Highlights
-
•
A multiplex mass spectrometric assay for human TDP-43 in biological matrices.
-
•
Suitable for complex biological matrices including human cell lines and brain tissue.
-
•
Enables higher structural resolution of TDP-43 compared to ligand binding methods.
-
•
Applications to the study of TDP-43 pathology in ALS and frontotemporal dementia.
Abbreviations: ALS, amyotrophic lateral sclerosis; ePAR, expected peak area ratio; FTLD, frontotemporal lobar degeneration; FTD, frontotemporal dementia; LC, liquid chromatography; MRM, multiple reaction monitoring; MS/MS, triple quadrupole mass spectrometer; p, detergent-insoluble fraction; PTM, post-translational modification; rec, recombinant; s, detergent-soluble fraction; S/N, signal to noise ration; TDP-43, transactive response DNA-binding protein 43 kDa
Keywords: Amyotrophic lateral sclerosis, Biomarkers, Frontotemporal dementia, Mass spectrometry, Multiple reaction monitoring, Transactive response DNA-binding protein 43 kDa
Abstract
Transactive response DNA-binding protein 43 kDa (TDP-43) is a highly conserved and widely expressed protein in human tissues that regulates nucleic acid processing. In frontotemporal dementia and amyotrophic lateral sclerosis, however, TDP-43 forms insoluble aggregates in central nervous tissues. These pathological deposits of TDP-43 have been primarily studied by ligand binding, namely western blot analysis, and, thus, methods with greater structural resolution are needed to aid in our understanding of the pathological processes associated with TDP-43 misfolding and aggregation. Toward this goal, we have developed a selective and multiplex method for the detection and characterization of TDP-43 using liquid chromatography tandem mass spectrometry. As proof-of-concept, the method was applied to the detection and characterization of TDP-43 in human cell lines and human brain tissue.
1. Introduction
Transactive response DNA-binding protein 43 kDa (TDP-43) is ubiquitously expressed in almost all human tissues where it functions to produce transcription variants and regulate RNA stability, transport, splicing, translation, and processing, along with other cellular functions [1], [2], [3], [4], [5]. In 2006, TDP-43 was discovered to be the main constituent of the tau-negative, ubiquitin-positive aggregates in frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS) [6], [7]. Thus, the pathology associated with FTD with TDP-43 inclusions is no longer defined by ubiquitin positivity, but by immunohistochemical detection of TDP-43 aggregates, a pathological finding now termed frontotemporal lobar degeneration with TDP-43 inclusions (FTLD-TDP).
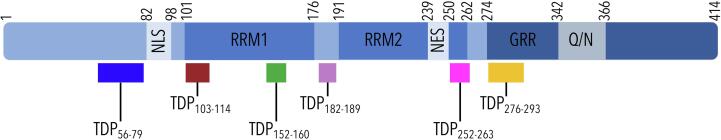
The role of TDP-43 in the initiation and/or progression of these neurodegenerative disorders has yet to be identified; however, abnormal processing and subsequent abnormal functioning of TDP-43 has been proposed. TDP-43 is a 414 residue protein that contains a nuclear localization and export signal sequence that enables nucleo-cytoplasmic shuttling [8]; two RNA recognition motifs (RRMs) [5]; and a glycine-rich region involved in protein-protein interactions [9] (Fig. 1). This glycine-rich region contains a prion-like domain in the asparagine- and glutamine-rich region, which is thought to contribute to the propensity of TDP-43 to aggregate [10], [11].
Fig. 1.
TDP-43 protein sequence and domains, with the location of proteotypic peptides included in the MRM LC–MS/MS method noted. (NLS: nuclear localization sequence; RRM: RNA recognition motif; NES: nuclear export sequence; GRR: glycine-rich region; Q/N: glutamine- and asparagine-rich region).
While the exact role of TDP-43 in the pathogenic mechanism of FTD and ALS remains elusive, post-translational modifications (PTMs) of TDP-43 in FTLD-TDP brain tissue have been identified. The existence of a range of phosphorylated, ubiquitinated, and truncated forms of TDP-43 in pathological specimens is supported predominately by western blot analyses and immunohistochemical staining [12], [13], [14], [15], [16]. Of these PTMs, truncated TDP-43 isoforms are among the most well-studied, however, much of this work was done in vitro and in mouse models. These truncated species of TDP-43 are thought to disrupt the function of normal RNA binding, as well as cellular trafficking, as truncation results in the loss of the nuclear localization signal, and thus disrupts TDP-43 nuclear import and export. Unfortunately, western blot analysis has revealed that several anti-TDP-43 antibodies demonstrate high cross-reactivity with human immunoglobulins (e.g., IgG heavy chain at ∼50 kDa and light chains at ∼25 kDa) and albumin (∼66 kDa) [17], [18], [19]. Given the importance of the identification of TDP-43 proteolytic fragments (TDP-43 molecules <43 kDa) and other PTMs that may result in an increase the molecular weight of TDP-43 (e.g., phosphorylation or ubiquitination), there is concern that the lack of specificity of anti-TDP-43 antibodies could be misinterpreted as the presence of TDP-43 PTMs.
As TDP-43 aggregates are the defining pathology in the majority of cases of FTD and ALS, it would be beneficial to achieve high sequence resolution of pathological TDP-43 in tissue samples and compare to controls. It would also be valuable to conduct this analysis in a routine manner on common analytical instrumentation. With the development of a method that detects multiple peptides (produced in vitro) spanning the TDP-43 sequence, the detection and relative ratios of these TDP-43 peptides could provide insight into the presence of PTMs. A TDP-43 peptide signature, based on unmodified peptides, is expected to be altered in the presence of non-uniform PTM of the protein sequence. For example, an increase in the amount of N-terminally truncated TDP-43 in vivo is hypothesized to result in a decrease in the number of in vitro proteolytic N-terminal peptides observed and the ratio of in vitro proteolytic peptides from the N-terminal domain to the central or C-terminal domains.
To address the need for selective and multiplex detection of TDP-43 isoforms from complex biological matrices, we have developed a targeted bottom-up TDP-43 high-performance liquid chromatography tandem mass spectrometry (LC–MS/MS) assay. As proof-of-concept, the method was applied to the detection of TDP-43 from human cell lysate, and brain tissue from an FTLD-TDP case and an unaffected individual.
2. Material and methods
2.1. Materials
2.1.1. Reagents
The following materials were obtained from the indicated commercial sources: formic acid [399388], N,N,N′,N′-tetramethylethylenediamine [T9281], Tween 20 [P1379], phosphate-buffered saline (PBS) [P4417], ammonium persulfate [A3678], ethanol [362808], sodium dodecyl sulfate (SDS) [L3771], sodium chloride (NaCl) [S7653], ethylenediaminetetraacetic acid (EDTA) [E4884], N-lauroylsarcosine (sarkosyl) [61745], urea [U5378], and Dulbecco’s Modified Eagle’s Medium [D6429], were obtained from Sigma-Aldrich (Canada). Alfa Aesar ammonium hydrogen carbonate (AHC) [A18566], acrylamide/bis-acrylamide solution [J63279], and Laemmli SDS sample buffer [J61337], Bio-Sciences Coomassie solution [786-497] and de-staining solution [786-499], Eppendorf 1.5 mL Protein LoBind tubes [022431081], Roche protease inhibitors [4693159001], acetonitrile (ACN) [BDH83640], tris [0826], CHAPS [0465], bovine serum albumin (BSA) [0332] and tris-buffered saline (TBS) [97063-680] were obtained from VWR (Canada). Nitrocellulose membrane [1620115] and Clarity Max ECL substrate [1705062] were obtained from Bio-Rad. Gibco fetal bovine serum [12483-020], penicillin-streptomycin [15070-063], and molecular weight protein ladder [26616] were purchased from Thermo Fisher Scientific (Canada). Methanol [A456-4] and filter paper [09-802-1A] were obtained from Fisher Scientific. Tosyl phenylalanyl chloromethyl ketone-treated (TPCK) trypsin [LS003744] was obtained from Worthington (USA). Lyophilized recombinant full-length human TDP-43, expressed in E. coli with an N-terminal 6*His-tag (referred to, herein, as recTDP-43) [Ag13119] was obtained from ProteinTech (USA). Anti-TDP-43 mouse monoclonal antibody [H00023435-M01] was obtained from Abnova (Taiwan). Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG antibody [sc-2005] was obtained from Santa Cruz Biotechnology (USA). The following unlabeled peptides were synthesized by New England Peptide (USA): GISVHISNAEPK, FTEYETQVK, and FGGNPGGFGNQGGFGNSR. C18 tips were obtained from Agilent [5188-5239] and Thermo Fisher [60109-412]. A 1 mL, 26-gauge needle [309597] was obtained from Becton, Dickinson and Company (NJ, USA). HeLa cells [ATCC CCL-2] were obtained from the American Type Culture Collection.
2.1.2. Instrumentation
Equipment utilized included: microvolume spectrophotometry (ND-8000, NanoDrop Technologies), centrifugal vacuum (Vacufuge plus, Eppendorf), and a gel imager (G:BOX Chemi XRQ, Syngene). For LC–MS/MS, samples were analyzed using an Aeris Peptide 3.6 µm XB-C18, 50 × 2.0 mm column (Phenomenex, USA) on a Shimadzu LC 20AD LC system coupled to a SCIEX 5500 triple quadrupole mass spectrometer.
2.1.3. Human specimens
This study was undertaken with University of British Columbia research ethics board approval. For the proof-of-concept analysis, frontal lobe brain tissue samples from an individual with immunohistochemistry-confirmed FTLD-TDP type A and from an unaffected individual, were obtained from the Neurodegenerative Brain Biobank at the University of British Columbia. Specimens were collected at autopsy, fresh-frozen, and stored at −70 °C until analysis.
HeLa cells were cultured in Dulbecco’s Modified Eagle’s Medium and supplemented with 10% fetal bovine serum and a penicillin/streptomycin cocktail (100 µg/mL).
2.2. Sample preparation
2.2.1. Tissue homogenization
Human frontal lobe brain tissue (0.2 g) was homogenized manually using a pestle for 2 min in 1 mL of tris-EDTA (TE) buffer (10 mM tris-HCL and 1 mM EDTA, pH 7.5, and protease inhibitor cocktail) containing 10% sucrose, 0.8 M NaCl, and 2% Tween 20, heated for 30 min at 37 °C, and centrifuged at 100,000×g for 30 min at 20 °C. The supernatant was collected (Tween-soluble fraction [s1]), and the pellet was homogenized (following the same steps as above) in TE buffer containing 1% sarkosyl. Again, the supernatant was collected (sarkosyl-soluble fraction [s2]), and the pellet was homogenized in TE buffer containing 1% CHAPS and centrifuged at 100,000×g. The final supernatant was collected (CHAPS-soluble fraction [s3]), and the resulting insoluble fraction (p) was combined with urea buffer (50 mM tris, pH 8.5, 8 M urea and 2% SDS) and sonicated in 1 s pulses, five times. All fractions were stored at −70 °C until analysis.
2.2.2. Cell lysis
HeLa cells were washed with PBS before the addition of ddH2O and incubation at 37 °C for 5 min. After incubation, the cells were left at room temperature for 5 min and then passed through a 26-gauge needle multiple times to rupture remaining intact cells. The resulting lysate was centrifuged at 13,000×g for 15 min, at 4 °C. The supernatant was collected, aliquoted, protein concentration determined by microvolume spectrophotometry, and stored at −70 °C until analysis.
2.2.3. Gel electrophoresis, western blot detection and in-gel digestion
RecTDP-43, HeLa cell lysate, and FTLD-TDP and unaffected homogenate fractions were separated on duplicate 10% polyacrylamide gels by electrophoresis under denaturing conditions. Proteins were transferred to a nitrocellulose membrane for western blot analysis. The membrane was blocked with 5% BSA, dissolved in TBS with Tween20 (0.05%) (TBS-T), for 1 h. The membrane was washed 3 times with TBS-T and probed with anti-TDP-43 antibody (1:1000 in 5% BSA in TBS-T) overnight at 4 °C. The membrane was then washed again and incubated in HRP-conjugated anti-mouse (1:10,000 in 5% BSA in TBS-T) for 1 h at room temperature. The blot was washed for a final time before being imaged using ECL substrate.
Prior to in-gel digestion, total protein was stained for one hour in Coomassie dye and then de-stained overnight prior to imaging. Bands were excised from the gel and placed into separate tubes. To prepare gel pieces for digestion, sequential incubations were performed at room temperature for 20 min in each of the following solutions: 50 mM AHC; 50% 25 mM AHC, 50% ACN; and 100% ACN. Gel pieces were then rehydrated in 50 mM AHC with 5 µg of TPCK-trypsin and incubated overnight at 37 °C. The digestion was halted by adding 1% formic acid to a final concentration of 0.1%. The supernatant was removed and saved for analysis. Remaining gel pieces were further extracted with 100 µL of 50% ACN and 0.1% formic acid in water and incubated at room temperature for 20 min. A final extraction using 100% ACN was performed. All extractions were combined, and the resulting mixture dried by vacuum centrifugation.
Samples were reconstituted in 0.5% formic acid and desalted using C18 tips (Agilent tips for HeLa cells and Thermo Fisher tips for brain tissue) according to the manufacturer’s protocol, with the substitution of formic acid for trifluoroacetic acid.
2.3. Proteotypic peptide selection and MRM method development
Tryptic peptides spanning TDP-43 residues 56–79, 103–114, 152–160, 182–189, 252–263, and 276–293 (referred to as TDP56-79, TDP103-114 TDP152-160, TDP182-189, TDP252-263, and TDP276-293, respectively), were selected based on previously developed criteria [20] and subsequent empirical evaluation of recTDP-43. The recTDP-43 was denatured at 95 °C for 10 min and incubated with TPCK-trypsin for 4 h at 37 °C, and halted by the addition of 1% formic acid – following our group’s previously developed approach to the design of digestion workflows suitable for clinical laboratory implementation [20]. For each of the six TDP peptides, the most abundant precursor ion and three product ions were selected for monitoring.
2.4. LC–MS/MS optimization
MS parameters were optimized using both tryptic digests of recTDP-43 in buffer and synthetic TDP-43 peptides. Collision energy (CE) and declustering potential (DP) were optimized for each MRM. Mobile phases A and B consisted of 0.1% formic acid in water and 0.1% formic acid in ACN, respectively. A flow rate of 0.25 mL/min and column temperature of 45 °C was used with the following gradient: 5% B from 0 to 1 min, 5–95% B from 1 to 7.5 min, 95% B from 7.5 to 8.5 min, and 5% B from 8.5 to 12 min for re-equilibration.
2.5. Figures of merit
The criteria for the lower limit of detection (LLOD) was a signal-to-noise (S/N) ratio ≥3 for the quantifier ion and transition peak area ratios (PARs) within 15% of the standard ratios for the qualifier ions. S/N was calculated as peak-to-peak where the maximal noise peak was considered within a 0.2 min retention time window of the signal peak.
Linearity was assessed by serially diluting a 1 μg/mL recTDP-43 tryptic digest solution, halving the concentration in 8 dilutions from 500 to 3.91 ng/mL using 50 mM AHC.
2.6. Data analysis
The observed m/z of peptide precursor and product ions were verified using Skyline. Each peptide sequence was assessed for its uniqueness for TDP-43 within the human proteome by protein BLAST search and the sequence explored for variants and potential PTMs including ubiquitination, phosphorylation, sumoylation, acetylation, glycosylation, and truncation using the Universal Protein Resource (Uniprot). LC–MS/MS data was analyzed using Analyst software (SCIEX v.1.6) and linear regressions performed using cp-R software [21].
3. Results
3.1. TDP-43 characterization, peptide selection & MRM development
The six peptides selected by in silico digestion were determined to be unique for TDP-43 within the human proteome (Fig. 1). Exploration of potential PTM sites and variants relevant to the tryptic peptide sequences monitored revealed reports of sumoylation of K79 and K263 [22], phosphorylation of S183 and S292 [23], methylation of R293 [24], and variants of P112H reported in a case of FTLD-TDP [25], K263E reported in a case of FTLD-TDP with supranuclear gaze palsy and chorea, G287S reported in a case of sporadic ALS, and G290A and S292N reported in cases of familial ALS [26].
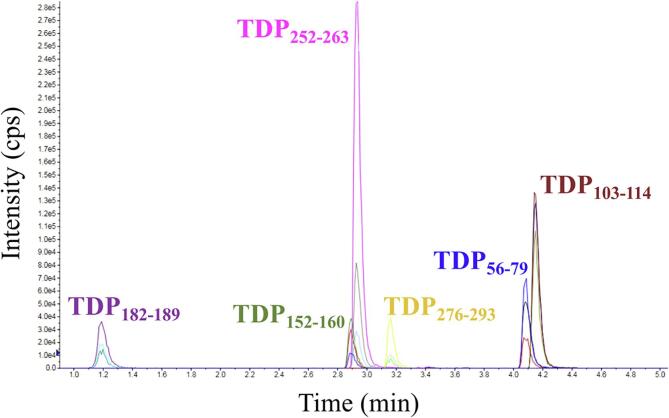
An MRM method was developed that monitored the six TDP-43 proteotypic peptides (Fig. 2). By empirical evaluation, retention times were confirmed and three precursor to product ion transitions per peptide were selected for monitoring (Fig. 3). Optimized MRM parameters are found in Table 1.
Fig. 2.
Representative chromatogram of the TDP-43 6-plex MRM assay.
Fig. 3.
Product ion scans of recTDP-43 tryptic peptides pre-optimization.
Table 1.
Optimized MRM parameters for proteotypic TDP-43 peptides.
| Peptide | Q1 ion (m/z) | Q1 charge state | Q3 ion (m/z)a | Fragment ion | DP (V) | CE (V) |
|---|---|---|---|---|---|---|
| TDP56-79 | 875.8 | 3+ | 822.5 | b16 | 100 | 30 |
| 882.3 | y7 | 100 | 30 | |||
| 981.8 | y8 | 100 | 30 | |||
| TDP103-114 | 671.6 | 2+ | 430.4 | y3 | 80 | 40 |
| 304.1 | b3 | 80 | 40 | |||
| 812.5 | y7 | 80 | 30 | |||
| TDP152-160 | 572.8 | 2+ | 869.3 | y7 | 40 | 26 |
| 767.5 | y6 | 40 | 30 | |||
| 604.7 | y5 | 40 | 30 | |||
| TDP182-189 | 486.7 | 2+ | 513.9 | y4 | 80 | 28 |
| 629.1 | y5 | 80 | 28 | |||
| 385.2 | y3 | 80 | 28 | |||
| TDP252-263 | 417.9 | 3+ | 541.4 | y10 | 80 | 23 |
| 448.5 | y8 | 80 | 22 | |||
| 758.6 | y7 | 80 | 20 | |||
| TDP276-293 | 863.9 | 2+ | 676.3 | y14 | 60 | 40 |
| 376.3 | y3 | 60 | 40 | |||
| 694.3 | y7 | 60 | 42 | |||
Listed in order of the quantifier ion first, followed by the two qualifier ions.
3.2. Sensitivity and linearity
Based on structural characterization and intensity in the LC–MS/MS, TDP252-263 was selected for assessment of assay sensitivity and linearity. Structurally, TDP252-263 is situated at the immediate C-terminal end of RRM2. Sumoylation of K263 at the C-terminal end of TDP252-263 has been reported; however, this is from a single, untargeted study analyzing the human sumoylation proteome with no confirmatory analysis performed [22]. A K263E variant has been reported in a single case of FTLD-TDP with supranuclear gaze palsy and chorea [26].
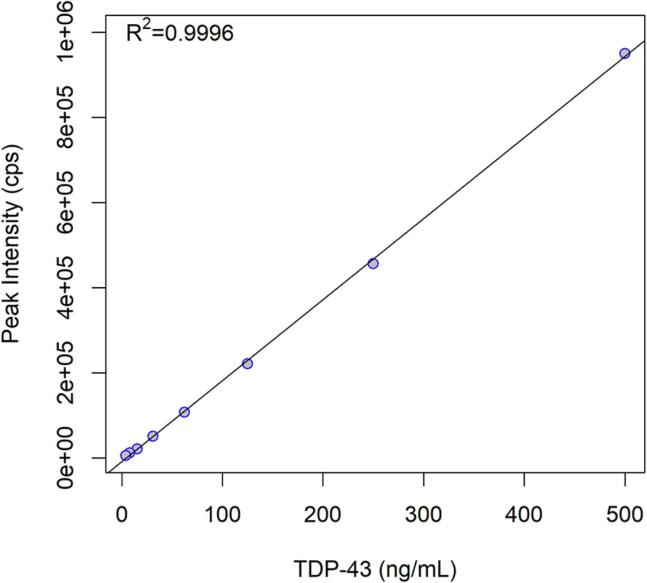
By analysis of a dilution series of trypsin-digested recTDP-43 in 50 mM AHC, the lower limit of detection for TDP-43 was determined to be 3.91 ng/mL (Table 2). LC–MS/MS analysis of the TDP252-263 MRM was linear over the range of 3.91 ng/mL to 500 ng/mL based on least squares regression analysis of the dilution series; R2 = 0.9996 (Fig. 4).
Table 2.
Comparison of recTDP-43 dilution series against detection criteria of S/N ≥ 3 and PAR within ±15% of expected (ePAR) for TDP252-263.
| TDP-43 (ng/mL) | S/N | % from ePARa |
|
|---|---|---|---|
| Qualifier-1 (448.5 m/z) | Qualifier-2 (758.6 m/z) | ||
| 500 | >100 | −4 | 0 |
| 250 | >100 | −4 | 0 |
| 125 | >100 | −11 | 0 |
| 62.5 | >100 | −14 | 0 |
| 31.25 | >100 | −4 | −11 |
| 15.63 | >100 | −11 | −11 |
| 7.81 | >73 | −11 | −11 |
| 3.91 | >35 | −11 | −11 |
| 1.95b | >22 | −14 | −56 |
| 0.98b | >16 | −4 | −22 |
| 0.49b | 8 | +36 | −22 |
| 0.24b | 3 | −25 | +11 |
Deviation from the expected peak area ratio for recTDP-43.
Failed detection criteria.
Fig. 4.
Linearity of the response of TDP252-263.
3.3. Detection of endogenous TDP-43 in HeLa cell lysate
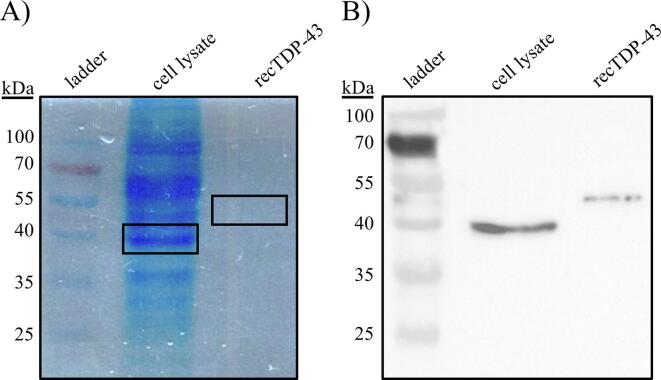
TDP-43 western blot analysis of HeLa cell lysate and recTDP-43 (with an N-terminal HIS-tag and a theoretical molecular mass of 48,284 Da) yielded bands in the expected molecular weight regions of 43 kDa and 49 kDa, respectively (Fig. 5).
Fig. 5.
(A) SDS-PAGE and (B) TDP-43 western blot analysis of HeLa cell lysate and recTDP-43. Boxes indicate gel excisions subjected to in-gel digest and LC–MS/MS analysis.
Gel regions where TDP-43 was detected by western blot were excised, digested, and analyzed by LC–MS/MS, which confirmed the presence of TDP-43. For HeLa cells, five TDP-43 tryptic peptides were observed, of which four (TDP56-79, TDP103-114, TDP152-160, and TDP252-263) satisfied the assay detection acceptance criteria (Table 3).
Table 3.
LC–MS/MS analysis of the in-gel digestion of HeLa cell lysate TDP-43 band.
| Peptide | Quantifier ion S/N | Qualifier ions (m/z) | % from ePARa |
|---|---|---|---|
| TDP56-79 | 40 | 882.3 | −15 |
| 981.8 | +15 | ||
| TDP103-114 | 49 | 304.1 | +7 |
| 812.3 | +1 | ||
| TDP152-160 | 18 | 767.4 | −11 |
| 604.3 | −13 | ||
| TDP252-263 | 37 | 448.2 | −11 |
| 758.4 | −15 | ||
| TDP276-293 | 22 | 376.3 | +7 |
| 694.3 | −24b |
Deviation from the expected peak area ratio for recTDP-43.
Failed acceptance criteria of ePAR within ±15%.
3.4. Detection of endogenous TDP-43 in human brain tissue
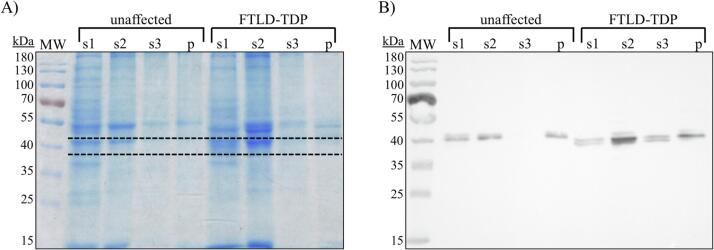
Human brain tissue from an unaffected individual and an individual with FTLD-TDP type A was homogenized, fractionated and analyzed by western blot and LC–MS/MS after in-gel digestion. Via western blot, TDP-43 was observed in all fractions for both specimens, except fraction s3 of the unaffected specimen (Fig. 6). Gel regions where TDP-43 was observed by western blot (around 43 kDa) were excised and subjected to in-gel digestion and LC–MS/MS analysis. In all western blot-positive gel pieces, LC–MS/MS identified multiple TDP-43 peptides. In these samples, LC–MS/MS identified four (unaffected s2 and FTLD-TDP s1) or five (unaffected s1 and pellet and FTLD-TDP s2, s3, and pellet) TDP-43 proteotypic peptides; and at least one peptide for each fraction met the pre-specified detection criteria (Table 4). Additionally, two TDP-43 peptides (TDP56-79 and TDP103-114, with the latter meeting the pre-defined detection criteria) were identified in the gel section from the unaffected s3 fraction, which was western blot-negative for TDP-43 (Table 4).
Fig. 6.
(A) SDS-PAGE and (B) western blot analysis of unaffected and FTLD-TDP type A human brain tissue (dashed line: gel region excised for in-gel digestion and LC–MS/MS analysis).
Table 4.
LC–MS/MS detection of TDP-43 in the insoluble fractions from the unaffected and FTLD-TDP type A frontal lobe tissue specimens.
| Peptide | Q3 ion (m/z) | Unaffected tissue |
FTLD-TDP tissue |
||
|---|---|---|---|---|---|
| Quantifier ion S/N | % from ePARa | Quantifier ion S/N | % from ePARa | ||
| TDP56-79 | 882.3 | 7 | −7 | 4 | −5 |
| 981.8 | +3 | +22b | |||
| TDP103-114 | 304.1 | 23 | −12 | 31 | +4 |
| 812.3 | −16b | −15 | |||
| TDP152-160 | 767.4 | 6 | +17b | 9 | +22b |
| 604.3 | +31b | +9 | |||
| TDP252-263 | 448.2 | 32 | +14 | 22 | +11 |
| 758.4 | 0 | +11 | |||
| TDP276-293 | 376.3 | 29 | +40b | 14 | +13 |
| 694.3 | +24b | +22b | |||
Deviation from the expected peak area ratio for recTDP-43.
Failed acceptance criteria of ePAR within ±15%.
4. Discussion
While TDP-43 has been identified as the defining pathological protein in the majority of FTD and ALS cases, there remain gaps in our knowledge about the structure of TDP-43 in these disease states. As such, there is great interest in detecting, characterizing and quantifying TDP-43 and its disease-related PTMs. To date, methods to study TDP-43 have largely relied on ligand-binding methods, which lack specificity for TDP-43 and the resolution to characterize sequence structure. To overcome this obstacle, we developed a multiplex LC–MS/MS method selective for proteotypic peptides of TDP-43. By shifting the detection of TDP-43 to the proteolytic peptide level, higher sequence level information can be obtained. This information can in turn be utilized to create TDP-43 peptide signatures (relative proteolytic peptides amounts) characteristic of TDP-43 proteinopathies compared to controls.
To assess detection of natively-folded endogenous TDP-43 in a complex biological sample, the LC–MS/MS method was applied to detection of endogenous TDP-43 in a human cell line. While analysis of recTDP-43 is helpful for method development, it is not an ideal surrogate for the natively structured protein. The recTDP-43 used had a non-cleavable 6*HIS-tag on the N-terminus and, based on several lots purchased and analyzed by western blot, was of varying purity; moreover, recTDP-43 is known to readily form aggregates in physiological buffers [27], which is not representative of natively folded endogenous TDP-43. As such, recTDP-43 is helpful in early method development, but ultimately endogenous TDP-43 from human sources should be tested.
Human cell lines are one such native source, and are particularly relevant due to the prevalence of FTD and ALS research conducted using human cell lines. While animal models are commonly studied in related neurodegenerative disorders, such as Alzheimer’s disease, there are challenges in creating animal models of TDP-43 pathology that fully recapitulate the findings in humans. To create an FTLD-TDP/ALS animal model, human TDP-43 must be transgenically overexpressed or knocked-in. While these two models can present with mild to moderate disease-associated phenotypes, the predominant pathological hallmark of the disease – neuronal cytoplasmic TDP-43 inclusions – are not always present [28]. Transgenic mice expressing similar amounts of human TDP-43 compared to humans develop age-dependent neurological phenotypes, but without neuronal loss, paralysis or reduced lifespan [28]. The use of human cell lines for studying TDP-43 pathogenesis has, thus, been an important focus of FTD/ALS research [29], [30], [31].
The LC–MS/MS method applied to the characterization of HeLa cells successfully detected proteolytic peptides from endogenous TDP-43. Detection post-in-gel-digest, yielded observation of five TDP-43 peptides of which TDP56-79, TDP103-114, TDP152-160, and TDP252-263 satisfied the detection acceptance criteria. The LC–MS/MS detected peptides had wide sequence representation covering multiple structural domains of TDP-43, including pathologically relevant sites. TDP-43 regions detected include the N-terminus and RNA binding domains, RRM1 and RRM2, which encompass the nuclear localization and export signal sequences. Detection of peptides from the RNA binding domains (residues 101–262) represent the functional domain of TDP-43 [5]. Pathologically relevant sites covered include disease-suspected 35 kDa and 25 kDa caspase cleavage products of TDP-43, that is, TDP-35 and TDP-25 spanning residues 90–414 and 220–414, respectively [32]. In cell lines, cleavage of TDP-43 by caspase 3 into TDP-35 and TDP-25 fragments, leads to redistribution of nuclear TDP-43 into the cytoplasm [32], [33] and can generate aggregation-prone fragments and form cytoplasmic toxic inclusion bodies [34]. While N-terminal and RNA-binding domains were detected, two peptides did not meet the detection criteria, including the one from the C-terminal domain. TDP182-189 was not observed, whereas TDP276-293 was observed, but failed one of the detection threshold criteria (i.e., a qualifier ion ratio). Peptides both N-terminal and C-terminal to TDP182-189 were detected, which along with relative signal intensities from recTDP-43, suggest inadequate sensitivity for these two peptides due to pre-analytical (e.g., proteolytic cleavage efficiency) and/or analytical factors (e.g., ionization efficiency). Notably, saturation of the C18 tips was observed in the analysis of the HeLa cell lysate (data not shown); this presents an opportunity for future improvements in sensitivity for all monitored tryptic peptides. As a result of this finding, C18 tips with a greater binding capacity were used for analysis of the brain tissue homogenates.
Common contemporary approaches for characterizing TDP-43 structure in brain tissue include immunohistochemical staining and western blot analyses, both of which are ligand binding methods dependent on antibody-antigen interactions. While this methodology has been valuable in the characterization of TDP-43 in disease, it has limited multiplexing capabilities, relies on indirect detection (resulting in a lack of specificity) and provides low-resolution structural information. The availability of a higher resolution method that directly detects the measurand of interest, e.g., MRM LC–MS/MS, would be helpful in routine characterization of tissues, and complement information obtained from immunometric approaches. As proof-of-concept, the ability of the TDP-43 MRM LC–MS/MS assay to detect endogenous TDP-43 in human brain tissue (immunohistochemical-confirmed FTLD-TDP type A and an unaffected control) was assessed.
In general, the low relative abundance of TDP-43 in complex matrices such as brain tissue (particularly the soluble fractions, which had a high non-TDP-43 protein content), presents a challenge for detection. With greater analytical sensitivity desired, the use of larger resin volumes for desalting and/or additional enrichment strategies, can be applied to further improve the sequence resolution by LC–MS/MS. Further enrichment may also result in sufficient purification of TDP-43 isoforms to enable alternate mass spectrometric approaches, such as top-down analysis. The current selective detection of TDP-43 by MRM LC–MS/MS and sequence coverage achieved for cell lysate and human tissue support the application of this targeted TDP-43 LC–MS/MS assay for routine characterization of FTLD-TDP and ALS pathological tissues, which, with ongoing method development, has the potential to provide even higher sequence resolution to aid in our understanding of structural modifications of TDP-43 associated with neurodegenerative disorders.
5. Conclusion
A targeted multiplex mass spectrometric method for the detection and characterization of TDP-43 was developed. The method enabled detection of TDP-43 in complex biological matrices, including human cell lines and human brain tissue specimens, and provides the opportunity for characterization of pathological forms of TDP-43 at higher resolution compared to ligand binding methods.
Declaration of Competing Interest
M.L.D. reports a grant from the Alzheimer's Drug Discovery Foundation and the Association for Frontotemporal Degeneration, and Y.Z.Z. reports a fellowship from the Alzheimer Society of Canada and the Firefly Foundation, during the conduct of the study. I.R.A.M. reports personal fees from Prevail Therapeutics, outside of the submitted work. T.D.P., L.M.F, and J.G.K.L. have nothing to disclose.
References
- 1.Buratti E. TDP-43 post-translational modifications in health and disease. Expert. Opin. Ther. Targets. 2018;22:279–293. doi: 10.1080/14728222.2018.1439923. [DOI] [PubMed] [Google Scholar]
- 2.Lee E.B., Lee V.M., Trojanowski J.Q. Gains or losses: molecular mechanisms of TDP43-mediated neurodegeneration. Nat. Rev. Neurosci. 2011;13:38–50. doi: 10.1038/nrn3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strong M.J., Volkening K., Hammond R., Yang W., Strong W., Leystra-Lantz C., Shoesmith C. TDP43 is a human low molecular weight neurofilament (hNFL) mRNA-binding protein. Mol. Cell Neurosci. 2007;35:320–327. doi: 10.1016/j.mcn.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Wang H.Y., Wang I.F., Bose J., Shen C.K. Structural diversity and functional implications of the eukaryotic TDP gene family. Genomics. 2004;83:130–139. doi: 10.1016/s0888-7543(03)00214-3. [DOI] [PubMed] [Google Scholar]
- 5.Buratti E., Baralle F.E. Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. J. Biol. Chem. 2001;276:36337–36343. doi: 10.1074/jbc.M104236200. [DOI] [PubMed] [Google Scholar]
- 6.Arai T., Hasegawa M., Akiyama H., Ikeda K., Nonaka T., Mori H., Mann D., Tsuchiya K., Yoshida M., Hashizume Y., Oda T. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 7.Neumann M., Sampathu D.M., Kwong L.K., Truax A.C., Micsenyi M.C., Chou T.T., Bruce J., Schuck T., Grossman M., Clark C.M., McCluskey L.F., Miller B.L., Masliah E., Mackenzie I.R., Feldman H., Feiden W., Kretzschmar H.A., Trojanowski J.Q., Lee V.M. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 8.Ayala Y.M., Zago P., D’Ambrogio A., Xu Y.F., Petrucelli L., Buratti E., Baralle F.E. Structural determinants of the cellular localization and shuttling of TDP-43. J. Cell. Sci. 2008;121:3778–3785. doi: 10.1242/jcs.038950. [DOI] [PubMed] [Google Scholar]
- 9.B. Rogelj, K.S. Godin, C.E. Shaw, J. A Ule. The functions of the glycine-rich regions in TDP-43, FUS and related RNA-binding proteins. RNA Binding Proteins. ISBN, (2011).
- 10.Guo W., Chen Y., Zhou X., Kar A., Ray P., Chen X., Rao E.J., Yang M., Ye H., Zhu L., Liu J., Xu M., Yang Y., Wang C., Zhang D., Bigio E.H., Mesulam M., Shen Y., Xu Q., Fushimi K., Wu J.Y. An ALS-associated mutation affecting TDP-43 enhances protein aggregation, fibril formation and neurotoxicity. Nat. Struct. Mol. Biol. 2011;18:822–830. doi: 10.1038/nsmb.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Budini M., Buratti E., Stuani C., Guarnaccia C., Romano V., De Conti L., Baralle F.E. Cellular model of TAR DNA-binding protein 43 (TDP-43) aggregation based on its C-terminal Gln/Asn-rich region. J. Biol. Chem. 2012;287:7512–7525. doi: 10.1074/jbc.M111.288720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasegawa M., Arai T., Nonaka T., Kametani F., Yoshida M., Hashizume Y., Beach T.G., Buratti E., Baralle F., Morita M., Nakano I., Oda T., Tsuchiya K., Akiyama H. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann. Neurol. 2008;64:60–70. doi: 10.1002/ana.21425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackenzie I.R., Neumann M., Bigio E.H., Cairns N.J., Alafuzoff I., Kril J., Kovacs G.G., Ghetti B., Halliday G., Holm I.E., Ince P.G., Kamphorst W., Revesz T., Rozemuller A.J., Kumar-Singh S., Akiyama H., Baborie A., Spina S., Dickson D.W., Trojanowski J.Q., Mann D.M. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol. 2009;117:15–18. doi: 10.1007/s00401-008-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neumann M. Molecular neuropathology of TDP-43 proteinopathies. Int. J. Mol. Sci. 2009;10:232–246. doi: 10.3390/ijms10010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neumann M., Mackenzie I.R., Cairns N.J., Boyer P.J., Markesbery W.R., Smith C.D., Taylor J.P., Kretzschmar H.A., Kimonis V.E., Forman M.S. TDP-43 in the ubiquitin pathology of frontotemporal dementia with VCP gene mutations. J. Neuropathol. Exp. Neurol. 2007;66:152–157. doi: 10.1097/nen.0b013e31803020b9. [DOI] [PubMed] [Google Scholar]
- 16.Neumann M., Tolnay M., Mackenzie I.R. The molecular basis of frontotemporal dementia. Expert. Rev. Mol. Med. 2009;11 doi: 10.1017/S1462399409001136. [DOI] [PubMed] [Google Scholar]
- 17.Feneberg E., Steinacker P., Lehnert S., Schneider A., Walther P., Thal D.R., Linsenmeier M., Ludolph A.C., Otto M. Limited role of free TDP-43 as a diagnostic tool in neurodegenerative diseases. Amyotroph. Lateral. Scler. Frontotemporal. Degener. 2014;15:351–356. doi: 10.3109/21678421.2014.905606. [DOI] [PubMed] [Google Scholar]
- 18.Kasai T., Tokuda T., Ishigami N., Sasayama H., Foulds P., Mitchell D.J., Mann D.M., Allsop D., Nakagawa M. Increased TDP-43 protein in cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Acta Neuropathol. 2009;117:55–62. doi: 10.1007/s00401-008-0456-1. [DOI] [PubMed] [Google Scholar]
- 19.Steinacker P., Hendrich C., Sperfeld A.D., Jesse S., von Arnim C.A., Lehnert S., Pabst A., Uttner I., Tumani H., Lee V.M., Trojanowski J.Q., Kretzschmar H.A., Ludolph A., Neumann M., Otto M. TDP-43 in cerebrospinal fluid of patients with frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Arch. Neurol. 2008;65:1481–1487. doi: 10.1001/archneur.65.11.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng Y.Z., DeMarco M.L. Manipulating trypsin digestion conditions to accelerate proteolysis and simplify digestion workflows in development of protein mass spectrometric assays for the clinical laboratory. Clin. Mass Spectrometry. 2017;6:1–12. [Google Scholar]
- 21.Holmes D.T. cp-R, an interface the R programming language for clinical laboratory method comparisons. Clin. Biochem. 2015;48:192–195. doi: 10.1016/j.clinbiochem.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Hendriks I.A., Lyon D., Young C., Jensen L.J., Vertegaal A.C., Nielsen M.L. Site-specific mapping of the human SUMO proteome reveals co-modification with phosphorylation. Nat. Struct. Mol. Biol. 2017;24:325–336. doi: 10.1038/nsmb.3366. [DOI] [PubMed] [Google Scholar]
- 23.Zhou H., Di Palma S., Preisinger C., Peng M., Polat A.N., Heck A.J., Mohammed S. Toward a comprehensive characterization of a human cancer cell phosphoproteome. J. Proteome Res. 2013;12:260–271. doi: 10.1021/pr300630k. [DOI] [PubMed] [Google Scholar]
- 24.Guo A., Gu H., Zhou J., Mulhern D., Wang Y., Lee K.A., Yang V., Aguiar M., Kornhauser J., Jia X., Ren J., Beausoleil S.A., Silva J.C., Vemulapalli V., Bedford M.T., Comb M.J. Immunoaffinity enrichment and mass spectrometry analysis of protein methylation. Mol. Cell Proteomics. 2014;13:372–387. doi: 10.1074/mcp.O113.027870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreno F., Rabinovici G.D., Karydas A., Miller Z., Hsu S.C., Legati A., Fong J., Schonhaut D., Esselmann H., Watson C., Stephens M.L., Kramer J., Wiltfang J., Seeley W.W., Miller B.L., Coppola G., Grinberg L.T. A novel mutation P112H in the TARDBP gene associated with frontotemporal lobar degeneration without motor neuron disease and abundant neuritic amyloid plaques. Acta Neuropathol. Commun. 2015;3:19. doi: 10.1186/s40478-015-0190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackenzie I.R., Rademakers R., Neumann M. TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet. Neurol. 2010;9:995–1007. doi: 10.1016/S1474-4422(10)70195-2. [DOI] [PubMed] [Google Scholar]
- 27.Fang Y.S., Tsai K.J., Chang Y.J., Kao P., Woods R., Kuo P.H., Wu C.C., Liao J.Y., Chou S.C., Lin V., Jin L.W., Yuan H.S., Cheng I.H., Tu P.H., Chen Y.R. Full-length TDP-43 forms toxic amyloid oligomers that are present in frontotemporal lobar dementia-TDP patients. Nat. Commun. 2014;5:4824. doi: 10.1038/ncomms5824. [DOI] [PubMed] [Google Scholar]
- 28.Dawson T.M., Golde T.E., Lagier-Tourenne C. Animal models of neurodegenerative diseases. Nature Neurosci. 2018;21:1370–1379. doi: 10.1038/s41593-018-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Igaz L.M., Kwong L.K., Chen-Plotkin A., Winton M.J., Unger T.L., Xu Y., Neumann M., Trojanowski J.Q., Lee V.M. Expression of TDP-43 C-terminal fragments in vitro recapitulates pathological features of TDP-43 proteinopathies. J. Biol. Chem. 2009;284:8516–8524. doi: 10.1074/jbc.M809462200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ling S.C., Albuquerque C.P., Han J.S., Lagier-Tourenne C., Tokunaga S., Zhou H., Cleveland D.W. ALS-associated mutations in TDP-43 increase its stability and promote TDP-43 complexes with FUS/TLS. Proc. Natl. Acad. Sci. U.S.A. 2010;107:13318–13323. doi: 10.1073/pnas.1008227107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newell K., Paron F., Mompean M., Murrell J., Salis E., Stuani C., Pattee G., Romano M., Laurents D., Ghetti B., Buratti E. Dysregulation of TDP-43 intracellular localization and early onset ALS are associated with a TARDBP S375G variant. Brain Pathol. 2018;29:397–413. doi: 10.1111/bpa.12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y.J., Xu Y.F., Dickey C.A., Buratti E., Baralle F., Bailey R., Pickering-Brown S., Dickson D., Petrucelli L. Progranulin mediates caspase-dependent cleavage of TAR DNA binding protein-43. J. Neurosci. 2007;27:10530–10534. doi: 10.1523/JNEUROSCI.3421-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dormann D., Capell A., Carlson A.M., Shankaran S.S., Rodde R., Neumann M., Kremmer E., Matsuwaki T., Yamanouchi K., Nishihara M., Haass C. Proteolytic processing of TAR DNA binding protein-43 by caspases produces C-terminal fragments with disease defining properties independent of progranulin. J. Neurochem. 2009;110:1082–1094. doi: 10.1111/j.1471-4159.2009.06211.x. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y.J., Xu Y.F., Cook C., Gendron T.F., Roettges P., Link C.D., Lin W.L., Tong J., Castanedes-Casey M., Ash P., Gass J., Rangachari V., Buratti E., Baralle F., Golde T.E., Dickson D.W., Petrucelli L. Aberrant cleavage of TDP-43 enhances aggregation and cellular toxicity. Proc. Natl. Acad. Sci. U.S.A. 2009;106:7607–7612. doi: 10.1073/pnas.0900688106. [DOI] [PMC free article] [PubMed] [Google Scholar]