Abstract
Background
Neurogenic pulmonary oedema (NPO) and Takotsubo cardiomyopathy are rare complications of ischaemic stroke. They are considered to be due to an excess catecholamine release after sympathetic nervous stimulation following stroke onset. Among the different types of Takotsubo cardiomyopathy, apical ballooning is recognized as the typical form, but three atypical patterns have been described (midventricular, basal, and focal) which are more commonly observed in patients with neurological disorders.
Case summary
A 78-year-old woman was treated with intravenous alteplase and underwent mechanical thrombectomy for ischaemic stroke. During the procedure, her respiratory condition quickly worsened requiring invasive mechanical ventilation because of a wide and persistent reduction of the inspiratory oxygen fraction/arterial partial oxygen pressure ratio. Transthoracic echocardiography revealed moderate left ventricular systolic dysfunction with akinesis of the septal-apical and inferior-apical segments. Coronary angiography excluded obstructive lesions and/or evidence of acute plaque rupture. Ventriculography confirmed akinesis/dyskinesis of the inferior segment of the left ventricular apex associated with normal kinesis of the remaining segments. Chest X-ray revealed an infiltrative shadow on both lungs. After 24 h from NPO onset, her respiratory function improved and she was finally discharged on Day 7 without neurological defects. Left ventricular systolic dysfunction was reversible and ejection fraction normalized in 3 months.
Discussion
It is a very rare case of simultaneous NPO and Takotsubo cardiomyopathy following ischaemic stroke. Moreover, it is unique in that it is the first observation of NPO associated with an atypical pattern of Takotsubo cardiomyopathy, which is more frequent in patients with neurological disorders. A rapid recognition and treatment are essential for patient survival.
Keywords: Stroke, Neurogenic pulmonary oedema, Takotsubo cardiomyopathy, Catecholamines, Case report
Learning points
Neurogenic pulmonary oedema (NPO) and Takotsubo cardiomyopathy are rare complications of ischaemic stroke. Atypical Takotsubo cardiomyopathy is more commonly observed in patients with neurological disorders.
Few cases of concomitant NPO and Takotsubo cardiomyopathy have been reported in the literature. The peculiar aspect of this case report, described for the first time in the literature, consists in an atypical focal form Takotsubo cardiomyopathy associated with NPO in an ischaemic stroke patient.
Rapid recognition allows rapid management of NPO preventing the occurrence of complications and resulting in better patient outcome.
Introduction
Neurogenic pulmonary oedema (NPO) is an acute pulmonary oedema associated with subarachnoid haemorrhage and other brain diseases in up to 25% of cases.1 Most likely NPO depends on systemic and pulmonary vasoconstriction due to sympathetic nervous system hyperstimulation with excess catecholamine release in the blood. NPO may worsen patient’s neurological status and outcome for global hypoperfusion and hypoxia.2 NPO morbidity and mortality could be reduced with early diagnosis and rapid management. NPO pathognomonic elements are (i) an early onset, usually within 24 h of the acute cerebral event, (ii) a marked reduction of arterial oxygen partial pressure requiring high-flow oxygen invasive mechanical ventilation, and (iii) a rapid resolution in 1–3 days.
Moreover, circulating catecholamine may also cause myocardial damage, which consists in acute left ventricular systolic dysfunction, called Takotsubo cardiomyopathy, reversible in few months.3 The absence of obstructive coronary disease and/or acute plaque rupture and a previous physical or emotional trigger are diagnostic. Apical ballooning with akinesis of the apical and midventricular segments and hyperkinesis of the basal segments are the most frequent phenotype of Takotsubo cardiomyopathy.4 Variants including midventricular, basal, or focal ballooning are increasingly recognized (called atypical Takotsubo). They are overall uncommon, but more frequent in patients with neurological diseases.5
Timeline
| Symptoms | Diagnosis | Diagnostic method | Specific treatment | Outcome/hospital discharge | |
|---|---|---|---|---|---|
| Day 1 | Dysarthria and left hemiparesis | Ischaemic stroke |
|
|
Resolved (Day 1) |
| Day 1, 4.5 h after stroke onset | Worsening dyspnoea | Acute pulmonary oedema |
|
|
Resolved (Day 1 in 24 h) |
| Day 1, 4.5 h after stroke onset | Cardiorespiratory arrest | Takotsubo cardiomyopathy |
|
|
Partially resolved (Day 7) |
| Day 1, 16 h after stroke onset | Palpitations | Paroxysmal atrial fibrillation |
|
|
Resolved (Day 1 in 1 h) |
| Day 7 | — | — | — | — | Hospital discharge |
| Month 3 | — | Takotsubo cardiomyopathy |
|
|
Resolved (Month 3) |
Case presentation
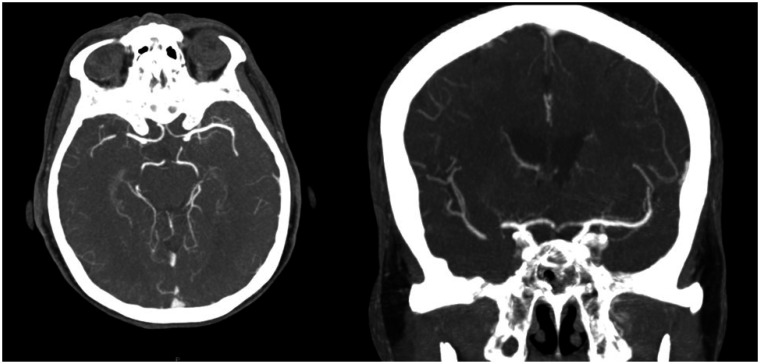
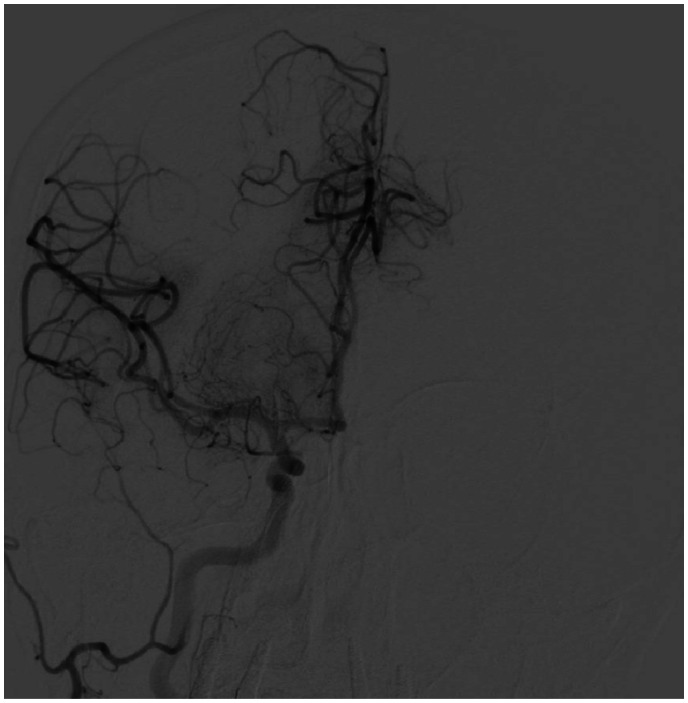
A 78-year-old woman was admitted to the emergency department for dizziness, dysarthria, and left hemiparesis with an NIHSS = 5. Medical history reported arterial hypertension treated with angiotensin-converting enzyme inhibitor, hypercholesterolaemia treated with statin, and paroxysmal atrial fibrillation (AF) (1 episode documented). The patient was treated with aspirin 100 mg/day, although oral anticoagulation is recommended. In the past, she underwent gall bladder removal and quadrant resection for breast cancer, followed by radiotherapy without cardiac damage at echocardiogram. Ranking score according to the modified Rankin scale was = 0. Electrocardiogram showed sinus rhythm, PR, QRS, and QTc intervals and ventricular repolarization within normal limits. Brain computed tomography (CT) scan was performed immediately showing light hypodensity of the posterior portion of the right lenticular nucleus (ASPECT = 9). The perfusion study revealed a penumbra in the right inferior temporal–occipital–insular area in the right middle cerebral artery territory. Cerebral angiography showed segmental thrombosis of the right M1 middle tract with signs of collateralization (Figure 1). Intravenous thrombolytic (alteplase) was started. The patient underwent mechanical thrombectomy ∼4.5 h after symptom onset. Cerebral angiography documented complete recanalization of the right M1 and of two branches of M2 with residual occlusion of the third proximal tract of M2. During the procedure the patient developed worsening dyspnoea and evolved to acute pulmonary oedema (at arterial blood gas analysis: pO2 = 66 mmHg, pCO2 = 83 mmHg, pH = 6.97, P/F ratio = 83) with cyanosis. Oxygen was promptly administered through Venturi mask, but was ineffective with a persistent reduction of the inspiratory oxygen fraction/arterial partial oxygen pressure ratio. The patient was switched to invasive mechanical ventilation but she suffered a 2 min cardiorespiratory arrest, which was resolved with external cardiac massage and intravenous adrenaline. Electrocardiogram documented sinus rhythm, slight ST-segment elevation, and negative T waves in D1 and aVL (Figure 2). Transthoracic echocardiography showed akinesis of the septal-apical and inferior-apical segments of the left ventricle without wall thinning and a marked reduction of ejection fraction (=35%). The following coronary angiography excluded obstructive coronary disease and/or acute plaque rupture (Figure 3). Ventriculography revealed akinesis/dyskinesis of the inferior segment of the left ventricular apex associated with normal kinesis of the remaining segments (Figure 4, Videos 1–2). Afterwards cerebral angiography documented complete recanalization of the right middle cerebral artery (Figure 5). Chest X-ray showed bilateral widespread interstitial-alveolar oedema. The patient was hospitalized in the emergency department. Brain CT scan control resulted negative for bleedings and ischaemic lesions. Medium-high rate paroxysmal AF was detected. The patient was maintained on mechanical ventilation for 24 h and was given intravenous diuretic with progressive normalization of respiratory parameters. Chest X-ray control demonstrated complete reabsorption of interstitial-alveolar oedema. Transthoracic echocardiography confirmed limited akinesis of the septal and inferior apex with a left ventricular ejection fraction at the lower limits of the normal range (= 50%). High sensitivity troponin I elevation was detected (625.5 ng/L, n.v. <11.6 ng/L). The patient was transferred to the Stroke Unit with an NIHSS = 0. The rate control of AF was obtained with a beta-blocker and oral anticoagulation was started instead of antiplatelet therapy. The patient was discharged on Day 7 without neurological defects and symptoms/signs of heart failure. After 3 months, left ventricular segmental kinetics and ejection fraction were completely normalized.
Figure 1.
Cerebral angiography: segmental thrombosis of the right M1 middle tract with signs of collateralization.
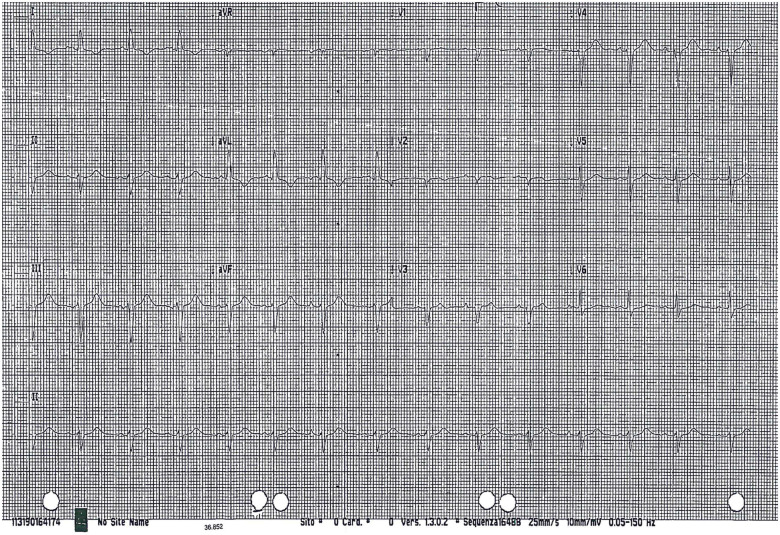
Figure 2.
Electrocardiogram: sinus rhythm, slight ST-segment elevation and negative T waves in D1 and in aVL.
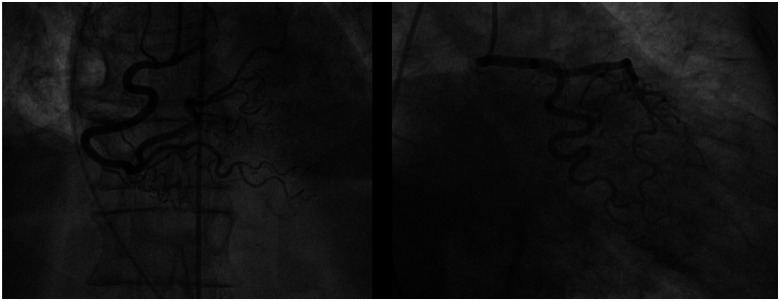
Figure 3.
Coronary angiography: no obstructive coronary lesions and/or acute plaque rupture.
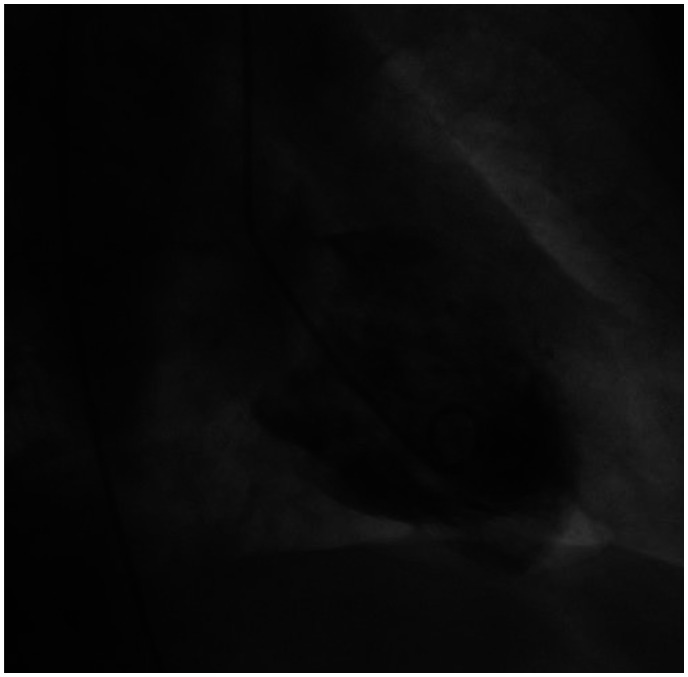
Figure 4.
Ventriculography: akinesis/dyskinesis of the inferior segment of the left ventricular apex and normal kinesis of the remaining segments.
Figure 5.
Cerebral angiography control: complete recanalization of the right middle cerebral artery.
Discussion
This case report describes the unique combination of NPO and an atypical pattern of Takotsubo cardiomyopathy in a patient with acute ischaemic stroke, due to sympathetic nervous system activation and serum catecholamine release after an emotional or physical trigger such as ischaemic stroke.3,6
Previously few cases of NPO associated with typical variant of Takotsubo cardiomyopathy have been reported. Kitagawa et al. described the case of a 79-year-old patient affected by ischaemic stroke due to right middle cerebral artery occlusion. Suddenly after alteplase infusion, NPO and left ventricular systolic dysfunction with apical ballooning appeared.7 Recently the same authors described another case with combination of these two events during carotid endarterectomy.8 An 88-year-old man with severe left internal carotid artery stenosis developed NPO and typical Takotsubo cardiomyopathy during surgery immediately after vessel clamping. The authors concluded that massive catecholamine release in the blood was due to both reperfusion damage and sympathetic nervous system stimulation for the contralateral propagation of left insular cortex ischaemia through the dorsal commissure of the hippocampus. It is known the right insular cortex stimulation is associated with sympathetic nervous system activation, while left insular cortex stimulation with the parasympathetic nervous system.9 In the paediatric setting, concomitant NPO and Takotsubo cardiomyopathy have been reported in one case of epidural haemorrhage.10
The peculiar aspect of this case report, described for the first time in the literature, consists in acute left ventricular systolic dysfunction limited to the apical inferior-septal segment, called atypical focal Takotsubo cardiomyopathy, associated with NPO.4 A higher prevalence of atypical Takotsubo cardiomyopathy is reported in patients with neurological disorders like ischaemic stroke.5 The prognosis of atypical Takotsubo cardiomyopathy does not differ from the typical one, although the focal type has a more favourable course without cardiogenic shock or in-hospital death. As in all Takotsubo presentations, the modest increase of troponin serum levels, without myocardial creatine kinase elevation, suggests a limited and transient myocardial damage.11
In our case, the rapid recognition and the consequent rapid management of NPO reduced any complications related to hypoxia, and only the administration of high-flow oxygen through invasive mechanical ventilation allowed to improve and normalize the patient’s gas exchange in a short time with complete resolution of interstitial-alveolar oedema. Furthermore, the echocardiogram, promptly performed, allowed to detect myocardial dysfunction. The concomitant focal type of Takotsubo cardiomyopathy improved from the first days following the event in relation to catecholamine short half-life and later regressed as expected. Finally, ischaemic stroke, which was the reason for patient’s hospitalization and the trigger for sympathetic nervous system hyperstimulation, completely recovered without residual ischaemic lesion at brain imaging after intravenous thrombolytic associated with mechanical thrombectomy, which represents the gold-standard procedure for ischaemic stroke according to current European and American guideline recommendations. Oral anticoagulation was started instead of antiplatelet therapy as recommended in patients affected by ischaemic stroke with AF documentation.
The main limitation is that serum catecholamine levels were not measured. Thus, we could not demonstrate sympathetic nervous system hyperstimulation due to ischaemic stroke. However, based on the known pathophysiological mechanism, we consider it plausible and responsible for the combined NPO and atypical Takotsubo cardiomyopathy.
Conclusions
This case report represents the first observation in the literature of combined NPO and atypical Takotsubo cardiomyopathy in a patient with ischaemic stroke and warrants a close neurology and cardiology interaction in this setting. Furthermore, atypical Takotsubo cardiomyopathy is more frequent in subjects with neurological disorders.
Lead author biography

Letizia Riva is a consultant cardiologist covering the role of cardiologist dedicated to Stroke Unit in Maggiore Hospital in Bologna (Italy). She has experience in the management of stroke, atrial fibrillation, cardiomyopathies and oral anticoagulation.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Funding: none declared.
Supplementary Material
References
- 1.Baumann A, Audibert G, McDonnell J, Mertes PM.. Neurogenic pulmonary edema. Acta Anaesthesiol Scand 2007;51:447–455. [DOI] [PubMed] [Google Scholar]
- 2.Muroi C, Keller M, Pangalu A, Fortunati M, Yonekawa Y, Keller E.. Neurogenic pulmonary edema in patients with subarachnoid hemorrhage. J Neurosurg Anesthesiol 2008;20:188–192. [DOI] [PubMed] [Google Scholar]
- 3.Chen Z, Venkat P, Seyfried D, Chopp M, Yan T, Chen J.. Brain-heart interaction: cardiac complications after stroke. Circ Res 2017;121:451–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurst TR, Prasad A, Askew JW III, Sengupta PP, Tajik AJ.. Takotsubo cardiomyopathy: a unique cardiomyopathy with variable ventricular morphology. JACC Cardiovasc Imaging 2010;3:641–649. [DOI] [PubMed] [Google Scholar]
- 5.Ghadri JR, Cammann VL, Napp LC, Jurisic S, Diekmann J, Bataiosu DR. et al. ; International Takotsubo (InterTAK) Registry. Differences in the clinical profile and outcomes of typical and atypical Takotsubo syndrome: data from the International Takotsubo Registry. JAMA Cardiol 2016;1:335–340. [DOI] [PubMed] [Google Scholar]
- 6.Riva L, Urbinati S, Di Pasquale G.. Heart-brain interactions. G Ital Cardiol 2019;20:265–278. [DOI] [PubMed] [Google Scholar]
- 7.Kitagawa T, Yamamoto J, Kureshima M, Maeda H, Nishizawa S.. Takotsubo cardiomyopathy and neurogenic pulmonary edema following fibrinolytic therapy for embolic stroke: a case report. No Shinkei Geka 2018;46:21–25. [DOI] [PubMed] [Google Scholar]
- 8.Kitagawa T, Ishikawa H, Yamamoto J, Ota S.. Takotsubo cardiomyopathy and neurogenic pulmonary edema after carotid endarterectomy. World Neurosurg 2019;124:157–160. [DOI] [PubMed] [Google Scholar]
- 9.Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC.. Cardiovascular effects of human insula cortex stimulation. Neurology 1992;42:1727–1732. [DOI] [PubMed] [Google Scholar]
- 10.Nakamori Y, Miyazawa N, Yoshitani K, Yamamoto S.. A pediatric case with Takotsubo cardiomyopathy and neurogenic pulmonary edema due to an epidural hemorrhage. J Neurosurg Anesthesiol 2018;30:279–280. [DOI] [PubMed] [Google Scholar]
- 11.Scantlebury DC, Prasad A.. Diagnosis of Takotsubo cardiomyopathy. Circ J 2014;78:2129–2139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.