Abstract
OBJECTIVE
To explore the effect of discontinuing continuous glucose monitoring (CGM) after 8 months of CGM use in adults with type 2 diabetes treated with basal without bolus insulin.
RESEARCH DESIGN AND METHODS
This multicenter trial had an initial randomization to either real-time CGM or blood glucose monitoring (BGM) for 8 months followed by 6 months in which the BGM group continued to use BGM (n = 57) and the CGM group was randomly reassigned either to continue CGM (n = 53) or discontinue CGM with resumption of BGM for glucose monitoring (n = 53).
RESULTS
In the group that discontinued CGM, mean time in range (TIR) 70–180 mg/dL, which improved from 38% before initiating CGM to 62% after 8 months of CGM, decreased after discontinuing CGM to 50% at 14 months (mean change from 8 to 14 months −12% [95% CI −21% to −3%], P = 0.01). In the group that continued CGM use, little change was found in TIR from 8 to 14 months (baseline 44%, 8 months 56%, 14 months 57%, mean change from 8 to 14 months 1% [95% CI −11% to 12%], P = 0.89). Comparing the two groups at 14 months, the adjusted treatment group difference in mean TIR was −6% (95% CI −16% to 4%, P = 0.20).
CONCLUSIONS
In adults with type 2 diabetes treated with basal insulin who had been using real-time CGM for 8 months, discontinuing CGM resulted in a loss of about one-half of the initial gain in TIR that had been achieved during CGM use.
Introduction
The clinical benefits of real-time continuous glucose monitoring (CGM) have been well established in people with type 1 or type 2 diabetes receiving intensive insulin therapy with either multiple daily insulin injections or insulin pump, as evidenced by a decrease in HbA1c, increase of time spent in target range, and decrease in hypoglycemia (1–5). However, the use of CGM in people with type 2 diabetes not on intensive therapy has been less studied. Recently, we published the results of the Continuous Glucose Monitoring in T2D Basal Insulin Users: The MOBILE Study (MOBILE), an 8-month randomized, multicenter clinical trial comparing CGM versus blood glucose monitoring (BGM) in 175 adults with type 2 diabetes treated with basal insulin without bolus insulin and managed by primary care providers (6). In this study, the use of CGM resulted in an HbA1c reduction from 9.1% (76 mmol/mol) to 8.0% (64 mmol/mol) at 8 months compared with 9.0% (75 mmol/mol) to 8.4% (68 mmol/mol) in the BGM group (P = 0.02). Furthermore, there was an increase in time in range (TIR) 70–180 mg/dL of 3.6 h per day (P < 0.001) and a decrease in time spent in hyperglycemia >250 mg/dL by 3.8 fewer hours per day (P < 0.001). While the novel approach of this study reveals the benefits of CGM therapy in adults with type 2 diabetes treated with basal insulin and primarily managed in the primary care setting, how well these benefits persist long term or what the effects of the discontinuation of CGM therapy in this population would be is not known. These questions were explored in a 6-month extension of the study.
Research Design and Methods
The 14-month study consisted of an initial 8-month randomized trial comparing real-time CGM versus BGM monitoring followed by a 6-month second phase in which the BGM group continued to use BGM and the CGM group was randomly reassigned either to continue CGM or to discontinue CGM with resumption of BGM for glucose monitoring. The study was conducted at 15 centers in the U.S. The protocol and informed consent form were approved by a central institutional review board for 14 centers and a local board for 1 center. Written informed consent was obtained from each participant on entering the initial randomized trial. Methods for the initial 8-month randomized trial have been reported (6,7). Key aspects of the protocol are summarized below.
Participants were recruited from primary care practices and could not be under the care of a diabetes specialist for their diabetes management. Major eligibility criteria included age ≥30 years, diagnosis of type 2 diabetes treated with one or two daily injections of long- or intermediate-acting basal insulin for at least 6 months, locally measured HbA1c of 7.8% (62 mmol/mol) to 11.5% (102 mmol/mol) (lower limit changed during the study from 8.0% [64 mmol/mol] to 7.8% [62 mmol/mol]), self-reported blood glucose meter testing averaging three or more times per week, and availability of a smartphone compatible with the CGM device for data uploading. Participants could be using any other antidiabetic medication in addition to basal insulin, provided that the regimen had been stable for at least 3 months, but not prandial insulin.
Before randomization for the 8-month trial (referred to as baseline), each participant used a Dexcom G6 Pro blinded sensor for up to 10 days and then was randomly assigned 2:1 to either the CGM group or the BGM group. Participants in both groups were provided with a Bluetooth-enabled blood glucose meter (OneTouch Verio Flex) and test strips. Participants in the BGM group were asked to perform fasting and postprandial BGM testing one to three times daily. Participants in the CGM group were provided with a Dexcom G6 CGM System and instructed to use CGM continuously. Follow-up visits for both treatment groups to review glucose data and self-titration of insulin occurred after 2 weeks, 4 weeks, and 8 months, and phone contacts were made after 2, 4, and 6 months. The study investigator served as an advisor to the primary care provider with respect to making changes in diabetes therapy, such as the addition of new glucose-lowering medications, which could include prandial insulin, unless immediate changes were deemed necessary because of excessive hypoglycemia. After each clinic or virtual visit, the study investigator sent the glucose data record and management suggestions to the patient’s primary care provider. HbA1c was measured at a central laboratory (University of Minnesota Advanced Research Diagnostic Laboratory) at the time of randomization and after 3 and 8 months using a Tosoh G8 HPLC Analyzer (Tosoh Bioscience, Inc., South San Francisco, CA).
Upon completion of the 8-month visit for the initial randomized trial, participants in the CGM group were randomly assigned on the study website from a computer-generated sequence to either discontinue CGM (discontinue CGM group) or continue CGM (continue CGM group) in a 1:1 ratio, using a permuted block design (random block sizes of two and four). Participants in the discontinued CGM group were asked to perform BGM fasting and postprandial testing one to three times daily. Participants in the continue CGM group continued to use a study-provided Dexcom G6 CGM System and were instructed to use CGM continuously. Participants in the BGM group continued without change in their glucose monitoring.
For all three treatment groups, a virtual visit by phone occurred after 3 months (11 months after the start of the randomized trial), and a single study follow-up clinic visit occurred after 6 months (14 months from the start of the initial randomized trial). The discontinue CGM group and the BGM group had an additional visit before the 14-month visit to place a blinded CGM sensor. HbA1c was measured at the central laboratory at 14 months. Adverse event occurrence was solicited throughout the study. Severe hypoglycemia was defined as an event that required assistance from another person to administer carbohydrates or other resuscitative action. Diabetic ketoacidosis diagnosis was based on the criteria established for the Diabetes Control and Complications Trial (DCCT) (8).
The coronavirus disease 2019 pandemic impacted the ability to complete in-clinic study visits beginning in March 2020 through the end of the study. When an in-clinic 14-month visit could not occur, a virtual visit was completed (for 35 participants) to collect study data. Participants were sent a kit for a fingerstick capillary blood draw that was processed by the same central laboratory for HbA1c measurement using the same method as the venous samples. This method has been determined to have comparable accuracy to venous blood draws (9).
Statistical Analysis
The sample size was statistically determined for the randomized trial that preceded this phase of the study. This phase of the study was not statistically powered for hypothesis testing comparing treatment groups. The primary outcome was CGM-measured TIR. Additional outcomes included HbA1c and other CGM metrics for hyperglycemia (mean glucose, time >180 mg/dL, time >250 mg/dL, time >300 mg/dL, area under curve 180 mg/dL), hypoglycemia (time <70 mg/dL, time <54 mg/dL, hypoglycemia event rate), and variability (coefficient of variation). Analyses assessed the change in outcomes from month 8 to month 14 as well as comparisons of the three treatment arms as defined in the extension phase.
For continuous outcomes, mean change from month 8 to month 14 within each treatment group was estimated using repeated-measures linear regression models, with study visit as a fixed effect and clinic site as a random effect. A similar approach was used to compare change from baseline to month 14. For treatment group comparisons of continuous outcomes, mean treatment group difference at 14 months was calculated using a longitudinal mixed-effects linear regression model that included visit and treatment-by-visit interaction as fixed effects and clinic site as a random effect. All models used to assess between-group and within-group differences included phase 1 randomization, month 8, and month 14 in the response. The models handled missing data using direct likelihood analysis, which maximizes the likelihood function integrated over all possible values of the missing data. Models for body weight adjusted for age and sex, and models for blood pressure and cholesterol adjusted for age, sex, and BMI at phase 1 randomization.
Statistical significance was defined as P < 0.05 for the primary outcome analyses. For all other analyses, CIs and P values were adjusted to control the false discovery rate using the two-stage Benjamini-Hochberg procedure. Analyses were conducted using SAS 9.4 statistical software (SAS Institute, Cary, NC). All P values are two-sided.
Results
The analysis of the initial randomized trial included 175 participants, with 116 in the CGM group and 59 in the BGM group. Of the 108 participants in the original randomized trial CGM group who completed the 8-month visit, 106 continued in the study and were randomly assigned to either the continue CGM group (n = 53) or discontinue CGM group (n = 53). In the BGM group, 57 participants completed phase 1 and continued in the study. Mean age at the time of the 8-month visit was 58 ± 9 years. Mean HbA1c was 9.1 ± 0.9% (75 ± 9.8 mmol/mol) in the overall cohort at the time of the phase 1 randomization, while at 8 months (phase 2 baseline), it was 7.9 ± 1.4% (63 ± 15.3 mmol/mol) in the discontinue CGM group, 8.2 ± 1.4% (66 ± 15.3 mmol/mol) in the continue CGM group, and 8.4 ± 1.3% (68 ± 14.2 mmol/mol) in the BGM group. Characteristics of the participants in the three groups at the time of the 8-month visit are shown in Supplementary Table 1. The 14-month visit (6 months from the discontinue CGM vs. continue CGM randomization) was completed by all participants in the discontinue CGM and continue-CGM groups and by all but two in the BGM group (Supplementary Fig. 1).
Use of Glucose-Lowering Medications
At the time of the 8-month visit, basal insulin was being used by all participants in the discontinue CGM group, by all but one participant in the continue CGM group, and by all participants in the BGM group. All but two participants in the discontinue CGM group, all participants in the continue CGM group, and all but four in the BGM group were using one or more other glucose-lowering medications or bolus insulin in addition to basal insulin (Supplementary Tables 1 and 2).
Between 8 and 14 months, no glucose-lowering medications were added or stopped by 47 (89%) participants in the discontinue CGM group, 38 (72%) of the continue CGM group, and 44 (77%) of the BGM group (Supplementary Table 3). Bolus insulin was added between 8 and 14 months by one (2%) participant in the discontinue CGM group, four (8%) in the continue CGM group, and three (5%) in the BGM group. Total daily insulin dose showed minimal change between month 8 and month 14 (mean change −0.01 ± 0.20 in the discontinue CGM group, −0.04 ± 0.17 in the continue CGM group, −0.01 ± 0.14 in the BGM group) (Supplementary Table 4).
Use of CGM and BGM
In the continue CGM group, median CGM use was 6.2 days per week (interquartile range 5.0–6.7) at month 14; seven participants did not have CGM data in month 14 (three of whom indicated that they were using CGM, but a data download was not available) (Supplementary Table 5). One participant in the discontinue CGM group and no participants in the BGM group used an unblinded CGM between month 8 and month 14.
In the discontinue CGM group, the frequency of BGM (from meter downloads) averaged 1.5 times per day before the initial randomized trial, decreasing to 0.8 at the end of the initial randomization trial at 8 months and then increasing to 1.1 at 14 months (6 months after discontinuation of CGM). In the continue CGM group, the daily frequencies averaged 1.5, 0.5, and 0.5, respectively, and in the BGM Group, the daily frequencies averaged 1.6, 1.6, and 1.5, respectively (Supplementary Table 6).
Glycemic Outcomes
TIR
At 14 months, the adjusted treatment group difference in mean TIR between the discontinue CGM group and continue CGM group was −6% (95% CI −16% to 4%, P = 0.20); between the discontinue CGM and BGM groups, 6% (95% CI −6% to 17%, P = 0.58); and between the continue CGM and BGM groups, 10% (95% CI −4% to 23%, P = 0.08) (Table 1). Results of a per-protocol analysis and an analysis limited to participants who completed the 14-month visit before 13 March 2020 (the date a national emergency was declared related to coronavirus disease 2019) did not show meaningful differences from the overall analyses. TIR outcomes in the three groups according to baseline characteristics are shown in Supplementary Table 7, and outcomes separated by daytime and nighttime are shown in Supplementary Table 8.
Table 1.
Treatment group comparisons for glycemic outcomes at month 14
| Difference at month 14* | |||||||
|---|---|---|---|---|---|---|---|
| Discontinue CGM vs. continue CGM |
Discontinue CGM vs. BGM | Continue CGM vs. BGM | |||||
| Mean (95% CI) | P | Mean (95% CI) | P | Mean (95% CI) | P | ||
| CGM outcomes | |||||||
| TIR 70–180 mg/dL (%) | −6 (−16 to 4) | 0.20 | 6 (−6 to 17) | 0.58 | 10 (−4 to 23) | 0.08 | |
| Mean glucose (mg/dL) | 15 (−9 to 39) | 0.27 | −9 (−32 to 14) | 0.58 | −15 (−42 to 11) | 0.12 | |
| Glucose CV (%) | 1 (−2 to 4) | 0.36 | −1 (−4 to 2) | 0.58 | −2 (−5 to 1) | 0.08 | |
| Time >180 mg/dL (%) | 6 (−8 to 20) | 0.36 | −5 (−16 to 7) | 0.58 | −9 (−23 to 5) | 0.11 | |
| Time >250 mg/dL† (%) | 6 (−3 to 16) | 0.27 | −3 (−12 to 6) | 0.58 | −8 (−17 to 1) | 0.06 | |
| Time >300 mg/dL† (%) | 4.8 (−0.5 to 10.9) | 0.27 | −1.8 (−6.9 to 3.9) | 0.58 | −5.9 (−10.9 to −1.2) | 0.05 | |
| AUC 180 mg/dL† | 12 (−3 to 25) | 0.27 | −4 (−17 to 9) | 0.58 | −13 (−26 to 0) | 0.06 | |
| Time <70 mg/dL† (%) | 0.05 (−0.20 to 0.30) | 0.79 | −0.33 (−0.79 to 0.03) | 0.58 | −0.50 (−1.06 to 0.01) | 0.08 | |
| Time <54 mg/dL† (%) | 0.00 (−0.06 to 0.06) | 0.96 | −0.11 (−0.32 to 0.03) | 0.58 | −0.13 (−0.37 to 0.04) | 0.31 | |
| Hypo event rate (per week)† | 0.01 (−0.12 to 0.16) | 0.79 | −0.05 (−0.22 to 0.10) | 0.58 | −0.08 (−0.29 to 0.11) | 0.31 | |
| HbA1c outcomes | |||||||
| % | 0.23 (−0.42 to 0.87) | 0.48 | −0.27 (−0.93 to 0.39) | 0.58 | −0.35 (−1.10 to 0.40) | 0.23 | |
| mmol/mol | 2.5 (−12.1 to 9.5) | −3.0 (−10.2 to 4.3) | −3.8 (−12 to 4.4) | ||||
AUC, area under the curve; CV, coefficient of variance; Hypo, hypoglycemia.
For continuous outcomes, the mean differences, 95% CIs, and P values are estimated from a mixed-effects linear regression model adjusting for a random site effect. For binary outcomes, a mixed-effects logistic regression model was fitted, adjusting for the baseline value of the outcome and a random site effect. CIs and nominal (uncorrected) P values were adjusted for multiple comparisons using the adaptive two-stage group Benjamini-Hochberg method.
Winsorized at the 10th and 90th percentiles before reporting summary statistics. P values and CIs were estimated using a bootstrap.
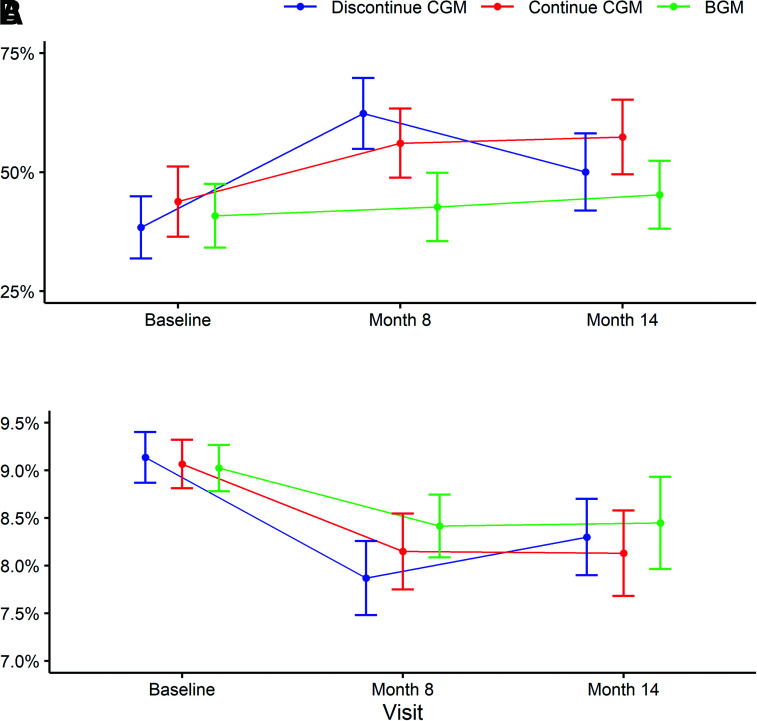
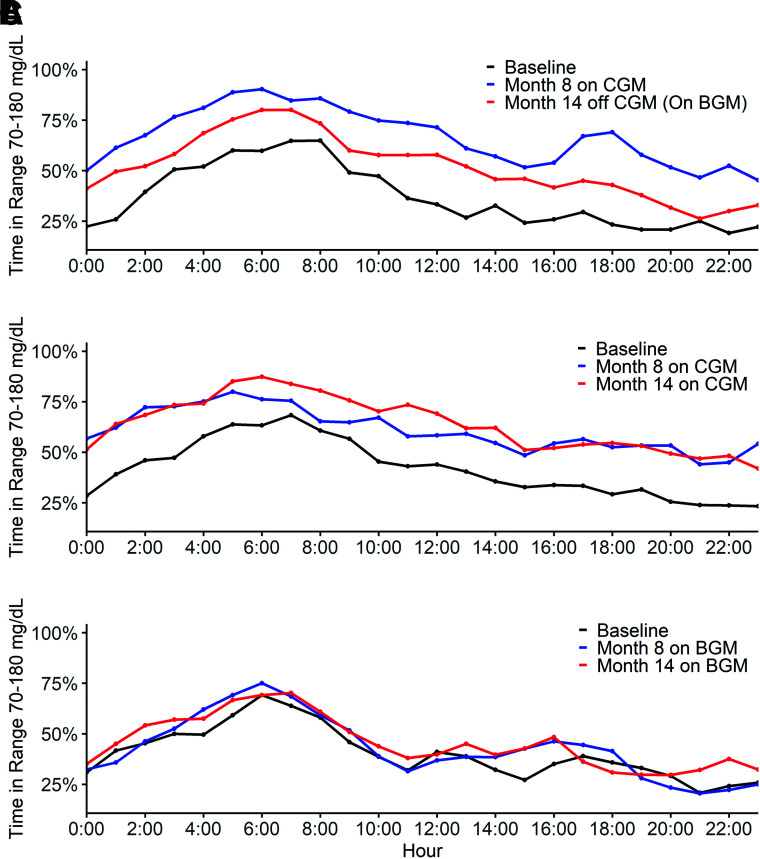
In the discontinue CGM group, mean TIR, which had increased from 38% before initiating CGM (original randomization trial baseline) to 62% at 8 months while using CGM, decreased to 50% at 14 months, 6 months after discontinuing CGM (mean change from 8 months to 14 months −12% [95% CI −21% to −3%], P = 0.01) (Table 2). In the continue CGM group, mean TIR, which was 44% before initiating CGM and 56% at 8 months, was 57% at 14 months (mean change from 8 months to 14 months 1% [95% CI −11% to 12%], P = 0.89). In the BGM group, mean TIR, which was 41% before the initial randomized trial and 43% at 8 months, was 45% at 14 months (mean change from 8 months to 14 months 3% [95% CI −9% to 14%], P = 0.70). Mean TIR over the 14 months of phase 1 plus phase 2 in each treatment group is shown in Fig. 1. In both the discontinue CGM group and the continue CGM group, mean TIR, although appearing lower in the discontinue CGM group, followed a similar pattern over the 24 h of the day, with a peak at ∼6:00 a.m. and a gradual decline through the day until 12:00 a.m., after which it gradually increased until 6:00 a.m. (Fig. 2 and Supplementary Fig. 2). The pattern of TIR over the course of the day in the discontinue CGM group is compared between the CGM use period and the BGM use period in Supplementary Fig. 3.
Table 2.
Glycemic Outcomes
| Discontinue CGM | Continue CGM | BGM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Month 8 | Month 14 | Baseline | Month 8 | Month 14 | Baseline | Month 8 | Month 14 | |
| CGM outcomes, n | 53 | 44 | 49 | 51 | 49 | 46 | 57 | 53 | 53 |
| TIR 70–180 mg/dL (%) | 38 ± 24 | 62 ± 24 | 50 ± 28 | 44 ± 26 | 56 ± 25 | 57 ± 26 | 41 ± 25 | 43 ± 26 | 45 ± 26 |
| Meeting target ≥70%, n (%) | 4 (8) | 19 (43) | 12 (24) | 7 (14) | 14 (29) | 18 (39) | 8 (14) | 10 (19) | 11 (21) |
| Mean glucose (mg/dL) | 213 ± 46 | 173 ± 38 | 196 ± 57 | 201 ± 47 | 184 ± 47 | 181 ± 44 | 204 ± 45 | 206 ± 53 | 201 ± 57 |
| Glucose CV (%) | 29 ± 8 | 27 ± 5 | 27 ± 7 | 28 ± 7 | 27 ± 7 | 26 ± 5 | 28 ± 7 | 29 ± 6 | 29 ± 7 |
| Time >180 mg/dL (%) | 61 ± 24 | 37 ± 25 | 50 ± 28 | 56 ± 27 | 44 ± 26 | 42 ± 27 | 58 ± 26 | 57 ± 27 | 53 ± 27 |
| Time >250 mg/dL* (%) | 28 ± 21 | 9 ± 11 | 20 ± 23 | 24 ± 22 | 12 ± 12 | 12 ± 15 | 23 ± 19 | 27 ± 24 | 22 ± 21 |
| Time >300 mg/dL* (%) | 13 ± 13 | 3 ± 5 | 10 ± 15 | 10 ± 13 | 4 ± 6 | 3 ± 5 | 10 ± 10 | 12 ± 14 | 10 ± 13 |
| AUC 180 mg/dL* | 47 ± 30 | 19 ± 17 | 35 ± 35 | 40 ± 32 | 23 ± 17 | 22 ± 21 | 39 ± 26 | 43 ± 34 | 37 ± 30 |
| Time <70 mg/dL* (%) | 0.19 ± 0.34 | 0.28 ± 0.42 | 0.30 ± 0.43 | 0.49 ± 0.88 | 0.22 ± 0.41 | 0.29 ± 0.47 | 0.47 ± 0.94 | 0.50 ± 0.84 | 0.79 ± 1.36 |
| Time <4 mg/dL* (%) | 0.01 ± 0.03 | 0.02 ± 0.04 | 0.05 ± 0.11 | 0.03 ± 0.06 | 0.01 ± 0.03 | 0.05 ± 0.09 | 0.10 ± 0.19 | 0.13 ± 0.28 | 0.25 ± 0.60 |
| Hypo event rate (per week)* | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.11 ± 0.27 | 0.08 ± 0.20 | 0.00 ± 0.00 | 0.10 ± 0.25 | 0.15 ± 0.30 | 0.16 ± 0.37 | 0.20 ± 0.47 |
| HbA1c, n | 52 | 52 | 48 | 53 | 51 | 51 | 56 | 51 | 51 |
| % | 9.1 ± 1.0 | 7.9 ± 1.4 | 8.2 ± 1.4 | 9.1 ± 0.9 | 8.2 ± 1.4 | 8.1 ± 1.6 | 9.0 ± 0.9 | 8.4 ± 1.3 | 8.5 ± 1.8 |
| mmol/mol | 76 ± 10.9 | 63 ± 15.3 | 66 ± 15.3 | 76 ± 9.8 | 66 ± 15.3 | 65 ± 17.5 | 75 ± 9.8 | 68 ± 14.2 | 69 ± 19.7 |
| Mean change | 95% CI | P | Mean change | 95% CI | P | Mean change | 95% CI | P | |
| Change from month 8 to 14† | |||||||||
| TIR 70–180 mg/dL (%) | −12 | −21 to −3 | 0.01 | 1 | −11 to 12 | 0.89 | 3 | −9 to 14 | 0.70 |
| Mean glucose (mg/dL) | 21 | 5–37 | 0.01 | −2 | −19 to 15 | 0.89 | −7 | −31 to 17 | 0.70 |
| Glucose CV (%) | 1 | −1 to 3 | 0.29 | −1 | −4 to 1 | 0.43 | 0 | −3 to 3 | 0.95 |
| Time >180 mg/dL (%) | 11 | 1–21 | 0.02 | −1 | −12 to 11 | 0.89 | −3 | −15 to 9 | 0.70 |
| Time >250 mg/dL (%) | 10 | 4–17 | 0.005 | −1 | −7 to 6 | 0.89 | −4 | −16 to 9 | 0.70 |
| Time >300 mg/dL (%) | 7 | 2–12 | 0.006 | −1 | −8 to 5 | 0.76 | −2 | −11 to 8 | 0.74 |
| AUC 180 mg/dL | 17 | 6–27 | 0.005 | −2 | −14 to 10 | 0.88 | −5 | −24 to 15 | 0.70 |
| Time <70 mg/dL (%) | 0.09 | −0.20 to 0.39 | 0.50 | 0.07 | −0.24 to 0.39 | 0.76 | 0.45 | −0.56 to 1.45 | 0.70 |
| Time <54 mg/dL (%) | 0.02 | −0.09 to 0.12 | 0.72 | 0.04 | −0.05 to 0.13 | 0.44 | 0.29 | −0.30 to 0.88 | 0.70 |
| Hypo event rate (per week) | 0.04 | −0.10 to 0.18 | 0.57 | 0.07 | −0.07 to 0.21 | 0.32 | 0.11 | −0.30 to 0.51 | 0.70 |
| HbA1c | |||||||||
| % | 0.43 | −0.02 to 0.87 | 0.06 | −0.03 | −0.50 to 0.43 | 0.89 | 0.01 | −0.58 to 0.60 | 0.95 |
| mmol/mol | 4.7 | −0.2 to 9.5 | −0.3 | −5.5 to 4.7 | 0.1 | −6.3 to 6.6 | |||
| Change from baseline to month 14† | |||||||||
| TIR 70–180 mg/dL (%) | 11 | 1–21 | 0.02 | 12 | −1 to 25 | 0.08 | 4 | −8 to 17 | 0.70 |
| Mean glucose (mg/dL) | −16 | −33 to 1 | 0.06 | −17 | −40 to 6 | 0.12 | −3 | −26 to 20 | 0.88 |
| Glucose CV (%) | −1 | −3 to 1 | 0.31 | −2 | −4 to 1 | 0.12 | 1 | −2 to 4 | 0.70 |
| Time >180 mg/dL (%) | −11 | −21 to −2 | 0.02 | −12 | −25 to 1 | 0.08 | −5 | −17 to 8 | 0.70 |
| Time >250 mg/dL (%) | −6 | −15 to 3 | 0.15 | −9 | −20 to 3 | 0.12 | 0 | −11 to 10 | 0.95 |
| Time >300 mg/dL (%) | −2 | −8 to 3 | 0.35 | −4 | −11 to 3 | 0.32 | 2 | −7 to 11 | 0.74 |
| AUC 180 mg/dL | −9 | −21 to 4 | 0.15 | −12 | −29 to 5 | 0.12 | 1 | −17 to 18 | 0.95 |
| Time <70 mg/dL (%) | 0.09 | −0.25 to 0.43 | 0.56 | −0.28 | −0.88 to 0.33 | 0.40 | 0.4 | −0.9 to 1.8 | 0.70 |
| Time <54 mg/dL (%) | 0.03 | −0.05 to 0.10 | 0.41 | 0.00 | −0.11 to 0.12 | 0.95 | 0.17 | −0.53 to 0.87 | 0.70 |
| Hypo event rate (per week) | 0.06 | −0.04 to 0.16 | 0.20 | −0.02 | −0.20 to 0.17 | 0.89 | −0.02 | −0.55 to 0.52 | 0.95 |
| HbA1c | |||||||||
| % | −0.85 | −1.30 to −0.40 | 0.001 | −0.92 | −1.53 to −0.32 | <0.001 | −0.58 | −1.31 to 0.15 | 0.31 |
| mmol/mol | −9.3 | −14.2 to −4.4 | −10.1 | −16.7 to −3.5 | −6.3 | −14.3 to 1.6 | |||
AUC, area under the curve; CV, coefficient of variance; Hypo, hypoglycemia.
Winsorized at the 10th and 90th percentiles before reporting summary statistics.
Mean differences, 95% CIs, and P values are estimates from a mixed-effects linear regression model adjusting for a random site effect. CIs and nominal (uncorrected) P values were adjusted for multiple comparisons using the adaptive two-stage group Benjamini-Hochberg method.
Figure 1.
Glycemic outcomes by treatment and visit. Mean TIR 70–180 mg/dL (A) and mean HbA1c (B) at baseline, month 8, and month 14. The continue CGM group showed CGM for the full 14 months, and the BGM group used BGM for the full 14 months. The discontinue CGM group used CGM through month 8 and then discontinued CGM and used BGM through month 14. Error bars represent 95% CIs.
Figure 2.
Twenty-four-hour plots of phase 2 TIR at baseline, month 8, and month 14 for each treatment group. A plot of TIR 70–180 mg/dL is shown for the discontinue CGM group (A), continue CGM group (B), and BGM group (C) at baseline, month 8, and month 14 according to time of day. Symbols denote the hourly median values for TIR.
The percentages of participants with a worsening in TIR of ≥5% between 8 and 14 months were 51% in the discontinue CGM group, 41% in the continue CGM group, and 31% in the BGM group. The percentages with decreasing TIR by ≥15% were 39%, 22%, and 20%, respectively (Supplementary Table 9).
Other CGM Metrics
In the discontinue CGM group, mean glucose increased from 173 to 196 mg/dL during the 6 months after discontinuing CGM (P = 0.01), while it decreased in the continue CGM group from 184 to 181 mg/dL (P = 0.89) and in the BGM Group from 206 to 201 mg/dL (P = 0.70) (Table 2). Mean glucose over the 24 h of the day in each treatment group can be seen in Supplementary Fig. 2.
Time >250 mg/dL increased from 9% to 20% after discontinuing CGM (P = 0.005), was unchanged at 12% at both 8 and 14 months in the continue CGM group (P = 0.89), and was 27% and 22% at 8 months and 14 months, respectively, in the BGM group (P = 0.70) (Table 2). The amount of hypoglycemia was very low while using CGM and remained low after CGM was discontinued (Table 2).
HbA1c Outcomes
In the discontinue CGM group, mean HbA1c, which had decreased from 9.1% (76 mmol/mol) before initiating CGM to 7.9% (63 mmol/mol) after 8 months of CGM use, increased to 8.2% (66 mmol/mol) 6 months after discontinuing CGM (P = 0.06 comparing 8 and 14 months) (Table 2). In contrast, in the continue CGM group, mean HbA1c, which had decreased from 9.1% (76 mmol/mol) before initiating CGM to 8.2% (66 mmol/mol) after 8 months of CGM use, was 8.1% (65 mmol/mol) at 14 months. In the BGM group, mean HbA1c was 9.0% (75 mmol/mol) before the original randomization, 8.4% (68 mmol/mol) at 8 months, and 8.5% (69 mmol/mol) at 14 months (Table 2). Mean HbA1c over the 14 months of phase 1 plus phase 2 in each treatment group is shown in Fig. 1. Other HbA1c outcomes are shown in Supplementary Table 10.
Metabolic and Safety Outcomes
Change in body weight, blood pressure, or non–HDL cholesterol appeared similar between groups (Supplementary Table 11). Between 8 and 14 months, two severe hypoglycemic events occurred in one participant in the continue CGM group and none in discontinue CGM group or the BGM group. Details of these two events are provided in Supplementary Table 12. There were no occurrences of diabetic ketoacidosis.
Conclusions
The MOBILE randomized trial demonstrated the beneficial effects of real-time CGM among racially and socioeconomically diverse adults with poorly controlled type 2 diabetes treated with basal insulin without prandial insulin and managed in a primary care setting. Approximately one-half of the cohort was of a minority race or ethnicity and approximately one-half had no more than a high school education. In this extension of the MOBILE trial, discontinuation of CGM after 8 months of use resulted in a substantial worsening of glycemic control over the next 6 months, with approximately one-half of the 24% improvement in TIR (from 38% to 62%) during 8 months of CGM use being lost after CGM was discontinued (from 62% to 50%). Since few glucose-lowering medications were added or discontinued and only minimal changes occurred in total daily insulin doses between months 8 and 14, the reduction in TIR, increase in mean glucose, and increase in time above range >250 mg/dL after CGM discontinuation can be attributed mainly to behavioral changes triggered by the lack of CGM cues regarding glucose levels. The fact that the drop in TIR after discontinuing CGM did not fully revert to levels before starting CGM suggests that there could be potential lasting benefits of the 8 months of CGM use possibly as a result of lifestyle and diet modifications or improved medication adherence.
This worsening of TIR after discontinuing CGM is clinically relevant, as evidence is emerging that TIR is associated with the risk of diabetes-related complications in type 1 and type 2 diabetes. In an analysis of the DCCT data, Beck et al. (10) demonstrated that TIR, measured from seven blood samples on 1 day every 3 months, had a strong association with the risk of development and/or progression of retinopathy and development of microalbuminuria. For every 10% lower TIR (roughly equivalent to the reduction in TIR in the current study when CGM was discontinued), the rate of development or worsening of retinopathy increased by 64%, and the rate of development of microalbuminuria increased by 40%. In a longitudinal study of type 2 diabetes, Lu et al. (11) demonstrated an association between lower TIR and an increased risk of all-cause and cardiovascular disease mortality. Several cross-sectional studies have demonstrated the association of TIR with diabetic polyneuropathy, carotid intimal-media thickness, cardiovascular autonomic neuropathy, retinopathy, and nephropathy (12–18).
To our knowledge, the effects of CGM discontinuation in adults with type 2 diabetes has not been previously evaluated. However, the effects of CGM discontinuation have been previously reported in type 1 diabetes. In the Sensing With Insulin Pump Therapy to Control HbA1c (SWITCH) crossover trial of 153 children and adults with type 1 diabetes using an insulin pump, the treatment arm that initially used CGM had improvement in mean HbA1c from 8.5% (69 mmol/mol) at baseline to 7.9% (63 mmol/mol) after 6 months. When CGM was discontinued, mean HbA1c increased to 8.3% (67 mmol/mol) after 4 months (19). Similarly, in the GOLD study (a randomized trial of the effect of CGM in individuals with type 1 diabetes treated with multiple daily insulin injections) of 142 adults using multiple daily injections of insulin, HbA1c improved from 8.5% (69 mmol/mol) at baseline to 7.9% (63 mmol/mol) at 6 months while using CGM followed by an increase to 8.4% (68 mmol/mol) 4 months after CGM was discontinued. Additionally, time <54 mg/dL improved with CGM from ∼2.2% to 0.8% after 6 months and then increased to 1.7% 4 months after CGM was discontinued (20,21). The worsening of HbA1c when CGM was withdrawn in these studies of type 1 diabetes may be greater than what was observed in our type 2 diabetes cohort.
In addition to evaluating the effect of discontinuation of CGM, the current study provided the opportunity to evaluate the use of CGM in this population over 14 months. The benefits in CGM seen after 8 months in TIR, other CGM metrics, and HbA1c were sustained after an additional 6 months of CGM use, and use of CGM remained high despite limited contact with the participants between 8 and 14 months.
A strength of this study was that it included a racially and socioeconomically diverse population receiving diabetes management in a primary care setting, with a substantial proportion of participants being non-White, with less than a college degree, and without private insurance. As such, the study results should be generalizable to most patients with type 2 diabetes using basal insulin without prandial insulin. A limitation in interpreting the results is that sufficient data were not available to evaluate how quickly the benefit of CGM was lost when it was discontinued. Additionally, the extension study was underpowered for treatment group comparisons since the sample size was defined for the original randomized trial and in the extension study, the group continuing CGM was only one-half the size it was in the original randomized trial. By chance, there was an imbalance in both the baseline and the 8-month TIR between the discontinue CGM group and continue CGM group. As a result, there could be a component of regression to the mean impacting the discontinue CGM group relative to the continue CGM group. Finally, despite continued elevations in glucose levels, adjustments were not made by primary care providers and/or participants to bring the values into the target range. Barriers to improvements need to be sought and overcome to help individuals to further improve their glycemic status.
In conclusion, the study results have demonstrated that the benefit of real-time CGM in patients with type 2 diabetes using basal insulin generally in combination with other medications but not bolus insulin is sustained through 14 months. When CGM is discontinued, much, but not all, of the benefit of CGM on glycemic outcomes is lost. Together, findings of the original randomized trial and this extension study demonstrate that for patients with type 2 diabetes with poor glycemic control using basal insulin without prandial insulin and managed in a primary care setting, CGM use is well accepted with a high degree of perseverance of use after 14 months and provides a nonpharmacologic modality to improve glycemic management. Continuation of CGM long term is necessary for the glycemic benefits to be sustained and ultimately contribute to risk reduction for long-term complications.
Article Information
Funding. Study funding and study devices were provided by Dexcom, Inc.
Dexcom had no approval authority for the manuscript before submission, including no right to veto publication and no control on the decision regarding to which journal the manuscript was submitted.
Duality of Interest. All authors received grant funding from Dexcom to their institution for the conduct of the submitted study. G.A. reports grants from AstraZeneca, Dexcom, Eli Lilly, Insulet, and Novo Nordisk and personal fees from Dexcom and Insulet. R.W.B. reports that his institution has received on his behalf grant funding and study supplies from Tandem Diabetes Care, Beta Bionics, and Dexcom; study supplies from Medtronic, Ascencia, and Roche; consulting fees and study supplies from Eli Lilly and Novo Nordisk; and consulting fees from Insulet, Bigfoot Biomedical, vTv Therapeutics, and Diasome. K.J.R. reports that her employer has received grant support from Beta Bionics and Tandem Diabetes Care. P.C. is a former Dexcom employee, and his current employer has received consulting payments on his behalf from vTv Therapeutics, Beta Bionics, Dexcom, and Diasome. A.L.P. reports serving on advisory boards for Abbott Diabetes Care, Eli Lilly, Medscape, Novo Nordisk, and Zealand; nonfinancial study supplies from Abbott Diabetes Care; and ownership of stock options for Omada Health and Teladoc. R.P.-B. reports serving on advisory boards for Averitas, Bayer, Boehringer Ingelheim, Nevro, and Novo Nordisk, and her institution received grants on her behalf from AstraZeneca. A.P.-T. reports that her employer has received funds on her behalf for research support, education support, consulting, or serving on the scientific advisory boards for Abbott Diabetes Care, Dexcom, Johnson & Johnson, Eli Lilly, Medscape, Medtronic, Novo Nordisk, Roche, Sanofi, and UnitedHealthCare. S.B. reports research funding paid to her institution, from Dexcom, Novo Nordisk, Mylan, AstraZeneca, and Bristol-Myers Squibb. G.U. reports research funding paid to his institution from Dexcom, Novo Nordisk, and AstraZeneca. G.D. reports funding paid to her institution from Insulet. D.K. reports grants and personal fees from Dexcom, consulting and research funds from Abbott Diabetes, consulting and speaking fees from Eli Lilly, consulting fees from Sanofi, speaker fees from Xeris Pharmaceuticals, and speaking, consulting, and research funding from Novo Nordisk. A.B. reports research grant–related funding from Abbott Diabetes, AbbVie, Amgen, Boehringer Ingelheim, Boston Therapeutics, Covance, Dexcom, Eli Lilly, Gan and Lee Pharmaceuticals, Insulet, Janssen, Kowa Pharmaceuticals, Madrigal Pharmaceuticals, Medtronic, Merck, Mylan, Novo Nordisk, Poxel, Quintiles, Sanofi, Senseonics, Tolerion, and Viking. L.Y. reports grant funds to her institution from Boehringer Ingelheim, Eli Lilly, Sanofi US, Tolerion, Novo Nordisk, Dexcom, and Bayer Health Care. J.B.B. reports that his employer has received funds on his behalf for consulting and travel from Adocia, AstraZeneca, Dance Biopharm, Dexcom, Eli Lilly, Fractyl, GI Dynamics, Intarcia Therapeutics, Lexicon, MannKind, Metavention, NovaTarg, Novo Nordisk, Orexigen, PhaseBio, Sanofi, Senseonics, vTv Therapeutics, and Zafgen and research grants and travel support from AstraZeneca, Eli Lilly, Intarcia Therapeutics, Johnson & Johnson, Lexicon, Medtronic, NovaTarg, Novo Nordisk, Sanofi, Theracos, Tolerion, and vTv Therapeutics. J.B.M. reports advisory board fees from Bayer, Eli Lilly, Metavant, and Salix; consultancy fees from Boehringer Ingelheim; and grants paid to her employer from Dexcom, Medtronic, and Novo Nordisk. T.M. reports funds paid to his nonprofit employer on his behalf for research support, speaking, or consulting from Abbott Diabetes Care, Dexcom, Insulet, Eli Lilly, Medtronic, Novo Nordisk, Medscape, and Bigfoot Biomedical. Q.T.N.’s employer has received funds on his behalf for research support, consulting, or serving on the scientific advisory boards for AstraZeneca, Sanofi, Novo Nordisk, Eli Lilly, Boehringer Ingelheim, MannKind, and Dexcom. W.B. reports research grant–related funding from Roche Diabetes Care, Novo Nordisk, Mylan, Gan and Lee Pharmaceuticals, and Dexcom. W.H.P. reports consultancy fees from Dexcom, Abbott Diabetes Care, Sanofi, Eli Lilly, Novo Nordisk, Boehringer Ingelheim, Provention Bio, Insulet, Adocia, and Intuity and research support from Dexcom and Abbott Diabetes Care. D.P. is an employee of Dexcom and reports holding stock in the company. R.M.B. reports that his employer has received funds on his behalf for research support, consulting, or service on the scientific advisory boards for Abbott Diabetes Care, Ascenia, Bigfoot Biomedical, Dexcom, Hygieia, Johnson & Johnson, Eli Lilly, Medscape, Medtronic, Novo Nordisk, Onduo, Roche, Sanofi, and UnitedHealthCare. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. G.A. and R.W.B. wrote the first draft of the manuscript and all other authors contributed to the final version. R.B. and P.C. conducted the statistical analyses. D.P., an employee of Dexcom, was involved in the review of the manuscript and interpretation of the data before submission for publication. R.W.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. R.W.B. and K.J.R. provided study coordination and oversight. G.A., A.L.P., R.P.-B., A.P.-T., S.B., G.U., G.D., D.K., A.B., L.Y., J.B.B., J.B.M., T.M., Q.T.N., I.O., W.B., K.J.L., and R.M.B. served as site investigators responsible for the conduct of the protocol at their sites. W.H.P. provided guidance with respect to quality of life measures. D.P. served as medical monitor and sponsor representative.
Prior Presentation. Parts of this study were included in an oral presentation at the 14th International Conference on Advanced Technologies & Treatments for Diabetes, Virtual, 2–5 June 2021.
Footnotes
Clinical trial reg. no. NCT03566693, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.16607969.
A complete list of the MOBILE Study Group can be found in the supplementary material online.
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
Contributor Information
Collaborators: Type 2 Diabetes Basal Insulin Users: The Mobile Study (MOBILE) Study Group:, Thomas Martens, Anders Carlson, Richard M. Bergenstal, Sharon Chambers, Shoua Yang, Laura Young, John Buse, M. Sue Kirkman, Alexander Kass, Rachael Fraser, Davida Kruger, Terra Cushman, Georgia Davis, Clementina Ramos, Guillermo Umpierrez, Anne L. Peters, Maria Magar, Martha Walker, Sara Serafin-Dokhan, Janet B. McGill, Maamoun Salam, Stacy Hurst, Mary Jane Clifton, Grazia Aleppo, Jelena Kravarusic, Anupam Bansal, Candice Fulkerson, Rodica Pop-Busui, Lynn Ang, Caroline Richardson, Kara Mizokami-Stout, Jake Reiss, Virginia Leone, Anuj Bhargava, Kirstie Stifel, Athena Philis-Tsimikas, George Dailey, Amy Change, James McCallum, Maria Isabel Garcia, Shichun Bao, Dianne Davis, Cynthia Lovell, Connie Root, William Biggs, Freida Toler, Lori Wilhelm, Robin Eifert, Lorena Murguia, Becky Cota, Quang T. Nguyen, Loida Nguyen, Randie Lipski, Ian Orozco, Mary Katherine Lawrence, Adelle Fournier, Matthew Carter, K. Jean Lucas, Stephanie Hoover, Roy W. Beck, Katrina J. Ruedy, Peter Calhoun, Ryan Bailey, Nathan Cohen, Thomas Mouse, Jessica Rusnak, Tiffany Campos, David Price, Nelly Njeru, Tom Arant, Stayce E. Beck, and Andrew Balo
References
- 1. Beck RW, Riddlesworth T, Ruedy K, et al.; DIAMOND Study Group . Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA 2017;317:371–378 [DOI] [PubMed] [Google Scholar]
- 2. Beck RW, Riddlesworth TD, Ruedy K, et al.; DIAMOND Study Group . Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med 2017;167:365–374 [DOI] [PubMed] [Google Scholar]
- 3. Pratley RE, Kanapka LG, Rickels MR, et al.; Wireless Innovation for Seniors With Diabetes Mellitus (WISDM) Study Group . Effect of continuous glucose monitoring on hypoglycemia in older adults with type 1 diabetes: a randomized clinical trial. JAMA 2020;323:2397–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ruedy KJ, Parkin CG, Riddlesworth TD, Graham C; DIAMOND Study Group . Continuous glucose monitoring in older adults with type 1 and type 2 diabetes using multiple daily injections of insulin: results from the DIAMOND trial. J Diabetes Sci Technol 2017;11:1138–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tamborlane WV, Beck RW, Bode BW, et al.; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group . Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 [DOI] [PubMed] [Google Scholar]
- 6. Martens T, Beck RW, Bailey R, et al.; MOBILE Study Group . Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA 2021;325:2262–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peters A, Cohen N, Calhoun P, et al. Glycaemic profiles of diverse patients with type 2 diabetes using basal insulin: MOBILE study baseline data. Diabetes Obes Metab 2021;23:631–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 9. Beck RW, Bocchino LE, Lum JW, et al. An evaluation of two capillary sample collection kits for laboratory measurement of HbA1c. Diabetes Technol Ther 2021;23:537–545 [DOI] [PubMed] [Google Scholar]
- 10. Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care 2019;42:400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lu J, Wang C, Shen Y, et al. Time in range in relation to all-cause and cardiovascular mortality in patients with type 2 diabetes: a prospective cohort study. Diabetes Care 2021;44:549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guo Q, Zang P, Xu S, et al. Time in range, as a novel metric of glycemic control, is reversely associated with presence of diabetic cardiovascular autonomic neuropathy independent of HbA1c in Chinese type 2 diabetes. J Diabetes Res 2020;2020: 5817074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim MY, Kim G, Park JY, et al. The association between continuous glucose monitoring-derived metrics and cardiovascular autonomic neuropathy in outpatients with type 2 diabetes. Diabetes Technol Ther 2021;23:434–442 [DOI] [PubMed] [Google Scholar]
- 14. Lu J, Ma X, Shen Y, et al. Time in range is associated with carotid intima-media thickness in type 2 diabetes. Diabetes Technol Ther 2020;22:72–78 [DOI] [PubMed] [Google Scholar]
- 15. Lu J, Ma X, Zhou J, et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care 2018;41:2370–2376 [DOI] [PubMed] [Google Scholar]
- 16. Mayeda L, Katz R, Ahmad I, et al. Glucose time in range and peripheral neuropathy in type 2 diabetes mellitus and chronic kidney disease. BMJ Open Diabetes Res Care 2020;8:e000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ranjan AG, Rosenlund SV, Hansen TW, Rossing P, Andersen S, Nørgaard K. Improved time in range over 1 year is associated with reduced albuminuria in individuals with sensor-augmented insulin pump-treated type 1 diabetes. Diabetes Care 2020;43:2882–2885 [DOI] [PubMed] [Google Scholar]
- 18. Yoo JH, Choi MS, Ahn J, et al. Association between continuous glucose monitoring-derived time in range, other core metrics, and albuminuria in type 2 diabetes. Diabetes Technol Ther 2020;22:768–776 [DOI] [PubMed] [Google Scholar]
- 19. Battelino T, Conget I, Olsen B, et al.; SWITCH Study Group . The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia 2012;55:3155–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA 2017;317:379–387 [DOI] [PubMed] [Google Scholar]
- 21. Ólafsdóttir AF, Polonsky W, Bolinder J, et al. A randomized clinical trial of the effect of continuous glucose monitoring on nocturnal hypoglycemia, daytime hypoglycemia, glycemic variability, and hypoglycemia confidence in persons with type 1 diabetes treated with multiple daily insulin injections (GOLD-3). Diabetes Technol Ther 2018;20:274–284 [DOI] [PMC free article] [PubMed] [Google Scholar]