This cohort study evaluates the risk factors for incident dementia in older individuals, including blood pressure levels.
Key Points
Question
Is the association between systolic blood pressure and dementia U-shaped, and do age and comorbidity play a role in this association?
Findings
In this cohort study of 7 studies with a total of 17 286 participants, higher systolic blood pressure was associated with a lower dementia risk in older individuals, and a U-shaped association occurred only in the oldest age groups. These associations were not attributable to longer survival with lower systolic blood pressure.
Meaning
The findings suggest that systolic blood pressure levels conveying the lowest dementia risk may differ between age groups and thus warrant further trials of personalized blood pressure targets that consider individual life expectancy and health context.
Abstract
Importance
The optimal systolic blood pressure (SBP) to minimize the risk of dementia in older age is unknown.
Objective
To investigate whether the association between SBP and dementia risk is U-shaped and whether age and comorbidity play a role in this association.
Design, Setting, and Participants
This cohort study used an individual participant data approach to analyze 7 prospective, observational, population-based cohort studies that were designed to evaluate incident dementia in older adults. These studies started between 1987 and 2006 in Europe and the US. Participants had no dementia diagnosis and had SBP and/or diastolic blood pressure (BP) data at baseline and incident dementia status during follow-up. Data analysis was conducted from November 7, 2019, to October 3, 2021.
Exposures
Baseline systolic BP.
Main Outcomes and Measures
All-cause dementia (defined using Diagnostic and Statistical Manual of Mental Disorders [Third Edition Revised] or Diagnostic and Statistical Manual of Mental Disorders [Fourth Edition] and established at follow-up measurements or in clinical practice), mortality, and combined dementia and mortality were the outcomes. Covariates included baseline antihypertensive medication use, sex, educational level, body mass index, smoking status, diabetes, stroke history, myocardial infarction history, and polypharmacy. Cox proportional hazards regression models were used, and nonlinear associations were explored using natural splines.
Results
The study analyzed 7 cohort studies with a total of 17 286 participants, among whom 10 393 were women (60.1%) and the mean (SD) baseline age was 74.5 (7.3) years. Overall, dementia risk was lower for individuals with higher SBP, with the lowest risk associated with an SBP of approximately 185 mm Hg (95% CI, 161-230 mm Hg; P = .001). Stratified by overlapping 10-year baseline age groups, the lowest dementia risk was observed at somewhat lower systolic BP levels in those older than 75 years (158 [95% CI, 152-178] mm Hg to 170 [95% CI, 160-260] mm Hg). For mortality, there was a clear U-shaped association, with the lowest risk at 160 mm Hg (95% CI, 154-181 mm Hg; P < .001). This U-shape occurred across all age groups, with the lowest dementia risk associated with an SBP of 134 mm Hg (95% CI, 102-149 mm Hg; P = .03) in those aged 60 to 70 years and increasing to between 155 mm Hg (95% CI, 150-166 mm Hg; P < .001) and 166 mm Hg (95% CI, 154-260 mm Hg; P = .02) for age groups between 70 and 95 years. Combined dementia and mortality risk curves closely resembled those for mortality. Associations of diastolic BP with dementia risk were generally similar but were less distinct.
Conclusions and Relevance
This cohort study found that dementia risk was lower for older individuals with higher SBP levels and that more distinctly U-shaped associations appeared for those older than 75 years, but these associations cannot be explained by SBP-associated changes in mortality risk. The findings may warrant future trials on tailored BP management in older age groups that take life expectancy and health context into consideration.
Introduction
Midlife hypertension is associated with an approximately 60% increased risk of dementia.1 However, in late life, this association disappears, with few studies finding associations with increased risk and most studies reporting neutral or even decreased risks associated with hypertension.2,3,4 Potentially explaining this heterogeneity, some studies have reported that U-shaped associations in late life exist, with both high and low blood pressure (BP) signaling increased dementia risk.4,5 However, studies of these U-shaped associations are scarce and lack the necessary details.2,5,6 It is unknown whether these U-shaped associations are generalizable in older populations, how they develop with aging, and with which comorbidities. Identifying relevant subgroups may be important given that opposite associations (association of higher BP with higher dementia risk in one group vs association of higher BP with lower dementia risk in another group) in those with vs those without a particular comorbidity may yield U-shaped associations when analyzed together. Addressing the competing risk of death is essential because increased mortality in individuals who are hypertensive may be a factor in the decline of dementia incidence.7,8 Such mechanisms could change the shape of the association between BP and incident dementia.
These U-shaped associations fuel concerns that lowering BP beyond a certain level in older age might be detrimental, especially for specific subgroups of individuals with comorbidities.6,9,10 Although randomized clinical trials (RCTs) have suggested that lowering BP in older people with hypertension may be beneficial overall, the inclusion criteria represent only one-third of the general older population,11,12 and the generalizability of the findings is hotly debated.12,13,14 Knowledge of the consistency of these U-shaped curves, their association with age and comorbidity, and the BP associated with the lowest risk of dementia that takes mortality into account is essential for future trial design for optimal personalized BP management in late life. Individual studies lack statistical power to comprehensively explore these associations, and their external validity is difficult to ascertain.
In this study, we used an individual participant data approach, combining data from multiple population-based cohorts to evaluate how BP values associated with the lowest risk of dementia differed in older age groups and how this association was affected by comorbidity and risk of death. Specifically, we investigated whether the association between systolic BP (SBP) and dementia risk is U-shaped and whether age and comorbidity play a role in this association.
Methods
All included studies received approval from their respective local ethical committees, and written informed consent was obtained from all participants in each study. The present study used anonymized data from these studies and thus required no approval and sought no waiver of informed consent. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Population
In this analysis, we included prospective, observational, population-based cohort studies that were designed to evaluate incident dementia in older people. Inclusion criteria were the availability of BP measurements in participants without dementia and data on subsequent incident dementia. Five of the 9 eligible cohorts from the 21st Century EURODEM consortium15 participated.16,17,18,19,20 Two additional studies were included to increase power, cover a more evenly distributed baseline age range, and minimize the impact of single studies within specific age ranges (eMethods 1 in the Supplement).21,22 The following 7 studies were selected, began between 1987 and 2006, and were conducted in Europe and the US: ACT (Adult Changes in Thought), H70 (Gothenburg H70 Birth Cohort Study), Kungsholmen Project, LEILA 75+ (Leipzig Longitudinal Study of the Aged), PreDIVA (Prevention of Dementia by Intensive Vascular Care), SNAC-K (Swedish National Study of Aging and Care in Kungsholmen), and ZARADEMP (Zaragoza Dementia Depression Project). Table 1 provides the characteristics of these studies.
Table 1. Characteristics of Included Studies.
| Study (location) | Period | Setting | Recruitment | Follow-up | Dementia | Mortality | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Response rate, % | Included or excluded individuals | Source of outcome data | Assessment | Maximum follow-up time, y | Diagnosis | Lost to follow-up, % | Maximum follow-up time, y | Verification | |||
| ZARADEMP (Zaragoza, Spain)16 | 1994-1999 | Community dwelling and institutionalized | Random census sample stratified by age and sex | 79 | Included: aged ≥55 y | Follow-up cognitive screening and municipal death registry | Every 2 y | 4 | Psychiatrist-confirmed DSM-IV diagnosisa | 10 | 13 | Verified by death records |
| SNAC-K (Stockholm, Sweden)17 | 2001-2020 | Community dwelling and institutionalized | Random population sample stratified by age; multiple cohorts | 73 | Included: aged ≥60 y | Follow-up cognitive screening, medical registries, informant interviews, and death certificates |
|
12 | 2nd independent physician–confirmed DSM-III-R diagnosis | 11 | 16 | Verified by death records |
| Kungsholmen Project (Stockholm, Sweden)18 | 1987-1996 | Community dwelling and institutionalized | Kungsholmen district (75+) | 76 | Included: aged ≥75 y | Follow-up cognitive screening, medical registries, informant interviews, and death certificates | Every 3 y | 9 | 2nd independent physician–confirmed DSM-III-R diagnosis | 12 | 11 | Verified by death records |
| LEILA 75+ (Leipzig, Germany)19 | 1997-2014 | Community dwelling and institutionalized | Representative population sample | 75 | Included: aged ≥75 y | Follow-up cognitive screening, death certificates, and relative interviews | Every 1.5 y | 16 | Expert panel–confirmed DSM-III-R or DSM-IV diagnosis | 9 | 16 | Verified by death records |
| H70 (Gothenburg, Sweden)20 | 2000-2012 | Community dwelling and institutionalized | Representative population samples; 1930 cohorts | 72 | Included: aged 70, 75, and 79 y | Follow-up cognitive screening, medical registries, informant interviews, and death records | Every 5 y | 12 | DSM-III-R diagnosis | 0 | 12 | Verified by death records |
| PreDIVA (Amsterdam, the Netherlands)21 | 2006-2015 | Community dwelling only | General practice populations (>98% Dutch population is registered with a general practitioner) | 53 |
|
Follow-up cognitive screening, EHRs, and municipal death records | Every 2 y | 9 | Expert panel–confirmed DSM-IV diagnosis | 2 | 9 | Verified by death records |
| ACT (Seattle, Washington)22 | 1994-2020 | Community dwelling only | Random health insurance sample (representative of local population) | 48 |
|
Follow-up cognitive screening, medical records, and death certificates | Every 2 y | 25 | Expert panel–confirmed DSM-IV diagnosis | 3 | 25 | Verified by death records |
Abbreviations: ACT, Adult Changes in Thought; DSM-III-R, Diagnostic and Statistical Manual of Mental Disorders (Third Edition Revised); DSM-IV, Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition); EHR, electronic health record; H70, Gothenburg H70 Birth Cohort Study; LEILA 75+, Leipzig Longitudinal Study of the Aged; PreDIVA, Prevention of Dementia by Intensive Vascular Care; SNAC-K, Swedish National Study of Aging and Care in Kungsholmen; ZARADEMP, Zaragoza Dementia Depression Project.
No dementia information was available for individuals between last assessment and death or study dropout.
Exposure and Outcome
Analyses included all study participants without a dementia diagnosis who had SBP and/or diastolic BP (DBP) measurements at baseline (ie, study entry) and incident dementia status at follow-up. Dementia was defined using the Diagnostic and Statistical Manual of Mental Disorders (Third Edition Revised) and Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria or clinical diagnoses from medical records that were verified by the study investigators (Table 1; eMethods 2 in the Supplement). Covariates included baseline antihypertensive medication use (yes or no), sex, educational level, body mass index (calculated as weight in kilograms divided by height in meters squared), smoking status (never, former, or current), diabetes, stroke history, myocardial infarction history, and the number of medications (as a proxy for frailty or multimorbidity). Data on race and ethnicity were not collected because these variables were not available for all cohorts and differed greatly between the different countries participating in this individual participant data analysis.
Statistical Analyses
The associations between baseline BP and incident dementia were assessed using mixed-effects Cox proportional hazards regression models, with dementia diagnosis or censoring age as the timescale and baseline age as the entry time. We modeled the BP values adjusted for the major potential confounders of sex and antihypertensive medication use (yes, no, or unknown) as fixed effects, with study-specific random baseline hazards (eMethods 3 in the Supplement). Proportional hazards assumptions were assessed by goodness-of-fit tests and visual inspection of Schoenfeld residuals. Systolic BP and DBP were evaluated independently in separate models. Potential nonlinear associations were examined using natural splines, with 2 to 4 degrees of freedom according to optimal fit (eMethods 3 in the Supplement). From the model, the BP associated with the lowest dementia risk (lowest risk point) was recorded, with 95% CIs calculated as the 2.5 and 97.5 percentiles from 1000 bootstraps. These intervals can be asymmetrical and denote the 95% CI within which the risk is similar to that at the lowest risk point. Extreme values suggest uncertainty that the risk is higher, extending from the lowest risk point (eMethods 4 in the Supplement). Linear models were also fitted and then compared with the nonlinear model using the Akaike information criterion (AIC) and log-likelihood tests. Comparisons between nonlinear and linear models are provided in eTables 2 through 5 and eTables 7 through 11 in the Supplement. Competing risk of death was evaluated using a cause-specific hazard approach, repeating all analyses with mortality and combined dementia and mortality as outcomes.7
To assess our a priori hypothesis that the lowest risk point for dementia increases with higher baseline age, we performed subgroup analyses for 10-year age groups from 65 to 90 years, shifting 5 years per group, to ensure sufficient participants per age group while gradually shifting the age and population composition (eMethods 5 in the Supplement). To evaluate whether confounding changed the association shapes, we performed analyses adjusted for body mass index, diabetes, smoking status, myocardial infarction history, stroke history, polypharmacy (≥4 medications), APOE (OMIM 107741) genotype (any ε4 allele vs none), and educational level (based on tertiles within studies). We assessed the effect modification by comorbidity in predefined subgroups for stroke history, myocardial infarction history, diabetes, and polypharmacy as well as for APOE genotype in accordance with previous findings.5 Interactions were evaluated using the AIC and P values from log-likelihood tests, with lower AICs and P < .05 considered to be relevant. Individuals with missing data were omitted per analysis (deleted pairwise).
We conducted several sensitivity analyses. First, we repeated analyses as stratified by baseline antihypertensive medication use (yes or no). Second, because BP may gradually decline in the decade preceding the dementia diagnosis,23,24 possibly as a prodrome, we repeated the analyses in similarly powered subgroups by short-term (<5 years), medium-term (5-10 years), and long-term (>10 years) follow-up. Because the likelihood of short-term events increases with aging, we repeated these analyses with stratification by baseline age. Third, to evaluate the impact of individual studies, we repeated the analyses and excluded 1 study at a time. Fourth, to assess multiple confounders at once, we repeated the analyses, adjusting for (1) the largest number of confounders feasible to maintain an acceptable sample size; (2) all confounders, categorizing missing values as unknown; and (3) missing value–adapted propensity scores.25 Fifth, to assess how competing mortality risk would change expected cumulative dementia incidence, we repeated the main analyses using Fine-Gray models. Sixth, to assess whether dementia-limited life expectancy was associated with mortality, we repeated mortality analyses using dementia-free mortality.
We used R packages coxme and splines, version 3.6.2 (R Foundation for Statistical Computing). A 2-sided P < .05 was considered to be statistically significant. Data analysis was conducted from November 7, 2019, to October 3, 2021.
Results
The combined population of the 7 studies comprised 17 286 participants, among whom 10 393 were women (60.1%) and 6893 were men (39.9%) with a mean (SD) baseline age of 74.5 (7.3) years. Of these individuals, 2799 (16.2%) had incident dementia with a median (IQR) time to diagnosis of 7.3 (5.2-11.0) years, representing 136 473 person-years (Table 2). Three studies contributed data for individuals across the full baseline age range of interest from 65 to 95 years or older16,17,22; 2 studies contributed data from individuals aged approximately 75 to 95 years or older18,19; and 2 studies contributed data for individuals aged 69 to 81 years20,21 (eFigures 1-2 in the Supplement).
Table 2. Population Characteristics for the Total Combined Population and the Individual Contributing Studies.
| Characteristic | No. (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| All studies | H7020 | PreDIVA21 | Kungsholmen Project18 | LEILA 75+19 | SNAC-K17 | ZARADEMP16 | ACT22 | |
| No. | 17 286 | 982 | 3454 | 1301 | 967 | 2671 | 2365 | 5546 |
| Age, mean (SD), y | 74.5 (7.3) | 72.9 (3.0) | 74.4 (2.5) | 81.5 (5.0) | 81.6 (4.9) | 73.1 (10.5) | 70.7 (8.3) | 74.2 (6.4) |
| Female sex | 10 393 (60.1) | 583 (59.4) | 1885 (54.6) | 976 (75.0) | 718 (74.3) | 1683 (63.0) | 1320 (55.8) | 3228 (58.2) |
| Male sex | 6893 (39.9) | 399 (40.6) | 1569 (45.4) | 325 (25.0) | 249 (25.7) | 988 (37.0) | 1045 (44.2) | 2318 (41.8) |
| Educational level, mean (SD), y | 11.9 (4.2) | 10.1 (3.6) | 11 (2.7) | 9.8 (3.1) | 11.9 (1.8) | NA | 7.7 (4.1) | 14.9 (3.2) |
| DBP, mean (SD), mm Hg | 79.3 (11.7) | 82.4 (10.9) | 81.4 (11.0) | 81 (10.8) | 86.3 (17.4) | 81.4 (10.7) | 79.5 (11.0) | 74.7 (10.3) |
| SBP, mean (SD), mm Hg | 146.6 (22.0) | 153.4 (21.9) | 155.3 (21.3) | 155.4 (21.7) | 158.5 (24.1) | 143.7 (19.9) | 140.6 (18.6) | 139.6 (20.7) |
| Hypertension | 12 966 (76.4) | 807 (82.5) | 3079 (89.2) | 1155 (89.7) | 745 (88.1) | 2072 (77.7) | 1578 (70.0) | 3530 (64.4) |
| Antihypertensive medication use | 7104 (42.2) | 317 (32.8) | 1891 (54.7) | 589 (45.3) | 176 (31.8) | 1117 (41.8) | 777 (32.9) | 2237 (40.5) |
| BMI, mean (SD) | 27.3 (4.8) | 26.8 (4.3) | 27.5 (4.1) | NA | NA | NA | 27.1 (5.5) | 27.4 (5.0) |
| Diabetes | 1801 (13.9) | 89 (9.1) | 634 (18.4) | NA | 219 (22.7) | NA | 282 (12.0) | 577 (11.2) |
| Smoking status | ||||||||
| Never | 6423 (48.4) | 431 (44.7) | 1124 (32.6) | NA | 644 (67.4) | NA | 1532 (64.8) | 2692 (48.7) |
| Former | 5593 (42.2) | 397 (41.2) | 1873 (54.3) | NA | 243 (25.4) | NA | 511 (21.6) | 2569 (46.4) |
| Current | 1246 (9.4) | 136 (14.1) | 450 (13.1) | NA | 69 (7.2) | NA | 320 (13.5) | 271 (4.9) |
| MI | 2191 (16.6) | 108 (11.0) | 1013 (29.5) | NA | 81 (8.4) | NA | 61 (2.6) | 928 (16.9) |
| Stroke | 732 (5.6) | 63 (6.6) | 338 (9.9) | NA | 60 (6.2) | NA | 110 (4.8) | 161 (2.9) |
| No. of medications | ||||||||
| 0-1 | 1469 (25.9) | 104 (21.1) | 346 (12.3) | NA | NA | NA | 1019 (43.1) | NA |
| 2-3 | 1794 (31.6) | 176 (35.7) | 784 (27.8) | NA | NA | NA | 834 (35.3) | NA |
| ≥4 | 2414 (42.5) | 213 (43.2) | 1689 (59.9) | NA | NA | NA | 512 (21.6) | NA |
| APOE ε4 genotype | 3278 (27.1) | 256 (27.1) | 799 (27.5) | 281 (28.5) | 40 (16.1) | 723 (28.7) | 0 | 1179 (26.2) |
| Dementia | 2799 (16.2) | 79 (8.0) | 233 (6.7) | 440 (33.8) | 214 (22.1) | 425 (15.9) | 138 (5.8) | 1270 (22.9) |
| Time to dementia diagnosis/censoring, y | ||||||||
| Age, mean (SD) | 82.4 (7.4) | 81.2 (2.4) | 80.7 (2.8) | 86.5 (4.8) | 86.4 (5.1) | 81.2 (10.4) | 80.9 (7.3) | 83.3 (8.3) |
| Time, median (IQR) | 7.3 (5.2-11.0) | 7.7 (6.7-11.7) | 6.6 (6.0-7.2) | 4.7 (1.9-8.2) | 4.4 (2.1-6.8) | 8.1 (6.4-9.8) | 11.2 (10.0-11.7) | 8.0 (4.0-13.0) |
| Mortality | 7920 (45.8) | 516 (52.5) | 553 (16.0) | 880 (67.6) | 519 (53.7) | 1300 (48.7) | 1083 (45.8) | 3069 (55.3) |
| Time to mortality/censoring, y | ||||||||
| Age, mean (SD) | 83.5 (7.2) | 86.0 (4.4) | 80.8 (2.7) | 87.4 (4.9) | 87.7 (5.7) | 85.4 (8.4) | 81.1 (7.4) | 83.3 (8.3) |
| Time, median (IQR) | 8.0 (6.0-12.2) | 13.6 (9.5-18.8) | 6.7 (6.1-7.3) | 6.0 (3.2-8.7) | 5.2 (3.0-7.5) | 16.0 (8.4-16.0) | 11.2 (10.3-11.7) | 8.0 (4.0-13.0) |
Abbreviations: ACT, Adult Changes in Thought; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DBP, diastolic blood pressure; H70, Gothenburg H70 Birth Cohort Study; LEILA 75+, Leipzig Longitudinal Study of the Aged; MI, myocardial infarction; NA, not applicable; PreDIVA, Prevention of Dementia by Intensive Vascular Care; SBP, systolic blood pressure; SNAC-K, Swedish National Study of Aging and Care in Kungsholmen; ZARADEMP, Zaragoza Dementia Depression Project.
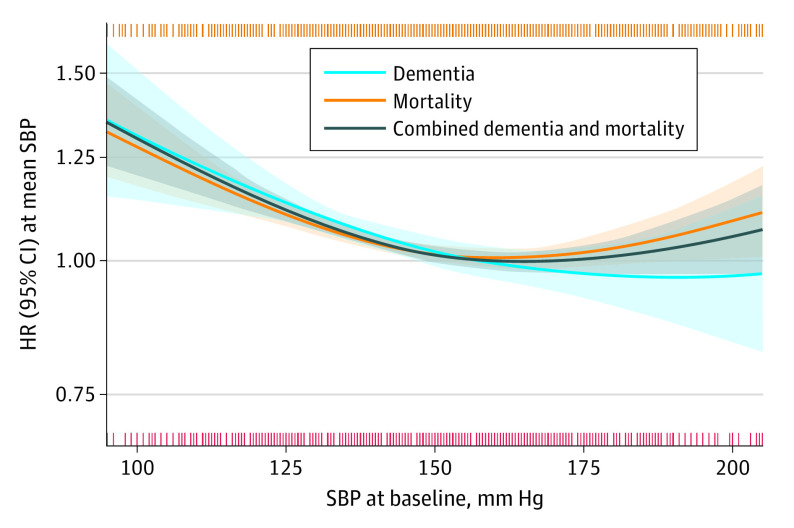
The associations of baseline SBP with incident dementia, mortality, and combined dementia and mortality varied (Figure 1, Table 3). Overall, SBP and dementia risk approached an inverse linear association (ie, the low point of the U-shape was at a high BP level, nearly suggesting that the higher the BP, the lower the risk), with an SBP of 185 mm Hg (95% CI, 161-230 mm Hg; P = .001) associated with the lowest dementia risk. The lowest risk point was 160 mm Hg (95% CI, 154-181 mm Hg; P < .001) for mortality and 163 mm Hg (95% CI, 158-197 mm Hg; P < .001) for combined dementia and mortality.
Figure 1. Associations With Dementia, Mortality, and Combined Dementia and Mortality According to Systolic Blood Pressure (SBP) at Baseline.
The lines indicate relative hazard ratios (HRs); shaded areas, 95% CIs; orange vertical stripes (top), 1 or multiple mortality cases at that specific SBP; red vertical stripes (bottom), 1 or multiple dementia cases at that specific SBP. The y-axis denotes that the HR is 1.00 at the mean SBP. Models were fitted using natural splines, with degrees of freedom that were selected from 1 (linear model) to a maximum of 4 (knots at 25th, 50th, and 75th percentile), based on the optimal model fit according to the Akaike information criterion. Models were adjusted for sex and antihypertensive medication use.
Table 3. Association of Systolic Blood Pressure With Risk of Dementia, Mortality, and Combined Dementia and Mortalitya.
| Outcome | Baseline age, y | No. of studies | No. of cases | Total No. | Lowest risk point (95% CI), mm Hg | P value |
|---|---|---|---|---|---|---|
| Dementia | All age groups | 7 | 2738 | 16 873 | 185 (161-230) | .001 |
| 60-70 | 5 | 230 | 3720 | 220 (150-245) | .41 | |
| 65-75 | 6 | 786 | 7656 | 197 (101-230) | .77 | |
| 70-80 | 7 | 1352 | 9602 | 224 (107-260) | .09 | |
| 75-85 | 7 | 1390 | 6449 | 170 (160-260) | .004 | |
| 80-90 | 7 | 947 | 2922 | 158 (152-178) | .001 | |
| 85-95 | 5 | 535 | 1504 | 162 (153-240) | .01 | |
| >90 | 5 | 121 | 345 | 160 (87.8-231) | .93 | |
| Mortality | All age groups | 7 | 7698 | 16 869 | 160 (154-181) | <.001 |
| 60-70 | 5 | 941 | 3720 | 134 (102-149) | .03 | |
| 65-75 | 6 | 2547 | 7655 | 146 (130-169) | .04 | |
| 70-80 | 7 | 3914 | 3914 | 166 (154-260) | .02 | |
| 75-85 | 7 | 3587 | 6446 | 163 (156-194) | .001 | |
| 80-90 | 7 | 2309 | 2922 | 155 (150-166) | <.001 | |
| 85-95 | 5 | 1320 | 1504 | 162 (154-230) | .01 | |
| >90 | 5 | 328 | 345 | 160 (154-220) | .13 | |
| Combined dementia and mortality | All age groups | 7 | 8375 | 16 871 | 163 (158-197) | <.001 |
| 60-70 | 5 | 1015 | 3720 | 136 (86-215) | .17 | |
| 65-75 | 6 | 2805 | 7655 | 149 (139-205) | .06 | |
| 70-80 | 7 | 4357 | 9600 | 169 (159-260) | .003 | |
| 75-85 | 7 | 3943 | 6448 | 164 (157-192) | <.001 | |
| 80-90 | 7 | 2461 | 2922 | 157 (151-167) | <.001 | |
| 85-95 | 5 | 1380 | 1504 | 165 (153-240) | .01 | |
| >90 | 5 | 333 | 345 | 229 (109-231) | .20 |
Results are from nonlinear models in age-based subgroups. For each outcome, the blood pressure associated with the lowest risk point estimated from the model is given, with 95% CI that was based on 1000 bootstraps. Full data for comparison with linear models are provided in eTable 2 in the Supplement. Associations are shown for the complete population (overall) and within 10-year age subgroups.
The associations were similar for DBP but were less distinct (eTable 1 in the Supplement). The nonlinear model for the association between DBP and incident dementia was not significant but approached an inversely linear shape, with the highest measurement in the distribution being associated with the lowest dementia risk (139 mm Hg; 95% CI, 80-139 mm Hg; P = .16). The lowest risk point was 84 mm Hg (95% CI, 80-97 mm Hg; P = .002) for mortality and 82 mm Hg (95% CI, 79-93 mm Hg; P = .01) for combined dementia and mortality.
Complete results of the comparison of linear with nonlinear models are provided in eTables 2 and 3 in the Supplement. These results show that, overall, the linear models fit slightly better for dementia for both SBP and DBP but not for mortality and combined dementia and mortality.
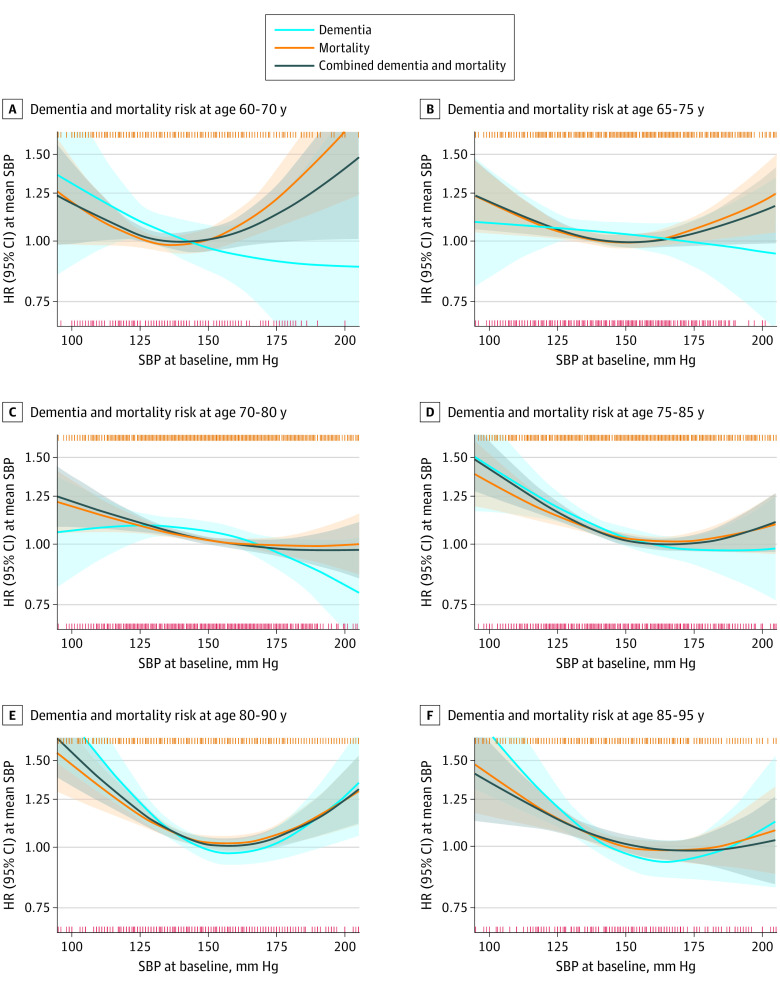
Age Groups
For SBP and incident dementia in baseline age groups, the nonlinear models were not significant for up to 70 to 80 years (Table 3, Figure 2) but approached inverse linear associations, with high SBP values as the lowest risk points (range, 197-220 mm Hg). In groups aged 75 to 95 years, the associations were more distinctly U-shaped, with the lowest risk points of approximately 165 mm Hg (range, 158 mm Hg [95% CI, 152-178 mm Hg; P < .001] to 170 mm Hg [95% CI, 160-260 mm Hg; P = .004]).
Figure 2. Associations Between Systolic Blood Pressure (SBP) and Risk of Combined Dementia and Mortality .
Results of the optimal nonlinear models are presented in 10-year age groups in the combined study population aged 60 to 95 years. The lines indicate relative hazard ratios (HRs); shaded areas, 95% CIs; orange vertical stripes (top), 1 or multiple mortality cases at that specific SBP; red vertical stripes (bottom), 1 or multiple dementia cases at that specific SBP. The y-axis denotes that the HR is 1.00 at the mean SBP. Models were fitted using natural splines, with degrees of freedom that were selected from 1 (linear model) to a maximum of 4 (knots at 25th, 50th, and 75th percentile), based on the optimal model fit according to the Akaike information criterion. Models were adjusted for sex and antihypertensive medication use.
For mortality, the lowest risk points increased with age, from 134 mm Hg (95% CI, 102-149 mm Hg; P = .03) in those between 60 and 70 years to approximately 160 mm Hg in those aged 70 years or older (range, 155 mm Hg [95% CI, 150-166 mm Hg; P < .001] to 166 mm Hg [95% CI, 154-260 mm Hg; P = .02]). Combined dementia and mortality risk curves resembled those for mortality.
Limited data precluded inferences in participants who were older than 90 years (Table 3). Associations were less distinct for DBP (eTable 1 and eFigure 3 in the Supplement).
Antihypertensive Medication
For SBP, we found significant interactions with baseline antihypertensive medication use for mortality (AIC, –5.1; P for interaction = .01) and combined dementia and mortality (AIC, –2.2; P for interaction = .04) but not for dementia (AIC, 1.4; P for interaction = .27).
For mortality, the overall lowest risk point was slightly higher in users of antihypertensive medication (164 mm Hg; 95% CI, 156-183 mm Hg; P < .001) than in nonusers (156 mm Hg; 95% CI, 144-225 mm Hg; P = .05) (eTable 4 in the Supplement). Among users of antihypertensive medication stratified by age, the lowest risk points increased with age from 145 mm Hg in those aged 60 to 70 years to 160 to 170 mm Hg in those aged 70 to 95 years, but only the models for the following age groups were significant: 65 to 75 years (lowest risk point, 157 mm Hg; 95% CI, 147-169 mm Hg; P = .01) and 80 to 90 years (lowest risk point, 158 mm Hg; 95% CI, 146-176 mm Hg; P = .03). In nonusers, there were no associations found for those younger than 75 years, but associations had relatively low lowest risk points (134-135 mm Hg). In older age groups, the lowest risk points were higher, but only the models for following age groups were significant: 75 to 85 years (160 mm Hg; 95% CI, 139-225 mm Hg; P = .04) and 80 to 90 years (150 mm Hg; 95% CI, 135-220 mm Hg; P = .01). Results for combined dementia and mortality were similar (eTable 4 in the Supplement).
For DBP, the only significant interaction was for mortality (AIC, –302; P < .001) (eTable 5 in the Supplement). Overall, the lowest risk point was relatively high in users of antihypertensive medication and approached an inversely linear association (105 mm Hg; 95% CI, 80-124 mm Hg; P = .14), whereas a U-shaped association was more distinct in nonusers (81 mm Hg; 95% CI, 76-90 mm Hg; P = .01).
Confounding and Modification
Adjustment for baseline diabetes, body mass index, polypharmacy, myocardial infarction history, stroke history, smoking status, educational level, or APOE genotype did not substantially alter association shapes (eFigure 4 in the Supplement). There were no significant differences in subgroup analyses for dementia for either SBP or DBP, except for stroke history (AIC, –1.88; P = .048) (eTable 6 in the Supplement). The lowest dementia risks were in those with a history of stroke at the lowest SBP ranges (lowest risk point, 100 mm Hg; 95% CI, 100-216 mm Hg; P = .24) and those without a history of stroke at the highest SBP ranges (lowest risk point, 229 mm Hg; 95% CI, 161-229 mm Hg; P = .03) (eTable 7 in the Supplement).
Sensitivity Analyses
Results were similar in analyses that excluded 1 study at a time, except for overall associations of SBP with incident dementia (eTable 8 in the Supplement). Relatively low lowest risk point estimates were found when leaving out the ACT study22 (lowest risk point, 167 mm Hg; 95% CI, 157-219 mm Hg; P = .001) and PreDIVA study21 (lowest risk point, 182 mm Hg; 95% CI, 158-230 mm Hg; P = .004). This finding did not affect the age-stratified associations.
Time-to-event subgroups suggested that nonlinear associations with relatively low lowest risk points may be specific for dementia that was diagnosed less than 5 years after baseline (lowest risk point, 160 mm Hg; 95% CI, 150-200 mm Hg; P = .01), with high lowest risk points approaching an association between higher SBP and lower dementia risks in the longer terms (lowest risk points, 225-230 mm Hg) (eTable 9 in the Supplement). A distinctly U-shaped association with dementia was observed in the subgroup of those 80 years or older who were diagnosed less than 5 years after baseline (eFigure 5 in the Supplement). For mortality and combined dementia and mortality, the lowest risk points were more consistently in the relatively low range of 150 to 180 mm Hg. Adjusting for multiple confounders in a single model did not change the results, regardless of the missing data strategy used (eTable 10 in the Supplement).
Results from the Fine-Gray model were similar to those of the main analyses, but with slightly attenuated associations (eTable 11 in the Supplement). The overall lowest risk point for dementia was relatively high (lowest risk point, 195 mm Hg; 95% CI, 158-230 mm Hg; P = .03). Relatively low lowest risk points were only observed in the older age groups, with the lowest risk point of approximately 160 mm Hg in those aged 80 to 95 years. The associations with DBP were similarly shaped as the associations with SBP (ie, relatively low risk points were observed only in the older age groups), with lowest risk points of approximately 100 mm Hg in the 80 to 95 years age group. Results of the analysis that considered dementia-free mortality were similar to those of overall mortality.
Discussion
We found that, overall, incident dementia risk was lower for individuals with higher baseline BP. U-shaped associations were only observed in older age groups, with an SBP of approximately 160 to 170 mm Hg being associated with the lowest dementia risk in those 75 years or older. For combined dementia and mortality, distinctly U-shaped associations were consistently observed throughout age groups, with lowest risk points of approximately 135 mm Hg in those aged 60 to 70 years, increasing to approximately 160 to 165 mm Hg in those older than 70 years. These results largely reflected the association of SBP with mortality. Single studies did not dominate the combined results, but the results showed the heterogeneity in individual studies and small subgroups. This heterogeneity suggests that population characteristics may be especially influential with moderate sample sizes, underlining the benefit of the large-scale combined data approach we used.
Previous reports on U-shaped associations of BP with dementia risk have varied.2,5,6 The findings of the present study suggest that U-shaped associations may reflect differences in participant age, study design, follow-up time intervals, stroke history, and small subgroups, but less so antihypertensive medication use. A recent UK Biobank study reported a direct dose-response relationship between SBP and dementia risk in women and a U-shaped association in men (lowest risk point, 150-160 mm Hg).26 We did not find such sex-based differences, but the Biobank population was much younger (mean [SD] age, 56 [8] years). A recent meta-analysis that modeled study-level aggregated data found direct linear associations of SBP with dementia risk and a U-shaped association for DBP only.2 However, when divided into groups of older and younger than 75 years, the findings from the previous meta-analysis were similar to those of the present study, with an inverse linear association between SBP and dementia risk in younger individuals and a U-shaped association in older individuals. Ecological fallacy may explain the difference in overall results, highlighting the benefit of the individual participant data approach that we used.
The results of this study suggest that lower SBP in older people overall may indicate a higher dementia risk, U-shaped associations only occur in older age groups, and these associations cannot be explained by lower mortality owing to lower SBP. The results might be interpreted as suggesting an optimal SBP level that balances the lowest risk of dementia and mortality, which increases with aging. However, RCT findings have suggested that lowering BP in individuals with hypertension may decrease mortality, cardiovascular events, and possibly dementia.27,28 For example, SPRINT-MIND (Systolic Blood Pressure Intervention Trial—Memory and Cognition in Decreased Hypertension) reported a significant 21% lower mild cognitive impairment and nonsignificant 17% lower probable dementia risk for reduced-SBP targets of less than 120 mm Hg vs less than 140 mm Hg in individuals older than 50 years and potentially even those older than 75 years.29,30 Furthermore, studies that evaluated antihypertensive medication discontinuation did not find (short-term) cognitive benefits.31,32 Compared with our findings, this result may seem paradoxical.
One explanation might be that the observed associations of low BP with poor outcomes were noncausal. Some studies reported that BP values decreased before dementia onset and mortality,23,24,33,34 possibly reflecting an overarching advanced aging phenomenon, which also involved other cardiovascular risk factors.35,36 When examining a single time point, such associations might create inverse or U-shaped associations: high BP as a causal risk factor, and low BP as a marker of increased risk. However, contrary to the findings of the present study, long-term associations would approach associations of higher BP with higher dementia risks, suggesting that other mechanisms may be at play. As a potential complication, antihypertensive medication classes might differentially alter dementia risk.37
Population selection may also play a role.12,13 Benefits of BP reduction in SPRINT (Systolic Blood Pressure Intervention Trial) extended to older and frailer subgroups29,38 but not to the oldest, cognitively vulnerable individuals.39 Older patients with complex comorbidity, polypharmacy, and/or limited life expectancy are unlikely to participate in RCTs, and subgroup analyses cannot overcome inclusion bias.10,12,13 Therefore, many guidelines remain cautious about low-SBP targets for these groups.40,41,42 A propagated hesitancy regarding SPRINT-MIND was its selected population of individuals with an SBP of 130 mm Hg or higher and elevated cardiovascular risk but limited comorbidity and its eligibility criteria that were estimated to exclude more than two-thirds of older people and three-quarters of frail patients.10,12,13 Furthermore, follow-up duration was 6 years (with 3 years of intervention), possibly negating the long-term implications for dementia incidence. The associations of high SBP with lower dementia risk in the present study specifically concerned the long term (>5 years).
All of the factors discussed may reconcile the ostensibly counterintuitive differences between observational evidence that associated higher dementia risk with lower SBP and RCT evidence that indicated lower risk resulting from SBP reduction. Clinically, RCT findings must take precedence, but observational data may identify crucial knowledge gaps in more inclusive populations, approximating real-world conditions, over a longer follow-up duration. Hybrid designs may be needed,43,44 especially in dementia prevention, wherein the challenges in RCT design to control the risk factors in large and representative older populations for a sufficient duration may be nearly insurmountable.45
Implications
We believe that the findings from this study have important implications. First, the reasons for these inversely linear and U-shaped associations remain unknown and puzzling in the context of the RCT evidence of BP-lowering treatments reducing dementia risks. Their clarification is essential to a better understanding of the implications of low BP for older individuals in the general population.14,40,41,42,46 In addition, future studies are needed to verify these findings in lower- and middle-income countries and in racially and ethnically as well as socioeconomically diverse populations with limited access to health care. Currently, the results accentuate concerns about the potential harms of low BP in advanced age.40,41,42,46 They warrant RCTs that test the implications of deprescribing antihypertensive medications for older individuals with BP that is far below the treatment thresholds and supporting more personalized BP management targets that take age, life expectancy, and health context into account.14,47,48 Second, dementia risk calculators assume that elevated BP increases dementia risk49,50 but inadequately estimate the risk for dementia in older people.51,52 The results of this study suggest that predictive models that are tailored to older age groups and that can differentiate between short-term and long-term risks are needed. Third, future RCTs of BP management to lower dementia risk need to consider an age- and health-tailored BP management approach and test personalized target BP values in older participants.
Strengths and Limitations
This study has strengths. To our knowledge, it was the first study to comprehensively assess potential nonlinear associations between BP and incident dementia, combining multiple cohorts, systematically assessing the role of confounders or comorbidities, and evaluating the role of mortality. All of the studies included in the analysis were designed to detect incident dementia, with regular cognitive screening, short follow-up time intervals, and few participants who were lost to follow-up that minimized missed cases. They used strict expert-confirmed dementia criteria, which increased the diagnostic certainty. Cases may have been missed, particularly between the last follow-up assessment and death, wherein studies mostly depended on diagnoses in medical records, that potentially hampered adherence to strict diagnostic criteria. However, potential misclassification is unlikely to be associated with BP levels, possibly weakening but not changing association shapes. In assessing multiple cohorts, we illustrated the heterogeneity in the findings and the importance of sample size as well as minimized publication bias, which is a major risk because mentioning an investigation of nonlinear associations may depend on their presence. Evaluating confounders separately and in combination using several missing value strategies implied that confounding did not change association shapes. However, residual confounding remains possible. Systematically investigating predefined subgroups limited the risk of spurious results. Bootstrapping allowed the assessment of CIs of the lowest risk points and the uncertainty of nonlinear associations. The narrow lower intervals for most of the lowest risk points suggested relative certainty that lower BPs may be associated with increased risk, and the wide upper intervals suggested that the associations for higher BPs may be neutral or inverse. Comparisons between nonlinear and linear model results provided a clear indication of when the associations approached linear associations of lower dementia risk with higher SBP (eTables 2-5 and 7-11 in the Supplement).
This study also has limitations. Studies were conducted in different periods and countries and involved differing BP-lowering practices, population disease burden, and life expectancy, which potentially affected the risk associations. Cohorts originated from Western countries with advanced, accessible health care, which potentially limited the generalizability in other parts of the world. Furthermore, we examined BP and covariates at baseline. Antihypertensive medications may have been initiated subsequently, and covariates may evolve over time. The findings of associations for dementia at older age that were distinctly U-shaped may have been affected by specific studies in specific age groups, although the findings were robust in leave-one-out analyses. The data used were observational, which precluded us from drawing inferences regarding causality.
Conclusions
Overall, incident dementia risk was lower for individuals with higher BP at baseline. U-shaped associations between SBP and dementia risk were observed only in older participants. Future RCTs may be needed to test BP management that is tailored to one’s age, life expectancy, and health context.
eMethods 1. Study Recruitment
eMethods 2. Dementia Ascertainment and Follow-up in Participating Studies
eMethods 3. Models and Model Selection
eMethods 4. Interpretation of Confidence Intervals
eMethods 5. Choice of Age-Bands and Shift in Analysis
eFigure 1. Participants per 5-Year Age Categories per Study
eFigure 2. Percentage Contribution per Study per 5-Year Age Category
eTable 1. Relations for Diastolic Blood Pressure With Risk of Dementia, Mortality, and Dementia/Mortality
eTable 2. Complete Results Comparing Linear and Non-Linear Models for Systolic Blood Pressure
eTable 3. Complete Results Comparing Linear and Non-Linear Models for Diastolic Blood Pressure
eFigure 3. Relations Between Diastolic Blood Pressure and Risk of Dementia/Mortality Combined
eTable 4. Relations for Systolic Blood Pressure With Risk of Dementia, Mortality, and Dementia/Mortality in Age Groups, Stratified According to Antihypertensive Medication (AHM) Use
eTable 5. Relations for Diastolic Blood Pressure With Risk of Dementia, Mortality, and Dementia/Mortality in Age Groups, Stratified According to Antihypertensive Medication (AHM) Use
eFigure 4. Analyses of Systolic Blood Pressure and Dementia/Mortality Risk Adjusted and Not Adjusted for Potentially Relevant Confounders
eTable 6. Model Improvement Stratified According to Subgroups
eTable 7. Subgroup Analyses in Individuals With/Without a History of Stroke
eTable 8. Leave-One-Out Analyses
eTable 9. Relations for Systolic and Diastolic Blood Pressure With Risk of Dementia, Mortality, and Dementia/Mortality According to Time to Event in the Total Population and Within Age Subgroups
eFigure 5. Analyses for Systolic Blood Pressure According to Time to Event in the Total Population and Within Age Subgroups
eTable 10. Sensitivity Analyses Adjusting for Multiple Confounders in Together in Single Models
eTable 11. Results for Fine-Gray Analyses of Dementia Accounting for the Competing Risk of Mortality in the Overall Population and Baseline Age Groups
References
- 1.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413-446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ou YN, Tan CC, Shen XN, et al. Blood pressure and risks of cognitive impairment and dementia: a systematic review and meta-analysis of 209 prospective studies. Hypertension. 2020;76(1):217-225. doi: 10.1161/HYPERTENSIONAHA.120.14993 [DOI] [PubMed] [Google Scholar]
- 3.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4(8):487-499. doi: 10.1016/S1474-4422(05)70141-1 [DOI] [PubMed] [Google Scholar]
- 4.Kennelly SP, Lawlor BA, Kenny RA. Blood pressure and the risk for dementia: a double edged sword. Ageing Res Rev. 2009;8(2):61-70. doi: 10.1016/j.arr.2008.11.001 [DOI] [PubMed] [Google Scholar]
- 5.Rajan KB, Barnes LL, Wilson RS, Weuve J, McAninch EA, Evans DA. Blood pressure and risk of incident Alzheimer’s disease dementia by antihypertensive medications and APOE ε4 allele. Ann Neurol. 2018;83(5):935-944. doi: 10.1002/ana.25228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iadecola C, Yaffe K, Biller J, et al. ; American Heart Association Council on Hypertension; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council . Impact of hypertension on cognitive function: a scientific statement from the American Heart Association. Hypertension. 2016;68(6):e67-e94. doi: 10.1161/HYP.0000000000000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. 2017;36(27):4391-4400. doi: 10.1002/sim.7501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Dalen JW, Moll van Charante EP, Richard E, van Gool WA. Antihypertensive drugs, incident dementia, and the competing risk of death. J Am Med Dir Assoc. 2018;19(11):1026-1027. doi: 10.1016/j.jamda.2018.07.025 [DOI] [PubMed] [Google Scholar]
- 9.Tadic M, Cuspidi C, Hering D. Hypertension and cognitive dysfunction in elderly: blood pressure management for this global burden. BMC Cardiovasc Disord. 2016;16(1):208. doi: 10.1186/s12872-016-0386-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benetos A, Petrovic M, Strandberg T. Hypertension management in older and frail older patients. Circ Res. 2019;124(7):1045-1060. doi: 10.1161/CIRCRESAHA.118.313236 [DOI] [PubMed] [Google Scholar]
- 11.Bress AP, Tanner RM, Hess R, Colantonio LD, Shimbo D, Muntner P. Generalizability of SPRINT results to the U.S. adult population. J Am Coll Cardiol. 2016;67(5):463-472. doi: 10.1016/j.jacc.2015.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheppard JP, Lown M, Burt J, et al. Generalizability of blood pressure lowering trials to older patients: cross-sectional analysis. J Am Geriatr Soc. 2020;68(11):2508-2515. doi: 10.1111/jgs.16749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Deudekom FJ, Postmus I, van der Ham DJ, et al. External validity of randomized controlled trials in older adults, a systematic review. PLoS One. 2017;12(3):e0174053. doi: 10.1371/journal.pone.0174053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheppard JP, Stevens S, Stevens R, et al. Benefits and harms of antihypertensive treatment in low-risk patients with mild hypertension. JAMA Intern Med. 2018;178(12):1626-1634. doi: 10.1001/jamainternmed.2018.4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.EU Joint Programme—Neurodegenerative Disease Research (JPND). 21st Century EURODEM. Report of a JPND Working Group on Longitudinal Cohorts. Accessed February 10, 2020. https://www.neurodegenerationresearch.eu/wp-content/uploads/2015/10/JPND-Report-Brayne.pdf
- 16.Lobo A, Lopez-Anton R, Santabárbara J, et al. Incidence and lifetime risk of dementia and Alzheimer’s disease in a Southern European population. Acta Psychiatr Scand. 2011;124(5):372-383. doi: 10.1111/j.1600-0447.2011.01754.x [DOI] [PubMed] [Google Scholar]
- 17.Qiu C, von Strauss E, Bäckman L, Winblad B, Fratiglioni L. Twenty-year changes in dementia occurrence suggest decreasing incidence in central Stockholm, Sweden. Neurology. 2013;80(20):1888-1894. doi: 10.1212/WNL.0b013e318292a2f9 [DOI] [PubMed] [Google Scholar]
- 18.Qiu C, von Strauss E, Fastbom J, Winblad B, Fratiglioni L. Low blood pressure and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Arch Neurol. 2003;60(2):223-228. doi: 10.1001/archneur.60.2.223 [DOI] [PubMed] [Google Scholar]
- 19.Riedel-Heller SG, Schork A, Matschinger H, Angermeyer MC. Recruitment procedures and their impact on the prevalence of dementia. Results from the Leipzig Longitudinal Study of the Aged (LEILA75+). Neuroepidemiology. 2000;19(3):130-140. doi: 10.1159/000026248 [DOI] [PubMed] [Google Scholar]
- 20.Rydberg Sterner T, Ahlner F, Blennow K, et al. The Gothenburg H70 birth cohort study 2014-16: design, methods and study population. Eur J Epidemiol. 2019;34(2):191-209. doi: 10.1007/s10654-018-0459-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moll van Charante EP, Richard E, Eurelings LS, et al. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (PreDIVA): a cluster-randomised controlled trial. Lancet. 2016;388(10046):797-805. doi: 10.1016/S0140-6736(16)30950-3 [DOI] [PubMed] [Google Scholar]
- 22.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144(2):73-81. doi: 10.7326/0003-4819-144-2-200601170-00004 [DOI] [PubMed] [Google Scholar]
- 23.Wagner M, Helmer C, Tzourio C, Berr C, Proust-Lima C, Samieri C. Evaluation of the concurrent trajectories of cardiometabolic risk factors in the 14 years before dementia. JAMA Psychiatry. 2018;75(10):1033-1042. doi: 10.1001/jamapsychiatry.2018.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peters R, Peters J, Booth A, Anstey KJ. Trajectory of blood pressure, body mass index, cholesterol and incident dementia: systematic review. Br J Psychiatry. 2020;216(1):16-28. doi: 10.1192/bjp.2019.156 [DOI] [PubMed] [Google Scholar]
- 25.Blake HA, Leyrat C, Mansfield KE, et al. Propensity scores using missingness pattern information: a practical guide. Stat Med. 2020;39(11):1641-1657. doi: 10.1002/sim.8503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gong J, Harris K, Peters SAE, Woodward M. Sex differences in the association between major cardiovascular risk factors in midlife and dementia: a cohort study using data from the UK Biobank. BMC Med. 2021;19(1):110. doi: 10.1186/s12916-021-01980-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes D, Judge C, Murphy R, et al. Association of blood pressure lowering with incident dementia or cognitive impairment: a systematic review and meta-analysis. JAMA. 2020;323(19):1934-1944. doi: 10.1001/jama.2020.4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding J, Davis-Plourde KL, Sedaghat S, et al. Antihypertensive medications and risk for incident dementia and Alzheimer’s disease: a meta-analysis of individual participant data from prospective cohort studies. Lancet Neurol. 2020;19(1):61-70. doi: 10.1016/S1474-4422(19)30393-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williamson JD, Pajewski NM, Auchus AP, et al. ; SPRINT MIND Investigators for the SPRINT Research Group . Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA. 2019;321(6):553-561. doi: 10.1001/jama.2018.21442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adler A; Blood Pressure Lowering Treatment Trialists’ Collaboration . Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet. 2021;397(10285):1625-1636. doi: 10.1016/S0140-6736(21)00590-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moonen JEF, Foster-Dingley JC, de Ruijter W, et al. Effect of discontinuation of antihypertensive treatment in elderly people on cognitive functioning—the DANTE Study Leiden: a randomized clinical trial. JAMA Intern Med. 2015;175(10):1622-1630. doi: 10.1001/jamainternmed.2015.4103 [DOI] [PubMed] [Google Scholar]
- 32.van Dalen JW, Moll van Charante EP, van Gool WA, Richard E. Discontinuation of antihypertensive medication, cognitive complaints, and incident dementia. J Am Med Dir Assoc. 2019;20(9):1091-1097.e3. doi: 10.1016/j.jamda.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 33.Wang R, Vetrano DL, Liang Y, Qiu C. The age-related blood pressure trajectories from young-old adults to centenarians: a cohort study. Int J Cardiol. 2019;296:141-148. doi: 10.1016/j.ijcard.2019.08.011 [DOI] [PubMed] [Google Scholar]
- 34.Delgado J, Bowman K, Ble A, et al. Blood pressure trajectories in the 20 years before death. JAMA Intern Med. 2018;178(1):93-99. doi: 10.1001/jamainternmed.2017.7023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdelhafiz AH, Loo BE, Hensey N, Bailey C, Sinclair A. The U-shaped relationship of traditional cardiovascular risk factors and adverse outcomes in later life. Aging Dis. 2012;3(6):454-464. [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmadi SF, Streja E, Zahmatkesh G, et al. Reverse epidemiology of traditional cardiovascular risk factors in the geriatric population. J Am Med Dir Assoc. 2015;16(11):933-939. doi: 10.1016/j.jamda.2015.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.den Brok MGHE, van Dalen JW, Abdulrahman H, et al. Antihypertensive medication classes and the risk of dementia: a systematic review and network meta-analysis. J Am Med Dir Assoc. 2021;22(7):1386-1395.e15. doi: 10.1016/j.jamda.2020.12.019 [DOI] [PubMed] [Google Scholar]
- 38.Peters R, Beckett N, Forette F, et al. ; HYVET investigators . Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial Cognitive Function Assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol. 2008;7(8):683-689. doi: 10.1016/S1474-4422(08)70143-1 [DOI] [PubMed] [Google Scholar]
- 39.Pajewski NM, Berlowitz DR, Bress AP, et al. Intensive vs standard blood pressure control in adults 80 years or older: a secondary analysis of the systolic blood pressure intervention trial. J Am Geriatr Soc. 2020;68(3):496-504. doi: 10.1111/jgs.16272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillips RA, Arnold RM, Peterson LE. Hypertension guidelines: the threads that bind them. J Am Coll Cardiol. 2018;72(11):1246-1251. doi: 10.1016/j.jacc.2018.07.014 [DOI] [PubMed] [Google Scholar]
- 41.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams B, Mancia G, Spiering W, et al. ; ESC Scientific Document Group . 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021-3104. doi: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 43.Ioannidis JPA, Adami HO. Nested randomized trials in large cohorts and biobanks: studying the health effects of lifestyle factors. Epidemiology. 2008;19(1):75-82. doi: 10.1097/EDE.0b013e31815be01c [DOI] [PubMed] [Google Scholar]
- 44.Golfam M, Beall R, Brehaut J, et al. Comparing alternative design options for chronic disease prevention interventions. Eur J Clin Invest. 2015;45(1):87-99. doi: 10.1111/eci.12371 [DOI] [PubMed] [Google Scholar]
- 45.Richard E, Andrieu S, Solomon A, et al. Methodological challenges in designing dementia prevention trials - the European Dementia Prevention Initiative (EDPI). J Neurol Sci. 2012;322(1-2):64-70. doi: 10.1016/j.jns.2012.06.012 [DOI] [PubMed] [Google Scholar]
- 46.Conroy SP, Westendorp RGJ, Witham MD. Hypertension treatment for older people-navigating between Scylla and Charybdis. Age Ageing. 2018;47(4):505-508. doi: 10.1093/ageing/afy053 [DOI] [PubMed] [Google Scholar]
- 47.Morrissey Y, Bedford M, Irving J, Farmer CK. Older people remain on blood pressure agents despite being hypotensive resulting in increased mortality and hospital admission. Age Ageing. 2016;45(6):783-788. doi: 10.1093/ageing/afw120 [DOI] [PubMed] [Google Scholar]
- 48.Sheppard JP, Burt J, Lown M, et al. ; OPTIMISE Investigators . Effect of antihypertensive medication reduction vs usual care on short-term blood pressure control in patients with hypertension aged 80 years and older: the OPTIMISE randomized clinical trial. JAMA. 2020;323(20):2039-2051. doi: 10.1001/jama.2020.4871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schiepers OJG, Köhler S, Deckers K, et al. Lifestyle for Brain Health (LIBRA): a new model for dementia prevention. Int J Geriatr Psychiatry. 2018;33(1):167-175. doi: 10.1002/gps.4700 [DOI] [PubMed] [Google Scholar]
- 50.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5(9):735-741. doi: 10.1016/S1474-4422(06)70537-3 [DOI] [PubMed] [Google Scholar]
- 51.Licher S, Yilmaz P, Leening MJG, et al. External validation of four dementia prediction models for use in the general community-dwelling population: a comparative analysis from the Rotterdam Study. Eur J Epidemiol. 2018;33(7):645-655. doi: 10.1007/s10654-018-0403-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vos SJB, van Boxtel MPJ, Schiepers OJG, et al. Modifiable risk factors for prevention of dementia in midlife, late life and the oldest-old: validation of the LIBRA index. J Alzheimers Dis. 2017;58(2):537-547. doi: 10.3233/JAD-161208 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Study Recruitment
eMethods 2. Dementia Ascertainment and Follow-up in Participating Studies
eMethods 3. Models and Model Selection
eMethods 4. Interpretation of Confidence Intervals
eMethods 5. Choice of Age-Bands and Shift in Analysis
eFigure 1. Participants per 5-Year Age Categories per Study
eFigure 2. Percentage Contribution per Study per 5-Year Age Category
eTable 1. Relations for Diastolic Blood Pressure With Risk of Dementia, Mortality, and Dementia/Mortality
eTable 2. Complete Results Comparing Linear and Non-Linear Models for Systolic Blood Pressure
eTable 3. Complete Results Comparing Linear and Non-Linear Models for Diastolic Blood Pressure
eFigure 3. Relations Between Diastolic Blood Pressure and Risk of Dementia/Mortality Combined
eTable 4. Relations for Systolic Blood Pressure With Risk of Dementia, Mortality, and Dementia/Mortality in Age Groups, Stratified According to Antihypertensive Medication (AHM) Use
eTable 5. Relations for Diastolic Blood Pressure With Risk of Dementia, Mortality, and Dementia/Mortality in Age Groups, Stratified According to Antihypertensive Medication (AHM) Use
eFigure 4. Analyses of Systolic Blood Pressure and Dementia/Mortality Risk Adjusted and Not Adjusted for Potentially Relevant Confounders
eTable 6. Model Improvement Stratified According to Subgroups
eTable 7. Subgroup Analyses in Individuals With/Without a History of Stroke
eTable 8. Leave-One-Out Analyses
eTable 9. Relations for Systolic and Diastolic Blood Pressure With Risk of Dementia, Mortality, and Dementia/Mortality According to Time to Event in the Total Population and Within Age Subgroups
eFigure 5. Analyses for Systolic Blood Pressure According to Time to Event in the Total Population and Within Age Subgroups
eTable 10. Sensitivity Analyses Adjusting for Multiple Confounders in Together in Single Models
eTable 11. Results for Fine-Gray Analyses of Dementia Accounting for the Competing Risk of Mortality in the Overall Population and Baseline Age Groups