Abstract
BACKGROUND
This review was commissioned by the World Health Organization and presents a summary of the latest research evidence on the impact of coronavirus disease 2019 (COVID-19) on people with diabetes (PWD).
PURPOSE
To review the evidence regarding the extent to which PWD are at increased risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and/or of suffering its complications, including associated mortality.
DATA SOURCES
We searched the Cochrane COVID-19 Study Register, Embase, MEDLINE, and LitCOVID on 3 December 2020.
STUDY SELECTION
Systematic reviews synthesizing data on PWD exposed to SARS-CoV-2 infection, reporting data on confirmed SARS-CoV-2 infection, admission to hospital and/or to intensive care unit (ICU) with COVID-19, and death with COVID-19 were used.
DATA EXTRACTION
One reviewer appraised and extracted data; data were checked by a second.
DATA SYNTHESIS
Data from 112 systematic reviews were narratively synthesized and displayed using effect direction plots. Reviews provided consistent evidence that diabetes is a risk factor for severe disease and death from COVID-19. Fewer data were available on ICU admission, but where available, these data also signaled increased risk. Within PWD, higher blood glucose levels both prior to and during COVID-19 illness were associated with worse COVID-19 outcomes. Type 1 diabetes was associated with worse outcomes than type 2 diabetes. There were no appropriate data for discerning whether diabetes was a risk factor for acquiring SARS-CoV-2 infection.
LIMITATIONS
Due to the nature of the review questions, the majority of data contributing to included reviews come from retrospective observational studies. Reviews varied in the extent to which they assessed risk of bias.
CONCLUSIONS
There are no data on whether diabetes predisposes to infection with SARS-CoV-2. Data consistently show that diabetes increases risk of severe COVID-19. As both diabetes and worse COVID-19 outcomes are associated with socioeconomic disadvantage, their intersection warrants particular attention.
Introduction
In the context of the coronavirus disease 2019 (COVID-19) pandemic, the World Health Organization (WHO) and WHO Member States are requesting information and guidance on key topics related to COVID-19 and the virus that causes the disease, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This review of reviews was commissioned to address specific key questions for the WHO to provide high-quality, evidence-informed information around COVID-19.
This review presents a summary of the synthesized research evidence on the effects of COVID-19 in people with diabetes (PWD).
At the outset of the pandemic, PWD were assumed to be at increased risk from COVID-19. During 2020, emerging data signaled increased risk of adverse outcomes in PWD, likely dependent on a range of different factors (1,2). It is important to establish the risks COVID-19 poses to PWD in order to enable informed decision-making by PWD, their carers, health care providers, and policymakers.
Therefore, in this review of reviews, we set out to synthesize the evidence regarding the extent to which PWD are at increased risk of SARS-CoV-2 infection and/or from suffering its complications, including associated mortality. In particular, we set out to analyze evidence on the following questions:
Is diabetes associated with increased risk of acquiring SARS-CoV-2?
Is diabetes associated with hospitalization with COVID-19?
Is diabetes associated with the severity (including intensive care unit [ICU] admission, death, and other composite measures of severity) of COVID-19 outcomes?
Are there differences in outcomes of SARS-CoV-2 infection within the population of PWD?
Research Design and Methods
A protocol was agreed to in advance with the WHO and published online (3). Methods follow a general framework for a suite of reviews commissioned by the WHO with respect to their scientific briefs on COVID-19 and selected noncommunicable diseases. As prespecified by the WHO, systematic reviews were first identified; primary studies were then to be reviewed only if insufficient systematic reviews were found.
Data Sources and Searches
We searched the Cochrane COVID-19 Study Register, Embase, MEDLINE, and LitCOVID on 3 December 2020 for published literature or literature accepted for publication but not yet published, in any language (see Appendix 1 in the Supplementary Material for search strategies).
Study Selection
Two reviewers screened titles and abstracts, with discrepancies resolved by discussion or referral to a third reviewer. One reviewer screened full texts. We selected systematic reviews (defined as any review in which at least one database was systematically searched) according to the following inclusion criteria, defined using PECO (population; exposure; comparator; outcome):
Population: people diagnosed with any type of diabetes, with no limitations by age, disease severity, or duration, excluding people with prediabetes (e.g., impaired glycemic control that does not meet the clinical threshold for diabetes diagnosis) and gestational diabetes.
Exposure: SARS-CoV-2 infection.
- Comparator: questions 1 to 3 (described above), people without diabetes; question 4 (described above), PWD according to the following comparisons as specified in advance by the WHO:
- Type 1 versus type 2 diabetes
- Controlled versus uncontrolled glycemia (by HbA1c, whichever definition of control has been used)
- Previously diagnosed diabetes versus diabetes first diagnosed at COVID-19 diagnosis
- People treated with metformin versus people not treated with metformin
- People treated with dipeptidyl peptidase 4 inhibitors (DPP-4i) versus people not treated with DPP-4i
- People treated with insulin versus people not treated with insulin
- People with cardiovascular disease (CVD)/hypertension/chronic kidney disease versus people without
- Low socioeconomic status versus high socioeconomic status.
Outcome: Rates of confirmed SARS-CoV-2 infection; admission to hospital and/or to ICU with COVID-19; death with COVID-19.
Data Extraction and Quality Assessment
One reviewer appraised and extracted data from systematic reviews in relation to the above-described review questions; data were checked by a second. We included any systematic reviews that met the above criteria. Quality was assessed using the AMSTAR-2 (A MeaSurement Tool to Assess systematic Reviews 2) checklist but focusing only on critical domains, namely, protocol registered before commencement, adequacy of literature search, justification for excluding individual studies, risk of bias from individual studies, appropriateness of meta-analytical methods, consideration of risk of bias when interpreting results, and assessment of presence and likely impact of publication bias (4). Domains were assessed according to AMSTAR-2 guidance (4). We considered reviews judged as yes or partial yes for six or seven out of the seven critical domains of AMSTAR-2 to be higher quality and those reviews judged as no for at least two critical domains to be of lower quality. Appraisal was not used as a basis for excluding reviews but was used when considering certainty in the findings from the reviews.
Data Synthesis and Analysis
Data from contributing systematic reviews were narratively synthesized by review question, with effect direction plots used where appropriate. 95% CIs and I2 values are presented alongside all point estimates, where available.
Results
Search Results
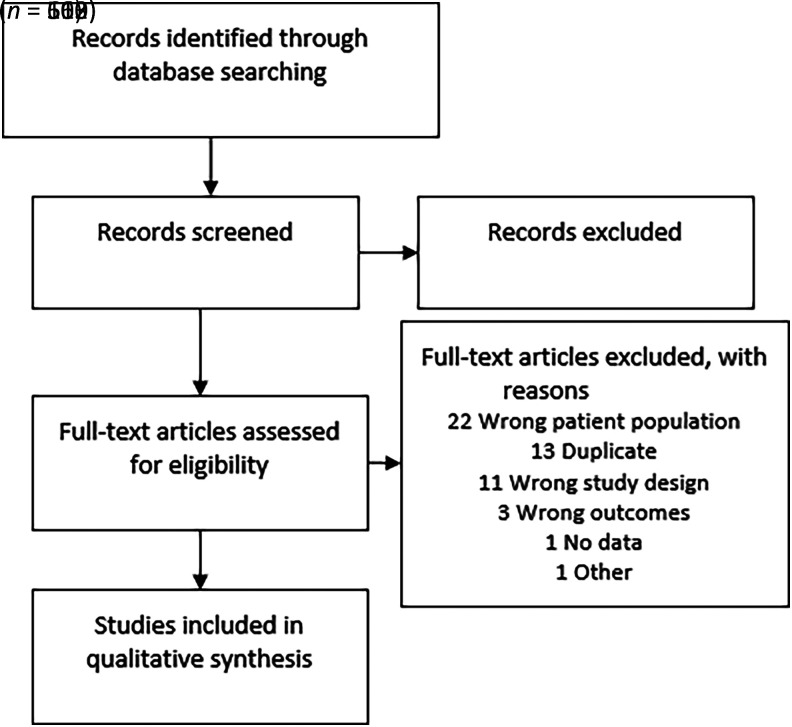
After removing duplicates, our searches for systematic reviews returned 663 references, 112 of which met our PECO criteria. The most common reason for exclusion at full-text stage was “wrong patient population” (Fig. 1). As we identified sufficient systematic reviews, we did not search further for primary literature (per the process set out in our protocol) (3).
Figure 1.
PRISMA diagram of study flow.
Characteristics of Included Reviews
Of the included reviews, 42 evaluated percentage of PWD in cohorts with COVID-19; 80 evaluated severity of COVID-19 outcomes in PWD compared with people without diabetes, including various definitions of severity, ICU admission (16 references), and/or mortality (56 references); and 45 looked at determinants of risk from COVID-19 within PWD (note that some reviews contributed data on more than one review question). Date of search ranged from March 2020 to November 2020. Supplementary Table 1 lists full citations for the included reviews; Supplementary Table 2 shows key characteristics of included reviews. Unless stated otherwise, reviews did not specify type of diabetes they included.
AMSTAR-2 judgements (conducted for critical domains only) are summarized by domain in Supplementary Table 3 and are provided in more detail in Supplementary Table 4. Five reviews (Tan et al. 2020; Sathish et al. 2020; Espinosa et al. 2020; Mesas et al. 2020; Izcovich et al. 2020) scored yes or partial yes across all seven domains. A further 14 scored yes or partial yes across six domains, and 21 scored yes or partial yes on five of the seven domains. Where systematic reviews provided conflicting answers for the same review question, we prioritized results from the higher-scoring reviews. Within the included reviews, included studies were mainly retrospective observational cohort studies of people hospitalized with COVID-19. None of the reviews identified relevant randomized controlled trials.
Is Diabetes Associated With an Increased Risk of Acquiring SARS-CoV-2 and/or of Hospitalization With COVID-19?
Forty-two reviews reported some data on percentage of PWD within COVID-19 cohorts (Table 1). Six of these were judged to be higher quality (yes or partial yes on at least six of seven AMSTAR-2 critical domains). The most recent search data within this set of reviews was August 2020. As asymptomatic community testing for COVID-19 remains limited, the vast majority of the data come from hospitalized or at least symptomatic cohorts. Therefore, questions on acquiring SARS-CoV-2 and hospitalization with COVID-19 are discussed together here. Few reviews looked at differences between prevalence of diabetes according to setting, but, as described below, one review indicated higher prevalence of diabetes in hospitalized than nonhospitalized (but symptomatic) cohorts. However, certainty in this finding was limited (5).
Table 1.
Prevalence of diabetes in people with COVID-19
| Authors, year | Prevalence [% (95% CI)] |
|---|---|
| Abdi et al., 2020 | 14.5 (10.4–19.9) |
| Bajgain et al., 2020 | 17.40 (NR) |
| Baradaran et al., 2020 | 10 (NR) |
| Barrera et al., 2020 | Across all studies: 12 (10–15) |
| Severe COVID-19 only: 18 (16–20) | |
| Bennett et al., 2020 | 9.2 (NR) |
| Del Sole et al., 2020 | 10.1 (NR) |
| Desai et al., 2020 | In studies in patients with mean age >50 years: 13.2 (9.7–17.1) |
| In studies in patients with mean age <50 years: 9.0 (5.1–13.5) | |
| Du et al., 2020 | In all COVID-19 patients: 10 (7–15) |
| In severe patients: 17 (14–20) | |
| In nonsevere patients: 6 (5–8) | |
| In patients dying with COVID-19: 30 (13–46) | |
| In patients surviving COVID-19: 8 (2–15) | |
| Emami et al., 2020 | 7.87 (6.57–9.28) |
| Espinosa et al., 2020** | 22 (21–23) |
| Fadini et al., 2020 | 10.3 (NR)* |
| Faghir-Gangi et al., 2020 | 14 (11–17) |
| Gold et al., 2020 | 9.65 (6.83–13.48) |
| Guler and Ozturk, 2020 | 7.7 (NR) |
| Hu et al., 2020 | 7.7 (6.1–9.3) |
| Hussain et al., 2020 | Overall: 15 (12–18) |
| In U.S. only: 21 (6–35) | |
| In China only: 14 (12–16) | |
| Kaur et al., 2020 | 12.80 (NR) |
| Khan et al., 2020 | 25.2 (NR) |
| Khateri et al., 2020** | 14 (NR) |
| Kumar et al., 2020 (1)** | 11.2 (9.5–13.0)† |
| Liu et al., 2020 (1) | 10.0 (8.0–12.0) |
| Liu et al., 2020 (2) | 8.5 (5.5–11.4) |
| Mair et al., 2020 | 8 hospitalized; 4 nonhospitalized (NR) |
| Mantovani et al., 2020 | Overall: 14.34 (12.62–16.06) |
| Patients aged >60 years: 23.30 (19.65–26.94) | |
| Patients aged <60 years: 8.79 (7.56–10.02) | |
| Non-Asian countries: 23.34 (16.40–30.28) | |
| Asian countries: 11.06 (9.73–12.39) | |
| Matsushita et al., 2020 | 5–58 (pooled results NR) |
| Meng et al., 2020** | Overall: 12.55 (not provided) |
| Severe patients: 20.50 (not provided) | |
| Miller et al., 2020 | 14.40 (not provided) |
| Nandy et al., 2020 | 13 (10–17) |
| Patel et al., 2020 (1) | 10 (not provided) |
| Patel et al., 2020 (2) | 15.4 (12–19.4) |
| Pinedo-Torres et al., 2020** | 10.8 (5.9–16.6) |
| Sacks et al., 2020 | Pooled result NR; report range within China of 5–20 |
| Sales-Peres et al., 2020 | 30.3 (not provided; in people who also had obesity) |
| Sanyaolu et al., 2020 | Report range: 9.4–23.8 |
| Sayed, 2020 | Report range: 1.7–39.7 |
| Tadic et al., 2020 | Report range: 3–21 |
| Tian et al., 2020 | 23.80 (not provided) |
| Venkata and Kiernan, 2020 | 23 (not provided) |
| Wang et al., 2020 (3) | Overall: 9 (6–12) |
| In moderately severe COVID patients: 7 (4–10) | |
| In severe COVID patients: 17 (13–21) | |
| Zaki et al., 2020 | Report range: 12–22 |
| Zhou et al., 2020 (2)** | In severe or fatal COVID-19 cases: 17 (15–20) |
Full reference citations are available in Supplementary Table 1. Data represent pooled prevalence unless indicated otherwise.
Considered higher quality (judged as yes or partial yes for at least six of seven critical AMSTAR-2 domains).
For comparison, the review states nationwide prevalence of diabetes in China in 2013 was 10.9% overall and 12.3% among people aged 40–59 years.
Meta-regression showed proportion of diabetes in patients with COVID-19 was influenced by age (with studies with higher patient age having higher proportion of diabetes, P < 0.001), type of composite end point (with studies reporting mortality end point having higher proportion of diabetes, P = 0.004), and country of study (with studies outside China having higher proportion of diabetes, P = 0.006). There was no influence of number of patients in studies or quality score of studies.
Estimates of percentages of PWD within cohorts of people with COVID-19 were highly heterogeneous, but on the whole, PWD were overrepresented in COVID-19 cases compared with population averages (note that population averages may also be underrepresentations of true diabetes prevalence due to selective diabetes screening within communities) (Table 2). Estimates from individual studies included in the retrieved systematic reviews ranged from 1.7% to 40% PWD within COVID-19 confirmed cases. The pooled estimates in the systematic reviews of PWD within COVID-19 confirmed cases ranged from 7.7% to 23%. In a cohort of people with obesity and COVID-19, this increased to 30.3% (6). Estimates of diabetes prevalence from the six higher-quality reviews ranged from 10.8% to 22% when looking at all cases and from 17% to 20% in subgroups with severe disease (7–12).
Table 2.
Association between diabetes and severe outcomes with COVID-19
| Authors, year | ICU admission and direction of effect | Mortality and direction of effect | Other measures of severity and direction of effect | |||
|---|---|---|---|---|---|---|
| Findings | Direction | Findings | Direction | Findings | Direction | |
| Abdi et al., 2020 | NR | Death rate higher among patients with both diabetes and COVID-19. No pooled data. | Severe symptoms higher among patients with both diabetes and COVID-19. No pooled data. | |||
| Aggarwal et al., 2020 | NR | Diabetes in people not surviving COVID-19 vs. those surviving COVID-19: OR 2.03 (95% CI 1.29–3.20). 4 studies, 307/618 died, of which 96 (15.5%) had diabetes. I2 = 0%. | ↑↑ | Diabetes in COVID-19 patients with or without severe disease: OR 2.60 (95% CI 1.96–3.45). 12 studies. 754/2,564 severe cases where 265 (10.3%) have diabetes. I2 = 56% and not explored/explained. | ↑↑ | |
| Apicella et al., 2020 | Reports estimates from 3 other reviews, all of which are included here. No new data. | Reports data from 2 other reviews, both of which are included here. No new data. Also reports data from large primary studies in type 1 and 2 diabetes (Barron et al. [1]): n = 23,804, 32% type 2, OR 2.03 (1.97, 2.09); 1.5% type 1, OR 3.5 (3.15, 3.89). | NR | |||
| Awortwe and Cascorbi, 2020 | Risk difference for ICU vs. non-ICU 0.01 (−0.33–0.34), P = 0.98, I2 = 84.8 (n studies/participants NR) | ↑ | Risk difference for surviving COVID-19 vs. not surviving: 0.14 (0.08–0.19), P < 00000.1, I2 = 21%. (n studies/participants NR) | ↑↑ | Risk difference for severe vs. mild (not defined): 0.08 (0.02–0.14), P = 0.002, I2 = 56. n studies/participants NR. | ↑↑ |
| Bajgain et al., 2020 | NR | 66 fatal, 172 nonfatal with diabetes. In studies showing only fatal cases: 33.2% fatality for diabetes and COVID-19. | NR | |||
| Barrera et al., 2020 | Unadjusted RR, 3 studies, n = 8,890, RR 1.96 (95% CI 1.19–3.22), I2 = 80% | ↑↑ | Unadjusted RR, 4 studies, n = 2,058, RR 2.78 (95% CI 1.39–5.58), I2 = 75% | ↑↑ | Unadjusted RR for severe COVID-19 (not defined): 1.50 (95% CI 0.90–2.50). I2 = 74%, 6 studies, n = 1,991. | ↑ |
| Chidambaram et al., 2020 | NR | PWD in those who died vs. those who survived: RR 1.59 (95% CI 1.41–1.78). 27 studies, n = 16,263, I2 = 23. | ↑↑ | PWD in severe vs. nonsevere cases: OR 2.09 (95% CI 1.66–2.64), I2 = 40%, 36 studies, n = 7,552 | ↑↑ | |
| Chowdhury and Goswami, 2020 | NR | Only reports data from Barron et al. (1) primary studies: 3.50 (3.15–3.89) greater odds of dying in hospital with COVID-19 in PWD compared with those without diabetes. Attenuated to 2.86 when adjusted for previous hospital admissions with coronary heart disease, cerebrovascular disease, or heart failure. | NR | |||
| Costa et al., 2020 | NR | Report results from 3 individual studies (no synthesis), all of which show greater risk in PWD. Cite Chinese CFR of 7.3% in PWD compared with 2.3% in total population. | NR | |||
| de Almeida-Pititto et al., 2020 | NR | 10 studies, 4,247 patients, 532 PWD. Compared diabetes rate in people who died vs. people who survived. OR 2.50 (95% CI 1.74–3.59). Random effects model (I2 = 50.72). No publication bias was detected. | ↑↑ | Severity (defined as ICU admission or need for mechanical ventilation or low O2 saturation <90%): 18 studies, 4,305 patients, 564 PWD. Compared diabetes rate in severe vs. nonsevere. OR 2.35 (95% CI 1.80–3.06), random effects model (I2 = 34.78), with no significant publication bias detected. | ↑↑ | |
| Del Sole et al., 2020 | NR | NR | Severity (ARDS, ICU admission, death): OR 2.78 (95% CI 2.09–7.72), 10 studies, n = 2,794. I2 NR. | ↑↑ | ||
| Deravi et al., 2020 | NR | NR | Narrative only. “Individuals with diabetes mellitus and hypertension are reported to be at higher risks for the late viral clearance of the coronavirus, and the worsened prognosis in SARS, MERS, and COVID-19 infections.” | |||
| Deshmukh et al., 2020 | NR | Narrative only. “These reports indicate that severe or critically ill COVID-19 patients with concurrent hypertension, diabetes and cardiovascular disease have a significantly higher risk of mortality and require special attention during their hospitalization.” | NR | |||
| Du et al., 2020 | Diabetes was not found to be significantly associated with admission to ICU (RR 1.16, 95% CI 0.15–9.11). 2 studies, n = 1,631. I2 = 78%. Suspected publication bias. | ↑ | Compared with patients without diabetes, the risk of death (RR 3.16, 95% CI 2.64–3.78, I2 = 34%) was higher in COVID-19 patients with diabetes. No evidence of publication bias. 4 studies, n = 46,654. | ↑↑ | Compared with patients without diabetes, the risks of severe cases (RR = 2.13, 95% CI 1.76–2.56, I2 = 49%) was higher in COVID-19 patients with diabetes. No evidence of publication bias. 7 studies, n = 2,662. | ↑↑ |
| Espinosa et al., 2020** | NR | 19% prevalence of PWD within deaths with COVID-19, 95% CI 16–22, number of studies NS, no comparisons made | 17% prevalence of PWD within ICU admission with COVID-19, 95% CI 15–19, number of studies NS, no comparisons made | |||
| Fadini et al., 2020 | NR | NR | Narrative only. “Based on these data, we conclude that diabetes may not increase the risk of SARS-CoV-2 infection but can worsen the outcome of this new coronavirus disease.” | |||
| Fang et al., 2020** | 5 studies, 3,747 total cases, RR 1.88 (1.10–3.23), I2 = 51%. Calculated as rates of diabetes in people in ICU vs. those not in ICU. | ↑↑ | Using 10 studies, with 4,748 cases, the RR of death with COVID-19 and comorbid diabetes was 1.75 (1.27–2.41), I2 = 23% | ↑↑ | Severity (American Thoracic Society guidelines for community-acquired pneumonia or the new coronavirus pneumonia prevention and control guidelines of China): RR 1.95 (1.60–2.36). Data from 23 studies, n = 7,739, I2 = 43%. Statistically and clinically significant increases in risk for ARDS and invasive ventilation also found in pooled data. | ↑↑ |
| Figliozzi et al., 2020** | NR | NR | “Adverse prognosis” composite (death, severe COVID-19 infection, hospitalization in ICU and/or use of mechanical ventilation and progression of the disease): OR 2.34, 95% CI 1.64–3.33, I2 = 80%, n = 15,953, 34 studies. | ↑↑ | ||
| Flaherty et al., 2020 | NR | NR | Narrative only. “Patients with diabetes have up to a 50% greater chance of a fatal outcome from COVID-19 than non-diabetic infected individuals.” | |||
| Gold et al., 2020 | NR | Diabetes was more prevalent among fatal cases (24.89% [95% CI 18.80–32.16%]) compared with total cases (9.65% [95% CI 6.83–13.48%]) | NR | |||
| Guler and Ozturk, 2020 | NR | Narrative only, discusses increased risk per Costa et al., 2020 (cite increased CFR in PWD) | NR | |||
| Guo et al., 2020 | NR | NR | Severity (indication of respiratory rate >30 breaths/min, or oxygen saturation <93% on room air, ratio of partial pressure of arterial oxygen to fractional concentration of oxygen inspired air (PaO2/FiO2) ≤300 mmHg, or critical complication [respiratory failure, septic shock, and or multiple organ dysfunction/failure] or death). Nine studies, 1,070/8,807 with diabetes, pooled RR 2.96 (95% CI 2.31–3.79; P < 0.001), I2 = 23%. No evidence of publication bias. | ↑↑ | ||
| Hartmann-Boyce et al., 2020 | Narrative only, cite data showing higher risk of death across individual studies | Narrative only. “…current data suggest that COVID-19 is associated with worse outcomes in PWD.” | ||||
| Hu et al., 2020 | NR | No comparisons. Within PWD, the risks of severity and mortality rate ranged from 12.6–23.5% and from 2.0–4.4%, with pooled estimates at 18.0 and 3.2%, respectively. | No comparisons. The percentage of severe cases (not defined but includes ARDS and acute cardiac injury) in diabetes was 44.5% (95% CI 27.0–61.9). | |||
| Huang et al., 2020 | RR 1.47 (0.38–5.67), P = 0.57; I2 = 63%. 10 studies, n = 1,985 | ↑ | RR 2.12 (1.44–3.11), P < 0.001; I2 = 72%. 10 studies, n = 1,985. | ↑↑ | Severe COVID: 1) respiratory distress (>30 breaths per min); 2) oxygen saturation at rest <93%; 3) ratio of partial pressure of arterial oxygen (PaO2) to fractional concentration of oxygen inspired air (FiO2) <300 mmHg; or 4) critical complication (respiratory failure, septic shock, and or multiple organ dysfunction/failure): RR 2.45 (1.79–3.35), P < 0.001; I2 = 45%, 13 studies, n = 3,561). Composite poor outcome: including mortality, severe COVID-19, ARDS, need for ICU care, and disease progression: RR 2.38 (1.88–3.03), P < 0.001; I2 = 62%, P < 0.001. Qualitatively symmetrical inverted funnel plot for the association between diabetes and composite poor outcome. | ↑↑ |
| Hussain et al., 2020 | RR 1.88 (95% CI 1.20–2.93). 5 studies, n = 7,484, I2 = 75%. Calculated as rates of diabetes in people in ICU compared with those not in ICU. | ↑↑ | Mortality risk was found to be significantly higher in COVID-19 patients with diabetes than in COVID-19 patients without diabetes with a pooled RR of 1.61 (95% CI 1.16–2.25). I2 = 93%. 11 studies, n = 7,093. | ↑↑ | NR | |
| Izcovich et al., 2020** | NR | 13.6% with diabetes, 7.9% without. OR 1.84 (95% CI 1.61–2.1). n = 30,303, 52 studies, I2 = 33%. High-certainty evidence according to GRADE. Estimated absolute risks: 5.6% increase in mortality, between 4.3% more and 7% more. | ↑↑ | “Severe COVID-19 disease” (severity as reported by primary study authors or ICU requirement, invasive mechanical ventilation, or ARDS): OR 2.51 (95% CI 2.2–2.87). n = 21,381, 97 studies. I2 = 32%. High-certainty evidence according to GRADE. Estimated absolute risks: 13.2% increase severe COVID-19 disease, between 11% more and 15.5% more. | ↑↑ | |
| Javanmardi et al., 2020 | NR | No comparison. 26% (21–31%) of those dying with COVID-19 had diabetes. | NR | |||
| Khan et al., 2020 | NR | Not calculated for diabetes specifically. For all immune and metabolic disorders, OR 2.46, 95% CI 2.03–2.85. | NR | |||
| Kumar et al., 2020 (1)** | NR | Presence of diabetes was found to be significantly associated with mortality due to COVID-19 (OR 1.90 [95% CI 1.37–2.64], I2 = 32%). 9 studies, total n NR. | ↑↑ | Severity of disease: 24 studies. End points: predefined criteria (16 studies); ICU requirement vs. no requirement (2 studies); invasive ventilation requirement vs. no requirement (2 studies); progressive disease vs. stable disease (2 studies); refractory disease vs. responsive disease (1 study); and ARDS vs. no ARDS (1 study). Presence of diabetes was found to be significantly associated with severe COVID-19 (OR 2.75 [95% CI 2.09–3.62], I2 = 63%). Total n not provided. | ↑↑ | |
| Kumar et al., 2020 (2) | NR | NR | Composite “severe clinical course”: 1) requiring ICU care; 2) developing ARDS, shock, respiratory failure, or those requiring mechanical ventilation; 3) categorized as severe or critical groups according to the diagnostic and treatment guideline for SARS-CoV-2 issued by the National Health Commission of the People’s Republic of China (version 3–5); or 4) not surviving. OR 3.11 (95% CI 1.99–4.88). I2 = 48%; 14 studies. | ↑↑ | ||
| Li et al., 2020 (1) | NR | Nonsurvivors of COVID-19 were significantly more likely to have diabetes than survivors (24.8%, 95% CI 18.7–32.0 vs. 13.9%, 95% CI 10.5–18.1, P = 0.003) | NR | |||
| Li et al., 2020 (3) | Diabetes accounted for 11.7% of ICU/severe cases but 4.0% of non-ICU/severe cases | NR | “The result indicated a higher proportion of diabetes in ICU/severe patients but without statistical significance.” RR 2.21, 95% CI 0.88–5.57, I2 = 67%. 5 studies, n = 1,514. | ↑ | ||
| Liu et al., 2020 (1) | NR | NR | “Increased risk of disease severity”: OR 2.61, 95% CI 1.93–3.52, I2 = 26.7%; 10 studies, n NR | ↑↑ | ||
| Lu et al., 2020 | NR | OR 2.63, 95% CI 1.45–4.76. 5 studies, n = 2,307. I2 NR. | ↑↑ | NR | ||
| Luo et al., 2020** | NR | In-hospital mortality OR 2.09, 95% CI 1.80–2.42 (did not report total n, studies or I2 for this analysis as part of larger analysis [124 studies]) | ↑↑ | OR 2.54, 95% CI 1.89–3.41 (total n, studies, or I2 NR for this analysis as part of larger analysis (124 studies) | ↑↑ | |
| Mahumud et al., 2020 | NR | NR | Chronic comorbid conditions (e.g., hypertension, diabetes, CVD, respiratory disease, and other chronic diseases) were identified as high-risk factors. | |||
| Mantovani et al., 2020 | NR | “Pre-existing diabetes was significantly associated with a ∼three-fold greater risk of in-hospital mortality associated with COVID-19” (n = 15 studies included random-effects, OR 2.68, 95% CI 2.09–3.44, I2 = 46.7%). Publication bias judged unlikely. | ↑↑ | Severe disease: “Patients with established diabetes had an approximate two-fold greater risk of severe/critical COVID-19 illness compared to their counterparts without diabetes” (22 studies, OR 2.10, 95% CI 1.71–2.57; I2 = 41.5%). Publication bias judged unlikely. | ↑↑ | |
| Mehraeen et al., 2020 | NR | OR 1.34, 95% CI 1.10–1.64 (114 studies, 310,494 participants, I2 NR) | ↑↑ | NR | ||
| Mesas et al., 2020** | NR | OR 2.12, 95% CI 1.79–2.52, I2 = 77.9. 38 studies, n = 25,498 | ↑↑ | NR | ||
| Miller et al., 2020 | NR | In meta-regression, “each 1% increase in diabetes prevalence was associated with a 1.5% absolute increase in the mortality rate (P < 0.001)” | ↑↑ | NR | ||
| Moula et al., 2020 | NR | Comparing mortality in PWD vs. those without diabetes, RR 1.59, 95% CI 1.25–2.02; 28 studies, total n NR, I2 NR | ↑↑ | NR | ||
| Mudatsir et al., 2020** | NR | NR | OR 2.10, 95% CI 1.33–3.34 (severity not defined). 17 studies, n = 3,120, I2 = 63%. | ↑↑ | ||
| Nandy et al., 2020 | NR | “Patients with diabetes mellitus had a higher risk of mortality than non-diabetic patients” (OR 2.28, 95% CI 1.40–5.55) | ↑↑ | NR | ||
| Noor and Islam | NR | The prevalence of mortality among COVID-19 patients with diabetes was 49%. RR 1.87, 95% CI 1.23–2.84. | ↑↑ | NR | ||
| Pal et al., 2020 | NR | In-hospital mortality rate: 45% in PWD | NR | |||
| Palaiodimos et al., 2020 | NR | PWD compared with people without diabetes: OR 1.65, 95% CI 1.35–1.96; I2 = 77.4%, 14 studies. Possible presence of publication bias. | ↑↑ | NR | ||
| Parohan et al., 2020 | NR | OR 2.41, 95% CI 1.05–5.51, I2 = 93.6% | ↑↑ | NR | ||
| Parveen et al., 2020 | OR 0.78, 95% CI 0.06–9.34; I2 = 75.9%. 2 studies, 179 patients, 49 ICU admissions | ↓ | Odds of survival: 2 studies, 465 patients, 167 deaths. “The pooled estimate (OR 0.56, 95% CI 0.35–0.90; I2 = 0.0%) suggested that diabetes was significantly lower in the survivors.” | ↑↑ | 3 studies, 1,374 patients, 271 severe (defined as having respiratory distress, respiratory rate >30 breaths per min in a resting state, mean oxygen saturation of <93%, and PaO2/FiO2 <300 mmHg). “The pooled estimate of two cohort studies and one case-series suggested significant association between diabetes and severity (OR 1.66; 95% CI 1.20–2.30; I2 = 0.0%).” | ↑↑ |
| Patel et al., 2020 (2) | NR | Meta-regression model: mortality OR 1.02 (0.94–1.11), age adjusted for diabetes | ↑ | “Diabetes was not found to be associated with need for invasive mechanical ventilation” | ||
| Pinedo-Torres et al., 2020** | ICU admission, 1 study, n = 138, prevalence 57.97% (95% CI 25.36–111.03) | Death, 2 studies, n = 716, prevalence 96.33% (95% CI 61.36–137.66), I2 = 0% | NR | |||
| Plasencia-Urizarri et al., 2020** | NR | NR | Severe clinical presentations: OR 3.53, 95% CI 2.79–4.47. 13 studies, n NR. I2 = 59%. | ↑↑ | ||
| Qui et al., 2020 | NR | “The pooled prevalence of diabetes in COVID-19 death patients was estimated to be 22.2% (95% CI 19.30, ∼25.10%). The heterogeneity of the study was low (I2 = 28.4%, P = 0.1519).” | NR | |||
| Radwan et al., 2020 | NR | NR | “Severity” (ICU admission, mechanical ventilation, and death): OR 2.46, 95% CI 1.53–3.96, 7 studies, n = 1,885, I2 = 31% | ↑↑ | ||
| Rod et al., 2020 | NR | NR | No synthesis, but diabetes identified as one of the main predictors of COVID-19 severity | |||
| Roncon et al., 2020 | “PWD had a significant increased risk of ICU admission” (OR 2.79, 95% CI 1.85–4.22, P < 0.0001, I2 = 46%), 4 studies | ↑↑ | “PWD had higher mortality risk” (OR 3.21, 95% CI 1.82–5.64, P < 0.0001, I2 = 16%), 4 studies | ↑↑ | NR | |
| Sacks et al., 2020 | Narrative only, cites mixed evidence of increased risk | Narrative only, cites consistent evidence of increased risk | NR | |||
| Sayed et al., 2020 | NR | China observational report (1,023 deaths/4,462 confirmed cases) overall CFR 2.3%, CRF in PWD 7.3%; U.S., 1,122 patients in 88 hospitals, 38.5% had either diabetes or uncontrolled hyperglycemia, had “a more than 4 times higher mortality rate compared to no diabetes/hyperglycemia” | NR | |||
| Sepandi et al., 2020 | NR | Type 2 diabetes OR 2.42 (95% CI 1.06–5.52), 9 studies, I2 = 90%. Number of participants NS. | ↑↑ | NR | ||
| Shang et al., 2020 | 28 studies. “COVID-19 patients with diabetes had higher mortality rate compared with those non-diabetic patients (28.5 vs. 13.3%, P < 0.01). COVID-19 patients with diabetes had a higher risk of death (pooled OR 2.21, 95% CI 1.83–2.66, P < 0.001; I2 50%, P < 0.01).” No evidence of publication bias. | ↑↑ | “COVID-19 patients with diabetes had higher severe infection rate compared with those non-diabetic patients (21.4 vs. 10.6%, P < 0.01). Diabetes was found to be associated with a significantly greater risk of severe COVID-19 infection (pooled OR 2.38, 95% CI 2.05–2.78, P < 0.001; I2 = 39%, P < 0.01).” 54 studies. No evidence of publication bias. | ↑↑ | ||
| Shoar et al., 2020 | NR | OR 1.7, 95% CI 1.04–2.78, I2 = 47%. n studies/participants NR for this analysis, 12 studies (n = 3,257) included overall | ↑ | NR | ||
| Singh et al., 2020 (1) | NR | No new analyses; cites existing studies finding increased risk | NR | |||
| Singh et al., 2020 (2)** | NR | Estimated pooled RR of mortality from COVID-19 with a comorbidity vs. without. RR 1.83 (95% CI 0.89–3.73), P = 0.100, 2 studies, total n not stated, I2 = 0%. | RR 2.11 (95% CI 1.40–3.19), 7 studies (total n not stated), I2 = 84.6%, possible publication bias detected | ↑↑ | ||
| Singh et al., 2020 (3) | NR | Increased risk of mortality in patients with comorbidities; however, this is a literature review with no pooled-effect data | NR | |||
| Ssentongo et al., 2020** | NR | Risk of mortality in PWD compared with people without diabetes: RR 1.48 (95% CI 1.02–2.15), 16 studies, I2 = 84%, n NR | ↑↑ | NR | ||
| Tadic et al., 2020 | Narrative only, cites studies finding increased risk of admission to ICU | “In most of the studies exists the trend toward higher prevalence of diabetes among non-survivors, but in majority of studies, it did not reach statistical significance due to the small sample size” | NR | |||
| Tan et al., 2020** | Prevalence of diabetes within COVID patients admitted to ICU/HDU: 26.6% (95% CI 22.7–30.8), I2 = 84, P < 0.01 | NR | NR | |||
| Tian et al., 2020 | NR | “Of 1,103 that died from COVID-19, 31.2% had diabetes. Of 3,212 that survived COVID-19, 21.2% had diabetes.” OR 1.97 (95% CI 1.67–2.31). 12 studies. I2 = 0%. | ↑↑ | NR | ||
| Varikasuvu et al., 2020** | NR | “Diabetic proportions were 259/879 and 429/3,292 in mortal and survival groups of COVID-19…diabetes related significantly with COVID-19 disease mortality (OR 2.52, 95% CI 1.93–3.30, Z = 6.79, P < 0.00001, I2 = 31%, P = 0.08).” 22 studies. | ↑↑ | “The diabetic proportions were 750/2,894 and 931/6,203 in severe and nonsevere groups of COVID-19 cases… diabetes related significantly with COVID-19 disease severity (OR 2.20, 95% CI 1.69–2.86, Z = 5.82, P < 0.00001, I2 = 58%, P < 0.0001).” Severe not defined. 35 studies. | ↑↑ | |
| Wang et al., 2020 (2)** | NR | NR | 1,558 patients with COVID-19 in 6 studies. Risk of exacerbation: OR 2.47, 95% CI 1.67–3.66, I2 = 39. | ↑↑ | ||
| Wu et al., 2020 | NR | OR 1.75 (95% CI 1.31–2.36). 6 studies, n = 1,471, I2 = 5%. | ↑↑ | NR | ||
| Xu et al., 2020 | NR | NR | Narrative only, states PWD more likely to develop severe COVID-19 | |||
| Yanai, 2020 | NR | NR | “Metabolic syndrome and its components are significantly associated with the susceptibility to SARS-CoV-2 infection and severity of COVID-19.” | |||
| Yang et al., 2020 | NR | NR | Diabetes in severe vs. nonsevere group: OR 2.07, 95% CI 0.89–4.82. 4 studies, total n NR, I2 = 62%. | ↑ | ||
| Zaki et al., 2020 | NR | NR | “Diabetes, hypertension, and cholesterol levels possess an apparent relation to COVID-19 severity.” | |||
| Zhao et al., 2020 (1) | NR | NR | NR | |||
| Zhao et al., 2020 (2) | RR 1.26, 95% CI 0.11–14.42; I2 = 80%. 2 studies, n = 179. | ↑ | NR | NR | ||
| Zheng et al., 2020 | NR | NR | Diabetes incidence significantly higher in “critical/mortal” patients compared with “noncritical,” OR 3.68, 95% CI 2.68–5.03. 11 studies, I2 = 45%, total n = 2,579. | ↑↑ | ||
| Zhou et al., 2020 (1) | NR | “The death group had significantly higher proportions of patients with diabetes (OR 2.51, 95% CI 1.86–3.35, I2 = 87.32%).” | ↑↑ | NR | ||
| Zhou et al., 2020 (2)** | 4 studies recorded ICU admission (a sample of 6,652 patients, 1,138 [17.1%] of whom were classified as admitted to the ICU). Regarding diabetes, OR 2.98 (95% CI 1.49–5.98), I2 = 48. | ↑↑ | 5 studies compared the rates of comorbidities in survivors vs. nonsurvivors, with a sample of 3,436 patients, 1,624 (47.3%) of whom died. In subgroup analysis based on severe clinical outcomes associated with COVID-19, 4 studies used for diabetes (n NR). OR 2.08 (95% CI 1.38–3.15), I2 = 0.0%. | ↑↑ | NR | |
Full reference citations are available in Supplementary Table 1. Boldface indicates pooled estimates. ↑, increased risk in PWD, not statistically significant; ↑↑, statistically significant increased risk in PWD; ↓, lower risk in PWD, not statistically significant. Severity is per definitions of individual study authors unless otherwise specified.
Higher quality (six or seven yes or partial yes on AMSTAR-2). CFR, case fatality ratio; HDU, high dependency unit; NS, not specified; OR, odds ratio; RR, risk ratio.
Multiple reviews flagged the presence of heterogeneity between studies. As acknowledged by the reviewers, some of this heterogeneity will be driven by different practices in recording diabetes status (e.g., on admission with COVID-19 or from previous health care records), but other reasons have also been investigated. Kumar et al. (9) (judged to be higher quality) conducted the most thorough investigation of between-study heterogeneity. Their meta-regression showed that the proportion of diabetes in patients with COVID-19 was influenced by age (with studies with higher patient age having a higher proportion of diabetes, P < 0.001), type of composite end point (with studies reporting mortality end point having a higher proportion of diabetes, P = 0.004), and country of study (with studies outside China having a higher proportion of diabetes, P = 0.006) (9). All other reviews that investigated these potential causes of heterogeneity found the same patterns. Desai et al. (13) and Mantovani et al. (14) also found the percentage of PWD was higher in older than younger patients (as would be expected given trends in diabetes prevalence in the general population). Hussain et al. (15) also found the percentage of PWD was higher in studies conducted outside China, and Mantovani et al. (14) found the percentage of PWD was greater in non-Asian than in Asian countries. Barrera et al. (16), Du et al. (17), Hartmann-Boyce et al. (2), Mair et al. (5), Meng et al. (10), and Wang et al. (18) also found the percentage of PWD was higher in patients with severe COVID-19 manifestations, indicating a relationship between diabetes and increased COVID-19 severity, which is explored further below (3).
Only one review directly compared percentage of PWD in hospitalized versus nonhospitalized cohorts with COVID-19. Mair et al. (5) found higher rates of diabetes in hospitalized (8%; 95% CI 5–10%, 13 studies, n not stated) versus community (4%; 95% CI 1–7%, 3 studies, n not stated) cohorts with COVID-19 and concluded that once clinically ill, PWD are more likely to be admitted to the hospital (5). However, this finding should be interpreted with caution: the review was judged to have several critical weaknesses according to AMSTAR-2, CIs are compatible with no difference, and between-study heterogeneity does not appear to have been investigated.
Conclusions
Because of a lack of widespread systematic, population-based asymptomatic community testing, data are insufficient to conclude whether or not diabetes predisposes to infection with SARS-CoV-2. Data on prevalence of diabetes in symptomatic/hospitalized COVID-19 cases are heterogeneous but, on the whole, suggest PWD are overrepresented, particularly in hospitalized cohorts. Heterogeneity may in part be driven by age of sample, with older cohorts having a higher prevalence of diabetes and multimorbidity; geographic location, with some indication of lower estimates of prevalence of diabetes in hospitalized COVID patients in Asia compared with outside Asia; and severity of COVID-19, with estimates being higher in severe COVID-19 cohorts. There are some data from an indirect comparison that indicate that, once clinically ill with COVID-19, PWD are more likely to be hospitalized; this is consistent with some studies suggesting PWD are overrepresented in hospitalized cohorts.
Is Diabetes Associated With the Severity of COVID-19 Outcomes?
Eighty reviews evaluated data related to this question. Of these, 15 were considered to be of higher quality (six or seven of seven AMSTAR-2 critical domains as yes or partial yes). The latest search date was August 2020. Where investigated, all of the reviews identified increased risk of mortality and severity of COVID-19 in PWD.
Where data were pooled across studies, outcomes were most commonly calculated as risk ratios (RRs) or odds ratios (ORs), with data given on number of PWD with and without outcome and number of people without diabetes with and without outcome. However, at times, effect estimates were extracted from individual studies and numeric data were not available. In addition to possible variation in the way they were calculated, pooled outcomes were subject to some limitations, including statistical heterogeneity and possible publication bias in some instances. The detection of high statistical heterogeneity or suspected publication bias is noted in Table 2 and, where relevant, discussed below. Most analyses were based on unadjusted estimates. However, results of individual studies that provided adjusted estimates are consistent with those from meta-analyses containing unadjusted data.
ICU Admission
Sixteen reviews evaluated ICU admission (Table 2). Of these, three were considered to be of higher quality (six or seven of seven AMSTAR-2 critical domains as yes or partial yes). Of those 10 that reported pooled effect estimates, 5 found point estimates indicating increased association of ICU admission with COVID-19 in PWD, with CIs excluding no difference. A further four found point estimates signaling increased association of ICU admission in PWD but with wide CIs that spanned no difference and are also compatible with a lower rate. One meta-analysis of two studies (n = 179) found lower rates of ICU admission in PWD, but here again the CIs were very wide, with the difference in risk being compatible with a 94% reduction to a greater than 900% increase (19).
The two higher-quality reviews that evaluated this outcome both found increases in admission for PWD, with the 95% CIs being compatible with a 10–500% increase, and moderate levels of statistical heterogeneity. Fang et al. (20) compared rates of diabetes in people in ICU versus those not in ICU and found an RR of 1.88 (95% CI 1.10–3.23, I2 = 51%, 5 studies, n = 3,747). Zhou et al. (2) conducted the same comparison using pooled data from 4 studies (n = 6,652) and found an OR of 2.98 (95% CI 1.49–5.98, I2 = 48%) (12).
Mortality
Fifty-six reviews evaluated mortality (death with COVID-19). Of those 34 that reported a pooled estimate, all found a point estimate suggesting increased risk of death with COVID-19 in PWD; 31 of these 34 pooled estimates had increases in CIs ranging from 1.02 to 5.58.
Nine of the reviews that calculated pooled effect estimates were considered higher quality according to the AMSTAR-2 critical domains; eight of the nine detected a statistically significant increase in risk when comparing mortality in PWD to mortality in people without diabetes. In the ninth review, Singh et al. (24), the pooled estimate from only 2 studies resulted in wide CIs (RR 1.88, 95% CI 0.89–3.73) (9,12,20–26). Point estimates for pooled RRs ranged from 1.48 to 1.83 and for ORs ranged from 1.84 to 2.52. Where I2 values were reported, these were in the range of those not considered to indicate significant heterogeneity (<40%), with the exception of Ssentongo et al. (25) (I2 = 84%). Authors of the largest meta-analysis in this group, Izcovich et al. (21), were also the only ones to use GRADE (grading of recommendations assessment, development, and evaluation) to evaluate certainty in the evidence and estimate absolute risks. In their meta-analysis of 52 studies (n = 30,303), diabetes increased odds of mortality by an OR of 1.84 (95% CI 1.61–2.1, I2 = 33%). This translated to an absolute estimated increased risk of a 5.6% increase in mortality (95% CI 4.3–7%). They judged the evidence to be of high certainty. Although some of the contributing studies were judged to be at high risk of bias, sensitivity analysis showed that the pooled estimate was not sensitive to the removal of studies at high risk of bias and/or those that did not report adjusted estimates.
Other Measures of Severity
Thirty reviews evaluated “severity” as a construct in and of itself; of these, 10 were considered higher quality. Severity had a broad definition. In some reviews, it was not defined or authors relied on categorizations from original study authors. In other reviews, severity was a composite score derived from set criteria, most commonly including elements such as ICU admission, mortality, oxygen levels, acute respiratory distress syndrome (ARDS), and the need for mechanical ventilation. More detail can be found in Table 2.
Of the 20 reviews that calculated a pooled estimate for severity, all found point estimates suggesting increased risk of severe disease in PWD compared with risk in people without diabetes. In 19 of 20 reviews, this effect was statistically significant (the one estimate that did not detect a statistically significant difference contained 4 studies [total n not reported (NR)] and found an OR of 2.07, 95% CI 0.89–4.82) (27). Point estimate ORs ranged from 1.66 to 3.68; RRs ranged from 1.50 to 2.96. I2 values tended to indicate moderate statistical heterogeneity, but with some variation. The 10 higher-quality reviews all found statistically significant increases equating to, on average, over a doubling in risk of severe disease in PWD compared with people without diabetes (9,20–22,24,26,28–31). Again, Izcovich et al. (21) was the largest analysis and also used GRADE to evaluate certainty and calculate absolute risks. In their meta-analysis of 97 studies (n = 21,381), in which severity was defined as reported by study authors or on the basis of ARDS or the requirement of ICU or invasive mechanical ventilation, they found a pooled OR of 2.51 (95% CI 2.2–2.87, I2 = 32%) and judged the evidence to be of high certainty. Estimated absolute risks were a 13.2% increase in severe COVID-19 disease (95% CI 11–15.5%) in PWD compared with people without diabetes (21).
Conclusions
There is consistent evidence across many systematic reviews that diabetes increases risks of severe COVID-19 disease, including ICU admissions, and of death with COVID-19. Most data are from retrospective cohort studies of people hospitalized with COVID-19. The largest review used GRADE to evaluate certainty and judged the evidence to be of high certainty regarding increased risk of severe COVID-19 and increased risk of death with COVID-19 in PWD; restricting analyses to studies at low risk of bias also showed increased risk for both outcomes in PWD. Estimates for severe disease suggest a greater than doubling increase in risk; for death, estimates suggest a slightly less than twofold increase in risk. There is some evidence of between-study heterogeneity, suggesting the magnitude of increase will vary by study population/characteristics. Data on ICU admission were more limited, with fewer reviews reporting this as an outcome, but they again suggested increased risk in PWD.
Are There Differences in Outcomes of SARS-CoV-2 Infection Within the Population of PWD?
Systematic reviews that contained analyses or data regarding our prespecified characteristics within PWD are discussed below. Only one review contained any data on socioeconomic status: Boddu et al. (32) reported on data from a U.K. cohort study that found that within PWD (as well as in the general population without diabetes), COVID-19 outcomes were worse in people from less advantaged groups. There were no data on ethnicity beyond analyses cited above, which looked at country in which research was conducted (this was not one of our prespecified outcomes but may inform future research needs).
Type of Diabetes
The majority of studies in this field and, hence, of reviews aggregating those studies do not delineate between diabetes types. To some extent, this may be due to issues with recording diabetes status in the hospital. Regardless, it is an area that warrants better reporting. Two reviews contained some data explicitly comparing risks in type 1 versus type 2 diabetes; both were judged to have two or more critical weaknesses according to AMSTAR-2, and neither conducted meta-analyses. No reviews explicitly considered differential risks in other types of diabetes (note that this was not something we set out to investigate). Both Apicella et al. (33) and Boddu et al. (32) cited data from a large U.K. cohort study (n = 6,141,447) that used population data collected from medical records independent of COVID-19 status (34). Adjusted for age, sex, deprivation, ethnicity, and geographical region and compared with people without diabetes, the risk of in-hospital COVID-19-related death was markedly higher in people with type 1 than with type 2 diabetes (type 1, OR 3.51 [95% CI 3.16–3.90]; type 2, OR 2.03 [95% CI 1.97–2.09]) (34).
Newly Diagnosed Diabetes
Two reviews contained some data on diabetes diagnosed at the time of COVID-19 infection. Sathish et al. (35), judged to be of higher quality, conducted a meta-analysis of eight studies (n = 3,700) to estimate the prevalence of newly diagnosed diabetes in hospitalized COVID-19 patients. They estimated a pooled proportion of 14.4% (95% CI 5.9–25.8%), but data were highly heterogeneous (I2 = 98%). Of note, this area may also be particularly prone to publication bias, as reports with higher-than-expected levels of newly diagnosed diabetes may be more likely to be written and subsequently published. Boddu et al. (32), which was judged to have two or more critical weaknesses according to AMSTAR-2, did not conduct meta-analysis but noted that SARS-CoV-2 can trigger severe diabetic ketoacidosis at presentation in people with new-onset diabetes (32). The authors note that at present there is no evidence that SARS-CoV-2 induces diabetes of its own accord. Acute infection, stress, and steroids all can also raise blood glucose. Distinguishing between new diabetes caused by COVID-19 and newly diagnosed diabetes that was already present prior to COVID-19 infection but was exacerbated and/or detected due to measurements taken at the hospital also is a challenge. A global registry of patients with COVID-19-related diabetes (https://www.e-dendrite.com/node/268) has been set up to monitor this.
Glucose Control
Eight systematic reviews contained some data on glucose control; all were judged to have two or more critical weaknesses according to AMSTAR-2 (2,32,33,36–40). A major challenge for this characteristic is temporality; glucose at admission may be an inappropriate proxy for glucose control over time.
Chen et al. (36) set out to assess the impact of COVID-19 on blood glucose, meaning measures were those when admitted with COVID-19. The authors pooled data from three studies (n = 222) in PWD comparing blood glucose or glycated hemoglobin (HbA1c) levels between patients classed as having severe versus mild disease (definition not provided). The pooled mean difference (MD) in blood glucose was 2.21 mmol/L (95% CI 1.30–3.13, I2 = 0%), indicating a statistically significantly greater elevation in blood glucose in patients with severe disease. HbA1c, representing longer-term glucose control, was also higher in patients with severe disease, but the estimate was also compatible with no difference (MD 0.29%, 95% CI −0.59, 1.16, I2 = 68%) when pooling the two small studies providing data (n = 179). Lee et al. (38) set out to determine the effects of hyperglycemia on complications of COVID-19 and did not specify at which points these measures were taken. They pooled results from 8 studies (including 681 PWD) and found that hyperglycemia was associated with worse COVID-19 prognosis in both PWD and people without diabetes. Pooled results showed an increased association of admission to ICU (OR 2.7, 95% CI 0.98–7.35, I2 NR) and of death with COVID-19 (OR 7.2, 95% CI 2.7–19.2, I2 NR) in PWD with hyperglycemia compared with those with “controlled blood glucose” (not defined).
The remaining six reviews did not conduct meta-analyses relevant to this question, but all described an association between higher blood glucose and worse COVID-19 outcomes, citing individual studies to support these assertions (2,32,33,37,39,40). As infection and steroids can, in themselves, raise blood glucose levels, determining the direction of association between high blood glucose when hospitalized with COVID-19 and worse COVID-19 outcomes is challenging. A number of reviews also cited data from large (mainly U.K.-based) population-based cohort studies that used last-measured HbA1c, taken prior to COVID-19 infection, providing a better picture of longer-term blood glucose control and its impact on COVID-19 risk. These studies also found significant associations between higher HbA1c (defined as >10% [86 mmol/mol]) and worse COVID-19 outcomes, including ICU admission and ARDS (1,34).
Selected Medications
We focused on metformin, DPP-4i, and insulin. No systematic reviews identified studies that evaluated the relationship between insulin–treated versus non-insulin–treated diabetes and COVID-19 outcomes.
Two reviews, both of which were considered to have two or more critical weaknesses according to AMSTAR-2, considered DPP-4i; neither conducted formal analyses. Apicella et al. (33) noted that although there is speculation that DPP-4i could reduce virulence (by acting as a coreceptor for a subset of coronaviruses and, hence, interfering with binding), there is no clinical evidence of this. They cite two studies that found no associations between glucose-lowering drugs (as prescribed/taken prior to COVID-19 illness) and COVID-19 outcomes in PWD hospitalized with COVID-19. Flaherty et al. (41) also sounds a note of possible optimism regarding the role of DPP-4i as possible receptors for SARS-CoV-2 but calls for further research to investigate their role.
Four reviews, all of which were considered to have two or more critical weaknesses according to AMSTAR-2, considered the role of metformin in COVID-19 outcomes. Three of these conducted meta-analyses, all of which found a clinically and statistically significant association between metformin use prior to COVID-19 diagnosis and reduction in death with COVID-19:
Hariyanto and Kurniawan (42) pooled 5 studies and found an RR of 0.54 (95% CI 0.32–0.90, I2 = 54%, n = 6,937) for metformin use in PWD. The authors caution that confounding was not taken into account in most studies, and that none of the studies stated the dose or duration of metformin treatment in their samples. In addition, all five studies were retrospective.
Kow and Hasan (43) pooled 5 studies (n = 8,121), all of which were in PWD and four of which were identified by the authors as reliable given their large scale and adjustments for multiple confounding factors. They found an OR of 0.62 (95% CI 0.43–0.89, I2 = 29%).
Lukito et al. (44) pooled 9 studies (n = 10,233), including both PWD and people without diabetes. They tested sensitivity between nonadjusted and adjusted models and found that regardless of model used, metformin was associated with a reduction in death with COVID-19 (nonadjusted model, OR 0.45, 95% CI 0.25–0.81, I2 = 63.9%; adjusted model, OR 0.64, 95% CI 0.43–0.97; I2 = 52.1%). However, there was some indication of small study effects.
Of note, metformin is consistently shown to be associated with lower mortality in a range of conditions (e.g., breast cancer [45], not just COVID-19). These associations are not presumed to be causal, and these findings should not be immediately interpreted as suggesting that metformin has a protective effect in COVID-19 illness without further investigation. Flaherty et al. (41) did not conduct meta-analyses but suggested metformin be discontinued in PWD with severe COVID-19 to reduce risk of developing lactic acidosis, although at first glance this seems to contradict the findings above, which relate, where specified, to prehospital use of metformin, not to the use of metformin when hospitalized with severe disease.
Selected Comorbidities
We focus here on PWD with concurrent CVD, hypertension, or chronic kidney disease. Considering the high prevalence of these comorbidities in PWD, there was a notable paucity of data in this area.
Three reviews considered comorbidities relevant to our review. All were considered to have two or more critical weaknesses according to AMSTAR-2. Only one conducted a meta-analysis that included investigation of comorbidities. Huang et al. (46) evaluated the impact of diabetes on a composite poor outcome in people with COVID-19 pneumonia and found a statistically and clinically significant association (13 studies, n = 3,561; Table 2). They used meta-regression to test whether the association between diabetes and worse outcomes was impacted by age, gender, CVD, hypertension, and chronic obstructive pulmonary disorder. In unadjusted models, gender, cardiovascular disease, and comorbid chronic obstructive pulmonary disorder did not statistically significantly influence the relationship with poor outcome within PWD. However, the association with composite poor outcome was influenced by age (weaker association in studies with median age >55 years, P = 0.003) and prevalence of comorbid hypertension (weaker association in populations with greater hypertension prevalence, P < 0.001). In studies where prevalence of comorbid hypertension was >25%, the RR was 1.93 (95% CI 1.48–2.52, I2 = 58%) compared with 3.06 in studies with prevalence of comorbid hypertension of <25% (95% CI 2.19–4.26; I2 = 33%). However, in multivariable meta-regression, including both age and comorbid hypertension, the association was attenuated for both comorbid hypertension (P = 0.107, RRs NR) and age (P = 0.334), suggesting the observed differences are dependent on each other.
The other two reviews provide very little data. Barerra et al. (16) report an unadjusted RR from 1 study of 22 people showing a high point estimate for risk of severe COVID-19 in PWD with hypertension, but, due to the small sample size, CIs are very wide (RR 10, 95% CI 0.94–105.2). Boddu et al. (32) cite the same large U.K., population-based cohort study mentioned above (34) and observe that the relationship between diabetes and COVID-19 mortality is particularly pronounced in older age groups with preexisting renal or cardiac disease. They interpret the low absolute risk of in-hospital death with COVID-19 in PWD under 40 years old as an indication that comorbidities contribute significantly to increased risk of death with COVID-19 in PWD. Of note, in Holman et al. (34), adjusting for previous hospital admissions with coronary heart disease, cerebrovascular disease, or heart failure somewhat attenuated the observed increase in risk of death with COVID-19 in PWD, but a clear increase in risk remained for both types of diabetes (type 1, OR 2.86, 95% CI 2.58–3.18; type 2, OR 1.80, 95% CI 1.75–1.86).
Conclusions
Individual studies, including a very large population-based study in the U.K., show that type 1 diabetes is associated with higher risks of COVID-19 mortality than type 2 diabetes. We did not find any meta-analyses evaluating this.
There is no evidence of differences in risk between new-onset and preexisting diabetes during COVID-19. Whether COVID-19 causes new-onset diabetes is unclear and is under investigation, including in a global registry.
Higher blood glucose levels, both in the immediate and longer terms, are associated with worse COVID-19 outcomes. As high blood glucose can be caused by infection and/or steroids to treat said infection, it is difficult to determine the causal relationship between worse COVID-19 outcomes and measures of blood glucose control taken when ill with COVID-19. However, general practice and national health services databases using HbA1c measured prior to COVID-19 show a clear association between glucose control and COVID-19 outcomes, with higher HbA1c prior to illness increasing risk from said illness. In the literature, HbA1c of 10% (86 mmol/mol) or 7.5% (58 mmol/mol) is commonly used as the cutoff for defining high risk.
Metformin use prior to hospitalization with COVID-19 was associated with a clinically meaningful reduction in the risk of death with COVID-19, as evidenced in three meta-analyses, but these all were judged to have critical weaknesses and none included studies that could establish causality. The use of metformin is cautioned against while patients are hospitalized with severe disease due to concerns over inducing lactic acidosis. Data on DPP-4i and insulin use are lacking in the context of COVID-19.
There is very little evidence regarding the role of comorbidities in increasing risk of worse outcomes from COVID-19 in PWD.
Conclusions
This overview of reviews provides consistent evidence from multiple meta-analyses that diabetes is a risk factor for severe disease and death from COVID-19. Fewer data were available on ICU admission as an outcome, but where available, these data also signaled increased risk in PWD. Within PWD, higher blood glucose levels were associated with worse COVID-19 outcomes. Type 1 diabetes was associated with worse outcomes than type 2, but these data come from individual studies; we did not find any meta-analyses evaluating this.
Due to the nature of the review questions, the majority of data contributing to included reviews came from retrospective observational studies. Reviews varied in the extent to which they assessed risk of bias. In the one review that used the GRADE framework to evaluate certainty, the authors judged the evidence on the association between diabetes and increased risk of worse outcomes and death from COVID-19 to be of high certainty (21). Although the majority of studies contributing to these analyses were judged to be at high risk of bias, results remained consistent when removing studies at high risk of bias and those that did not provide adjusted estimates.
We were unable to reach any firm conclusions on whether PWD were more likely to be infected with SARS-CoV-2. This is unsurprising and reflects limited data, especially a lack of widespread community asymptomatic testing for both SARS-CoV-2 and diabetes. Additionally, other complex issues may be at play that determine whether or not someone is tested. This includes country-level variations in testing capacity but also individual-level considerations. For example, it may be that PWD are more likely to get tested than others (if they feel or are a priori perceived as more vulnerable), but given links between deprivation and diabetes, it may also be that PWD are less likely to be tested, given reports from health care providers that some symptomatic patients are refusing to be tested or isolate because they cannot afford to miss work. As with all overviews of reviews, a further limitation to this work is that lack of data availability for some outcomes and associations may be because this evidence has yet to be included in a systematic review, as opposed to reflecting a lack of primary studies.
There are, of course, other well-established differences in risks for COVID-19 outcomes beyond those investigated here. It is worth noting that risk factors that exist in the wider population also exist in PWD, e.g., older age, deprivation, obesity, non-White ethnicity, and being male all confer greater risk both within and outside PWD (1). Some of these risk factors for COVID-19 severity are also risk factors for diabetes (2). To the extent to which reviews and individual studies have been able to adjust for these, associations have been only somewhat attenuated. In a nationwide analysis in England, arguably the largest study of its type to contribute data on COVID-19 risks in PWD, authors adjusted for age, sex, deprivation, ethnicity, and geographic region and still found increased ORs for in-hospital COVID-19-related death of approximately twofold for people with type 2 diabetes and greater than threefold for people with type 1 diabetes (1). As both diabetes and worse COVID-19 outcomes are associated with socioeconomic disadvantage, their intersection is likely to further exacerbate existing health disparities. This warrants increased research and syntheses in this area. The consistent data found in this overview of systematic reviews showing increased risks from SARS-CoV-2 in PWD should inform policy and practice moving forward.
A Note Regarding Pediatric Populations
Age was not a prespecified characteristic for this review due to clear evidence that COVID-19 risk increases with age. Risk of severe disease in children and adolescents from COVID-19 is low in the general population, and none of the systematic reviews suggested otherwise in children and adolescents with diabetes. Although a lack of evidence typically connotes uncertainty, if COVID-19 posed a substantial risk to children and adolescents with diabetes, it may be reasonable to assume that evidence would have started to emerge by now. D’Annunzio et al. (47), who focused on type 1 diabetes, note that, at present, COVID-19 infection in children and adolescents with type 1 diabetes is clinically different from that of adults, without increased morbidity and mortality. They state that there are no reports suggesting diabetes is a comorbidity associated with poor COVID-19 outcomes in children and adolescents and advise that, as with any suspected infection in PWD, careful glycemic management is required.
Article Information
Editor’s Note. After this article was originally published, the authors updated the review with literature through October 2022 at the request of the World Health Organization. In October 2023, the review with updated data was added as supplementary material to this article and can be accessed at https://doi.org/10.2337/figshare.16629145. For more information, please see the summary of updates detailed in the published letter by Hartmann-Boyce et al. (https://doi.org/10.2337/dc23-1365).
Acknowledgments. The authors thank Nia Roberts, subject librarian, Bodeleian Health Care Libraries, University of Oxford, for designing and conducting searches.
Funding. WHO commissioned and financially supported this work. K.K. is supported by the National Institute for Health Research Applied Research Collaboration East Midlands and the National Institute for Health Research Leicester Biomedical Research Centre. E.M. and C.G. are supported by Wellcome Trust Doctoral Research Fellowships (grant number 203921).
Duality of Interest. K.K. reports payment to institution from Boehringer Ingelheim, AstraZeneca, Novartis, Novo Nordisk, Sanofi, Lilly, and Merck Sharp & Dohme and individual payment from Bayer, NAPP, Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, Roche, Berlin-Chemie AG/Menarini Group, Sanofi, Servier, and Boehringer Ingelheim. He is chair of the Ethnicity Subgroup of SAGE and member of Independent SAGE. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. J.H.-B., E.M.M., C.G., S.S., and K.K. conceived the review. J.H.-B., K.R., J.C.P., S.A.K., E.M.M., C.G., A.A.O., O.E.J., and N.R.S. screened reviews, extracted data, and conducted quality assessment. J.H.-B. wrote the manuscript. All authors contributed to and approved the final version of the manuscript. J.H.-B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.16629145.
This article is part of a special article collection available at https://care.diabetesjournals.org/collection/diabetes-and-COVID19.
References
- 1. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol 2020;8:813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hartmann-Boyce J, Morris E, Goyder C, et al. Diabetes and COVID-19: risks, management, and learnings from other national disasters. Diabetes Care 2020;43:1695–1703 [DOI] [PubMed] [Google Scholar]
- 3. Centre for Evidence-Based Medicine . PROTOCOL rapid reviews of evidence for WHO scientific briefs on COVID-19 and selected noncommunicable diseases (NCDs), 2020. Accessed 10 May 2021. Available from https://www.cebm.net/covid-19/protocol-rapid-reviews-of-evidence-for-who-scientific-briefs-on-covid-19-and-selected-noncommunicable-diseases-ncds/
- 4. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mair M, Singhavi H, Pai A, et al. A meta-analysis of 67 studies with presenting symptoms and laboratory tests of COVID-19 patients. Laryngoscope 2020 [DOI] [PubMed] [Google Scholar]
- 6. Sales-Peres SHC, de Azevedo-Silva LJ, Bonato RCS, Sales-Peres MC, Pinto ACDS, Santiago Junior JF. Coronavirus (SARS-CoV-2) and the risk of obesity for critically illness and ICU admitted: meta-analysis of the epidemiological evidence. Obes Res Clin Pract 2020;14:389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Espinosa OA, Zanetti ADS, Antunes EF, Longhi FG, Matos TA, Battaglini PF. Prevalence of comorbidities in patients and mortality cases affected by SARS-CoV2: a systematic review and meta-analysis. Rev Inst Med Trop São Paulo 2020;62:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khateri S, Mohammadi H, Khateri R, Moradi Y. The prevalence of underlying diseases and comorbidities in COVID-19 patients; an updated systematic review and meta-analysis. Arch Acad Emerg Med 2020;8:e72. [PMC free article] [PubMed] [Google Scholar]
- 9. Kumar A, Arora A, Sharma P, et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr 2020;14:535–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meng M, Zhao Q, Kumar R, Bai C, Deng Y, Wan B. Impact of cardiovascular and metabolic diseases on the severity of COVID-19: a systematic review and meta-analysis. Aging (Albany NY) 2020;12:23409–23421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pinedo-Torres I, Flores-Fernández M, Yovera-Aldana M, et al. Prevalence of diabetes mellitus and its associated unfavorable outcomes in patients with acute respiratory syndromes due to coronaviruses infection: a systematic review and meta-analysis. Clin Med Insights Endocrinol Diabetes 2020;13:1179551420962495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou Y, Yang Q, Chi J, et al. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: a systematic review and meta-analysis. Int J Infect Dis 2020;99:47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Desai R, Singh S, Parekh T, Sachdeva S, Sachdeva R, Kumar G. COVID-19 and diabetes mellitus: a need for prudence in elderly patients from a pooled analysis. Diabetes Metab Syndr 2020;14:683–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mantovani A, Byrne CD, Zheng MH, Targher G. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: a meta-analysis of observational studies. Nutr Metab Cardiovasc Dis 2020;30:1236–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hussain S, Baxi H, Chand Jamali M, Nisar N, Hussain MS. Burden of diabetes mellitus and its impact on COVID-19 patients: a meta-analysis of real-world evidence. Diabetes Metab Syndr 2020;14:1595–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barrera FJ, Shekhar S, Wurth R, Moreno-Pena PJ, Ponce OJ, Hajdenberg M, et al. Prevalence of diabetes and hypertension and their associated risks for poor outcomes in Covid-19 patients. J Endocr Soc 2020;4:bvaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Du M, Lin YX, Yan WX, Tao LY, Liu M, Liu J. Prevalence and impact of diabetes in patients with COVID-19 in China. World J Diabetes 2020;11:468–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang X, Wang S, Sun L, Qin G. Prevalence of diabetes mellitus in 2019 novel coronavirus: a meta-analysis. Diabetes Res Clin Pract 2020;164:108200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parveen R, Sehar N, Bajpai R, Agarwal NB. Association of diabetes and hypertension with disease severity in covid-19 patients: a systematic literature review and exploratory meta-analysis. Diabetes Res Clin Pract 2020;166:108295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fang X, Li S, Yu H, et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging (Albany NY) 2020;12:12493–12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Izcovich A, Ragusa MA, Tortosa F, et al. Prognostic factors for severity and mortality in patients infected with COVID-19: a systematic review. PLoS One 2020;15:e0241955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luo L, Fu M, Li Y, et al. The potential association between common comorbidities and severity and mortality of coronavirus disease 2019: a pooled analysis. Clin Cardiol 2020;43:1478–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mesas AE, Cavero-Redondo I, Álvarez-Bueno C, et al. Predictors of in-hospital COVID-19 mortality: a comprehensive systematic review and meta-analysis exploring differences by age, sex and health conditions. PLoS One 2020;15:e0241742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singh AK, Gillies CL, Singh R, et al. Prevalence of co-morbidities and their association with mortality in patients with COVID-19: a systematic review and meta-analysis. Diabetes Obes Metab 2020;22:1915–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ssentongo P, Ssentongo AE, Heilbrunn ES, Ba DM, Chinchilli VM. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: a systematic review and meta-analysis. PLoS One 2020;15:e0238215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Varikasuvu SR, Dutt N, Thangappazham B, Varshney S. Diabetes and COVID-19: a pooled analysis related to disease severity and mortality. Prim Care Diabetes 2021;15:24–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis 2020;94:91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Figliozzi S, Masci PG, Ahmadi N, et al. Predictors of adverse prognosis in COVID-19: a systematic review and meta-analysis. Eur J Clin Invest 2020;50:e13362. [DOI] [PubMed] [Google Scholar]
- 29. Mudatsir M, Fajar JK, Wulandari L, et al. Predictors of COVID-19 severity: a systematic review and meta-analysis. F1000 Res 2020;9:1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Plasencia-Urizarri TM, Aguilera-Rodríguez R, Almaguer-Mederos LE. Comorbidities and clinical severity of COVID-19: systematic review and meta-analysis. Rev Habanera Cienc Méd 2020:e3389-e [Google Scholar]
- 31. Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY) 2020;12:6049–6057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boddu SK, Aurangabadkar G, Kuchay MS. New onset diabetes, type 1 diabetes and COVID-19. Diabetes Metab Syndr 2020;14:2211–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol 2020;8:782–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol 2020;8:823–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sathish T, Kapoor N, Cao Y, Tapp RJ, Zimmet P. Proportion of newly diagnosed diabetes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Obes Metab 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen J, Wu C, Wang X, Yu J, Sun Z. The impact of COVID-19 on blood glucose: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 2020;11:574541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chowdhury S, Goswami S. COVID-19 and type 1 diabetes: dealing with the difficult duo. Int J Diabetes Dev Ctries. 14 July 2020 [Epub ahead of print]. DOI: 10.1007/s13410-020-00846-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee MH, Wong C, Ng CH, Yuen DCW, Lim AYL, Khoo CM. Effects of hyperglycaemia on complications of COVID-19: a meta-analysis of observational studies. Diabetes Obes Metab 2021;23:287–289 [DOI] [PubMed] [Google Scholar]
- 39. Sacks LJ, Pham CT, Fleming N, Neoh SL, Ekinci EI. Considerations for people with diabetes during the coronavirus disease (COVID-19) pandemic. Diabetes Res Clin Pract 2020;166:108296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yanai H. Metabolic syndrome and COVID-19. Cardiol Res 2020;11:360–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Flaherty GT, Hession P, Liew CH, et al. COVID-19 in adult patients with pre-existing chronic cardiac, respiratory and metabolic disease: a critical literature review with clinical recommendations. Trop Dis Travel Med Vaccines 2020;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hariyanto TI, Kurniawan A. Metformin use is associated with reduced mortality rate from coronavirus disease 2019 (COVID-19) infection. Obes Med 2020;19:100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kow CS, Hasan SS. Mortality risk with preadmission metformin use in patients with COVID-19 and diabetes: a meta-analysis. J Med Virol 2021;93:695–697 [DOI] [PubMed] [Google Scholar]
- 44. Lukito AA, Pranata R, Henrina J, Lim MA, Lawrensia S, Suastika K. The effect of metformin consumption on mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. Diabetes Metab Syndr 2020;14:2177–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee KN, Torres MA, Troeschel AN, He J, Gogineni K, McCullough LE. Type 2 diabetes, breast cancer specific and overall mortality: associations by metformin use and modification by race, body mass, and estrogen receptor status. PLoS One 2020;15:e0232581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr 2020;14:395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. d’Annunzio G, Maffeis C, Cherubini V, et al. Caring for children and adolescents with type 1 diabetes mellitus: Italian Society for Pediatric Endocrinology and Diabetology (ISPED) statements during COVID-19 pandemia. Diabetes Res Clin Pract 2020;168:108372. [DOI] [PMC free article] [PubMed] [Google Scholar]