Abstract
Background and Objectives
Neurofilament light chain (NfL) is a biomarker for neuroaxonal damage and has been found to be elevated proportionally to the degree of neuronal damage in neurologic diseases. The objective of this study was to determine the prognostic accuracy of NfL concentrations on unfavorable outcome in adults with community-acquired bacterial meningitis.
Methods
We measured NfL concentration CSF samples from a prospective cohort study of adults with community-acquired bacterial meningitis in The Netherlands and determined associations between NfL CSF concentrations, clinical characteristics, and outcome in multivariate analyses. We identified independent predictors of an unfavorable outcome (Glasgow Outcome Scale scores 1–4) by logistic regression.
Results
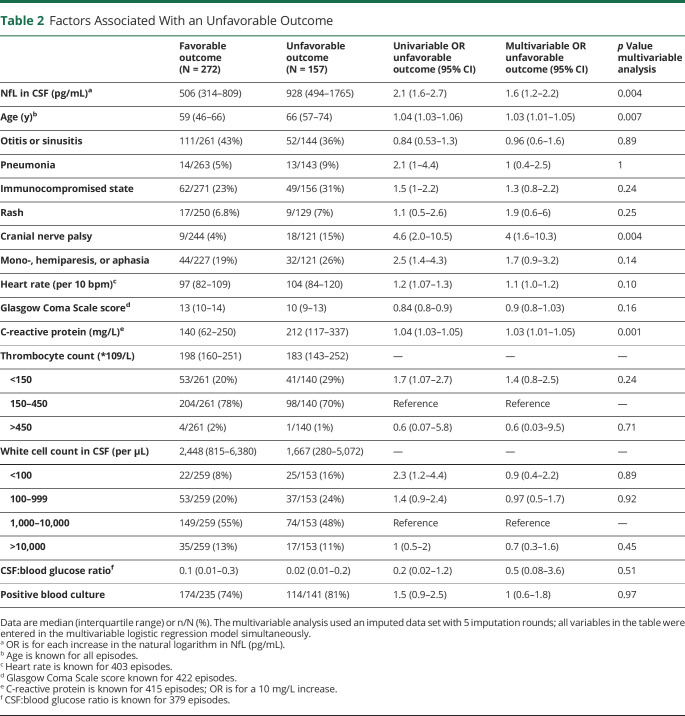
CSF NfL concentrations were evaluated in 429 episodes of 425 patients with community-acquired bacterial meningitis. The median age of 429 episodes was 62 years (interquartile range, 50–69 years). Of note, 290 of 422 (68%) episodes presented with an altered mental status (Glasgow Coma Scale score < 14). Most common causative pathogens were Streptococcus pneumoniae (73%), Neisseria meningitidis (7%), and Listeria monocytogenes (5%). The overall case fatality rate was 62 of 429 (15%), and unfavorable outcome occurred in 57 (37%) of 429 episodes. In multivariate analysis, predictors of unfavorable outcome were older age (OR 1.03, 95% CI 1.01–1.05), cranial nerve palsy (OR 4, 95% CI 1.6–10.3), high serum C-reactive protein concentration (OR 1.3, 95% CI 1.01–1.05), and high CSF NfL concentration (OR 1.5, 95% CI 1.07–2.00). CSF NfL concentrations were higher in patients presenting with focal cerebral deficits (717 pg/mL [416–1,401] vs 412 pg/mL [278–731]; p < 0.001). The area under the curve (AUC) for predicting unfavorable outcome in bacterial meningitis of CSF NfL concentration was 0.69 (95% CI, 0.64–0.74).
Discussion
CSF NfL concentration is independently associated with unfavorable outcome in adults with community-acquired bacterial meningitis, suggesting that CSF NfL concentration may be a useful biomarker for prognostic assessment in bacterial meningitis.
Classification of Evidence
Can the level of NfL in CSF (the index test) predict unfavorable outcome in patients with bacterial meningitis, in a cohort of bacterial meningitis patients with a favorable and unfavorable outcome? This study provides Class II evidence that NfL level in CSF is a moderate predictor, with the AUC for predicting unfavorable outcome in bacterial meningitis being 0.69 (95% CI, 0.64–0.74).
Bacterial meningitis is a life-threatening disease associated with high mortality and morbidity and is predominantly caused by Streptococcus pneumoniae.1-3 About half of survivors of pneumococcal meningitis have neurologic sequelae, including hearing loss, focal cerebral deficits, and cognitive impairment.4-7 Unfavorable outcome after meningitis is mainly caused by an excessive inflammation reaction of the host's immune system in reaction to the bacterial infection, leading to injury to the CNS.8,9 Brain autopsy of pneumococcal meningitis cases has shown that vascular damage is key in the process of cortical necrosis and brain damage.10 The use of an objective and dynamic fluid biomarker for neuronal damage might provide clinicians with an additional tool for prognostic assessment.
Neurofilaments are cylindrical proteins that are exclusively located in the neuronal cytoplasm and form an integral component of the axonal cytoskeleton.11 Neurofilaments consist of 3 subunits: neurofilament light chain (NfL), heavy chain, and medium chain. As NfL is the backbone of neurofilaments, it can be robustly measured in body fluids. Although low concentrations of NfL are continuously being released in an age-dependent manner from axons, a wide variety of CNS diseases that cause axonal damage result in elevated NfL concentrations proportionally to the degree of axonal damage.12 This includes multiple sclerosis (MS), frontotemporal dementia (FTD), and amyotrophic lateral sclerosis (ALS), in which NfL has been suggested to be valuable not only in diagnosis (ALS) but also as a serum marker of disease progression (FTD and ALS) and treatment response (MS).11-14 Few studies have described neurofilaments in CNS infections,15,16 with a study in children with bacterial meningitis showing that peak concentrations of neurofilament heavy chain in CSF were higher in patients with neurologic sequelae than in those without.17 In an experimental pneumococcal rat model, CSF NfL concentrations were increased after injection with experimental S. pneumoniae compared with mock-infected rats.18
Our objective was to determine the prognostic accuracy of NfL in bacterial meningitis in adults. We hypothesized that NfL in CSF might be increased in patients with bacterial meningitis and that the quantification of neuronal damage could allow for prognostic assessment. In this prospective nationwide cohort study, we evaluated the prognostic value of concentrations of NfL in CSF in adults with community-acquired bacterial meningitis.
Methods
Patients
The MeninGene study is an ongoing nationwide cohort study in The Netherlands that started in 2006 to identify host and pathogen factors influencing the risk and outcome of bacterial meningitis.2,19,20 Detailed methods of the cohort have been described previously.2 In summary, patients aged 16 years or older with community-acquired bacterial meningitis were prospectively included following a report from the Netherlands Reference Laboratory for Bacterial Meningitis. This laboratory receives bacterial strains from over 85% of all patients with bacterial meningitis in the Netherlands and notifies the investigators with the name of the treating physicians, who are then contacted to include the patient in the study. Written informed consent was obtained from participating patients or their legal representatives.
Inclusion and Exclusion Criteria
Patients were included in the MeninGene study if they had a positive CSF culture or if the CSF showed at least 1 individual finding predictive of bacterial meningitis according to the Spanos criteria (glucose <1.9 mmol/L, CSF serum glucose ratio <0.23, protein concentration >2.20 g/L, white cell count >2,000 cells/mm3, or CSF neutrophil count >1,180 cells/mm3)21 in combination with a positive CSF PCR, CSF antigen, or blood culture. Patients who developed meningitis while in hospital or within 1 week after discharge, following head trauma or neurosurgery in the previous month, or with a neurosurgical device in situ were excluded.22
Neurofilament Light Chain (NfL) Measurement in CSF
CSF from the diagnostic lumbar puncture (at presentation or on admission) was collected in a central biobank in Amsterdam UMC and was available for ±60% of included episodes. After withdrawal, the samples were centrifuged, frozen, and stored in 96-well plates at −80°C until further analysis. For the current measurements, 6 CSF plates were randomly selected. NfL in CSF (5 µL) was measured using the Simoa NF-light Advantage Kit (ref. 103186) on an HD-X instrument (Quanterix, Billerica, MA) at the Neurochemistry Laboratory at Amsterdam UMC, location VUmc, according to the manufacturer's instructions.
Quality of the assay is monitored using 3 serum pools from patients, having different NfL levels spanning the measurement range. The interassay coefficient of variability over 146 runs was 7.7%. The intra-assay variation of the assays has been extensively tested, being consistently <3%.
Clinical Data Collection and Definitions
Data on patients' baseline characteristics, symptoms on admission, clinical course, treatment, and (neurologic) outcome were prospectively collected with a secured online case record form. Immunocompromised state was defined as having active cancer, a splenectomy, diabetes mellitus, HIV, alcoholism, or the use of immunosuppressive drugs. At discharge, neurologic examination was assessed using the Glasgow Outcome Scale (GOS) score, ranging from a score of 1 = death to a score of 5 = mild or no disability (able to return to work or school). An unfavorable outcome was defined as a GOS score of 1–4, and a favorable outcome was defined as a score of 5.
Statistical Analysis
Categorical variables are expressed as counts and proportions, and continuous variables are expressed as median with interquartile range (IQR). CSF NfL concentrations between groups were compared using the Mann-Whitney U test or the Kruskal-Wallis test. Logistic regression was used to analyze the association between CSF NfL level with unfavorable outcome, providing ORs and 95% CIs. We chose possible confounding variables for NfL and predictors of an unfavorable outcome on the basis of previous research2 and pathophysiologic interest. Linearity of the association between continuous predictors and outcome was assessed with the Hosmer-Lemeshow goodness of fit test and by visual inspection. If there was no linear relationship, the continuous predictor was either transformed or categorized for further analysis. We estimated both univariable and multivariable ORs corrected for all other variables in the model. We used multiple imputation for missing data in the multivariable analysis and used all predictors together to impute missing values. For the multivariable model, missing values in the selected prognostic factors (median 4.9% per prognostic factor [IQR 3.9–6.6]) were imputed, subsequently combining the coefficients of each data set (N = 5) according to Rubin rule.23 All tests were 2 tailed, and a p value <0.05 was considered significant. Statistical analysis was conducted using IBM SPSS Statistics data Editor (v.26).
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the Medical Ethical Review Committee of the Amsterdam UMC (number METC 2013_043). Written informed consent was obtained from all participants or their representatives.
Data Availability
Anonymized data not published within the article will be shared on the request from any qualified investigator.
Results
Between 2006 and July 2018, a total of 2,116 episodes of bacterial meningitis were included in our cohort study. For the current analyses, we used 429 episodes with an available CSF sample from the diagnostic lumbar puncture (eFigure 1, links.lww.com/NXI/A673). Characteristics between selected and nonselected patients for this analysis were similar (eTable 1, links.lww.com/NXI/A673). The 429 episodes occurred in 425 patients; 4 patients had recurrent meningitis within the cohort study period.
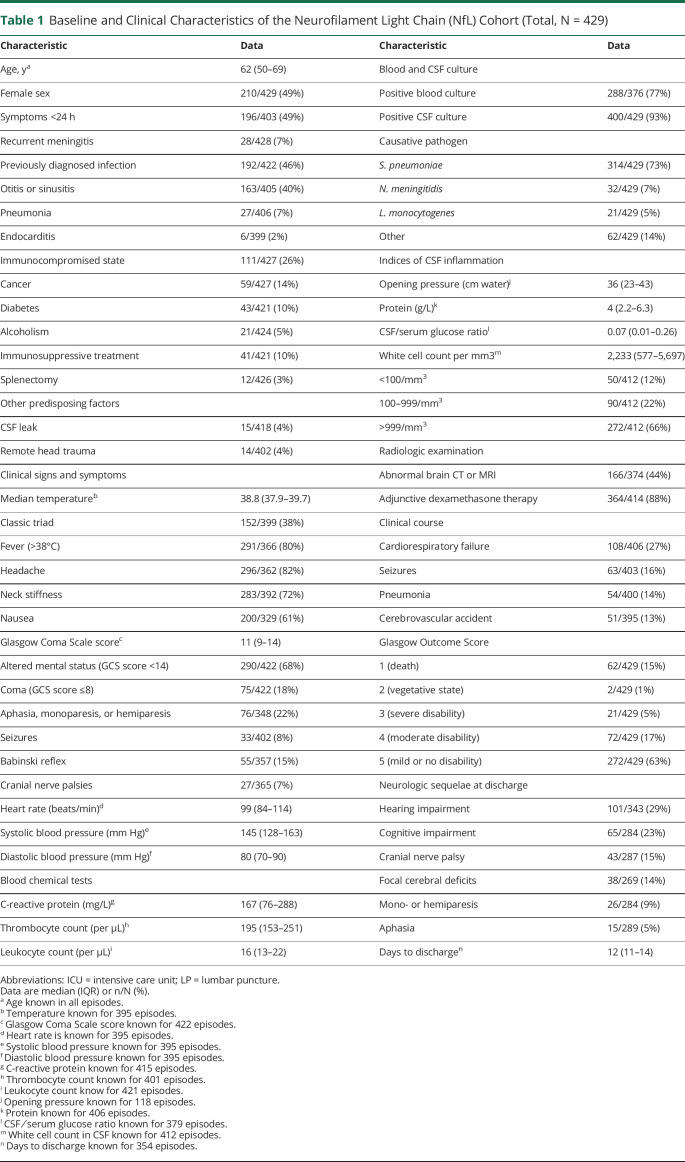
The median patient age in the 429 episodes was 62 years (IQR 50–69 years), and 210 of 429 episodes occurred in females (49%; Table 1). Immunocompromising conditions were present in 111 of 427 (26%) of episodes, predisposing infection foci in 192 of 422 (46%) episodes, and 15 of 418 (4%) of episodes had a CSF leak. An altered mental status (defined as Glasgow Coma Scale [GCS] score <14) was present on admission in 290 of 422 (68%) episodes, of whom 75 episodes presented with coma (GCS score <8). The classical triad consisting of fever, altered mental status, and neck stiffness was present in 152 of 399 episodes (38%). Streptococcus pneumoniae and Neisseria meningitidis were the most common causative pathogens causing 314 of 429 (73%) and 32 of 429 (7%) of the cases. An unfavorable outcome occurred in 157 of 429 (37%) of episodes, and 62 of 429 (15%) patients died. Most common neurologic sequelae at discharge were hearing impairment (101 of 343 episodes, 29%), cognitive impairment (65 of 284 episodes, 23%), cranial nerve palsies (43 of 287 episodes, 15%), and focal cerebral deficits (38 of 269 episodes, 14%).
Table 1.
Baseline and Clinical Characteristics of the Neurofilament Light Chain (NfL) Cohort (Total, N = 429)
The median CSF NfL concentration was 600 pg/mL (IQR 348–1,047 pg/mL). A moderate correlation was found between CSF NfL concentrations and age (r = 0.6, p < 0.001), and a weak correlation between CSF NfL and CSF protein concentrations (r = 0.14, p = 0.009) and CSF NfL and CSF leukocyte counts (r = -0.2, p < 0.001). NfL concentrations were higher in patients presenting with focal cerebral deficits (717 pg/mL [416–1,401] vs 412 pg/mL [278–731] without focal cerebral deficits; p < 0.001) and those presenting with a GCS score <14 (NfL 654 pg/mL [IQR 397–1,083 pg/mL] vs 513 pg/mL [IQR 271–873 pg/mL] in those presenting with a GCS score of 14 and 15; p = 0.002). CSF NfL concentrations differed between causative pathogens (Figure 1). CSF NfL concentrations were higher in patients with pneumococcal meningitis compared with nonpneumococcal meningitis (median NfL 616 pg/mL [IQR 361–1,043 pg/mL] vs 541 pg/mL [IQR 319–1,054 pg/mL], after correcting for age p = 0.048).
Figure 1. Comparison of NfL Level (Median) in CSF Between Different Causative Pathogens.
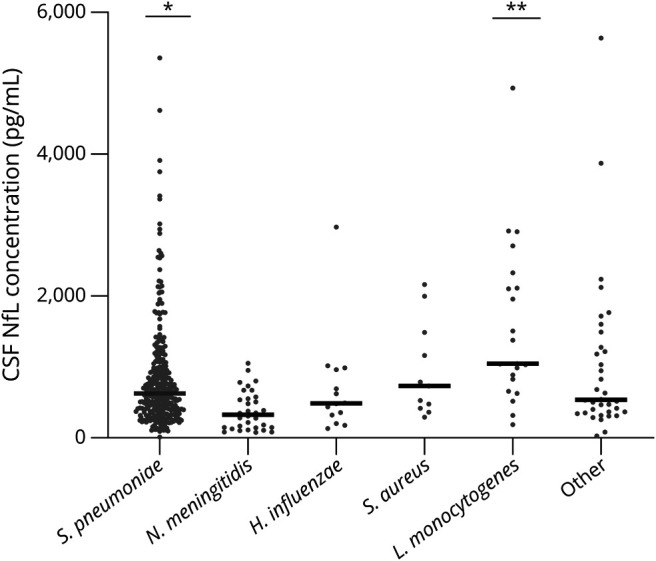
*Significant difference in NfL level between pneumococcal (N = 314) vs nonpneumococcal (N = 115) cases, after correction for age (respectively, 616 pg/mL [IQR 361–1,043 pg/mL] and 541 pg/mL [IQR 319–1,054 pg/mL], F[1,426] = 3.93, p = 0.048). **Significant difference in NfL level between Listeria monocytogenes (N = 21) vs non-Listeria (N = 408) cases, after correction for age (respectively, 1,049 pg/mL [IQR 741–2,219 pg/mL] and 572 pg/mL [IQR 342–1,007 pg/mL], F[1,426] = 6.29, p = 0.013). Haemophilus influenzae (N = 13) and Staphylococcus aureus (N = 11). Other pathogens: Streptococcus agalactiae (N = 7), Streptococcus pyogenes (N = 11), Streptococcus suis (N = 3), Streptococcus salivarius (N = 2), Streptococcus anginosus (N = 3), Streptococcus dysgalactiae (N = 1), Streptococcus intermedius (N = 2), Streptococcus oralis (N = 1), Escherichia coli (N = 2), Klebsiella pneumoniae (N = 1), Campylobacter fetus (N = 1), Nocardia farcinica (N = 1), Group C streptococcus not other specified (N = 1), viridans group streptococci not other specified (N = 1), and Fusobacterium necrophorum (N = 1). IQR = interquartile range; NfL = neurofilament light chain.
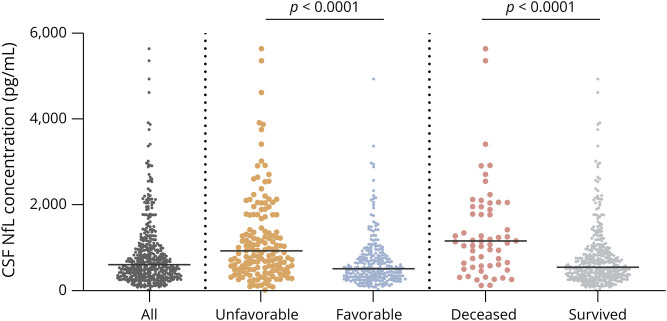
CSF NfL concentrations in patients who died were more than 2-fold higher than in surviving patients (1,152 pg/mL [IQR 594–2043 pg/mL] vs 541 pg/mL [IQR 335–924 pg/mL], p < 0.001; Figure 2). CSF NfL concentrations were higher in patients with an unfavorable outcome (928 pg/mL [IQR 494–1765 pg/mL] vs 506 pg/mL [IQR 314–809 pg/mL], p < 0.001). In a multivariable analysis, older age, cranial nerve palsy, high serum C-reactive protein concentration, and high CSF NfL concentration were associated with an unfavorable outcome in bacterial meningitis due to any pathogen (Table 2). The association between CSF NfL concentration and outcome remained robust when including the causative pathogen (eTable 2, links.lww.com/NXI/A673). The area under the curve (AUC) for predicting unfavorable outcome in bacterial meningitis was 0.69 (95% CI 0.64–0.74). The optimal cutoff point in CSF NfL concentration according to the Youden index was 681 pg/mL (sensitivity 63% and specificity 67%). The AUC for predicting mortality in bacterial meningitis was 0.72 (95% CI 0.65–0.79), with an optimal cutoff point of 926 pg/mL (sensitivity 67% and specificity 75%).
Figure 2. Comparison of NfL Level Between Total Cohort (N = 429) and Different Outcome Groups (Median, Interquartile Range).
NfL level in deceased (N = 62) vs alive (N = 367) patients (respectively, 1,152 pg/mL [IQR 594–2043 pg/mL] and 541 pg/mL [IQR 335–924 pg/mL]; p < 0.001). NfL level in patients with unfavorable (N = 157) vs favorable outcome (N = 272) (respectively, 928 pg/mL [IQR 494–1765 pg/mL] and 506 pg/mL [IQR 314–809 pg/mL); p < 0.001]. IQR = interquartile range; NfL = neurofilament light chain.
Table 2.
Factors Associated With an Unfavorable Outcome
Discussion
Our study shows that CSF NfL concentration independently predicts unfavorable outcome in adults with community-acquired bacterial meningitis. Previous studies have identified several predictors for unfavorable outcome in bacterial meningitis2,3,24,25: a low level of consciousness on admission, cranial nerve palsy, a low CSF white cell count, factors predictive of pneumococcal infection (advanced age; presence of otitis or sinusitis, pneumonia, or immunocompromised status; and absence of rash) or systemic compromise (high heart rate and high serum C-reactive protein concentration), and infection by a penicillin-resistant S. pneumoniae.26 As neuronal damage plays a central role in the pathophysiology of bacterial meningitis,9 the use of a body fluid biomarker for neuronal damage such as NfL might provide clinicians with an additional tool for better prognostic assessment and could aid to identify those patients at risk for an unfavorable outcome.
In bacterial meningitis, outcome is in part determined by an excessive inflammation reaction of the host's immune system in reaction to the bacterial infection,8,9 causing vascular and neuronal damage.10 In previous studies, NfL was shown to predict outcome and disease progression in a wide variety of neurologic disorders such as Guillain-Barre syndrome,27 amyotrophic lateral sclerosis,28,29 critical illness neuropathy in intensive care patients,30 Alzheimer disease,31 FTD,32 chronic inflammatory demyelinating polyneuropathy,33 and MS.34,35 Furthermore, plasma NfL concentrations demonstrated excellent prognostic accuracy in comatose patients after out-of-hospital cardiac arrest.36,37 The prognostic value of neurofilaments in CNS infections has been studied previously. Two studies showed higher serum and CSF NfL levels in patients with varicella zoster virus encephalitis compared with varicella zoster virus meningitis.15,16 One study showed higher CSF concentrations of neurofilament heavy chain in 26 children with bacterial meningitis compared with controls.17
Results of this study show that the diagnostic accuracy of CSF NfL concentrations for predicting death or unfavorable outcome on an individual patient level is limited. Although NfL levels are higher in patients with an unfavorable outcome, the ranges overlap to an extent that makes it difficult to define a pathologic cutoff at the individual patient level, which is also reflected by the moderate AUC. Nevertheless, NfL in CSF might be a potential useful variable in a prediction model for unfavorable outcome in bacterial meningitis. Several previous studies have researched such prediction models and prognostics factors in bacterial meningitis.38,39 Improving the stratification of patients with bacterial meningitis at presentation with respect to the risk for unfavorable outcome remains an important tool for physicians to predict clinical deterioration, but can also be a valuable tool for targeting intervention strategies, e.g., as stratification marker or outcome measure. The use of a marker for neuroaxonal damage such as NfL might be specifically interesting, as unfavorable outcome in bacterial meningitis is mainly caused by intracranial complications, and thus, the marker likely reflects downstream biological effects.1,4,9 Future studies should therefore consider to evaluate the added value of NfL in predictor models for bacterial meningitis.
Our study has limitations. First, NfL was not measured in all patients included in the MeninGene cohort, but only in a limited number of samples. CSF samples from the diagnostic lumbar puncture are stored in about 60% of patients included in the cohort. However, baseline characteristics between episodes selected and not selected for the study were similar, indicating that the influence of selection bias on our results is limited. Second, NfL concentrations were measured in the CSF of the diagnostic lumbar puncture only. For better prognostic assessment, measuring several time points, for example, in plasma, might provide additional information on disease progression, as has been shown in some previous studies.29,31,34,40 For several neurodegenerative diseases, a strong correlation between CSF and plasma NfL has been demonstrated.14,35 Also, in an experimental pneumococcal meningitis model, CSF and plasma NfL showed strong correlation.18 Future studies should therefore evaluate the prognostic value of serial plasma NfL measurement in bacterial meningitis.17,18 Third, the exact time to clinical presentation from symptom onset in our cohort is not known, which would possibly be of interest as the level of NfL might differ over time.
In conclusion, CSF NfL concentrations are independently associated with unfavorable outcome in adults with community-acquired bacterial meningitis. Future studies should evaluate the added value of NfL in prediction models for bacterial meningitis.
Glossary
- ALS
amyotrophic lateral sclerosis
- AUC
area under the curve
- FTD
frontotemporal dementia
- GCS
Glasgow Coma Scale
- GOS
Glasgow Outcome Scale
- IQR
interquartile range
- MS
multiple sclerosis
- NfL
neurofilament light chain
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Contributor Information
Nora Chekrouni, Email: n.chekrouni@amsterdamumc.nl.
Thijs M. van Soest, Email: t.vansoest@amsterdamumc.nl.
Matthijs C. Brouwer, Email: m.c.brouwer@amsterdamumc.nl.
Eline A.J. Willemse, Email: e.willemse@amsterdamumc.nl.
Charlotte E. Teunissen, Email: c.teunissen@amsterdamumc.nl.
Diederik van de Beek, Email: d.vandebeek@amsterdamumc.nl.
Study Funding
This work was supported by the Netherlands Organization for Health Research and Development (ZonMw; NWO-Vidi Grant [917.17.308] to M.C.B; NWO-Vici-Grant [grant number 918.19.627 to D. B.]); the Academic Medical Center (AMC Fellowship to D. B); and the European Research Council (ERC Consolidator grant to M.C.B., ERC Starting grant to D. B). The Netherlands Reference Laboratory for Bacterial Meningitis is supported by the National Institute of Public Health and the Environmental Protection, Bilthoven.
Disclosure
None. Go to Neurology.org/NN for full disclosures.
References
- 1.van de Beek D, Brouwer M, Hasbun R, Koedel U, Whitney CG, Wijdicks E. Community-acquired bacterial meningitis. Nat Rev Dis Primers. 2016;2:16074. [DOI] [PubMed] [Google Scholar]
- 2.Bijlsma MW, Brouwer MC, Kasanmoentalib ES, et al. Community-acquired bacterial meningitis in adults in The Netherlands, 2006–14: a prospective cohort study. Lancet Infect Dis. 2016;16(3):339-347. [DOI] [PubMed] [Google Scholar]
- 3.van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351(18):1849-1859. [DOI] [PubMed] [Google Scholar]
- 4.Lucas MJ, Brouwer MC, van de Beek D. Neurological sequelae of bacterial meningitis. J Infect. 2016;73(1):18-27. [DOI] [PubMed] [Google Scholar]
- 5.Hoogman M, van de Beek D, Weisfelt M, de Gans J, Schmand B. Cognitive outcome in adults after bacterial meningitis. J Neurol Neurosurg Psychiatry. 2007;78(10):1092-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van de Beek D, de Gans J, Tunkel AR, Wijdicks EFM. Community-acquired bacterial meningitis in adults. New Engl J Med. 2006;354(1):44-53. [DOI] [PubMed] [Google Scholar]
- 7.Kloek AT, Brouwer MC, Schmand B, Tanck MWT, van de Beek D. Long-term neurologic and cognitive outcome and quality of life in adults after pneumococcal meningitis. Clin Microbiol Infect. 2020;26(10):1361-1367. [DOI] [PubMed] [Google Scholar]
- 8.Koelman DLH, Brouwer MC, van de Beek D. Targeting the complement system in bacterial meningitis. Brain. 2019;142(11):3325-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mook-Kanamori BB, Geldhoff M, van der Poll T, van de Beek D. Pathogenesis and pathophysiology of pneumococcal meningitis. Clin Microbiol Rev. 2011;24(3):557-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelen-Lee JY, Brouwer MC, Aronica E, van de Beek D. Pneumococcal meningitis: clinical-pathological correlations (MeninGene-Path). Acta Neuropathol Commun. 2016;4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry. 2019;90(8):870-881. [DOI] [PubMed] [Google Scholar]
- 12.Bridel C, van Wieringen WN, Zetterberg H, et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta-analysis. JAMA Neurol. 2019;76(9):1035-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Disanto G, Barro C, Benkert P, et al. Serum Neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81(6):857-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14(10):577-589. [DOI] [PubMed] [Google Scholar]
- 15.Grahn A, Hagberg L, Nilsson S, Blennow K, Zetterberg H, Studahl M. Cerebrospinal fluid biomarkers in patients with varicella-zoster virus CNS infections. J Neurol. 2013;260(7):1813-1821. [DOI] [PubMed] [Google Scholar]
- 16.Tyrberg T, Nilsson S, Blennow K, Zetterberg H, Grahn A. Serum and cerebrospinal fluid neurofilament light chain in patients with central nervous system infections caused by varicella-zoster virus. J Neurovirol. 2020;26(5):719-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsushige T, Ichiyama T, Kajimoto M, Okuda M, Fukunaga S, Furukawa S. Serial cerebrospinal fluid neurofilament concentrations in bacterial meningitis. J Neurol Sci. 2009;280(1-2):59-61. [DOI] [PubMed] [Google Scholar]
- 18.Le ND, Muri L, Grandgirard D, Kuhle J, Leppert D, Leib SL. Evaluation of neurofilament light chain in the cerebrospinal fluid and blood as a biomarker for neuronal damage in experimental pneumococcal meningitis. J Neuroinflammation. 2020;17(1):293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koopmans MM, Bijlsma MW, Brouwer MC, van de Beek D, van der Ende A. Listeria monocytogenes meningitis in The Netherlands, 1985-2014: a nationwide surveillance study. J Infect. 2017;75(1):12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brouwer MC, Heckenberg SG, de Gans J, Spanjaard L, Reitsma JB, van de Beek D. Nationwide implementation of adjunctive dexamethasone therapy for pneumococcal meningitis. Neurology. 2010;75(17):1533-1539. [DOI] [PubMed] [Google Scholar]
- 21.Spanos A, Harrell FE Jr, Durack DT. Differential diagnosis of acute meningitis. An analysis of the predictive value of initial observations. JAMA. 1989;262(19):2700-2707. [PubMed] [Google Scholar]
- 22.van de Beek D, Drake JM, Tunkel AR. Nosocomial Bacterial Meningitis. N Engl J Med. 2010;362(2):146-154. [DOI] [PubMed] [Google Scholar]
- 23.Rubin DB. Multiple Imputation for Nonresponse in Surveys. John Wiley & Sons: 2004; Vol 81. [Google Scholar]
- 24.Weisfelt M, Van De Beek D, Spanjaard L, Reitsma JB, De Gans J. Community-acquired bacterial meningitis in older people. J Am Geriatr Soc. 2006;54(10):1500-1507. [DOI] [PubMed] [Google Scholar]
- 25.Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev. 2010;23(3):467-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gouveia EL, Reis JN, Flannery B, et al. Clinical outcome of pneumococcal meningitis during the emergence of pencillin-resistant Streptococcus pneumoniae: an observational study. BMC Infect Dis. 2011;11(1):323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petzold A, Hinds N, Murray NF, et al. CSF neurofilament levels: a potential prognostic marker in Guillain–Barré syndrome. Neurology. 2006;67(6):1071-1073. [DOI] [PubMed] [Google Scholar]
- 28.Gagliardi D, Meneri M, Saccomanno D, Bresolin N, Comi GP, Corti S. Diagnostic and prognostic role of blood and cerebrospinal fluid and blood neurofilaments in amyotrophic lateral sclerosis: a review of the literature. Int J Mol Sci. 2019;20(17):4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu CH, Macdonald-Wallis C, Gray E, et al. Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology. 2015;84(22):2247-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisse AL, Pitarokoili K, Leppert D, et al. Serum neurofilament light chain as outcome marker for intensive care unit patients. J Neurol. 2021; 268(4):1323-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Preische O, Schultz SA, Apel A, et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer's disease. Nat Med. 2019;25(2):277-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meeter LHH, Vijverberg EG, Del Campo M, et al. Clinical value of neurofilament and phospho-tau/tau ratio in the frontotemporal dementia spectrum. Neurology. 2018;90(14):e1231-e1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Lieverloo GGA, Wieske L, Verhamme C, et al. Serum neurofilament light chain in chronic inflammatory demyelinating polyneuropathy. J Peripher Nerv Syst. 2019;24(2):187-194. [DOI] [PubMed] [Google Scholar]
- 34.Barro C, Benkert P, Disanto G, et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain. 2018;141(8):2382-2391. [DOI] [PubMed] [Google Scholar]
- 35.Kapoor R, Smith KE, Allegretta M, et al. Serum neurofilament light as a biomarker in progressive multiple sclerosis. Neurology. 2020;95(10):436-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wihersaari L, Ashton NJ, Reinikainen M, et al. Neurofilament light as an outcome predictor after cardiac arrest: a post hoc analysis of the COMACARE trial. Intensive Care Med. 2021;47(1):39-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moseby-Knappe M, Mattsson N, Nielsen N, et al. Serum neurofilament light chain for prognosis of outcome after cardiac arrest. JAMA Neurol. 2019;76(1):64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weisfelt M, van de Beek D, Spanjaard L, Reitsma JB, de Gans J. A risk score for unfavorable outcome in adults with bacterial meningitis. Ann Neurol. 2008;63(1):90-97. [DOI] [PubMed] [Google Scholar]
- 39.Bijlsma MW, Brouwer MC, Bossuyt PM, et al. Risk scores for outcome in bacterial meningitis: systematic review and external validation study. J Infect. 2016;73(5):393-401. [DOI] [PubMed] [Google Scholar]
- 40.Kester MI, Scheffer PG, Koel-Simmelink MJ, et al. Serial CSF sampling in Alzheimer's disease: specific versus non-specific markers. Neurobiol Aging. 2012;33(8):1591-1598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within the article will be shared on the request from any qualified investigator.