Abstract
Purpose of review
Bioengineering the lung based on its natural extracellular matrix (ECM) offers novel opportunities to overcome the shortage of donors, to reduce chronic allograft rejections, and to improve the median survival rate of transplanted patients. During the last decade, lung tissue engineering has advanced rapidly to combine scaffolds, cells, and biologically active molecules into functional tissues to restore or improve the lung’s main function, gas exchange. This review will inspect the current progress in lung bioengineering using decellularized and recellularized lung scaffolds and highlight future challenges in the field.
Recent findings
Lung decellularization and recellularization protocols have provided researchers with tools to progress toward functional lung tissue engineering. However, there is continuous evolution and refinement particularly for optimization of lung recellularization. These further the possibility of developing a transplantable bioartificial lung.
Summary
Bioengineering the lung using recellularized scaffolds could offer a curative option for patients with end-stage organ failure but its accomplishment remains unclear in the short-term. However, the state-of-the-art of techniques described in this review will increase our knowledge of the lung ECM and of chemical and mechanical cues which drive cell repopulation to improve the advances in lung regeneration and lung tissue engineering.
Keywords: decellularization, lung regeneration, lung scaffold, recellularization
INTRODUCTION
Lung bioengineering and tissue regeneration is an exciting and rapidly progressing area in the biomedical field. The increasing demand for transplantable organs in end-stage lung failures [1] has motivated researchers to investigate creative alternatives. One such approach involves the cellularization of natural extracellular matrix (ECM) lung scaffolds obtained via decellularization, a strategy that might allow researchers to leverage a large pool of lungs currently not suitable for transplantation [2,3]. The decellularization process consists of removing all cellular content from the scaffold while preserving its complex natural three-dimensional structure, the protein and molecular composition, and the macromechanical and nanomechanical properties of the source tissue. The decellularized scaffolds will then ideally support colonization of patient sourced stem/progenitor cells, and produce a transplantable, bioengineered lung, with significantly reduced risk of rejection and immunosuppression requirements [4,5].
Over the last several years, investigators have combined scaffolds, cells, and biologically active molecules into semifunctional tissues able to perform some level of short-term gas exchange, the primary function of the lung. However, full gas exchange and the creation of long-lasting bioartificial lungs remains an elusive goal. This review will discuss the current progress and challenges in lung decellularization and recellularization based on the available techniques that have and will continue to contribute to increased knowledge of lung ECM, chemical composition, and mechanical characterization.
REFINING THE TECHNIQUE: DECELLULARIZATION AND RECELLULARIZATION
There is a substantial literature on lung decellularization and recellularization [6,7▪,8]. However, intermediate steps for ECM characterization, devices for tissue development, and functional/quality characterization are continuously evolving.
Decellularization
A variety of approaches have been utilized including one or more of a combination of physical [9], enzymatic, and chemical agents [10] (Fig. 1a). The most common decellularization protocols are detergent-based and include combined use of nonionic and ionic detergents, Triton X-100 and sodium deoxycholate (SDC) [7▪,11] or anionic surfactant such as SDS, alone [6,12]. There is no standardized procedure, in part as the lungs, in contrast to other organs, can be perfused with decellularization solutions through either the airways, the vasculature, or both simultaneously; all three approaches have been utilized. Moreover, different concentrations of each reagent, specific order of administration, and routes of perfusion are described in the literature [8,13,14]. Further refinements [15] have attempted to define the most effective way to perfuse these lungs. For example, some approaches have utilized either physiological flow or pressure through the vasculature [6,16], whereas others have utilized incremental perfusion rates, systematically alternated between airway and vasculature [11,17]. Moreover, different lengths of exposure times for detergent and other decellularization reagents, ranging from several hours to several days, have been utilized [10,13].
FIGURE 1. Characterization of the decellularized lung scaffold.
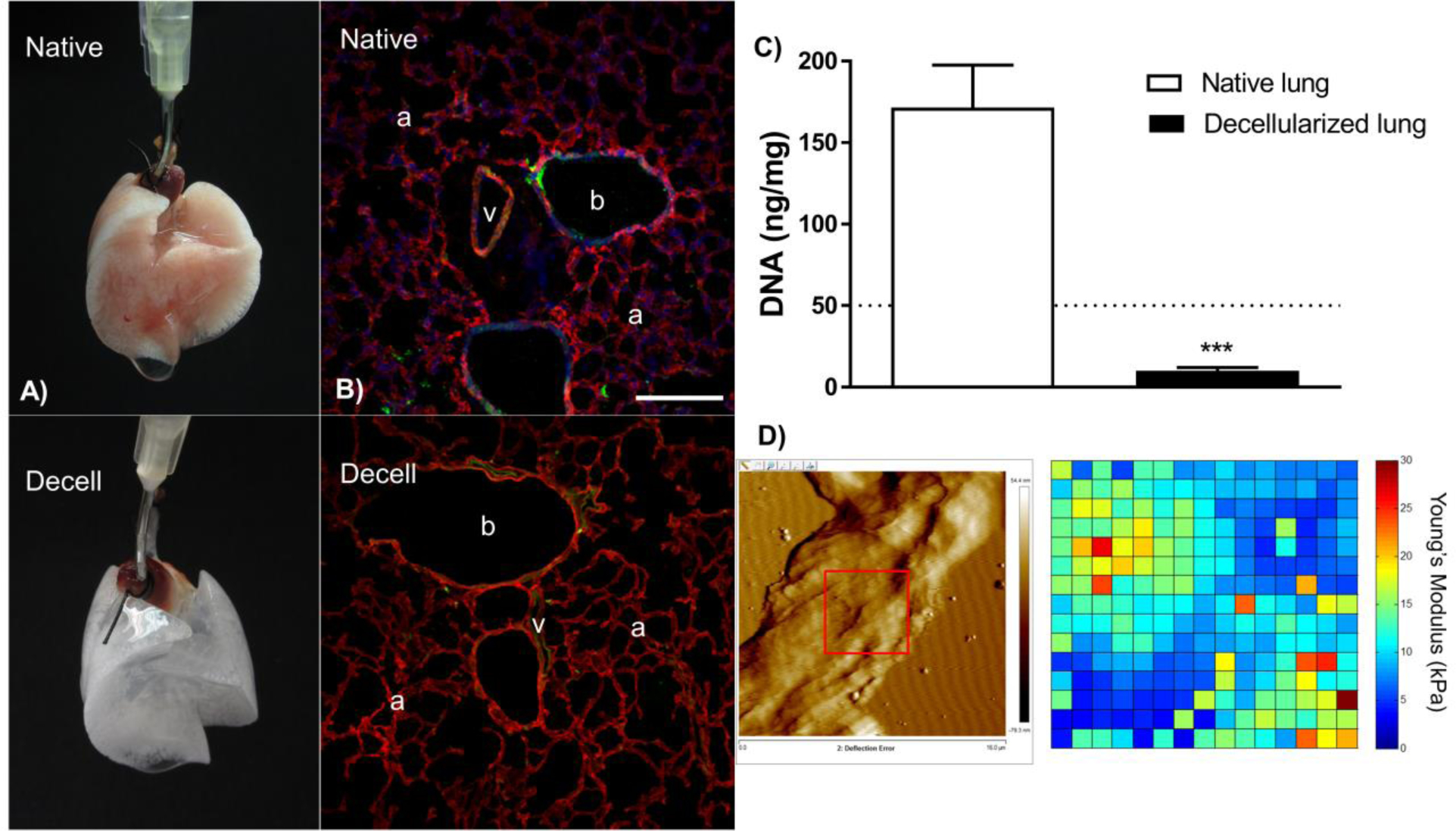
The panel depicts in (a) a native and a decellularized murine lung which preserves its three-dimensional structure. (b) Chemical composition of the extracellular matrix, assessed by immunofluorescence and confocal microscopy, determines that cell nuclear components are absent in the decellularized scaffold, whereas collagen I and elastin are present after the procedure. The bronchial space (b), vasculature (v) and alveoli (a) are depicted in the images. Bar scale is 200 μm. (c) The DNA content remaining after decellularization is below the threshold of 50 ng/mg of dry tissue total protein suggested in the literature [18]. Data are presented as means ± SD and n = 4. (d) The nanomechanical properties of the acellular lung scaffold are depicted by a topographical image and a force map obtained on an alveolar wall of the lung parenchyma. Pyramidal cantilever, nominal spring constant of 0.03 N/m. The panel is constituted by original images obtained in collaborative work of D.J.W. at the University of Vermont and Prof Dr R. Farré at the University of Barcelona.
Characterization of the decellularized scaffold
The decellularization technique is particularly challenging requiring a balance between preservation of the structural and chemical composition of the ECM (Fig. 1b) with total cellular content removal (Fig. 1c). Despite the different protocols, most investigators achieve some degree of decellularization, which can be defined as [7▪,18] macrostructure and microstructure preservation, minimal residual DNA, preservation of the native ECM composition, and preservation of relevant mechanical properties. Suggested criteria for successful decellularized include absence of cellular content and nuclear material by routine histologic (Hematoxylin & Eosin, DAPI) staining levels of double-stranded DNA below 50 ng/mg dry tissue, and absence of DNA fragments above 200 bp on DNA gels [19]. Based on our experience, using Triton X-100 and SDC preserves the structure while removing cellular components better than methods using SDS or CHAPS detergent (3-((3-cholamidopropyl) dimethylammonio)-1-propane-sulfonate). Pressure-controlled decellularization protocols pose the risk of insufficient delivery of detergents to atelectatic areas of the lung especially in larger organs from pigs and humans. Small lungs from rodents for example are therefore usually easier to decellularize. As the diffusion length during the incubation steps is shorter even improper detergent distribution can usually be overcome. We believe that volume controlled methods are a better approach standardizing the amount of liquid used to the tidal volume of the respective organ to be decellularized.
Visualization of the decellularized scaffold and assessment of structural integrity generally involves light microscopy, immunofluorescent staining of residual ECM proteins such as collagens, elastin, laminin, fibrillin, or either ECM components including glycosaminoglycans (GAGs), and electron micrographic techniques [20]. Although histologic and immunofluorescent approaches provide important information on overall decellularized lung structure, electron microscopy, while providing important ultrastructural datais limited to a few individual regions and does not give an overall evaluation of the scaffold in its entirety [9,21].
The chemical composition of the ECM is commonly assessed by immunofluorescent staining and quantitative measurements of specific structural components. However, the use of quantitative techniques such as western blotting is limited to the assessment of few selected proteins [6,7▪,11,22]. Moreover, the ECM composition can vary depending on the protocol that has been utilized [7▪,23,24▪▪]. Therefore, more quantitative methods like protein arrays [25] and mass spectrometry with semiquantitative and quantitative proteomics [23,24▪▪,26▪,27,28▪] are employed. These global approaches allow better comparative assessments of the residual protein content left behind [29▪] by different methods of decellularization.
The mechanical properties of the decellularized lung scaffolds, including stiffness, elastance, airways resistance, and others, are critically important but not as well studied. Approaches utilizing mechanical ventilation and tensile strength testing of lung strips are the most commonly used approaches [12,30]. Recent investigations utilizing atomic force microscopy have provided more detailed assessments of the nanomechanical properties of decellularized lung tissues [31,32] (Fig. 1d). However, further research in this field is needed.
Current decellularization approaches have proven the possibility to isolate the lung natural scaffold. To move lung tissue engineering toward clinical application based on acellular scaffolds all described methods for scaffold characterization (Fig. 1) need to be performed to certify the accomplishment of the procedure.
Recellularization
Before starting the recellularization process, it is necessary to assure that residual detergents are properly removed, as they are detrimental to cell survival. Available techniques to confirm proper detergent removal by invasive [6] and noninvasive detergent detection methods [33] should be widely used as quality control.
A wide variety of cell types have been seeded into decellularized models including rodent, pig, and human lungs. These have included mixtures of fetal lung homogenates, differentiated lung epithelial, pulmonary vascular endothelial, and stromal cells, and a variety of endogenous lung progenitor and stem cell populations including lung epithelial and other cells derived from induced pluripotent stem cells [2,3,11,34▪▪–36▪▪,37▪]. These have also included xenogeneic approaches, for examples, human cells into decellularized rat or pig lungs [38▪,39▪▪]. A variety of seeding strategies have been employed as well including pressure vs. flow drive airway vs. vascular inoculations. These approaches have resulted in a range of results showing ability to differentially seed different compartments (airways vs. vasculature) and have provided important information on bioreactor techniques necessary to sustain and promote cell growth [4,5,34▪▪,35▪▪,37▪,40▪,41,42▪–44▪]. However, there is no clear advantage to any particular type of seeding methods or range and combinations of inoculated cells. Further, the ultimate goal of recreating the lung cellular environment with properly functioning airway and alveolar epithelial cells as well as pulmonary vascular endothelial cells and stromal cells all in their proper compartments remains difficult to achieve. As such, decellularized lungs can certainly be populated with cells but creation of functional gas exchange units capable of long-term use remains as elusive today as it did following the first reports of these approaches in 2010 [3–5,36▪▪,42▪,45,46].
Further knowledge about the nature of the ECM is critical for improving recellularization as ECM affects cellular behavior including stem cell differentiation [25,47–49]. Moreover, the bioreactor technologies utilized to culture the recellularizing lungs, consisting of sophisticated isolation chambers, continuous perfusion systems, environmental control, and physiological ventilation, are continuously evolving [40▪,50].
There are numerous emerging applications stemming from this singular pursuit, already beginning to provide a return on these investments. These include the bioengineering of individual lung structures for transplantation, development of gas-exchange assist technologies, high-throughput three-dimensional tissue models, for testing promising drug and therapeutic strategies, research tool for studying lung developmental biology and disease mechanisms, material sourcing for development of lung ECM derived biomaterials [51,52].
APPLICATIONS, CHALLENGES, AND OPPORTUNITIES
The bioengineering of the whole-organ, particularly the lung is an exciting evolving field in biomedical engineering [37▪,44▪] (Fig. 2). The research related to lung tissue regeneration has rapidly progressed in recent years. However, the lung is a very complex organ composed of numerous cell types, chemical ECM components and intrinsic mechanical properties [53], and this complexity offers many challenges. For example, the most common techniques used to decellularize lungs involves perfusion of chemical and biological agents, often in combination with a physical method, which may alter or destroy important ECM components impacting the structure and the possibility for future cells to adhere and proliferate [54–56]. Currently understudied areas are GAG and lipid composition of scaffolds. GAGs are particularly important to investigate as they can bind matrikines and can therefore critically influence cellular behavior [28▪]. New advanced bioreactor systems must be designed to provide the bioengineered lung with nutrition, oxygen, and mechanical ventilation, but also real-time measurements of for example electrolytes, pH, glucose, and lactate [50]. Standardized sterilization processes which do not negatively affect the cells or scaffolds need to be optimized [22,57–59]. Although a functional gas-exchange unit is the final goal, decellularized lungs can provide valuable research tools with which to study ECM and cell-matrix interactions in normal and in diseased lungs.
FIGURE 2. Procedure to bioengineer the lung.
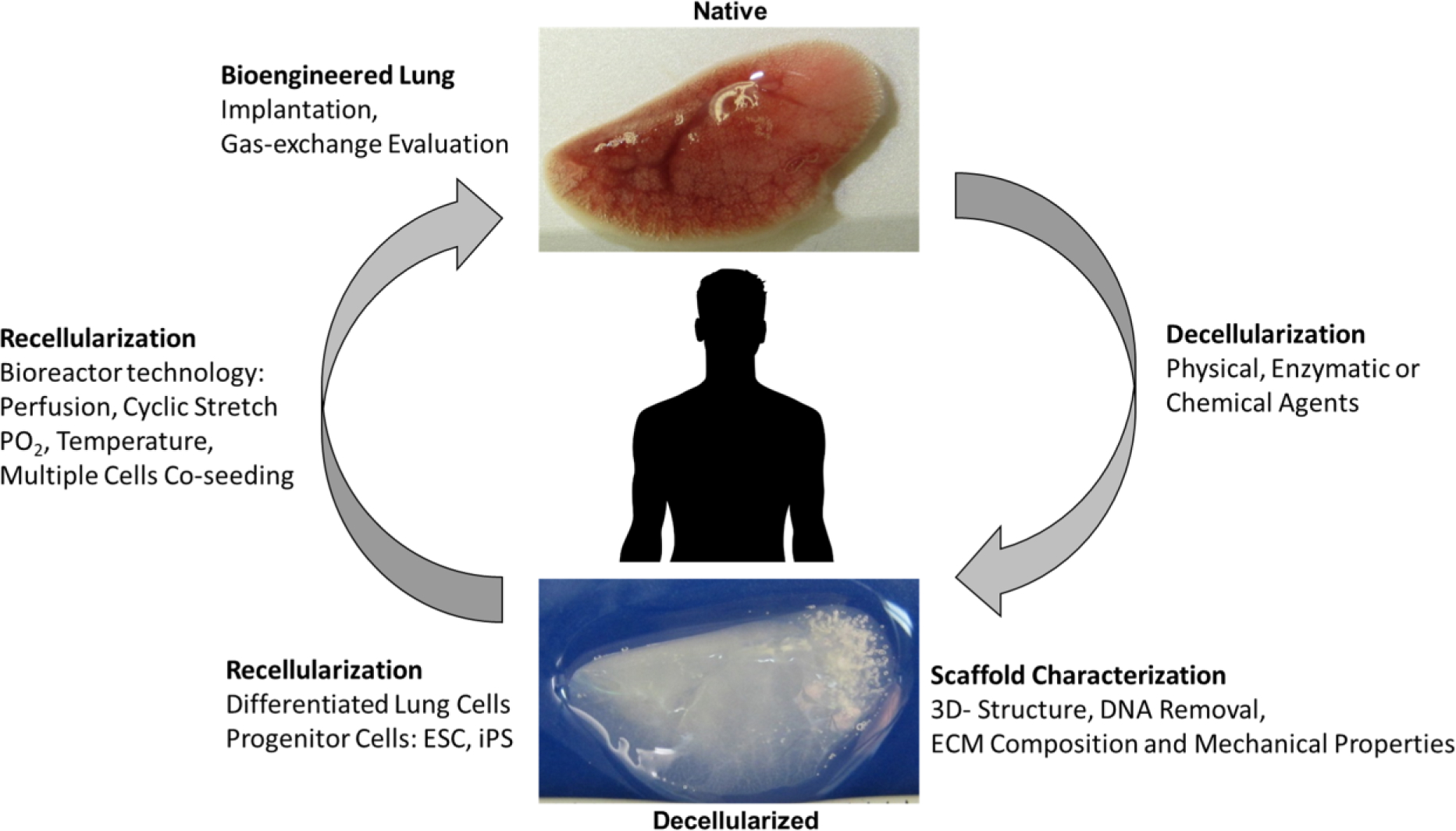
Schematic for the procedure for bioengineering the lung based on the decellularization and recellularization process. This figure is based on previous published work [4].
CONCLUSION
Eight years after the initial reports of bioengineered lungs, the decellularization techniques and ECM characterization continue to be refined as do modifications in cell seeding conditions and bioreactor technologies. Further investigations need to focus on large-area coverage, cell coculture, differentiation, surfactant production, and long-term gas-exchange to finally achieve transplantable bioengineered lungs based on decellularized and recellularized scaffolds.
KEY POINTS.
The ECM, known as scaffold, can be obtained by the decellularization process maintaining three-dimensional structure, chemical composition, and mechanical properties.
The acellular lung scaffold can be repopulated with different cell types particularly stem and lung progenitor cells. However, new approaches are needed in terms of complete surface coverage and gas-exchange.
The interest in lung bioengineering is increasing in the regenerative medicine field, due to the current shortage of donors for organ transplantation and the possibility to develop engraftments obtained from allogenic and xenogeneic donors that can be translated into the clinics.
Bioengineering the lung is at an early stage; however, initial results of gas-exchange are motivation to the scientific community to finally achieve transplantable bioengineered lungs.
Acknowledgements
The authors want to thank Drs Ramon Farré and Daniel Navajas at the University of Barcelona for their collaboration in the preparation of the figures.
Financial support and sponsorship
The authors are supported by the following grants: J.J.U. by the NIH RO1 HL12714401 (D.J.W.), F.E.U. by the Knut and Alice Wallenberg Foundation fellowship of her supervisor Dr Meissner, S.E.R.E. by a Marie Curie Post-doctoral Research Fellowship (RESPIRE3) from the European Respiratory Society and the European Union’s H2020 research and innovation programme (Marie Sklodowska-Curie grant agreement no. 713406), R.A.P. by the NHBLI Lung Biology Training grant T32. Research support: HL076122 (D.J.W.), NHLBI HL127144-01 (D.J.W.), EB024329, the Cystic Fibrosis Foundation, and United Therapeutics Inc.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Lund LH, Khush KK, Cherikh WS, et al. The registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Heart Transplantation Report – 2017; focus theme: allograft ischemic time. J Heart Lung Transplant 2017; 36:1037–1046. [DOI] [PubMed] [Google Scholar]
- 2.Ott HC, Matthiesen TS, Goh S-K, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med 2008; 14:213–221. [DOI] [PubMed] [Google Scholar]
- 3.Petersen TH, Calle EA, Zhao L, et al. Tissue-engineered lungs for in vivo implantation. Science 2010; 329:538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner DE, Bonvillain RW, Jensen T, et al. Can stem cells be used to generate new lungs? Ex vivo lung bioengineering with decellularized whole lung scaffolds. Respirology 2013; 18:895–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren X, Moser PT, Gilpin SE, et al. Engineering pulmonary vasculature in decellularized rat and human lungs. Nat Biotechnol 2015; 33:1097–1102. [DOI] [PubMed] [Google Scholar]
- 6.Gilpin SE, Guyette JP, Gonzalez G, et al. Perfusion decellularization of human and porcine lungs: bringing the matrix to clinical scale. J Hear Lung Transplant 2014; 33:298–308. [DOI] [PubMed] [Google Scholar]
- 7.▪.Uhl FE, Wagner DE, Weiss DJ. Preparation of decellularized lung matrices for cell culture and protein analysis. New York, NY: Humana Press; 2017; 253–283. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors define quality control assessments for decellularized and recellularized scaffolds, including mass spectrometry for protein analysis.
- 8.Keane TJ, Saldin LT, Badylak SF. Decellularization of mammalian tissues: preparing extracellular matrix bioscaffolds. In: Characterisation and design of tissue scaffolds. Waltham, MA, USA: Elsevier; 2016. pp. 75–103. [Google Scholar]
- 9.Nonaka PN, Campillo N, Uriarte JJ, et al. Effects of freezing/thawing on the mechanical properties of decellularized lungs. J Biomed Mater Res A 2014; 102:413–419. [DOI] [PubMed] [Google Scholar]
- 10.Keane TJ, Swinehart IT, Badylak SF. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 2015; 84:25–34. [DOI] [PubMed] [Google Scholar]
- 11.Wagner DE, Bonenfant NR, Sokocevic D, et al. Three-dimensional scaffolds of acellular human and porcine lungs for high throughput studies of lung disease and regeneration. Biomaterials 2014; 35:2664–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nonaka PN, Uriarte JJ, Campillo N, et al. Mechanical properties of mouse lungs along organ decellularization by sodium dodecyl sulfate. Respir Physiol Neurobiol 2014; 200:1–5. [DOI] [PubMed] [Google Scholar]
- 13.Badylak SF, Taylor D, Uygun K. Whole organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng 2010; 13:110301095218061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Wang Z, Yu Q, et al. Comparative study of two perfusion routes with different flow in decellularization to harvest an optimal pulmonary scaffold for recellularization. J Biomed Mater Res A 2016; 104:2567–2575. [DOI] [PubMed] [Google Scholar]
- 15.Dorrello NV, Guenthart BA, O’Neill JD, et al. Functional vascularized lung grafts for lung bioengineering. Sci Adv 2017; 3:e1700521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials 2006; 27:3675–3683. [DOI] [PubMed] [Google Scholar]
- 17.Wagner DE, Bonenfant NR, Parsons CS, et al. Comparative decellularization and recellularization of normal versus emphysematous human lungs. Biomaterials 2014; 35:3281–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials 2011; 32:3233–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilbert TW, Freund JM, Badylak SF. Quantification of DNA in biologic scaffold materials. J Surg Res 2009; 152:135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urbano JJ, da Palma RK, de Lima FM, et al. Effects of two different decellularization routes on the mechanical properties of decellularized lungs. PLoS One 2017; 12:e0178696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balestrini JL, Gard AL, Liu A, et al. Production of decellularized porcine lung scaffolds for use in tissue engineering. Integr Biol (Camb) 2015; 7:1598–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uriarte JJ, Nonaka PN, Campillo N, et al. Mechanical properties of acellular mouse lungs after sterilization by gamma irradiation. J Mech Behav Biomed Mater 2014; 40:168–177. [DOI] [PubMed] [Google Scholar]
- 23.Hill RC, Calle EA, Dzieciatkowska M, et al. Quantification of extracellular matrix proteins from a rat lung scaffold to provide a molecular readout for tissue engineering. Mol Cell Proteomics 2015; 14:961–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.▪▪.Calle EA, Hill RC, Leiby KL, et al. Targeted proteomics effectively quantifies differences between native lung and detergent-decellularized lung extracellular matrices. Acta Biomater 2016; 46:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper compares two decellularization methods and includes the application of mass spectrometry technique to identify key proteins in the ECM. The authors also propose the protein analysis as a quality control for acellular scaffolds.
- 25.Shojaie S, Ermini L, Ackerley C, et al. Acellular lung scaffolds direct differentiation of endoderm to functional airway epithelial cells: requirement of matrix-bound HS proteoglycans. Stem Cell Rep 2015; 4:419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.▪.Gilpin SE, Li Q, Evangelista-Leite D, et al. Fibrillin-2 and Tenascin-C bridge the age gap in lung epithelial regeneration. Biomaterials 2017; 140:212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors quantify proteins in neonatal lung ECM and identify an increase in developmentally associated proteins FBN-2 and TN-C, potential mediators to enhance re-epithelization in decellularized lung scaffolds.
- 27.Schiller HB, Fernandez IE, Burgstaller G, et al. Time- and compartment-resolved proteome profiling of the extracellular niche in lung injury and repair. Mol Syst Biol 2015; 11:819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.▪.Li Q, Uygun BE, Geerts S, et al. Proteomic analysis of naturally-sourced biological scaffolds. Biomaterials 2016; 75:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]; This research shows the proteome of acellular organs and tracks of specific protein losses during the decellularization process, highlighting the importance of thoroughly characterizing acellular scaffolds.
- 29.▪.Wrenn SM, Griswold ED, Uhl FE, et al. Avian lungs: a novel scaffold for lung bioengineering. PLoS One 2018; 13:e0198956. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors describe decellularized avian lungs, recellularized with human lung cells as a potential bioscaffolds for use as a lung assist device.
- 30.Wallis JM, Borg ZD, Daly AB, et al. Comparative assessment of detergent-based protocols for mouse lung de-cellularization and re-cellularization. Tissue Eng Part C Methods 2012; 18:420–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melo E, Garreta E, Luque T, et al. Effects of the decellularization method on the local stiffness of acellular lungs. Tissue Eng Part C Methods 2014; 20:412–422. [DOI] [PubMed] [Google Scholar]
- 32.Liu F, Tschumperlin DJ. Micro-mechanical characterization of lung tissue using atomic force microscopy. J Vis Exp 2011; pii: e2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zvarova B, Uhl FE, Uriarte JJ, et al. Residual detergent detection method for nondestructive cytocompatibility evaluation of decellularized whole lung scaffolds. Tissue Eng Part C Methods 2016; 22:418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.▪▪.Scarritt ME, Pashos NC, Motherwell JM, et al. Re-endothelialization of rat lung scaffolds through passive, gravity-driven seeding of segment-specific pulmonary endothelial cells. J Tissue Eng Regen Med 2018; 12:e786–e806. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors explore the recellularization of rat lung scaffolds through passive, gravity-driven seeding.
- 35.▪▪.Ghaedi M, Le AV, Hatachi G, et al. Bioengineered lungs generated from human iPSCs-derived epithelial cells on native extracellular matrix. J Tissue Eng Regen Med 2018; 12:e1623–e1635. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this manuscript, the authors investigate the recellularization of human lung and rat lungs with iPSC-derived epithelial progenitor cells in order to explore the feasibility of producing engineered lungs from stem cells.
- 36.▪▪.Doi R, Tsuchiya T, Mitsutake N, et al. Transplantation of bioengineered rat lungs recellularized with endothelial and adipose-derived stromal cells. Sci Rep 2017; 7:8447. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors explore the possibility to transplant in rats engineered decellularized lungs repopulated with adipose-derived stem/stromal cells.
- 37.▪.Gilpin SE, Charest JM, Ren X, et al. Regenerative potential of human airway stem cells in lung epithelial engineering. Biomaterials 2016; 108:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors describe extensive recellularization with basal epithelial stem cell in rat scaffolds, indicating dynamic compliance of the tissue and simple gas exchange capacity.
- 38.▪.Platz J, Bonenfant NR, Uhl FE, et al. Comparative decellularization and recellularization of wild-type and alpha 1,3 galactosyltransferase knockout pig lungs: a model for ex vivo xenogeneic lung bioengineering and transplantation. Tissue Eng Part C Methods 2016; 22:725–739. [DOI] [PMC free article] [PubMed] [Google Scholar]; This research investigates whether decellularized α-gal KO pig lungs recellularized with human cells might present a viable option for lung transplantation.
- 39.▪▪.Zhou H, Kitano K, Ren X, et al. Bioengineering human lung grafts on porcine matrix. Ann Surg 2018; 267:590–598. [DOI] [PubMed] [Google Scholar]; The authors surgically implant bioengineered lung grafts, which were able to withstand physiological blood flow and short-term gas exchange.
- 40.▪.Engler AJ, Le AV, Baevova P, Niklason LE. Controlled gas exchange in whole lung bioreactors. J Tissue Eng Regen Med 2018; 12:e119–e129. [DOI] [PMC free article] [PubMed] [Google Scholar]; This manuscript describes improvements in bioreactor equipment based on mathematical modeling and implementation of controllable oxygen parameters.
- 41.Barkauskas CE, Cronce MJ, Rackley CR, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest 2013; 123:3025–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.▪.Nichols JE, La Francesca S, Niles JA, et al. Production and transplantation of bioengineered lung into a large-animal model. Sci Transl Med 2018; 10:eaao3926. [DOI] [PubMed] [Google Scholar]; The authors describe their study in decellularized and recellularized lung scaffold and the transplantation of bioengineered lungs in a porcine model.
- 43.▪.Niklason LE. Understanding the extracellular matrix to enhance stem cell-based tissue regeneration. Cell Stem Cell 2018; 22:302–305. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this manuscript, the authors discuss novel tools for characterizing ECM to optimize the decellularization process, recellularization techniques and the application of the scaffolds.
- 44.▪.De Santis MM, Bölükbas DA, Lindstedt S, Wagner DE. How to build a lung: latest advances and emerging themes in lung bioengineering. Eur Respir J 2018; 52:; pii: 1601355. [DOI] [PubMed] [Google Scholar]; The authors discuss latest advances and promising ideas within the field of lung bioengineering.
- 45.Ott HC, Clippinger B, Conrad C, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med 2010; 16:927–933. [DOI] [PubMed] [Google Scholar]
- 46.Kitano K, Schwartz DM, Zhou H, et al. Bioengineering of functional human induced pluripotent stem cell-derived intestinal grafts. Nat Commun 2017; 8:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balestrini JL, Gard AL, Gerhold KA, et al. Comparative biology of decellularized lung matrix: implications of species mismatch in regenerative medicine. Biomaterials 2016; 102:220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burgstaller G, Sengupta A, Vierkotten S, et al. Distinct niches within the extracellular matrix dictate fibroblast function in (cell free) 3D lung tissue cultures. Am J Physiol Lung Cell Mol Physiol 2018; 314:L708–L723. [DOI] [PubMed] [Google Scholar]
- 49.Zhou Y, Horowitz JC, Naba A, et al. Extracellular matrix in lung development, homeostasis and disease. Matrix Biol 2017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Panoskaltsis-Mortari A Bioreactor development for lung tissue engineering. Curr Transplant Rep 2015; 2:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pouliot RA, Link PA, Mikhaiel NS, et al. Development and characterization of a naturally derived lung extracellular matrix hydrogel. J Biomed Mater Res A 2016; 104:1922–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beachley V, Ma G, Papadimitriou C, et al. Extracellular matrix particle-glycosaminoglycan composite hydrogels for regenerative medicine applications. J Biomed Mater Res A 2018; 106:147–159. [DOI] [PubMed] [Google Scholar]
- 53.Franks TJ, Colby TV, Travis WD, et al. Resident cellular components of the human lung: current knowledge and goals for research on cell phenotyping and function. Proc Am Thorac Soc 2008; 5:763–766. [DOI] [PubMed] [Google Scholar]
- 54.Burgess JK, Mauad T, Tjin G, et al. The extracellular matrix – the under-recognized element in lung disease? J Pathol 2016; 240:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bellezzia MA, Cruz FF, Martins V, et al. Impact of different intratracheal flows during lung decellularization on extracellular matrix composition and mechanics. Regen Med 2018; 13:519–530. [DOI] [PubMed] [Google Scholar]
- 56.da Palma RK, Campillo N, Uriarte JJ, et al. Pressure- and flow-controlled media perfusion differently modify vascular mechanics in lung decellularization. J Mech Behav Biomed Mater 2015; 49:69–79. [DOI] [PubMed] [Google Scholar]
- 57.Kajbafzadeh AM, Javan-Farazmand N, Monajemzadeh M, Baghayee A. Determining the optimal decellularization and sterilization protocol for preparing a tissue scaffold of a human-sized liver tissue. Tissue Eng Part C Methods 2013; 19:642–651. [DOI] [PubMed] [Google Scholar]
- 58.Bonenfant NR, Sokocevic D, Wagner DE, et al. The effects of storage and sterilization on de-cellularized and re-cellularized whole lung. Biomaterials 2013; 34:3231–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fidalgo C, Iop L, Sciro M, et al. A sterilization method for decellularized xenogeneic cardiovascular scaffolds. Acta Biomater 2018; 67:282–294. [DOI] [PubMed] [Google Scholar]


