Abstract
Certain chronic comorbidities, including diabetes, are highly prevalent in people with coronavirus disease 2019 (COVID-19) and are associated with an increased risk of severe COVID-19 and mortality. Mild glucose elevations are also common in COVID-19 patients and associated with worse outcomes even in people without diabetes. Several studies have recently reported new-onset diabetes associated with COVID-19. The phenomenon of new-onset diabetes following admission to the hospital has been observed previously with other viral infections and acute illnesses. The precise mechanisms for new-onset diabetes in people with COVID-19 are not known, but it is likely that a number of complex interrelated processes are involved, including previously undiagnosed diabetes, stress hyperglycemia, steroid-induced hyperglycemia, and direct or indirect effects of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on the β-cell. There is an urgent need for research to help guide management pathways for these patients. In view of increased mortality in people with new-onset diabetes, hospital protocols should include efforts to recognize and manage acute hyperglycemia, including diabetic ketoacidosis, in people admitted to the hospital. Whether new-onset diabetes is likely to remain permanent is not known, as the long-term follow-up of these patients is limited. Prospective studies of metabolism in the setting of postacute COVID-19 will be required to understand the etiology, prognosis, and treatment opportunities.
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that results in the clinical disease coronavirus disease 2019 (COVID-19) was first reported in December 2019 in Wuhan, China, and has claimed over 2 million lives globally (1). Certain chronic comorbidities, such as hypertension, cardiovascular disease, obesity, diabetes, and kidney disease, are highly prevalent in people with COVID-19. While these comorbidities do not appear to increase the risk of developing COVID-19, they are associated with an increased risk of a more severe case of the condition as well as mortality (2).
Hyperglycemia and New-Onset Diabetes Associated With COVID-19
Severe hyperglycemia is common in critically ill patients and is often seen as a marker of disease severity (3). Several studies over the course of the pandemic have reported that COVID-19 is associated with hyperglycemia in people with and without known diabetes (4,5). One study from Wuhan of hospitalized, mainly elderly COVID-19 patients reported that 21.6% had a history of diabetes, and, based on the first glucose measurement upon admission, 20.8% were newly diagnosed with diabetes (fasting admission glucose ≥7.0 mmol/L and/or HbA1c ≥6.5%), and 28.4% were diagnosed with dysglycemia (fasting glucose 5.6–6.9 mmol/L and/or HbA1c 5.7–6.4%) (5).
A number of studies have reported new-onset diabetes (that phenotypically could be classified as either type 1 diabetes [T1D] or type 2 diabetes [T2D]) as being associated with the presence of COVID-19 (Table 1). A study from London, U.K., reported 30 children aged 23 months to 16.8 years with new-onset T1D (6). Of these, 70% presented with diabetic ketoacidosis (DKA), 52% with severe DKA, and 15% with a positive COVID-19 test (6). The authors concluded that this represented an 80% increase in new-onset T1D during the pandemic compared with previous years (6). Further, it would also appear that the severity of presentation of youth with T1D is increased (7). Conflicting results have also been reported, however, with data from 216 pediatric diabetes centers in Germany showing no increase in the number of children diagnosed with T1D during the early months of the pandemic (8). However, the same centers reported data on 532 children and adolescents with newly diagnosed T1D and found significant increases in DKA and severe ketoacidosis at diagnosis during the same time period (9).
Table 1.
Studies reporting new-onset diabetes
| Reference | Country | Design | Population | Results |
|---|---|---|---|---|
| Li et al. (5) | China | Retrospective observational | 453 patients with laboratory-confirmed SARS-CoV-2 infection aged 61 (IQR 49, 68) years | 94 patients (21%) were newly diagnosed with diabetes (fasting admission glucose ≥7.0 mmol/L and/or HbA1c ≥6.5%) |
| Unsworth et al. (6) | U.K. | Cross-sectional | 33 children aged 10.9 (IQR 6.8) years, 68% male, 36% White European | 30 children (91%) presented with new-onset T1D; 5 children tested positive for SARS-CoV-2; 70% presented with DKA and 52% with severe DKA |
| Ebekozien et al. (17) | U.S. | Cross-sectional | 64 children and adults aged 20.9 (SD 14.84) years, 61% female, 48.4% non-Hispanic White | 6 patients (9.8%) had new-onset T1D, with 5 (15.6%) in the COVID-19–positive group |
| Tittel et al. (8) | Germany | Prospective study | Pediatric T1D patients with onset age between 6 months and <18 years diagnosed between 13 March and 13 May in each year between 2011 and 2020 (from German Diabetes Registry data) | T1D incidence (per 100,000 patient-years) increased from 16.4 in 2011 to 22.2 in 2019; the incidence in 2020 (23.4) did not significantly differ from the predicted value |
| Armeni et al. (14) | U.K. | Retrospective case series | 35 patients with SARS-CoV-2 infection aged 60 (IQR 45, 70) years, 22.9% female, 20% Caucasian; inclusion criteria were 1) hospitalization with confirmed COVID-19 diagnosis, 2) DKA and/or hyperosmolar hyperglycemic state at presentation, 3) known or new diagnosis of diabetes and presence of ketonemia, and 4) Glasgow coma scale of at least 12 on admission | 28 (80%) patients had T2D, and 2 (5.7%) were new presentations of diabetes |
| Sathish et al. (16) | China, Italy, U.S. | Systematic review and meta-analysis | From 8 studies, 3,711 COVID-19 patients, aged between 47 and 64.9 years, 53.3–80.0% male | 492 patients had newly diagnosed diabetes, and random-effect meta-analysis estimated a pooled prevalence of new-onset diabetes of 14.4% (95% CI 5.9–25.8%) |
| Wang et al. (64) | China | Retrospective study | 605 patients with SARS-CoV-2 infection, aged 59.0 (IQR 47.0, 68.0) years, 46.8% female; exclusion criteria were 1) no definitive 28-day outcome since transfer to another hospital, 2) missing key clinical information, 3) no FBG data available at admission, and 4) having previously diagnosed diabetes | 176 patients (29.1%) with new-onset/newly detected diabetes |
| Yang et al. (65) | China | Retrospective cohort study | 69 patients with laboratory-confirmed SARS-CoV-2 infection aged 61 (IQR 52, 57) years, 49.3% male; exclusion criteria were patients receiving glucocorticoid treatment or with a history of diabetes, myocardial infarction, heart failure, dialysis, renal transplant, or cirrhosis and patients missing basic medical information | In critical and moderate + severe patients the prevalence of new-onset diabetes was 53.85% and 13.95%, respectively |
| Fadini et al. (66) | Italy | Retrospective study | 413 patients with SARS-CoV-2 infection aged 64.9 (SD 15.4) years, 59.3% male | 21 patients (5%) with new-onset/newly detected diabetes |
| Wu et al. (46) | Australia | Retrospectively analyzed | 8 patients with T2D were admitted to the intensive care unit with COVID-19; 5 had preexisting diabetes | Within patients with newly diagnosed diabetes, C-peptide levels and negative anti-GAD antibodies were found, consistent with T2D, and HbA1c ranged from 11.1% to 12.4% (98 to 112 mmol/mol) |
| Ghosh et al. (22) | India | Retrospective cohort | 555 patients with new-onset diabetes were included, with 282 with new-onset diabetes prior to the COVID-19 pandemic (19 September to 20 February) and 273 with new-onset diabetes during COVID-19 (April–October 20) | Patients with new-onset diabetes during the COVID-19 pandemic had higher fasting and postprandial blood glucose, glycated hemoglobin levels, and C-peptide vs. patients with new-onset diabetes prior to pandemic; no differences were seen in C-peptide or glycemic outcomes in the patients with new-onset diabetes between those who tested positive or negative for COVID-19 (antibody test) |
| Zhang et al. (67) | China | Retrospective study | 312 patients with COVID-19 with a mean age of 57 (IQR 38, 66) years; 55% were female, 84 had diabetes, and 36 were new diagnoses (57 had fasting glucose levels ≥7.0 mmol/L, including 30 without and 27 with a known history of diabetes); exclusion criteria included no positive COVID-19 test, patients remaining in hospital, and missing information on clinical outcomes because of transfer to other hospitals | Diabetes at admission was associated with higher risks of adverse outcomes among patients with COVID-19 (irrespective of whether or not the diagnosis was new) |
| Smith et al. (68) | U.S. | Retrospective study | 184 patients hospitalized for COVID-19, aged 64.4 years (range 21–100), 67.7% female | 6 patients without diabetes and with normal HbA1c levels also had repeatedly elevated fasting blood glucose; these 29 patients had fasting blood glucose levels consistent with new-onset diabetes and temporally associated with recent acquisition of SARS-CoV-2 infection |
| Liu et al. (69) | China | Retrospective study | In total, 233 patients were included in the final analysis; 80 (34.3%) patients had diabetes, among whom 44 (55.0%) were previously diagnosed and 36 (45.0%) were newly defined as having undiagnosed diabetes with an HbA1c level ≥6.5% (48 mmol/mol) at admission | Risk of in-hospital death was significantly increased in all patients with diabetes (HR 3.80, 95% CI 1.71–8.47), those with diagnosed diabetes (HR 4.03, 95% CI 1.64–9.91), and those with undiagnosed diabetes who were newly defined by HbA1c testing at admission (HR 1.89, 95% CI 1.18–3.05) compared with those without diabetes |
IQR, interquartile range.
A few studies have also observed that DKA and hyperosmolar hyperglycemic state are unusually common in COVID-19 patients with known diabetes (10–13). In a Chinese study, 42 patients had COVID-19 and ketoacidosis, and 27 had no prior diagnosis of diabetes (12). A study from London, U.K., included 35 patients with COVID-19 who presented with DKA (31.4%), mixed DKA and hyperosmolar hyperglycemic state (HSS; 31.7%), HSS (5.7%), or hyperglycemic ketoacidosis (25.7%) (14). Overall, 80% had T2D. Of those with T2D, the prevalence of DKA was high, indicating insulinopenia in people with COVID-19. In addition, 5.7% of the 35 patients with COVID-19 had newly diagnosed diabetes. DKA was protracted in people with COVID-19 compared with previous reports of those with non–COVID-19 DKA (35 h vs. 12 h), and they had a higher insulin requirement (14). Another recent U.S. study of 5,029 patients (mean age 47 years) from 175 hospitals found that patients with COVID-19 had higher BMI, higher insulin requirement, prolonged time to resolution of DKA, and higher mortality than those without COVID-19 (15). A U.K. study reported that children presented more frequently with DKA than during the prepandemic period (10% severe prepandemic vs. 47% during the first wave of the pandemic) and had higher HbA1c (13% vs. 10.4%) (7).
A number of studies have also reported that preexisting diabetes as well as newly diagnosed diabetes with a first glucose measurement on hospital admission are both associated with an increased risk of all-cause mortality in hospitalized patients with COVID-19. In a systematic review of 3,711 COVID-19 patients from 8 studies (492 patients with new-onset diabetes), the pooled prevalence of new-onset diabetes was 14.4% (95% CI 5.9–25.8%) from a random-effect meta-analysis (16). Worryingly, the risk of mortality appears to be higher in people with new-onset diabetes than with COVID-19 patients with known diabetes (5,17). An Italian study of 271 people admitted with COVID-19, 20.7% of whom had preexisting diabetes, found that hyperglycemia was independently associated with mortality (hazards ratio [HR] 1.80, 95% CI 1.03–3.15). The study also showed that people with diabetes and hyperglycemia had worse inflammatory profiles (18). In a study from Wuhan, China, patients with newly diagnosed diabetes were more likely to be admitted to the intensive care unit, require invasive mechanical ventilation, have a high prevalence of acute respiratory distress syndrome, acute kidney injury, or shock, and have the longest hospital stays (5). The study also reported data showing that glucose levels at hospital admission in people with newly diagnosed diabetes and in those with a history of diabetes were both associated with the increased risk of all-cause mortality (5). Patients with newly diagnosed diabetes had a higher mortality than COVID-19 patients with known diabetes, hyperglycemia (fasting glucose 5.6–6.9 mmol/L and/or HbA1c 5.7–6.4%) or normal glucose (HR 9.42, 95% CI 2.18–40.7). This is one of a few studies where HbA1c was measured on admission to determine whether newly diagnosed diabetes was present in asymptomatic patients prior to admission or whether those who developed it did so following admission (5).
Type of Diabetes
It is currently unclear whether the new-onset diabetes associated with COVID-19 is type 1, type 2, or a complex subtype of diabetes. Although in T1D insulin deficiency is usually the result of an autoimmune process, in SARS-CoV-2 infection it could be due to destruction of the β-cells. Unfortunately, studies of islet cell antibodies in people with new-onset diabetes have been limited to a few case reports (19,20). Multiple studies have reported a high number of incidents of DKA in people with and without COVID-19, suggesting a direct effect of SARS-CoV-2 on pancreatic β-cells. One study of hospitalized patients with SARS-CoV-1 infection showed that immunostaining for angiotensin-converting enzyme 2 (ACE2) protein was strong in pancreatic islets but weak in exocrine tissues (21). However, a recent study from India compared new-onset diabetes in hospitalized patients prior to COVID-19 with new-onset diabetes during COVID-19 and found worse glycemic parameters in new-onset diabetes during COVID-19 and diabetes but no difference in symptoms, phenotype, or C-peptide levels (22).
Potential Mechanisms for New-Onset Diabetes
The precise mechanisms behind the development of new-onset diagnosis in people with COVID-19 are not known, but it is likely that a number of complex, interrelated etiologies are responsible, including impairments in both glucose disposal and insulin secretion, stress hyperglycemia, preadmission diabetes, and steroid-induced diabetes (Fig. 1). One recent article reported an increase in the number of children admitted to pediatric intensive care unit with new-onset T1D with severe DKA and a smaller increase in incidence of new-onset T1D (23). Overall, 7/20 (35%) of the children diagnosed in 2020 were tested for SAR-CoV-2, with all being negative. The authors suggested that the increase in incidence and severity were due to altered presentation during the pandemic rather than direct effects of COVID-19. Current data also suggest a bidirectional relationship between T2D and COVID-19 (24), but whether there is a bidirectional relationship between hyperglycemia and COVID-19 is not known (Fig. 2). The following sections give more detailed discussions of some of the proposed mechanisms for new-onset diabetes associated with COVID-19.
Figure 1.
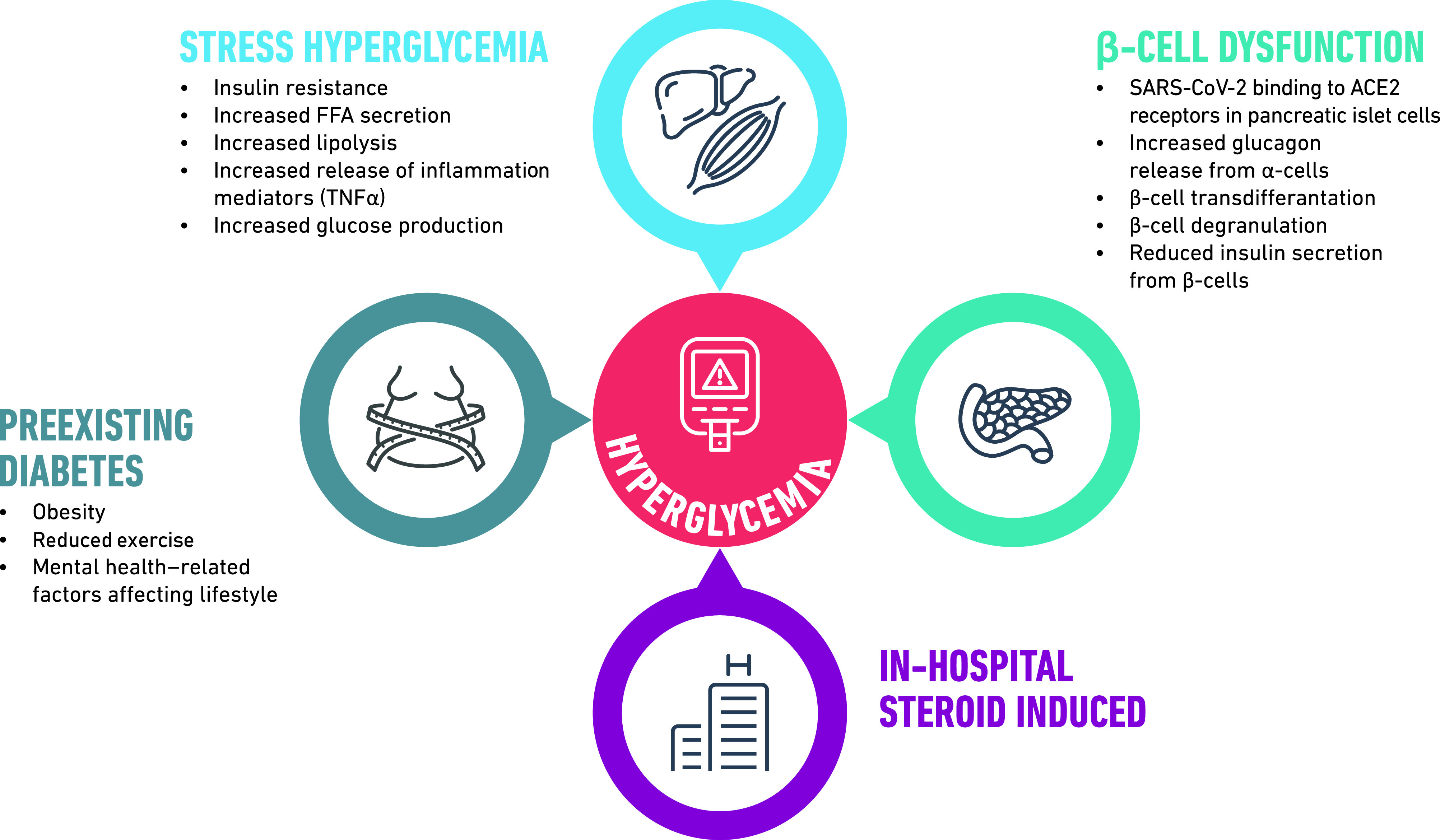
Potential mechanisms for development of new-onset diabetes in people with COVID-19.
Figure 2.
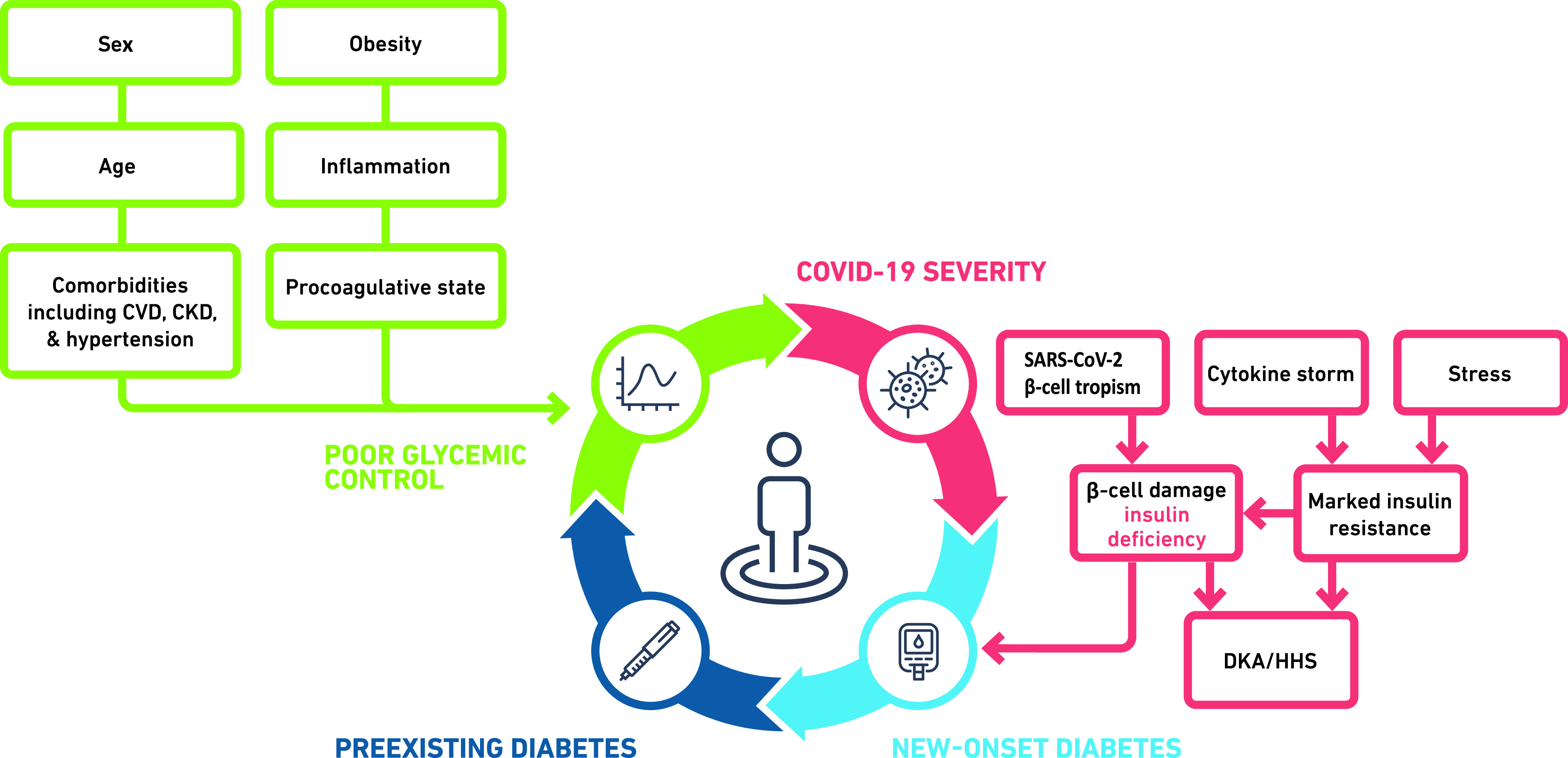
Bidirectional relationship between T2D, hyperglycemia, and COVID-19. CVD, cardiovascular disease; CKD, chronic kidney disease; HHS, hyperosmolar hyperglycemic syndrome.
Preexisting Undiagnosed Diabetes
One reason for new-onset diabetes is that these patients may have had undetected diabetes prior to admission, potentially as a consequence of recent weight gain due to changes in lifestyle and worsening of hyperglycemia mainly due to self-isolation, social distancing, reduced physical activity, and poor diets as a result of mental health issues. For example, a recent survey of 155 countries showed that 53% of individuals had reduced their preventative- and service-level access for noncommunicable diseases either partly or completely (25). These lifestyle changes could lead to insulin resistance, which would further trigger inflammatory pathways, leading to new-onset diabetes.
Stress Hyperglycemia and New-Onset Diabetes Following Acute Illness
The phenomenon of hyperglycemia and new-onset diabetes following admission to the hospital with acute illness is not new and was previously observed during the SARS-CoV-1 outbreak, where new-onset diabetes without glucocorticoid use on admission was also associated with increased mortality (26). Stress hyperglycemia is a sign of relative insulin deficiency, which is associated with increased lipolysis and increased circulating free fatty acids seen in acute illness such as myocardial infarction or severe infections (27). In COVID-19, stress hyperglycemia may be even more severe due to the cytokine storm.
Studies have shown that patients with newly diagnosed diabetes have higher levels of inflammatory markers such as C-reactive protein, erythrocyte sedimentation rate, and white blood cells (5). Acute inflammation seen in cytokine storm may worsen insulin resistance (10), with one study showing neutrophils, d-dimers, and inflammatory markers being significantly higher in those with hyperglycemia than in those with normal glucose (18). People with obesity are also at risk for diabetes and severe outcomes related to COVID-19 (28), with adiposity being a driver for impaired glucose metabolism, immune responses, and inflammation (10).
Previous studies have reported stress hyperglycemia after several acute conditions, including myocardial infarction. However, there have been difficulties in interpretation of these studies due to the variable definitions used to define new-onset diabetes and stress hyperglycemia. One systematic review of 15 studies of patients admitted with myocardial infarction without diabetes with a glucose level in the range 6.1–8.2 mmol/L was associated with a 3.5-fold (95% CI 2.9–5.4) higher risk of death than that for patients without diabetes with lower glucose concentrations (27). This meta-analysis also reported that glucose values in the range of 8.0–10.0 mmol/L on admission were associated with an increased risk of congestive heart failure or cardiogenic shock in people without diabetes (27), and the risk of death was increased by 70% (relative risk 1.7, 95% CI 1.2–2.4) (27). Stress hyperglycemia following myocardial infarction has also been shown to be associated with an increased risk of in-hospital mortality in patients with and without diabetes (27). Another systematic review of 43 studies totaling 536,476 patients showed that stress hyperglycemia was associated with increased mortality, intensive care unit admission, hospital length of stay, and mechanical ventilation (29).
Although stress-related hyperglycemia in acutely ill hospitalized patients occurs in many settings, the data related to new-onset diabetes due to SARS-CoV-2 seem to suggest that the prevalence is disproportionate compared with data from populations admitted with other acute illnesses (16). A number of studies have reported stress hyperglycemia following critical acute illness; however, only a few studies have followed these patients beyond hospitalization to determine if the stress hyperglycemia is transient or indicative of new-onset diabetes. One meta-analysis of four cohort studies with 2,923 participants included 698 (23.9%) people with stress hyperglycemia. On follow-up more than 3 months after hospital discharge, 131 cases or 18.8% of people with stress hyperglycemia were identified with newly diagnosed diabetes, and stress hyperglycemia was associated with an increased incidence of diabetes (odds ratio [OR] 3.48, 95% CI 2.02–5.98) (30). However, three studies defined stress hyperglycemia as blood glucose of ≥7.8 mmol/L, and one database study defined it as a glucose of >11.1 mmol/L. Furthermore, the timing of glucose measurement was not reported in any of these studies.
Viral Infections and New-Onset Diabetes
Viral infections may have a direct or indirect effect on the pancreas. Previous studies have reported acute inflammation in the pancreas due to other viruses, such as human immunodeficiency virus, mumps, measles, cytomegalovirus virus, herpes simplex virus, and hepatitis virus (13). A meta-analysis of 24 case-control studies showed that enterovirus infection was significantly associated with T1D-related autoimmunity (OR 3.7, 95% CI 2.1–6.8) and clinical T1D (OR 9.8, 95% CI 5.5–17.4) (31). Another meta-analysis of 34 studies showed that there was a significantly increased risk of T2D with hepatitis C viral infection compared with noninfected control subjects in both retrospective (OR 1.68, 95% CI 1.15–2.20) and prospective (OR 1.67, 95% CI 1.28–2.06) studies. The excess risk was also observed compared with hepatitis B virus–infected control subjects (OR 1.80, 95% CI 1.20–1.40) (32). Studies of human islet cells have shown that coxsackie B viruses cause functional impairment or β-cell death (33).
Acute hyperglycemia with coronavirus infection has been linked to the binding of the coronavirus to the ACE2 receptor in the pancreatic islet cells (30). ACE2 expression has been shown to be higher in the pancreas than the lungs and expressed in both exocrine glands and the islets of the pancreas, including β-cells (34,35). However, the evidence for ACE2 expression in pancreatic cells is conflicting, with studies suggesting ACE2 expression in a limited subset of β-cells (36). Data from human pancreatic tissues identified ACE2 expression in pancreatic ductal epithelium and microvasculature and concluded that SARS-CoV-2 infection of pancreatic endocrine cells (including β-cells) is unlikely to be a central mechanism related to diabetes (37). Alternatively, the proinflammatory cytokines and acute-phase reactants due to COVID-19 could directly cause inflammation and damage to pancreatic β-cells (38).
A cytokine storm in people infected with SARS-CoV-2 is a prothrombotic, highly inflammatory pathological state that can have direct and indirect effects on pancreatic β-cells. An autopsy study of three patients who died of COVID-19 in China reported they had degeneration of islets (39). A study from Wuhan of 121 COVID-19 patients showed that even patients with mild COVID-19 had increased levels of amylase and lipase (1.85%), although people with severe COVID-19 had much higher levels (17%) (34). Some patients also had symptoms of acute pancreatitis. In this study, computed tomography scans of people with severe COVID-19 showed changes in the pancreas that comprised mainly enlargement of the pancreas or dilation of the pancreatic duct without acute necrosis (34). A recent study of gene and protein expression in live human pancreatic cultures and postmortem pancreatic tissue from COVID-19 patients observed that SARS-CoV-2 can infect pancreatic cells and indicated that endocrine islets and exocrine acinar and ductal cells within the pancreas allow SAR-CoV-2 entry (40). Another study reported that the SARS-CoV-2 receptor and ACE2 and related entry factors are expressed in the pancreatic β-cells, and in COVID-19 patients they infect β-cells, attenuate pancreatic insulin levels and secretion, and induce β-cell apoptosis (41).
In-Hospital Steroid-Induced Hyperglycemia
Steroid-induced hyperglycemia is common in hospitalized patients. Previous studies show that 53–70% of individuals without diabetes develop steroid-induced hyperglycemia (42). An Australian study of 80 hospitalized people without diabetes reported that 70% of subjects had at least one blood glucose measurement of ≥10 mmol/L (43). A meta-analysis of 13 studies showed that overall, 32.3% of people developed glucocorticoid-induced hyperglycemia and 18.6% developed diabetes (44). Use of steroids, particularly following the publication of the RECOVERY trial with the use of dexamethasone in people admitted to the hospital with COVID-19, may therefore also be associated with an increased risk of developing diabetes, which again could be directly related to steroid-induced abnormalities with delayed or blunted recovery of βcell damage (10).
Management of People With New-Onset Diabetes Following COVID-19
As the precise mechanisms and epidemiology of new-onset diabetes related to COVID-19 are not known, it is difficult to guide management pathways for these patients. However, in view of increased mortality in people with new-onset diabetes and in those with elevated glucose at admission, hospital protocols should include management of acute hyperglycemia. It is also imperative to recognize new-onset diabetes and manage DKA in people admitted to the hospital to improve outcomes. These patients frequently also require higher doses of insulin than those with acute illness caused by other conditions or non–COVID-19 DKA (18,45,46).
Whether hospital admission of new-onset diabetes is likely to remain permanent is not known, as long-term follow-up of these patients is limited. People with stress hyperglycemia may revert to normoglycemia following the recovery from acute illness and, therefore, may not be classed as having diabetes or requiring any glucose-lowering medication; they will require follow-up to determine if the new-onset diabetes is indeed permanent.
Although there are no data on follow-up of newly diagnosed people with diabetes related to COVID-19, one systematic review of four cohort studies with a 3-month follow-up reported 18.8% with newly diagnosed diabetes in those who were diagnosed with in-hospital hyperglycemia. However, studies differed in their definitions of stress hyperglycemia, participants included, and follow-up (30). In another prospective study, 181 consecutive patients admitted with myocardial infection in Sweden with an admission glucose of ≥11.1 mmol/L had a 75-g oral glucose tolerance test at 3 months postdischarge (47). Overall, 35% and 40% of patients, respectively, had impaired glucose tolerance at discharge and at 3 months postdischarge, and 31% and 25%, respectively, had new-onset diabetes (47).
A recent case series from India reported that three individuals who had COVID-19 and developed acute-onset diabetes and DKA initially responded to treatment with intravenous fluid and insulin. They were then transitioned to multiple doses of subcutaneous insulin, and, at follow-up of 4–6 weeks, all had their insulin stopped and were initiated on oral glucose-lowering agents (20). Two patients had GAD antibody measured and were both negative. Although new-acute-onset diabetes with DKA in adults would normally indicate T1D, these case data suggest that these patients have had a transient insulinopenia.
Persistent diabetes in COVID-19 patients may also be related to “long COVID,” also known as post–COVID-19 syndrome or post-acute sequelae of COVID-19 (PASC), defined as persistence of symptoms beyond 3 months postinfection. It frequently affects multiple organ systems and is estimated to affect 10% of COVID-19 patients (48,49). Long COVID is complex due to varying symptoms and pathophysiology (48,49) but may be due to immune and inflammatory responses seen in many severe acute viral infections (49). The risks of cardiorenal complications are high in people admitted with COVID-19, and a meta-analysis of 44 studies showed that the prevalence of cardiorenal complications is high in people with long COVID, with acute cardiac injury occurring in 15%, venous thromboembolism in 15%, and acute kidney injury in 6% (50).
As risk factors for poor outcomes in people with COVID-19 include obesity, hyperglycemia, and cardiovascular and renal disease, glucose-lowering agents that improve metabolic function without weight gain would be preferable for long-term management of people following acute COVID-19 infection and sustained symptoms (i.e., long COVID). Novel therapeutic options include sodium–glucose cotransporter 2 inhibitors (SGLT2i) and glucagon-like peptide 1 receptor agonists (GLP-1RAs), particularly as cardiovascular outcome trials in people with T2D have confirmed benefits on weight, glycemic control, and cardiovascular events, including cardiovascular death and renal outcomes (51). SGLT2i have also been shown to reduce hospitalization for heart failure and may reduce the risk of death from noncardiovascular causes (51). However, data for these therapies in management of patients with long COVID are lacking.
The DARE-19 study investigating the safety of dapagliflozin in people admitted to the hospital with COVID-19 have recently been reported (52). The study showed that the primary end points were not achieved; namely, dapagliflozin did not prevent organ dysfunction (pulmonary, cardiac, or renal) or death and did not improve clinical recovery within 30 days following commencing the medication. However, DKA was reported in two patients with T2D of the 625 patients in the dapagliflozin arm, with the events being nonsevere and resolving after study medication discontinuation. Other therapeutic trials are ongoing with dipeptidyl peptidase 4 inhibitors, pioglitazone, and the GLP-1RA semaglutide (53–58).
Long-term follow-up of patients with COVID-19 and hyperglycemia will therefore be required to determine whether they would still need glucose-lowering agents. A recent study from China reported new-onset diabetes in 3.3% of 1,733 people at 6 months following discharge from hospital with COVID-19 (59). Another study from England of 47,780 people discharged from hospital following admission for COVID-19 showed 4.9% developed diabetes at a mean follow-up of 140 days (60). Another study using a national health care database of the U.S. Department of Veterans Affairs reported a higher burden of new-onset diabetes 6 months following COVID-19 (61). However, none of these studies reported any further details regarding new-onset diabetes, including type of diabetes. COVID-19–related hyperglycemia and new-onset diabetes are new findings and of great interest globally. However, it remains to be seen if hyperglycemia associated with COVID-19 is indeed associated with a higher prevalence of new-onset diabetes after acute and chronic illness. The diagnosis of diabetes will need to be based on fasting glucose, 2-h post–oral glucose tolerance test, or HbA1c as recommended by international guidelines (62). Previous studies have demonstrated that new-onset diabetes is associated with the level of in-hospital hyperglycemia. One systematic review of 18 studies (111,078 patients) admitted with acute or chronic illness reported new-onset diabetes in 4% (95% CI 2–7%), 12% (95% CI 9–15%), and 28% (95% CI 18–39%) of patients with in-hospital normoglycemia, mild hyperglycemia, and severe hyperglycemia, respectively (3). Studies in the meta-analysis had a mean follow-up of 3–60 months without significant effect on diabetes incidence.
It will also be important to continue long-term surveillance of people with new-onset diabetes to ensure their risk factors are managed and that they achieve good glycemic control, as many may also have other symptoms of long COVID. Stress hyperglycemia due to acute critical illness may also identify patients who are already at high risk of diabetes, and therefore early diagnosis, interventions, and long-term follow-up of complications are essential for these patients. Whether screening everyone following a diagnosis of COVID-19 for diabetes and prediabetes would identify a significant number of people or is cost-effective remains to be seen. However, there may be a case for this, as many international guidelines recommend screening high-risk populations for diabetes and prediabetes and, if identified, to then manage people with diabetes according to international guidelines or lifestyle intervention of people with prediabetes. In view of the associated cardiovascular and renal damage following COVID-19, these patients should have regular monitoring of cardiovascular and kidney risk factors with a view to tight risk factor control. These patients may also benefit from regular screening for microvascular and macrovascular complications.
Future Research Recommendations
New-onset diabetes in relation to COVID-19 is a new phenomenon and provides an opportunity to observe these patients longer term and conduct research studies that include epidemiological and interventional approaches. An international group of researchers have already established a global registry of patients with new-onset COVID-19–related diabetes, called the CoviDIAB Project, and will report on findings in future (63). However, further international collaborative research programs are urgently needed to understand the natural disease epidemiology of COVID-19.
Recommendations for future studies should include the following:
Multicenter prospective cohort studies following these patients for several years to assess the trajectory of new-onset diabetes with COVID-19 and quantify whether the risks of admission-related hyperglycemia and new-onset diabetes with COVID-19 are different from usual-onset diabetes.
Investigation of pathophysiology by cross-sectional and prospective studies to assess β-cell function and insulin resistance in people with COVID-19 related to new-onset diabetes.
Experimental studies of direct effects of SARS-CoV-2 on pancreatic β-cells and other islet cell types.
Assessment of inflammatory markers to get full understanding of new-onset COVID-19–related diabetes.
Development and validation of methods of screening for diabetes in people who have developed COVID-19–related hyperglycemia.
Modeling of cost-effectiveness of targeted screening of people following COVID-19.
Evaluation of management plans and models of care that may be appropriate to this phenomenon.
Determination of prevalence and impact of long COVID in people with new-onset diabetes.
Comparisons of longer-term outcomes of people with COVID-19–related new-onset diabetes with new-onset diabetes due to other acute illnesses (such as other infections and myocardial infarction).
Understanding of the benefits and cost-effectiveness of use of different therapeutic options, including novel therapies such as SGLT2i and GLP-1RAs.
Conclusions
Recently published studies suggest that COVID-19 is associated with new-onset diabetes; therefore, there is potential to identify and manage these people early, with the aim of improving long-term outcomes. Whether elevated glucose concentrations (in a non-diabetes range) or new-onset diabetes is due to immune-mediated and inflammatory responses, the direct effect of SARS-CoV-2 on β-cells, or a complex combination of mechanisms, is not known. The majority of studies have mainly assessed patients who have been hospitalized with COVID-19, and there are no or limited data on patients with milder illness managed in the community. There are also no data on long-term outcomes of people with diabetes and COVID-19 and their risk of long COVID. New-onset diabetes with SARS-CoV-2 infection also appears to be a complex syndrome associated with a number of pathophysiological mechanisms and, given we are still in the midst of a global COVID-19 pandemic, are likely to see even larger numbers of people globally with new-onset diabetes. International efforts need to be established to study COVID-19–associated new-onset diabetes with follow-up of large numbers of patients.
Article Information
Funding. K.K. is supported by the National Institute for Health Research (NIHR) Applied Research Collaboration East Midlands and the NIHR Leicester Biomedical Research Centre. J.B.B. is supported by grants from the National Institutes of Health (UL1TR002489 and P30DK124723). S.E.K. is supported by the United States Department of Veterans Affairs.
The views expressed are those of the authors and not necessarily those of the NIHR, National Health Service, or the Department of Health and Social Care.
Duality of Interest. K.K. has acted as a consultant or speaker or received grants for investigator-initiated studies for AstraZeneca, Novartis, Novo Nordisk, Sanofi, Lilly and Merck Sharp & Dohme, Boehringer Ingelheim, Bayer, Berlin-Chemie AG/Menarini Group, Janssen, and Napp. S.D.P. has served on the advisory panel for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Co., GlaxoSmithKline, Merck & Co., Novartis Pharmaceuticals, Novo Nordisk, Sanofi, and Takeda Pharmaceuticals. He received research support from AstraZeneca and Boehringer Ingelheim. C.M. serves or has served on the advisory panel for Novo Nordisk, Sanofi, Merck Sharp & Dohme, Eli Lilly and Co., Novartis, AstraZeneca, Boehringer Ingelheim, Roche, Medtronic, ActoBio Therapeutics, Pfizer, Insulet, and Zealand Pharma. Financial compensation for these activities has been received by KU Leuven. KU Leuven has received research support for C.M. from Medtronic, Novo Nordisk, Sanofi, and ActoBio Therapeutics. C.M. serves or has served on the speakers bureau for Novo Nordisk, Sanofi, Eli Lilly and Co., Boehringer Ingelheim, Astra Zeneca, and Novartis. Financial compensation for these activities has been received by KU Leuven. S.E.K. has served as a consultant and on advisory boards for Bayer, Boehringer Ingelheim, Casma Therapeutics, Eli Lilly and Co., Intarcia, Merck, Novo Nordisk, Pfizer, and Third Rock Ventures and as a speaker for Boehringer Ingelheim and Merck. R.A.G. is an advisor to Onduo, Vida Health, Lark, and HealthReveal. J.B.B.’s contracted consulting fees and travel support for contracted activities are paid to the University of North Carolina by Adocia, Novo Nordisk, Senseonics, and vTv Therapeutics, as well as grant support from Dexcom, NovaTarg, Novo Nordisk, Sanofi, Tolerion, and vTv Therapeutics. He is also a consultant to Anji, AstraZeneca, Boehringer Ingelheim, Cirius Therapeutics Inc., Eli Lilly and Co., Fortress Biotech, Glycadia, Glyscend, Janssen, Mellitus Health, Moderna, Pendulum Therapeutics, Praetego, Stability Health, and Zealand Pharma. He holds stock/options in Glyscend, Mellitus Health, Pendulum Therapeutics, PhaseBio, Praetego, and Stability Health.
Author Contributions. K.K. researched the data for this review. K.K. wrote the first draft of this manuscript, and all other authors contributed to and provided critical feedback and edits on the manuscript. K.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the review and its conclusions.
Footnotes
This article is part of a special article collection available at https://care.diabetesjournals.org/collection/diabetes-and-COVID19.
References
- 1. Worldometer . Worldometer COVID-19 data. Accessed 27 July 2020. Available from https://www.worldometers.info/coronavirus/about
- 2. Singh AK, Gillies CL, Singh R, et al. Prevalence of co-morbidities and their association with mortality in patients with COVID-19: a systematic review and meta-analysis. Diabetes Obes Metab 2020;22:1915–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jivanji CJ, Asrani VM, Windsor JA, Petrov MS. New-onset diabetes after acute and critical illness: a systematic review. Mayo Clin Proc 2017;92:762–773 [DOI] [PubMed] [Google Scholar]
- 4. Bode B, Garrett V, Messler J, et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol 2020;14:813–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li H, Tian S, Chen T, et al. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID-19. Diabetes Obes Metab 2020;22:1897–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Unsworth R, Wallace S, Oliver NS, et al. New-onset type 1 diabetes in children during COVID-19: multicenter regional findings in the U.K. Diabetes Care 2020;43:e170–e171 [DOI] [PubMed] [Google Scholar]
- 7. McGlacken-Byrne SM, Drew SEV, Turner K, Peters C, Amin R. The SARS-CoV-2 pandemic is associated with increased severity of presentation of childhood onset type 1 diabetes mellitus: a multi-centre study of the first COVID-19 wave. Diabet Med 2021;38:e14640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tittel SR, Rosenbauer J, Kamrath C, et al.; DPV Initiative . Did the COVID-19 lockdown affect the incidence of pediatric type 1 diabetes in Germany? Diabetes Care 2020;43:e172–e173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kamrath C, Mönkemöller K, Biester T, et al. Ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the COVID-19 pandemic in Germany. JAMA 2020;324:801–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Accili D. Can COVID-19 cause diabetes? Nat Metab 2021;3:123–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alraddadi BM, Watson JT, Almarashi A, et al. Risk factors for primary Middle East respiratory syndrome coronavirus illness in humans, Saudi Arabia, 2014. Emerg Infect Dis 2016;22:49–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab 2020;22:1935–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rawla P, Bandaru SS, Vellipuram AR. Review of infectious etiology of acute pancreatitis. Gastroenterol Res 2017;10:153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Armeni E, Aziz U, Qamar S, et al. Protracted ketonaemia in hyperglycaemic emergencies in COVID-19: a retrospective case series. Lancet Diabetes Endocrinol 2020;8:660–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pasquel FJ, Messler J, Booth R, et al. Characteristics of and mortality associated with diabetic ketoacidosis among US patients hospitalized with or without COVID-19. JAMA Netw Open 2021;4:e211091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sathish T, Kapoor N, Cao Y, Tapp RJ, Zimmet P. Proportion of newly diagnosed diabetes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Obes Metab 2021;23:870–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care 2020;43:e83–e85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coppelli A, Giannarelli R, Aragona M, et al.; Pisa COVID-19 Study Group . Hyperglycemia at hospital admission is associated with severity of the prognosis in patients hospitalized for COVID-19: the Pisa COVID-19 study. Diabetes Care 2020;43:2345–2348 [DOI] [PubMed] [Google Scholar]
- 19. Hollstein T, Schulte DM, Schulz J, et al. Autoantibody-negative insulin-dependent diabetes mellitus after SARS-CoV-2 infection: a case report. Nat Metab 2020;2:1021–1024 [DOI] [PubMed] [Google Scholar]
- 20. Kuchay MS, Reddy PK, Gagneja S, Mathew A, Mishra SK. Short term follow-up of patients presenting with acute onset diabetes and diabetic ketoacidosis during an episode of COVID-19. Diabetes Metab Syndr 2020;14:2039–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol 2010;47:193–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ghosh A, Anjana RM, Shanthi Rani CS, et al. Glycemic parameters in patients with new-onset diabetes during COVID-19 pandemic are more severe than in patients with new-onset diabetes before the pandemic: NOD COVID India study. Diabetes Metab Syndr 2021;15:215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salmi H, Heinonen S, Hästbacka J, et al. New-onset type 1 diabetes in Finnish children during the COVID-19 pandemic. Arch Dis Child 27 May 2021 [Epub ahead of print]. DOI: https://doi.org/10.1136/archdischild-2020-321220 [DOI] [PubMed] [Google Scholar]
- 24. Muniangi-Muhitu H, Akalestou E, Salem V, Misra S, Oliver NS, Rutter GA. Covid-19 and diabetes: a complex bidirectional relationship. Front Endocrinol (Lausanne) 2020;11:582936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. World Health Organization . COVID-19 significantly impacts health services for noncommunicable diseases. Published 1 June 2020. Accessed 12 March 2021. Available from https://www.who.int/news/item/01-06-2020- covid-19-significantly-impacts-health-services -for-noncommunicable-diseases
- 26. Yang JK, Feng Y, Yuan MY, et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med 2006;23:623–628 [DOI] [PubMed] [Google Scholar]
- 27. Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet 2000;355:773–778 [DOI] [PubMed] [Google Scholar]
- 28. Seidu S, Gillies C, Zaccardi F, et al. The impact of obesity on severe disease and mortality in people with SARS-CoV-2: a systematic review and meta-analysis. Endocrinol Diabetes Metab 2020;e00176:e00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Olariu E, Pooley N, Danel A, Miret M, Preiser JC. A systematic scoping review on the consequences of stress-related hyperglycaemia. PLoS One 2018;13:e0194952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ali Abdelhamid Y, Kar P, Finnis ME, et al. Stress hyperglycaemia in critically ill patients and the subsequent risk of diabetes: a systematic review and meta-analysis. Crit Care 2016;20:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yeung WC, Rawlinson WD, Craig ME. Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ 2011;342:d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. White DL, Ratziu V, El-Serag HB. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J Hepatol 2008;49:831–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roivainen M, Rasilainen S, Ylipaasto P, et al. Mechanisms of coxsackievirus-induced damage to human pancreatic beta-cells. J Clin Endocrinol Metab 2000;85:432–440 [DOI] [PubMed] [Google Scholar]
- 34. Liu F, Long X, Zhang B, Zhang W, Chen X, Zhang Z. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin Gastroenterol Hepatol 2020;18:2128–2130.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fignani D, Licata G, Brusco N, et al. SARS-CoV-2 receptor angiotensin I-converting enzyme type 2 (ACE2) is expressed in human pancreatic β-cells and in the human pancreas microvasculature. Front Endocrinol (Lausanne) 2020;11:596898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Atkinson MA, Powers AC. Distinguishing the real from the hyperglycaemia: does COVID-19 induce diabetes? Lancet Diabetes Endocrinol 2021;9:328–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kusmartseva I, Wu W, Syed F, et al. Expression of SARS-CoV-2 entry factors in the pancreas of normal organ donors and individuals with COVID-19. Cell Metab 2020;32:1041–1051.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ahlqvist E, Storm P, Käräjämäki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol 2018;6:361–369 [DOI] [PubMed] [Google Scholar]
- 39. Yao XH, Li TY, He ZC, et al. [A pathological report of three COVID-19 cases by minimal invasive autopsies]. Zhonghua Bing Li Xue Za Zhi 2020;49:411–417 [DOI] [PubMed] [Google Scholar]
- 40. Shaharuddin SH, Wang V, Santos RS, et al. Deleterious effects of SARS-CoV-2 infection on human pancreatic cells. Front Cell Infect Microbiol 2021;11:678482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu CT, Lidsky PV, Xiao Y, et al. SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment. Cell Metab 2021;33:1565–1576.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cheung NW. Steroid-induced hyperglycaemia in hospitalised patients: does it matter? Diabetologia 2016;59:2507–2509 [DOI] [PubMed] [Google Scholar]
- 43. Fong AC, Cheung NW. The high incidence of steroid-induced hyperglycaemia in hospital. Diabetes Res Clin Pract 2013;99:277–280 [DOI] [PubMed] [Google Scholar]
- 44. Liu XX, Zhu XM, Miao Q, Ye HY, Zhang ZY, Li YM. Hyperglycemia induced by glucocorticoids in nondiabetic patients: a meta-analysis. Ann Nutr Metab 2014;65:324–332 [DOI] [PubMed] [Google Scholar]
- 45. Bornstein SR, Rubino F, Khunti K, et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol 2020;8:546–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu L, Girgis CM, Cheung NW. COVID-19 and diabetes: insulin requirements parallel illness severity in critically unwell patients. Clin Endocrinol (Oxf) 2020;93:390–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Norhammar A, Tenerz A, Nilsson G, et al. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet 2002;359:2140–2144 [DOI] [PubMed] [Google Scholar]
- 48. Dennis A, Wamil M, Kapur S, et al. Multi-organ impairment in low-risk individuals with long COVID. medRxiv. 16 October 2020 [preprint]. DOI: https://doi.org/10.1101/2020.10.14.20212555 [Google Scholar]
- 49. Altmann DM, Boyton RJ. Decoding the unknowns in long COVID. BMJ 2021;372:n132. [DOI] [PubMed] [Google Scholar]
- 50. Potere N, Valeriani E, Candeloro M, et al. Acute complications and mortality in hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Crit Care 2020;24:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation 2019;139:2022–2031 [DOI] [PubMed] [Google Scholar]
- 52. Kosiborod MN, Esterline R, Furtado RHM, et al. Dapagliflozin in patients with cardiometabolic risk factors hospitalised with COVID-19 (DARE-19): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol 2021;9:586–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. U.S. National Library of Medicine . Effects of DPP4 inhibition on COVID-19. ClinicalTrials.gov identifier NCT04341935. Published 10 April 2020. Accessed 9 April 2021. Available from https://clinicaltrials.gov/ct2/show/NCT04341935
- 54. U.S. National Library of Medicine . Efficacy and safety of dipeptidyl peptidase-4 inhibitors in diabetic patients with established COVID-19. ClinicalTrials.gov identifier NCT04371978. Published 1 May 2020. Accessed 9 April 2021. Available from https://clinicaltrials.gov/ct2/show/NCT04371978
- 55. U.S. National Library of Medicine . The effect of sitagliptin treatment in COVID-19 positive diabetic patients (SIDIACO). ClinicalTrials.gov identifier NCT04365517. Published 28 April 2020. Accessed 9 April 2021. Available from https://clinicaltrials.gov/ct2/show/NCT04365517
- 56. U.S. National Library of Medicine . Metformin glycinate in patients with MS or DM2, hospitalized with COVID-19 and SARS secondary to SARS-CoV-2 (DMMETCOV19). ClinicalTrials.gov identifier NCT04626089. Published 12 November 2020. Accessed 9 April 2021. Available from https://clinicaltrials.gov/ct2/show/NCT04626089
- 57. U.S. National Library of Medicine . Effect of pioglitazone on T2DM patients with COVID-19 (PIOQ8). ClinicalTrials.gov identifier NCT04604223. Published 27 October 2020. Accessed 9 April 2021. Available from https://clinicaltrials.gov/ct2/show/NCT04604223
- 58. U.S. National Library of Medicine . Semaglutide to reduce myocardial injury in patients with COVID-19 (SEMPATICO). ClinicalTrials.gov identifier NCT04615871. Published 4 November 2020. Accessed 9 April 2021. Available from https://clinicaltrials.gov/ct2/show/NCT04615871
- 59. Huang C, Huang L, Wang Y, et al. 6-Month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021;397:220–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ayoubkhani D, Khunti K, Nafilyan V, et al. Post-covid syndrome in individuals admitted to hospital with COVID-19: retrospective cohort study. BMJ 2021;372:n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021;594:259–264 [DOI] [PubMed] [Google Scholar]
- 62. American Diabetes Association . 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S14–S31 [DOI] [PubMed] [Google Scholar]
- 63. Rubino F, Amiel SA, Zimmet P, et al. New-onset diabetes in Covid-19. N Engl J Med 2020;383:789–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang S, Ma P, Zhang S, et al. Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: a multi-centre retros pective study. Diabetologia 2020;63:2102–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yang J-K, Jin J-M, Liu S, et al. New onset COVID-19–related diabetes: an indicator of mortality. medRxiv. 26 June 2020 [preprint]. DOI: 10.1101/2020.04.08.20058040. [Google Scholar]
- 66. Fadini GP, Morieri ML, Boscari F, et al. Newly-diagnosed diabetes and admission hyperglycemia predict COVID-19 severity by aggravating respiratory deterioration. Diabetes Res Clin Pract 2020;168:108374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang J, Kong W, Xia P, et al. Impaired fasting glucose and diabetes are related to higher risks of complications and mortality among patients with coronavirus disease 2019. Front Endocrinol (Lausanne) 2020;11:525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Smith SM, Boppana A, Traupman JA, et al. Impaired glucose metabolism in patients with diabetes, prediabetes, and obesity is associated with severe COVID-19. J Med Virol 2021;93:409–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Liu Y, Lu R, Wang J, et al. Diabetes, even newly defined by HbA1c testing, is associated with an increased risk of in-hospital death in adults with COVID-19. BMC Endocr Disord 2021;21:56. [DOI] [PMC free article] [PubMed] [Google Scholar]


