Abstract
OBJECTIVE
To assess the relationship between the glucose management indicator (GMI) and HbA1c in non-White individuals with diabetes.
RESEARCH DESIGN AND METHODS
We performed a retrospective analysis of continuous glucose monitoring metrics in individuals with diabetes divided by race into non-White and White cohorts.
RESULTS
We evaluated 316 individuals (non-White n = 68; White n = 248). Although GMI was not different (7.6 vs. 7.7; P = not significant) between the cohorts, HbA1c was higher in the non-White cohort (8.7% vs. 8.1%; P = 0.004). HbA1c higher than GMI by ≥0.5% was more frequently observed in the non-White cohort (90% vs. 75%; P = 0.02). In the non-White cohort only, duration of hypoglycemia was longer among those with HbA1c higher than GMI by ≥0.5% compared with those with HbA1c and GMI within 0.5%.
CONCLUSIONS
A differential relationship between HbA1c and GMI in non-White versus White individuals with diabetes was observed. In non-White individuals, a greater difference between HbA1c and GMI was associated with higher risk of hypoglycemia.
Introduction
A discrepancy between laboratory HbA1c and mean glucose measured by both self-blood glucose monitoring and continuous glucose monitoring (CGM) (1,2) has been described in individuals with diabetes of different races (1,3,4). In addition, HbA1c tends to run higher in non-White individuals at the same mean glucose level (3).
The glucose management indicator (GMI), the CGM-derived metric reflecting the mean sensor glucose, has been introduced to address the discrepancy between laboratory HbA1c and estimated HbA1c using mean glucose value measured by CGM (5). The relationship between HbA1c and GMI in different races has not been described.
In this study, we assessed the relationship between laboratory HbA1c, GMI, and time spent in hypoglycemia in non-White versus White individuals with diabetes.
Research Design and Methods
We conducted a retrospective cross-sectional study on data collected at a tertiary diabetes center of patients undergoing professional CGM (proCGM) (FreeStyle Libre; Abbott Diabetes Care, Inc., Alameda, CA) between 1 January 2017 and 31 December 2020. Demographic and clinical data were retrieved from electronic health records. The study was institutional review board approved.
Data from adult patients (age ≥18 years) with type 1 diabetes (T1D) or T2D who were prescribed proCGM were collected. Patients receiving insulin (basal, basal/bolus, or pump) with CGM wear time of 14 days and ≥70% available data were included in this analysis. Patients with T2D not receiving insulin and those without recorded race were excluded.
CGM metrics were evaluated as per the international consensus on use of CGM (6). GMI was calculated as 3.31 + (0.02392 × mean glucose in mg/dL) (5,6). Laboratory HbA1c values, all performed at a single laboratory (Tina-quant HbA1c Gen. 3; Roche Diagnostics), were collected within 1 month of proCGM wear.
On the basis of the reported race in the electronic health record, patients were divided in two groups: non-White—including American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian, or Pacific Islander—and White.
For categorical variables, data are reported as number (n) and percentage (%) of the cohort. Data are reported as mean ± SD for data with normal distribution and as median and first and third interquartile (quartile 1, quartile 3) for data with nonnormal distribution. SAS version 9.4 software was used for all analyses and included Pearson correlations, Student t tests, Fisher exact tests, and simple linear regression modeling. A P value of <0.05 was considered statistically significant.
Results
We evaluated 316 patients (mean age 60 ± 18 years, 51% female, duration of diabetes 24 ± 15 years, and T1D 45% and T2D 55%) with diabetes receiving insulin. Insulin regimens included multiple daily injections (85%), pump (14%), and basal insulin only (<1%). Participants were divided into two cohorts identified by race as non-White (22%; n = 68) and White (78%; n = 248). The two cohorts did not differ in age, sex, duration of diabetes, or BMI. In the non-White cohort, more patients had T2D versus T1D (78% vs. 22%), while in the White cohort, the percentages of patients with T2D and T1D were similar (49% vs. 51%). CGM metrics did not differ between the non-White versus White cohort, including time in range (728 ± 34 vs. 722 ± 307 min/day; P = not significant [ns]), time spent in hypoglycemia (<70 mg/dL) (median 63 [interquartile range 17, 24] vs. 63 [14, 144] min/day; P = ns), coefficient of variation (39 ± 11% vs. 39 ± 10%; P = ns), and GMI (7.7 ± 1.5% vs. 7.6 ± 1.3%; P = ns). HbA1c was higher in the non-White compared with the White cohort (8.7 ± 1.6% vs. 8.1 ± 1.3%; P = 0.01).
Next, we looked at absolute and relative differences between HbA1c and GMI in the two racial cohorts. An absolute difference between HbA1c and GMI ≥0.5 (considered clinically significant) was present in 72% of the non-White cohort and 66% of the White cohort (P = 0.03). HbA1c higher than GMI by ≥0.5% was more frequent in the non-White versus White cohort (90% vs. 75%; P = 0.02); however, HbA1c lower than GMI by ≥0.5% did not differ between the two cohorts (10% vs. 25%; P = ns).
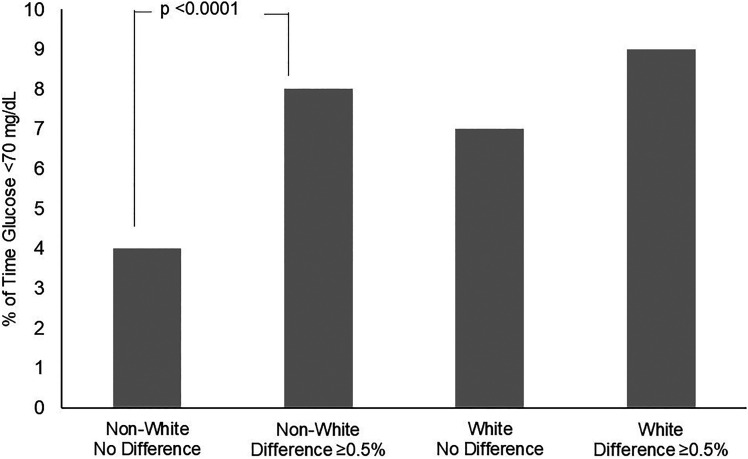
Next, we examined the percentage of time spent in hypoglycemia (<70 mg/dL). Participants in the non-White cohort spent more time in hypoglycemia when HbA1c was higher than GMI by ≥0.5% compared with when HbA1c and GMI values were within 0.5% (8% vs. 4%; P = 0.01). In the White cohort, the percentage of time spent in hypoglycemia did not differ, whether the difference between HbA1c and GMI was ≥0.5% or <0.5% (9% vs. 7%; P = ns) (Fig. 1).
Figure 1.
Percentage of time spent in hypoglycemia in non-White and White cohorts divided by difference between HbA1c and GMI within 0.5% (no difference) and HbA1c greater than GMI by ≥0.5%.
Conclusions
In this cross-sectional study, we found a differential relationship between laboratory HbA1c and GMI in a White versus non-White cohort of patients with diabetes receiving insulin. A clinically significant difference between laboratory HbA1c and GMI (≥0.5%) was observed more frequently in the non-White than White cohort. In addition, a relative difference of HbA1c greater than GMI by ≥0.5% was observed in 90% of the non-White cohort compared with 75% of the White cohort and was strongly associated with higher time spent in hypoglycemia in the non-White cohort only. To our knowledge, this is the first time that the relationship between HbA1c and GMI in a population of different races has been described.
The identified discrepancy between HbA1c and GMI and its strong correlation with time spent in hypoglycemia in the non-White cohort highlight the additional value of the use of CGM in non-White individuals with diabetes, in whom HbA1c tends to run higher than GMI. Obtaining CGM-derived metrics, along with the measurement of HbA1c, in non-White individuals with diabetes receiving insulin therapy can help to establish the relationship between GMI and HbA1c. This relationship remains constant over time within a patient (7,8) and can guide clinicians to develop a personalized patient-centric treatment plan that may allow a higher HbA1c goal in some non-White individuals in order to avoid hypoglycemia.
Our results are consistent with data in the literature, where HbA1c is found to be higher in the non-White versus White population, for the same mean glucose level (4). Furthermore, a clinically significant discrepancy (≥0.5%) between HbA1c and GMI was described in ∼19% of adults with diabetes (5) and in up to 50% of older adults with T1D. In the latter group, GMI was more closely associated with time spent in hypoglycemia and hyperglycemia compared with HbA1c (9). A small study suggested that the GMI formula may be influenced by CGM systems and patient race (5,10); however, the current CGM reports display the GMI value calculated using a standardized formula (5). The majority of the non-White cohort in our analysis was represented by individuals with T2D treated with insulin, in whom the use of CGM is currently limited (11). Larger studies about the impact of race on measures of glycemia in T1D and T2D are needed. Nonetheless, our data underscore the importance of CGM use in clinical practice, especially in non-White individuals receiving insulin therapy.
The limitations of our study include the retrospective single-center observational design, and therefore, causality cannot be assessed. Additionally, the FreeStyle Libre CGM may not be accurate in detecting hypoglycemia (12), and CGM data are from one-time-only wear and may not reflect long-term glycemic control.
In conclusion, these data suggest a racial difference in the relationship between GMI and HbA1c. GMI can provide additional information on glycemic control and time spent in hypoglycemia in non-White individuals that is not identified by HbA1c alone. Non-White patients receiving insulin therapy will benefit from CGM-derived measurements in addition to HbA1c.
Article Information
Acknowledgments. The authors thank the philanthropic donors of the Joslin Clinical Research Center.
Funding. This study was supported by the Joslin Clinical Research Center, a National Institutes of Health (NIH) DP3 grant (1DP3-DK-11221 4-01), and an NIH P30 grant (P30DK036836).
The funding source had no role in the design or conduct of the study, collection, management, analyses, interpretation of the data, or preparation or decision to submit manuscript for publication.
Duality of Interest. E.T. is a consultant for Medtronic. M.M. is a consultant for Sanofi and Eli Lilly. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. All authors contributed to data acquisition, analysis, and interpretation and drafted the manuscript. E.T., A.M., A.A., A.A.-C., C.S., and M.M. critically revised the manuscript for important intellectual content. E.T., C.S., A.A.-C., and M.M. contributed to study concept and design. E.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented virtually in oral abstract form at the 81st Scientific Sessions of the American Diabetes Association, 25–29 June 2021.
References
- 1. Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D; A1c-Derived Average Glucose Study Group . Translating the A1C assay into estimated average glucose values. Diabetes Care 2008;31:1473–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The fallacy of average: how using HbA1c alone to assess glycemic control can be misleading. Diabetes Care 2017;40:994–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kirk JK, D’Agostino RB Jr, Bell RA, et al. Disparities in HbA1c levels between African-American and non-Hispanic White adults with diabetes: a meta-analysis. Diabetes Care 2006;29:2130–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bergenstal RM, Gal RL, Beck RW. Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann Intern Med 2018;168:232–233 [DOI] [PubMed] [Google Scholar]
- 5. Bergenstal RM, Beck RW, Close KL, et al. Glucose Management Indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care 2018;41:2275–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care 2017;40:1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nayak AU, Holland MR, Macdonald DR, Nevill A, Singh BM. Evidence for consistency of the glycation gap in diabetes. Diabetes Care 2011;34:1712–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wilson DM, Xing D, Cheng J, et al. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group . Persistence of individual variations in glycated hemoglobin: analysis of data from the Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Randomized Trial. Diabetes Care 2011;34:1315–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Toschi E, Slyne C, Sifre K, et al. The relationship between CGM-derived metrics, A1C, and risk of hypoglycemia in older adults with type 1 diabetes. Diabetes Care 2020;43:2349–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Angellotti E, Muppavarapu S, Siegel RD, Pittas AG. The calculation of the glucose management indicator is influenced by the continuous glucose monitoring system and patient race. Diabetes Technol Ther 2020;22:651–657 [DOI] [PubMed] [Google Scholar]
- 11. Karter AJ, Parker MM, Moffet HH, Gilliam LK, Dlott R. Association of real-time continuous glucose monitoring with glycemic control and acute metabolic events among patients with insulin-treated diabetes. JAMA 2021;325:2273–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Galindo RJ, Migdal AL, Davis GM, et al. Comparison of the FreeStyle Libre Pro Flash Continuous Glucose Monitoring (CGM) System and point-of-care capillary glucose testing in hospitalized patients with type 2 diabetes treated with basal-bolus insulin regimen. Diabetes Care 2020;43:2730–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]