, 150 µL, 8 M) was added, followed by shaking (600 rpm) at room temperature for 10 min. The samples were diluted by an addition of 1 mL of water.
A molecular weight cut-off ultracentrifugation filter (Vivacon 2, 30 kDa, Sartorius) was used to isolate endogenous peptides. All centrifugation steps were performed at 20 °C and at a centrifugal rate of 2500 × g. Conditioning and equilibration of the filter was performed by sequential addition and centrifugation (2 × 20 min) of 2 mL of 1 M GdnHCl followed by 1.5 mL of 25 mM triethylammonium bicarbonate. The flow-through was discarded and the collection tube was rinsed with water. The sample was added to the filter and centrifuged (40 min), followed by addition of 1 mL of 25 mM triethylammonium bicarbonate and centrifugation (30 min) to recover remaining peptides. pH was adjusted to 3 by titration with 20% phosphoric acid.
The peptide filtrate was purified by solid phase extraction (SPE, 1 mL Sep-Pak C18, Waters) The SPE cartridges were attached to a vacuum manifold and operated according to the manufacturer, using the following solutions: conditioning: 1 mL of 84% acetonitrile, 0.1% formic acid; 1 mL of 1 M GdnHCl, and 1 mL of 84% acetonitrile, 0.1% formic acid; equilibration: 2 × 1 mL of 0.1% formic acid; washing: 2 × 1 mL of 0.1% formic acid; elution: 1 mL of 84% acetonitrile, 0.1% formic acid. The solvents were evaporated through vacuum centrifugation and the samples were subsequently stored at −80 °C pending analysis.
2.5. LC-Ms
Samples were dissolved in 6.5 µL 2% acetonitrile, 0.1% formic acid by gentle agitation for 20 min. 5 µL were loaded on a nano-flow HPLC (Ultimate 3000 RSLC-nano, Thermo), configured in trap column mode (trap column: Acclaim® PepMap 100, 75 µm × 2 cm, C18, 100 Å pore size, 3 µm particle size; separation column: Acclaim® PepMap C18 75 µm × 500 mm, 100 Å pore size, 2 µm particle size). Two aqueous mobile phases: A) 0.1% formic acid and B) 84% acetonitrile, 0.1% formic acid, were applied at a constant flow of 150 nL/min in a 70-minute gradient: t (min) = 0, B = 3%; t = 10, B = 2%; t = 45, B = 30%; t = 46, B = 80%; t = 50, B = 80%; t = 50.5, B = 3%; t = 70, B = 3%.
Mass analysis was performed on a high-resolution Orbitrap mass spectrometer (Fusion™ Tribrid™, Thermo) connected to the HPLC via a FlexiSpray™ nano-ESI source, using the following settings: spray voltage: 1700 V; transfer capillary temp: 275 °C; isolation window: 1.0 m/z; resolution: 60.000; AGC target: 1.0e5; maximum injection time: 118 ms.
2.6. Peptide quantification and statistical analysis
MS data was analysed using Skyline v3.7 [25]. Quantification was based on the calculated peak area-ratio between light and heavy isotopologues. Statistical analysis was performed using GraphPad Prism (v7.03).
2.7. Immunassay
CSF T-tau and P-tau concentrations were measured in both cohorts and Aβ1-42 concentrations were measured only in the validation cohort, using a sandwich enzyme linked immunosorbent assay (ELISA; INNOTEST®: β-AMYLOID 1–42, hTAU Ag and PHOSPHO-TAU 181P; Fujirebio, Belgium).
3. Results
3.1. Development of mass spectrometric assay
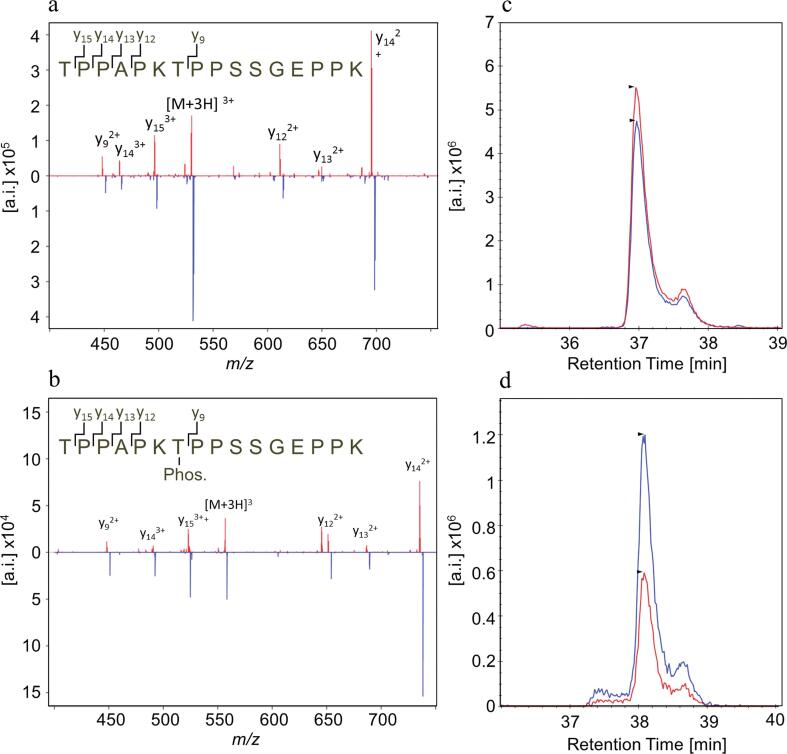
A mass spectrometric method based on PRM for measurement of tau 175–190 and P-tau 175–190 in CSF was established on a quadrupole Orbitrap mass spectrometer. SIL peptides labelled with 13C6–Lys were used as internal standards. For both peptides, the +3 ions were the most prominent charge state, and thus chosen as precursor ions. Fragmentation resulted in six prominent y-ions from the respective peptides (Fig. 1, Supplementary Table S1). The fragmentation patterns of the SIL peptide standard (blue) were identical to those of the respective endogenous peptides in CSF (red), confirming the identities of the peptides. For both peptides, y14++ was used for quantification, while the other fragments were used to verify the identity of the peptide.
Fig. 1.
PRM of tau 175–190 and P-tau 175–190. MS/MS spectra of the [M + 3H]3+ ion of (a) tau 175–190 and (b) P-tau 175–190. The six most prominent y ions are labelled in the spectra. PRM chromatograms of (c) tau 175–190 and (d) P-tau 175–190. The spectra and chromatograms of the endogenous form of the respective peptide coloured red and the SIL peptide is coloured blue.
Calibration curves constructed by spiking human plasma, diluted 1:200, with different amounts of unlabelled peptides and a fixed amount of SIL peptides (Supplementary Fig. S1) showed good linearity for tau 175–190 (R2 = 0.997) over the tested concentration range 8–40 fmol/ml (2–10 fmol on column) and for P-tau 175–190 the (R2 = 0.986) over the concentration range 2–20 fmol/mL (0.5–5 fmol on column), based on three measurements. The LLOQ (CV < 20%) for tau 175–190 was 2 fmol/mL, and for P-tau 175–190, 0.5 fmol/mL.
Method repeatability was evaluated by analysing five CSF samples prepared separately, resulting in a CV of 5.97% for tau 175–190 and 6.13% for P-tau 175–190.
3.2. The tau ratio and P-tau ratio improve the diagnostic accuracy of tau in a discovery cohort
To evaluate whether CSF tau 175–190 and P-tau 175–190 are altered in neurodegenerative disorders, CSF from 60 individuals clinically diagnosed with AD (n = 15), PD (n = 15), or PSP (n = 15), along with healthy control subjects (HC, n = 15) were analysed using the developed PRM method. The core AD biomarkers T-tau and P-tau were also measured, using ELISA (for demographics, see Supplementary Table S2).
No significant differences in the concentrations of either peptide were observed in any of the groups (Fig. 2a and b, Table 1), however, T-tau (measured by ELISA) correlated more strongly to tau 175–190 (measured by PRM) in the controls, as well as in PD and PSP compared to in AD (Fig. 2c, Supplementary Table S3). The same observation was made for P-tau (Fig. 2d).
Fig. 2.
CSF tau 175–190 and P-tau 175–190 concentrations in the discovery cohort. Scatter plots of (a) tau 175–190 and (b) P-tau 175–190 in patients with Alzheimer’s disease (AD), Parkinson’s disease (PD), supranuclear palsy (PSP), and healthy controls (HC). Scatterplots of the correlations of (c) tau 175–190 to T-tau and (d) P-tau 175–190 to P-tau.
Table 1.
T-tau, P-tau, endogenous peptides, and tau- and P-tau ratios in the discovery cohort. Shown are mean protein and peptide concentrations and mean molar T-tau and P-tau ratios, as well as effect sizes (Cohen’s d) and ROC curve analysis AUC values for the separations of Alzheimer’s disease (AD) versus healthy controls (HC), Parkinson’s disease (PD), supranuclear palsy (PSP), and all non-AD groups combined.
| AD | HC | PD | PSP | (HC + PD + PSP) | ||
|---|---|---|---|---|---|---|
| T-tau | mean [fmol/ml] | 14.48 | 5.88 | 5.00 | 6.09 | 5.66 |
| CV [%] | 39.19 | 24.69 | 27.84 | 36.72 | 31.81 | |
| Cohen's d | – | 1.72 | 1.89 | 1.68 | 1.76 | |
| AUC | – | 0.95 | 0.96 | 0.93 | 0.95 | |
| tau 175–190 | mean [fmol/ml] | 16.25 | 15.52 | 12.34 | 13.57 | 13.81 |
| CV [%] | 28.91 | 22.56 | 24.23 | 32.23 | 28.19 | |
| Cohen's d | – | 0.17 | 0.63 | 0.63 | 0.12 | |
| AUC | – | 0.56 | 0.73 | 0.68 | 0.66 | |
| T-tau/tau 175–190 | mean [fmol/ml] | 0.90 | 0.38 | 0.40 | 0.45 | 0.41 |
| CV [%] | 31.46 | 11.81 | 12.67 | 23.47 | 19.04 | |
| Cohen's d | – | 1.98 | 1.89 | 1.71 | 1.86 | |
| AUC | – | 1.00 | 1.00 | 0.97 | 0.99 | |
| P-tau | mean [fmol/ml] | 1.52 | 0.85 | 0.72 | 0.79 | 0.79 |
| CV [%] | 31.05 | 21.09 | 26.91 | 32.40 | 27.85 | |
| Cohen's d | – | 1.53 | 1.83 | 1.66 | 1.67 | |
| AUC | – | 0.92 | 0.94 | 0.91 | 0.92 | |
| P-tau 175–190 | mean [fmol/ml] | 3.61 | 3.21 | 2.48 | 2.77 | 2.82 |
| CV [%] | 32.97 | 19.81 | 20.45 | 29.72 | 25.99 | |
| Cohen's d | – | 0.43 | 1.21 | 0.90 | 0.85 | |
| AUC | – | 0.61 | 0.78 | 0.71 | 0.70 | |
| P-tau/P-tau 175–190 | mean [fmol/ml] | 0.44 | 0.26 | 0.29 | 0.29 | 0.28 |
| CV [%] | 28.50 | 10.72 | 13.98 | 22.99 | 17.50 | |
| Cohen's d | – | 1.72 | 1.50 | 1.48 | 1.57 | |
| AUC | – | 0.95 | 0.89 | 0.86 | 0.90 |
These observations suggested that the endogenous tau peptides may reflect the proportion of CSF tau that is secreted under physiological conditions, independent of pathological processes, and prompted us to test if AD-control group separation by T-tau and P-tau could be improved by normalizing T-tau and P-tau concentration to those of the respective endogenous peptides.
The plots in Fig. 3a and b show the group separations for T-tau and for the ratio of T-tau/tau 175–190. The effect size for separating AD from controls (Table 1) was larger for the T-tau ratio (Cohen’s d = 1.98) than for T-tau (1.72). Similarly, a larger effect size was observed for the P-tau ratio (d = 1.72) than for P-tau (d = 1.53; Fig. 3c, d). The ratio of T-tau/tau 175–190 also showed improved separation between AD, and all the other groups combined, compared to T-tau, while P-tau/P-tau 175–190 did not result in any improvement.
Fig. 3.
Scatter plots of group separation and ROC analysis of diagnostic accuracy in the discovery cohort. The ability to separate disease groups/healthy controls based on (a) t-tau concentrations, (b) t-tau/tau 175–190 M ratio, (c) P-tau concentrations and (d) P-tau/P-tau 175–190 M ratio. (e, f) ROC curve analysis showing the effect on diagnostic precision when separating AD from healthy controls based on protein concentration alone compared to protein/peptide ratio.
The molar mass of tau-441 (Isoform F; P10636-8, MW 45850 Da) was used to convert the T-tau and P-tau immunoassay data from pg/mL to fmol/mL. While this isoform is often taken to represent full-length CSF tau, the immunoassays can be expected to detect only the subset of processed tau forms that contain the amino acid sequence spanned by the antibody epitopes. While, therefore, it may not be possible to assign a precise molecular mass and molar concentration of CSF tau, we performed the conversion because the molar ratio provides a meaningful estimate of the stoichiometry of the endogenous peptides relative to intact tau protein and larger tau fragments.
The mean relative increase in AD for the T-tau ratio (139%; Supplementary Table S4) was similar to that of T-tau (146%), but the CVs for the tau ratios in both the AD (31.5%) and control (11.8%) groups were smaller compared to those of t-tau (Table 1; CVAD = 39.2; CVHC = 24.7%). Similar results were observed for the P-tau ratio (CVAD = 28.5; CVHC = 10.7%) compared to P-tau (CVAD = 31.1; CVHC = 21.1%).
Receiver operating characteristic (ROC) curve analysis showed improved diagnostic performance in separating AD from HC for both the T-tau ratio (area under the curve (AUC) = 100%) compared to t-tau (AUC = 95.1%), and for the P-tau ratio (AUC = 95.3%) compared to P-tau (AUC = 92%; Fig. 3 e and f).
The degree of phosphorylation was calculated, for the tau protein as the ratio of [P-tau]/[T-tau], and for the endogenous peptides as [P-tau 175–190]/[T-tau 175–190]. There was no apparent difference in the degree of phosphorylation for any disease group (Supplementary Table S5).
3.3. Verification of the results
To verify the findings from the discovery cohort, tau 175–190 and P-tau 175–190 were measured in a second cohort, consisting of CSF from 40 patients from a different medical centre, with clinical diagnosis of AD (n = 16) and HC (n = 24; for demographics, see Supplementary Table S6). The core biomarkers, T-tau, P-tau, and Aβ1-42 were determined by ELISA.
T-tau and P-tau showed a significant (p < 0.01) group separation (Fig. 4a and c), but with more overlap compared to the discovery cohort. The T-tau and P-tau ratios improved group separations (Fig. 4b and d), with similar mean relative increase in AD (Supplementary Table S7), and with lower intra-group variation (Table 2). The effect size for separating AD from controls was larger for the T-tau ratio (Cohen’s d = 1.59) than for T-tau (1.25). Similarly, a larger effect size was observed for the P-tau ratio (1.64) than for P-tau (1.03). As in the discovery cohort, neither tau 175–190 nor P-tau 175–190 differed between AD patients and controls (Supplementary Fig. S2).
Fig. 4.
Scatter plots of group separation and ROC analysis of diagnostic accuracy in the validation cohort. The ability to separate AD from healthy controls based on (a) t-tau concentrations, (b) t-tau/tau 175–190 M ratio, (c) P-tau concentrations and (d) P-tau/P-tau 175–190 M ratio. (e, f) ROC curve analysis showing the effect on diagnostic precision when separating AD from healthy controls based on protein concentration alone compared to protein/peptide ratio.
Table 2.
T-tau, P-tau, endogenous peptides, and tau- and P-tau ratios in the validation cohort. Shown are mean protein and peptide concentrations and mean molar T-tau and P-tau ratios, as well as effect sizes (Cohen’s d) and ROC curve analysis AUC values for the separations of Alzheimer’s disease (AD) and healthy controls (HC).
| AD | HC | ||
|---|---|---|---|
| T-tau | mean [fmol/ml] | 19.69 | 6.78 |
| CV [%] | 62.73 | 47.97 | |
| Cohen's d | – | 1.25 | |
| AUC | – | 0.88 | |
| tau 175–190 | mean [fmol/ml] | 13.14 | 13.39 |
| CV [%] | 39.89 | 28.86 | |
| Cohen's d | – | −0.05 | |
| AUC | – | 0.54 | |
| T-tau/t-tau 175–190 | mean [molar ratio] | 1.45 | 0.50 |
| CV [%] | 39.11 | 28.39 | |
| Cohen's d | – | 1.59 | |
| AUC | – | 0.97 | |
| P-tau | mean [fmol/ml] | 2.56 | 1.14 |
| CV [%] | 71.12 | 33.56 | |
| Cohen's d | – | 1.03 | |
| AUC | – | 0.83 | |
| P-tau 175–190 | mean [fmol/ml] | 3.50 | 3.54 |
| CV [%] | 44.42 | 26.82 | |
| Cohen's d | – | −0.03 | |
| AUC | – | 0.55 | |
| P-tau/P-tau 175–190 | mean [molar ratio] | 0.69 | 0.32 |
| CV [%] | 28.29 | 20.05 | |
| Cohen's d | – | 1.64 | |
| AUC | – | 0.98 |
Contrary to the discovery cohort, the correlation between T-tau and tau 175–190 was similar for AD patients and controls; the correlation between P-tau and P-tau 175–190 was even slightly higher in the AD group (Supplementary Fig.S2, Supplementary Table S8). ROC curve analysis (p = 0.01) showed improved group separation for the T-tau ratio (AUC = 0.97) and the P-tau ratio (AUC = 0.98) compared to t-tau (AUC = 0.88) and P-tau (AUC = 0.82; Fig. 4e, f). As in the discovery set, the degree of phosphorylation did not differ between the groups (Supplementary Table S9).
3.4. Alternative ratios
Because tau 175–190 and P-tau 175–190 were strongly correlated in both patients and healthy controls (Supplementary Table S8), we tested if tau 175–190 and P-tau 175–190 could be used interchangeably for normalization by calculating the ratios of T-tau/P-tau 175–190 and P-tau/tau 175–190 (Supplementary Figs. S3 and S4, and Supplementary Table S10 and S11). In both cases, little difference in group separation and effect size was observed compared to T-tau/tau 175–190 and P-tau/P-tau 175–190.
To investigate whether the endogenous tau peptides reflect a protein-specific variation or variation of a more general nature, we tested if ratios of Aβ1-42 to tau 175–190 and P-tau 175–190 would improve group separation in a manner similar to Aβ1-40 normalization. However, Aβ1-42/tau 175–190 and Aβ1-42/P-tau 175–190 both resulted in an increased intra group CV, decreased effect size and a decreased AUC for separating AD patients and controls, compared to using the Aβ1-42 concentration alone (Supplementary Fig. S5 and Supplementary Table S12).
The tau/Ab42 ratio has been proposed as a single AD marker, giving a better performance than the two biomarkers separately [26]. This finding was confirmed in our discovery cohort (AUCAβ1-42 = 87%, AUCT-tau/Aβ1-42 = 93%). Using (T-tau/tau 175–190)/Aβ1-42 resulted in further improvement (AUC = 96%). Similar results were obtained for P-tau (Supplementary Table S13).
4. Discussion
Herein, we have explored the potential of LC-MS-based biomarker development in neurodegenerative diseases through investigation of an endogenous CSF peptide from microtubule-associated protein tau, in a phosphorylated and non-phosphorylated state. By ‘endogenous peptides’ we mean peptides naturally present in the neat CSF, i.e., not produced by in vitro proteolysis of CSF proteins with trypsin, as is the procedure most commonly applied in proteomics.
The peptide chosen for the current study was one out of seven endogenous peptides from tau that were identified in an explorative peptidomic analysis [13]. In that study, endogenous CSF peptides were subjected to extensive fractionation and analysed by nano-LC-MS on a high-resolution mass spectrometer operated in the data-dependent mode, resulting in the identification of over 18,000 peptides in the molecular mass range 470 – 5900 Da. The peptides originated from 1918 proteins, many of which are associated with neurodegeneration, synaptic loss, axonal damage, or AD. The large number of endogenous peptides in CSF raises several questions: do their concentrations correlate to those of their parent proteins? Can they be used as disease biomarkers and, if so, do they relay information on pathologies different from that of their parent proteins?
The peptide tau 175–190 was selected for the current study because it spans threonine-181, phosphorylation of which is widely regarded as an AD-specific biomarker [14], [15], [16], [17], [18], [19]. Further, the peptide could be detected both in the phosphorylated and non-phosphorylated state, enabling measurement of the degree of phosphorylation at Thr-181. We observed, however, no difference in the concentration of either the phosphorylated or the non-phosphorylated form of tau 175–190, or in the degree of phosphorylation, in AD, PD or PSP patients, compared to healthy controls in the discovery cohort, or between AD and healthy controls in the validation cohort.
The observations that tau 175–190 and P-tau 175–190 did not increase in AD and correlate more strongly to ELISA measurements of T-tau and P-tau, respectively, in the healthy, PD and PSP patients, compared to AD, suggests that the endogenous peptides are shed into the CSF through processes different from those responsible for the increased CSF tau in AD. This observation prompted us to test the diagnostic performance of the ratio of T-tau to tau 175–190 (tau ratio) compared to t-tau alone. We found that the tau ratio improved the group separations. This finding was confirmed in a second, independent cohort. The effect was mainly attributed to a decreased intra-group variation in all study groups, leading to significantly reduced variation, particularly in the control group, while the mean relative difference between AD patients and controls was unchanged. These results thus indicate that variation in the concentration of the endogenous peptides is not related to AD.
In the discovery cohort, the diagnostic performance of T-tau (sensitivity 100%, specificity 93%) was higher than historical data for this biomarker (sensitivity 78–84%, specificity 84–90% [2], [27]). Similarly, the diagnostic performance of P-tau in the discovery set was higher (sensitivity 100%, specificity 80%) than comparative historical figures (sensitivity 85%, specificity 75% [19], [27]). For this reason, it is not possible to observe any significant improvement in ROC AUC for the T-tau/tau 175–190 ratio, however, it can be observed for the effect size.
The discovery cohort was analysed as a first pilot to explore if the endogenous tau peptides have any biomarker potential. Because the apparent benefit of the tau ratio was discovered while inspecting the data, i.e., was not hypothesized prior to the analysis, there is a risk that the model overfits the data. Therefore, the analysis was repeated in a second cohort, in which the effect of the tau ratio was the only hypothesis tested. The validation cohort was collected at a different clinical centre to avoid the risk of bias stemming from participant recruitment. In the validation cohort, the performance of both T-tau (sensitivity 81%, specificity 87%) and P-tau (sensitivity 81%, specificity 75%) are closer to previous reports, and the improvement of using the T-tau/tau 175–190-ratio (sensitivity 94%, specificity 92%) and P-tau/P-tau 175–190-ratio (sensitivity 94%, specificity 96%) are more pronounced.
Improving the diagnostic performance of a biomarker by taking the ratio of the biomarker to a non-biomarker endogenous peptide from the same protein has previously been reported [28], [29] and is well-established for the AD biomarker Aβ1-42 in using the Aβ1-42/1-40 ratio [30], [31], [32]. As with the tau ratio, the improvement observed for the Aβ1-42/Aβ1-40 ratio compared to Aβ1-42 alone, results from decreased intra group variation while the relative difference between AD and healthy do not change.
It is not known what the variation in Aβ1-40 accounts for; it has been suggested that it might reflect variability in the efficiency of processing the amyloid precursor protein or differences in the expression profile of APP on the cell surface [32]. Alternatively, the ratio of Aβ1-42 to Aβ1-40 may normalize for differences in CSF dynamics, e.g. production, flow along the spinal cord, and absorbance. Analogously for tau, one may speculate that there is a pool of secreted tau – or tau-derived peptides – different from that released into the CSF during the process of AD pathological neurodegeneration, that displays a normal variation in abundance and that is not affected in AD. Our observation that using tau 175–190 or P-tau 175–190 to normalize Aβ1-42 measurements resulted in poorer group separation than for Aβ1-42 alone (Supplementary Table S10), suggests that the variation in tau 175–190 and P-tau 175–190, at least to some extent, is protein specific.
Do tau 175–190 and P-tau 175–190 relay the same information? The peptides correlate strongly in both AD patients and healthy controls (Supplementary Table S11) and when using tau 175–190 to normalize P-tau and, vice versa, P-tau 175–190 to normalize t-tau, group separations were significantly improved compared to the non-normalized T-tau and P-tau results, in both cohorts (Supplementary Table S8 and S9). Comparing t-tau/tau 175–190 with t-tau/P-tau 175–190 or P-tau/P-tau 175–190 with P-tau/tau 175–190 showed relatively small differences.
At this point it is not clear where the tau peptides are formed. We do not believe that they are generated in vitro. Possibly, they are generated as part of the normal tau synthesis; for example, it is known that short peptides derived from most proteins are produced by the proteasome and presented on the cell surface by MHC-I as part of the cell’s self-declaration of protein synthesis.
5. Limitations of the study
While our study strongly indicates that the performance of T-tau and P-tau as AD biomarkers can be improved by normalizing their CSF concentrations to those of the endogenous peptides tau 175–190 and P-tau 175–190, these findings should be validated further in larger cohorts. Such studies should also include patients with mild cognitive impairment, other age spans, and other dementias to more fully evaluate the utility of the tau ratio. Another limitation of the study is that immunoassays (used to measure t-tau and P-tau) often do not correlate well with mass spectrometry (used to quantify the endogenous peptides), which may add inter-analytical variation to the tau ratio. Recently, mass spectrometric methods have been reported for quantification of CSF tau based on analysis of tau peptides produced by tryptic cleavage [33]. It is possible that measuring both tau protein and endogenous peptide concentrations with a mass spectrometric method would yield decreased analytical variation and, thereby, improved performance of the tau ratio as a biomarker of AD.
In this study, we present one of the first attempts at bridging the cleft between MS- and immunoprecipitation-based biomarker measurements. Although it might be considered a roundabout way of supporting biomarker measurement by LC-MS, the concept of normalising protein-level measurements by ELISA against endogenous peptides measured by LC-MS serves to showcase the usefulness of the former, albeit in a supportive role.
The fact that the current core biomarkers for AD – decreased CSF concentration of Aβ1-42 and increased CSF T-tau and P-tau – correspond to pathological features that have been known for decades, and have been measured in a large number of studies [34], yet are not implemented in clinical routine, suggests that introducing concepts such as endogenous peptides of unknown disease involvement as biomarker normalisers, will require much further study. This current study may, therefore, be seen as a proof of concept and a stepping stone towards introducing LC-MS into clinical routine measurement.
Declaration of Competing Interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clinms.2019.07.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Blennow K., de Leon M.J., Zetterberg H. Alzheimer's disease. Lancet. 2006;368(9533):387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 2.Blennow K., Hampel H., Weiner M., Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease Nature reviews. Neurology. 2010;6(3):131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 3.Weingarten M.D., Lockwood A.H., Hwo S.Y., Kirschner M.W. A protein factor essential for microtubule assembly. Proc. Nat. Acad. Sci. U. S. A. 1975;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drechsel D.N., Hyman A., Cobb M.H., Kirschner M. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol. Biol. Cell. 1992;3(10):1141–1154. doi: 10.1091/mbc.3.10.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feinstein H.E., Benbow S.J., LaPointe N.E., Patel N., Ramachandran S., Do T.D., et al. Oligomerization of the microtubule-associated protein tau is mediated by its N-terminal sequences: implications for normal and pathological tau action. J. Neurochem. 2016;137(6):939–954. doi: 10.1111/jnc.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seiberlich V., Bauer N.G., Schwarz L., Ffrench-Constant C., Goldbaum O., Richter-Landsberg C. Downregulation of the microtubule associated protein Tau impairs process outgrowth and myelin basic protein mRNA transport in oligodendrocytes. Glia. 2015;63(9):1621–1635. doi: 10.1002/glia.22832. [DOI] [PubMed] [Google Scholar]
- 7.Saftig P., Bovolenta P. Proteases at work: cues for understanding neural development and degeneration. Front. Mol. Neurosci. 2015:8.. doi: 10.3389/fnmol.2015.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mietelska-Porowska A., Wasik U., Goras M., Filipek A., Niewiadomska G. Tau Protein Modifications and Interactions: their Role in Function and Dysfunction. Int. J. Mol. Sci. 2014;15(3):4671–4713. doi: 10.3390/ijms15034671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson G.V., Stoothoff W.H. Tau phosphorylation in neuronal cell function and dysfunction. J. Cell Sci. 2004;117(24):5721–5729. doi: 10.1242/jcs.01558. [DOI] [PubMed] [Google Scholar]
- 10.Grundke-Iqbal I., Iqbal K., Tung Y.-C., Quinlan M., Wisniewski H.M., Binder L.I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. 1986;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bretteville A., Planel E. Tau aggregates: toxic, inert, or protective species? J. Alzheimers Dis. 2008;14(4):431–436. doi: 10.3233/jad-2008-14411. [DOI] [PubMed] [Google Scholar]
- 12.Meredith J.E., Jr., Sankaranarayanan S., Guss V., Lanzetti A.J., Berisha F., Neely R.J., et al. Characterization of novel CSF Tau and ptau biomarkers for Alzheimer's disease. PLoS ONE. 2013;8(10) doi: 10.1371/journal.pone.0076523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansson K.T., Skillbäck T., Pernevik E., Kern S., Portelius E., Höglund K., et al. Expanding the cerebrospinal fluid endopeptidome. Proteomics. 2017;17:5. doi: 10.1002/pmic.201600384. [DOI] [PubMed] [Google Scholar]
- 14.Vanmechelen E., Van Kerschaver E., Blennow K., De Deyn P., Gartner F., Parnetti L., et al. CSF-phospho-tau (181P) as a promising marker for discriminating Alzheimer’s disease from dementia with Lewy bodies. Alzheimer’s Disease: advances in Etiology. Pathogenesis Ther. 2001:285–291. [Google Scholar]
- 15.Vanmechelen E., Vanderstichele H., Davidsson P., Van Kerschaver E., Van Der Perre B., Sjögren M., et al. Quantification of tau phosphorylated at threonine 181 in human cerebrospinal fluid: a sandwich ELISA with a synthetic phosphopeptide for standardization. Neurosci. Lett. 2000;285(1):49–52. doi: 10.1016/s0304-3940(00)01036-3. [DOI] [PubMed] [Google Scholar]
- 16.Schönknecht P., Pantel J., Hunt A., Volkmann M., Buerger K., Hampel H., et al. Levels of total tau and tau protein phosphorylated at threonine 181 in patients with incipient and manifest Alzheimer's disease. Neurosci. Lett. 2003;339(2):172–174. doi: 10.1016/s0304-3940(02)01481-7. [DOI] [PubMed] [Google Scholar]
- 17.Lewczuk P., Esselmann H., Bibl M., Beck G., Maler J.M., Otto M., et al. Tau protein phosphorylated at threonine 181 in CSF as a neurochemical biomarker in Alzheimer’s disease. J. Mol. Neurosci. 2004;23(1):115–122. doi: 10.1385/JMN:23:1-2:115. [DOI] [PubMed] [Google Scholar]
- 18.Hampel H., Buerger K., Zinkowski R., Teipel S.J., Goernitz A., Andreasen N., et al. Measurement of phosphorylated Tau epitopes in the differential diagnosisof Alzheimer disease: a comparative cerebrospinal fluid study. Arch. Gen. Psychiatry. 2004;61(1):95–102. doi: 10.1001/archpsyc.61.1.95. [DOI] [PubMed] [Google Scholar]
- 19.Itoh N., Arai H., Urakami K., Ishiguro K., Ohno H., Hampel H., et al. Large-scale, multicenter study of cerebrospinal fluid tau protein phosphorylated at serine 199 for the antemortem diagnosis of Alzheimer's disease. Ann. Neurol. 2001;50(2):150–156. doi: 10.1002/ana.1054. [DOI] [PubMed] [Google Scholar]
- 20.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 21.Rabe-Jablonska J. A new draft of the mental disorders classification prepared by the American Psychiatric Association: diagnostic and statistical manual of mental disorders-IV, Options Book. Psychiatr. Pol. 1993;27(2):109–119. [PubMed] [Google Scholar]
- 22.Gelb D.J., Oliver E., Gilman S. Diagnostic criteria for Parkinson disease. Arch. Neurol. 1999;56(1):33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 23.Litvan I., Agid Y., Calne D., Campbell G., Dubois B., Duvoisin R.C., et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47(1):1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Dubois B., Feldman H.H., Jacova C., Hampel H., Molinuevo J.L., Blennow K., et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014;13(6):614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 25.MacLean B., Tomazela D.M., Shulman N., Chambers M., Finney G.L., Frewen B., et al. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 2010;26(7):966–968. doi: 10.1093/bioinformatics/btq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smach M.A., Charfeddine B., Ben Othman L., Lammouchi T., Dridi H., Nafati S., et al. Evaluation of cerebrospinal fluid tau/beta-amyloid(42) ratio as diagnostic markers for Alzheimer disease. Eur. Neurol. 2009;62(6):349–355. doi: 10.1159/000241881. [DOI] [PubMed] [Google Scholar]
- 27.Hampel H., Toschi N., Baldacci F., Zetterberg H., Blennow K., Kilimann I., et al. Alzheimer's disease biomarker-guided diagnostic workflow using the added value of six combined cerebrospinal fluid candidates: Abeta1-42, total-tau, phosphorylated-tau, NFL, neurogranin, and YKL-40. Alzheimer's Dement. 2018;14(4):492–501. doi: 10.1016/j.jalz.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Hansson O., Zetterberg H., Buchhave P., Andreasson U., Londos E., Minthon L., et al. Prediction of Alzheimer’s disease using the CSF Aβ42/Aβ40 ratio in patients with mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2007;23(5):316–320. doi: 10.1159/000100926. [DOI] [PubMed] [Google Scholar]
- 29.Wiltfang J., Esselmann H., Bibl M., Hüll M., Hampel H., Kessler H., et al. Amyloid β peptide ratio 42/40 but not Aβ42 correlates with phospho-Tau in patients with low-and high-CSF Aβ40 load. J. Neurochem. 2007;101(4):1053–1059. doi: 10.1111/j.1471-4159.2006.04404.x. [DOI] [PubMed] [Google Scholar]
- 30.Dumurgier J., Schraen S., Gabelle A., Vercruysse O., Bombois S., Laplanche J.-L., et al. Cerebrospinal fluid amyloid-β 42/40 ratio in clinical setting of memory centers: a multicentric study. Alzheimer's Res. Ther. 2015;7(1):30. doi: 10.1186/s13195-015-0114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janelidze S., Pannee J., Mikulskis A., Chiao P., Zetterberg H., Blennow K., et al. Concordance between different amyloid immunoassays and visual amyloid positron emission tomographic assessment. JAMA Neurol. 2017;74(12):1492–1501. doi: 10.1001/jamaneurol.2017.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewczuk P., Lelental N., Spitzer P., Maler J.M., Kornhuber J. Amyloid-β 42/40 cerebrospinal fluid concentration ratio in the diagnostics of Alzheimer's disease: validation of two novel assays. J. Alzheimers Dis. 2015;43(1):183–191. doi: 10.3233/JAD-140771. [DOI] [PubMed] [Google Scholar]
- 33.McAvoy T., Lassman M.E., Spellman D.S., Ke Z., Howell B.J., Wong O., et al. Quantification of tau in cerebrospinal fluid by immunoaffinity enrichment and tandem mass spectrometry. Clin. Chem. 2014;60(4):683–689. doi: 10.1373/clinchem.2013.216515. [DOI] [PubMed] [Google Scholar]
- 34.Olsson B., Lautner R., Andreasson U., Ohrfelt A., Portelius E., Bjerke M., et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15(7):673–684. doi: 10.1016/S1474-4422(16)00070-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.