Abstract
The energy intake exceeding energy expenditure (EE) results in a positive energy balance, leading to storage of excess energy and weight gain. Here, we investigate the potential of a newly synthesized compound as an inducer of EE for the management of diet-induced obesity and insulin resistance. Xanthohumol (XN), a prenylated flavonoid from hops, was used as a precursor for the synthesis of a pyrazole derivative tested for its properties on high-fat diet (HFD)-induced metabolic impairments. In a comparative study with XN, we report that 4-(5-(4-hydroxyphenyl)-1-methyl-1H-pyrazol-3-yl)-5-methoxy-2-(3-methylbut-2-en-1-yl)benzene-1,3-diol (XP) uncouples oxidative phosphorylation in C2C12 cells. In HFD-fed mice, XP improved glucose tolerance and decreased weight gain by increasing EE and locomotor activity. Using an untargeted metabolomics approach, we assessed the effects of treatment on metabolites and their corresponding biochemical pathways. We found that XP and XN reduced purine metabolites and other energy metabolites in the plasma of HFD-fed mice. The induction of locomotor activity was associated with an increase in inosine monophosphate in the cortex of XP-treated mice. Together, these results suggest that XP, better than XN, affects mitochondrial respiration and cellular energy metabolism to prevent obesity in HFD-fed mice.
Keywords: obesity, insulin resistance, energy expenditure, locomotor activity, xanthohumol, pyrazole
Obesity is often the result of an imbalance between energy intake and energy expenditure (EE), which influences daily energy homeostasis and ultimately leads to weight gain.1 Heat production in brown adipose tissue (BAT) contributes to cold defense, stress-induced increases in body temperature, and energy balance.2 The concept of managing obesity through the stimulation of thermogenesis and EE is a focus of considerable attention. Thermogenic and fat-oxidizing molecules are being investigated for their antiobesity properties and include methylxanthines, polyphenols, minerals, proteins/amino acids, carbohydrates/sugars, and fats/fatty acids.3 In recent studies, flavonoids have been found to induce white adipose tissue (WAT) browning and promote energy balance in humans and animals through non-shivering thermogenesis.4,5 The thermogenic potentials of these products range from marginal to modest, but a safe increase to 10–15% above daily EE is expected to have significant impact on weight management in humans.3
Xanthohumol (XN), a flavonoid from hops (Humulus lupulus), has been extensively studied for its health-promoting and antiobesity properties.6−9 Anti-inflammatory, antioxidant, antiangiogenic, antiproliferative, and apoptotic effects, mainly assessed in vitro, reasonably suggest a potential chemopreventive activity of XN.7 Several in vivo studies have shown that oral administration of XN attenuates weight gain in obese male Zucker fa/fa rats,10 in KK-A(y) mice,11 and to various extents in obese C57BL/6(J) mice depending on the diet, dose, and formulation.8,12,13 Furthermore, oral administration of XN has been shown to lower the hepatic triglyceride content in KK-A(y) mice14 and in diet-induced obese C57BL/6J mice.8 In these mouse models, XN treatment also improved glucose intolerance8,15 and cognitive performance.9 XN increases the thermogenic uncoupling protein UCP1 in preadipocytes, resulting in the upregulation of mitochondrial uncoupling and oxygen consumption in vitro.14,15 The mild mitochondrial uncoupling effect of XN observed in vitro(9,14) suggests that XN might also induce EE in vivo. However, due to the α,-unsaturated ketone in its chemical structure, XN can spontaneously form a stable isomer, isoxanthohumol (IX), the biological precursor to the potent phytoestrogen, 8-prenylnaringenin (8PN).16 As a result, estrogenic side effects might be associated with XN administration in humans, and the investigation of active XN derivatives unable to form 8PN in vivo is ongoing.
Here, we used XN as a precursor for the generation of 4-(5-(4-hydroxyphenyl)-1-methyl-1H-pyrazol-3-yl)-5-methoxy-2-(3-methylbut-2-en-1-yl)benzene-1,3-diol (XP), a novel XN pyrazole derivative (Figure 1) unable to convert into 8PN. Pyrazoles are five-membered heterocycles that constitute one of the most studied groups of compounds among the azole family.17 They have attracted a lot of attention due to their interesting pharmacological properties including central nervous system depressant, antihypertensive, analgesic, antidiabetic, and antimicrobial.18−20 Many pyrazole derivatives have found their application clinically as nonsteroidal anti-inflammatory drugs, antiobesity agents, and other applications such as erectile dysfunction and chronic gout.20,21 Therefore, these molecules represent valuable building blocks for the chemical synthesis of bioactive compounds. XP was designed using a simplified synthetic approach to retain pharmacological properties of XN. Nine-week-old male C57BL/6J mice were fed a high-fat diet (HFD) to induce obesity and supplemented with 30 mg/kg body weight/day of XP compared to a dose of 60 mg/kg body weight/day of XN. We measured markers of HFD-induced metabolic dysfunction, EE, thermogenic proteins, and performed untargeted metabolome profiling to formulate mechanistic hypotheses. Our findings indicated that XP had a greater potential than XN in the improvement of HFD-induced obesity and insulin resistance in mice. We provide evidence that XP depolarizes the mitochondrial transmembrane, induces EE, increases locomotor movement, and regulates energy metabolites. These findings have potentially important implications for the management of diet-induced obesity and associated comorbidities.
Figure 1.
Chemical structures of XN and XN methyl-pyrazole derivative (XP).
Results
Chemical Synthesis of XP
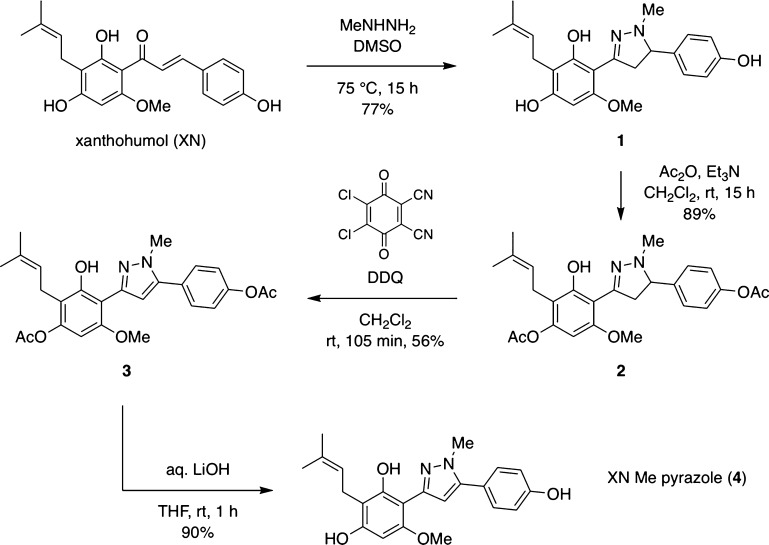
The synthesis of XP started with the treatment of XN with methylhydrazine in dimethyl sulfoxide (DMSO) at 70 °C to afford the corresponding pyrazoline 1 with 77% yield (Scheme 1). To obtain the oxidized derivative 4, we used DDQ as the oxidizing reagent after protection of the free phenolic moieties of pyrazoline 1, which are sensitive to oxidative conditions. Pyrazoline 1 was acetylated with acetic anhydride, in the presence of triethylamine, to obtain derivative 2. Interestingly, only two of the three free hydroxyl groups were acetylated. This was likely due to a strong hydrogen bond between the remaining hydroxyl group and the free nitrogen of the pyrazole derivative. Indeed, the presence of a far downfield signal in the 1H NMR (12.0–12.5 ppm) of XP and XP intermediates (Figures S1–S8) could suggest the formation of a highly conjugated enamine/orthoquinomethide tautomer (Figure S9). XN is also characterized by a strong hydrogen bond between one hydroxyl group and its carbonyl moiety, favoring the fully conjugated tautomer.22 Compound 2 was treated with DDQ in dichloromethane to afford derivative 3 with 56% yield. For the final deprotection, two strategic approaches were undertaken. Exposure of derivative 3 to potassium hydroxide, in methanol at 0 °C, resulted in product degradation. Therefore, the final compound 4 was obtained with 97% yield by hydrazinolysis by treating derivative 3 with hydrazine monohydrate. This method avoids strong basic reagents, which, in the presence of phenolic groups, could lead to the formation of reactive quinone derivatives, resulting in polymerization processes. However, hydrazine is known to be highly toxic. Hence, other conditions were tested, and exposure of compound 3 to lithium hydroxide afforded compound 4 with 90% yield.
Scheme 1. Chemical Synthesis of XP.
XP Decreases Weight and Improves Glucose Metabolism in HFD-Fed Mice
To evaluate the toxicity of the newly synthesized compound, we performed in vitro cell viability assays on human liver cancer cell line HepG2 and murine myoblast cell line C2C12. At concentrations lower than 50 μM, XP had no effect on the viability of HepG2 and C2C12 cells compared to the control (Figure S10A,B). We previously reported that XN is cytotoxic in HepG2 and C2C12 cells at concentrations above 25 μM.9 These results suggest that XN and XP have comparable toxicity profiles and that XP is safe to use in vivo at dosages similar to XN.
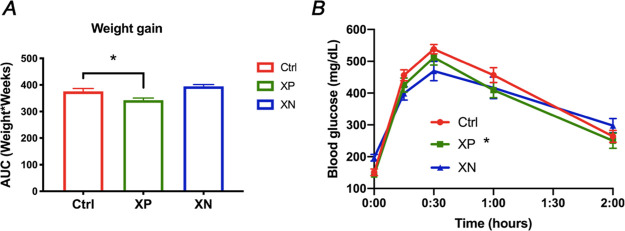
XN induces a dose-dependent decrease in hepatic triglycerides at 30 and 60 mg/kg/day.8 With in vivo safety profiles of XP being unknown, HFD-fed mice were treated with XP at a lower dose of 30 mg/kg body weight/day, while XN-treated mice were supplemented with XN at a high dose of 60 mg/kg body weight/day. Mice treated with XP gained significantly less weight over the period of the feeding experiment than control HFD-fed mice (p = 0.03, Figures 2A and S11A). On the other hand, mice treated with XN exhibited no change in weight compared to the control mice. We observed no significant differences in food intake among treatment groups (Figure S11B). After 4 weeks of feeding, we performed a glucose tolerance test (GTT) and found XP to improve glucose tolerance in HFD-fed mice (p = 0.03, Figure 2B), while XN had no effect on glucose clearance assessed by GTT. The homeostatic model assessment of insulin resistance (HOMA-IR), known as the insulin resistance score, assessed at week 11 was decreased by 77.9% (p = 0.01, Table 1) in HFD-fed XP-treated mice. These results suggest that XP improved insulin sensitivity and might lead to more effective regulation of blood glucose concentrations.
Figure 2.
XP decreases weight gain and improves glucose tolerance in HFD-fed mice. Body weight AUC (A) and glucose tolerance test (B) in HFD-fed male mice control treated with XP or treated with XN. Data is represented as mean ± SEM (n = 4–8 per group). *p < 0.05, one-way and repeated measures ANOVA.
Table 1. List of Metabolic Parameters Measured in Male Mice after 11 Weeks of HFD ± XP/XNa.
| HFD | HFD + XP | HFD + XN | |
|---|---|---|---|
| Final body weight (g) | 40.42 ± 1.94 | 37.87 ± 1.72 | 44.25 ± 1.97 |
| Fasting glucose (mg/dL) | 120.39 ± 13.50 | 128.98 ± 7.18 | 142.67 ± 6.29 |
| Insulin (μU/mL) | 77.71 ± 29.15 | 34.36 ± 3.73 | 71.17 ± 34.38 |
| HOMA-IR | 49.56 ± 18.19 | 10.94* ±1.52 | 26.76 ± 13.47 |
| AST (U/mL) | 0.45 ± 0.13 | 0.30 ± 0.06 | 0.16 ± 0.03 |
| ALT (U/mL) | 0.55 ± 0.22 | 0.38 ± 0.07 | 0.22 ± 0.06 |
| MCP1 (pg/mL) | 69.20 ± 14.07 | 45.00 ± 4.68 | 57.34 ± 15.74 |
| IL-6 (pg/mL) | 1.43 ± 0.53 | 5.78 ± 2.04 | 5.03 ± 3.3 |
| PCSK9 (ng/mL) | 211 ± 15.80 | 217.166 ± 26.88 | 215 ± 35.12 |
| Plasma TG (mg/dL) | 87.39 ± 13.17 | 81.81 ± 4.33 | 173.13** ± 23.24 |
| Plasma cholesterol (mg/dL) | 175.56 ± 5.70 | 160.47 ± 4.73 | 170.88 ± 6.76 |
Data displayed as mean ± SEM (n = 4–8 per group). Significant differences are marked as *p < 0.05, **p < 0.01, and ***p < 0.001 for the effect of treatment, one-way ANOVA.
We measured plasma concentrations of inflammatory cytokines often associated with obesity and insulin resistance. Although the monocyte chemoattractant protein-1 (MCP-1) was decreased by 35% and pro-inflammatory cytokine IL-6 was increased in XP-treated mice, the differences among treatment and control groups were not significant (Table 1). There were no changes in triglycerides (TGs) and total cholesterol concentrations in the plasma of XP-treated mice, while we observed an increase of plasma TGs in XN-treated mice (Table 1). The plasma concentrations of the proprotein convertase subtilisin/kexin type 9 (PCSK9) that induces LDL receptor degradation in hepatocytes and regulates plasma LDL-cholesterol23 were not affected by XP or XN (Table 1). To assess liver toxicity associated with treatment, we measured enzymatic activities of aspartate transaminase (AST) and alanine transaminase (ALT) in liver homogenates of control and treated mice (Table 1). XP-treated mice had 30% decrease in liver enzymes; the change was not significant and indicated that XP did not induce liver toxicity in vivo.
XP Increases EE in HFD-Fed Mice
We measured EE in XP-treated HFD-fed mice during three successive dark cycles and two successive light cycles using metabolic cages. The metabolic parameters were normalized on body weight to correct for weight variations within and among the treatment groups24 (Figure S12A). Mean EE, a measure of the amount of energy mice burn per hour, was increased by 20–27% in XP-treated mice (p = 0.0002) and by 4–8% in XN-treated mice (p = 0.01, Figures 3A and S12B). The mean respiratory exchange ratio (RER), which reflects the respiratory exchange of CO2 and O2, was increased in XP-treated mice (p < 0.0001) and XN-treated mice (p = 0.0004, Figure 3B), indicating higher ventilation rates in the treatment groups. As expected, the volumes of O2 consumed and CO2 produced were also increased in both treatment groups (Figure 3C,D). There was no change in food intake measured during the calorimetric experiment (Figure S12C). These data indicate that both XN and XP increased daily EE, tipping the balance between energy intake and energy output.
Figure 3.
XP and XN increase EE and RER in HFD-fed mice. Mean (A) EE, (B) RER, (C) O2 consumption volume, and (D) CO2 emission volume in HFD-fed mice control treated with XP or treated with XN. Data is represented as mean ± SEM (n = 4 per group). *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001, repeated measures ANOVA.
XP Modulates Plasma Metabolome in HFD-Fed Mice
We performed an untargeted plasma metabolomics analysis to identify metabolite alterations secondary to increased EE in treated mice and to gain more insight into XP mechanisms of action (Figure S13A and Table S1). We annotated 155 metabolites, which we categorized into major biological processes (Figure 4A). In the plasma of XP-treated mice, a non-significant yet uniform increase in amino acids, dipeptides, and TCA cycle metabolites was inferred by the presence of more intense red cells in the heatmap rows corresponding to analytes from these groups (Figure 4A). On the other hand, metabolites involved in adenosine triphosphate (ATP) anabolism and catabolism such as creatine, fatty acids (12-HETE), purine metabolites including adenosine monophosphate (AMP), inosine monophosphate (IMP), inosine, hypoxanthine, and xanthine were significantly decreased by XP. In XN-treated mice, 12-HETE, citrate, aconitate, creatine, and purine metabolites were also significantly decreased.
Figure 4.
XP and XN modulate plasma metabolome in HFD-fed mice. (A) Heatmap of relative abundances of individual plasma metabolites and (B) graphical representation of the purine degradation pathway with relative abundances of AMP, IMP, inosine, hypoxanthine, and xanthine in the plasma of HFD-fed mice treated with XP or XN. Features with significant differences among treatment groups are marked as *p < 0.05, **p < 0.01, and ***p < 0.001, one-way ANOVA. Abbreviations: ADP: adenosine diphosphate; AMP: adenosine monophosphate; ATP: adenosine triphosphate; ATPase: ATP synthase; ADK: adenylate kinase; AMPD: AMP deaminase; ADA: adenosine deaminase; GMP: guanosine monophosphate; GAH: guanine deaminase; HETE: hydroxy-eicosatetraenoic acid; IMP: inosine monophosphate; 5NT: 5′-nucleotidase; NAAG: N-acetyl-aspartyl-glutamate; PNP: purine nucleotide phosphorylase; SAM: S-adenosyl-methionine; XOR: xanthine Oxidoreductase.
A secondary cellular source of anaerobic ATP is the myokinase reaction converting 2 ADP into AMP and ATP. Therefore, intracellular purine nucleotides act as a reservoir that promotes ATP regeneration by converting excess AMP into IMP and driving forward the myokinase reaction.25,26 Out of the 11 metabolites significantly affected by XP or XN treatment, 5 metabolites belonged to purine metabolism pathways (Figures 4B and S14). These data indicate that increased EE is associated with the down-regulation of plasma metabolites involved in energy metabolism in XP- and XN-treated mice.
XP Uncouples Oxidative Phosphorylation in C2C12 Cells
Mitochondrial respiration is coupled to ATP synthesis through an electrochemical proton gradient. Proton leak across the inner membrane results in depolarization of the mitochondrial transmembrane without deleteriously lowering the ATP synthesis.27 Proton leak can be mediated by UCPs and leads to the dissipation of the energy derived from substrate oxidation as heat.28 Considering the XP and XN effect on EE, we evaluated the thermogenic activity by assessing changes in UCP1 protein expression in the skeletal muscle, BAT, and WAT of HFD-fed mice. Unexpectedly, XP had no effect in UCP1 in the skeletal muscle, BAT, or WAT of HFD-fed mice (Figure 5A). The UCP1 protein expression was decreased in the WAT of XN-treated mice, and we did not observe significant changes in the UCP1 protein expression in the skeletal muscle and BAT of these mice (Figure 5A). These data indicate that the XP and XN thermogenic effect is not mediated by UCP1 upregulation in HFD-fed mice.
Figure 5.

Effect of XP and XN on UCP1 and mitochondrial membrane potential. (A) Western blots and protein quantification of UCP1 in skeletal muscle, BAT, and WAT of HFD-fed mice control treated with XP or treated with XN. (B) Ratio of JC-1 aggregates and JC-1 monomers in C2C12 cells treated with FCCP, XP, or XN. Data is represented as mean ± SEM (n = 3 per group). *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001, one-way ANOVA.
Therefore, we investigated the XP intrinsic protonophore properties by measured variations of mitochondrial membrane potential (ΔψM) in C2C12 cells stained with JC-1 dye. In cells with low ΔψM, JC-1 remains in the monomeric form and exhibits green fluorescence, while in cells with high ΔψM, JC-1 aggregates to exhibit red fluorescence. In C2C12 cells treated for 60 min, XP and XN decreased the ratio of JC-1 aggregates to JC-1 monomers at concentrations as low as 1 μM (Figure 5B). The effects of XP and XN were dose-dependent and milder than that of protonophore FCCP, a potent uncoupler of oxidative phosphorylation. The decrease in the ratio of red to green fluorescence is indicative of a lower polarization of the mitochondrial membrane in XP-treated cells, suggesting that XP acts as a protonophore.
XP Increases Ambulatory Locomotor Activity in HFD-Fed Mice
With physical activity being an important component of EE measured via indirect calorimetry, we monitored motor activity along with EE during three successive dark cycles and two successive light cycles. All movements including direct locomotion and fine movements such as grooming and scratching were increased in XP-treated mice (p = 0.009) and XN-treated mice (p = 0.04, Figure 6A). The locomotor movement, an exclusive measure of direct locomotion, was increased by 75–135% in XP-treated mice (p = 0.009, Figure 6B) but not in XN-treated mice (p = 0.06). Percent ambulatory time—a sum of animal’s time spent in ambulatory locomotion over total cycle duration—was also increased in XP-treated mice (p = 0.02, Figure 6C). These data suggest that XP also has a central effect, resulting in increased physical activity to enhance the EE and prevent weight gain.
Figure 6.

XP increases locomotor movement in HFD-fed mice. Mean (A) all movement, (B) locomotor movement, and (C) percent ambulatory time in HFD-fed mice control, treated with XP or treated with XN. Data is represented as mean ± SEM (n = 4 per group). *p < 0.05 and **p < 0.01, repeated measures ANOVA.
To answer the question whether XP crossed the blood–brain barrier (BBB), we measured concentrations of XP in the cortex and plasma of treated mice by UPLC-MS (Table 2 and Figure S15). With glucuronidation being the predominant phase II metabolism for flavonoids including XN,29,30 we compared the concentrations of XP with and without glucuronidase digestion. In the plasma, total XP and XN pools measured following glucuronidase treatment were significantly more abundant than their respective aglycone pools. XP was detected in the cortex of XP-treated mice, where we observed no differences between unconjugated XP and total XP concentrations (Table 2). On the other hand, total XN pools were higher in some mice, indicating the presence of glucuronidated XN in the cortex, but there was no significant difference between unconjugated and total XN concentrations. These results suggest that a considerable portion of circulating XP is in the form of O-glucuronide, while non-conjugated XP is the form that crosses the BBB.
Table 2. Concentrations of XP and XN in the Plasma and Cortex of Treated Mice Following Glucuronidase Digestion or without Glucuronidase Digestiona.
| Plasma (ng/mL) |
Cortex (ng/g) |
|||
|---|---|---|---|---|
| Non-glucuronidated | Glucuronidated + non-glucuronidated | Non-glucuronidated | Glucuronidated + non-glucuronidated | |
| XP | 43.46 ± 3.54 | 193.81** ± 39.34 | 40.14 ± 2.8 | 39.85 ± 4.56 |
| XN | 13.23 ± 3.07 | 222.64* ± 37.99 | 59.70 ± 16.75 | 360 ± 188.35 |
Data displayed as mean ± SEM (n = 4–8 per group). Significant differences are marked as *p < 0.05, **p < 0.01, and ***p < 0.001 for comparisons between free and total pools, two-tailed unpaired t-test.
Effect of XP on Cortex Metabolites in HFD-Fed Mice
We performed an untargeted cortex metabolomics analysis to gain more insights into metabolic changes linked to the XP effect on locomotor activity (Figure S13B and Table S2). Unexpectedly, in the cortex, XN had the most significant effect on the metabolome, and the most annotated features were decreased (Figure 7A). Metabolites significantly decreased in both treatment groups including S-adenosylmethionine (SAM), creatine, 3-phosphoglycerate, reduced glutathione (GSH), and neurotransmitters such as aspartate (Figure 7A–E). Neurotransmitters such as dopamine and norepinephrine, known for their involvement in locomotor activity,31 were not detected using our untargeted metabolomics method. Other metabolites including N-acetyl-aspartate (NAA), choline, carnitine, and oxidized glutathione (GSSG) were also decreased in XN-treated mice. There were no significant changes in the GSH/GSSG ratio between the control and treatment groups (Figure S16). Among the metabolites affected by XP, but not XN treatment, IMP was the only metabolite significantly increased in XP-treated mice (p = 0.006, Figure S17). This observation was especially striking as the 76% increase in cortical IMP was inconsistent with the IMP decrease observed in the plasma, suggesting that IMP might be involved in the central effects of XP on locomotor activity.
Figure 7.
XP and XN modulate the cortex metabolome in HFD-fed mice. (A) Heatmap of relative abundances of individual metabolites in the cortex of HFD-fed mice treated with XP or XN. Relative abundances of (B) creatine, (C) 3-phospho-glycerate, (D) IMP, and (E) glutathione in the cortex of HFD-fed mice treated with XP or XN. Data displayed as mean ± SEM. Significant differences are marked as *p < 0.05, **p < 0.01, and ***p < 0.001 for effect of treatment, one-way ANOVA. Abbreviations: ADP: adenosine diphosphate; AMP: adenosine monophosphate; ATP: adenosine triphosphate; HETE: hydroxy-eicosatetraenoic acid; IMP: inosine monophosphate; GPC: glycerophosphocholine; NAAG: N-acetyl-aspartyl-glutamate; SAM: S-adenosyl-methionine; SAH: S-adenosyl-homocysteine; and UMP: uridine monophosphate.
Discussion and Conclusions
XN has been reported to have beneficial effects on obesity.8,10 However, due to its poor bioavailability and extensive metabolism, the clinical applications of XN remain limited. Studies have reported the low biological efficacy of XN native extracts administered orally,12,32 and stable derivatives of XN were shown to be more potent in vivo.9,33,34 Several factors might contribute to the discrepancies in XN-induced weight loss in this study in comparison to previous reports.8,12 Under the same feeding and housing conditions, some animals are more prone to develop obesity,35−38 and these phenotypic variabilities might have a significant impact on the pharmacodynamics of XN. Moreover, recently published data shows that XN requires the intestinal microbiota to improve glucose metabolism in diet-induced obese mice,39 suggesting that gut microbiota composition is another potential variable. In the current study, we synthesized and assessed the biological activity of a pyrazole derivative of XN that cannot be converted to 8-PN. XP improved metabolic markers in HFD-fed mice without inducing liver toxicity, as indicated by hepatic AST and ALT concentrations. XP reduced body weight gain, feed efficiency, and improved HFD-induced insulin resistance. Insulin resistance is a requirement for the development of type 2 diabetes, which is closely linked to obesity.40 Increased release of cytokines such as tumor necrosis factor-α, IL-6, and MCP-1 might also have a role in the development of insulin resistance.41 We observed no significant changes in cytokine levels in vivo, but XP improved the animals’ response to insulin secretion following a sudden increase in glucose as assessed by the GTT and the decreased HOMA-IR index.
We previously suggested that XN-mediated weight reduction is partially due to XN being a mitochondrial uncoupler.9 By acting as a protonophore dissipating the proton gradient necessary for ATP synthesis, XN reduces the efficiency of the oxidative phosphorylation.42 In fact, endogenous mitochondrial UCPs dissipate the proton gradient and are involved in various physiological processes including thermogenesis, autophagy, mitophagy, reactive oxygen species (ROS) production, and protein secretion.28 Therefore, mitochondrial uncoupling has been proposed as a mechanism to treat several human diseases, such as obesity and cardiovascular diseases.27,28 Our study reveals that XN depolarizes the mitochondrial membrane, which is associated with an increase in respiratory rate and EE in XN-treated C57BL/6J mice, independent from UCP1 protein expression. These data support that XN mitochondrial uncoupling properties are due to proton translocation by the flavonoid across the mitochondrial inner membrane.9,42 UCP1 protein expression in WAT was lower in XN-treated mice, contrary to previous reports of browning of white adipocytes by XN in vitro.15 This suggests a compensatory regulation of UCP levels in vivo to prevent complete impairment of the mitochondrial respiration. Although the protonophore effect of XP in vitro was comparable to XN, daily EE was higher in XP-treated mice than in XN-treated mice. The marked increase in EE in XP-treated mice was, at least partially, due to the increase in locomotor activity. In fact, daily EE consists of the basal metabolic rate, diet-induced thermogenesis, and energy cost of physical activity.43 During physical activity, EE matches the sum of heat loss and work output.44 As both thermogenesis and physical activity contribute to EE, we observed higher EE in XP-treated mice, with up to 27% increase in daily EE compared to control mice.
An induction in motor behavior, similar to that observed in XP-treated mice, is associated with the administration of dopamine agonists such as haloperidol. Haloperidol induces an increase in locomotor response as a result of the blockade of presynaptic auto-receptors, which disrupts negative feedback and increases release of dopamine.45,46 Several compounds with thermogenic and fat-oxidizing potentials also possess sympathomimetic stimulatory activity.3,47,48 We observed higher IMP levels in the cortex of XP-treated mice, which might be related to the changes in motor behavior. In fact, inosine and inosine metabolites including IMP activate adenosine receptors such as adenosine 2A receptor (A2AR) and stimulate A2AR-mediated intracellular cAMP production.49 The activation of adenosine receptors is known to induce anxiolytic effects in mice50,51 and A2AR mediates psychostimulant-mediated effects on locomotor activity.52 IMP also has a central effect on mood and behavior in mice by reversing HFD-induced excess in neuronal NO synthase in the cerebellum.53,54 These reports combined with our data suggest the involvement of IMP in increased locomotor activity in XP-treated mice. We suggest that a dual mechanism involving direct and indirect mitochondrial uncoupling is responsible for XP-mediated increase in EE in HFD-fed mice.
Untargeted metabolomics suggested that XP and XN influence cellular energy metabolism in HFD-fed mice, leading to decreased purine metabolites and creatine in the plasma. Purine metabolism consists of de novo synthesis, catabolism, and salvage pathways, and purine molecules form the scaffold of the key molecule for storing cellular energy. Although mitochondrial and glycolytic pathways are used to produce energy, instantaneous energy needs are satisfied through the phosphocreatine shuttle and the combined efforts of AMP deaminase (AMPD), AMP-activated protein kinase (AMPK), and adenylate kinase (ADK).25 These enzymes regulate AMP conversion to IMP to keep AMP levels low during times of high ATP utilization and to favor the production of ATP and AMP from two ADPs. IMP may then be degraded to inosine and then to hypoxanthine and potentially further degraded to xanthine and uric acid.
Several mechanisms might explain the decrease in purine metabolites observed in treated mice. First, the downregulation of the purine degradation pathway by XP and XN may reflect a decrease in oxidative stress. In fact, purine degradation is regulated by oxidative stress, and increased purine degradation serves as an indication of an increased inflammatory response.55 In inflammatory diseases such as periodontitis, purine metabolites are significantly increased at the disease sites.56 This hypothesis is further supported by the notion that other metabolites associated with oxidative stress such as 12-HETE in the plasma and glutathione metabolites in the brain were decreased by XP and XN. GSH scavenges ROS and reactive nitrogen species to protect cells from oxidative stress.57,58 Obesity has been associated with a decrease in GSH in the liver and kidney, but GSH was shown to be increased in cardiac tissues of obese rats in an effort to protect cells against oxidative damage.59,60 Moreover, there is evidence that under the right conditions, GSH depletion and ROS may prevent type 2 diabetes.14,61 The decreased abundances of both GSH and GSSG in the cortex of treated mice might indicate decreased oxidative stress in the brain of XP- and XN-treated mice. Second, the downregulation of plasma purine metabolites may be secondary to mitochondrial uncoupling and increased EE in XP-treated and XN-treated mice. Purine metabolite concentrations fluctuate with physical activity and acute muscular exercise results in increased plasma hypoxanthine levels.25 Paradoxically, long-lasting exercise and endurance training cause a decrease in pre- and post-exercise plasma hypoxanthine concentrations in periods of specific preparation and competition in male athletes.62 The constant mitochondrial uncoupling induced by chronic administration of XP and XN leads to enhanced consumption of fatty acids, sugars, and proteins to make ATP, creating a situation that could be comparable to mild endurance training.
Previous studies have shown that XN is detected in the brain of XN-treated mice.63 Similar to XN, we measured XP in the cortex of treated mice and the compounds might cross the BBB as aglycones. UDP-glucuronosyltransferases (UGTs) such as UGT1a6a64 and UGT2b3565 have been identified in mouse brain and might mediate the glucuronidation of xenobiotics in the brain. Together, these results demonstrate that XP improved HFD-induced metabolic dysfunction, increased EE, and locomotor activity in HFD-fed mice. XP did not cause liver damage in mice administered the compound at a dose of 30 mg/kg body weight/day, which is equivalent to a dose of 175 mg/day in a 70 kg person.66,67 As managing obesity by stimulating thermogenesis and EE remains a promising concept in the search of potent antiobesity agents, these data suggest that XP could represent a safe and promising option for the treatment of obesity-related metabolic impairments.
Materials and Methods
Cell Viability Assays
Cells were seeded in 96-well plates 24 h prior to adding the compound. HepG2 cells were seeded at a density of 5 × 104 cells/mL and C2C12 at 1 × 104 cells/mL in order to reach 80% confluency at the beginning of the experiment. After 24 h, the culture medium was replaced with fresh medium containing various concentrations ranging between 0 and 200 μM of XP. The cells were incubated in the presence of the compound for 24 h, at which time, the MTT viability assay was carried as previously described.9 MTT was dissolved at 0.5 mg/mL in serum-free Dulbecco’s modified Eagle’s medium, filter-sterilized and 100 μL was added to the cells. The cells were incubated for 3 h and then acidified with 100% isopropanol for 15 min before absorbance reading at 570 nm on a BioTek Synergy HT plate reader.
Animal Studies
All animal experiments were performed in accordance with institutional and National Health and Medical Research Council guidelines. The experimental protocol was approved by the Institutional Animal Care and Use Committee at Oregon State University and the studies were carried out in accordance with the approved protocol. Nine-week-old wild-type male C57BL/6J mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA). Mice were single-housed in ventilated cages under a 12–12 h light–dark cycle and fed a HFD (Dyets Inc., Bethlehem, PA, USA) containing 60, 20, and 20% total calories from fat, carbohydrate, and protein, respectively. XN (99% purity, Hopsteiner Inc., New York, NY, USA) and XP (99% purity) were mixed into the diet as described previously9 at a concentration of 0.066% for XN and 0.033% for XP to reach respective doses of 60 mg/kg body weight/day and 30 mg/kg body weight/day. The study included eight HFD-fed mice, eight XP-treated mice, and four XN-treated mice; food intake and body weights were recorded weekly. At week 4, glucose tolerance was tested after 6 h fasting. Following intraperitoneal bolus injection of 1.5 g/kg of d-glucose, blood glucose levels were measured at 0, 15, 30, 60, and 120 min using a One Touch UltraMini glucometer (LifeScan Inc., Milpitas, CA, USA). At the end of 11 weeks of feeding, mice were euthanized, and blood, liver, BAT, epididymal WAT, and cortex samples were collected for analyses.
Measure of Metabolic Activity
We performed metabolic determinations at week 6, with four mice from each treatment group housed in Promethion metabolic cages (Sable Systems, Las Vegas, NV, USA). The indirect calorimetry system consists of metabolic cages identical to regular cages with bedding, each equipped with food hoppers connected to load cells for food intake monitoring. Prior to data collection, all mice were acclimated to the cages for 8 h. A standard 12 h light/dark cycle was maintained throughout the calorimetry studies, and data was collected over three dark cycles and two light cycles. Metabolic measurements such as EE and respiratory exchange rate (calculated from the ratio of CO2 volume produced and O2 volume consumed) were measured. Motor activity and food intake were also recorded.
JC-1 Mitochondrial Membrane Potential Assay
C2C12 cells were seeded in 96-well plates at 1 × 104 cells/mL. After 24 h, the culture medium was replaced with fresh medium containing carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (FCCP) from Sigma-Aldrich (St Louis, MO, USA), XP, XN, or DMSO vehicle. Cells were incubated in the presence of the treatments for 1 h and 5,5′,6,6′-tetrachloro-1,1′,3,3′- tetraethylbenzimidazolylcarbocyanine iodide (JC-1) dye was added to each well following the manufacturer’s protocol (Cayman Chemical, Ann Arbor, MI, USA). JC-1 aggregates were detected with excitation and emission wavelengths of 540 and 570 nm. JC-1 monomers were detected with excitation and emission wavelengths of 485 and 535 nm on a BioTek Synergy HT plate reader.
Measure of Metabolic Parameters and Liver Enzymes
ALT and AST enzymatic activities and liver samples (n = 4–8 per group) were homogenized in 10 mL of 100 mM Tris (pH = 7.8) per gram of tissue. The homogenates were centrifuged at 10 000g for 15 min at 4 °C, and the supernatants were tested for ALT and AST activity using colorimetric assay kits purchased from Cayman Chemical (Ann Arbor, MI, USA). Triglycerides and total cholesterol were analyzed with commercially available kits (Cayman Chemical, Ann Arbor, MI, USA). Plasma glucose at week 11 was measured with a colorimetric assay, and plasma insulin, MCP-1, IL-6, and PCSK9 were analyzed by ELISA kits purchased from Abcam (Cambridge, UK).
Plasma and Cortex LC–MS Metabolomics
The left and right sides of the cortex were pooled and ground using liquid nitrogen. Cortex samples (approximately 30 mg) were extracted as previously described68 with slight modifications. Tissues were homogenized with 600 μL of cold 80% acetonitrile with 0.1% formic acid containing 0.2 μg/mL of dopamine-d4 (Sigma-Aldrich, St Louis, MO, USA) and 0.2 μg/mL of chlorpropamide (Sigma-Aldrich, St Louis, MO, USA) using a counter-top bullet blender for 2 min and centrifuged at 20 000rpm for 10 min. Supernatants were transferred to high-performance liquid chromatography (HPLC) vials for analysis.
Plasma metabolites were extracted with 400 μL of cold acetonitrile/methanol (1:1, v/v) containing 0.2 μg/mL of dopamine-d4 and 0.2 μg/mL of chlorpropamide per 50 μL of plasma. Samples were vortexed 30 s, incubated at −20 °C for 1 h, and centrifuged at 13 000rpm for 15 min, supernatants were evaporated under vacuum, and the dry extracts were reconstituted in 200 μL of 80% acetonitrile containing 0.1% formic acid. The samples were vortexed, centrifuged at 15 000rpm for 5 min, and supernatants were transferred to HPLC vials for analysis.
LC–high-resolution mass spectrometry/MS was performed as previously reported69 with some modifications. Data-dependent acquisition in both negative and positive ion mode was conducted using a Shimadzu Nexera UPLC system coupled to an AB SCIEX TripleTOF 5600 mass spectrometer (AB SCIEX, Toronto, Canada). Chromatographic separation was conducted using an Inertsil Phenyl-3 column (4.6 × 150 mm, 100 Å, 5 μm; GL Sciences, Tokyo, Japan). Spray voltage was −4200 V for negative ion mode and 4500 V for positive ion mode acquisition. The injection volume was 3 μL. Metabolite identifications were performed using Progenesis QI software (V2.4.6911) with METLIN plugin V1.0.6499 (NonLinear Dynamics, United Kingdom). Progenesis QI was used for peak picking, retention time correction, data normalization, peak alignment, and metabolite annotations.70,71 Metabolites were detected in both ion modes; the one with the lowest coefficient of variation of the QC was kept.
Quantification of XP and XN
For plasma extractions, ascorbic acid (5 μL, 10% m/m water, Sigma-Aldrich, St Louis, MO, USA) was added to 40 μL of plasma. For tissue extractions, 20 mg of cortex was extracted with 200 μL of 50% methanol in water and 10 μL of ascorbic acid (10% m/m water). Samples were incubated with or without 10 μL of glucuronidase (20 mg/mL, Sigma-Aldrich, St Louis, MO, USA) for 3 h at 37 °C. The samples were extracted with 200 μL of acetonitrile containing 100 ng/mL of [13C3]-XN.22 The supernatant was transferred to a new tube and vacuum-dried, and the pellet was reconstituted in 100 μL of 50% methanol in water. UPLC-MS was performed using a hybrid triple quadrupole linear ion trap mass spectrometer (4000 QTRAP, AB SCIEX, Toronto, Canada) as previously reported72 with some modifications. The elution profile was set as follows: 5% B from 0 to 0.5 min, 5 to 100% B from 0.5 to 3 min, 1 min hold at 100% B, followed by re-equilibration of the HPLC column at 5% B for 2 min. Selected reaction monitoring transitions used for quantification were m/z 353.1 > 119.1 for XN, m/z 379.3 > 321.3 for XP, and m/z 356.1 > 120.1 for the [13C3]-XN internal standard.
Western Blotting
Approximately 100 mg of frozen BAT and WAT was homogenized in RIPA buffer with protease and phosphatase inhibitors (Santa Cruz Biotechnology, Dallas, TX, USA). Tissues were sonicated for 30 s, and the homogenates were centrifuged at 15 700g for 10 min to collect the supernatants. Protein (30 μg) was separated by SDS-PAGE using 4–15% MP TGX Gels (Bio-Rad, Hercules, CA, USA) and blotted onto nitrocellulose membranes. The membranes were blocked with 5% nonfat milk for 90 min and incubated with antibodies against GAPDH (#0411, Santa Cruz Biotechnology, Dallas, TX, USA) or UCP1 (#10983, Genetex, Irvine, CA, USA) for 60 min. After a second reaction with secondary antibodies (horseradish peroxidase-conjugated IgG antibodies), the protein bands on the nitrocellulose membranes were visualized by enhanced chemiluminescence substrate on an electrochemical luminescence (ECL) imager (Thermo Fisher Scientific, Waltham, MA, USA). Band intensities were quantified by densitometry using Image J software.
Statistical Analyses
Bar graph values are represented as mean ± SEM (standard error of the mean). The data were analyzed using GraphPad Prism version 8.0 (San Diego, CA, USA). The statistical analyses compared each of the two treatment groups to the control group. A one-way ANOVA followed by Dunnett’s test was used for single measures among animals (i.e., liver and plasma metabolic measures) and a repeated-measures-in-time design ANOVA for repeated measures within animals (i.e., glucose tolerance test, EE, and locomotor activity). Two-tailed unpaired t-tests were used to compare unconjugated and total concentrations of XP and XN.
Acknowledgments
This work was supported by the Linus Pauling Institute, the National Institutes of Health (NIH grants S10RR022589 and S10RR027878), the OSU College of Pharmacy, and the OSU Foundation Buhler-Wang Research Fund.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.1c00161.
Detailed description of XP synthesis including NMR and MS/MS spectra of XP and intermediates, cell viability assays, and LC–MS untargeted metabolomics annotations (PDF)
Author Contributions
I.L.P., L.M.M., C.L.M., C.K., P.R.B., and J.F.S. designed the experiments. I.L.P., L.M.M., A.A.M., J.C., L.S.P., M.A.R, C.L.M., and P.R.B. performed the experiments and analyzed the results. I.L.P. and L.M.M. drafted the manuscript. All authors reviewed and edited the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Turner N.; Cooney G. J.; Kraegen E. W.; Bruce C. R. Fatty acid metabolism, energy expenditure and insulin resistance in muscle. J. Endocrinol. 2014, 220, T61. 10.1530/joe-13-0397. [DOI] [PubMed] [Google Scholar]
- Morrison S. F. Central Pathways Controlling Brown Adipose Tissue Thermogenesis. Physiology 2004, 19, 67–74. 10.1152/nips.01502.2003. [DOI] [PubMed] [Google Scholar]
- Dulloo A. G. The search for compounds that stimulate thermogenesis in obesity management: from pharmaceuticals to functional food ingredients. Obes. Rev. 2011, 12, 866–883. 10.1111/j.1467-789X.2011.00909.x. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Li X.; Fang H.; Guo F.; Li F.; Chen A.; Huang S. Flavonoids as inducers of white adipose tissue browning and thermogenesis: signalling pathways and molecular triggers. Nutr. Metab. 2019, 16, 47. 10.1186/s12986-019-0370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H.; Lee S.; Otieno D.; Ha K. Flavonoids, potential bioactive compounds, and non-shivering thermogenesis. Nutrients 2018, 10, 1168. 10.3390/nu10091168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi R. E.; Whyand T.; Caplin M. E. Benefits of Xanthohumol in Hyperlipidaemia, Obesity and Type 2 Diabetes Mellitus: A Review. J. Obes. Chron. Dis. 2019, 03, 14–18. 10.17756/jocd.2019-023. [DOI] [Google Scholar]
- Magalhães P. J.; Carvalho D. O.; Cruz J. M.; Guido L. F.; Barros A. A. Fundamentals and health benefits of xanthohumol, a natural product derived from hops and beer. Nat. Prod. Commun. 2009, 4, 1934578X0900400. 10.1177/1934578X0900400501. [DOI] [PubMed] [Google Scholar]
- Miranda C. L.; Elias V. D.; Hay J. J.; Choi J.; Reed R. L.; Stevens J. F. Xanthohumol improves dysfunctional glucose and lipid metabolism in diet-induced obese C57BL/6J mice. Arch. Biochem. Biophys. 2016, 599, 22–30. 10.1016/j.abb.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda C. L.; Johnson L. A.; de Montgolfier O.; Elias V. D.; Ullrich L. S.; Hay J. J.; Paraiso I. L.; Choi J.; Reed R. L.; Revel J. S.; Kioussi C.; Bobe G.; Iwaniec U. T.; Turner R. T.; Katzenellenbogen B. S.; Katzenellenbogen J. A.; Blakemore P. R.; Gombart A. F.; Maier C. S.; Raber J.; Stevens J. F. Non-estrogenic Xanthohumol Derivatives Mitigate Insulin Resistance and Cognitive Impairment in High-Fat Diet-induced Obese Mice. Sci. Rep. 2018, 8, 613. 10.1038/s41598-017-18992-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legette L. L.; Moreno Luna A. Y.; Reed R. L.; Miranda C. L.; Bobe G.; Proteau R. R.; Stevens J. F. Xanthohumol lowers body weight and fasting plasma glucose in obese male Zucker fa/fa rats. Phytochemistry 2013, 91, 236–241. 10.1016/j.phytochem.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Nozawa H. Xanthohumol, the chalcone from beer hops (Humulus lupulus L.), is the ligand for farnesoid X receptor and ameliorates lipid and glucose metabolism in KK-Ay mice. Biochem. Biophys. Res. Commun. 2005, 336, 754–761. 10.1016/j.bbrc.2005.08.159. [DOI] [PubMed] [Google Scholar]
- Mahli A.; Seitz T.; Freese K.; Frank J.; Weiskirchen R.; Abdel-Tawab M.; Behnam D.; Hellerbrand C. Therapeutic Application of Micellar Solubilized Xanthohumol in a Western-Type Diet-Induced Mouse Model of Obesity, Diabetes and Non-Alcoholic Fatty Liver Disease. Cells 2019, 8, 359. 10.3390/cells8040359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa R.; Rodrigues I.; Guardão L.; Rocha-Rodrigues S.; Silva C.; Magalhães J.; Ferreira-de-Almeida M.; Negrão R.; Soares R. Xanthohumol and 8-prenylnaringenin ameliorate diabetic-related metabolic dysfunctions in mice. J. Nutr. Biochem. 2017, 45, 39–47. 10.1016/j.jnutbio.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Kirkwood J. S.; Legette L. L.; Miranda C. L.; Jiang Y.; Stevens J. F. A Metabolomics-driven Elucidation of the Anti-obesity Mechanisms of Xanthohumol. J. Biol. Chem. 2013, 288, 19000–19013. 10.1074/jbc.M112.445452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels J. S.; Shashidharamurthy R.; Rayalam S. Novel anti-obesity effects of beer hops compound xanthohumol: role of AMPK signaling pathway. Nutr. Metab. 2018, 15, 42. 10.1186/s12986-018-0277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J.; Nikolic D.; Chadwick L. R.; Pauli G. F.; van Breemen R. B. Identification of human hepatic cytochrome P450 enzymes involved in the metabolism of 8-prenylnaringenin and isoxanthohumol from hops (Humulus lupulus L.). Drug Metab. Dispos. 2006, 34, 1152–1159. 10.1124/dmd.105.008250. [DOI] [PubMed] [Google Scholar]
- Karrouchi K.; Radi S.; Ramli Y.; Taoufik J.; Mabkhot Y.; Al-aizari F.; Ansar M. h. Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review. Molecules 2018, 23, 134. 10.3390/molecules23010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandgar B. P.; Gawande S. S.; Bodade R. G.; Gawande N. M.; Khobragade C. N. Synthesis and biological evaluation of a novel series of pyrazole chalcones as anti-inflammatory, antioxidant and antimicrobial agents. Bioorg. Med. Chem. 2009, 17, 8168–8173. 10.1016/j.bmc.2009.10.035. [DOI] [PubMed] [Google Scholar]
- Kumar V.; Kaur K.; Gupta G. K.; Sharma A. K. Pyrazole containing natural products: Synthetic preview and biological significance. Eur. J. Med. Chem. 2013, 69, 735–753. 10.1016/j.ejmech.2013.08.053. [DOI] [PubMed] [Google Scholar]
- Alam O.; Naim M.; Nawaz F.; Alam M. J.; Alam P. Current status of pyrazole and its biological activities. J. Pharm. BioAllied Sci. 2016, 8, 2–17. 10.4103/0975-7406.171694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria J. V.; Vegi P. F.; Miguita A. G. C.; dos Santos M. S.; Boechat N.; Bernardino A. M. R. Recently reported biological activities of pyrazole compounds. Bioorg. Med. Chem. 2017, 25, 5891–5903. 10.1016/j.bmc.2017.09.035. [DOI] [PubMed] [Google Scholar]
- Ellinwood D. C.; El-Mansy M. F.; Plagmann L. S.; Stevens J. F.; Maier C. S.; Gombart A. F.; Blakemore P. R. Total synthesis of [13C] 2-,[13C] 3-, and [13C] 5-isotopomers of xanthohumol, the principal prenylflavonoid from hops. J. Labelled Compd. Radiopharm. 2017, 60, 639–648. 10.1002/jlcr.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert G.; Charlton F.; Rye K.-A.; Piper D. E. Molecular basis of PCSK9 function. Atherosclerosis 2009, 203, 1–7. 10.1016/j.atherosclerosis.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Tschöp M. H.; Speakman J. R.; Arch J. R. S.; Auwerx J.; Brüning J. C.; Chan L.; Eckel R. H.; Farese R. V.; Galgani J. E.; Hambly C.; Herman M. A.; Horvath T. L.; Kahn B. B.; Kozma S. C.; Maratos-Flier E.; Müller T. D.; Münzberg H.; Pfluger P. T.; Plum L.; Reitman M. L.; Rahmouni K.; Shulman G. I.; Thomas G.; Kahn C. R.; Ravussin E. A guide to analysis of mouse energy metabolism. Nat. Methods 2012, 9, 57–63. 10.1038/nmeth.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T. A.; Jinnah H. A.; Kamatani N. Shortage of Cellular ATP as a Cause of Diseases and Strategies to Enhance ATP. Front. Pharmacol. 2019, 10, 98. 10.3389/fphar.2019.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipata P. L. Mechanism of ATP loss in nonoxidative contracting muscle. Adv. Physiol. Educ. 2011, 35, 92–94. 10.1152/advan.00102.2010. [DOI] [PubMed] [Google Scholar]
- Divakaruni A. S.; Brand M. D. The Regulation and Physiology of Mitochondrial Proton Leak. Physiology 2011, 26, 192–205. 10.1152/physiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- Demine S.; Renard P.; Arnould T. Mitochondrial Uncoupling: A Key Controller of Biological Processes in Physiology and Diseases. Cells 2019, 8, 795. 10.3390/cells8080795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmazer M.; Stevens J. F.; Buhler D. R. In vitro glucuronidation of xanthohumol, a flavonoid in hop and beer, by rat and human liver microsomes. FEBS Lett. 2001, 491, 252–256. 10.1016/S0014-5793(01)02210-4. [DOI] [PubMed] [Google Scholar]
- Boutin J. A.; Meunier F.; Lambert P.-H.; Hennig P.; Bertin D.; Serkiz B.; Volland J.-P. In vivo and in vitro glucuronidation of the flavonoid diosmetin in rats. Drug Metab. Dispos. 1993, 21, 1157–1166. [PubMed] [Google Scholar]
- Kuczenski R.; Segal D. S. Locomotor Effects of Acute and Repeated Threshold Doses of Amphetamine and Methylphenidate: Relative Roles of Dopamine and Norepinephrine. J. Pharmacol. Exp. Ther. 2001, 296, 876. [PubMed] [Google Scholar]
- Khayyal M. T.; El-Hazek R. M.; El-Sabbagh W. A.; Frank J.; Behnam D.; Abdel-Tawab M. Micellar solubilization enhances the anti-inflammatory effect of xanthohumol. Phytomedicine 2020, 71, 153233. 10.1016/j.phymed.2020.153233. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Bobe G.; Miranda C. L.; Lowry M. B.; Hsu V. L.; Lohr C. V.; Wong C. P.; Jump D. B.; Robinson M. M.; Sharpton T. J.; Maier C. S.; Stevens J. F.; Gombart A. F. Tetrahydroxanthohumol, a xanthohumol derivative, attenuates high-fat diet-induced hepatic steatosis by antagonizing PPARγ. Elife 2021, 10, e66398 10.7554/eLife.66398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraiso I. L.; Revel J. S.; Choi J.; Miranda C. L.; Lak P.; Kioussi C.; Bobe G.; Gombart A. F.; Raber J.; Maier C. S.; Stevens J. F. Targeting the Liver-Brain Axis with Hop-Derived Flavonoids Improves Lipid Metabolism and Cognitive Performance in Mice. Mol. Nutr. Food Res. 20202000341, 64, 2000341. 10.1002/mnfr.202000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koza R. A.; Nikonova L.; Hogan J.; Rim J.-S.; Mendoza T.; Faulk C.; Skaf J.; Kozak L. P. Changes in gene expression foreshadow diet-induced obesity in genetically identical mice. PLoS Genet. 2006, 2, e81 10.1371/journal.pgen.0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Francesco P. N.; Cornejo M. P.; Barrile F.; García Romero G.; Valdivia S.; Andreoli M. F.; Perello M. Inter-individual Variability for High Fat Diet Consumption in Inbred C57BL/6 Mice. Front. Nutr. 2019, 6, 67. 10.3389/fnut.2019.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Smith D. L. Jr; Keating K. D.; Allison D. B.; Nagy T. R. Variations in body weight, food intake and body composition after long-term high-fat diet feeding in C57BL/6J mice. Obesity 2014, 22, 2147–2155. 10.1002/oby.20811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burcelin R.; Crivelli V.; Dacosta A.; Roy-Tirelli A.; Thorens B. Heterogeneous metabolic adaptation of C57BL/6J mice to high-fat diet. Am. J. Physiol.: Endocrinol. Metab. 2002, 282, E834–E842. 10.1152/ajpendo.00332.2001. [DOI] [PubMed] [Google Scholar]
- Logan I. E.; Shulzhenko N.; Sharpton T. J.; Bobe G.; Liu K.; Nuss S.; Jones M. L.; Miranda C. L.; Vasquez-Perez S.; Pennington J. M.; Leonard S. W.; Choi J.; Wu W.; Gurung M.; Kim J. P.; Lowry M. B.; Morgun A.; Maier C. S.; Stevens J. F.; Gombart A. F. Xanthohumol Requires the Intestinal Microbiota to Improve Glucose Metabolism in Diet-Induced Obese Mice. Mol. Nutr. Food Res. 2021, 2100389. 10.1002/mnfr.202100389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn B. B.; Flier J. S. Obesity and insulin resistance. J. Clin. Invest. 2000, 106, 473–481. 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn S. E.; Hull R. L.; Utzschneider K. M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- Stevens J. F.Xanthohumol and Structurally Related Prenylflavonoids for Cancer Chemoprevention and Control. In Natural Products for Cancer Chemoprevention; Springer, 2020, pp 319–350. 10.1007/978-3-030-39855-2_10. [DOI] [Google Scholar]
- Westerterp K. R. Diet induced thermogenesis. Nutr. Metab. 2004, 1, 5. 10.1186/1743-7075-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerterp K. R. Control of energy expenditure in humans. Eur. J. Clin. Nutr. 2017, 71, 340–344. 10.1038/ejcn.2016.237. [DOI] [PubMed] [Google Scholar]
- Dias F. R. C.; de Matos L. W.; Sampaio M. d. F. d. S.; Carey R. J.; Carrera M. P. Opposite effects of low versus high dose haloperidol treatments on spontaneous and apomorphine induced motor behavior: Evidence that at a very low dose haloperidol acts as an indirect dopamine agonist. Behav. Brain Res. 2012, 229, 153–159. 10.1016/j.bbr.2011.12.042. [DOI] [PubMed] [Google Scholar]
- De la Casa L. G.; Cárcel L.; Ruiz-Salas J. C.; Vicente L.; Mena A. Conditioned increase of locomotor activity induced by haloperidol. PLoS One 2018, 13, e0200178 10.1371/journal.pone.0200178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson K. J.; Zahorska-Markiewicz B.; Pittet P.; Anantharaman K.; Jéquier E. Caffeine and coffee: their influence on metabolic rate and substrate utilization in normal weight and obese individuals. Am. J. Clin. Nutr. 1980, 33, 989–997. 10.1093/ajcn/33.5.989. [DOI] [PubMed] [Google Scholar]
- Fisone G.; Borgkvist A.; Usiello A. Caffeine as a psychomotor stimulant: mechanism of action. Cell. Mol. Life Sci. 2004, 61, 857–872. 10.1007/s00018-003-3269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welihinda A. A.; Kaur M.; Greene K.; Zhai Y.; Amento E. P. The adenosine metabolite inosine is a functional agonist of the adenosine A2A receptor with a unique signaling bias. Cell Signal. 2016, 28, 552–560. 10.1016/j.cellsig.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N.; Kemp N.; Adeyemo O.; Buchanan P.; Stone T. W. Anxiolytic activity of adenosine receptor activation in mice. Br. J. Pharmacol. 1995, 116, 2127–2133. 10.1111/j.1476-5381.1995.tb16421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraco R. A.; Martens K. A.; Parizon M.; Normile H. J. Adenosine A2a receptors in the nucleus accumbens mediate locomotor depression. Brain Res. Bull. 1993, 31, 397–404. 10.1016/0361-9230(93)90233-2. [DOI] [PubMed] [Google Scholar]
- Ballesteros-Yáñez I.; Castillo C. A.; Merighi S.; Gessi S. The Role of Adenosine Receptors in Psychostimulant Addiction. Front. Pharmacol. 2018, 8, 985. 10.3389/fphar.2017.00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiga Y.; Sakai K.; Nakashima S.; Uehara Y.; Kawanaka K.; Higaki Y. Effects of inosine monophosphate and exercise training on neuronal nitric oxide synthase in the mouse brain. Neurosci. Lett. 2020, 734, 135083. 10.1016/j.neulet.2020.135083. [DOI] [PubMed] [Google Scholar]
- Tomiga Y.; Yoshimura S.; Ito A.; Nakashima S.; Kawanaka K.; Uehara Y.; Tanaka H.; Higaki Y. Exercise training rescues high fat diet-induced neuronal nitric oxide synthase expression in the hippocampus and cerebral cortex of mice. Nitric Oxide 2017, 66, 71–77. 10.1016/j.niox.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Barnes V. M.; Kennedy A. D.; Panagakos F.; Devizio W.; Trivedi H. M.; Jönsson T.; Guo L.; Cervi S.; Scannapieco F. A. Global metabolomic analysis of human saliva and plasma from healthy and diabetic subjects, with and without periodontal disease. PLoS One 2014, 9, e105181 10.1371/journal.pone.0105181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes V. M.; Teles R.; Trivedi H. M.; Devizio W.; Xu T.; Mitchell M. W.; Milburn M. V.; Guo L. Acceleration of purine degradation by periodontal diseases. J. Dent. Res. 2009, 88, 851–855. 10.1177/0022034509341967. [DOI] [PubMed] [Google Scholar]
- Aquilano K.; Baldelli S.; Ciriolo M. R. Glutathione: new roles in redox signaling for an old antioxidant. Front. Pharmacol. 2014, 5, 196. 10.3389/fphar.2014.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galano A.; Alvarez-Idaboy J. R. Glutathione: mechanism and kinetics of its non-enzymatic defense action against free radicals. RSC Adv. 2011, 1, 1763–1771. 10.1039/C1RA00474C. [DOI] [Google Scholar]
- Noeman S. A.; Hamooda H. E.; Baalash A. A. Biochemical Study of Oxidative Stress Markers in the Liver, Kidney and Heart of High Fat Diet Induced Obesity in Rats. Diabetol. Metab. Syndr. 2011, 3, 17. 10.1186/1758-5996-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent H.; Powers S.; Dirks A.; Scarpace P. Mechanism for obesity-induced increase in myocardial lipid peroxidation. Int. J. Obes. 2001, 25, 378–388. 10.1038/sj.ijo.0801536. [DOI] [PubMed] [Google Scholar]
- Findeisen H. M.; Gizard F.; Zhao Y.; Qing H.; Jones K. L.; Cohn D.; Heywood E. B.; Bruemmer D. Glutathione depletion prevents diet-induced obesity and enhances insulin sensitivity. Obesity 2011, 19, 2429–2432. 10.1038/oby.2011.298. [DOI] [PubMed] [Google Scholar]
- Zieliński J.; Kusy K. Training-induced adaptation in purine metabolism in high-level sprinters vs. triathletes. J. Appl. Physiol. 2012, 112, 542–551. 10.1152/japplphysiol.01292.2011. [DOI] [PubMed] [Google Scholar]
- Zamzow D. R.; Elias V.; Legette L. L.; Choi J.; Stevens J. F.; Magnusson K. R. Xanthohumol improved cognitive flexibility in young mice. Behav. Brain Res. 2014, 275, 1–10. 10.1016/j.bbr.2014.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchihashi S.; Nishikawa M.; Sakaki T.; Ikushiro S.-i. Comparison of serotonin glucuronidation activity of UDP-glucuronosyltransferase 1a6a (Ugt1a6a) and Ugt1a6b: evidence for the preferential expression of Ugt1a6a in the mouse brain. Drug Metab. Pharmacokinet. 2013, 28, 260–264. 10.2133/dmpk.DMPK-12-NT-091. [DOI] [PubMed] [Google Scholar]
- Buckley D. B.; Klaassen C. D. Tissue-and gender-specific mRNA expression of UDP-glucuronosyltransferases (UGTs) in mice. Drug Metab. Dispos. 2007, 35, 121–127. 10.1124/dmd.106.012070. [DOI] [PubMed] [Google Scholar]
- Shin J.-W.; Seol I.-C.; Son C.-G. Interpretation of animal dose and human equivalent dose for drug development. J. Korean Med. 2010, 31, 1–7. [Google Scholar]
- Nair A.; Jacob S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27. 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgacsova A.; Galba J.; Garruto R. M.; Majerova P.; Katina S.; Kovac A. A novel liquid chromatography/mass spectrometry method for determination of neurotransmitters in brain tissue: Application to human tauopathies. J. Chromatogr. B 2018, 1073, 154–162. 10.1016/j.jchromb.2017.12.015. [DOI] [PubMed] [Google Scholar]
- Alcazar Magana A.; Reed R. L.; Koluda R.; Miranda C. L.; Maier C. S.; Stevens J. F. Vitamin C Activates the Folate-Mediated One-Carbon Cycle in C2C12 Myoblasts. Antioxidants 2020, 9, 217. 10.3390/antiox9030217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner L. W.; Amberg A.; Barrett D.; Beale M. H.; Beger R.; Daykin C. A.; Fan T. W.-M.; Fiehn O.; Goodacre R.; Griffin J. L.; Hankemeier T.; Hardy N.; Harnly J.; Higashi R.; Kopka J.; Lane A. N.; Lindon J. C.; Marriott P.; Nicholls A. W.; Reily M. D.; Thaden J. J.; Viant M. R. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. 10.1007/s11306-007-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viant M. R.; Kurland I. J.; Jones M. R.; Dunn W. B. How close are we to complete annotation of metabolomes?. Curr. Opin. Chem. Biol. 2017, 36, 64–69. 10.1016/j.cbpa.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraiso I. L.; Tran T. Q.; Magana A. A.; Kundu P.; Choi J.; Maier C. S.; Bobe G.; Raber J.; Kioussi C.; Stevens J. F. Xanthohumol ameliorates diet-induced liver dysfunction via farnesoid X receptor-dependent and independent signaling. Front. Pharmacol. 2021, 12, 749. 10.3389/fphar.2021.643857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.