Abstract
The orphan nuclear receptor tailless homologue (TLX) is expressed almost exclusively in neural stem cells acting as an essential factor for their survival and is hence considered as a promising drug target in neurodegeneration. However, few studies have characterized the roles of TLX due to the lack of ligands and limited functional understanding. Here, we identify xanthines including caffeine and istradefylline as TLX modulators that counteract the receptor’s intrinsic repressor activity. Mutagenesis of residues lining a cavity within the TLX ligand binding domain altered the activity of these ligands, suggesting direct interactions with helix 5. Using xanthines as tool compounds, we observed a ligand-sensitive recruitment of the co-repressor silencing mediator for retinoid or thyroid-hormone receptors, TLX homodimerization, and heterodimerization with the retinoid X receptor. These protein–protein interactions evolve as factors that modulate the TLX function and suggest an unprecedented role of TLX in directly repressing other nuclear receptors.
Keywords: transcription factor, tailless homologue, NR2E1, neurodegeneration, caffeine
The human homologue of the drosophila tailless gene tll, TLX (NR2E1), is a member of the nuclear receptor (NR) family, which acts as ligand-dependent transcriptional regulators. In adults, the expression of TLX is strongly limited to adult neural stem cells (NSCs) residing in the subventricular zone and dentate gyrus of the hippocampus as well as retinal progenitor cells.1,2 According to rodent models, TLX is required to maintain NSCs in an undifferentiated proliferating state, and its mutations cause the disruption of neurogenesis in NSCs.3 In mice and drosophila, TLX knockout results in severe aggressiveness, abnormal brain development, and retinal dystrophies.4,5 Moreover, TLX appears to play a crucial role in spatial learning and cognitive functions during adolescence and adulthood.6−8 Therefore, dysregulation of TLX has been associated with mental illness including bipolar disorders and schizophrenia.9 Beyond these roles in neuronal homeostasis and brain function, recent reports have suggested a potential tumorigenic activity of the orphan NR due to marked overexpression in glioblastoma and neuroblastoma cell lines.10,11 These lines of evidence suggest TLX as an attractive drug target for neurodegenerative diseases and malignant brain tumors. However, endogenous TLX ligands, forming an essential part of the NR function, remain elusive, and only few synthetic TLX modulators have been described to date.12−15 In light of its obvious therapeutic potential, further evaluation of the receptor’s function and the discovery of potent TLX ligands are imperative.
Here, we identify xanthines as TLX modulators. Based on caffeine which we discovered in TLX ligand screening as a lead compound, our structure–activity relationship study succeeded in tuning the potency of this TLX ligand chemotype, and we have employed xanthines as an early tool to characterize the modulation and binding of TLX by ligands on cellular and molecular levels. Mutagenesis studies defined a molecular epitope of TLX modulation by this class of TLX ligands in a region inside the ligand-binding domain (LBD) involving an interaction with helix 5. On a molecular level, we observed pronounced homo- and heterodimerization of TLX as well as recruitment of the NR co-repressor 2 (SMRT—silencing mediator for retinoid or thyroid-hormone receptors). These three protein–protein interactions of TLX turned out to be sensitive to xanthines aligning with their effects on cellular TLX activity. Our results provide a novel class of TLX modulators to study the receptor’s role in health and disease and as a chemical starting point to facilitate future TLX ligand discovery.
Results and Discussion
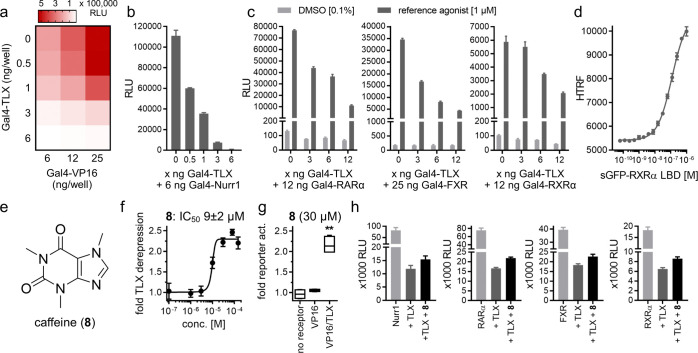
As an essential basis to identify and characterize TLX ligands, we have established a hybrid reporter gene assay to capture TLX activity in a bidirectional fashion by combining Gal4-TLX with the potent transcriptional inducer Gal4-VP1616 (Figure 1). The Gal4-hybrid technique, which employs fusion proteins composed of the Gal4-DNA-binding domain (DBD) and a NR LBD, has been successfully used for many NRs and provides several advantages.17 We used a Gal4-responsive firefly luciferase as a reporter gene and a constitutively expressed (SV40 promoter) renilla luciferase for normalization of the transfection efficiency and compound toxicity. A Gal4-DBD-TLX-LBD (aa. 150–385) clone was employed for the Gal4-based TLX assay system. Transfection of HEK293T cells with Gal4-DBD-TLX-LBD, Gal4-responsive firefly, and SV40-renilla, however, resulted in exceptionally low reporter expression and relative light units (RLUs) compared to the activity of other NRs in Gal4 format and even below the baseline activity of Gal4-responsive firefly luciferase in the absence of a Gal4-hybrid receptor. These results further confirmed that TLX—also as a chimeric receptor with Gal4-DBD—acts as a transcriptional repressor.18−21 Hence, to reflect this characteristic in a robust Gal4-based test system with a sufficient S/N ratio, a wide signal window, and the opportunity to observe bidirectional TLX modulation, we employed the hybrid protein Gal4-VP1616 (encoded by pECE-SV40-Gal4-VP1622) as a transcriptional activator, which is composed of the VP16 protein from herpes simplex virus and Gal4-DBD. Gal4-VP16 has been characterized as a potent, ligand-independent transcriptional inducer16 and, therefore, appeared well-suited to serve as a transcriptional activator for the envisioned hybrid assay with the transcriptional repressor Gal4-TLX. Expectedly, Gal4-VP16 alone caused a strong dose-dependent increase in the signal of Gal4-responsive firefly luciferase. Co-transfection with Gal4-TLX indeed revealed a marked and dose-dependent decrease in the Gal4-VP16-induced reporter signal (Figure 1a). Hence, the combination of Gal4-VP16 and Gal4-TLX provided a robust hybrid reporter gene assay setup, enabling the observation of TLX activation (resulting in stronger VP16 repression) and TLX derepression (resulting in reduced repression by TLX and hence higher reporter activity). Moreover, this setting enabled a highly informative control experiment by repeating activity tests on VP16 alone (in the absence of TLX) which in the absence of changes in Gal4-VP16-induced reporter activity confirm TLX-mediated effects.
Figure 1.
VP16/TLX reporter gene assay with caffeine as a TLX modulator. The schematic assay concept is shown in Figure S1. (a) Gal4-TLX represses the transcriptional inducer activity of Gal4-VP16 in a dose-dependent fashion. Heatmap shows mean RLU; n = 3. (b,c) Dose-dependent repressor activity of Gal4-TLX is also observed on human NRs in a Gal4 format including constitutively active receptors (b; Nurr1 as an example) and receptors with low intrinsic activity (c; RARα, FXR, and RXRα as examples). Data are mean ± SEM; n = 3. RARα (1 μM tretinoin), FXR (1 μM GW4064), and RXRα (1 μM bexarotene) are activated with reference agonists. (d) Recombinant LBDs of TLX and RXRα heterodimerized in a cell-free setting. Tb3+-cryptate-labeled TLX LBD was titrated with GFP-labeled RXRα LBD in a HTRF assay. Data are mean ± SD; N = 3. (e) Chemical structure of caffeine (8). (f) Dose–response curve of 8 in the VP16/TLX reporter gene assay. Data are mean ± SEM; n ≥ 3. The underlying reporter and control data are shown in Figure S2. (g) Control experiments (no Gal4 hybrid receptor and Gal4-VP16 alone) confirmed the TLX-mediated activity of 8. Boxplots show min–max fold reporter activation compared to 0.1% dimethyl sulfoxide (DMSO) in the respective setting; n = 4; **p < 0.01 (t-test). (h) Caffeine (8) showed a trend (p > 0.05 for 8, one-way ANOVA) to reduce the repressor activity of TLX on NRs. Data are mean ± SEM; n ≥ 3.
The ability of Gal4-TLX to block Gal4-VP16 activity suggested a potential direct repression of NRs by TLX. Co-transfection experiments of Gal4-TLX with other NRs in the Gal4-format supported this assumption. The transcriptional repressor markedly diminished the reporter activity induced by constitutively active (Gal4-Nurr1; Figure 1b) and agonist-activated [Gal4-retinoic acid receptor alpha (RARα), Gal4-farnesoid X receptor (FXR), Gal4-retinoic acid X receptor alpha (RXRα); Figure 1c] NRs in a dose-dependent fashion. To confirm this observation in a cell-free setting and to determine whether direct interactions were involved, we probed the dimerization of TLX with RXR by titrating a Tb3+-cryptate-labeled TLX LBD with a green fluorescent protein (GFP)-labeled RXRα LBD in a homogenous time-resolved fluorescence resonance energy transfer (HTRF) assay and detected strong RXR-TLX heterodimerization with an EC50 value of 120 nM (Figure 1d). The results of these cellular and cell-free experiments suggested an unprecedented direct repressor activity of TLX on other NRs and validated the VP16/TLX assay setting as representative and suitable to study TLX modulation.
Using the VP16/TLX reporter gene assay, we have screened a drug fragment library comprising 480 small organic molecules (MW range: 80–300 g/mol) for TLX modulatory activity.14 Therein, we have discovered 1-methylxanthine (1) as a TLX modulator that diminished TLX-mediated transcriptional repression with an IC50 value of 9 ± 3 μM (Table 1). Intrigued by this activity, we studied the structurally related xanthines 2–8 for TLX modulation. 2–4 and 7 were inactive on TLX, whereas theophylline (5), paraxanthine (6), and caffeine (8, Figure 1e,f) counteracted TLX-dependent transcriptional repression as well. Control experiments in the absence of the Gal4-TLX hybrid receptor revealed no unspecific effects of the xanthines, indicating TLX-mediated activity (Figure 1g).
Table 1. Activity of 1–15 on TLX in the VP16/TLX Settinga.

| ID | R1 | R2 | R3 | X–Y | R4 | IC50 (fold TLX repression) | |
|---|---|---|---|---|---|---|---|
| 2 | xanthine | H | H | H | N=C | H | inactive at 100 μM |
| 1 | 1-methylxanthine | Me | H | H | N=C | H | 9 ± 3 μM (2.3 ± 0.3) |
| 3 | 3-methylxanthine | H | Me | H | N=C | H | inactive at 100 μM |
| 4 | 7-methylxanthine | H | H | Me | N=C | H | inactive at 100 μM |
| 5 | theophylline | Me | Me | H | N=C | H | 10 ± 2 μM (2.6 ± 0.1) |
| 6 | paraxanthine | Me | H | Me | N=C | H | 11 ± 2 μM (2.4 ± 0.1) |
| 7 | theobromine | H | Me | Me | N=C | H | inactive at 100 μM |
| 8 | caffeine | Me | Me | Me | N=C | H | 9 ± 2 μM (2.3 ± 0.1) |
| 9 | uric acid | H | H | H | HN–C | =O | inactive at 100 μM |
| 10 | 1-methyluric acid | Me | H | H | HN–C | =O | 25 ± 3 μM (1.7 ± 0.1) |
| 11 | 1,7-dimethyluric acid | Me | H | Me | HN–C | =O | 44 ± 4 μM (3.0 ± 0.2) |
| 12 | 1,3,7-trimethyluric acid | Me | Me | Me | HN–C | =O | 24 ± 3 μM (2.7 ± 0.2) |
| 13 | 8-bromotheophylline | Me | Me | H | N=C | Br | 6 ± 3 μM (1.6 ± 0.1) |
| 14 | 8-chlorotheophylline | Me | Me | H | N=C | Cl | 17 ± 3 μM (2.1 ± 0.2) |
| 15 | 8-phenyltheophylline | Me | Me | H | N=C | Ph | 0.5 ± 0.3 μM (2.4 ± 0.2) |
Compounds were tested to a maximum concentration of 100 μM. Fold repression refers to the maximum fold increase in the reporter activity (compared to 0.1% DMSO) resulting from the inhibition of TLX. Control experiments on Gal4-VP16 in the absence of Gal4-TLX have excluded non-specific effects and confirmed TLX-mediated activity for all active compounds. All data are mean ± SEM, n ≥ 3.
We also probed whether caffeine (8) affected the repressor activity of TLX on other NRs and observed a partial derepression of TLX in the presence of 30 μM caffeine (8, Figure 1h). Such an activity was observed for constitutively active NRs (e.g., Gal4-Nurr1) and for NRs with low intrinsic activity (e.g., Gal4-RARα, Gal4-FXR, and Gal4-RXRα). Of note, we observed no effects of caffeine (8) on the activity of other Gal4-NR hybrid receptors (in absence of Gal4-TLX) in a uniform assay format (Figure S3) except for a weak additive activity with the reference agonist T0901317 on LXRs, providing further evidence that the effects of 8 were TLX-mediated.
As TLX modulators are very rare, the natural product caffeine (8) appeared attractive for structural optimization. The activity data of 1–8 demonstrated alkylation in 1-position as essential. Hence, we studied the biological activity of further derivatized xanthines (Table 1) focusing on the remaining free 8-position. The endogenous metabolite uric acid (9) turned out inactive on TLX while methylated uric acid derivatives 10–12 comprised weak activity. Similar as observed for the xanthines, uric acid derivatives required 1-methyl substitution to exhibit TLX modulation. Substituents in 8-position of xanthines (13–15) had pronounced effects on TLX modulatory activity. 8-Bromotheophylline (13) displayed slightly stronger potency compared to 5 while the 8-chlorine analogue 14 was less active, suggesting a preference for bulky moieties in this region. The extended 8-phenyltheophylline (15) indeed comprised remarkably enhanced, submicromolar activity on TLX, prompting us to study the potential of expanding the 8-phenyl residue with substituents (Table 2).
Table 2. Activity of 15–34 on TLX in the VP16/TLX Settinga.

Compounds were tested to a maximum concentration of 30 μM. Fold repression refers to the maximum fold increase in reporter activity (compared to 0.1% DMSO) resulting from the inhibition of TLX. Control experiments on Gal4-VP16 in the absence of Gal4-TLX have excluded non-specific effects and confirmed TLX-mediated activity for all active compounds. All data are mean ± SEM, n ≥ 3.
Synthesis of analogues 16–29 and 31–34 was accomplished by Suzuki coupling of 8-bromotheophylline with arylboronic acids or by cyclization of the respective 5,6-diamino-1,3-dialkylpyrimidine-2,4(1H,3H)-dione with arylcarboxylates (Supporting Information). We prepared all tolyl (16–18), chlorophenyl (19–21), methoxyphenyl (22–24), and biphenyl (25–27) regioisomers of 15 from which the activity data indicated a common preference for derivatizations of 15 in the meta-position (17, 20, and 23). The chlorine substituent (19–21) was generally less favored in terms of efficacy and the bulkier biphenyl analogues (25–27) exhibited lower potency. meta-Methoxy analogue 23 possessed the most favorable profile regarding the potency and efficacy. We hypothesized that this might be mimicked by furan analogues which simultaneously would promote solubility and thus characterized the furan derivatives 28 and 29 whose 3-furyl isomer 29 exhibited further enhanced effects on cellular TLX activity. The recently approved adenosine A2 (A2A) receptor antagonist istradefylline (30) shares the 8-substituted xanthine scaffold of this TLX ligand chemotype, prompting us to evaluate a potential TLX modulation by 30 and dose–response characterization of 30 in the VP16/TLX assay setting indeed revealed repression of TLX activity by 30, too. Transferring the 3,4-dimethoxystyryl moiety of 30 to the theophylline scaffold in 31 resulted in another strong TLX modulator with enhanced efficacy but with reduced potency compared to 30, suggesting that the bulkier 1- and 3-substituents in 30 contributed to its potency on TLX. Reduction of the styryl residue in 31 to a phenethyl substituent in 32 retained high efficacy but was not favored in terms of the IC50 value.
Eventually, we combined the 1,3-diethyl substitution pattern of the most active TLX ligand 30 with the favored 8-(furan-3-yl)theophylline motif (29) in compound 33, which exhibited equal potency but higher efficacy compared to 30. Further extension of the alkyl substituents to 1,3-dipropyl in 34 enhanced potency but was accompanied by a loss in efficacy compared to the diethyl analogue 33. In summary, our preliminary structure–activity relationship (SAR) evaluation of the xanthine scaffold for TLX modulation revealed modifications in the 8-position and the alkylation pattern as avenues to enhanced activity. In the 8-position, small heterocyclic residues were favored with the 3-furyl substituent as a preferred motif, and their activity tended to increase with larger alkyl substituents in 1- and 3-positions. Our observations on xanthines as TLX modulators present this chemotype as an attractive scaffold for the development of TLX modulators as urgently required tools. Xanthines are well-known adenosine receptor antagonists,23 however, and not selective for TLX which must be considered when using xanthines to study TLX modulation in a cellular context. The early SAR for TLX modulation by xanthines in several parts resembled the SAR of the chemotype as an adenosine receptor antagonist (see Table S1) but also revealed important exceptions: among the 8-phenyltheophylline derivatives 16–24, para-substituents (18, 21, 24) consistently diminished the activity on TLX compared to the meta-analogues (17, 20, 23) while the para-isomers are highly favored over the meta-analogues in terms of adenosine receptor antagonism (Table S1). In line with this, 8-(4-biphenyl)theophylline (27) has been reported as a very potent adenosine receptor antagonist (IC50 0.0035 μM)24 and is more than 1000 times less active as a TLX modulator (IC50 7 μM). Moreover, the ortho- and meta-chlorophenyltheophyllines 19 and 20 modulated TLX with sub-micromolar potencies but have been found inactive as adenosine receptor antagonists.25 Despite not exhibiting full TLX selectivity, 8-(2-chlorophenyl)theophylline (19) thus revealed a preference for TLX, which suggests the compound as a potential early tool for cellular studies on TLX modulation. Overall, the few but pronounced differences in the SAR of xanthines as TLX and adenosine receptor modulators support a direct mechanism by which xanthines modulate TLX. To exclude a potential crosstalk of adenosine receptor signaling and TLX modulation and to provide further evidence for direct TLX modulation by xanthines, we performed additional experiments.
To confirm a direct interaction of xanthines with TLX, we conducted isothermal titration calorimetry (ITC) experiments for key compounds of the series using recombinant TLX LBD protein (Figure 2). As the aqueous solubility of xanthines is low but markedly increases with higher temperature,23 we performed the ITC experiments at 37 °C, which demonstrated direct endothermal binding of caffeine (8), 19, and 33 to the recombinant TLX LBD at 37 °C with affinities aligning with the compounds’ potencies in the reporter gene assay. ITC also indicated the binding of istradefylline (30) to the TLX LBD but the compound’s solubility was too low for full ITC experiments despite an increased temperature. Further evidence for direct TLX modulation by xanthines evolved from 1H NMR-based binding experiments, which revealed chemical shift perturbations of 29, 30, and 33 in presence of the TLX LBD (Figure S5).
Figure 2.
ITC demonstrating the binding of caffeine (8, a), 19 (b), and 33 (c) to the recombinant TLX LBD. The isotherms of the compound–protein titrations, the fitting of the heat of binding, and the isotherms of the compound–buffer (bottom) titrations are shown. The isotherm of the buffer–protein titration is shown in Figure S4.
The VP16/TLX assay and control experiments on Gal4-VP16 alone (Figure 3a) demonstrated that TLX was required for the in vitro activity of xanthines since no effect on Gal4-VP16-induced reporter activity was observed in the absence of Gal4-TLX. Additionally, the xanthines were selective over multiple other NRs in Gal4 format (Figure S3). To probe adenosine receptor involvement in the effects on TLX activity, we evaluated the agonist adenosine in the VP16/TLX assay but observed no effect on reporter expression (Figure 3b). Moreover, adenosine showed no competitive effects with xanthines (Figure 3c) as the dose–response curves for TLX modulation by 8, 19, and 29 in the absence or presence of adenosine were congruent. In the case of adenosine receptor-mediated TLX modulation, the adenosine receptor agonist would be expected to compete with the xanthines and cause a shift of the dose–response curves. Altered cAMP levels as a key cellular effect of adenosine receptor modulation might also exhibit unspecific effects on reporter activity, but exogenous cAMP (1–100 μM) had no effect on VP16/TLX, too (Figure 3d). Hence, despite not excluding overlapping pathways, these data indicate no major involvement of adenosine receptor modulation in the observed effects of xanthines on TLX.
Figure 3.
Xanthines act as direct TLX modulators. (a) Control experiments on Gal4-VP16 without Gal4-TLX confirmed the TLX-mediated activity of 19, 29, 30, and 33. Boxplots show min–max fold reporter activation compared to 0.1% DMSO in the respective setting; n ≥ 4; ***p < 0.001 (t-test). (b) Activity of adenosine in the VP16/TLX assay. Key compounds 8, 19, 29, 30, and 33 for comparison. Adenosine had no effect on TLX activity up to 100 μM. Data are mean ± SEM fold TLX repression vs 0.1% DMSO; n ≥ 3; **p < 0.01, ***p < 0.001 (t-test vs DMSO). (c) Adenosine did not alter the effects of xanthines on TLX activity. Dose–response curves of 8, 19, and 29 in the VP16/TLX assay in the absence (yellow curves) and presence (blue curves) of 3 μM adenosine. Data are mean ± SEM fold TLX repression vs 0.1% DMSO; n ≥ 3. (d) Exogenous cAMP (up to 100 μM) had no effect in the VP16/TLX assay. 8 is shown for comparison. Data are mean ± SEM fold TLX repression vs 0.1% DMSO; n ≥ 3.
Based on their confirmed direct interaction with the TLX LBD, their effects on cellular TLX activity, and the control experiments demonstrating TLX but not adenosine receptor-mediated effects, xanthines appeared suitable as early tools for further studies on TLX modulation by ligands. In an attempt to define key residues involved in TLX modulation by xanthines as a basis for future TLX ligand discovery, we performed a preliminary mutagenesis study. From the only available TLX X-ray apo-structure 4XAJ,26 we hypothesized a potential binding region inside the TLX LBD clamped between residues A189, F226, I230, and L268 (Figure 4a). We used site-directed mutagenesis to mutate these residues in the Gal4-TLX construct and employed the resulting mutants in the VP16/TLX reporter gene assay setting with equal conditions as used for wt-TLX to evaluate the impact of the mutations on TLX activity (Figure 4b) and on TLX modulation by xanthines (Figure 4c).
Figure 4.
Mutagenesis experiments suggested potential residues involved in TLX modulation by xanthines. (a) TLX LBD (apo, PDB-ID: 4XAJ(26)) with mutated residues in red, helix 5 in yellow, and bound co-regulator in blue. (b) Activity of wt-TLX and mutants in the VP16/TLX assay. TLXA189E and TLXF226W/I230E acted as transcriptional repressors. Data are mean ± SEM; n = 3. (c) Dose–response curves of caffeine (8) and xanthine derivatives 19, 29, and 30 on wt-TLX (gray) and on the mutants TLXA189E (red) and TLXF226W/I230E (blue). Xanthines exhibited a higher activity on TLXF226W/I230E than on wt-TLX, while the A189E mutation had no effect on the activity of xanthines. Fold repression refers to the maximum fold increase in the reporter activity compared to 0.1% DMSO in the respective setting. Data are mean ± SEM; n ≥ 3. Table shows the mean ± SEM IC50 values and the max repression efficacy of 8, 19, 29, and 30 on wt-TLX and the mutants TLXA189E and TLXF226W/I230E.
TLXA189E retained the repressor activity of wt-TLX while TLXL268R turned out as almost inactive in terms of repressing VP16-induced reporter expression (Figure 4b). Combination of both mutations for a potential intramolecular salt bridge in TLXA189E/L268R led to inactivity, too. Mutagenesis of F226 and I230 in TLXF226W/I230E produced another functional mutant while the triple mutant TLXA189E/F226W/I230E was only a weak transcriptional repressor. These observations indicated that the mutations L268R, A189E/L268R, and A189E/F226W/I230E compromised the expression, stability, or activity of apo-TLX and were not suitable for further evaluation. The activity of mutants TLXA189E and TLXF226W/I230E, in contrast, resembled wt-TLX in the VP16/TLX assay, suggesting them as functional. TLXA189E and TLXF226W/I230E were hence employed to probe the effects of mutations on TLX modulation by xanthines. Dose–response profiling of caffeine (8) on the mutants in the VP16/TLX assay setting (Figure 4c) revealed equal activity on TLXA189E as on wt-TLX but almost 10-fold enhanced potency on TLXF226W/I230E. Consistently, the dose–response curves of the more potent xanthine derivatives 19, 29, and 30 on TLXA189E aligned with the effects on wt-TLX while all three compounds were more active on TLXF226W/I230E (Figure 4c). Despite not revealing the precise binding site of xanthines on TLX, these results provide further evidence for direct modulation of the TLX LBD by xanthines and suggest that residues in helix 5 of the TLX LBD are involved in TLX modulation by this ligand type.
Next, we probed the response of the recombinant TLX LBD on ligand binding in terms of co-regulator recruitment and dimerization to elucidate the potential mechanisms by which ligands modulate TLX activity. We employed various HTRF-based settings using Tb3+-cryptate or GFP-labeled NR LBDs and Tb3+-cryptate or fluorescein-labeled co-regulator peptides. An association of the TLX LBD with atrophin has been described previously prompting us to determine ligand effects on the affinity between TLX and the atrobox peptide.26 The TLX LBD robustly and specifically recruited the atrobox peptide,26 an interaction incompetent mutant27 was not bound (Figure S6a). The TLX–atrobox interaction was, however, not modulated by caffeine (8), 29, istradefylline (30) or the previously reported TLX ligand ccrp212 (Figure S6a). Hence, we hypothesized that other NR co-regulators might involve in TLX regulation and in the receptor’s response to ligands. We screened 29 canonical co-regulator interaction motifs (fluorescein-labeled) for recruitment to the TLX LBD (Tb3+-cryptate-labeled) in the apo-state or in the presence of xanthine-type ligands (Figure 5a). In this initial screening, we detected no ligand-sensitive co-regulator interaction of the TLX LBD for the selected xanthines 8, 19, 30, and 33. However, particularly high HTRF for apo-TLX in the presence of the SMRT (also termed NCoR2) indicated a potential interaction between SMRT and the TLX LBD (Figure 5b) aligning with the repressor role of TLX. Titration of SMRT with the TLX LBD in an optimized setting (SMRT: Tb3+-cryptate-labeled and TLX–LBD: GFP-labeled) confirmed this assumption, indicating similar or even higher affinity compared to the atrobox peptide (Figure 5c). We also detected a response of SMRT to ligand binding in this optimized HTRF setting for the TLX modulator 30 but not for ccrp212 (Figure 5d). However, the potency of 30 for SMRT displacement (IC50 10 μM) was considerably weaker compared to its cellular effects, suggesting that TLX modulation by xanthines is only partly mediated via the TLX–SMRT interaction and that further mechanisms are involved. Hence, based on the repressor activity of TLX, we also probed an interaction with the NR co-repressor 1 (NCoR1) in an optimized HTRF assay which was also bound to the TLX–LBD but showed no response to ligands (Figure S6b).
Figure 5.
Molecular contributions to TLX activity and modulation by ligands. (a) Screening of 29 canonical NR co-regulator peptides (fluorescein-labeled) for ligand-sensitive interactions with the TLX LBD (Tb3+-cryptate-labeled) revealed no effects of xanthines 8 (100 μM), 19 (10 μM), 30 (10 μM), or 33 (10 μM). Data are mean ± SD; N = 4. (b) The co-regulator recruitment screen on the apo-TLX LBD showed a high HTRF for SMRT, suggesting a potential binding. Data are mean ± SD; N = 4. (c) Titration of Tb3+-cryptate-labeled SMRT with the GFP-labeled TLX LBD confirmed a TLX–SMRT interaction. Data are mean ± SD; N = 3. (d) The TLX–SMRT interaction was responsive to 30 but not to ccrp2. Data are mean ± SD; N = 3. (e) TLX–RXR heterodimerization was responsive to 19, 29, and 30 and to ccrp2 and 8 with a weaker effect. All compounds were used at 10 μM. Data are mean ± SD; N = 3. (f) Gal4-TLX recruited a VP16-RXRα-LBD fusion protein as observed by a dose-dependent increase in the reporter activity, demonstrating TLX–RXR heterodimerization also in the cellular context. Data are mean ± SEM RLUs; n = 3. (g) The TLX–RXR interaction was responsive to 30 also in the cellular setting. Data are mean ± SEM reporter activity vs DMSO; n = 6. ***p < 0.001 (t-test vs DMSO). (h) The TLX LBD strongly homodimerized. Tb3+-cryptate-labeled TLX LBD titrated with GFP-labeled TLX LBD. Xanthine-based TLX modulators 8, 19, 29, and 30 as well as the reference ligand ccrp2 markedly enhanced the TLX homodimerization. Data are mean ± SD; N = 3. (i) The Xanthine-based TLX modulators 29 and 33 enhanced the expression of the TLX-regulated genes SIRT1, p21, and SLC1a1. The reference TLX ligand ccrp2 showed a trend to induce SIRT and SLC1a1. Data are mean ± SEM relative mRNA expression; GAPDH was used as reference gene; n = 4; #p < 0.1, *p < 0.05 vs DMSO (one-way ANOVA).
As we have detected strong dimerization of TLX with RXR (Figure 1d), we hypothesized this heterodimerization of TLX as a potential further mediator of ligand effects. When we titrated Tb3+-cryptate-labeled TLX LBD with the GFP-labeled RXRα LBD in the presence of TLX ligands, we observed indeed enhanced dimerization for xanthines (8, 19, 29, 30) and ccrp212 (Figure 5e). 19 and 30 (10 μM each) had the strongest effect and increased the dimerization affinity of the TLX LBD by approx. a factor of 5. The heterodimer stabilizing effects of caffeine (8) and ccrp212 were weaker (factor 2.5). In an attempt to confirm the relevance of this TLX–RXR interaction in cellular setting, we established another reporter gene assay using Gal4-TLX, a VP16-RXRα-LBD fusion construct lacking a DNA binding domain, Gal4-responsive firefly luciferase, and constitutive renilla luciferase. In the absence of Gal4-TLX, VP16-RXRα failed to induce reporter transcription but upon addition of Gal4-TLX, the reporter activity increased with increasing Gal4-TLX doses (Figure 5f), demonstrating that the interaction between the TLX and RXRα LBDs is relevant in cells. Moreover, addition of 30 increased the TLX–RXRα interaction as observed by elevated reporter activity (Figure 5g). These findings align with the observation of a direct TLX repressor activity on other NRs (Figure 1b,c) and support direct and ligand-sensitive LBD interactions involving in these effects. In addition to heterodimer formation, the activity of many NRs also involves homodimerization. Hence, we analyzed this type of oligomerization for TLX and indeed observed robust homodimer formation with slightly weaker affinity compared to the TLX–RXR dimer (Figure 5h). Remarkably, all evaluated xanthine-based TLX modulators (8, 19, 29, 30) as well as the reference ligand ccrp212 (10 μM each) strongly stabilized the TLX homodimer by up to a factor of 4. The molecular effects of xanthines on TLX homo- and heterodimerization may crucially alter the monomer–oligomer equilibria of TLX and the levels of free TLX which might importantly trigger TLX activity. The effects of xanthines on TLX dimerization also aligned with the ITC results showing unfavorable binding enthalpy that may result from major ligand-induced conformational changes required for dimerization.
To observe potential cellular effects of TLX modulation by xanthines, we analyzed the changes in TLX-regulated gene expression upon treatment of human glioblastoma cells (T98G) with xanthines. It should be noted, however, that in contrast to the cell-free experiments and the artificial reporter gene assays, experiments on TLX modulation by xanthines in native cells may be compromised by the lack of selectivity over adenosine receptors. Owing to the lack of a fully TLX-selective xanthine, we employed 29 and 33 for cellular experiments, which exhibited potent and efficient TLX repression in the VP16/TLX assay (Table 2). The previously reported TLX ligand ccrp212 served as a reference. Quantitative real-time PCR analysis (Figure 5i) demonstrated enhanced mRNA expression of NAD+-dependent deacetylase sirtuin-1 (SIRT1), cyclin-dependent kinase inhibitor 1 (p21), and solute carrier family 1 member 1 (SLC1a1) upon treatment with 29 (10 μM) or 33 (1 μM), all of which are known as TLX regulated.18,28−33 Importantly, the expression level of TLX (Figure S7) was not affected, supporting that direct effects of 29 and 33 altered TLX-regulated gene expression. The reference TLX ligand ccrp212 also revealed a tendency to induce SIRT1 and SLC1a1 but failed to modulate p21 expression, illustrating that further studies with selective tools are needed to obtain consistent understanding of TLX ligand effects on gene expression.
The orphan NR TLX is exclusively expressed in certain areas of the CNS and attracts attention for its potential as a therapeutic target in neurodegeneration, neurological disorders, and brain tumors.34 However, studies on TLX biology beyond knockout experiments are hindered by the lack of suitable TLX modulators as tools. Moreover, molecular mechanisms underlying TLX activity and modulation remain elusive which complicates the search for TLX ligands. To overcome these obstacles in characterization and target validation of TLX, we have established a robust screening system for TLX ligands, identified xanthines as TLX modulators, and employed them as tools for early functional studies.
Combining the transcriptional repressor Gal4-TLX with the potent ligand-independent transcriptional activator Gal4-VP16 revealed an ability of Gal4-TLX to counter Gal4-VP16-induced reporter gene activity in a dose-dependent fashion which allowed tuning of the test setup to observe bidirectional TLX modulation. Despite its artificial character, this cellular test system enabled the discovery and preliminary characterization of TLX ligands and might also be transferable to other repressive NRs such as the testicular receptors (TR). Importantly, Gal4-TLX also repressed Gal4-hybrids of human NRs, supporting suitability of the VP16/TLX setting for screening purposes. In addition, this observation unprecedentedly indicated that TLX acted as a direct repressor toward various NRs in vitro, which was further confirmed by an observed dimerization of the TLX and RXR LBDs in cell-free setting.
Using the VP16/TLX assay, we discovered xanthines including caffeine as TLX modulators that counteracted the repressor activity of TLX. Caffeine revealed an IC50 value of 9 μM and also reversed TLX-mediated repression of several human NRs. Systematic SAR elucidation of xanthines as TLX ligands revealed 8-aryl substituents as favored and led to the discovery of xanthine-based TLX modulators exceeding the activity of caffeine. Several cellular control experiments, ITC, NMR-based binding studies, and cell-free HTRF assays demonstrated direct TLX modulation by the xanthines but also indicated that additional mechanisms might be involved in the observed cellular effects. The majority of the TLX-modulating xanthines also exhibit adenosine receptor antagonism,23 and further studies will hence be needed to elucidate potentially overlapping mechanisms of adenosine receptor and TLX signaling as well as the individual contributions of both activities to the biological effects of xanthines. 8-(2-Chlorophenyl)theophylline (19) evolved as an important exception as it is bound to the TLX LBD and counteracted TLX activity but is no adenosine receptor antagonist.2519 may therefore be considered as most suitable xanthine to be used as an early tool for functional studies on TLX.
We have employed the xanthine-based TLX ligands to evaluate the molecular mechanisms of TLX activity and modulation. In a preliminary mutagenesis study, we observed enhanced potency of various xanthines on a TLXF226W/I230E mutant, supporting the assumption that the residues Phe226 and Ile230 located in helix 5 in the core region of the TLX LBD are involved in TLX modulation by xanthines. Moreover, consistent with previous studies,26 we observed a recruitment of the atrobox peptide to the TLX LBD, yet this interaction was not affected by xanthines. Hence, we screened a collection of canonical NR co-regulators and discovered the co-repressors NCoR1 and SMRT as further interaction partners of TLX. In contrast to TLX–NCoR1, the TLX–SMRT interaction was sensitive to the xanthine 30, suggesting its potential involvement in TLX modulation despite the weak potency of 30. As another contribution, we detected effects of xanthines on the homo- and heterodimerization of TLX, suggesting that ligand effects on the monomer–oligomer equilibria may evolve as a key mechanism of TLX modulation. In an orthogonal cellular system, we have observed that TLX–RXR dimerization also occurs in a cellular context. From these observations, we conclude that TLX modulation by xanthines likely results from several contributing effects on protein–protein interactions involving dimerization and co-regulator binding. With only a limited collection of potential interactors tested, we also hypothesize that further factors participate in this network. The presence of several contributing molecular effects may also provide an explanation for lower potency of the xanthines in the cell-free assays compared to the cellular context. Each of the HTRF-based systems used to study TLX modulation observed a single factor of a complex monomer–oligomer and co-regulator recruitment equilibrium. The individual contributions to TLX modulation by ligands likely affect each other, and, for example, ligand-induced dimerization might also enhance co-regulator binding.35 Higher potency in cellular settings may thus reflect the sum of weaker contributions on a molecular level.
Together with the few available TLX ligands,12−15 the xanthines will be a valuable tool to study the mechanisms of TLX modulation in cell-free systems and may also be useful as an early tool for experiments in a cellular context—particularly the TLX preferential xanthine 19. In addition, TLX modulation by xanthines may have pharmacological relevance. Several lines of evidence point to pharmacological effects of caffeine (8) in reducing the risk for Parkinson’s disease and Alzheimer’s disease.23 Moreover, the xanthine derivative istradefylline (30) holds approval for Parkinson’s disease treatment and its use in an animal model of Alzheimer’s disease improved memory and spatial learning.36 These observations align with the role of TLX as an essential regulator of NSC homeostasis, neurogenesis, and learning.3,6−8,33 Hence, our findings support the hypothesis that TLX modulation in addition to adenosine receptor antagonism may contribute to the pharmacological effects of xanthines in neurodegenerative diseases.
Experimental Section
Reporter Gene Assays
Plasmid Constructs
pFR-Luc was used as a reporter plasmid (Stratagene, San Diego, CA, U.S.A). pRL-SV40 (Promega, Fitchburg, WI, U.S.A.) was used for the normalization of cell growth and transfection efficiency and to observe test compound toxicity. pECE-SV40-Gal4-VP16 was a gift from Lea Sistonen (Addgene plasmid # 71728; http://n2t.net/addgene:71728; RRID:Addgene_71728; Addgene, Watertown, MA, U.S.A.) and was used as a transcriptional inducer of reporter gene expression. Gal4 hybrid clones of human NRs were obtained by inserting the respective protein sequence including the hinge region into pFA-CMV (Agilent Technologies). The Gal4-DBD fusion plasmid with TLX (NR2E1; UniProt entry: Q9Y466-1; residues 150–385) was constructed by integrating a cDNA fragment obtained from PCR amplification using the natural cDNA (TLX BC028031.1, purchased as I.M.A.G.E. cDNA clone #5242079 from Source BioScience, Nottingham, UK) as a template between the BamHI cleavage site of the pFA-CMV vector (Stratagene) and an afore inserted KpnI cleavage site. Variants with the mutated TLX LBD were generated by site-directed mutagenesis using Q5 high fidelity polymerase (New England Biolabs). The respective codons were changed according to the optimal codon usage in human cells. All plasmids were verified by sequencing of the entire Gal4-TLX open reading frame. Generation of pFA-CMV-hCAR-LBD,37 pFA-CMV-hFXR-LBD,38 pFA-CMV-hLXRα-LBD,38 pFA-CMV-hLXRβ-LBD,38 pFA-CMV-hPPARα-LBD,39 pFA-CMV-hPPARγ-LBD,39 pFA-CMV-hPPARδ-LBD,39 pFA-CMV-hRARα-LBD,37 pFA-CMV-hRARβ-LBD,37 pFA-CMV-hRARγ-LBD,37 pFA-CMV-hRXRα-LBD,37 pFA-CMV-hRXRβ-LBD,37 pFA-CMV-hRXRγ-LBD,37 pFA-CMV-hVDR-LBD,37 pFA-CMV-hNur77-LBD,40 pFA-CMV-hNurr1-LBD,40 and pFA-CMV-hNOR1-LBD40 has been described previously. To study the LBD heterodimer formation of TLX and RXRα in the cellular context, an RXRα construct was needed which itself does not bind the Gal4 response element but is capable of recruiting the transcription machinery. In the original plasmid pFA-CMV, the section encoding the Gal4-DBD (that starts with the eighth codon of the CMV-controlled ORF) was replaced by a DNA sequence coding for VP16 (α-trans-inducing factor; UniProt P06492; aa 413–490), followed by a Gly-Ser linker. The resulting plasmid was termed pFTI-CMV (fusion trans-inducing factor plasmid). The native cDNA sequence for human RXRα (aa 225–462) was inserted into this plasmid by the Gibson assembly between the restriction sites for BamHI and XbaI within the original multiple cloning site, resulting in pFTI-CMV-VP16-RXRα-LBD. Expression of the fusion protein MDYKDDVAST-[VP16 (aa 413–490)]-SSGGGGSSGGS-[RXRα LBD (aa 225–262)] is under the control of the CMV promoter.
Gal4-TLX/Gal4-VP16 Assay Procedure
HEK293T cells were grown in Dulbecco’s modified Eagle’s medium (DMEM, Thermo Fisher Scientific), high glucose with 10% fetal calf serum (FCS), sodium pyruvate (1 mM), penicillin (100 U/mL), and streptomycin (100 μg/mL) at 37 °C and 5% CO2. 24 h before transfection, cells were seeded in 96-well plates (30,000 cells/well) in DMEM with the above-mentioned supplements. Prior to transfection, medium was changed to Opti-MEM (Thermo Fisher Scientific) without supplements. Cells were then transiently transfected with plasmid mixtures containing 100 ng/well pFR-Luc, 1 ng/well pRL-SV40, 6 ng/well of pECE-SV40-Gal4-VP16, and 3 ng/well of pFA-CMV-Gal4-TLX (during the assay development, these plasmid amounts per well were systematically varied to optimize conditions allowing robust observation of bidirectional TLX modulation). Transient transfection was achieved using a Lipofectamine LTX reagent (Thermo Fisher Scientific) according to the manufacturer’s protocol. Five hours after transfection, cells were treated with Opti-MEM supplemented with penicillin (100 U/mL) and streptomycin (100 μg/mL) additionally containing 0.1% DMSO and the respective test compounds or 0.1% DMSO alone as a negative control. Each sample was tested in duplicates, and every experiment was conducted at least three times. After 14 h incubation, cells were lysed for luciferase luminescence detection using the Dual-Glo Luciferase Assay System (Promega) according to the manufacturer’s protocol. Luminescence was measured with an Tecan Spark luminometer (Tecan Deutschland GmbH). To consider transfection efficiency and cell growth, the obtained firefly luciferase signal was normalized by dividing firefly luciferase signals by renilla luciferase signals and multiplying by a factor of 1000 to obtain RLUs. Fold reporter activation or repression was obtained by dividing the mean RLU of a test compound at a respective concentration by the mean RLU of the 0.1% DMSO control. IC50 values were obtained by plotting fold reporter activation versus test compound concentrations and fitting the resulting sigmoidal curve with a four parameter logistic regression in SigmaPlot 12.5. Separate control experiments were performed following the same procedure with the exception that cells were only transfected with 100 ng/well pFR-Luc, 1 ng/well pRL-SV40, and 6 ng/well pECE-SV40-Gal4-VP16 to exclude unspecific cellular or VP16-mediated effects. Experiments with the TLX mutants were performed equally using the respective mutant clones pFA-CMV-Gal4-TLXA189E, pFA-CMV-Gal4-TLXL268R, pFA-CMV-Gal4-TLXA189E/L268R, pFA-CMV-Gal4-TLXF226W/I230E, or pFA-CMV-Gal4-TLXA189E/F226W/I230E instead of wild-type pFA-CMV-Gal4-TLX.
Gal4-TLX/Gal4-NR Assay Procedures
Interaction of Gal4-TLX with diverse human NRs in Gal4-format was studied in identical procedures to the Gal4-TLX/Gal4-VP16 experiments with varying concentrations of pFA-CMV-Gal4-TLX (0, 0.5, 1, 3, 6, 12 ng/well) and the respective Gal4-NR clones (fixed concentration). Amounts of the reporter (pFR-Luc, 100 ng/well) and control (pRL-SV40, 2 ng/well) were fixed. NRs with low intrinsic activity were activated by using reference agonists at 1 μM. Plasmid amounts and reference agonists were as follows: Gal4-CAR (25 ng/well, CITCO), Gal4-FXR (25 ng/well, GW4064), Gal4-LXRα (50 ng/well, T0901317), Gal4-LXRβ (50 ng/well, T0901317), Gal4-Nurr1 (6 ng/well), Gal4-PPARα (25 ng/well, GW7647), Gal4-PPARγ (25 ng/well, pioglitazone), Gal4-PPARδ (25 ng/well, L165041), Gal4-RARα (12 ng/well, tretinoin), Gal4-RARβ (12 ng/well, tretinoin), Gal4-RARγ (12 ng/well, tretinoin), Gal4-RORα (6 ng/well), Gal4-RXRα (12 ng/well, bexarotene), Gal4-RXRβ (12 ng/well, bexarotene), Gal4-RXRγ (12 ng/well, bexarotene), and Gal4-VDR (25 ng/well, calcitriol).
Gal4-TLX/VP16-RXRα Assay Procedure
Interaction of Gal4-TLX with VP16-RXRα was studied according to the Gal4-TLX/Gal4-VP16 experimental procedure with co-transfection of varying amounts of pFTI-CMV-VP16-RXRα (0, 0.5, 1, 3, and 6 ng/well). 3 or 12 ng/well Gal4-TLX, 100 ng/well pFR-Luc, and 1 ng/well pRL-SV40 were used.
Quantification of TLX and TLX-Regulated Gene Expression in Human Glioblastoma Cells
T98G cells (European Collection of Authenticated Cell Cultures) were cultured in DMEM (Thermo Fisher Scientific), high glucose supplemented with 10% FCS, sodium pyruvate (1 mM), penicillin (100 U/mL), and streptomycin (100 μg/mL) at 37 °C and 5% CO2. For the experiments, cells were seeded in six-well plates (1 × 106 cells/well). After 24 h, medium was changed to Minimal Essential Medium (MEM) supplemented with 1% charcoal-stripped FCS, penicillin (100 U/mL), streptomycin (100 μg/mL), and 2 mM l-glutamine. 24 h later T98G were incubated with ccrp212 (10 μM), 29 (10 μM) or 33 (1 μM) dissolved in the same medium additionally containing 0.1% DMSO or with 0.1% DMSO as untreated control for 8 h. Cells were then harvested, washed with phosphate-buffered saline, and used directly for mRNA extraction by the E.Z.N.A. Total RNA kit I (R6834-02, Omega Bio-Tek, Inc., Norcross, GA, USA). Extracted mRNA was reverse-transcribed into cDNA using a high-capacity RNA-to-cDNA kit (4387406, Thermo Fischer Scientific, Inc.). TLX target gene expression was analyzed by quantitative real-time PCR with a StepOnePlus System (Life Technologies, Carlsbad, CA, USA) using Power SYBR Green (Life Technologies). Each sample was analyzed in duplicates and repeated in at least eight independent experiments. Data were analyzed by the comparative ΔΔCT method with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a reference gene. The following primers were used for T98G cells (human genes): GAPDH:41 forward 5′—CCT GTT CGA CAG TCA GCC G—3′, reverse 5′—CGA CCA AAT CCG TTG ACT CC—3′ p21 (Origene, Rockville, MD, U.S.A.): forward 5′—AGG TGG ACC TGG AGA CTC TCA G—3′, reverse 5′—TCC TCT TGG AGA TCA GCC G—3′; SIRT1:28 forward 5′—GAA CCT TTG CCT CAT CTA CA—3′, reverse 5′—AGC CGC TTA CTA ATC TGC TC—3′; SLC1a1:13 forward 5′—CGA AAG AAC CCT TTC CGA TTT GC—3′, reverse 5′—GAA GGT GAC AGG CAG TGT TGC T—3′; TLX: forward 5′—CTA AGA GTG TGC CAG CCT TC—3′, reverse 5′—TGT TAG CAT CAA CCG GAA TGG—3′.
Protein Expression
TLX Protein Expression
The recombinant TLX LBD with an N-terminal His6-tag was expressed in Escherichia coli Rosetta. Cells were initially cultured in the TB medium at 37 °C to an OD600 of 2.8 prior to induction with 0.5 mM IPTG at 18 °C overnight. Cells were harvested and resuspended in a buffer containing 50 mM N-(2-hydroxyethyl)piperazine-N′-ethanesulfonic acid (HEPES) pH 7.5, 500 mM NaCl, 20 mM imidazole, 5% glycerol, and 1 mM TCEP and lysed by sonication. The recombinant TLX LBD was initially purified by Ni2+ affinity chromatography. The histidine tag was removed by TEV protease treatment, and the cleaved protein was separated by reverse Ni2+ affinity purification. The protein was further purified by size exclusion chromatography and stored in 20 mM Tris, pH 7.5, 150 mM NaCl, 0.2 mM TCEP and 5% glycerol.
Expression and Purification of Recombinant RXRα and TLX Fusion Proteins
The coding sequence for the RXRα LBD (UniProt entry: P19793-1, residues 226–462) and TLX LBD (UniProt entry: Q9Y466-1, residues 150–385) was codon optimized for E. coli and purchased from Geneart (Regensburg, Germany), respectively. For expression of the RXRα fusion protein with an N-terminal GFP, an expression construct based on pET29b was prepared. For this, the entire section between the original NdeI site and the fourth position following the His-Tag coding sequence of pET29b was replaced, hence essentially leaving only the vector backbone unmodified. The section was replaced by a sequence encoding a restriction site for NcoI (overlapping with the start codon) and an open reading frame for Met-Gly-[His10-Tag]-Asp-Tyr-Asp-Ile-Pro-Thr-Thr-[TEV site]-GFP,42 followed by restriction sites for BamHI (in frame) and XhoI. The sequence coding for the RXRα LBD followed by a stop codon was then introduced in frame between the afore-inserted restriction sites for BamHI and XhoI. For expression of the TLX LBD with an N-terminal GFP, the sequence encoding TLX residues 150–385 was cloned between the same sites. For generation of the biotinylated TLX LBD, the pMal vector system (New England Biolabs, NEB, Ipswich, MA, USA) was used. In pMal-c2E, the section between the sequence encoding 10× asparagine (Asn10) and the SalI restriction site was replaced with a sequence encoding Leu-Gly-Ile-Glu-Leu-Val-[His8-Tag]-Asp-Tyr-Asp-Ile-Pro-Gly-Thr-Leu-[TEV site], followed by an Avi-Tag and restriction sites for BamHI and XhoI. The sequence encoding TLX followed by two stop codons was cloned in a frame between these restriction sites. From this construct, a fusion protein is expressed with an N-terminal maltose-binding protein (MBP), followed by an Asn10 linker, a His8-Tag, a cleavage site for TEV protease, an Avi-Tag, and the TLX LBD with unmodified C-terminus. For expression, E. coli T7 expressing cells (NEB) were co-transformed with pGro7 (TAKARA Bio Inc., Kusatsu, Japan) and the aforementioned expression construct and selected overnight at 37 °C on LB (Luria Broth) agar containing 34 μg/mL chloramphenicol and either 100 μg/mL ampicillin (for pMal) or 35 μg/mL kanamycin (for pET). Culture in liquid LB was inoculated and grown at 37 °C with constant shaking at 180 rpm until optical density at 600 nm (OD600) reached 0.7. At this time point, expression of the chaperone GroEL/ES from pGro7 was induced with 1 g/L l(+)-arabinose, and the temperature was reduced to 20 °C. At OD600 = 1, the expression of the target protein was induced by the addition of 0.5 mM IPTG. After 12–16 h, cells were harvested by centrifugation and resuspended in buffer A (400 mM NaCl, 20 mM NaPi pH 7, 8, 10% (w/v) glycerol and 20 mM ß-mercaptoethanol). Cells were kept on ice and disrupted in the presence of 1 mM ATP, DNAse I, RNAse A, 20 mM MgSO4, and EDTA-free cOmplete protease inhibitor cocktail (F. Hoffmann-La Roche AG, Basel, Switzerland) by the addition of lysozyme and 10 passages through an Invensys APV-1000 homogenizer (APV Systems, Silkeborg, Denmark). Cell debris was removed by centrifugation at 16,500g for 20 min at 4 °C.
Purification was achieved by immobilized metal affinity chromatography (IMAC) using columns packed with Ni Sepharose 6 Fast Flow resin on an ÄKTApurifier FPLC system (GE Healthcare, Chicago, IL, USA). After washing with buffer supplemented with 50 mM imidazole, the protein was eluted with 300 mM imidazole. Afterward, GFP fusion proteins were processed with His tagged TEV protease overnight while imidazole content was reduced to 10 mM by dialysis against buffer A in order to allow for reverse IMAC. The flow through was concentrated and applied to size exclusion chromatography using a 16/60 Superdex200 column equilibrated and run in HTRF assay buffer [25 mM HEPES pH 7.5, 150 mM KF, 10% (w/v) glycerol, 5 mM dithiothreitol (DTT)]. Following the initial IMAC purification step, the MBP fusion protein for the generation of the biotin-labeled TLX LBD was processed with MBP-tagged TEV protease during overnight dialysis against buffer A. Afterward, uncleaved fusion protein, free MBP-Tag, and TEV protease were removed by passaging through a gravity flow column packed with amylose high flow resin (NEB). The flow through was then supplemented with 0.5 mM biotin, 0.5 mM ATP, 5 mM MgCl2, and E. coli biotin ligase birA at a molar ratio of approx. 1:10 for enzymatic conjugation of biotin to the lysine residue in the avitag. After overnight incubation at 4 °C, the solution was subjected to a column packed with 5 ml monomeric avidin UltraLink resin (Pierce Biotechnology Inc., Rockford, IL, USA). Unlabeled protein and birA were removed by washing for 10 column volumes with buffer A before the biotin-labeled TLX LBD was eluted using buffer A supplemented with 2 mM biotin. The product was then concentrated and subjected to size exclusion chromatography using a 10/30 Superdex75 column equilibrated and run with the HTRF assay buffer.
Isothermal Titration Calorimetry
ITC was conducted on an Affinity ITC system (TA Instruments, New Castle, DE, USA). Experiments were performed at 37 °C, and the stirring rate was set to 75 rpm. 10–40 μM of TLX–LBD protein in buffer containing 1% DMSO (20 mM Tris pH 7.5, 150 mM NaCl, 0.2 mM TCEP, 5% glycerol) was titrated with the compounds 8, 19, and 33 (60–100 μM in same buffer containing 1% DMSO, 31 injections: 1 × 1 μL and 30 × 3 μL). The injection interval was set to 300 s. As control experiments, the compounds (at the same concentrations) were titrated into buffer and buffer was titrated to the TLX–LBD protein with otherwise identical conditions. The heat rates of the compound–TLX titrations were analyzed using an independent binding model by NanoAnalyze Software (TA Instruments, New Castle, DE, USA.).
NMR Spectroscopy
Spectra acquisition was carried out on a Bruker 600 MHz AVIIIHD spectrometer equipped with a 5 mm nitrogen-cooled triple resonance probe 1H/19F [13C,15N]-TCI (Prodigy) and high throughput sample changer (SampleJet) for 579 samples with temperature option for sample storage. All spectra were acquired and processed using Bruker software Topspin 3.6.2 and Topspin 4.0.9, respectively. For the TLX–ligand interaction studies, two samples (with and without protein) were prepared. 1H-1D and water-suppressed proton 1D (zgesgppe,43 water suppression using excitation sculpting with gradients using perfect echo) were acquired for each of the sample. Interaction studies were performed at a ratio of 1:1 with respect to TLX and the ligand. The final [protein]–ligand concentration was 50 μM. A sample volume of 200 μL in buffer (see ITC) with 5% D2O (NMR lock solvent) and 100 μM of TMSP-Na as an NMR internal reference (chemical shift reference; DMSO-d6 in the case of 33) was prepared and transferred to a 3 mm NMR tube for measurements. The samples were stored at 4 °C and measured at 298 K.
HTRF Assays
Co-Regulator Preference Screen
Recruitment of co-regulator peptides to the recombinant TLX–LBD was studied in a HTRF assay system. Terbium cryptate as a streptavidin conjugate (Tb–SA; Cisbio assays, France) was coupled to biotinylated recombinant TLX–LBD protein and served as a FRET donor. Fluorescein-labeled co-regulator peptides as FRET acceptors were purchased (Thermo Fisher Scientific). Assay solutions were prepared in HEPES buffer [25 mM HEPES pH 7.5, 150 mM KF, 10% (w/v) glycerol, 5 mM DTT, 0.1% (w/v) 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS)] and contained recombinant biotinylated TLX–LBD (3 nM), Tb–SA (3 nM), and the respective fluorescein-labeled co-regulator peptide (100 nM) as well as 1% DMSO and the test compound or DMSO alone as negative control. After 2 h incubation at RT, fluorescence intensities (FI) after excitation at 340 nm were recorded at 520 nm for fluorescein acceptor fluorescence and 620 nm for Tb–SA donor fluorescence on a Tecan SPARK luminometer (Tecan Group Ltd.). FI520nm was divided by FI620nm and multiplied with 10,000 to give a dimensionless HTRF signal.
Co-Regulator Affinity Assay
The strength and modulation of affinity for individual co-regulators were investigated by titration of the GFP-TLX LBD against a FRET donor coupled co-regulator peptide. Assay solutions were prepared in a HTRF assay buffer supplemented with 0.1% (w/v) CHAPS as well as 1% DMSO with test compounds at 10 μM or DMSO alone as a negative control. The FRET donor complex formed from the biotinylated co-regulator peptide wild-type atrobox (biotin-PPYADTPALRQLSEYARPHVAFSP), recruitment deficient mutant atrobox (biotin-PPYADTPAARQASEYARPHVAFSP), NCOR1 (ID2) (biotin-GHSFADPASNLGLEDIIRKALMGSFD), or SMRT (ID2) (biotin-SQAVQEHASTNMGLEAIIRKALMGKYDQW) coupled to Tb–SA (12 nM) was kept constant, while the concentration of GFP-TLX LBD was varied starting with 4 μM as the highest concentration. Free GFP was added to keep the total GFP content stable at 4 μM throughout the entire series in order to suppress artefacts from changes in the degree of diffusion-enhanced FRET. After 1 h of incubation at RT, FIs after excitation at 340 nm were recorded at 520 nm for GFP acceptor fluorescence and 620 nm for Tb–SA donor fluorescence on a SPARK plate reader (Tecan Group Ltd.). FI520nm was divided by FI620nm and multiplied with 10,000 to give a dimensionless HTRF signal. Modulation of recruitment of SMRT by increasing concentrations of ccrp2 and 30 was assayed with a constant concentration of 100 nM (ccrp2) or 200 nM (30) GFP-TLX LBD.
TLX Dimerization
Strength and modulation of the formation of the dimers composed of the LBDs of TLX and RXRα was investigated by titration of the GFP-RXRα LBD or GFP-TLX LBD against a fixed concentration of the FRET donor labeled TLX LBD. Assay solutions were prepared in HTRF assay buffer supplemented with 0.1% (w/v) CHAPS as well as 1% DMSO with test compounds at 100 μM or DMSO alone as negative control. The FRET donor complex formed from biotinylated TLX LBD (final concentration 0.375 nM) and Tb–SA (0.75 nM) was kept constant, while the concentration of a GFP-RXRα LBD (highest concentration 600 nM) or GFP-TLX LBD (highest concentration 1200 nM) was varied. The total GFP was again kept constant (600 or 1200 nM) throughout the entire experiment by supplementation with free GFP, and measurement was performed as described before for the co-regulator affinity assay.
Acknowledgments
D.M. is grateful for the support by the Aventis Foundation. This work was supported by the research funding program LOEWE of the State of Hesse, Research Center for Translational Medicine and Pharmacology TMP. Work at BMRZ was supported by the State of Hesse. A.C., X.N., and S.K. are grateful for support by the SGC, a registered charity (no: 1097737) that receives funds from AbbVie, Bayer AG, Boehringer Ingelheim, Canada Foundation for Innovation, Eshelman Institute for Innovation, Genentech, Genome Canada through Ontario Genomics Institute [OGI-196], EU/EFPIA/OICR/McGill/KTH/Diamond, Innovative Medicines Initiative 2 Joint Undertaking [EUbOPEN grant 875510], Janssen, Merck KGaA, Pfizer, São Paulo Research Foundation-FAPESP, and Takeda. Gal4-VP16 was a gift from Lea Sistonen (Addgene plasmid # 71728).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.1c00195.
Schematic representation of the VP16/TLX assay; reporter (firefly luciferase) and control (renilla luciferase) data for the key compounds 8, 20, 30 and 33; selectivity profiles of 8, 20, and 30; representative control ITC experiment; NMR spectra of TLX ligands; titration of Tb3+-cryptate-labeled NCoR1 with GFP-labeled TLX; relative TLX mRNA expression; in vitro potencies of xanthine derivatives as adenosine receptor antagonists according to literature; synthesis of xanthine derivatives; and supplementary methods (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Shi Y.; Chichung Lie D.; Taupin P.; Nakashima K.; Ray J.; Yu R. T.; Gage F. H.; Evans R. M. Expression and Function of Orphan Nuclear Receptor TLX in Adult Neural Stem Cells. Nature 2004, 427, 78–83. 10.1038/nature02211. [DOI] [PubMed] [Google Scholar]
- Miyawaki T. Tlx, an Orphan Nuclear Receptor, Regulates Cell Numbers and Astrocyte Development in the Developing Retina. J. Neurosci. 2004, 24, 8124–8134. 10.1523/jneurosci.2235-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.-K.; Belz T.; Bock D.; Takacs A.; Wu H.; Lichter P.; Chai M.; Schutz G. The Nuclear Receptor Tailless Is Required for Neurogenesis in the Adult Subventricular Zone. Genes Dev. 2008, 22, 2473–2478. 10.1101/gad.479308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahams B. S.; Kwok M. C. H.; Trinh E.; Budaghzadeh S.; Hossain S. M.; Simpson E. M. Pathological Aggression in “Fierce” Mice Corrected by Human Nuclear Receptor 2E1. J. Neurosci. 2005, 25, 6263–6270. 10.1523/jneurosci.4757-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S. M.; Thomas A. L.; Nomie K. J.; Huang L.; Dierick H. A. Tailless and Atrophin Control Drosophila Aggression by Regulating Neuropeptide Signalling in the Pars Intercerebralis. Nat. Commun. 2014, 5, 3177. 10.1038/ncomms4177. [DOI] [PubMed] [Google Scholar]
- O’Leary J. D.; Kozareva D. A.; Hueston C. M.; O’Leary O. F.; Cryan J. F.; Nolan Y. M. The Nuclear Receptor Tlx Regulates Motor, Cognitive and Anxiety-Related Behaviours during Adolescence and Adulthood. Behav. Brain Res. 2016, 306, 36–47. 10.1016/j.bbr.2016.03.022. [DOI] [PubMed] [Google Scholar]
- Kozareva D. A.; O’Leary O. F.; Cryan J. F.; Nolan Y. M. Deletion of TLX and Social Isolation Impairs Exercise-Induced Neurogenesis in the Adolescent Hippocampus. Hippocampus 2018, 28, 3–11. 10.1002/hipo.22805. [DOI] [PubMed] [Google Scholar]
- O’Leary J. D.; O’Leary O. F.; Cryan J. F.; Nolan Y. M. Regulation of Behaviour by the Nuclear Receptor TLX. Gene Brain Behav. 2018, 17, e12357 10.1111/gbb.12357. [DOI] [PubMed] [Google Scholar]
- Kumar R. A.; McGhee K. A.; Leach S.; Bonaguro R.; Maclean A.; Aguirre-Hernandez R.; Abrahams B. S.; Coccaro E. F.; Hodgins S.; Turecki G.; Condon A.; Muir W. J.; Brooks-Wilson A. R.; Blackwood D. H.; Simpson E. M. Initial Association of NR2E1 with Bipolar Disorder and Identification of Candidate Mutations in Bipolar Disorder, Schizophrenia, and Aggression through Resequencing. Am. J. Med. Genet., Part B 2008, 147B, 880–889. 10.1002/ajmg.b.30696. [DOI] [PubMed] [Google Scholar]
- Zou Y.; Niu W.; Qin S.; Downes M.; Burns D. K.; Zhang C.-L. The Nuclear Receptor TLX Is Required for Gliomagenesis within the Adult Neurogenic Niche. Mol. Cell. Biol. 2012, 32, 4811–4820. 10.1128/mcb.01122-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavali P. L.; Saini R. K. R.; Zhai Q.; Vizlin-Hodzic D.; Venkatabalasubramanian S.; Hayashi A.; Johansson E.; Zeng Z.-j.; Mohlin S.; Påhlman S.; Hansford L.; Kaplan D. R.; Funa K. TLX Activates MMP-2, Promotes Self-Renewal of Tumor Spheres in Neuroblastoma and Correlates with Poor Patient Survival. Cell Death Dis. 2014, 5, e1502 10.1038/cddis.2014.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benod C.; Villagomez R.; Filgueira C. S.; Hwang P. K.; Leonard P. G.; Poncet-Montange G.; Rajagopalan S.; Fletterick R. J.; Gustafsson J.-Å.; Webb P. The Human Orphan Nuclear Receptor Tailless (TLX, NR2E1) Is Druggable. PLoS One 2014, 9, e99440 10.1371/journal.pone.0099440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffett K.; Bedia-Diaz G.; Hegazy L.; de Vera I. M. S.; Wanninayake U. S.; Billon C.; Koelblen T.; Wilhelm M. L.; Burris T. P. The Orphan Nuclear Receptor TLX Is a Receptor for Synthetic and Natural Retinoids. Cell Chem. Biol. 2020, 27, 1272–1284. 10.1016/j.chembiol.2020.07.013. [DOI] [PubMed] [Google Scholar]
- Faudone G.; Bischoff-Kont I.; Rachor L.; Willems S.; Zhubi R.; Kaiser A.; Chaikuad A.; Knapp S.; Fürst R.; Heering J.; Merk D. Propranolol Activates the Orphan Nuclear Receptor TLX to Counteract Proliferation and Migration of Glioblastoma Cells. J. Med. Chem. 2021, 64, 8727–8738. 10.1021/acs.jmedchem.1c00733. [DOI] [PubMed] [Google Scholar]
- Dueva E.; Singh K.; Kalyta A.; LeBlanc E.; Rennie P.; Cherkasov A. Computer-Aided Discovery of Small Molecule Inhibitors of Transcriptional Activity of TLX (NR2E1) Nuclear Receptor. Molecules 2018, 23, 2967. 10.3390/molecules23112967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski I.; Ma J.; Triezenberg S.; Ptashne M. GAL4-VP16 Is an Unusually Potent Transcriptional Activator. Nature 1988, 335, 563–564. 10.1038/335563a0. [DOI] [PubMed] [Google Scholar]
- Heering J.; Merk D. Hybrid Reporter Gene Assays: Versatile In Vitro Tools to Characterize Nuclear Receptor Modulators. Methods Mol. Biol. 2019, 1966, 175–192. 10.1007/978-1-4939-9195-2_14. [DOI] [PubMed] [Google Scholar]
- Sun G.; Yu R. T.; Evans R. M.; Shi Y. Orphan Nuclear Receptor TLX Recruits Histone Deacetylases to Repress Transcription and Regulate Neural Stem Cell Proliferation. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 15282–15287. 10.1073/pnas.0704089104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama A.; Takezawa S.; Schüle R.; Kitagawa H.; Kato S. Transrepressive Function of TLX Requires the Histone Demethylase LSD1. Mol. Cell. Biol. 2008, 28, 3995–4003. 10.1128/mcb.02030-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.-L.; Zou Y.; Yu R. T.; Gage F. H.; Evans R. M. Nuclear Receptor TLX Prevents Retinal Dystrophy and Recruits the Corepressor Atrophin1. Genes Dev. 2006, 20, 1308–1320. 10.1101/gad.1413606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estruch S. B.; Buzón V.; Carbó L. R.; Schorova L.; Lüders J.; Estébanez-Perpiñá E. The Oncoprotein BCL11A Binds to Orphan Nuclear Receptor TLX and Potentiates Its Transrepressive Function. PLoS One 2012, 7, e37963 10.1371/journal.pone.0037963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budzyński M. A.; Puustinen M. C.; Joutsen J.; Sistonen L. Uncoupling Stress-Inducible Phosphorylation of Heat Shock Factor 1 from Its Activation. Mol. Cell. Biol. 2015, 35, 2530–2540. 10.1128/MCB.00816-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faudone G.; Arifi S.; Merk D. The Medicinal Chemistry of Caffeine. J. Med. Chem. 2021, 64, 7156–7178. 10.1021/acs.jmedchem.1c00261. [DOI] [PubMed] [Google Scholar]
- Hamilton H. W.; Ortwine D. F.; Worth D. F.; Badger E. W.; Bristol J. A.; Bruns R. F.; Haleen S. J.; Steffen R. P. Synthesis of Xanthines as Adenosine Antagonists, a Practical Quantitative Structure-Activity Relationship Application. J. Med. Chem. 1985, 28, 1071–1079. 10.1021/jm00146a016. [DOI] [PubMed] [Google Scholar]
- Bruns R. F.; Daly J. W.; Snyder S. H. Adenosine Receptor Binding: Structure-Activity Analysis Generates Extremely Potent Xanthine Antagonists. Proc. Natl. Acad. Sci. U.S.A. 1983, 80, 2077–2080. 10.1073/pnas.80.7.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi X.; Zhou X. E.; He Y.; Searose-Xu K.; Zhang C.-L.; Tsai C.-C.; Melcher K.; Xu H. E. Structural Basis for Corepressor Assembly by the Orphan Nuclear Receptor TLX. Genes Dev. 2015, 29, 440–450. 10.1101/gad.254904.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corso-Díaz X.; de Leeuw C. N.; Alonso V.; Melchers D.; Wong B. K.; Houtman R.; Simpson E. M. Co-Activator Candidate Interactions for Orphan Nuclear Receptor NR2E1. BMC Genom. 2016, 17, 832. 10.1186/s12864-016-3173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahara N.; Hisahara S.; Hayashi T.; Horio Y. Transcriptional Activation of NAD+-Dependent Protein Deacetylase SIRT1 by Nuclear Receptor TLX. Biochem. Biophys. Res. Commun. 2009, 386, 671–675. 10.1016/j.bbrc.2009.06.103. [DOI] [PubMed] [Google Scholar]
- Wu D.; Yu S.; Jia L.; Zou C.; Xu Z.; Xiao L.; Wong K.-B.; Ng C.-F.; Chan F. L. Orphan Nuclear Receptor TLX Functions as a Potent Suppressor of Oncogene-Induced Senescence in Prostate Cancer via Its Transcriptional Co-Regulation of the CDKN1A (P21WAF1/CIP1) and SIRT1 Genes. J. Pathol. 2015, 236, 103–115. 10.1002/path.4505. [DOI] [PubMed] [Google Scholar]
- O’Loghlen A.; Martin N.; Krusche B.; Pemberton H.; Alonso M. M.; Chandler H.; Brookes S.; Parrinello S.; Peters G.; Gil J. The Nuclear Receptor NR2E1/TLX Controls Senescence. Oncogene 2015, 34, 4069–4077. 10.1038/onc.2014.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan A. P.; Bock D.; Gass P.; Schwger A.; Wolfer D. P.; Lipp H.-P.; Schütz G. Defective Limbic System in Mice Lacking the Tailless Gene. Nature 1997, 390, 515–517. 10.1038/37364. [DOI] [PubMed] [Google Scholar]
- Yu R. T.; Chiang M.-Y.; Tanabe T.; Kobayashi M.; Yasuda K.; Evans R. M.; Umesono K. The Orphan Nuclear Receptor Tlx Regulates Pax2 and Is Essential for Vision. Proc. Natl. Acad. Sci. U.S.A. 2000, 97, 2621–2625. 10.1073/pnas.050566897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M. M.; Zhang C.-L. TLX A Master Regulator for Neural Stem Cell Maintenance and Neurogenesis. Biochim. Biophys. Acta, Gene Regul. Mech. 2015, 1849, 210–216. 10.1016/j.bbagrm.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems S.; Zaienne D.; Merk D. Targeting Nuclear Receptors in Neurodegeneration and Neuroinflammation. J. Med. Chem. 2021, 64, 9592–9638. 10.1021/acs.jmedchem.1c00186. [DOI] [PubMed] [Google Scholar]
- Kilu W.; Merk D.; Steinhilber D.; Proschak E.; Heering J. Heterodimer Formation with Retinoic Acid Receptor RXRα Modulates Coactivator Recruitment by Peroxisome Proliferator-Activated Receptor PPARγ. J. Biol. Chem. 2021, 297, 100814. 10.1016/j.jbc.2021.100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr A. G.; Lo I.; Schumacher H.; Ho K.; Gill M.; Guo W.; Kim D. H.; Knox A.; Saito T.; Saido T. C.; Simms J.; Toddes C.; Wang X.; Yu G.-Q.; Mucke L. Istradefylline Reduces Memory Deficits in Aging Mice with Amyloid Pathology. Neurobiol. Dis. 2018, 110, 29–36. 10.1016/j.nbd.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch D.; Cheung S.-Y.; Schmidt J.; Gabler M.; Heitel P.; Kramer J.; Kaiser A.; Hartmann M.; Lindner M.; Lüddens-Dämgen K.; Heering J.; Lamers C.; Lüddens H.; Wurglics M.; Proschak E.; Schubert-Zsilavecz M.; Merk D. Non-Acidic Farnesoid X Receptor Modulators. J. Med. Chem. 2017, 60, 7199–7205. 10.1021/acs.jmedchem.7b00903. [DOI] [PubMed] [Google Scholar]
- Heitel P.; Achenbach J.; Moser D.; Proschak E.; Merk D. DrugBank Screening Revealed Alitretinoin and Bexarotene as Liver X Receptor Modulators. Bioorg. Med. Chem. Lett. 2017, 27, 1193–1198. 10.1016/j.bmcl.2017.01.066. [DOI] [PubMed] [Google Scholar]
- Rau O.; Wurglics M.; Paulke A.; Zitzkowski J.; Meindl N.; Bock A.; Dingermann T.; Abdel-Tawab M.; Schubert-Zsilavecz M. Carnosic Acid and Carnosol, Phenolic Diterpene Compounds of the Labiate Herbs Rosemary and Sage, Are Activators of the Human Peroxisome Proliferator-Activated Receptor Gamma. Planta Med. 2006, 72, 881–887. 10.1055/s-2006-946680. [DOI] [PubMed] [Google Scholar]
- Willems S.; Kilu W.; Ni X.; Chaikuad A.; Knapp S.; Heering J.; Merk D. The Orphan Nuclear Receptor Nurr1 Is Responsive to Non-Steroidal Anti-Inflammatory Drugs. Commun. Chem. 2020, 3, 85. 10.1038/s42004-020-0331-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J.; Klingler F.-M.; Proschak E.; Steinhilber D.; Schubert-Zsilavecz M.; Merk D. NSAIDs Ibuprofen, Indometacin, and Diclofenac Do Not Interact with Farnesoid X Receptor. Sci. Rep. 2015, 5, 14782. 10.1038/srep14782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pédelacq J. D.; Cabantous S.; Tran T.; Terwilliger T. C.; Waldo G. S. Engineering and Characterization of a Superfolder Green Fluorescent Protein. Nat. Biotechnol. 2006, 24, 79–88. 10.1038/nbt1172. [DOI] [PubMed] [Google Scholar]
- Adams R. W.; Holroyd C. M.; Aguilar J. A.; Nilsson M.; Morris G. A. “Perfecting” WATERGATE: Clean Proton NMR Spectra from Aqueous Solution. Chem. Commun. 2013, 49, 358–360. 10.1039/c2cc37579f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.