Highlights
-
•
Parathyroid Hormone Related Protein (PTHrP) in Alzheimer’s disease (AD)
-
•
PTHrP and calcium measured in CSF samples of AD patients.
-
•
PTHrP correlates with AD biomarkers.
-
•
PTHrP in CSF likely reflects neuronal functionality and CSF turnover.
Abbreviations: AD, Alzheimer’s disease; CSF, cerebrospinal fluid; CNS, central nervous system; Ca, calcium; MS, mass spectrometry; LP, lumbar puncture; LC-MS/MS, liquid chromatography tandem mass spectrometry; ICP-MS, inductively coupled plasma mass spectrometry; PTHrP, parathyroid hormone-related protein; 15N PTHrP, parathyroid hormone related protein containing isotopically labelled nitrogen; PTH, parathyroid hormone; RIA, radioimmunoassay; Aβ42, β-Amyloid; T-tau, total tau protein; P-tau, phosphorylated tau protein
Keywords: Alzheimer’s disease, Parathyroid hormone-related protein, PTHrP, Mass spectrometry, Tau protein, Phosphorylated tau protein, Beta-amyloid
Abstract
Background
Parathyroid hormone-related protein (PTHrP) is involved in intracellular calcium regulation, neural cell proliferation and synaptic transmission. To date, no studies have been performed to evaluate the potential of PTHrP concentrations in cerebrospinal fluid (CSF) as a biomarker of brain pathophysiology. In this study we evaluated the association between CSF concentrations of PTHrP with the core CSF biomarkers of Alzheimer’s disease (AD).
Methods
PTHrP and calcium were analysed using validated mass spectrometry based methods in a set of CSF samples that tested positive (n = 45) and negative (n = 45) for the AD biomarkers, including total tau protein (T-tau), phosphorylated tau protein (P-tau) and amyloid-β 42 (Aβ42). The measured CSF concentrations of PTHrP and calcium (Ca) were evaluated for association with AD CSF biomarkers.
Results
PTHrP and Ca concentrations in CSF samples ranged between 25 and 137 pmol/L and 0.92–1.53 mmol/L, respectively. Higher concentrations of PTHrP were observed in association with increased concentrations of T-tau and P-tau in the AD and the control group; while a stronger correlation was observed in the control group (ρ = 0.6, p < 0.0001; and ρ = 0.72, p < 0.0001, for T-tau and P-tau, respectively). Negative correlation was observed between concentrations of PTHrP and Aβ42 in the AD group (ρ = 0.27, p = 0.015). A statistically significantly lower ratio Aβ42/PTHrP was observed in the AD group (p < 0.0001).
Conclusion
In the current study, we observed an association of PTHrP concentrations with concentrations of clinically used CSF biomarkers of AD. Concentrations of PTHrP were positively correlated with concentrations of T-tau and P-tau, suggesting an association with neuronal secretion and function, which is reduced upon progression to AD pathology. Our data suggest potential utility of the Aβ42/PTHrP ratio in assessment of AD progression.
1. Introduction
Alzheimer’s disease (AD) is the most prevalent neurodegenerative disorder characterized by cognitive decline as a result of progressive degeneration and loss of neurons and their synapses [1]. The neuropathological hallmarks of AD include formation of protein deposits in the brain, including intercellular neurofibrillary tangles consisting of hyper-phosphorylated tau protein and extracellular plaques formed by aggregated beta-amyloid peptides (Aβ) [1]. The exact mechanisms underlying AD pathogenesis are still not fully understood, hampering development of therapeutic treatment strategies, while the leading hypothesis places amyloid aggregation and deposition upstream of neurodegeneration and tau pathology [2].
Advanced methods, including mass spectrometry based targeted proteomics, are essential for the discovery of novel biomarkers and identification of targets for therapeutic intervention. Limited availability of sensitive and specific diagnostic markers is a major issue in developing therapies and providing timely treatment for AD patients [3]. The CSF biomarkers, total tau protein (T-tau), phosphorylated tau protein (P-tau) and the 42 amino acid variant of Aβ (Aβ42) allow diagnosis of AD in the early stages of the disease [4]; these biomarkers are widely used in clinical practice [5], [6], [7], [8]. Psychiatric symptoms occurring in the elderly can be caused by various disorders; patients with behavioral disorders can be misdiagnosed, since some of the symptoms could be confused with AD [3]. Considering that the timing between onset of psychiatric symptoms and cognitive decline is variable among individuals, new biomarkers are needed to detect AD earlier, prior to the appearance of cognitive symptoms, as well as to distinguish AD from other neuropathologies [9], [10]. The availability of new, reliable AD biomarkers could allow diagnosis early in the disease process and offer an improved selection of treatment strategies, which is expected to be important in the future as more effective treatments for neurologic diseases become available.
Parathyroid hormone related protein (PTHrP) is known to be involved in intracellular calcium (Ca) regulation [11]. It has been reported that PTHrP regulates calcium influx in neurons, and is involved in neural cell proliferation, differentiation and maintenance of normal neuronal synaptic transmission [12]. Despite these findings, which suggest the importance of PTHrP in the brain and central nervous system (CNS), studies aimed at direct measurement of PTHrP in CSF either did not detect PTHrP [13], or reported PTHrP concentrations up to 100 times lower than those observed in blood [14].
Liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) has been increasingly used for routine diagnostic measurement of peptide and protein biomarkers in biological samples [15], [16], [17], [18]. Recently, we developed and validated a method for measurement of PTHrP [17]; the method is currently in routine use in a clinical diagnostic reference laboratory (ARUP Laboratories, Salt Lake City, USA). Earlier, Kushnir et al. demonstrated poor analytical specificity and sensitivity of commercial radioimmunoassay (RIA) for measurement of PTHrP in plasma [17]. This could explain the inability to detect PTHrP in CSF in the earlier studies [13], [14].
In a separate study [19], we presented data on concentrations of PTHrP and Ca in paired serum and CSF samples of individuals without neurologic diseases, and established reference intervals for PTHrP and Ca in CSF samples. The observed CSF PTHrP concentrations were 20–150 times higher than in serum, and were associated with age and albumin index, suggesting that PTHrP may play a role in age-related physiologic changes in the brain and in neuropathological diseases.
In this exploratory study, we analyzed PTHrP and calcium in a set of CSF samples from patients with a biochemically defined AD diagnosis versus a control group. The aims of this study were to evaluate the association of CSF-PTHrP and calcium concentrations with currently utilized CSF AD biomarkers, and to determine whether measurement of PTHrP and calcium concentrations in CSF could have utility in diagnosing AD, as well as contribute to understanding its underlying pathology.
2. Material and methods
2.1. Patient samples
Lumbar CSF samples were obtained from 45 patients (referred to as the AD group, 22 men and 23 women, median age (range) 74 (57–87) years) and 45 controls (referred to as the control group, 23 men and 22 women, median age (range) 68 (38–88) years). The CSF samples were collected following a standardized protocol following recommendations by the Clinical Neurochemistry Laboratory [3]. Lumbar puncture (LP) with CSF collection was performed following a standardized protocol [3]. LP was performed in the morning, in the L3–L4 or L4–L5 lumbar interspace. In the case of minor bleeding, the first 1–2 mL of CSF was discarded, and then 10–12 mL of CSF was collected by the gravity drip technique in a polypropylene tube to avoid absorption of hydrophobic proteins to the tube walls. The samples were sent directly to the laboratory, centrifuged, aliquoted into 1.5 mL polypropylene cryovials, and stored at −80 °C until analysis.
Participants of the AD group had cognitive deterioration and a CSF biomarker profile indicative of AD (according to cut-off values published in [20]), including increased T-tau (>350 ng/L) and P-tau (>60 ng/L) and low Aβ42 (<530 ng/L). In samples of from the control group, concentrations of all three CSF AD biomarkers were within the reference intervals (T-tau <350 ng/L, P-tau <60 ng/L, Aβ42 >530 ng/L); individuals included in this group did not have evidence of AD pathology [20].
The samples were analyzed as two sets, the first set contained CSF samples of 25 AD patients (14 men and 11 women) and 25 controls (16 men and 9 women), the second set contained CSF samples of 20 AD patients (8 men and 12 women) and 20 controls (7 men and 13 women). The first and the second set of samples were analyzed for PTHrP eight months apart. The CSF samples used for the present study were de-identified left-over aliquots from routine clinical samples. The study was approved by the Ethics Committee at University of Gothenburg (Gothenburg, Sweden). A summary of the demographic characteristics and the ranges of the observed concentrations of T-tau, P-tau, Aβ42, PTHrP, ratio Aβ42/PTHrP and Ca are shown in Table 1.
Table 1.
Demographic characteristics of the participants and concentrations of the measured biomarkers.*
| AD group (n = 45) | Control group (n = 45) | p-value | |
|---|---|---|---|
| Age, years | 73 (57–87) | 68 (38–88) | 0.00378 |
| Gender (men/women) | 22/23 | 23/22 | na |
| Total Tau (T-tau), ng/L | 660 (450–1330) | 244 (123–367) | <0.0001 |
| Phospho Tau (P-tau), ng/L | 75 (60–138) | 39 (21–56) | <0.0001 |
| Aβ42, ng/L | 390 (205–573) | 718 (416–1032) | <0.0001 |
| PTHrP, pmol/L | 60 (25–137) | 57 (27–107) | 0.51 |
| Calcium, mmol/L | 1.20 (0.92–1.45) | 1.20 (0.98–1.52) | 0.38 |
| Ratio Aβ42/PTHrP | 1.38 (0.42–4.7) | 2.88 (1.36–5.78)) | <0.0001 |
Among the samples of AD group 43 out of 45 samples had a PTHrP concentration within the reference interval for CSF [19]. In two samples, the concentration was above the upper end of the reference interval; in all samples of the control group PTHrP concentrations were within the reference interval [19], [25].
Values represented median (central 95th percent of the distribution).
2.2. AD CSF biomarker measurements
CSF concentrations of T-tau, P-tau and Aβ42 were measured by ELISA, as described previously [21], [22], [23]. The AD CSF biomarker analyses were performed at the Clinical Neurochemistry Laboratory (Sahlgrenska University Hospital, Mölndal, Sweden) using protocols accredited by the Swedish Board for Accreditation and Conformity Assessment (SWEDAC) by board-certified laboratory technologists who were blinded to the clinical data.
2.3. Measurement of PTHrP
CSF PTHrP analysis [17] was performed at ARUP Laboratories (Salt Lake City, UT, USA). PTHrP concentrations were determined using an immune-precipitation LC-LC-ESI-MS/MS method [17]; sample preparation and analysis was performed as follows: To a 200 μL aliquot of CSF, 600 μL of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer (HEPES, pH 7.4) and 20 μL of the internal standard (recombinant 15N PTHrP) were added, and the samples were incubated for 15 min. After the incubation, 5 μL of magnetic beads with conjugated anti-PTHrP antibody were added, and the samples were incubated with agitation at 10 °C for 3 h. The beads were washed with HEPES, trypsin was added to the samples, and the samples were incubated at 37 °C for 3 h. After protein digestion, the supernatants were transferred into a 96-well plate, and aliquots were analyzed on the LC-MS/MS instrument.
LC-MS/MS analysis was performed using a system consisting of 1260 and 1290 series HPLC pumps (Agilent Technologies, Santa Clara, CA), and an AB 6500 triple quadrupole mass spectrometer (AB Sciex, Framingham, MA) equipped with a V-spray ionization source running in positive ion mode using multiple reaction monitoring (MRM). The mass transitions monitored for PTHrP-specific peptide (105YLTQETNK112) were m/z 498.75 → 720.35 and 499.25 → 721.35; mass transitions of the internal standard were m/z 504.25 → 729.35 and 504.75 → 730.25. Qualitative confirmation of PTHrP was assessed through the ratio of PTHrP concentrations determined from two mass transitions of the targeted peptide and the internal standard. The acceptability range was ±30% [17], [24]. Detailed information on materials and the method is provided in the Supplemental Materials.
The assay was fully validated for plasma samples according to the CLSI guidelines; performance characteristics of the method for CSF samples were established [17], [25]. The limit of quantitation of the assay was 0.3 pmol/L; the upper limit of linearity was 1100 pmol/L; the total imprecision of the assay at concentrations of 1.3 and 600 pmol/L was 9.3% and 5.8%, respectively. Five calibration standards and five quality controls were analyzed along with every set of samples.
2.4. Calcium measurement
Total Ca was measured in a subset of samples (25 AD patients and 25 controls) using a 7900 ICP-MS (Agilent, Santa Clara, CA) equipped with a Teledyne MVX-7100 autosampler (Teledyne CETAC, Omaha, NB). Sample preparation consisted of diluting 50 μL of CSF with 450 μL of 1% nitric acid containing ethylenediaminetetraacetic acid (EDTA), Triton-X, and an internal standard (Iridium). The quantitation of Ca was performed using Ca isotope m/z 43. Calibrators and controls were from an FDA approved assay for Ca measurements in serum (Roche Diagnostics, Indianapolis, IN). The imprecision of the assay at 6.0 mg/dL, 9.5 and 11.6 mg/dL was <5%.
2.5. Statistical analyses
Baseline characteristics of the study population are summarized in Table 1. For the data analysis, patients were classified as AD or non-AD based on the concentrations of T-tau, P-tau and Aβ42 according to cut-off criteria specified by Hansson et al. [20]. All values are expressed as medians and central 95th percentiles of the distribution. The between-group comparisons were performed using nonparametric Wilcoxon two-group tests for continuous variables; correlation between the variables was assessed using a Pearson regression analysis. A p-value threshold of 0.05 was used for assessment of the statistical significance. Statistical analyses were performed using JMP12 software (SAS Institute, Cary, NC, USA).
3. Results
3.1. Distribution of PTHrP and Ca concentrations
In this study we measured PTHrP concentrations in CSF samples using a novel validated mass spectrometry based method [17]. The specificity of the PTHrP measurements was assessed in every sample by monitoring two mass transitions of the measured PTHrP peptide and the internal standard. In all study samples the ratio of the mass transitions was within the acceptance range utilized in the assay. Extracted ion chromatograms and product ion mass spectra of the PTHrP specific peptide and internal standard utilized in the method are shown on Supplemental Fig. 1. The median concentrations and central 95th percent of the distributions are summarized in Table 1 and Supplemental Fig. 2.
3.2. Association of PTHrP concentrations with concentrations of the AD biomarkers
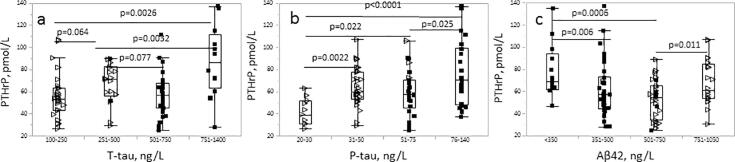
The associations between CSF concentrations of PTHrP and the AD biomarkers (T-tau, P-tau, Aβ42); were evaluated in a sample set from patients diagnosed biochemically with AD [20], [26] and in a control group that had a negative AD biomarker profile and no evidence of AD pathology. Distributions of PTHrP and Ca concentrations were not significantly different between the AD and the control group (Table 1). CSF-PTHrP concentrations were positively associated with CSF concentrations of T-tau (Fig. 1A) and P-tau (Fig. 1B); and negatively associated with concentrations of Aβ42 (Fig. 1C). The association between concentrations of P-tau and Aβ42 remained significant (p = 0.017 and p = 0.011, respectively) after correcting for age of the participants.
Fig. 1.
Box plots with distribution of PTHrP concentrations in association with concentrations of (a) T-tau, (b) P-tau, and (c) Aβ-42. The p-values shown represent the statistical significance for the between-group differences. Filled markers correspond to patients diagnosed with AD.
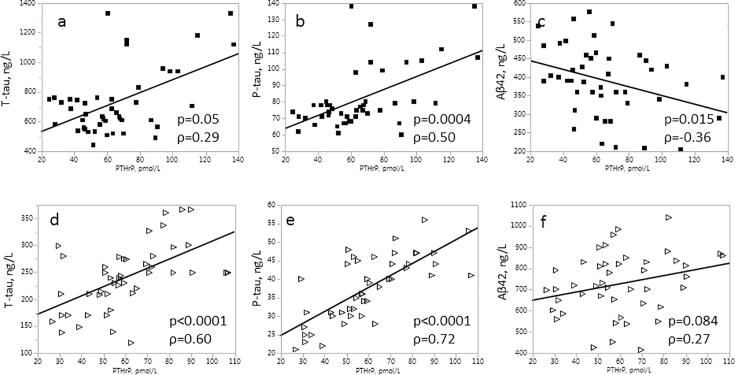
In the control group, concentrations of PTHrP correlated strongly with T-tau (p < 0.0001, ρ = 0.60), P-tau (p < 0.0001, ρ = 0.72) but not with Aβ42 (p = 0.084, ρ = 0.27) (Fig. 2). In contrast, the AD group displayed a weak correlation of PTHrP with T-tau (p = 0.050 ρ = 0.29); and P-tau (p = 0.0004, ρ = 0.50), while a negative correlation was observed with Aβ42 (p = 0.015, ρ = −0.36) (Fig. 2).
Fig. 2.
Association between concentrations of PTHrP and (a, d) T-tau, (b, e) P-tau, and (c, f) Aβ-42 in AD (a, b, c) (n = 45) and in control (d, e, f) (n = 45) groups.
3.3. Association of PTHrP and Ca concentrations with age
No statistically significant association was observed between PTHrP concentrations and the age of the participants in the entire study group; a trend towards a positive association of PTHrP concentration with age was observed in the AD group (Supplemental Fig. 3a). A slight negative trend was observed between Aβ42 and age in the AD (p = 0.16) and the control group (p = 0.024) (Supplemental Fig. 4a,c). No statistically significant difference was observed in the distribution of T-tau, P-tau, Aβ42 or PTHrP concentrations between samples from men and women (data not shown). A trend towards a positive association between CSF Ca with age was observed in the control group (p = 0.09, ρ = 0.35), but not in the AD group (p = 0.99, ρ = −0.001, data not shown).
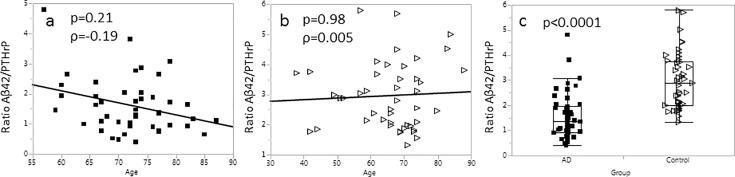
Due to the fact that Aβ42 correlated inversely with PTHrP (Fig. 2c) and PTHrP, in the AD group, correlated positively with age (Supplemental Fig. 3), we examined the ability of the ratio A42/PTHrP to distinguish samples of the AD and the control groups, as well as association of the ratio with age. Lower Aβ42/PTHrP ratios (p < 0.0001, Fig. 3c) were observed in the AD group. A trend towards lower Aβ42/PTHrP ratios in association with age (p = 0.19) was observed in the samples of the AD group (Fig. 3a); when the Aβ42/PTHrP ratios were normalized to the median value observed in the study, the association became significant (p = 0.039 Supplemental Fig. 4b). No association with age was observed in the control group (Fig. 3b and Supplemental Fig. 4d).
Fig. 3.
Association of Aβ42/PTHrP ratio with age; a – AD group (n = 45); b – control group (n = 45); c – box plot with distribution of the Aβ42/PTHrP ratio in the AD and the control group.
Ratios Aβ42/T-tau and Aβ42/P-tau were also evaluated for their ability to distinguish samples from the AD and the control groups. Lower Aβ42/T-tau ratios and higher Aβ42/P-tau (both p < 0.0001, Supplemental Fig. 5c,f) were observed in samples of the AD group. No association with age was observed for Aβ42/T-tau in the AD and the control groups; a positive association with age was observed for Aβ42/P-tau in the AD (p = 0.0077, ρ = −0.393) and the control groups (p = 0.028, ρ = 0.328; Supplemental Fig. 5d,e).
4. Discussion
CSF biomarkers play important role in the assessment of patients with neurological symptoms [27], [28], [29]. The aim of this study was to investigate the association of CSF concentrations of PTHrP with concentrations of the AD biomarkers T-tau, P-tau and Aβ42; and to determine whether measurement of PTHrP concentrations in CSF could have utility in diagnosing AD. In our earlier study [19] we determined that PTHrP is a normal constituent of human CSF, with concentrations, on average, 50 times higher than serum-PTHrP. CSF-PTHrP concentrations were significantly associated with serum-PTHrP, were higher in older individuals, and lower in individuals with higher albumin indices. Considering the above, we hypothesized that PTHrP concentrations in CSF could be associated with age-related physiologic changes in the brain and with neurologic conditions.
4.1. CSF-PTHrP
In the present study, we used an LC-MS/MS method [17] to determine PTHrP concentrations in CSF samples from AD patients and a control group and observed a trend to positive association of PTHrP concentrations with concentrations of T-tau and P-tau; and a trend to negative association with Aβ42 (Fig. 1a–c). No statistically significant difference was observed in the distribution of PTHrP concentrations between the AD and control groups; while PTHrP concentrations were higher in the AD group (Table 1).
The presence of PTHrP in CSF could be a consequence of the release of intracellular PTHrP from neurons and might, therefore, reflect neuronal secretion, general cellular integrity and neuronal function. In earlier studies, mRNA encoding PTHrP was found to be expressed within nerve cells in the brain [30]. PTHrP mRNA was detected in neurons within the cerebral and cerebellar cortex, as well as in dentate granule cells, cornus ammonis pyramidal neurons and large dentate hilar interneurons of the hippocampal formation [30], all of which are involved in memory formation circuits. All of these neuronal cell types are characterized by high electric activity [31] and generation of transmembrane calcium flux [12], [32], which may explain the high PTHrP concentrations observed in CSF samples in this study. Interestingly, the correlation of PTHrP concentrations with concentrations of T-tau and P-Tau was stronger in the control group than in the AD group. This suggests that CSF-PTHrP may reflect neuronal secretion and CSF turnover dynamics, which is reduced in AD. While these are affected in AD, the data suggest that the observed correlation of PTHrP in CSF with T-tau and P-tau is not specific for AD pathology. CSF-PTHrP may, therefore, be a housekeeping protein, and could be useful for assessment of neuronal function and CSF turnover.
Based on the current clinical practice, the main application of diagnostic measurements of PTHrP is confirmation of suspected hypercalcemia of malignancy. A high prevalence of hypercalcemia was reported in AD patients [33], [34], which, based on observations from this study, could be a consequence of higher CSF-PTHrP concentrations in AD patients in combination with a compromised blood-brain barrier [35].
The study samples were analyzed in two batches, with an 8 month interval between the analyses; similar trends in the between-group comparison were observed when data analysis was performed for the first and the second set of samples, separately. In order to assess the potential bias caused by measurements performed 8 months apart, we evaluated the between-run/day imprecision of PTHrP measurements for two quality control samples analyzed in 20 routine batches of samples. The between-run/day imprecision for measurements in samples containing 4.4 and 11.8 pmol/L of PTHrP was 13.4% and 12.2%, respectively.
In our earlier study [19], we evaluated PTHrP storage stability in CSF samples; no changes in PTHrP concentrations were observed after CSF sample storage at ambient conditions for 72 h.
4.2. CSF-CA
Earlier it was shown that PTHrP is involved in Ca regulation in various regions of brain [14], [36], [37], [38]. Considering this, we analyzed a subset of the study samples for Ca using ICP-MS. No difference in CSF-Ca concentrations was observed between the AD and the control groups, suggesting that CSF-Ca concentrations are independent of the AD pathology. A trend towards positive association of Ca concentrations with age was observed in the control group, while not in the AD group. No association of CSF-Ca with CSF-PTHrP and AD biomarkers was observed in the analyzed samples, suggesting that concentrations of PTHrP in extracellular space are not associated with a known functional role.
4.3. Ratio CSF-Aβ42/CSF-PTHrP
In individuals without AD, Aβ42 concentrations are higher than in AD patients [20], while a trend towards higher CSF-PTHrP was observed in AD patients (Table 1). The fact, that in AD, concentrations of Aβ42 and PTHrP are inversely associated (Fig. 2C), suggests that the release of PTHrP could be related to neuronal degeneration. In consideration of this, we evaluated the Aβ42/PTHrP ratio for its ability to distinguish patients of the AD and the control groups. The Aβ42/PTHrP ratio was found to be significantly lower in the AD group (Fig. 3C).
Based on the above findings, and the fact that Aβ42 is inversely associated with T-tau and P-tau, we evaluated the Aβ42/T-tau and Aβ42/P-tau ratios for the ability to distinguish samples from the AD and the control groups. Lower Aβ42/T-tau and higher Aβ42/P-tau ratios (Supplemental Fig. 5c,f) were observed in samples of the AD group.
4.4. Association of CSF-PTHrP and ratio CSF-Aβ42/CSF-PTHrP with age
Our data demonstrate opposite trends in association of PTHrP and Aβ42 concentrations with age in AD patients, with higher PTHrP and lower Aβ42 in older individuals (Supplemental Figs. 3 and 4a,c). A statistically significant negative association was observed between the Aβ42/PTHrP ratio and age in the AD group, while there was no association in the control group (Supplemental Fig. 4b,d). No association with age was observed for the Aβ42/T-tau ratio; a positive association with age was observed for Aβ42/P-tau in both, the AD and the control groups (Supplemental Fig. 5d,e). Considering the above, the Aβ42/PTHrP ratio could be useful for diagnosing AD onset and progression in older individuals.
4.5. Strength and limitations of the study
In this study, the samples were analyzed for PTHrP using clinically and analytically validated mass spectrometry based method [17]. At the time of the analysis the samples were blinded and no information on either the diagnoses of the participants or concentrations of the CSF biomarkers was available until the testing was completed. One limitation of the study is that patients in the AD group were significantly older than the patients in the control group, although applying a correction for the participants age did not reduce the level of statistical significance for the observed associations.
5. Conclusions
In this study, PTHrP and Ca concentrations were measured in CSF samples of AD patients and controls. PTHrP and Ca quantification was performed using mass spectrometry based methods. Concentrations of PTHrP were positively associated with concentrations of T-tau and P-tau in both the AD and the control group, although the degree of association was not as strong in the AD group. Neither PTHrP nor Ca concentrations were significantly different between the AD group and the control group. In the AD group, concentrations of PTHrP and Aβ42 were inversely associated, with lower Aβ42/PTHrP concentration ratios observed in the AD group than in the controls. Higher PTHrP concentrations and lower Aβ42 concentrations were observed in older AD patients, suggesting potential utility of the Aβ42/PTHrP ratio in confirmation of AD diagnosis and as a marker of disease progression in older individuals. While, based on the data from this study, PTHrP is not a specific marker for AD pathology, it has the potential to serve as a complementary marker for monitoring neuronal function and integrity. More studies are needed to assess the clinical utility of PTHrP in association with AD and other neurodegenerative diseases.
Conflict of interest
All authors report no conflicts of interest relevant to this article. The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
Acknowledgments
Acknowledgements
We thank the ARUP® Institute for Clinical and Experimental Pathology for supporting this project (MMK, ALR, FGS).
Funding
The Swedish Research Council VR (#2014-6447, JH; #2017-00915, KB), the Royal Society of Arts and Sciences in Gothenburg (KVVS, JH), Alzheimerfonden (JH, KB), Hjärnfonden (KB), Demensfonden (JH), Jeanssons Stiftelsen (JH), Ahlén Stiftelsen (JH), Åke Wiberg Stiftelsen (JH), Stiftelsen Gamla Tjänarinnor (JH, KB, WM), Stohnes Stiftelse (JH, WM), and the Torsten Söderberg Foundation (KB) and Stiftelsen Wilhelm och Martina Lundgrens Vetenskapsfond (JH) are acknowledged for financial support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clinms.2018.10.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Blennow K., de Leon M.J., Zetterberg H. Alzheimer’s disease. Lancet. 2006;368(9533):387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 2.Hardy J.A., Higgins G.A. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 3.Blennow K., Hampel H., Weiner M., Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat. Rev. Neurol. 2010;6(3):131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 4.Olsson B., Blennow K., Zetterberg H. The clinical value of fluid biomarkers for dementia diagnosis – author’s reply. Lancet Neurol. 2016;15(12):1204–1205. doi: 10.1016/S1474-4422(16)30247-2. [DOI] [PubMed] [Google Scholar]
- 5.Troussiere A.C., Wallon D., Mouton-Liger F., Yatimi R., Robert P., Hugon J., Hannequin D., Pasquier F., Paquet C. Who needs cerebrospinal biomarkers? A national survey in clinical practice. J. Alzheimers Dis. 2014;40(4):857–861. doi: 10.3233/JAD-132672. [DOI] [PubMed] [Google Scholar]
- 6.Tapiola T., Alafuzoff I., Herukka S.K., Parkkinen L., Hartikainen P., Soininen H., Pirttila T. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch. Neurol. 2009;66(3):382–389. doi: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- 7.Strozyk D., Blennow K., White L.R., Launer L.J. CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology. 2003;60(4):652–656. doi: 10.1212/01.wnl.0000046581.81650.d0. [DOI] [PubMed] [Google Scholar]
- 8.Sancesario G.M., Toniolo S., Chiasserini D., Di Santo S.G., Zegeer J., Bernardi G., Musicco M., Caltagirone C., Parnetti L., Bernardini S. The clinical use of cerebrospinal fluid biomarkers for Alzheimer’s disease diagnosis: the Italian selfie. J. Alzheimers Dis. 2017;55(4):1659–1666. doi: 10.3233/JAD-160975. [DOI] [PubMed] [Google Scholar]
- 9.Kvartsberg H., Duits F.H., Ingelsson M., Andreasen N., Ohrfelt A., Andersson K., Brinkmalm G., Lannfelt L., Minthon L., Hansson O., Andreasson U., Teunissen C.E., Scheltens P., Van der Flier W.M., Zetterberg H., Portelius E., Blennow K. Cerebrospinal fluid levels of the synaptic protein neurogranin correlates with cognitive decline in prodromal Alzheimer's disease. Alzheimers Dement. 2015;11(10):1180–1190. doi: 10.1016/j.jalz.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Wellington H., Paterson R.W., Portelius E., Tornqvist U., Magdalinou N., Fox N.C., Blennow K., Schott J.M., Zetterberg H. Increased CSF neurogranin concentration is specific to Alzheimer disease. Neurology. 2016;86(9):829–835. doi: 10.1212/WNL.0000000000002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodda C.P., Kubota M., Heath J.A., Ebeling P.R., Moseley J.M., Care A.D., Caple I.W., Martin T.J. Evidence for a novel parathyroid hormone-related protein in fetal lamb parathyroid glands and sheep placenta: comparisons with a similar protein implicated in humoral hypercalcaemia of malignancy. J. Endocrinol. 1988;117(2):261–271. doi: 10.1677/joe.0.1170261. [DOI] [PubMed] [Google Scholar]
- 12.Gu Z., Liu Y., Zhang Y., Jin S., Chen Q., Goltzman D., Karaplis A., Miao D. Absence of PTHrP nuclear localization and carboxyl terminus sequences leads to abnormal brain development and function. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0041542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akino K., Ohtsuru A., Nakashima M., Ito M., Ting-Ting Y., Braiden V., Kawawaki T., Baba J., Yamashita S., Iwahori N. Distribution of the parathyroid hormone-related peptide and its receptor in the saccus vasculosus and choroid plexus in the red stingray (Dasyatis akajei: Elasmobranch) Cell. Mol. Neurobiol. 1998;18(3):361–368. doi: 10.1023/A:1022509300758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buhler G., Balabanova S., Milowski S., Rosenthal J., Antoniadis G., Mohr K., Richter H.P. Detection of immunoreactive parathyroid hormone-related protein in human cerebrospinal fluid. Exp. Clin. Endocrinol. Diabetes. 1997;105(6):336–340. doi: 10.1055/s-0029-1211775. [DOI] [PubMed] [Google Scholar]
- 15.Kushnir M.M., Rockwood A.L., Roberts W.L., Abraham D., Hoofnagle A.N., Meikle A.W. Measurement of thyroglobulin by liquid chromatography-tandem mass spectrometry in serum and plasma in the presence of antithyroglobulin autoantibodies. Clin. Chem. 2013;59(6):982–990. doi: 10.1373/clinchem.2012.195594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kushnir M.M., Rockwood A.L., Straseski J.A., Meikle A.W. Comparison of LC-MS/MS to immunoassay for measurement of thyroglobulin in fine-needle aspiration samples. Clin. Chem. 2014;60(11):1452–1453. doi: 10.1373/clinchem.2014.227504. [DOI] [PubMed] [Google Scholar]
- 17.Kushnir M.M., Rockwood A.L., Strathmann F.G., Frank E.L., Straseski J.A., Meikle A.W. LC-MS/MS measurement of parathyroid hormone-related peptide. Clin. Chem. 2016;62(1):218–226. doi: 10.1373/clinchem.2015.244012. [DOI] [PubMed] [Google Scholar]
- 18.Hoofnagle A.N., Whiteaker J.R., Carr S.A., Kuhn E., Liu T., Massoni S.A., Thomas S.N., Townsend R.R., Zimmerman L.J., Boja E., Chen J., Crimmins D.L., Davies S.R., Gao Y., Hiltke T.R., Ketchum K.A., Kinsinger C.R., Mesri M., Meyer M.R., Qian W.J., Schoenherr R.M., Scott M.G., Shi T., Whiteley G.R., Wrobel J.A., Wu C., Ackermann B.L., Aebersold R., Barnidge D.R., Bunk D.M., Clarke N., Fishman J.B., Grant R.P., Kusebauch U., Kushnir M.M., Lowenthal M.S., Moritz R.L., Neubert H., Patterson S.D., Rockwood A.L., Rogers J., Singh R.J., Van Eyk J.E., Wong S.H., Zhang S., Chan D.W., Chen X., Ellis M.J., Liebler D.C., Rodland K.D., Rodriguez H., Smith R.D., Zhang Z., Zhang H., Paulovich A.G. Recommendations for the generation, quantification, storage, and handling of peptides used for mass spectrometry-based assays. Clin. Chem. 2016;62(1):48–69. doi: 10.1373/clinchem.2015.250563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kushnir M.M., Peterson L.K., Strathmann F.G. Parathyroid hormone related protein concentration in human serum and CSF correlates with age. Clin. Biochem. 2018;52:56–60. doi: 10.1016/j.clinbiochem.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Hansson O., Zetterberg H., Buchhave P., Londos E., Blennow K., Minthon L. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5(3):228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 21.Blennow K., Wallin A., Agren H., Spenger C., Siegfried J., Vanmechelen E. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease? Mol. Chem. Neuropathol. 1995;26(3):231–245. doi: 10.1007/BF02815140. [DOI] [PubMed] [Google Scholar]
- 22.Andreasen N., Hesse C., Davidsson P., Minthon L., Wallin A., Winblad B., Vanderstichele H., Vanmechelen E., Blennow K. Cerebrospinal fluid beta-amyloid(1–42) in Alzheimer disease: differences between early- and late-onset Alzheimer disease and stability during the course of disease. Arch. Neurol. 1999;56(6):673–680. doi: 10.1001/archneur.56.6.673. [DOI] [PubMed] [Google Scholar]
- 23.Vanmechelen E., Vanderstichele H., Davidsson P., Van Kerschaver E., Van Der Perre B., Sjogren M., Andreasen N., Blennow K. Quantification of tau phosphorylated at threonine 181 in human cerebrospinal fluid: a sandwich ELISA with a synthetic phosphopeptide for standardization. Neurosci. Lett. 2000;285(1):49–52. doi: 10.1016/s0304-3940(00)01036-3. [DOI] [PubMed] [Google Scholar]
- 24.Kushnir M.M., Rockwood A.L., Nelson G.J., Yue B., Urry F.M. Assessing analytical specificity in quantitative analysis using tandem mass spectrometry. Clin. Biochem. 2005;38(4):319–327. doi: 10.1016/j.clinbiochem.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 25.M.M. Kushnir, A.L. Rockwood, F.G. Strathmann. LC-MS/MS measurements of Parathyroid Hormone-Related Protein (PTHrP): negative correlation between age and PTHrP concentrations in CSF. MSACL Conference, Palm Springs, USA, 2016.
- 26.Carmona P., Molina M., Toledano A. Blood-based biomarkers of Alzheimer's disease: diagnostic algorithms and new technologies. Curr. Alzheimer Res. 2016;13(4):450–464. doi: 10.2174/1567205013666151116130301. [DOI] [PubMed] [Google Scholar]
- 27.Gill S.S., Bronskill S.E., Normand S.L., Anderson G.M., Sykora K., Lam K., Bell C.M., Lee P.E., Fischer H.D., Herrmann N., Gurwitz J.H., Rochon P.A. Antipsychotic drug use and mortality in older adults with dementia. Ann. Intern. Med. 2007;146(11):775–786. doi: 10.7326/0003-4819-146-11-200706050-00006. [DOI] [PubMed] [Google Scholar]
- 28.Kleijer B.C., Koek H.L., van Marum R.J., Jansen P.A., Egberts T.C., Heerdink E.R. Risk of acute coronary syndrome in elderly users of antipsychotic drugs: a nested case-control study. Heart. 2012;98(15):1166–1171. doi: 10.1136/heartjnl-2012-301801. [DOI] [PubMed] [Google Scholar]
- 29.Schneider L.S., Dagerman K.S., Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934–1943. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- 30.Weir E.C., Brines M.L., Ikeda K., Burtis W.J., Broadus A.E., Robbins R.J. Parathyroid hormone-related peptide gene is expressed in the mammalian central nervous system. PNAS. 1990;87(1):108–112. doi: 10.1073/pnas.87.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cortes R., Supavilai P., Karobath M., Palacios J.M. Calcium antagonist binding sites in the rat brain: quantitative autoradiographic mapping using the 1,4-dihydropyridines [3H]PN 200–110 and [3H]PY 108–068. J. Neural Transm. 1984;60(3–4):169–197. doi: 10.1007/BF01249092. [DOI] [PubMed] [Google Scholar]
- 32.Llinas R.R. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988;242(4886):1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- 33.Ward B.K., Magno A.L., Walsh J.P., Ratajczak T. The role of the calcium-sensing receptor in human disease. Clin. Biochem. 2012;45(12):943–953. doi: 10.1016/j.clinbiochem.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi K. Cognitive, function and calcium. The link between dementia and bone and calcium metabolism disorders. Clin. Calcium. 2015;25(2):189–194. [PubMed] [Google Scholar]
- 35.Elfakhri K.H., Duong Q.V., Langley C., Depaula A., Mousa Y.M., Lebeouf T., Cain C., Kaddoumi A. Characterization of hit compounds identified from high-throughput screening for their effect on blood-brain barrier integrity and amyloid-beta clearance: In Vitro and In Vivo Studies. Neuroscience. 2018;379:269–280. doi: 10.1016/j.neuroscience.2018.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balabanova S., King O., Teller W.M., Reinhardt G. Distribution and concentration of immunoreactive parathyroid hormone in brain and pituitary of sheep. Klin. Wochenschr. 1985;63(9):419–422. doi: 10.1007/BF01733667. [DOI] [PubMed] [Google Scholar]
- 37.Weaver D.R., Deeds J.D., Lee K., Segre G.V. Localization of parathyroid hormone-related peptide (PTHrP) and PTH/PTHrP receptor mRNAs in rat brain. Brain Res. Mol. Brain Res. 1995;28(2):296–310. doi: 10.1016/0169-328x(94)00222-z. [DOI] [PubMed] [Google Scholar]
- 38.Balabanova S., Peter J., Reinhardt G. Parathyroid hormone secretion by brain and pituitary of sheep. Klin. Wochenschr. 1986;64(4):173–176. doi: 10.1007/BF01713458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.