Abstract
OBJECTIVE
Irregular menstrual cycles are associated with increased cardiovascular mortality. Polycystic ovary syndrome (PCOS) is characterized by androgen excess and irregular menses; androgens are drivers of increased metabolic risk in women with PCOS. Combined oral contraceptive pills (COCPs) are used in PCOS both for cycle regulation and to reduce the biologically active androgen fraction. We examined COCP use and risk of dysglycemia (prediabetes and type 2 diabetes) in women with PCOS.
RESEARCH DESIGN AND METHODS
Using a large U.K. primary care database (The Health Improvement Network [THIN]; 3.7 million patients from 787 practices), we carried out a retrospective population-based cohort study to determine dysglycemia risk (64,051 women with PCOS and 123,545 matched control subjects), as well as a nested pharmacoepidemiological case-control study to investigate COCP use in relation to dysglycemia risk (2,407 women with PCOS with [case subjects] and without [control subjects] a diagnosis of dysglycemia during follow-up). Cox models were used to estimate the unadjusted and adjusted hazard ratio, and conditional logistic regression was used to obtain adjusted odds ratios (aORs).
RESULTS
The adjusted hazard ratio for dysglycemia in women with PCOS was 1.87 (95% CI 1.78–1.97, P < 0.001; adjustment for age, social deprivation, BMI, ethnicity, and smoking), with increased rates of dysglycemia in all BMI subgroups. Women with PCOS and COCP use had a reduced dysglycemia risk (aOR 0.72, 95% CI 0.59–0.87).
CONCLUSIONS
In this study, limited by its retrospective nature and the use of routinely collected electronic general practice record data, which does not allow for exclusion of the impact of prescription-by-indication bias, women with PCOS exposed to COCPs had a reduced risk of dysglycemia across all BMI subgroups. Future prospective studies should be considered for further understanding of these observations and potential causality.
Introduction
Polycystic ovary syndrome (PCOS) is the most prevalent endocrine disorder in women of reproductive age (1) and is defined by irregular menses and androgen excess. While previously mostly perceived as a reproductive disorder, PCOS is now recognized as a lifelong metabolic disorder with an increased prevalence of the cardiovascular risk factors insulin resistance, dyslipidemia, and hypertension (2–4). An increased risk of type 2 diabetes in women with PCOS has been described in both cross-sectional (5) and cohort studies (5,6), the latter reporting a two- to fourfold increased risk, with type 2 diabetes diagnosed on average 4 years earlier in women with PCOS than in the background population (7). In a population-based cohort study, women with PCOS were also found to have an increased risk of nonalcoholic fatty liver disease (NAFLD), a significant hepatic complication of metabolic syndrome (3). In a recently published prospective cohort study (8) of nearly 80,000 women with a follow-up period of 24 years, investigators described an increased risk of premature mortality, primarily due to cardiovascular disease, in women with irregular and long menstrual cycles, suggestive of PCOS as the major underlying risk factor.
Androgen excess is a cardinal feature of PCOS (1), and its severity has been shown to correlate with insulin resistance in cross-sectional studies (9–11). In the general population, type 2 diabetes risk in women increases with circulating androgen concentrations and decreasing concentrations of sex hormone binding globulin (SHBG) (12–15). Endogenous androgen concentrations in women have also been identified as a risk factor for the development of NAFLD in PCOS, independent of body weight (3). Combined oral contraceptive pills (COCPs) are widely prescribed in women with PCOS for menstrual cycle regulation. In addition, COCPs can exert antiandrogen effects through two distinct mechanisms. The estrogen component in COCPs increases the production of SHBG in the liver, thereby reducing the concentration of free testosterone capable of binding and activating the androgen receptor in target tissues of androgen action (16). Furthermore, some progestins used in COCPs can convey additional antiandrogenic action through androgen receptor blockade, namely, cyproterone and drospirenone, while other progestins are proandrogenic or exert no effect on the androgen receptor.
Data on the impact of COCP prescription on glucose metabolism in women are conflicting. Clinicians have previously raised concerns that COCP intake may adversely impact on glucose metabolism (17); however, available evidence is limited by a lack of prospective studies and by the confounding issue of higher ethinylestradiol content in historical formulations. Furthermore, a wide diversity of combined oral contraceptive formulations available, including differences in progestin components, makes an accurate assessment of the impact of COCP prescription on glycemia very challenging. In a recent Cochrane library review the authors concluded that current evidence suggests no significant impact on carbohydrate metabolism in women without PCOS, highlighting a paucity of large-scale prospective studies to adequately address the question (18). Conversely, it has been hypothesized that the impact of COCP on carbohydrate metabolism may be protective against incident dysglycemia, due to the impact both of raising SHBG levels and of partial androgen receptor blockade in selected formulations containing antiandrogenic progestins.
Here we tested the hypothesis that the use of COCPs decreases the risk of type 2 diabetes in women with PCOS. To this end, undertaking a population-based cohort study, we first determined the risk of incident dysglycemia, i.e., a composite outcome combining prediabetes and type 2 diabetes, in women with PCOS and then examined in a nested pharmacoepidemiological case-control study whether COCP intake impacts on this risk.
Research Design and Methods
Data Source
Data sets were derived from a U.K. primary care database. There are >17 million patient records from 787 general practices in the IQVIA Medical Research Data (also known as The Health Improvement Network [THIN] database) (19). THIN uses Read codes, a hierarchical coding system for recording symptoms and diagnoses, and is highly suited for assessment of chronic health conditions (3,12,20).
Study Population
Our study population was comprised of women aged 18–50 years during the study period (1 January 2000–31 January 2017). Women were eligible 1 year after registration with their general practice or from the time their practice became eligible for THIN participation (3).
Study Designs
PCOS and Incident Dysglycemia
This matched cohort study considered women with PCOS as exposed. Exposure was ascertained by Read codes for “Polycystic ovary syndrome (PCOS)” or “Polycystic ovaries (PCO)” (3), as this composite code list reflects community prevalence (21). Each exposed woman was matched with up to two women without PCOS within the same general practice for age (±2 years) and BMI (±2 kg/m2) (22,23).
Follow-up start date or the index date for the exposed patients was set to PCOS diagnosis date for incident PCOS patients or patient eligibility date for prevalent PCOS patients (patients with a diagnosis ahead of cohort entry). The index date for a matched control subject was set to the corresponding index date of the exposed patient to mitigate immortality time bias (24). Follow-up occurred until the earliest occurrence of 1) outcome, 2) study end, or 3) patient censorship denoted by death, deregistration from the practice, or practice withdrawing from the THIN database.
Outcomes were type 2 diabetes and dysglycemia, with the latter defined as the composite outcome of prediabetes and type 2 diabetes, which were ascertained by Read codes and laboratory results (type 2 diabetes, HbA1c ≥6.5% [48 mmol/mol], fasting blood glucose ≥7 mmol/L; dysglycemia, HbA1c ≥6.0% (42 mmol/mol), fasting blood glucose ≥6 mmol/L, random blood glucose ≥11.1 mmol/L, and 2-h oral glucose tolerance test [OGTT] result indicated as “abnormal” or “high”). Patients with a recording of the outcome of interest (dysglycemia) or glucose-lowering drug prescription at baseline were not eligible.
Risk Factors of Type 2 Diabetes and Dysglycemia Among Women With PCOS
To identify potential risk factors for the development of type 2 diabetes and dysglycemia within the PCOS cohort, we examined demographic risk factors, BMI, clinical features of androgen excess, and prescription of COCPs at baseline as candidate risk factors.
Combined Oral Contraceptives and Incident Dysglycemia Among Women With PCOS
To define the impact of COCPs on dysglycemia risk, we conducted a nested case-control study. Women who developed dysglycemia during the follow-up period were case subjects, and the remaining women were potential control subjects. One control subject per case subject was randomly selected after matching for age (±2 years), BMI (±2 kg/m2), PCOS diagnosis date (±2 years), and whether PCOS was diagnosed before or after the patient became eligible to take part in the study. Index date was assigned as the date of diagnosis of dysglycemia for the case subjects, and the same date was assigned to the corresponding control, ensuring a comparable exposure window for matched case-control pairs and, therefore, avoiding time-window bias (25).
The exposure window was prespecified and extended from 1 year prior to cohort entry, to avoid disregarding valid prescriptions in the immediate period after patient registration, and 6 months prior to index date, to exclude prescriptions that cannot be validly attributed to the development of dysglycemia.
COCP prescription was initially considered as a binary variable. COCP prescription was then categorized according to whether the respective progestin component exerts antiandrogen activity. Patients with no prescription of COCP formed the reference groups for both the categorical exposure variables.
Analysis
Crude incidence rates of the primary and secondary composite outcome (type 2 diabetes and dysglycemia) were estimated per 10,000 person-years. Unadjusted and adjusted hazard ratios were obtained using Cox models. Covariates for adjustment were selected based on biological plausibility for confounding. Covariates include age, BMI, socioeconomic status, ethnicity, smoking status, and record of hypertension, hypothyroidism, and prescription of lipid-lowering medications. Socioeconomic status was presented with use of Townsend score (26–28). Ethnicity was categorized based on U.K. 2011 census classification. Smoking status was categorized as currently smoking, discontinued, and never smoked. Selection of Read code lists exposure, outcome, and covariates was based on methods and codes set out in previous publications (3,12,20,29) (Supplementary Table 1). BMI was categorized as per World Health Organization guidelines, with nonstandard BMI categorization of South Asian women as per the recommended guidelines (30).
Sensitivity Analyses
Sensitivity analyses were performed to assess the extent of misclassification and survival bias. The exposure was restricted, firstly, to women with PCOS-specific diagnostic codes and, secondly, to those with a PCOS diagnosis during the study period (incident patients) (31).
In addition, in the nested-case control study, we investigated whether control selection based on risk set sampling altered our findings, allowing a patient to serve as a control subject for multiple patients diagnosed with dysglycemia, while patients not diagnosed with dysglycemia at a similar time of follow-up could serve as control subjects before they developed dysglycemia.
Subgroup Analyses
To check whether risks of type 2 diabetes and dysglycemia are independent of BMI status, we conducted subgroup analyses within each BMI category.
Analyses for Predictors of Dysglycemia
In the cohort restricted to women with PCOS, Cox regression analysis was used to identify statistically significant predictors of type 2 diabetes and dysglycemia. In addition to covariates mentioned in the primary analysis, prescription of COCPs and variables characteristic of androgen excess and prescription of antiandrogen therapy with single agent drugs were also considered as candidate predictors.
Analysis of Nested Case-Control Study
Conditional logistic regression was performed to obtain unadjusted and adjusted ORs for dysglycemia based on exposure to COCP. The adjusted model included all covariates in the primary analysis, plus prescription of metformin and antiandrogen therapy.
Results
Study Population Characteristics
A total of 64,051 women with PCOS and 123,545 women without PCOS and matched for age, sex, and general practice were included in the study (Supplementary Fig. 1 and Table 1). The median follow-up period was 3.5 years (interquartile range [IQR] 1.4–7.2). Mean (SD) age of the whole cohort was 30.5 (7.1) years and median BMI 25.6 kg/m2 (IQR 22.1–31.4). Age, BMI, deprivation quintiles (Townsend index), and smoking status had no apparent imbalance in distribution between the two groups. Women with PCOS were more likely to be documented as South Asian (4.8% vs. 2.9%), hypothyroid (3.4% vs. 2.1%), and hypertensive (2.2% vs. 1.6%) at baseline (Table 1). COCPs were prescribed for 43.4% of the PCOS-exposed women before the index date; 22.5% of the women with PCOS were prescribed COCPs with an antiandrogenic progestin component (drospirenone or cyproterone acetate) (Table 1).
Table 1.
Baseline characteristics of participants of the population-based cohort study, with stratification by PCOS exposure status
| Women with PCOS (n = 64,051) | Women without PCOS (n = 123,545) | |
|---|---|---|
| Age, years, mean (SD) | 30.4 (7.0) | 30.5 (7.1) |
| BMI, kg/m2, median (IQR) | 25.9 (22.2–31.9) | 25.4 (22.0–30.8) |
| BMI categories, n (%)* | ||
| Normal/underweight | 23,490 (36.6) | 48,360 (39.1) |
| Overweight | 12,734 (19.8) | 25,229 (20.4) |
| Obese | 17,591 (27.5) | 29,907 (24.2) |
| Missing | 10,236 (16.0) | 20,049 (16.2) |
| Smoking status, n (%) | ||
| Nonsmoker | 37,311 (58.3) | 71,114 (57.6) |
| Discontinued | 9,044 (14.1) | 16,285 (13.2) |
| Smoker | 14,674 (22.9) | 28,284 (22.9) |
| Missing | 3,022 (4.7) | 7,862 (6.4) |
| Ethnicity, n (%) | ||
| Caucasian | 30,597 (47.8) | 50,206 (40.6) |
| Black | 1,464 (2.3) | 2,636 (2.1) |
| Chinese | 582 (0.91) | 883 (0.7) |
| South Asian | 3,085 (4.8) | 3,517 (2.9) |
| Mixed race | 897 (1.4) | 1,645 (1.3) |
| Missing | 27,426 (42.8) | 64,658 (52.3) |
| Townsend deprivation score, n (%) | ||
| 1 (least deprived) | 11,270 (17.6) | 21,839 (17.7) |
| 2 | 10,280 (16.1) | 19,866 (16.1) |
| 3 | 12,064 (18.8) | 23,471 (19.0) |
| 4 | 11,530 (18.0) | 22,623 (18.3) |
| 5 (most deprived) | 8,182 (12.8) | 16,186 (13.1) |
| Missing | 10,725 (16.7) | 19,560 (15.8) |
| Baseline comorbidity, n (%) | ||
| Hypothyroidism | 2,172 (3.4) | 2,585 (2.1) |
| Hypertension | 1,420 (2.22) | 2,030 (1.64) |
| Baseline medication, n (%) | ||
| Any COCP | 27,768 (43.4) | 66,332 (53.7) |
| COCP without antiandrogenic progestin | 25,481 (39.8) | 64,157 (51.9) |
| COCP with antiandrogenic progestin | 14,437 (22.5) | 12,336 (10.0) |
| Drospirenone | 4,944 (7.7) | 6,550 (5.3) |
| Cyproterone | 11,069 (17.3) | 7,305 (5.9) |
| Single-agent antiandrogen therapy† | ||
| Cyproterone | 444 (0.69) | |
| Other antiandrogen drugs^ | 42 (0.07) | |
| Lipid-lowering medication | 410 (0.64) | 534 (0.43) |
Normal/underweight, <23.5 kg/m2 for patients of South Asian ethnicity and <25 kg/m2 for patients of all other ethnic groups; overweight, 23.5–27.5 kg/m2 for patients of South Asian ethnicity and 25–30 kg/m2 for patients of all other ethnic groups; and obese, ≥27.5 kg/m2 for patients of South Asian ethnicity and ≥30 kg/m2 for patients of all other ethnic groups.
Includes dutasteride, enzalutamide, finasteride, flutamide, and spironolactone.
PCOS-relevant variables summarized only for the PCOS-exposed cohort; note that patients with impaired glucose regulation or glucose-lowering drug prescription at baseline were not included in the cohort.
Risk of Type 2 Diabetes and Dysglycemia
In the primary analysis, the incidence rate of type 2 diabetes among the exposed and the unexposed was 48.7 and 22.8 per 10,000 person-years during a median follow-up of 3.39 years (IQR 1.34–7.16) and 3.47 years (IQR 1.39–7.18), respectively, equating to a doubling in risk of type 2 diabetes among women with PCOS (hazard ratio 2.13, 95% CI 1.98–2.29, P < 0.001). Adjustment for age, deprivation quintiles, BMI category, ethnicity, smoking status, and hypothyroidism did not alter the estimated hazard ratio (adjusted hazard ratio [aHR] 2.04, 95% CI 1.89–2.20, P < 0.001) (Supplementary Table 2).
In analysis of the effect of PCOS on the composite outcome (dysglycemia), a similar effect was observed (aHR 1.87, 95% CI 1.78–1.97, P < 0.001). The incidence rates of dysglycemia were 96.3 and 49.4 per 10,000 person-years among women with and without PCOS during a median follow-up of 3.32 years (IQR 1.32–7.03) and 3.44 years (IQR 1.38–7.11), respectively (Supplementary Table 2).
Sensitivity Analysis
The strength of association between PCOS and type 2 diabetes did not decrease when the analysis was restricted to women with incident diagnosis of PCOS (aHR 1.98, 95% CI 1.70–2.31, P < 0.001) and to women with PCOS-specific codes (aHR 2.17, 95% CI 1.88–2.51, P < 0.001). This was similarly observed for dysglycemia (incident cohort, aHR 1.95, 95% CI 1.76–2.16, P < 0.001; PCOS-specific cohort, aHR 1.93, 95% CI 1.75–2.13, P < 0.001) (Supplementary Table 2).
Subgroup Analysis With Stratification by BMI
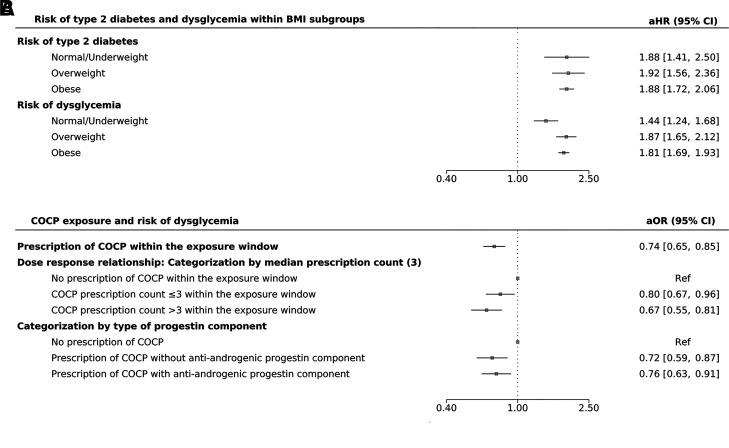
In subgroup analyses, women with PCOS had an increased risk of type 2 diabetes in all BMI categories compared with women without PCOS in the same BMI category (normal/underweight category, BMI <23 kg/m2 among women of South Asian ethnicity and <25 kg/m2 among women of all other ethnic groups, aHR 1.88, 95% CI 1.42–2.51, P < 0.001; overweight category, BMI 23–27.5 kg/m2 among women of South Asian ethnicity and 25–29.9 kg/m2 among women of all other ethnic groups, aHR1.92, 95% CI 1.56–2.35, P < 0.001; and obesity category, BMI ≥27.5 kg/m2 among women of South Asian ethnicity and ≥30 kg/m2 among women of all other ethnic groups, aHR 1.88, 95% CI 1.72–2.06, P < 0.001) (Fig. 1A). Similar findings were observed for the composite outcome (dysglycemia) (Fig. 1A).
Figure 1.
Risk of type 2 diabetes and dysglycemia among 64,051 women with PCOS compared with 123,545 matched control subjects and according to BMI subgroup (population-based cohort study [A]). aOR for risk of dysglycemia according to the prescription of COCPs (B) overall and according to prescription counts and type of progestin component, respectively, in the nested pharmacoepidemiological case-control study (2,407 women with PCOS with a diagnosis of dysglycemia during follow-up [case subjects] and 2,407 women with PCOS without a diagnosis of dysglycemia [control subjects]). Normal/underweight, <23.5 kg/m2 for patients of South Asian ethnicity and <25 kg/m2 for patients of all other ethnic groups; overweight, 23.5–27.5 kg/m2 for patients of South Asian ethnicity and 25–30 kg/m2 for patients of all other ethnic groups; and obese, ≥27.5 kg/m2 for patients of South Asian ethnicity and ≥30 kg/m2 for patients of all other ethnic groups. Ref, reference.
Risk Factors for Type 2 Diabetes and Dysglycemia Among Women With PCOS
In analysis of the cohort of women with PCOS to identify risk factors for type 2 diabetes, PCOS-specific variables emerged as significant risk factors, namely, anovulation (aHR 1.21, 95% CI 1.08–1.35, P = 0.001) and hirsutism (aHR 1.20, 95% CI 1.05–1.36, P = 0.007). Conversely, prescription of COCPs emerged as a protective factor, with similar effects observed for COCPs with (aHR 0.84, 95% CI 0.73–0.97, P = 0.020) and without (aHR 0.83, 95% CI 0.72–0.94, P = 0.005) an antiandrogenic progestin component. The same risk factors and protective factors were observed for the composite dysglycemia outcome (Supplementary Table 3).
Nested Case-Control Analysis: The Effect of Oral Contraceptives on Risk of Dysglycemia
Of the 64,051 women with PCOS in the base cohort, 0.45% (n = 2,885) developed dysglycemia during follow-up, who were assigned as the case subjects in the nested case-control study (Table 2). The remaining 61,166 (95.5%) women were considered as potential control subjects. A total of 478 case subjects could not be matched to a control subject on the basis of age, BMI, PCOS diagnosis date, and incident/prevalent status of PCOS diagnosis. Therefore, our final analysis included 2,407 case subjects and corresponding 2,407 matched control subjects.
Table 2.
Baseline characteristics of women with PCOS included in the nested case-control study
| Variable | Women with PCOS and a diagnosis of dysglycemia (case subjects), n = 2,407 | Women with PCOS and without a diagnosis of dysglycemia (control subjects), n = 2,407 |
|---|---|---|
| Age at index date (dysglycemia diagnosis for cases), years, mean (SD) | 38.89 (8.32) | 38.84 (8.27) |
| Age at PCOS diagnosis, years, mean (SD) | 28.84 (14.43) | 28.76 (14.00) |
| BMI, kg/m2, mean (SD) | 32.72 (6.98) | 32.59 (7.03) |
| BMI categories, n (%) | ||
| Normal/underweight | 270 (11.2) | 305 (12.7) |
| Overweight | 439 (18.2) | 437 (18.2) |
| Obese | 1,322 (54.9) | 1,289 (53.5) |
| Missing | 376 (15.6) | 376 (15.6) |
| Townsend deprivation score, n (%) | ||
| 1 (least deprived) | 351 (14.6) | 481 (20.0) |
| 2 | 359 (14.9) | 436 (18.1) |
| 3 | 473 (19.7) | 457 (19.0) |
| 4 | 471 (19.6) | 420 (17.5) |
| 5 (most deprived) | 408 (17.0) | 295 (12.3) |
| Missing | 345 (14.3) | 318 (13.2) |
| Smoking status, n (%) | ||
| Nonsmoker | 1,306 (54.3) | 1,354 (56.3) |
| Discontinued | 295 (12.3) | 362 (15.0) |
| Smoker | 639 (26.6) | 501 (20.8) |
| Missing | 167 (6.9) | 190 (7.9) |
| Ethnicity, n (%) | ||
| Caucasian | 999 (41.5) | 1,099 (45.7) |
| Mixed race | 38 (1.6) | 21 (0.87) |
| Chinese/Middle Eastern/other | 21 (0.87) | 13 (0.54) |
| Black | 80 (3.3) | 40 (1.7) |
| South Asian | 241 (10.0) | 77 (3.2) |
| Missing | 1,028 (42.7) | 1,157 (48.1) |
| Concurrent conditions at baseline, n (%) | ||
| Hypothyroidism | 256 (10.6) | 188 (7.8) |
| Hypertension | 623 (25.88) | 179 (11.59) |
| Prescription of drugs within the exposure time window, n (%) | ||
| Contraceptives | ||
| No pill | 1,728 (71.8) | 1,592 (66.1) |
| COCP without antiandrogenic progestin | 301 (12.5) | 389 (16.2) |
| COCP with antiandrogenic progestin* | 378 (15.7) | 426 (17.7) |
| Single-agent antiandrogen therapy^ | 41 (1.7) | 23 (0.96) |
| Metformin | 417 (17.3) | 330 (13.7) |
| Lipid-lowering medication | 150 (6.23) | 119 (4.94) |
Case and control subjects are matched women with and without a diagnosis of dysglycemia during follow-up, respectively.
Normal/underweight, <23.5 kg/m2 for patients of South Asian ethnicity and <25 kg/m2 for patients of all other ethnic groups; overweight, 23.5–27.5 kg/m2 for patients of South Asian ethnicity and 25–30 kg/m2 for patients of all other ethnic groups; obese, ≥27.5 kg/m2 for patients of South Asian ethnicity and ≥30 kg/m2 for patients of all other ethnic groups.
Cyproterone acetate/drospirenone.
Cyproterone acetate/flutamide/finasteride.
Mean (SD) age at index date was 38.9 (8.3) years and mean age at PCOS diagnosis was 28.8 (14.4) years, which were similar between case subjects and control subjects. BMI at cohort entry was similarly distributed between case and control subjects (mean [SD] 32.7 [7.0] vs. 32.6 [7.0] kg/m2). Compared with control subjects, case subjects were more likely to be from a deprived background (Townsend deprivation score level 5 [deprived]: 17.0% vs. 12.3%), smokers (26.6% vs. 20.8%), and of South Asian ethnicity (10.0% vs. 3.2%). At cohort entry, there was also a higher proportion of case subjects with concurrent hypothyroidism (10.6% vs. 7.8% of control subjects). Altogether, 679 case subjects (28.2%) and 815 control subjects (33.9%) were prescribed COCPs during the exposure window. Among those prescribed COCPs, the median COCP prescription count per person during the exposure window was 3 (IQR 1–7).
With adjustment for age, smoking status, BMI category, ethnicity, Townsend score, baseline hypothyroidism, hypertension, and prescription of isolated antiandrogen drugs, metformin, and lipid-lowering medication at baseline, women with PCOS exposed to COCP were seen to have a reduced risk of dysglycemia (adjusted odds ratio [aOR] 0.74, 95% CI 0.65–0.85, P < 0.001). For every issued COCP prescription recorded within the exposure window, there was a 2% reduction in the odds of dysglycemia (aOR 0.98, 95% CI 0.96–0.99, P = 0.004) (Fig. 1B).
When COCP prescription issue count was categorized as 1) no prescription, 2) prescription count of three or fewer, and 3) prescription count of more than three within the exposure window, a dose-responsive reduction in the risk of dysglycemia was observed (in reference to no prescription of COCP, aOR of dysglycemia with prescription count of three or fewer = 0.80, 95% CI 0.67–0.96, P = 0.017, and aOR with prescription count of more than three = 0.67, 95% CI 0.55–0.81, P < 0.001) (Fig. 1B).
Women with PCOS exposed to COCPs had a reduced risk of dysglycemia irrespective of the type of progestin component (COCPs with antiandrogenic progestin, aOR 0.76, 95% CI 0.63–0.91, P = 0.003; COCPs with progestin without antiandrogen activity, aOR 0.72, 95% CI 0.59–0.87; P < 0.001) (Fig. 1B and Supplementary Table 4).
Metformin prescription within the exposure window period was associated with increased risk of dysglycemia (aOR 1.50, 95% CI 1.24–1.81, P < 0.001), suggestive of possible prescription-by-indication bias for those at increased risk. Findings in the sensitivity analysis with incorporation of a risk set sampling approach showed a similar result (aOR 0.76, 95% CI 0.63–0.91, P = 0.003).
Conclusions
With use of a rigorous nested case-control pharmacoepidemiological analysis, we found that women with PCOS exposed to COCPs had a reduced risk of developing dysglycemia across all BMI subgroups. Our study is also the largest with reporting of glycemic outcomes in a primary care cohort of women with PCOS, demonstrating a twofold increased risk of incident type 2 diabetes and dysglycemia in women with PCOS of any BMI.
Our finding of an increased type 2 diabetes risk in women with PCOS is consistent with results of recent population studies from Denmark and Finland (7,32) and hospitalization data from Australia (33), all reporting a two- to fourfold increased type 2 diabetes risk in PCOS. Using the Australian Longitudinal Study on Women’s Health, Kakoly et al. (34) demonstrated that a diagnosis of PCOS was one of the most influential predictors of incident type 2 diabetes in women, even after adjusting for BMI and family history. Few population studies have looked specifically at the composite outcome of dysglycemia, which takes into account a spectrum of impaired glucose regulation ranging from impaired glucose tolerance and impaired fasting glucose through to overt hyperglycemia (35,36). Crucially, our data highlight that normal-weight women with PCOS were also at increased risk of type 2 diabetes and dysglycemia. This parallels our previous finding of increased NAFLD risk in normal-weight women with PCOS (3), further challenging the notion that PCOS-related metabolic complications are only relevant in the context of obesity.
These data suggest that, rather than obesity in isolation, PCOS-specific factors, including androgen excess, underpin the increased metabolic risk. We found that those women with PCOS and hirsutism, a clinical feature of androgen excess, had a further increased risk of dysglycemia. In a population-based cohort study, using the same primary care population database, we previously documented an independent link between serum testosterone and incident diabetes risk in women (12). We demonstrated that the risk of incident type 2 diabetes increased significantly in women with serum testosterone level >1.5 nmol/L compared with the reference cohort with levels <1 nmol/L; the risk was twofold higher in women with serum testosterone values >3.5 nmol/L. We also demonstrated in a small cross-sectional cohort study that women with increased circulating androgen concentrations had a higher risk of an abnormal OGTT result, with the OGTT-derived insulin sensitivity index correlating inversely with circulating androgen burden (9). In a recent meta-analysis (37) it was demonstrated that women with increased serum testosterone had a 60% higher risk of type 2 diabetes than women with normal testosterone levels. Furthermore, a recent large-scale genome association study in 425,097 participants of the UK Biobank demonstrated that the risk of type 2 diabetes in women increased in line with increasing circulating testosterone concentrations (15).
The association of female androgen excess, insulin resistance, and type 2 diabetes is undoubtedly complex. Insulin resistance promotes androgen excess by upregulating ovarian androgen generation and peripheral androgen activation in adipose tissue (38,39); the latter increases lipid accumulation in the adipocyte and, once adipocyte lipid storage capacity is exhausted, fatty acid overspill (39), which is intricately linked to metabolic dysfunction. Abnormalities in skeletal muscle metabolic function have also been described in PCOS, with altered muscle mitochondrial energy biogenesis in the context of androgen excess likely to drive disturbances in glucose metabolism (40,41). Rodent-based studies also support a direct role for androgens in pancreatic β-cell dysfunction, driving insulin hypersecretion, oxidative injury, and consequent β-cell failure (42). These data have recently been underpinned by a study using human pancreatic islets, demonstrating that intracrine activation of testosterone to the most potent androgen, 5α-dihydrotestosterone, increases glucose-stimulated insulin secretion (43).
A recent cohort study (8) in nearly 80,000 women with a follow-up period of 24 years described an increased risk of premature mortality, primarily due to cardiovascular disease, in women with irregular cycles. COCPs are routinely used for menstrual cycle regulation in women with PCOS. Our study is the first population-based study investigating the hypothesis that COCPs might mitigate the risk of dysglycemia in women with PCOS, with antiandrogen activity conferred by an estrogen-mediated increase in SHBG as the proposed mechanism. Studies examining the impact of COCPs on glucose metabolism have reported conflicting results, and most are limited by small participant numbers and significant heterogeneity in COCP use. In a 2016 Korean population study of 6,554 postmenopausal women, investigators found that those who took the COCP during their reproductive years for >6 months had a 37% increased risk of type 2 diabetes (44). However, in a more recent study with examination of the National Health and Nutrition Examination Survey (NHANES) database between 2007 and 2018 it was found that COCP use in >6,000 women aged 35–50 years who met matching criteria was associated with a 29% reduced risk of type 2 diabetes compared with the risk of never users (45). A further limitation is the tendency in previous studies to extrapolate data from otherwise healthy female patient groups to women with PCOS, who are likely to manifest a biologically distinct set of risk factors for dysglycemia. For the first cohort of the Nurses’ Health Study, 2,276 healthy women were followed for a median of 12 years from 1976, with findings that risk of type 2 diabetes was increased by 10% in women with previous COCP use compared with those who never took the medication (46); however, these data reflect the use of older COCP preparations with higher ethinylestradiol concentrations between the 1970s and 1990s. In a recent Cochrane Library review no convincing evidence was found of glycemic risk associated with COCP prescription among women without PCOS (18), while a 2011 meta-analysis of the limited evidence in women with PCOS suggested neither adverse nor beneficial impact of COCPs on glucose homeostasis (47). A 2017 systematic review and meta-analysis highlighted the urgent need for further studies to understand the relationship between glucose metabolism and COCP use in both lean and obese women with PCOS (48). The results of our study improve our understanding in this regard and indicate the need for prospective, randomized controlled trials on the impact of COCPs on the risk of type 2 diabetes and dysglycemia. We found that following adjustment for confounding factors, women with PCOS and COCP use had a 27% reduction in the relative risk of incident dysglycemia, with the highest reduction in patients receiving higher numbers of COCP prescriptions. When analyzed separately, women with PCOS and COCP use had a similarly reduced risk of dysglycemia when exposed to COCPs with and without antiandrogenic progestin components, suggesting that the estrogen-induced increase in SHBG may be the primary driver of the risk-mitigating effect. However, this finding is potentially limited by the lower number of patients receiving antiandrogenic COCPs. Cyproterone acetate and drospirenone are progestins with antiandrogenic properties, as opposed to progestins such as desogestrel or levonorgestrel, which have neutral or proandrogenic effects (49). While cyproterone acetate and drospirenone exert antiandrogen activity via androgen receptor blockade, their antiandrogen activity is considerably lesser than that of recently approved novel antiandrogens mainly used in the treatment of prostate cancer (50).
Our finding that women using metformin and women using single-agent antiandrogen therapy had an increased risk of incident dysglycemia is very likely reflective of a confounding-by-indication bias (51). Accordingly, the women with PCOS at highest risk of dysglycemia based on metabolic or androgen phenotype may have been systematically prescribed metformin and single agent antiandrogen therapy. It is possible that our observation of reduced dysglycemia risk in women with PCOS on COCPs may also reflect a prescription-by-indication bias, whereby those women with cardiovascular risk factors such as obesity, dyslipidemia, and hypertension were less likely to have been prescribed the COCP. However, we believe that this is less likely from closer review of the data; in our nested pharmacoepidemiological study, 26% of women had a BMI in the obese range and one-quarter of women with a BMI >35 kg/m2 took COCPs during the follow-up period. We also carefully adjusted our analysis for metabolic phenotype by including BMI, hypertension, and dyslipidemia as variables.
Our study has a number of notable limitations, including the above-mentioned prescription-by-indication bias issues and others that are common to studies of retrospective data with use of electronic general practice databases. The definition of women with no PCOS was based on the absence of any Read code in relation to PCOS and not on systematic diagnostic assessment to exclude PCOS. Therefore, the proportion of women with PCOS was also much lower than the published community prevalence data for PCOS (52). Another limitation is that we used the Read code for polycystic ovaries (PCO) as indicative of PCOS. However, in a sensitivity analysis limited to women with PCOS Read codes, we documented similar findings, excluding the use of the PCO Read code as a significant limitation. Higher testing rates for type 2 diabetes among women with PCOS may also have resulted in overestimating the effect size; however, the effect size observed for type 2 diabetes in our study is similar to that of existing literature (53). It was also not possible to adjust for more specific lifestyle factors such as physical activity, energy intake, or fiber consumption within a large population database as used in the current study. To explore the possibility of right censoring bias, the median follow-up and the loss to follow-up pattern was compared between patients with and without PCOS. There was no systematic difference observed between the two groups, and therefore the assumption of noninformative censoring was reasonable for the time-to-event analysis in this study, limiting the possibility of right censoring bias.
In conclusion, we demonstrated that women with PCOS have a significantly increased risk of dysglycemia that persisted after adjustment for BMI, corroborating the recommendation that women with PCOS should be systematically screened for type 2 diabetes irrespective of body weight category. In our nested pharmacoepidemiology study, we found that women with PCOS and exposure to COCPs had a lower risk of incident dysglycemia. Though the limitations of our study design preclude ascertainment of causality, we hypothesize that a beneficial effect of COCPs might be conveyed by an estrogen-induced increase in hepatic SHBG production. This increase would result in a decrease in the biologically active, unbound circulating androgen fraction, and this reduction in androgen excess could have metabolically beneficial effects including a decrease in risk of dysglycemia. However, to definitively establish causality a large-scale randomized trial evaluating the efficacy of COCPs in reducing the risk of dysglycemia in women with PCOS would be required, with careful comparison of the potential additional benefit of COCPs containing antiandrogenic progestin components.
Article Information
Funding. This work was supported by the Wellcome Trust (Investigator Grant WT209492/Z/17/Z [to W.A.]) and the Health Research Board (Emerging Clinician Scientist Award ECSA-FA-2020-001 [to M.W.O.]). K.N. is a UK Research and Innovation (UKRI)/Health Data Research (HDR) UK Innovation Clinical Fellow. W.A. receives support from the NIHR Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham (grant BRC-1215-20009).
The views expressed are those of the authors and not necessarily those of the National Institute for Health Research or the Department of Health and Social Care of the U.K.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. B.K., M.W.O., W.A., and K.N. developed the research question and designed the study. D.Š. and L.A. contributed to the design of the study. B.K., M.W.O., A.S., W.A., and K.N. designed the analysis, interpreted the results, and drafted the manuscript. D.Š. and L.A. contributed to the design of the study. All authors reviewed and revised the manuscript. W.A. and K.N. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
B.K., M.W.O., and A.S. contributed equally to this work.
W.A. and K.N. are joint senior authors.
This article contains supplementary material online at https://doi.org/10.2337/figshare.16608068.
References
- 1. Teede HJ, Misso ML, Costello MF, et al.; International PCOS Network . Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril 2018;110:364–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Randeva HS, Tan BK, Weickert MO, et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev 2012;33:812–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kumarendran B, O’Reilly MW, Manolopoulos KN, et al. Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: a longitudinal study based on a United Kingdom primary care database. PLoS Med 2018;15:e1002542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Legro RS, Arslanian SA, Ehrmann DA, et al.; Endocrine Society . Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2013;98:4565–4592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pelanis R, Mellembakken JR, Sundström-Poromaa I, et al. The prevalence of type 2 diabetes is not increased in normal-weight women with PCOS. Hum Reprod 2017;32:2279–2286 [DOI] [PubMed] [Google Scholar]
- 6. Morgan CL, Jenkins-Jones S, Currie CJ, Rees DA. Evaluation of adverse outcome in young women with polycystic ovary syndrome versus matched, reference controls: a retrospective, observational study. J Clin Endocrinol Metab 2012;97:3251–3260 [DOI] [PubMed] [Google Scholar]
- 7. Rubin KH, Glintborg D, Nybo M, Abrahamsen B, Andersen M. Development and risk factors of type 2 diabetes in a nationwide population of women with polycystic ovary syndrome. J Clin Endocrinol Metab 2017;102:3848–3857 [DOI] [PubMed] [Google Scholar]
- 8. Wang YX, Arvizu M, Rich-Edwards JW, et al. Menstrual cycle regularity and length across the reproductive lifespan and risk of premature mortality: prospective cohort study. BMJ 2020; 371:m3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O’Reilly MW, Taylor AE, Crabtree NJ, et al. Hyperandrogenemia predicts metabolic phenotype in polycystic ovary syndrome: the utility of serum androstenedione. J Clin Endocrinol Metab 2014;99: 1027–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Münzker J, Hofer D, Trummer C, et al. Testosterone to dihydrotestosterone ratio as a new biomarker for an adverse metabolic phenotype in the polycystic ovary syndrome. J Clin Endocrinol Metab 2015;100:653–660 [DOI] [PubMed] [Google Scholar]
- 11. Luotola K, Piltonen TT, Puurunen J, Morin-Papunen LC, Tapanainen JS. Testosterone is associated with insulin resistance index independently of adiposity in women with polycystic ovary syndrome. Gynecol Endocrinol 2018;34:40–44 [DOI] [PubMed] [Google Scholar]
- 12. O’Reilly MW, Glisic M, Kumarendran B, et al. Serum testosterone, sex hormone-binding globulin and sex-specific risk of incident type 2 diabetes in a retrospective primary care cohort. Clin Endocrinol (Oxf) 2019;90:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2006;295:1288–1299 [DOI] [PubMed] [Google Scholar]
- 14. Rasmussen JJ, Selmer C, Frøssing S, et al. Endogenous testosterone levels are associated with risk of type 2 diabetes in women without established comorbidity. J Endocr Soc 2020;4:a050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ruth KS, Day FR, Tyrrell J, et al.; Endometrial Cancer Association Consortium . Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med 2020;26:252–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Amiri M, Nahidi F, Bidhendi-Yarandi R, Khalili D, Tohidi M, Ramezani Tehrani F. A comparison of the effects of oral contraceptives on the clinical and biochemical manifestations of polycystic ovary syndrome: a crossover randomized controlled trial. Hum Reprod 2020;35:175–186 [DOI] [PubMed] [Google Scholar]
- 17. Diamanti-Kandarakis E, Baillargeon JP, Iuorno MJ, Jakubowicz DJ, Nestler JE. A modern medical quandary: polycystic ovary syndrome, insulin resistance, and oral contraceptive pills. J Clin Endocrinol Metab 2003;88:1927–1932 [DOI] [PubMed] [Google Scholar]
- 18. Lopez LM, Grimes DA, Schulz KF. Steroidal contraceptives: effect on carbohydrate metabolism in women without diabetes mellitus. Cochrane Database Syst Rev 2012;18:CD006133. [DOI] [PubMed] [Google Scholar]
- 19. Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care 2011;19:251–255 [DOI] [PubMed] [Google Scholar]
- 20. Jadhakhan F, Marshall T, Ryan R, Gill P. Risk of chronic kidney disease in young adults with impaired glucose tolerance/impaired fasting glucose: a retrospective cohort study using electronic primary care records. BMC Nephrol 2018;19:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ding T, Baio G, Hardiman PJ, Petersen I, Sammon C. Diagnosis and management of polycystic ovary syndrome in the UK (2004-2014): a retrospective cohort study. BMJ Open 2016;6:e012461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Azziz R. Does the risk of diabetes and heart disease in women with polycystic ovary syndrome lessen with age? Fertil Steril 2017;108:959–960 [DOI] [PubMed] [Google Scholar]
- 23. Echiburú B, Pérez-Bravo F, Galgani JE, et al. Enlarged adipocytes in subcutaneous adipose tissue associated to hyperandrogenism and visceral adipose tissue volume in women with polycystic ovary syndrome. Steroids 2018;130:15–21 [DOI] [PubMed] [Google Scholar]
- 24. Karim ME, Gustafson P, Petkau J; Long-Term Benefits and Adverse Effects of Beta-Interferon for Multiple Sclerosis (BeAMS) Study Group . Comparison of statistical approaches for dealing with immortal time bias in drug effectiveness studies. Am J Epidemiol 2016;184:325–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suissa S, Dell’aniello S, Vahey S, Renoux C. Time-window bias in case-control studies: statins and lung cancer. Epidemiology 2011;22:228–231 [DOI] [PubMed] [Google Scholar]
- 26. Townsend P. Deprivation. J Soc Policy 1987;16:125–146 [Google Scholar]
- 27. Adams J, Ryan V, White M. How accurate are Townsend Deprivation Scores as predictors of self-reported health? A comparison with individual level data. J Public Health (Oxf) 2005;27:101–106 [DOI] [PubMed] [Google Scholar]
- 28. Riley J, Antza C, Kempegowda P, et al. Social deprivation and incident diabetes-related foot disease in patients with type 2 diabetes: a population-based cohort study. Diabetes Care 2021;44:731–739 [DOI] [PubMed] [Google Scholar]
- 29. Davé S, Petersen I. Creating medical and drug code lists to identify cases in primary care databases. Pharmacoepidemiol Drug Saf 2009;18:704–707 [DOI] [PubMed] [Google Scholar]
- 30. Nishida C, Barba C, Cavalli-Sforza T, et al.; WHO Expert Consultation . Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363:157–163 [DOI] [PubMed] [Google Scholar]
- 31. Benchimol EI, Smeeth L, Guttmann A, et al.; RECORD Working Committee . The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med 2015;12:e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ollila M-ME, West S, Keinänen-Kiukaanniemi S, et al. Overweight and obese but not normal weight women with PCOS are at increased risk of Type 2 diabetes mellitus-a prospective, population-based cohort study. Hum Reprod 2017;32:423–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hart R, Doherty DA. The potential implications of a PCOS diagnosis on a woman’s long-term health using data linkage. J Clin Endocrinol Metab 2015;100:911–919 [DOI] [PubMed] [Google Scholar]
- 34. Kakoly NS, Earnest A, Teede HJ, Moran LJ, Joham AE. The impact of obesity on the incidence of type 2 diabetes among women with polycystic ovary syndrome. Diabetes Care 2019;42:560–567 [DOI] [PubMed] [Google Scholar]
- 35. Li HWR, Lam KSL, Tam S, et al. Screening for dysglycaemia by oral glucose tolerance test should be recommended in all women with polycystic ovary syndrome. Hum Reprod 2015;30: 2178–2183 [DOI] [PubMed] [Google Scholar]
- 36. Cheung LP, Ma RCW, Lam PM, et al. Cardiovascular risks and metabolic syndrome in Hong Kong Chinese women with polycystic ovary syndrome. Hum Reprod 2008;23:1431–1438 [DOI] [PubMed] [Google Scholar]
- 37. Yao Q-M, Wang B, An X-F, Zhang J-A, Ding L. Testosterone level and risk of type 2 diabetes in men: a systematic review and meta-analysis. Endocr Connect 2018;7:220–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ehrmann DA, Schneider DJ, Sobel BE, et al. Troglitazone improves defects in insulin action, insulin secretion, ovarian steroidogenesis, and fibrinolysis in women with polycystic ovary syndrome. J Clin Endocrinol Metab 1997;82: 2108–2116 [DOI] [PubMed] [Google Scholar]
- 39. O’Reilly MW, Kempegowda P, Walsh M, et al. AKR1C3-mediated adipose androgen generation drives lipotoxicity in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2017;102: 3327–3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Skov V, Glintborg D, Knudsen S, et al. Reduced expression of nuclear-encoded genes involved in mitochondrial oxidative metabolism in skeletal muscle of insulin-resistant women with polycystic ovary syndrome. Diabetes 2007;56:2349–2355 [DOI] [PubMed] [Google Scholar]
- 41. Hansen SL, Svendsen PF, Jeppesen JF, et al. Molecular mechanisms in skeletal muscle underlying insulin resistance in women who are lean with polycystic ovary syndrome. J Clin Endocrinol Metab 2019;104:1841–1854 [DOI] [PubMed] [Google Scholar]
- 42. Navarro G, Allard C, Morford JJ, et al. Androgen excess in pancreatic β cells and neurons predisposes female mice to type 2 diabetes. JCI Insight 2018;3:e98607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xu W, Schiffer L, Qadir MMF, et al. Intracrine testosterone activation in human pancreatic β-cells stimulates insulin secretion. Diabetes 2020;69:2392–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim SW, Jeon JH, Lee WK, et al. Long-term effects of oral contraceptives on the prevalence of diabetes in post-menopausal women: 2007-2012 KNHANES. Endocrine 2016;53:816–822 [DOI] [PubMed] [Google Scholar]
- 45. Yao W, Dong X, Yu X, Luo J, Zhang D. The use of oral contraceptive is inversely associated with the risk of type 2 diabetes mellitus among middle-aged women. Gynecol Endocrinol 2021;37: 758–763 [DOI] [PubMed] [Google Scholar]
- 46. Rimm EB, Manson JE, Stampfer MJ, et al. Oral contraceptive use and the risk of type 2 (non-insulin-dependent) diabetes mellitus in a large prospective study of women. Diabetologia 1992;35:967–972 [DOI] [PubMed] [Google Scholar]
- 47. Halperin IJ, Kumar SS, Stroup DF, Laredo SE. The association between the combined oral contraceptive pill and insulin resistance, dysglycemia and dyslipidemia in women with polycystic ovary syndrome: a systematic review and meta-analysis of observational studies. Hum Reprod 2011;26:191–201 [DOI] [PubMed] [Google Scholar]
- 48. Luque-Ramírez M, Nattero-Chávez L, Ortiz Flores AE, Escobar-Morreale HF. Combined oral contraceptives and/or antiandrogens versus insulin sensitizers for polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 2018;24:225–241 [DOI] [PubMed] [Google Scholar]
- 49. Mathur R, Levin O, Azziz R. Use of ethinylestradiol/drospirenone combination in patients with the polycystic ovary syndrome. Ther Clin Risk Manag 2008;4:487–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rice MA, Malhotra SV, Stoyanova T. Second-generation antiandrogens: from discovery to standard of care in castration resistant prostate cancer. Front Oncol 2019;9:801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Daniel S, Koren G, Lunenfeld E, Levy A. NSAIDs and spontaneous abortions - true effect or an indication bias? Br J Clin Pharmacol 2015;80:750–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod 2016;31:2841–2855 [DOI] [PubMed] [Google Scholar]
- 53. Kakoly NS, Khomami MB, Joham AE, et al. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: a systematic review and meta-regression. Hum Reprod Update 2018;24:455–467 [DOI] [PubMed] [Google Scholar]