Abstract
OBJECTIVE
Sulfonylureas, the first available drugs for the management of type 2 diabetes, remain widely prescribed today. However, there exists significant variability in glycemic response to treatment. We aimed to establish heritability of sulfonylurea response and identify genetic variants and interacting treatments associated with HbA1c reduction.
RESEARCH DESIGN AND METHODS
As an initiative of the Metformin Genetics Plus Consortium (MetGen Plus) and the DIabetes REsearCh on patient straTification (DIRECT) consortium, 5,485 White Europeans with type 2 diabetes treated with sulfonylureas were recruited from six referral centers in Europe and North America. We first estimated heritability using the generalized restricted maximum likelihood approach and then undertook genome-wide association studies of glycemic response to sulfonylureas measured as HbA1c reduction after 12 months of therapy followed by meta-analysis. These results were supported by acute glipizide challenge in humans who were naïve to type 2 diabetes medications, cis expression quantitative trait loci (eQTL), and functional validation in cellular models. Finally, we examined for possible drug-drug-gene interactions.
RESULTS
After establishing that sulfonylurea response is heritable (mean ± SEM 37 ± 11%), we identified two independent loci near the GXYLT1 and SLCO1B1 genes associated with HbA1c reduction at a genome-wide scale (P < 5 × 10−8). The C allele at rs1234032, near GXYLT1, was associated with 0.14% (1.5 mmol/mol), P = 2.39 × 10−8), lower reduction in HbA1c. Similarly, the C allele was associated with higher glucose trough levels (β = 1.61, P = 0.005) in healthy volunteers in the SUGAR-MGH given glipizide (N = 857). In 3,029 human whole blood samples, the C allele is a cis eQTL for increased expression of GXYLT1 (β = 0.21, P = 2.04 × 10−58). The C allele of rs10770791, in an intronic region of SLCO1B1, was associated with 0.11% (1.2 mmol/mol) greater reduction in HbA1c (P = 4.80 × 10−8). In 1,183 human liver samples, the C allele at rs10770791 is a cis eQTL for reduced SLCO1B1 expression (P = 1.61 × 10−7), which, together with functional studies in cells expressing SLCO1B1, supports a key role for hepatic SLCO1B1 (encoding OATP1B1) in regulation of sulfonylurea transport. Further, a significant interaction between statin use and SLCO1B1 genotype was observed (P = 0.001). In statin nonusers, C allele homozygotes at rs10770791 had a large absolute reduction in HbA1c (0.48 ± 0.12% [5.2 ± 1.26 mmol/mol]), equivalent to that associated with initiation of a dipeptidyl peptidase 4 inhibitor.
CONCLUSIONS
We have identified clinically important genetic effects at genome-wide levels of significance, and important drug-drug-gene interactions, which include commonly prescribed statins. With increasing availability of genetic data embedded in clinical records these findings will be important in prescribing glucose-lowering drugs.
Introduction
Sulfonylureas are potent glucose-lowering drugs that reduce HbA1c by an average of 1.5% (18 mmol/mol) (1). Despite an increasing trend to use more modern, expensive treatments, sulfonylureas remain commonly prescribed in the U.K., making up 27% of new prescriptions, second only to metformin (2). Due to their very low cost, they are extensively used in low- and middle-income countries. However, considerable variation exists in response to sulfonylureas, with 10–20% of people with diabetes not responding at initiation of sulfonylurea therapy and 30–35% failing to respond to monotherapy after 5 years (3,4). It is likely that a combination of genetic and nongenetic modifying factors underlies the clinical variability of glycemic response to sulfonylureas. While many clinical risk factors such as baseline HbA1c, sex, duration of diabetes, and dose are associated with glycemic response to sulfonylureas (5–7), modulatory genetic factors remain largely unexplored, with the exception of a few proof of concept studies with use of a candidate gene approach (8–12).
Glycemic response to metformin is heritable, with 34% of the variance in response explainable by common genetic variants (13–15). There have been no similar estimates for sulfonylurea response, and to date, no genome-wide association studies (GWAS) of glycemic response to sulfonylurea treatment have been reported, so the genetic contribution to how patients respond to sulfonylureas and clinical implication of this genetic variation have not been systematically studied. As an initiative of the Metformin Genetics Plus Consortium (MetGen Plus) and the DIabetes REsearCh on patient straTification (DIRECT) consortium, we report here the first genome-wide meta-analysis of glycemic response to sulfonylureas, measured as HbA1c reduction after 12 months of therapy. Based on these findings we then explore the impact of interacting drugs and identify clinically important genotype-dependent statin-sulfonylurea interactions for this important class of diabetes therapies.
Research Design and Methods
List of abbreviations used throughout this article and their corresponding explanations are shown in Supplementary Table 1.
Study Design and Participants
We established an international consortium allowing recruitment of 5,485 unrelated individuals of European ancestry from six referral centers in Europe and North America as part of MetGen Plus and the DIRECT consortium (Supplementary Table 2). Included participants had a clinical diagnosis of type 2 diabetes and were treated with sulfonylureas as monotherapy or as an add-on to metformin. This study was approved by respective research ethics review boards, and participants provided written informed consent.
Sample Ascertainment
Clinical, prescription, and biochemical data were retrieved from the electronic medical record systems. Participants with type 2 diabetes aged >35 years at diagnosis who used sulfonylureas with no history of insulin use were identified. They were stably treated with sulfonylureas for at least 6 months with no other glucose-lowering drug started or stopped within the study period. The baseline HbA1c was between 7% (53.0 mmol/mol) and 14% (129.5 mmol/mol) at sulfonylurea initiation.
Measurement of Glycemic Response and Definition of Variables
Participants’ glycemic response to sulfonylurea was modeled as the quantitative phenotype of HbA1c reduction between baseline HbA1c and treatment HbA1c while the patients were maintained on stable treatment. Baseline HbA1c was defined as the HbA1c measure closest to sulfonylurea initiation and within 6 months before and 7 days after this date. The treatment HbA1c was the HbA1c measure closest to 12 months after initiation of sulfonylureas (between 6 and 15 months).
In all the studies, covariates were selected based on previous reports and univariate association between the outcome variable (HbA1c reduction) and explanatory variables. The best fit linear regression model was determined using stepwise backward elimination. Accordingly, baseline HbA1c, sex, age at diagnosis, baseline BMI, average daily dose, time between baseline HbA1c and treatment HbA1c, and drug group (sulfonylurea monotherapy or sulfonylurea added to metformin) were considered in the final model as available in each cohort (Supplementary Table 3). Average daily dose was calculated as the mean daily dose of prescriptions filled during the study period (mean of percentage of each sulfonylurea divided by maximum prescribable according to the British National Formulary). Baseline weight was the measure nearest to the sulfonylurea start date (index date) and within 180 days on either side of the index date. Each study was adjusted for the top n principal components (PCs) to account for 80–90% of the variation in population structure.
The final response model was as follows: HbA1c reduction ∼ baseline HbA1c + PCs + study-specific covariates.
Genome-Wide Array Genotyping, Quality Control, and Imputation
For each respective cohort genome-wide genotyping was performed on a variety of arrays as illustrated in Supplementary Table 3. Genotyping and quality control procedures for the Genetics of Diabetes Audit and Research in Tayside Scotland (GoDARTS), Hoorn Diabetes Care System (DCS), and Pharmacogenomics of Metformin (PMET) cohorts have previously been described (13,15,16). Genotyping data for each platform were individually cleaned by each study center. Standard postgenotyping quality control procedures were applied to each data set (Supplementary Fig. 1). Monomorphic single nucleotide polymorphisms (SNPs) with minor allele frequency (MAF) <1%, call rate <98%, or Hardy-Weinberg equilibrium <10−6 were removed. Samples with genotyping calls <98% or heterozygosity >3 SDs from the mean or correlated with another sample (identity by descent >0.125) were filtered out. All genetic variants were mapped to and reported with Genome Reference Consortium Human genome build 37 (GRCh37). Each data set was then imputed to the 1000 Genomes CEU reference panel (phase 1, version 3) with IMPUTE software (17), except PMET2 and Geisinger where imputation was performed with the HRC.r1-1 EUR reference genome (GRCh37 build) using the Michigan server. Postimputation, SNPs with poor imputation quality (INFO < 0.6), monomorphic variants, or MAF <5% were excluded (Supplementary Fig. 1).
Genome-Wide Association Analysis
Following imputation, GWAS was conducted for each respective cohort under an additive genetic model for assessment of the role of common variants (MAF ≥5%) in glycemic response to sulfonylureas. Each SNP was tested for association with quantitative measure of sulfonylurea-related HbA1c reduction with SNPTEST v2.536 (18) using multiple linear regression correcting for baseline HbA1c, genotypic PCs, and other study-specific variables (Supplementary Table 3). Genome-wide association analyses were carried out separately by respective study centers. Prior to meta-analysis, we performed post-GWAS harmonization and quality control of GWAS results from each cohort to track possible errors in the study-specific analyses. We used the standard protocol accompanied by the EasyQC R package (19). Specifically, we removed SNPs with MAF <5%, low imputation quality (<0.6), large absolute values of β-coefficients and SEs (≥10), low call rate (<0.98), and deviations from Hardy-Weinberg equilibrium (P < 10−6). Meta-analysis was then performed with use of an inverse variance–weighted fixed-effects model, implemented in GWAMA v2.1.34 (20). Post–meta-analysis, SNPs with MAF <5%, available in fewer than six studies, with large absolute values of β-coefficients and SEs (≥10) were excluded (Supplementary Fig. 1). Heterogeneity was assessed with the I2 metric from the complete study-level meta-analysis. Between-study heterogeneity was tested with the Cochran Q statistic and considered significant at P < 0.1. We used the commonly accepted threshold of 5.0 × 10−8 for joint P values to determine statistical significance. Nominal significance was considered to be P < 0.05. The CMplot package (21) in R was used to generate Manhattan and quantile-quantile plots. Regional plots around genome-wide or suggestive genes were visualized using LocusZoom (22). The final meta-analysis included 5,385,635 common autosomal SNPs from 5,485 independent individuals of European ancestors treated with sulfonylureas (λ = 1.008) (Supplementary Fig. 2).
Common Variant Heritability
We used the generalized restricted maximum likelihood approach under the LDAK assumptions using SumHer v5.1 (23) to estimate how much of the variance in HbA1c reduction after sulfonylurea treatment could be attributed to common genetic variants (SNP-based heritability [h2 SNP]). This method is a valid approach for estimating heritability in studies in which acquisition of data of family members with the same diagnosis who have received the same medication and were assessed with use of the same treatment outcome is not feasible. In addition, SumHer uses GWAS summary without requiring individual-level data (23). Therefore, we estimated the SNP heritability using summary statistics from the meta-GWAS. To avoid the impact of extreme linkage disequilibrium (LD) regions and disproportionately large effect size SNPs on heritability estimates, we exclude SNPs within the MHC (chromosome 6: 25–34 Mb) and SNPs that individually explain >1% of phenotypic variation and SNPs in LD with these (within 1 cM).
Conditional Analysis
Given rs10770791 is in partial LD with previously established nonsynonymous variants, rs4149056 (*5; V174A, D′ = 1; r2 = 0.17) and rs2306283 (*1B; N130D, D′ = 0.98; r2 = 0.63), we performed conditional analysis by including these SNPs in the model together. This analysis was carried out with individual-level data from the GoDARTS and PMET cohorts (65% of the total population) and baseline HbA1c, PCs, and other study-specific covariates.
Biochemical Response to Glipizide
To test weather meta-GWAS–identified genetic variants are associated with trough glucose levels, we performed a lookup using data from the Study to Understand the Genetics of the Acute Response to Metformin and Glipizide in Humans (SUGAR-MGH). SUGAR-MGH enrolled 1,000 participants at risk for antidiabetes therapy in the future or individuals with lifestyle-controlled type 2 diabetes naïve to treatment. Participants received a single dose of 5 mg glipizide followed by measurement of glucose and insulin levels at 30, 60, 90, 120, 180, and 240 min. This was used to construct phenotypes of acute glipizide response. The association of rs1234032 and rs10770791 with glipizide response was assessed with linear regression with baseline glucose, age, sex, and the first 10 PCs as a covariate (see Supplementary Notes).
Drug-Drug-Gene Interaction Analysis
Given we have identified a genetic variant in the SLCO1B1 (a gene encoding hepatic transporter of statins) associated with glycemic response to sulfonylureas, we checked for interaction between SLCO1B1 rs10770791 and statin use in a drug-drug-gene interaction model using linear regression, with HbA1c reduction as the dependent variable. This analysis was performed with use of individual-level data from the GoDARTS and PMET cohorts where we have access to prescription data.
Statin-treated case subjects were recipients of sulfonylureas who were also prescribed statins for at least the 3 months prior to the measurement of treatment HbA1c. Statin untreated control subjects were those recipients of sulfonylureas who did not receive a statin prescription for at least 1 year prior to measurement of the treatment HbA1c.
Expression Quantitative Trait Locus Lookups
Expression quantitative trait loci (eQTL) analysis seeks to identify genetic variants that affect the expression of one or more genes: a gene-SNP pair for which the expression of the gene is associated with the allelic configuration of the SNP is referred to as an eQTL. eQTL lookups were performed in human liver and whole blood samples for rs10770791 and rs1234032, respectively. Additional lookups were performed using publicly available data from the Genotype-Tissue Expression (GTEx) consortium.
The human liver eQTL lookups were carried out using data from a previous study performed by the group of F.I. (24). In brief, this eQTL study was performed with 1,183 liver samples, combined from four data sets (24). We looked up the top associated SNP, rs10770791, from this study, as it is in the SLCO1B1 and SLCO1B3 region, which are genes that are abundantly expressed in the liver.
The human whole blood eQTL lookup was performed with use of data from the DIRECT consortium in a total of 3, 029 subjects at high risk of developing type 2 diabetes or with recently diagnosed type 2 diabetes (25). A detailed explanation of the eQTL analysis has previously been published (26), and summary statistics are available (DOI: 10.5281/zenodo.4475681).
Cell Culture and In Vitro Transport and Inhibition Studies
Human embryonic kidney (HEK)-293 Flp-In cells stably expressing empty vector (EV), OATP1B1, and OATP1B3, were used for performance of in vitro transport and inhibition studies to establish the potency of inhibitors as IC50 (i.e., concentration of inhibitor required to inhibit 50% uptake of a particular OATP1B1 and OATP1B3 substrate). Stably transfected HEK-293 Flp-In cells were maintained in DMEM H-21 supplemented with 10% FBS, 100 units/mL penicillin, 100 units/mL streptomycin, and 500 µg/mL geneticin. For transport studies, 150,000 cells/well were seeded the day before the experiment on a poly-d-lysine–coated 48-well plate. After 16–24 h, media were removed and cells were incubated at 37°C for 5–10 min in 0.5 mL Hanks’ balanced salt solution (HBSS) (Thermo Fisher Scientific). Uptake studies were initiated after removal of 0.3 mL of the HBSS above and addition of 0.15 mL HBSS containing a trace amount of 3H-glyburide (NET1024250UC; PerkinElmer), 3H-glipizide (MT1855; Moravek), or 3H-esterone sulfate (as positive control, NET203250UC; PerkinElmer). After 5 min, radioactive substrates were removed and washed twice with 1 mL ice-cold HBSS. For inhibition studies, the same methods above were used, where 3H-glyburide was used as substrate and various concentrations of atorvastatin (Cayman Chemical) or simvastatin (Cayman Chemical) were added together with 3H-glyburide. For comparison of the uptake of 3H-glyburide and 3H-glipizide in OATP1B1 reference and OATP1B1-174A (*5)–expressing cells, studies were performed using stable and transiently transfected cells. The stable and transient experiments were carried out with HEK-293 Flp-In cell lines expressing EV, OATP1B1 reference, and OATP1B1-174A (*5), previously established by our group (27). These cell lines were used to determine the uptake of 3H-glyburide, 3H-glipizide, and 3H-esterone sulfate (as positive control). In brief, each well was transfected with 200 ng DNA vector with 0.4 µL Lipofectamine LTX transfection reagent (Thermo Fisher Scientific) in a 48-well poly-d-lysine–coated plate. Uptake studies were then performed after 48 h with the methods described above and in triplicate wells.
Data and Resource Availability
Summary-level data that underlie the results reported in this article are available upon request to the corresponding author.
Results
Glycemic Response to Sulfonylureas Is Heritable
The SNP heritability estimate (h2) for a model-adjusted absolute reduction in HbA1c was mean ± SEM 37 ± 11%, comparable with our previous estimate for metformin (h2 = 34%) (14). This suggests that approximately one-third of the total variance of glycemic response to sulfonylureas is due to the additive effects of common variants.
GWAS Identifies Two Variants Associated With Altered Glycemic Response to Sulfonylureas
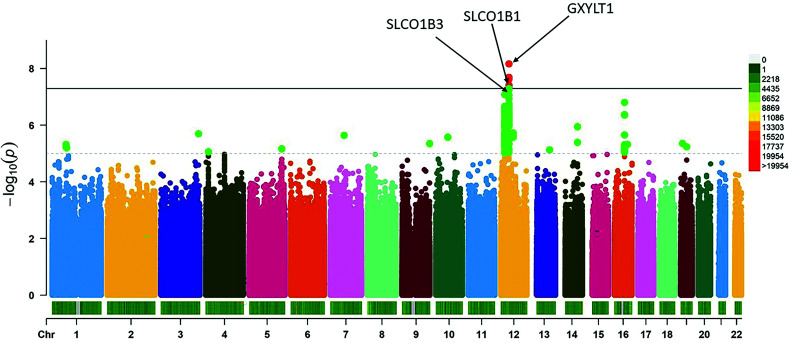
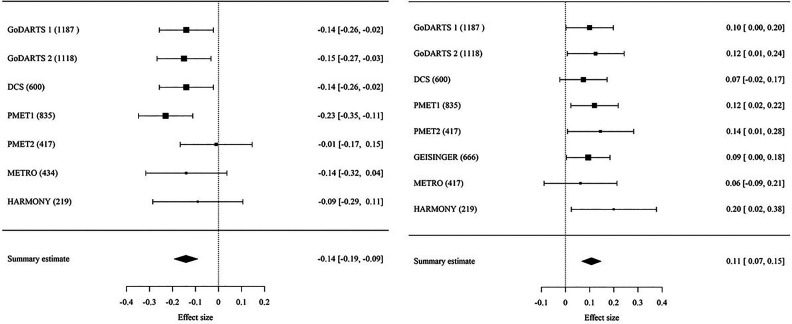
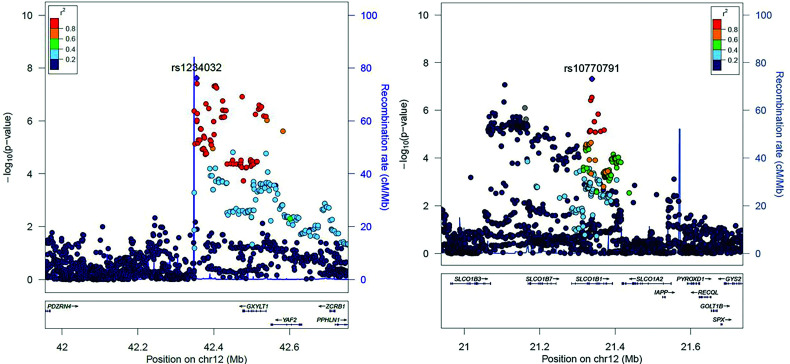
Meta-GWAS identified two genome-wide significant variants, rs1234032 and rs10770791, both on chromosome 12 (Fig. 1, Supplementary Fig. 2, and Table 1). The most significant association was obtained for rs1234032, with a mean ± SEM −0.14 ± 0.03% (−1.5 ± 0.3 mmol/mol) difference in HbA1c reduction per C allele; P = 2.39 × 10−8. No statistical evidence for difference in effect size between studies was observed (P for heterogeneity [Phet] = 0.55) (Fig. 3). We then examined data from a healthy volunteer population (SUGAR-MGH, N = 857) given a single dose of glipizide (28) and found that the C allele of rs1234032 was associated with higher postdose glucose trough levels (β = 1.61, P = 0.005), and thus worse response, consistent with our GWAS findings. rs1234032 is an intergenic SNP, near GXYLT1 (Fig. 2 and Fig. 3), a gene that encodes a xylose transferase. rs1234032 is a cis eQTL to GXYLT1 in the whole blood with use of 3,029 samples from the DIRECT consortium, with the C allele being associated with increased expression (β = 0.21, P = 2.04 × 10−58). rs1234032 also showed a significant association with GXYLT1 expression in multiple tissues including adipose subcutaneous (P = 8.1 × 10−5), artery tibial (P = 2.8 × 10−9), artery aorta (P = 3.4 × 10−6), nerve tibial (P = 3.6 × 10−6) and whole blood (P = 0.01) from the GTEx consortium (29), with the C allele associated with increased expression. These significant eQTL analyses could be due to strong linkage of rs1234032 (D′ = 1 and R2 = 0.95) to rs7958582, which is within the cis-regulatory elements (https://screen.wenglab.org/). The C allele of rs1234032 is also in LD with the A allele of rs7964383 (D′ = 0.98, r2 = 0.41), which is highly associated with increased whole blood gene expression (P = 1.7 × 10−4) (29) and circulating protein levels of GXYLT1 (30). Both rs7958582 (β per G allele = −0.10, P = 1.84 × 10−06) and rs7964383 (β per A allele = −0.06, P = 0.003) were also nominally associated with glycemic response to sulfonylureas.
Figure 1.
Manhattan plot of genome-wide results from single marker association with glycemic response to sulfonylureas with use of an additive genetic model in a meta-analysis consisting of 5,485 individuals with type 2 diabetes on sulfonylureas.
Table 1.
Results for index variants in the top 15 independent loci (P < 1.0 × 10−5) associated with glycemic response
| rsID | Chr | Position | Nearest gene | EA | NEA | EAF | β§ | SE | P | N studies | P het | N samples |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs1234032 | 12 | 42354629 | GXYLT1 | C | T | 0.252 | −0.141429 | 0.025 | 2.39 × 10−8 | 7 | 0.55 | 4,810 |
| rs10770791 | 12 | 21338406 | SLCO1B1 | C | T | 0.498 | 0.107475 | 0.020 | 4.80 × 10−8 | 8 | 0.93 | 5,476 |
| rs2217693 | 12 | 21107376 | SLCO1B3–SLCO1B7 | G | A | 0.925 | −0.188639 | 0.037 | 8.40 × 10−8 | 8 | 0.34 | 5,479 |
| rs8062936 | 16 | 52475969 | TOX3 | G | A | 0.371 | 0.122292 | 0.023 | 1.57 × 10−7 | 7 | 0.39 | 4,810 |
| rs7965567 | 12 | 21161025 | SLCO1B3–SLCO1B7 | T | G | 0.051 | 0.251377 | 0.051 | 7.81 × 10−7 | 6 | 0.57 | 4,591 |
| rs7703659 | 5 | 83222316 | LOC107986386 | A | G | 0.132 | −0.14596 | 0.030 | 1.15 × 10−6 | 8 | 0.30 | 5,478 |
| rs1900362 | 13 | 85059600 | LINC00333 | G | A | 0.339 | −0.102358 | 0.021 | 1.26 × 10−6 | 8 | 0.69 | 5,475 |
| rs11816402 | 10 | 61491043 | MRLN | T | C | 0.082 | −0.217113 | 0.046 | 2.66 × 10−6 | 7 | 0.39 | 4,810 |
| rs11667346 | 19 | 8817909 | NFILZ | G | A | 0.099 | −0.194814 | 0.042 | 4.39 × 10−6 | 6 | 0.37 | 4,591 |
| rs59012839 | 9 | 138419280 | LCN1 | G | A | 0.097 | −0.216643 | 0.047 | 4.51 × 10−6 | 6 | 0.56 | 4,210 |
| rs12928694 | 16 | 10067543 | GRIN2A | A | C | 0.159 | −0.123792 | 0.027 | 5.52 × 10−6 | 8 | 0.56 | 5,475 |
| rs58013952 | 19 | 29917652 | LOC284395 | T | C | 0.115 | 0.160896 | 0.036 | 5.78 × 10−6 | 7 | 0.56 | 4,810 |
| rs75553467 | 1 | 74014130 | LINC02238 | C | G | 0.059 | −0.233071 | 0.052 | 6.31 × 10−6 | 6 | 0.48 | 4,591 |
| rs73239453 | 4 | 14122932 | LINC01085 | T | C | 0.106 | 0.160255 | 0.036 | 8.69 × 10−6 | 7 | 0.93 | 4,810 |
| rs10250448 | 7 | 33489223 | BBS9 | G | A | 0.10 | 0.15 | 0.03 | 8.94 × 10−6 | 8 | 0.78 | 5,479 |
Data shown are for index variants identified in a GWAS meta-analysis of sulfonylurea users with type 2 diabetes. Chr, chromosome; EA, effective allele; EAF, effective allele frequency; NEA, noneffective allele; rsID, reference SNP cluster identifier.
Negative β value implies that the effective allele is associated with reduced response to sulfonylureas.
Figure 3.
Forest plot of the meta-analysis of the association of HbA1c reduction with rs1234032 (left) and rs10770791 (right) variants after sulfonylurea treatment. Information on the various cohorts can be found in Supplementary Data. The numbers in parentheses indicate the number of individuals in each of the cohorts. The last column shows the effect size [95% CI].
Figure 2.
Regional association plots around genome-wide significant SNPs, rs1234032 (left) and rs10770791 (right) locus at chromosome 12, for the meta-GWAS. The purple diamonds in both plots indicate the top SNPs in the locus.
The second variant, rs10770791, is located in an intron of SLCO1B1 (Fig. 2), and each copy of the C allele (frequency 49.8%) was associated with a mean ± SEM 0.11 ± 0.02% (1.2 ± 0.2 mmol/mol) greater HbA1c reduction; P = 4.80 × 10−8. Stratified analyses showed a consistent direction of association across cohorts with similar effect sizes with no significant heterogeneity (Phet = 0.94) (Fig. 3). rs10770791 genotype was not significantly associated with sulfonylurea dose modification (P = 0.16) or drug group (the likelihood of being on mono- or dual therapy) (P = 0.29). No significant association between rs10770791 and postglipizide trough glucose concentration was observed in healthy volunteers given glipizide in SUGAR-MGH (β = −0.37, P = 0.46).
rs10770791 Is an eQTL for SLCO1B1 That Encodes OATP1B1, a Transporter of Sulfonylureas
Focusing on the SLCO1B1 locus, we performed locus-wide meta-analysis to identify the candidate causal gene (Fig. 2). We also examined two established common nonsynonymous variants in SLCO1B1, rs4149056 (*5; V174A) and rs2306283 (*1B; N130D) (30). rs4149056 (D′ = 1; r2 = 0.17) and rs2306283 (D′ = 0.98; r2 = 0.63) were in partial LD with rs10770791, with both rs4149056 (mean ± SEM β = 0.10 ± 0.03% [1.1 ± 0.3 mmol/mol], P = 2.72 × 10 −4) and rs2306283 (β = 0.08 ± 0.02% [0.9 ± 0.2 mmol/mol], P = 4.32 × 10−5) nominally associated with sulfonylurea response. However, in a conditional analysis where we have individual-level data from the GoDARTS and PMET cohorts, n = 3,557 (65% of the total population), only rs10770791 remained strongly associated with sulfonylurea response (β = 0.15 ± 0.05% [2 ± 0.4 mmol/mol], P = 1.4 × 10−3), with rs4149056 (β = 0.03 ± 0.05% [0.3 ± 0.4 mmol/mol], P = 0.58) and rs2306283 (β = 0.06 ± 0.05% [0.7 ± 0.4 mmol/mol], P = 0.19) not significant.
We then undertook eQTL lookups of SLCO1B1 expression in 1,183 liver samples of European ancestry (24) and demonstrated that the C-allele of rs10770791 was associated with decreased SLCO1B1 expression (β = −5.24, P = 1.61 × 10−7) and, marginally, with decreased SLCO1B3 expression (β = −2.46, P = 0.01). We found directionally consistent but nonsignificant associations in the 208 liver samples examined in the GTEx project (β = −0.06, P = 0.13 for SLCO1B1).
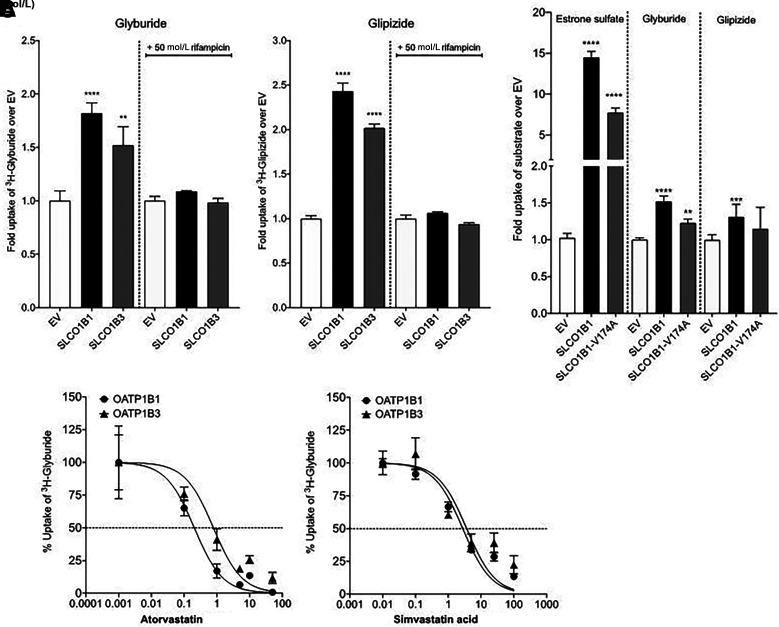
Glyburide is a substrate of both OATP1B1 and OATP1B3 (31–35), whereas there are conflicting reports about glipizide, which has been shown to be a substrate of OATP1B3 but not OATP1B1 (31). We therefore undertook functional studies on sulfonylurea transport and observed that both glyburide and glipizide were substrates of OATP1B1 and OATP1B3 in HEK-293 cells recombinantly expressing the transporters (Fig. 4A). Further, we observed that OATP1B1 Ala174 (c.521C) had a significantly lower uptake of glyburide (P < 0.002) and a trend toward a lower uptake of glipizide (P = 0.06) compared with OATP1B1 Val174 (c.521T) (Fig. 4B).
Figure 4.
Glyburide and glipizide uptake in HEK-293 Flp-ln cells recombinantly expressing SLCO1B1 or SLCO1B3. A: Uptake of [3H]-glyburide and [3H]-glipizide in HEK-293 Flp-ln stable cells expressing EV, SLCO1B1, or SLCO1B3. Rifampicin (50 μmol/L) is used as a canonical inhibitor of SLCO1B1 and SLCO1B3 P values, representing significance from EV, were determined by one-way ANOVA followed by Dunnett two-tailed test. ****P < 0.0001; ***P < 0.001; **P < 0.01; *P < 0.05. Bars represent the mean ± SEM uptake from three wells. Values shown are from a representative experiment of at least three independent studies. B: Uptake of [3H]-estrone sulfate, [3H]- glyburide, and [3H]-glipizide in HEK-293 Flp-ln stable cells expressing EV, SLCO1B1, and SLCO1B1 V174A. Estrone sulfate is a canonical substrate of SLCO1B1 and is used as a positive control in this assay. P values, for significance from EV, were determined by one-way ANOVA followed by Dunnett two-tailed test. ****P < 0.0001; ***P < 0.001; **P < 0.01; *P < 0.05. Bars represent the mean ± SEM uptake from four wells from a representative experiment. The uptake values for [3H]-glyburide and [3H]-glipizide shown are from at least four independent studies with three or four replicates per study. C: Inhibition of [3H]-glyburide uptake by atorvastatin and simvastatin acid in HEK-293 Flp-ln stable cells expressing SLCO1B1 and SLCO1B3. Each point represents the mean ± SEM uptake from four wells. Values shown are from a representative experiment of two independent studies.
Statins Inhibit Sulfonylurea Transport via OATP1B1; Genetically Reduced OATP1B1 Transport Has a Large Effect in Nonstatin Users
Given the high frequency with which hypercholesterolemia and diabetes co-occur, statins are often taken concomitantly with sulfonylureas. OATP1B1, expressed on the basolateral membrane of human hepatocytes (36), contributes to the hepatic uptake of sulfonylureas and statins from portal blood (37). We therefore sought to examine whether the initiation of statins in patients receiving sulfonylurea is associated with glycemic response in a drug-drug-gene interaction model with a sample of 3,566 adults, where we have access to individual-level data. On the basis of retrospective data from the GoDARTS and PMET cohorts, 2,096 (59%) sulfonylurea users were coprescribed statins and 1,470 (41%) were not. In a multiple linear regression model adjusted for baseline HbA1c, statin cotreatment was associated with greater HbA1c reduction on initiation of sulfonylurea, but only with adjustment for rs10770791 (mean ± SEM 0.22 ± 0.09% [2 ± 1.0 mmol/mol], P = 0.02). These results highlight a significant interaction between statin use and SLCO1B1 genotype (rs10770791) (P = 0.001) (Supplementary Table 4). In support of these results, we show that atorvastatin acid and simvastatin acid inhibited OATP1B1- and OATP1B3-mediated uptake of glyburide, with IC50 values ranging between 0.2 and 2.9 μmol/L (Supplementary Table 5), consistent with previous studies showing that these two statins inhibit OATP1B1-mediated uptake of estradiol-17β-glucuronide (38).
We then performed stratified analysis to see whether statin use modifies the association between rs10770791 and sulfonylurea-related HbA1c reduction using a similar model. We observed that the effect of rs10770791 was abolished in sulfonylurea users prescribed statins (mean ± SEM β = 0.053 ± 0.03% [0.6 ± 0.3 mmol/mol)], P = 0.11). However, among users of sulfonylureas without statins, we found a pronounced HbA1c reduction associated with the C allele of rs10770791 (β = 0.23 ± 0.049% [2.4 ± 0.6 mmol/mol], P = 3.1 × 10−6) (Supplementary Table 6). C allele homozygotes at rs10770797 had a 0.48 ± 0.12% (5.2 ± 1.26 mmol/mol) greater absolute HbA1c reduction than T allele homozygotes.
Conclusions
We report the first meta-GWAS on glycemic response to sulfonylureas and establish that this trait is heritable with a 37% heritability estimate. We have identified two novel loci at chromosome 12 and confirmed a potential involvement of the GXYLT1 and SLCO1B1 genes in glycemic response to sulfonylureas. We report large clinical effects of variants in SLCO1B1, which encodes a transporter for sulfonylureas in the liver where it is metabolized, and report interaction with coprescription of statins.
The SNP rs1234032 is an eQTL for GXYLT1 in multiple tissues including whole blood. GXYLT1 adds the first xylose to O-glucose–modified residues in NOTCH1 (31), which is a major determinant of pancreatic islet cell mass and insulin secretion and is a risk factor for diabetes (32). The C allele at rs1234032 was associated with increased expression of GXYLT1. Transgenic overexpression of human GXYLT1 was previously shown to impair Notch signaling (39). Notch signaling pathway is known to play an important role in regulating development of pancreas and also shown to be expressed in adult pancreas (40). In a recent study, Eom et al. (40) compared glucose levels, insulin secretion, and islet and β-cell masses in Notch1 antisense transgenic (NAS) and control mice after intraperitoneal glucose tolerance test. Higher glucose levels, lower insulin secretion, and decreased total islet and β-cell masses were shown in NAS in comparison with control mice. In line with this, we have shown increased trough glucose concentration with the C allele in healthy volunteers who were naïve to type 2 diabetes medications who received a glipizide challenge and, hence, worse response.
The C allele at rs10770791 was significantly associated with reduced expression of SLCO1B1 mRNA in the liver and worse glycemic response to sulfonylureas. SLCO1B1 encodes the organic anion-transporting polypeptide, OATP1B1, which facilitates the hepatic uptake of clinically relevant drugs such as statins. Gliclazide, glipizide, glyburide (glibenclamide), glimepiride, tolazamide, and tolbutamide were prescribed for the subjects in this study. Approximately 90% of the prescriptions in GoDARTS were for gliclazide, and glipizide was the main sulfonylurea in the PMET cohorts. While gliclazide and glimepiride are substrates of OATP1B1 (31,34), glyburide has been shown to be a substrate of both OATP1B1 and OATP1B3 (31,34–36,41,42). However, there are conflicting reports about glipizide, which has been shown to be a substrate of OATP1B3 but not OATP1B1 (36). Here we show that both glyburide and glipizide were substrates of OATP1B1 and OATP1B3. Further, we observed a significantly lower uptake of glyburide (P < 0.002) and a trend toward a lower uptake of glipizide (P = 0.06) for OATP1B1 Ala174 (c.521C) compared with OATP1B1 Val174 (c.521T). Examination of other known missense variants (rs60140950 [p.Gly256Ala], rs11045681 [p.Tyr311Ser], and rs11045819 [p.Pro155Thr]) in the SLCO1B3 and SLCO1B3–SLCO1B7 regions that are in partial LD with rs10770791 showed no significant association. Taken together these results suggest that the pharmacogenetic mechanism for the effect of rs10770791 on sulfonylurea response is primarily a result of altered hepatic expression of SLCO1B1 and, to a lesser extent, SLCO1B3. Partial LD of rs10770791 with various missense variants may contribute to its effect on sulfonylurea response; however, conditional analysis demonstrated association of rs10770791 with glycemic response independent of the missense variants. The reduced SLCO1B1 expression likely results in less OATP1B1-mediated transport of sulfonylurea into the liver and potentially higher plasma concentrations available at the site of action (pancreas).
There is a high prevalence of multimorbidity and subsequent polypharmacy in type 2 diabetes, highlighting a need to consider drug-drug as well as drug-drug-gene interactions in prediction models of glycemic response to sulfonylureas. Given that statins are often taken concomitantly with sulfonylureas, with both being substrates of OATP1B1, we examined for a possible drug-drug-gene interaction and showed a significant interaction between statin use and SLCO1B1 genotype (rs10770791) (P = 0.001). Stratified analysis by statin use showed differential effects of rs10770791 in statin users and nonusers. While the association between rs10770791 and glycemic response to sulfonylureas was abolished in statin users, it was more pronounced in statin nonusers. In those not treated with statins nearly one-quarter of the population who carry two C alleles at rs10770791 had a 0.48% (5.2 mmol/mol) greater HbA1c reduction compared with T allele homozygotes. These large effects are the equivalent of those in starting a dipeptidyl peptidase 4 inhibitor (43) and equated to a dose difference of 28 mg gliclazide. Our findings suggest that the previous reported observational association between statins and hypoglycemia in sulfonylurea users (44) may be explained by interactions at SLCO1B1, depending on the underlying genotype. The findings are consistent with previous studies in healthy volunteers and rodents demonstrating that atorvastatin administration is associated with increased levels of glimepiride (45) and glyburide (46), respectively. Given that there is a strong recommendation to use statins by recent guidelines, statin use is increasing among people with diabetes (47). Therefore, integrating comedications with genetic data could improve optimization of polypharmacy regimens.
This study has some limitations. First, the modest sample size does not have sufficient power to detect the contribution of rare and low-frequency variants in heritability estimation and/or glycemic response to sulfonylureas. However, this is the first GWAS and largest pharmacogenomic study on sulfonylureas response so far. Second, this study was conducted in Whites of European descent, and therefore the results may not generalize to other populations. Third, even though we have performed several validation studies, direct replication of the findings in an independent study is warranted. Finally, further studies need to be done to elucidate the biological mechanism of the identified associations, especially for GXYLT1.
In conclusion, we have established that common genetic variants contribute to the variation in glycemic response to sulfonylureas, with an estimated heritability of 37%. This result shows that a moderate proportion of the variance in glycemic response is genetic, with an important role for common genetic variation in glycemic response to sulfonylureas. We report that a variant that modulates gene expression and circulating GXYLT1 reduces response to sulfonylureas. We have also revealed a robust association between rs10770791, a cis eQTL for SLCO1B1 expression in the liver, and glycemic response to sulfonylureas, with reduced SLCO1B1 expression associating with increased response to sulfonylureas. Our results suggest the potential of rs10770791 to be a biomarker for stratified medicine in diabetes. In addition, we have highlighted significant drug-drug-gene interactions for sulfonylurea, statin use, and rs10770791, with clinically actionable genetic effects with pronounced differences in HbA1c reduction in a subgroup of patients treated with sulfonylureas without statins. Over the next 5 years we will see an ever-increasing availability of genotype or sequence data embedded in the medical records; given replication, the SLCO1B1-statin interaction could be clinically actionable and will need to be taken into account at the point of prescribing sulfonylureas.
Article Information
Acknowledgments. The authors acknowledge the following individuals for their contributions in providing information required for the studies: Dana Fraser from Parexel, who completed imputation for the Metformin Response (METRO) cohort; Dilrini Ranatunga from Kaiser Permanente Northern California Division of Research, for providing the phenotype and genotype data for the PMET1 cohort and phenotype data for PMET2 cohort; Sara R. Rashkin Center for Applied Bioinformatics, St. Jude Children’s Research Hospital, Memphis, TN, for providing advice related to computational coding and analyses required for the PMET cohorts; and Xujia Zhou, University of California, San Francisco, CA, for experimental assistance required for this study. The authors also acknowledge Jennifer L. Aponte, Genomic Medicine, Parexel; Margaret G. Ehm, Target Sciences, GlaxoSmithKline; and Dawn M. Waterworth, Target Sciences, GlaxoSmithKline for their contribution to data analysis and revising the manuscript. The authors also acknowledge clinicalstudydatarequest.com for access to the HARMONY data. Finally, the authors thank all study participants.
Funding and Duality of Interest. The work leading to this publication has received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115317 (DIRECT), resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007-2013) and European Federation of Pharmaceutical Industries (EFPIA) companies’ in kind contribution. Funding was in part from the National Institutes of Health (NIH), R01-GM117163, to J.C.F., M.M.H., and K.M.G. E.R.P. holds a Wellcome New Investigator Award (102820/Z/13/Z). Funding for SUGAR-MGH was provided by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), NIH, R01-DK088214. J.H.L. is supported by NIDDK, NIH, T32-DK007028. J.C.F. is supported by NIDDK, NIH, K24-DK110550. Geisinger MyCode type 2 diabetes project was supported by the Geisinger Health Plan Quality Pilot Fund (Principal Investigator: M.T.M.L.). E.R.P. has received honoraria for speaking from Lilly and Sanofi. J.C.F. has received honoraria for speaking at scientific conferences from Novo and for consulting from Goldfinch Bio. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. A.Y.D, S.W.Y, L.M.‘t.H., K.M.G., and E.R.P. contributed to conception and design of the study. A.Y.D., S.W.Y., N.V.L., Y.Z., M.K.S., A.E., F.I, F.X., J.H.L., R.C.S., and Y.-C.C. contributed to data analysis. S.W.Y., A.E., F.I., J.M.M, V.K., J.S.W., M.T.M.L., Y.K., Y.M., M.K., C.N.A.P., J.C.F., M.M.H., and L.M.‘t.H. contributed to data collection and genotyping. A.Y.D., S.W.Y., K.M.G., and E.R.P. contributed to manuscript writing, with contributions from all authors on the final version. A.Y.D. S.W.Y, K.M.G., and E.R.P are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
A.Y.D. and S.W.Y. are joint first authors.
K.M.G. and E.R.P. are joint senior authors.
This article contains supplementary material online at https://doi.org/10.2337/figshare.16593023.
Contributor Information
Collaborators: MetGen Plus investigators:, Ewan Pearson, Adem Dawed, Kaixin Zhou, Rury Holman, Ruth Coleman, Leen ‘t Hart, Roderick Slieker, Joline Beulens, Amber van der Heijden, Giel Nijpels, Petra Elders, Femke Rutters, Bruno Stricker, Fariba Ahmadizar, Catherine de Keyser, Adriaan Koov, Mattijs Out, Jānis Kloviņš, Linda Zaharenko, Martin Javorsky, Ivan Tkac, Jose Florez, Kathy Giacomini, Sook Wah Yee, Monique Hedderson, Michiaki Kubo, Alison Motsinger-Reif, Michael Wagner, Sabina Semiz, Tanja Dujic, Mette Christensen, Kim Brøsen, Dawn Waterworth, Meg Ehm, Ronald Ma, Bruce Psaty, and James Floyd
References
- 1. Hirst JA, Farmer AJ, Dyar A, Lung TW, Stevens RJ. Estimating the effect of sulfonylurea on HbA1c in diabetes: a systematic review and meta-analysis. Diabetologia 2013;56:973–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Curtis HJ, Dennis JM, Shields BM, et al. Time trends and geographical variation in prescribing of drugs for diabetes in England from 1998 to 2017. Diabetes Obes Metab 2018;20:2159–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kahn SE, Haffner SM, Heise MA, et al.; ADOPT Study Group . Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 4. Pearson ER. Pharmacogenetics and future strategies in treating hyperglycaemia in diabetes. Front Biosci 2009;14:4348–4362 [DOI] [PubMed] [Google Scholar]
- 5. Martono DP, Lub R, Lambers Heerspink HJ, Hak E, Wilffert B, Denig P. Predictors of response in initial users of metformin and sulphonylurea derivatives: a systematic review. Diabet Med 2015;32:853–864 [DOI] [PubMed] [Google Scholar]
- 6. Pearson ER, Donnelly LA, Kimber C, et al. Variation in TCF7L2 influences therapeutic response to sulfonylureas: a GoDARTs study. Diabetes 2007;56:2178–2182 [DOI] [PubMed] [Google Scholar]
- 7. Dennis JM, Henley WE, Weedon MN, et al.; MASTERMIND Consortium . Sex and BMI alter the benefits and risks of sulfonylureas and thiazolidinediones in type 2 diabetes: a framework for evaluating stratification using routine clinical and individual trial data. Diabetes Care 2018;41:1844–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Becker ML, Visser LE, Trienekens PH, Hofman A, van Schaik RH, Stricker BH. Cytochrome P450 2C9 *2 and *3 polymorphisms and the dose and effect of sulfonylurea in type II diabetes mellitus. Clin Pharmacol Ther 2008;83:288–292 [DOI] [PubMed] [Google Scholar]
- 9. Suzuki K, Yanagawa T, Shibasaki T, Kaniwa N, Hasegawa R, Tohkin M. Effect of CYP2C9 genetic polymorphisms on the efficacy and pharmacokinetics of glimepiride in subjects with type 2 diabetes. Diabetes Res Clin Pract 2006;72:148–154 [DOI] [PubMed] [Google Scholar]
- 10. Zhou K, Donnelly L, Burch L, et al. Loss-of-function CYP2C9 variants improve therapeutic response to sulfonylureas in type 2 diabetes: a Go-DARTS study. Clin Pharmacol Ther 2010;87:52–56 [DOI] [PubMed] [Google Scholar]
- 11. Chen L, Li JH, Kaur V, et al. The presence of two reduced function variants in CYP2C9 influences the acute response to glipizide. Diabet Med 2020;37:2124–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Srinivasan S, Kaur V, Chamarthi B, et al. TCF7L2 genetic variation augments incretin resistance and influences response to a sulfonylurea and metformin: the Study to Understand the Genetics of the Acute Response to Metformin and Glipizide in Humans (SUGAR-MGH). Diabetes Care 2018;41:554–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou K, Bellenguez C, Spencer CC, et al.; GoDARTS and UKPDS Diabetes Pharmacogenetics Study Group; Wellcome Trust Case Control Consortium 2; MAGIC investigators . Common variants near ATM are associated with glycemic response to metformin in type 2 diabetes. Nat Genet 2011;43:117–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou K, Donnelly L, Yang J, et al.; Wellcome Trust Case Control Consortium 2 . Heritability of variation in glycaemic response to metformin: a genome-wide complex trait analysis. Lancet Diabetes Endocrinol 2014;2:481–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou K, Yee SW, Seiser EL, et al.; MetGen Investigators; DPP Investigators; ACCORD Investigators . Variation in the glucose transporter gene SLC2A2 is associated with glycemic response to metformin. Nat Genet 2016;48:1055–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morris AP, Voight BF, Teslovich TM, et al.; Wellcome Trust Case Control Consortium; Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC) Investigators; Genetic Investigation of ANthropometric Traits (GIANT) Consortium; Asian Genetic Epidemiology Network–Type 2 Diabetes (AGEN-T2D) Consortium; South Asian Type 2 Diabetes (SAT2D) Consortium; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium . Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet 2012;44:981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet 2009;5:e1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marchini J, Howie B, Myers S, McVean G, Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet 2007;39:906–913 [DOI] [PubMed] [Google Scholar]
- 19. Winkler TW, Day FR, Croteau-Chonka DC, et al.; Genetic Investigation of Anthropometric Traits (GIANT) Consortium . Quality control and conduct of genome-wide association meta-analyses. Nat Protoc 2014;9:1192–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mägi R, Morris AP. GWAMA: software for genome-wide association meta-analysis. BMC Bioinformatics 2010;11:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yin L, Zhang H, Tang Z, et al. rMVP: a memory-efficient, visualization-enhanced, and parallel-accelerated tool for genome-wide association study. 20 August 2020 [preprint]. bioRxiv:2020.08.20.258491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pruim RJ, Welch RP, Sanna S, et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 2010;26:2336–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Speed D, Balding DJ. SumHer better estimates the SNP heritability of complex traits from summary statistics. Nat Genet 2019;51:277–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Etheridge AS, Gallins PJ, Jima D, et al. A new liver expression quantitative trait locus map from 1,183 individuals provides evidence for novel expression quantitative trait loci of drug response, metabolic, and sex-biased phenotypes. Clin Pharmacol Ther 2020;107:1383–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koivula RW, Forgie IM, Kurbasic A, et al.; IMI DIRECT Consortium . Discovery of biomarkers for glycaemic deterioration before and after the onset of type 2 diabetes: descriptive characteristics of the epidemiological studies within the IMI DIRECT Consortium. Diabetologia 2019;62:1601–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Viñuela A, Brown AA, Fernandez J, et al. Genetic analysis of blood molecular phenotypes reveals regulatory networks affecting complex traits: a DIRECT study. medRxiv. 2021:2021.03.26.21254347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yee SW, Giacomini MM, Hsueh CH, et al. Metabolomic and genome-wide association studies reveal potential endogenous biomarkers for OATP1B1. Clin Pharmacol Ther 2016;100:524–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Walford GA, Colomo N, Todd JN, et al. The study to understand the genetics of the acute response to metformin and glipizide in humans (SUGAR-MGH): design of a pharmacogenetic resource for type 2 diabetes. PLoS One 2015;10:e0121553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. GTEx Consortium . The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 2020;369:1318–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Michalski C, Cui Y, Nies AT, et al. A naturally occurring mutation in the SLC21A6 gene causing impaired membrane localization of the hepatocyte uptake transporter. J Biol Chem 2002;277:43058–43063 [DOI] [PubMed] [Google Scholar]
- 31. Chen Y, Chen L, Zhang H, Huang S, Xiong Y, Xia C. Interaction of sulfonylureas with liver uptake transporters OATP1B1 and OATP1B3. Basic Clin Pharmacol Toxicol 2018;123:147–154 [DOI] [PubMed] [Google Scholar]
- 32. Koenen A, Köck K, Keiser M, Siegmund W, Kroemer HK, Grube M. Steroid hormones specifically modify the activity of organic anion transporting polypeptides. Eur J Pharm Sci 2012;47:774–780 [DOI] [PubMed] [Google Scholar]
- 33. Meyer Zu Schwabedissen HE, Boettcher K, Steiner T, et al. OATP1B3 is expressed in pancreatic β-islet cells and enhances the insulinotropic effect of the sulfonylurea derivative glibenclamide. Diabetes 2014;63:775–784 [DOI] [PubMed] [Google Scholar]
- 34. Yang F, Xiong X, Liu Y, et al. CYP2C9 and OATP1B1 genetic polymorphisms affect the metabolism and transport of glimepiride and gliclazide. Sci Rep 2018;8:10994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zheng HX, Huang Y, Frassetto LA, Benet LZ. Elucidating rifampin’s inducing and inhibiting effects on glyburide pharmacokinetics and blood glucose in healthy volunteers: unmasking the differential effects of enzyme induction and transporter inhibition for a drug and its primary metabolite. Clin Pharmacol Ther 2009;85:78–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee HH, Ho RH. Interindividual and interethnic variability in drug disposition: polymorphisms in organic anion transporting polypeptide 1B1 (OATP1B1; SLCO1B1). Br J Clin Pharmacol 2017;83:1176–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Y, Panfen E, Fancher M, Sinz M, Marathe P, Shen H. Dissecting the contribution of OATP1B1 to hepatic uptake of statins using the OATP1B1 selective inhibitor estropipate. Mol Pharm 2019;16:2342–2353 [DOI] [PubMed] [Google Scholar]
- 38. Chen C, Mireles RJ, Campbell SD, et al. Differential interaction of 3-hydroxy-3-methylglutaryl-coa reductase inhibitors with ABCB1, ABCC2, and OATP1B1. Drug Metab Dispos 2005;33:537–546 [DOI] [PubMed] [Google Scholar]
- 39. Lee TV, Sethi MK, Leonardi J, et al. Negative regulation of notch signaling by xylose. PLoS Genet 2013;9:e1003547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eom YS, Gwon AR, Kwak KM, et al. Notch1 has an important role in β-cell mass determination and development of diabetes. Diabetes Metab J 2021;45:86–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Holstein A, Beil W, Kovacs P. CYP2C metabolism of oral antidiabetic drugs--impact on pharmacokinetics, drug interactions and pharmacogenetic aspects. Expert Opin Drug Metab Toxicol 2012;8:1549–1563 [DOI] [PubMed] [Google Scholar]
- 42. Zharikova OL, Fokina VM, Nanovskaya TN, et al. Identification of the major human hepatic and placental enzymes responsible for the biotransformation of glyburide. Biochem Pharmacol 2009;78:1483–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gan S, Dawed AY, Donnelly LA, et al. Efficacy of modern diabetes treatments DPP-4i, SGLT-2i, and GLP-1RA in White and Asian patients with diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care 2020;43:1948–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leonard CE, Bilker WB, Brensinger CM, et al. Severe hypoglycemia in users of sulfonylurea antidiabetic agents and antihyperlipidemics. Clin Pharmacol Ther 2016;99:538–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sana T, Aslam B, Aslam N, et al. Therapeutic effect of atorvastatin on kidney functions and urinary excretion of Glimepiride in healthy adult human male subjects. Pak J Pharm Sci 2016;29(Suppl.):2321–2326 [PubMed] [Google Scholar]
- 46. Neerati P, Gade J. Influence of atorvastatin on the pharmacokinetics and pharmacodynamics of glyburide in normal and diabetic rats. Eur J Pharm Sci 2011;42:285–289 [DOI] [PubMed] [Google Scholar]
- 47. Brennan MB, Huang ES, Lobo JM, et al. Longitudinal trends and predictors of statin use among patients with diabetes. J Diabetes Complications 2018;32:27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]