Abstract
OBJECTIVE
To determine whether metformin or lifestyle modification can lower rates of all-cause and cause-specific mortality in the Diabetes Prevention Program and Diabetes Prevention Program Outcomes Study.
RESEARCH DESIGN AND METHODS
From 1996 to 1999, 3,234 adults at high risk for type 2 diabetes were randomized to an intensive lifestyle intervention, masked metformin, or placebo. Placebo and lifestyle interventions stopped in 2001, and a modified lifestyle program was offered to everyone, but unmasked study metformin continued in those originally randomized. Causes of deaths through 31 December 2018 were adjudicated by blinded reviews. All-cause and cause-specific mortality hazard ratios (HRs) were estimated from Cox proportional hazards regression models and Fine-Gray models, respectively.
RESULTS
Over a median of 21 years (interquartile range 20–21), 453 participants died. Cancer was the leading cause of death (n = 170), followed by cardiovascular disease (n = 131). Compared with placebo, metformin did not influence mortality from all causes (HR 0.99 [95% CI 0.79, 1.25]), cancer (HR 1.04 [95% CI 0.72, 1.52]), or cardiovascular disease (HR 1.08 [95% CI 0.70, 1.66]). Similarly, lifestyle modification did not impact all-cause (HR 1.02 [95% CI 0.81, 1.28]), cancer (HR 1.07 [95% CI 0.74, 1.55]), or cardiovascular disease (HR 1.18 [95% CI 0.77, 1.81]) mortality. Analyses adjusted for diabetes status and duration, BMI, cumulative glycemic exposure, and cardiovascular risks yielded results similar to those for all-cause mortality.
CONCLUSIONS
Cancer was the leading cause of mortality among adults at high risk for type 2 diabetes. Although metformin and lifestyle modification prevented diabetes, neither strategy reduced all-cause, cancer, or cardiovascular mortality rates.
Introduction
Adults with prediabetes are at a higher risk for all-cause, cardiovascular, and cancer mortality (1–3). While metformin and lifestyle interventions to achieve weight loss and increase physical activity have been shown to lower the risk of type 2 diabetes in adults with impaired glucose tolerance (4), limited data from randomized clinical trials exist on whether these interventions can reduce mortality rates.
Both metformin and lifestyle interventions have evidence for life span and health span extension in animal models, potentially through nutrient-sensing and stress-response pathways (5). In addition, numerous observational studies suggest that metformin may lower the risk of all-cause, cardiovascular, and cancer mortality among populations with type 2 diabetes (6–14). There are also observational studies that suggest that self-reported intentional weight loss in people with overweight and obesity is associated with lower mortality rates (15–17). However, evidence for mortality benefits of metformin and lifestyle modification from clinical trials is more limited.
The UK Prospective Diabetes Study (UKPDS) is a clinical trial of adults with newly diagnosed diabetes that demonstrated the efficacy of metformin in lowering the risk of all-cause mortality compared with conventional control or intensive control with chlorpropamide, glibenclamide, or insulin (18). However, no trial has evaluated the effect of metformin on all-cause or cause-specific mortality in a population of adults at high risk for developing type 2 diabetes. A meta-analysis of randomized behavioral weight loss trials in adults with obesity concluded that weight loss is associated with a lower risk of all-cause mortality, but many of the trials were of limited duration (19). In the Da Qing Diabetes Prevention Outcome Study, a randomized controlled trial of lifestyle interventions administered over 6 years to Chinese adults with impaired glucose tolerance and a mean baseline BMI of 26 kg/m2, all-cause and cardiovascular mortality rates were reduced after 20–30 years’ follow-up (20,21). However, it is unknown whether these findings will be replicated in the Diabetes Prevention Program (DPP) study population with higher mean age and BMI at baseline after 21 years’ follow-up. Impaired glucose tolerance is associated with a higher risk of cancer and cancer deaths, the second leading cause of mortality in the U.S (3,22). No trials exist, however, to evaluate the efficacy of lifestyle modification in lowering the risk of cancer mortality among adults with impaired glucose tolerance.
DPP was designed to evaluate the efficacy of metformin and intensive lifestyle interventions to prevent diabetes, and the long-term follow-up of enrolled DPP participants in the Diabetes Prevention Program Outcomes Study (DPPOS) focused on their effects on development of microvascular complications, cancer, and cardiovascular disease. Because obesity and diabetes increase the risk for all-cause, cancer, and cardiovascular disease mortality (23,24), and because metformin and lifestyle interventions were effective in lowering weight and preventing diabetes in the DPP (4), we hypothesized that metformin and lifestyle modification may also lower the risk for all-cause, cancer, and cardiovascular disease mortality. While mortality was not a primary outcome in DPP or DPPOS, mortality was assessed with rigorous adjudication of causes to account for the competing risk of deaths on key outcomes measured in DPPOS. Therefore, these high-quality mortality data, the long duration of the metformin intervention, and the long follow-up period in DPPOS provide a unique opportunity to conduct a secondary data analysis to evaluate the effects of metformin and intensive lifestyle modification interventions on all-cause and cause-specific mortality in a population of adults at high risk of type 2 diabetes.
Research Design and Methods
Study Population
From 1996 to 1999, 3,234 adults ages ≥25 years were enrolled in DPP at 27 clinical sites in the U.S. Written informed consent was obtained from all participants before screening, consistent with the Declaration of Helsinki and the guidelines of each center’s institutional review board. For inclusion of a study population at high risk for developing type 2 diabetes, inclusion criteria included a BMI ≥24 kg/m2 (≥22 kg/m2 for Asian Americans), fasting plasma glucose 95–125 mg/dL (≤125 mg/dL for American Indians), and 2-h glucose 140–199 mg/dL after a 75-g oral glucose load. Key exclusions were significant cardiovascular or renal disease, cancer requiring treatment in the past 5 years (except cancer considered cured or associated with a good prognosis, such as nonmelanoma skin cancer, papillary thyroid carcinoma, and cervical carcinoma in situ), hepatitis, and other medical conditions likely to limit life span or increase the risk of the interventions, as previously described (25).
Study Design and Interventions
Participants were randomized to one of three groups: an intensive lifestyle intervention (lifestyle) focused on achieving at least 150 min physical activity weekly and ≥7% body weight loss, metformin 850 mg twice daily with standard diet and exercise recommendations, or a placebo twice daily with standard diet and exercise recommendations, as previously described (Consolidated Standards of Reporting Trials [CONSORT] diagram [Supplementary Fig. 1]) (26). The masked intervention phase of the study was stopped on 1 July 2001 when efficacy for the primary outcome of diabetes prevention was achieved. In 2002, after a bridge period during which all participants received a modified group lifestyle intervention, 2,779 participants continued in DPPOS and were offered quarterly lifestyle sessions. Those originally randomized to lifestyle were offered additional lifestyle reinforcement semiannually, and those randomized to metformin continued to receive open-label metformin 850 mg twice daily. Study metformin and instructions to take it were provided if participants did not develop a contraindication to the study drug, until plasma glucose worsened to ≥140 mg/dL in the DPP, or when hemoglobin A1c (HbA1c) was ≥7% during the DPPOS, at which time study metformin was discontinued and diabetes management was transferred to the participant’s health care provider. Participants were instructed to bring any unused study metformin to study visits for pill counts to track adherence, as previously described (27).
Outcomes
The primary outcome of all-cause mortality was ascertained for all participants enrolled in DPP through regular surveillance of the study population at annual visits and two National Death Index (NDI) searches through 31 December 2018. No deaths were counted after that date, and anyone not known to be deceased or found in the NDI search by that closing date was considered to be alive. An adjudication committee that was blinded to treatment assignment used medical records, death certificates, and NDI cause of death codes to assign the underlying cause of death for the secondary outcomes of cause-specific mortality. Participants had previously signed medical release forms allowing for the acquisition of their medical records. Categories of cause-specific mortality for this manuscript comprise cardiovascular disease, cancer, and other causes. Demographics, medical history, and lifestyle factors were assessed via questionnaire; standardized exams that included weight, height, and blood pressure; and laboratory specimens from fasting subjects that were assayed for glucose, lipids, and HbA1c, as previously described (26). In addition, all participants were instructed to bring medications (lists, prescriptions, and containers) to their annual study visit for an inventory of concomitant medications taken within the prior 2 weeks, on the basis of which use of antihypertensive medications, lipid-lowering agents, and out-of-study metformin use was assessed.
Statistical Analysis
Hazard ratios (HRs) for all-cause mortality associated with metformin and lifestyle compared with placebo were estimated from Cox proportional hazards regression models with adjustments for baseline age, race/ethnicity, and sex, and the assumption of proportional hazards was confirmed. Time-to-event Fine-Gray models accounting for the competing risk of other causes of mortality were used to determine the risk for cause-specific mortality associated with metformin and lifestyle compared with placebo with adjustments for baseline age, race/ethnicity, and sex for causes with ≥50 deaths. Differences in the effects of the DPP randomized interventions on mortality in prespecified subgroups were explored by testing of interactions by age, sex, race/ethnicity, and BMI in these models without adjustment for multiplicity, and P values <0.05 were considered statistically significant. Sensitivity analyses using alternative models were performed to account for potential factors that may have affected the effects of treatment: 1) multivariable models to account for time-varying characteristics collecting during follow-up (including diabetes status and duration, BMI, glycemic exposure, and cardiovascular risk factors), since these covariates are influenced by DPP interventions and are risk factors for mortality; 2) multivariable models to account for drop-in use of out-of-study metformin in the three arms of DPP with adjustment for time-varying, out-of-study metformin use in the above time-to-event Fine-Gray models; and 3) marginal structural models with truncated inverse probability stability weights to estimate the etiologic effect of metformin use on all-cause mortality in the presence of out-of-study metformin and discontinuation of and adherence to randomized metformin (28). All statistical analyses were performed with SAS 9.4 and R 3.5.2.
Data and Resource Availability
In accordance with the National Institutes of Health (NIH) Public Access Policy, we continue to provide all manuscripts to PubMed Central including this manuscript. DPP/DPPOS has provided the protocols and lifestyle and medication intervention manuals to the public through its public website (https://www.dppos.org). The DPPOS abides by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) data sharing policy and implementation guidance as required by the NIH/NIDDK (https://www.niddkrepository.org/studies/dppos/).
Results
The study population enrolled in the DPP had a mean ± SD age of 50.6 ± 10.7 years and BMI 34.0 ± 6.7 kg/m2, 68% were female, and 55% were non-Hispanic White. With regard to other cardiovascular risk factors at baseline, 41% were former or current smokers, 29% had hypertension or were treated for hypertension, and 69% had hyperlipidemia or were on treatment with lipid-lowering medications. There were no significant differences in baseline demographic or clinical characteristics across the three randomized groups (Table 1). However, by 31 December 2018, there were differences in the prevalence of diabetes in the metformin (55%), lifestyle (53%), and placebo (60%) groups (P = 0.003).
Table 1.
Baseline characteristics of participants by DPP randomized groups
| Characteristic | Total (n = 3,234) | Placebo (n = 1,082) | Metformin (n = 1,073) | Lifestyle (n = 1,079) |
|---|---|---|---|---|
| Age (years) | 50.6 ± 10.7 | 50.3 ± 10.4 | 50.9 ± 10.3 | 50.6 ± 11.3 |
| Women | 2,191 (68) | 747 (69) | 710 (66) | 734 (68) |
| Race/ethnicity | ||||
| White | 1,768 (55) | 586 (54) | 602 (56) | 580 (54) |
| African American | 645 (20) | 220 (20) | 221 (21) | 204 (19) |
| Hispanic | 508 (16) | 168 (16) | 162 (15) | 178 (16) |
| American Indian | 171 (5) | 59 (6) | 52 (5) | 60 (6) |
| Asian American | 142 (4) | 49 (4) | 36 (3) | 57 (5) |
| Education | ||||
| Primary | 130 (4) | 51 (5) | 36 (3) | 43 (4) |
| High school | 704 (22) | 234 (22) | 233 (22) | 237 (22) |
| College | 1,556 (48) | 520 (48) | 514 (48) | 522 (48) |
| Graduate school | 844 (26) | 277 (26) | 290 (27) | 277 (26) |
| Income >$50,000 | 1,328 (41) | 429 (40) | 463 (43) | 436 (40) |
| Smoking | ||||
| Never | 1,897 (59) | 635 (59) | 634 (59) | 628 (58) |
| Former | 1,111 (34) | 363 (34) | 367 (34) | 381 (35) |
| Current | 226 (7) | 84 (8) | 72 (7) | 70 (7) |
| Weekly alcohol use | ||||
| <1 drink | 2,380 (75) | 796 (75) | 784 (74) | 800 (76) |
| 1–7 drinks | 647 (20) | 224 (21) | 220 (21) | 203 (19) |
| >7 drinks | 148 (5) | 44 (4) | 53 (5) | 51 (5) |
| BMI (kg/m2) | 34.0 ± 6.7 | 34.1 ± 6.7 | 33.9 ± 6.6 | 33.9 ± 6.8 |
| Hypertension* | 925 (29) | 301 (28) | 312 (29) | 312 (29) |
| Hyperlipidemia† | 2,244 (69) | 769 (71) | 741 (70) | 734 (68) |
| Fasting glucose (mg/dL) | 106.5 ± 8.3 | 106.7 ± 8.4 | 106.5 ± 8.5 | 106.3 ± 8.1 |
| HbA1c (%) | 5.9 ± 0.5 | 5.9 ± 0.5 | 5.9 ± 0.5 | 5.9 ± 0.5 |
| HbA1c (mmol/mol) | 41.0 ± 5.5 | 41.0 ± 5.5 | 41.0 ± 5.5 | 41.0 ± 5.5 |
Baseline characteristics are described as means ± SD or n (%) as appropriate.
Hypertension is defined as blood pressure of at least 140/90 mmHg or use of antihypertensive medications.
Hyperlipidemia is defined as LDL cholesterol ≥130 mg/dL, triglyceride ≥150 mg/dL, or lipid-lowering medications.
Over a median follow-up of 21 years (interquartile range 20–21), 453 (14%) of the 3,234 participants died. The cause of death was unknown in 7% due to missing records or other documentation needed for accurate adjudication of the cause. Cancer was the most common cause of death (37%), followed by cardiovascular disease (29%). The fractions of deaths from chronic respiratory diseases, infection, neurologic disease, renal disease, trauma, and other causes were all <10% (Table 2).
Table 2.
Adjudicated causes of death by DPP randomized groups
| Cause of death | Total | Placebo | Metformin | Lifestyle |
|---|---|---|---|---|
| Cancer | 170 (37) | 53 (37) | 57 (37) | 60 (38) |
| Cardiovascular disease | 131 (29) | 38 (27) | 44 (29) | 49 (31) |
| Neurologic (nonstroke) | 36 (8) | 12 (8) | 12 (8) | 12 (8) |
| Unknown | 32 (7) | 14 (10) | 7 (5) | 11 (7) |
| Infection | 25 (5) | 8 (6) | 11 (7) | 6 (4) |
| Other* | 22 (5) | 5 (3) | 10 (7) | 7 (4) |
| Trauma | 20 (4) | 8 (6) | 6 (4) | 6 (4) |
| Chronic respiratory disease | 9 (2) | 3 (2) | 3 (2) | 3 (2) |
| Renal disease | 8 (2) | 2 (1) | 2 (1) | 4 (3) |
| Total | 453 | 143 | 152 | 158 |
Data are n or n (%).
Other causes of death include cardiac arrest, multiorgan failure, hepatic failure, suicide, acute pancreatitis, myelofibrosis, upper gastrointestinal bleed, or ventriculitis.
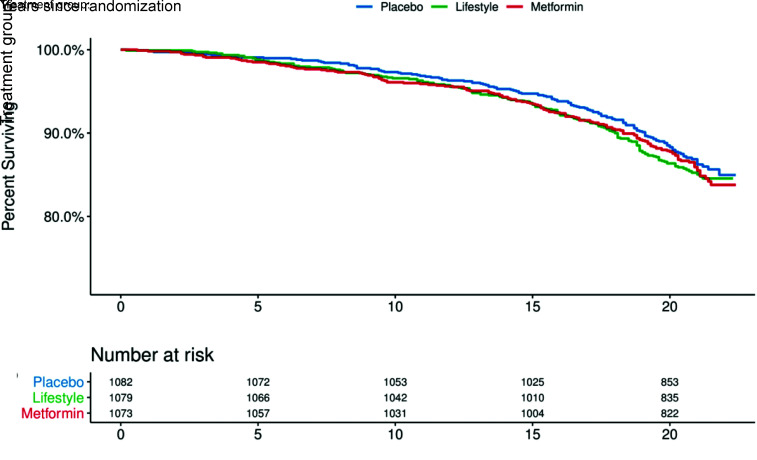
Mortality rates were similar across groups randomized to metformin (7.1 deaths/1,000 person-years), lifestyle (7.4 deaths/1,000 person-years), and placebo (6.6 deaths/1,000 person-years (Fig. 1). There was no difference between metformin and placebo groups in the risk of all-cause (HR 0.99 [95% CI 0.79, 1.25]), cardiovascular (HR 1.08 [95% CI 0.70], 1.66), cancer (HR 1.04, [95% CI 0.72, 1.52]), or other (HR 0.94 [95% CI 0.64, 1.38]) mortality (Table 3). There was also no difference between lifestyle and placebo groups in the risk of all-cause (HR 1.02 [95% CI 0.81, 1.28]), cardiovascular (HR 1.18 [95% CI 0.77, 1.81]), cancer (HR 1.07 [95% CI 0.74, 1.55]) or other (HR 0.85 [95% CI 0.58, 1.26]) mortality (Table 3).
Figure 1.
Kaplan-Meier survival curves for metformin, lifestyle, and placebo groups. The figure shows the survival by randomization to metformin, lifestyle, and placebo.
Table 3.
Rates and risk for all-cause and cause-specific mortality associated with metformin and lifestyle compared with placebo
| Number of events | Event rate/1,000 person-years | Metformin versus placebo | Lifestyle versus placebo | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Causes of death | Placebo | Metformin | Lifestyle | Placebo | Metformin | Lifestyle | HR (95% CI)* | P | HR (95% CI)* | P |
| All cause | 143 | 152 | 158 | 6.59 | 7.13 | 7.37 | 0.99 (0.79, 1.25) | 0.95 | 1.02 (0.81, 1.28) | 0.87 |
| Cancer | 53 | 57 | 60 | 2.45 | 2.67 | 2.80 | 1.04 (0.72, 1.52) | 0.83 | 1.07 (0.74, 1.55) | 0.71 |
| CVD | 38 | 44 | 49 | 1.75 | 2.06 | 2.28 | 1.08 (0.70, 1.66) | 0.74 | 1.18 (0.77, 1.81) | 0.44 |
| Other | 52 | 51 | 49 | 2.40 | 2.39 | 2.28 | 0.94 (0.64, 1.38) | 0.74 | 0.85 (0.58, 1.26) | 0.43 |
HRs associated with metformin or lifestyle compared with placebo are adjusted for age, race/ethnicity, and sex in Cox proportional hazards models for all-cause mortality and in Fine-Gray models accounting for competing risk of other deaths for cause-specific mortality.
The risks of all-cause mortality associated with metformin or lifestyle compared with placebo did not differ by age, sex, race/ethnicity, or BMI (Supplementary Table 1). In sensitivity analyses with adjustment for time-varying out-of-study metformin use in all three randomized groups, the risk of all-cause mortality associated with metformin compared with placebo remained unchanged (HR 0.97 [95% CI 0.77, 1.23] [Supplementary Table 2]). Multivariable models exploring the potential mediating effects of diabetes status and duration, changes in BMI, cumulative glycemic exposure, and cardiovascular risk factors also did not materially change the risk for all-cause mortality associated with lifestyle or metformin compared with placebo (Supplementary Table 2). With use of a marginal structural model to account for metformin discontinuation and adherence and use of out-of-study metformin due to diabetes development and HbA1c, the estimated risk of all-cause mortality associated with randomized metformin compared with placebo appeared lower, but the conclusion remained unchanged (HR 0.78 [95% CI 0.55, 1.11]).
Conclusions
Among DPP participants at high risk for type 2 diabetes at study entry, all-cause mortality did not differ for those randomized to metformin or lifestyle compared with placebo over a median observation time of 21 years. Although metformin and lifestyle were associated with reductions in several risk factors for cardiovascular disease (29,30), these interventions did not lower cardiovascular mortality compared with placebo. Cancer was the leading cause of death in this study population, but neither metformin nor lifestyle reduced the risk of cancer mortality compared with placebo.
To our knowledge there has been a paucity of epidemiologic data on cause-specific mortality in people with prediabetes in the U.S. Prior reports of cause-specific mortality among people with diabetes in the U.S. have described cardiovascular disease as the leading cause of death, albeit declining over time (31,32). This may be due to improved cardiovascular risk factor control among people with and without diabetes in the U.S. between 1988 and 2014 (33). A recent publication found that a decline in vascular disease death rates has now resulted in a predominance of deaths due to cancer among individuals with diabetes in England (34). Our finding of cancer being the leading cause of death in this study population of prediabetes is consistent with this and the Centers for Disease Control and Prevention reports of malignant neoplasms being the leading cause of death for U.S. adults age 45–64 years (35).
This trial is unique in its ability to examine the effect of metformin on all-cause and cause-specific mortality in a study population at high risk for type 2 diabetes. While metformin lessened major risk factors for mortality by lowering weight and reducing the risk of diabetes in DPP and longer follow-up in DPPOS (4,36), it did not reduce the risk of all-cause mortality or deaths due to cancer and cardiovascular disease. Throughout DPP and DPPOS, study participants who developed diabetes in all randomized groups were frequently prescribed metformin as first-line therapy for type 2 diabetes by their health care providers. It is possible that the ability to detect an effect of randomization to metformin was hampered by crossover use of metformin in the other groups or by residual confounding due to unmeasured factors introduced during follow-up. Nevertheless, sensitivity analyses to account for out-of-study metformin use, discontinuation of study metformin, and confounding related to metformin treatment and randomization over time did not show any material difference in the risk for all-cause mortality.
Numerous observational studies have suggested that metformin may help lower the risk of all-cause, cardiovascular, and cancer mortality in patients with type 2 diabetes (6–14). Past observational findings may differ from our results due to potential confounding by indication with patients on metformin being healthier than comparison groups treated with other antidiabetes medications for more advanced diabetes or because of a contraindication to metformin, such as severe renal impairment. Although the observational studies attempted to account for confounding by indication and other biases, there still could also be residual confounding by diabetes duration, severity, and complications since patients on metformin may not have progressed further in their disease to require medication change or intensification. Nevertheless, the findings in this study also differ from more definitive evidence from UKPDS showing the lower risk of all-cause mortality with metformin in participants with new-onset type 2 diabetes (18). In both the observational studies and in UKPDS, the ability to detect an effect of metformin on mortality may have been stronger given a higher underlying mortality risk in the population of adults potentially due to preexisting cardiovascular disease or cancer, which were exclusion criterion at DPP enrollment. For example, crude cancer mortality rates reported among adults with prediabetes enrolled in European observational cohorts (4.64 per 1,000 person-years) were much higher than those in the placebo arm of DPPOS (2.40 per 1,000 person-years), though it is difficult to compare, since mortality rates are not age or sex standardized and most of the European cohorts had a higher mean age and greater proportion of males than our study population (3). Albeit not age or sex standardized, mortality rates were certainly higher in metformin-treated participants in UKPDS after 10.7 years (13.5 per 1,000 person-years) than in those in DPPOS after 21 years (7.13 per 1,000 person-years). It is also possible that the greater glycemic difference that was attained with metformin use in the study population of newly diagnosed diabetes enrolled in the UKPDS explains the survival benefit, whereas in DPPOS, baseline dysglycemia was lower and the reduction in HbA1c achieved with metformin was much less (29). Nevertheless, in this study population of adults at risk for developing type 2 diabetes, metformin does not appear to lower mortality rates.
Obesity and diabetes are conditions that confer a higher risk of all-cause, cardiovascular, and cancer mortality (23,24). Although the lifestyle intervention was more efficacious in decreasing the incidence of diabetes and reducing body weight than placebo or metformin in DPP, it did not result in a lower risk of all-cause, cardiovascular, or cancer mortality for adults at high risk of type 2 diabetes. These findings are similar to reports from the Finnish Diabetes Prevention Study despite the longer median follow-up time in DPPOS (21 vs. 10.6 years) and higher total mortality rate in the DPP lifestyle (7.37/1,000 person-years) and placebo (6.59/1,000 person-years) groups compared with the Finnish Diabetes Prevention Study lifestyle (2.2/1,000 person-years) and control (3.8/1,000 person-years) arms (37). However, these findings differ from mortality outcomes published in two other lifestyle intervention trials. The Malmö Preventive Project found a lower risk of all-cause mortality (relative risk 0.45 [95% CI 0.23, 0.85]) for men with impaired glucose tolerance receiving a prevention program of dietary therapy and physical exercise compared with a standard care control group (38). The variance in results between this study and ours may be due to the longer duration of the lifestyle intervention (6 years) and higher-risk study population (males only) in the Malmö Preventive Project, but it may also be due to the fact that the Malmö Preventive Project was not a randomized study, had participants in the control group who were excluded from the intervention group due to contraindications, and could not control for differences in intensity, quality of care, and cardiovascular disease risk factor management between the intervention and control groups during this pragmatic trial. Regardless, the Da Qing Diabetes Prevention Outcome Study was a randomized clinical trial that reported significant reductions in the risk of all-cause and cardiovascular disease mortality in adults with impaired glucose tolerance associated with their lifestyle intervention at 23 and 30 years’ follow-up (20,21). While the study population enrolled in the Da Qing Diabetes Prevention Outcome Study was younger at baseline with a lower mean BMI compared with the study population enrolled in DPP, they had other risk factors at baseline that may have contributed to mortality, such as a higher proportion of current smokers and higher mean fasting plasma glucose and systolic and diastolic blood pressure (39). In fact, incident diabetes (for diet and exercise: 96 cases of diabetes per 1,000 person-years at 6-year follow-up) and the mortality rate (intervention group: 14.3 deaths per 1,000 person-years at 23-year follow-up) were much higher in the Da Qing Diabetes Prevention Outcome Study than in DPPOS (lifestyle: 59 cases of diabetes per 1,000 person-years at 10-year follow-up and 7.37 deaths per 1,000 person-years at 20-year follow-up). It is also possible that the underlying pathophysiology contributing to mortality in those with impaired glucose tolerance is different in Asians and that diet and exercise changes are more effective in targeting that defect for Asians. The point estimate from subgroup analysis in our study indicates a 32% lower mortality rate with lifestyle in Asian Americans (Supplementary Table 1), but the very wide CI around this estimate includes the null value and reflects the small sample size and number of deaths in this subgroup.
There are several notable strengths to this study. This current trial has an extended follow-up of participants over a long duration with rigorous adjudication of cause-specific mortality from death certificates, medical records, and NDI cause of death codes. Follow-up for mortality was censored at a fixed closing date (31 December 2018) when ascertainment of vital status was complete, as previously recommended (40). This approach avoids potential biases inherent in other censoring schemes, such as censoring each person’s follow-up at last encounter or death date after a fixed closing date.
There are also several limitations to note. While this trial has one of the longest metformin interventions in a population at high risk for type 2 diabetes, the drop-in use of provider-prescribed metformin in all randomized groups when participants developed diabetes may not have been fully controlled for in our sensitivity analyses. Furthermore, there may have been effects of the ethically justified modification of the protocol to offer lifestyle sessions to all participants at the end of DPP. There were also differences in weight, incident diabetes, diabetes duration, and cardiovascular risks over time between the randomized groups, reflecting the positive intervention effects. Multivariable adjustment was performed to account for these differences with no material changes in the findings. The enrolled DPP study population had to meet strict inclusion and exclusion criteria to ensure safety of the trial interventions, so this was a fairly healthy population at enrollment and results may not generalize to sicker study populations with significant cardiovascular disease, active cancer, or other conditions that would limit their immediate life span. Our healthier study population may account for why this study had much lower mortality rates than other studies of metformin use in populations with type 2 diabetes and lifestyle modification in other populations with impaired glucose tolerance. In addition, secular changes in health care demonstrating improved cardiovascular risk factor control during the majority of our study period may have contributed to the lower total and cardiovascular mortality rates in our study compared with prior studies (33). However, it is difficult to make conclusions from comparisons of crude mortality rates due to potential confounding from differences between our study population and other cohorts like age and sex. The lower mortality rates in DPPOS may have limited the precision of our effect estimates, particularly for smaller subgroup analyses. The all-cause mortality HRs of 0.99 and 1.02 for metformin versus placebo and lifestyle versus placebo, respectively, are the single best estimates of effect of these interventions, but the associated 95% CIs indicate that the true effect for the all-cause mortality may range from 0.79 to 1.25 for metformin versus placebo and 0.81 to 1.28 for lifestyle versus placebo (Table 3), consistent with potential modest beneficial or harmful effects of either intervention. CIs were much wider for specific causes of death (Table 3) and subgroups of participants (Supplementary Fig. 2A and B).
In summary, while reductions in weight, cardiovascular risk factors, and incident diabetes were achieved with metformin and lifestyle interventions in DPP, these interventions did not lower the risk of all-cause or cause-specific mortality over 20 years’ follow-up. Because cancer was found to be the leading cause of death among participants at high risk of type 2 diabetes and others have shown that adults with prediabetes have an increased risk for cancer mortality, dedicated research efforts are needed to better understand how to prevent excess morbidity and mortality from cancer in this population.
Article Information
Acknowledgments. The Diabetes Prevention Program Research Group Research Group gratefully acknowledges the commitment and dedication of the participants of DPP and DPPOS.
Funding. Research reported in this publication was supported by the NIDDK of the NIH under award numbers U01 DK048489, U01 DK048339, U01 DK048377, U01 DK048349, U01 DK048381, U01 DK048468, U01 DK048434, U01 DK048485, U01 DK048375, U01 DK048514, U01 DK048437, U01 DK048413, U01 DK048411, U01 DK048406, U01 DK048380, U01 DK048397, U01 DK048412, U01 DK048404, U01 DK048387, U01 DK048407, U01 DK048443, and U01 DK048400, through providing funding during DPP and DPPOS to the clinical centers and the Coordinating Center for the design and conduct of the study, and through collection, management, analysis, and interpretation of the data. Funding was also provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute on Aging, the National Eye Institute, the National Heart, Lung, and Blood Institute, the National Cancer Institute, the Office of Research on Women’s Health, the National Institute on Minority Health and Health Disparities, the Centers for Disease Control and Prevention, and the American Diabetes Association. The Southwestern American Indian Centers were supported directly by the NIDDK, including its Intramural Research Program, and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources, and the Department of Veterans Affairs supported data collection at many of the clinical centers. Merck KGaA provided medication for DPPOS. DPP/DPPOS have also received donated materials, equipment, or medicines for concomitant conditions from Bristol-Myers Squibb, Parke-Davis, and LifeScan, Health o Meter, Hoechst Marion Roussel, Merck-Medco Managed Care, Merck and Co., Nike Sports Marketing, SlimFast, and The Quaker Oats Company. The sponsor of this study was represented on the Steering Committee and played a part in study design, how the study was done, and publication.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. All authors in the writing group had access to all data. The opinions expressed are those of the study group and do not necessarily reflect the views of the funding agencies.
Duality of Interest. K.M.G. received research grants from AstraZeneca and BioKier. McKesson BioServices, Matthews Media Group, and Henry M. Jackson Foundation for the Advancement of Military Medicine provided support services under subcontract with the Coordinating Center. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. C.G.L. and M.T. wrote the manuscript and researched the data. B.H., D.D., K.M.G., P.P., E.J.B., W.C.K., and J.P.C. researched data, edited and reviewed the manuscript, and contributed to discussion. D.E., L.F., X.P., and A.W. reviewed and edited the manuscript. M.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. nos. NCT00004992 and NCT00038727, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.16652587.
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
A complete list of centers, investigators, and staff for the Diabetes Prevention Program Research Group can be found in the supplementary material online.
Contributor Information
Collaborators: Diabetes Prevention Program Research Group:, George A. Bray, Kishore M. Gadde, Iris W. Culbert, Jennifer Arceneaux, Annie Chatellier, Amber Dragg, Catherine M. Champagne, Crystal Duncan, Barbara Eberhardt, Frank Greenway, Fonda G. Guillory, April A. Herbert, Michael L. Jeffirs, Betty M. Kennedy, Erma Levy, Monica Lockett, Jennifer C. Lovejoy, Laura H. Morris, Lee E. Melancon, Donna H. Ryan, Deborah A. Sanford, Kenneth G. Smith, Lisa L. Smith, Julia A. St. Amant, Richard T. Tulley, Paula C. Vicknair, Donald Williamson, Jeffery J. Zachwieja, Kenneth S. Polonsky, Janet Tobian, David A. Ehrmann, Margaret J. Matulik, Karla A. Temple, Bart Clark, Kirsten Czech, Catherine DeSandre, Brittnie Dotson, Ruthanne Hilbrich, Wylie McNabb, Ann R. Semenske, Jose F. Caro, Kevin Furlong, Barry J. Goldstein, Pamela G. Watson, Kellie A. Smith, Jewel Mendoza, Marsha Simmons, Wendi Wildman, Renee Liberoni, John Spandorfer, Constance Pepe, Richard P. Donahue, Ronald B. Goldberg, Ronald Prineas, Jeanette Calles, Anna Giannella, Patricia Rowe, Juliet Sanguily, Paul Cassanova-Romero, Sumaya Castillo-Florez, Hermes J. Florez, Rajesh Garg, Lascelles Kirby, Olga Lara, Carmen Larreal, Valerie McLymont, Jadell Mendez, Arlette Perry, Patrice Saab, Bertha Veciana, Steven M. Haffner, Helen P. Hazuda, Maria G. Montez, Kathy Hattaway, Juan Isaac, Carlos Lorenzo, Arlene Martinez, Monica Salazar, Tatiana Walker, Dana Dabelea, Richard F. Hamman, Patricia V. Nash, Sheila C. Steinke, Lisa Testaverde, Jennifer Truong, Denise R. Anderson, Larry B. Ballonoff, Alexis Bouffard, Brian Bucca, B. Ned Calonge, Lynne Delve, Martha Farago, James O. Hill, Shelley R. Hoyer, Tonya Jenkins, Bonnie T. Jortberg, Dione Lenz, Marsha Miller, Thomas Nilan, Leigh Perreault, David W. Price, Judith G. Regensteiner, Emily B. Schroeder, Helen Seagle, Carissa M. Smith, Brent VanDorsten, Edward S. Horton, Medha Munshi, Kathleen E. Lawton, Sharon D. Jackson, Catherine S. Poirier, Kati Swift, Ronald A. Arky, Marybeth Bryant, Jacqueline P. Burke, Enrique Caballero, Karen M. Callaphan, Barbara Fargnoli, Therese Franklin, Om P. Ganda, Ashley Guidi, Mathew Guido, Alan M. Jacobsen, Lyn M. Kula, Margaret Kocal, Lori Lambert, Kathleen E. Lawton, Sarah Ledbury, Maureen A. Malloy, Roeland J.W. Middelbeek, Maryanne Nicosia, Cathryn F. Oldmixon, Jocelyn Pan, Marizel Quitingon, Riley Rainville, Stacy Rubtchinsky, Ellen W. Seely, Jessica Sansoucy, Dana Schweizer, Donald Simonson, Fannie Smith, Caren G. Solomon, Jeanne Spellman, James Warram, Steven E. Kahn, Basma Fattaleh, Brenda K. Montgomery, Celeste Colegrove, Wilfred Fujimoto, Robert H. Knopp, Edward W. Lipkin, Michelle Marr, Ivy Morgan-Taggart, Anne Murillo, Kayla O’Neal, Dace Trence, Lonnese Taylor, April Thomas, Elaine C. Tsai, Samuel Dagogo-Jack, Abbas E. Kitabchi, Mary E. Murphy, Laura Taylor, Jennifer Dolgoff, William B. Applegate, Michael Bryer-Ash, Debra Clark, Sandra L. Frieson, Uzoma Ibebuogu, Raed Imseis, Helen Lambeth, Lynne C. Lichtermann, Hooman Oktaei, Harriet Ricks, Lily M.K. Rutledge, Amy R. Sherman, Clara M. Smith, Judith E. Soberman, Beverly Williams-Cleaves, Avnisha Patel, Ebenezer A. Nyenwe, Ethel Faye Hampton, Boyd E. Metzger, Mark E. Molitch, Mariana K. Johnson, Daphne T. Adelman, Catherine Behrends, Michelle Cook, Marian Fitzgibbon, Mimi M. Giles, Deloris Heard, Cheryl K.H. Johnson, Diane Larsen, Anne Lowe, Megan Lyman, David McPherson, Samsam C. Penn, Thomas Pitts, Renee Reinhart, Susan Roston, Pamela A. Schinleber, Amisha Wallia, David M. Nathan, Charles McKitrick, Heather Turgeon, Mary Larkin, Marielle Mugford, Kathy Abbott, Ellen Anderson, Laurie Bissett, Kristy Bondi, Enrico Cagliero, Jose C. Florez, Linda Delahanty, Valerie Goldman, Elaine Grassa, Lindsery Gurry, Kali D’Anna, Fernelle Leandre, Peter Lou, Alexandra Poulos, Elyse Raymond, Valerie Ripley, Christine Stevens, Beverly Tseng, Jerrold M. Olefsky, Elizabeth Barrett-Connor, Sunder Mudaliar, Maria Rosario Araneta, Mary Lou Carrion-Petersen, Karen Vejvoda, Sarah Bassiouni, Madeline Beltran, Lauren N. Claravall, Jonalle M. Dowden, Steven V. Edelman, Pranav Garimella, Robert R. Henry, Javiva Horne, Marycie Lamkin, Simona Szerdi Janesch, Diana Leos, William Polonsky, Rosa Ruiz, Jean Smith, Jennifer Torio-Hurley, F. Xavier Pi-Sunyer, Blandine Laferrere, Jane E. Lee, Susan Hagamen, David B. Allison, Nnenna Agharanya, Nancy J. Aronoff, Maria Baldo, Jill P. Crandall, Sandra T. Foo, Kim Kelly-Dinham, Jose A. Luchsinger, Carmen Pal, Kathy Parkes, Mary Beth Pena, Ellen S. Rooney, Gretchen E.H. Van Wye, Kristine A. Viscovich, Mary de Groot, David G. Marrero, Kieren J. Mather, Melvin J. Prince, Susie M. Kelly, Marcia A. Jackson, Gina McAtee, Paula Putenney, Ronald T. Ackermann, Carolyn M. Cantrell, Yolanda F. Dotson, Edwin S. Fineberg, Megan Fultz, John C. Guare, Angela Hadden, James M. Ignaut, Marion S. Kirkman, Erin O’Kelly Phillips, Kisha L. Pinner, Beverly D. Porter, Paris J. Roach, Nancy D. Rowland, Madelyn L. Wheeler, Vanita Aroda, Michelle Magee, Robert E. Ratner, Gretchen Youssef, Sue Shapiro, Natalie Andon, Catherine Bavido-Arrage, Geraldine Boggs, Marjorie Bronsord, Ernestine Brown, Holly Love Burkott, Wayman W. Cheatham, Susan Cola, Cindy Evans, Peggy Gibbs, Tracy Kellum, Lilia Leon, Milvia Lagarda, Claresa Levatan, Milajurine Lindsay, Asha K. Nair, Jean Park, Maureen Passaro, Angela Silverman, Gabriel Uwaifo, Debra Wells-Thayer, Renee Wiggins, Mohammed F. Saad, Karol Watson, Maria Budget, Sujata Jinagouda, Medhat Botrous, Anthony Sosa, Sameh Tadros, Khan Akbar, Claudia Conzues, Perpetua Magpuri, Kathy Ngo, Amer Rassam, Debra Waters, Kathy Xapthalamous, Julio V. Santiago, Samuel Dagogo-Jack, Neil H. White, Angela L. Brown, Samia Das, Prajakta Khare-Ranade, Tamara Stich, Ana Santiago, Edwin Fisher, Emma Hurt, Tracy Jones, Michelle Kerr, Lucy Ryder, Cormarie Wernimont, Sherita Hill Golden, Christopher D. Saudek, Vanessa Bradley, Emily Sullivan, Tracy Whittington, Caroline Abbas, Adrienne Allen, Frederick L. Brancati, Sharon Cappelli, Jeanne M. Clark, Jeanne B. Charleston, Janice Freel, Katherine Horak, Alicia Greene, Dawn Jiggetts, Deloris Johnson, Hope Joseph, Kimberly Loman, Nestoras Mathioudakis, Henry Mosley, John Reusing, Richard R. Rubin, Alafia Samuels, Thomas Shields, Shawne Stephens, Kerry J. Stewart, LeeLana Thomas, Evonne Utsey, Paula Williamson, David S. Schade, Karwyn S. Adams, Janene L. Canady, Carolyn Johannes, Claire Hemphill, Penny Hyde, Leslie F. Atler, Patrick J. Boyle, Mark R. Burge, Lisa Chai, Kathleen Colleran, Ateka Fondino, Ysela Gonzales, Doris A. Hernandez-McGinnis, Patricia Katz, Carolyn King, Julia Middendorf, Amer Rassam, Sofya Rubinchik, Willette Senter, Debra Waters, Jill Crandall, Harry Shamoon, Janet O. Brown, Gilda Trandafirescu, Danielle Powell, Elsie Adorno, Liane Cox, Helena Duffy, Samuel Engel, Allison Friedler, Angela Goldstein, Crystal J. Howard-Century, Jennifer Lukin, Stacey Kloiber, Nadege Longchamp, Helen Martinez, Dorothy Pompi, Jonathan Scheindlin, Elissa Violino, Elizabeth A. Walker, Judith Wylie-Rosett, Elise Zimmerman, Joel Zonszein, Trevor Orchard, Elizabeth Venditti, Rena R. Wing, Susan Jeffries, Gaye Koenning, M. Kaye Kramer, Marie Smith, Susan Barr, Catherine Benchoff, Miriam Boraz, Lisa Clifford, Rebecca Culyba, Marlene Frazier, Ryan Gilligan, Stephanie Guimond, Susan Harrier, Louann Harris, Andrea Kriska, Qurashia Manjoo, Monica Mullen, Alicia Noel, Amy Otto, Jessica Pettigrew, Bonny Rockette-Wagner, Debra Rubinstein, Linda Semler, Cheryl F. Smith, Valarie Weinzierl, Katherine V. Williams, Tara Wilson, Marjorie K. Mau, Narleen K. Baker-Ladao, John S. Melish, Richard F. Arakaki, Renee W. Latimer, Mae K. Isonaga, Ralph Beddow, Nina E. Bermudez, Lorna Dias, Jillian Inouye, Kathy Mikami, Pharis Mohideen, Sharon K. Odom, Raynette U. Perry, Robin E. Yamamoto, William C. Knowler, Harelda Anderson, Norman Cooeyate, Charlotte Dodge, Mary A. Hoskin, Carol A. Percy, Alvera Enote, Camille Natewa, Kelly J. Acton, Vickie L. Andre, Rosalyn Barber, Shandiin Begay, Peter H. Bennett, Mary Beth Benson, Evelyn C. Bird, Brenda A. Broussard, Brian C. Bucca, Marcella Chavez, Sherron Cook, Jeff Curtis, Matthew S. Doughty, Roberta Duncan, Cyndy Edgerton, Jacqueline M. Ghahate, Justin Glass, Martia Glass, Dorothy Gohdes, Wendy Grant, Robert L. Hanson, Ellie Horse, Louise E. Ingraham, Merry Jackson, Priscilla Jay, Roylen S. Kaskalla, Karen Kavena, David Kessler, Kathleen M. Kobus, Jonathan Krakoff, Jason Kurland, Catherine Manus, Cherie McCabe, Sara Michaels, Tina Morgan, Yolanda Nashboo, Julie A. Nelson, Steven Poirier, Evette Polczynski, Christopher Piromalli, Mike Reidy, Jeanine Roumain, Debra Rowse, Robert J. Roy, Sandra Sangster, Janet Sewenemewa, Miranda Smart, Chelsea Spencer, Darryl Tonemah, Rachel Williams, Charlton Wilson, Michelle Yazzie, Raymond Bain, Sarah Fowler, Marinella Temprosa, Michael D. Larsen, Tina Brenneman, Sharon L. Edelstein, Solome Abebe, Julie Bamdad, Melanie Barkalow, Joel Bethepu, Tsedenia Bezabeh, Anna Bowers, Nicole Butler, Jackie Callaghan, Caitlin E. Carter, Costas Christophi, Gregory M. Dwyer, Mary Foulkes, Yuping Gao, Robert Gooding, Adrienne Gottlieb, Kristina L. Grimes, Nisha Grover-Fairchild, Lori Haffner, Heather Hoffman, Kathleen Jablonski, Steve Jones, Tara L. Jones, Richard Katz, Preethy Kolinjivadi, John M. Lachin, Yong Ma, Pamela Mucik, Robert Orlosky, Qing Pan, Susan Reamer, James Rochon, Alla Sapozhnikova, Hanna Sherif, Charlotte Stimpson, Ashley Hogan Tjaden, Fredricka Walker-Murray, Elizabeth M. Venditti, Andrea M. Kriska, Linda Semler, Valerie Weinzierl, Santica Marcovina, F. Alan Aldrich, Jessica Harting, John Albers, Greg Strylewicz, R. Eastman, Judith Fradkin, Sanford Garfield, Christine Lee, Edward Gregg, Ping Zhang, Dan O’Leary, Gregory Evans, Matthew Budoff, Chris Dailing, Elizabeth Stamm, Ann Schwartz, Caroline Navy, Lisa Palermo, Pentti Rautaharju, Ronald J. Prineas, Teresa Alexander, Charles Campbell, Sharon Hall, Yabing Li, Margaret Mills, Nancy Pemberton, Farida Rautaharju, Zhuming Zhang, Elsayed Z. Soliman, Julie Hu, Susan Hensley, Lisa Keasler, Tonya Taylor, Barbara Blodi, Ronald Danis, Matthew Davis, Larry Hubbard, Ryan Endres, Deborah Elsas, Samantha Johnson, Dawn Myers, Nancy Barrett, Heather Baumhauer, Wendy Benz, Holly Cohn, Ellie Corkery, Kristi Dohm, Amitha Domalpally, Vonnie Gama, Anne Goulding, Andy Ewen, Cynthia Hurtenbach, Daniel Lawrence, Kyle McDaniel, Jeong Pak, James Reimers, Ruth Shaw, Maria Swift, Pamela Vargo, Sheila Watson, Jose A. Luchsinger, Jennifer Manly, Elizabeth Mayer-Davis, Robert R. Moran, Ted Ganiats, Kristin David, Andrew J. Sarkin, Erik Groessl, Naomi Katzir, Helen Chong, William H. Herman, Michael Brändle, Morton B. Brown, Jose C. Florez, David Altshuler, Liana K. Billings, Ling Chen, Maegan Harden, Robert L. Hanson, William C. Knowler, Toni I. Pollin, Alan R. Shuldiner, Kathleen Jablonski, Paul W. Franks, and Marie-France Hivert
References
- 1. Huang Y, Cai X, Chen P, et al. Associations of prediabetes with all-cause and cardiovascular mortality: a meta-analysis. Ann Med 2014;46:684–692 [DOI] [PubMed] [Google Scholar]
- 2. Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ 2016;355:i5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou XH, Qiao Q, Zethelius B, et al.; DECODE Study Group . Diabetes, prediabetes and cancer mortality. Diabetologia 2010;53:1867–1876 [DOI] [PubMed] [Google Scholar]
- 4. Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing Res Rev 2012;11:390–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnson JA, Majumdar SR, Simpson SH, Toth EL. Decreased mortality associated with the use of metformin compared with sulfonylurea monotherapy in type 2 diabetes. Diabetes Care 2002;25:2244–2248 [DOI] [PubMed] [Google Scholar]
- 7. Johnson JA, Simpson SH, Toth EL, Majumdar SR. Reduced cardiovascular morbidity and mortality associated with metformin use in subjects with Type 2 diabetes. Diabet Med 2005;22:497–502 [DOI] [PubMed] [Google Scholar]
- 8. Campbell JM, Bellman SM, Stephenson MD, Lisy K. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: a systematic review and meta-analysis. Ageing Res Rev 2017;40:31–44 [DOI] [PubMed] [Google Scholar]
- 9. Bergmark BA, Bhatt DL, McGuire DK, et al.; SAVOR-TIMI 53 Steering Committee and Investigators . Metformin use and clinical outcomes among patients with diabetes mellitus with or without heart failure or kidney dysfunction: observations from the SAVOR-TIMI 53 trial. Circulation 2019;140:1004–1014 [DOI] [PubMed] [Google Scholar]
- 10. Vistisen D, Witte DR, Brunner EJ, et al. Risk of cardiovascular disease and death in individuals with prediabetes defined by different criteria: the Whitehall II study. Diabetes Care 2018;41:899–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gandini S, Puntoni M, Heckman-Stoddard BM, et al. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res (Phila) 2014;7:867–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Landman GW, Kleefstra N, van Hateren KJ, Groenier KH, Gans RO, Bilo HJ. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care 2010;33:322–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Han Y, Xie H, Liu Y, Gao P, Yang X, Shen Z. Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: a systematic review and an updated meta-analysis. Cardiovasc Diabetol 2019;18:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cao X, Wu Y, Wang J, Liu K, Wang X. The effect of metformin on mortality among diabetic cancer patients: a systematic review and meta-analysis. JNCI Cancer Spectr 2017;1:pkx007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williamson DF, Thompson TJ, Thun M, Flanders D, Pamuk E, Byers T. Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care 2000;23:1499–1504 [DOI] [PubMed] [Google Scholar]
- 16. Gregg EW, Gerzoff RB, Thompson TJ, Williamson DF. Intentional weight loss and death in overweight and obese U.S. adults 35 years of age and older. Ann Intern Med 2003;138:383–389 [DOI] [PubMed] [Google Scholar]
- 17. Murphy RA, Patel KV, Kritchevsky SB, et al. Weight change, body composition, and risk of mobility disability and mortality in older adults: a population-based cohort study. J Am Geriatr Soc 2014;62:1476–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. UK Prospective Diabetes Study (UKPDS) Group . Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 19. Kritchevsky SB, Beavers KM, Miller ME, et al. Intentional weight loss and all-cause mortality: a meta-analysis of randomized clinical trials. PLoS One 2015;10:e0121993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li G, Zhang P, Wang J, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol 2014;2:474–480 [DOI] [PubMed] [Google Scholar]
- 21. Gong Q, Zhang P, Wang J, et al.; Da Qing Diabetes Prevention Study Group . Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol 2019;7:452–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang Y, Cai X, Qiu M, et al. Prediabetes and the risk of cancer: a meta-analysis. Diabetologia 2014;57:2261–2269 [DOI] [PubMed] [Google Scholar]
- 23. Di Angelantonio E, Bhupathiraju ShN, Wormser D, et al.; Global BMI Mortality Collaboration . Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016;388:776–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rao Kondapally Seshasai S, Kaptoge S, Thompson A, et al.; Emerging Risk Factors Collaboration . Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rubin RR, Fujimoto WY, Marrero DG, et al.; DPP Research Group . The Diabetes Prevention Program: recruitment methods and results. Control Clin Trials 2002;23:157–171 [DOI] [PubMed] [Google Scholar]
- 26. The Diabetes Prevention Program . The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 1999;22:623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Diabetes Prevention Program Research Group . Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care 2012;35:731–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grafféo N, Latouche A, Le Tourneau C, Chevret S. ipcwswitch: an R package for inverse probability of censoring weighting with an application to switches in clinical trials. Comput Biol Med 2019;111:103339. [DOI] [PubMed] [Google Scholar]
- 29. Orchard TJ, Temprosa M, Barrett-Connor E, et al.; Diabetes Prevention Program Outcomes Study Research Group . Long-term effects of the Diabetes Prevention Program interventions on cardiovascular risk factors: a report from the DPP Outcomes Study. Diabet Med 2013;30:46–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Knowler WC, Fowler SE, Hamman RF, et al.; Diabetes Prevention Program Research Group . 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gregg EW, Cheng YJ, Srinivasan M, et al. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet 2018;391:2430–2440 [DOI] [PubMed] [Google Scholar]
- 32. Kim D, Li AA, Cholankeril G, et al. Trends in overall, cardiovascular and cancer-related mortality among individuals with diabetes reported on death certificates in the United States between 2007 and 2017. Diabetologia 2019;62:1185–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sun X, Du T. Trends in cardiovascular risk factors among U.S. men and women with and without diabetes, 1988-2014. BMC Public Health 2017;17:893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pearson-Stuttard J, Bennett J, Cheng YJ, et al. Trends in predominant causes of death in individuals with and without diabetes in England from 2001 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol 2021;9:165–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Data and Statistics (WISQARS) , 2018. Accessed 13 October 2020. Available from https://www.cdc.gov/injury/wisqars
- 36. Diabetes Prevention Program Research Group . Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol 2015;3:866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Uusitupa M, Peltonen M, Lindström J, et al.; Finnish Diabetes Prevention Study Group . Ten-year mortality and cardiovascular morbidity in the Finnish Diabetes Prevention Study--secondary analysis of the randomized trial. PLoS One 2009;4:e5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eriksson KF, Lindgärde F. No excess 12-year mortality in men with impaired glucose tolerance who participated in the Malmö Preventive Trial with diet and exercise. Diabetologia 1998;41:1010–1016 [DOI] [PubMed] [Google Scholar]
- 39. Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 40. Lesko CR, Edwards JK, Cole SR, Moore RD, Lau B. When to censor? Am J Epidemiol 2018;187:623–632 [DOI] [PMC free article] [PubMed] [Google Scholar]