Abstract
Objectives
In patients with recurrent laryngeal nerve (RLN) injury after thyroid surgery, unrecovered vocal fold motion (VFM) and subjective voice impairment cause extreme distress. For surgeons, treating these poor outcomes is extremely challenging. To enable early treatment of VFM impairment, this study evaluated prognostic indicators of non-transection RLN injury and VFM impairment after thyroid surgery and evaluated correlations between intraoperative neuromonitoring (IONM) findings and perioperative voice parameters.
Methods
82 adult patients had postoperative VFM impairment after thyroidectomy were enrolled. Demographic characteristics, RLN electromyography (EMG), and RLN injury mechanism were compared. Multi-dimensional voice program, voice range profile and Index of voice and swallowing handicap of thyroidectomy (IVST) were administered during I-preoperative; II-immediate, III-short-term and IV-long-term postoperative periods. The patients were divided into R/U Group according to the VFM was recovered/unrecovered 3 months after surgery. The patients in U Group were divided into U1/U2 Group according to total IVST score change was <4 and ≥4 during period-IV.
Results
Compared to R Group (42 patients), U Group (38 patients) had significantly more patients with EMG >90% decrease in the injured RLN (p<0.001) and thermal injury as the RLN injury mechanism (p=0.002). Voice parameter impairments were more severe in U Group compared to R Group. Compared to U1 group (19 patients), U2 Group (19 patients) had a significantly larger proportion of patients with EMG decrease >90% in the injured RLN (p=0.022) and thermal injury as the RLN injury mechanism (p=0.017). A large pitch range decrease in period-II was a prognostic indicator of a moderate/severe long-term postoperative subjective voice impairment.
Conclusion
This study is the first to evaluate correlations between IONM findings and voice outcomes in patients with VFM impairment after thyroid surgery. Thyroid surgeons should make every effort to avoid severe type RLN injury (e.g., thermal injury or injury causing EMG decrease >90%), which raises the risk of unrecovered VFM and moderate/severe long-term postoperative subjective voice impairment. Using objective voice parameters (e.g., pitch range) as prognostic indicators not only enables surgeons to earlier identify patients with low voice satisfaction after surgery, and also enable implementation of interventions sufficiently early to maintain quality of life.
Keywords: thyroid surgery, intraoperative neuromonitoring (IONM), vocal fold motion, recurrent laryngeal nerve (RLN), index of voice and swallowing handicap of thyroidectomy (IVST), subjective/objective voice analysis
Introduction
Recurrent laryngeal nerve (RLN) injury during thyroid surgery is the most common etiology of vocal fold motion (VFM) impairment (1) and morbidity after this procedure and a leading cause of medico-legal litigation after thyroid surgery (2). To avoid RLN injury during thyroid surgery, adjunct use of intraoperative neuromonitoring (IONM) has gained widespread use for identifying the RLN early in thyroid surgery and for elucidating nerve injury mechanism (3–6). The incidence of RLN paralysis may be underestimated if VFM is not routinely examined (7). Previous works report that permanent RLN palsy occurs in 1–3% of thyroid surgeries and that temporary RLN palsy occurs in 1.4-38% of these procedures (8, 9). For comprehensive evaluation of RLN function before and after thyroid surgery, current practice guidelines recommend performing pre- and post-operative laryngofiberscopy routinely (6, 10).
Patients who had a RLN injury during thyroid surgery are expected to have VFM impairment after surgery and may also experience severe dysphonia, dyspnea, and aspiration (11). In patients with temporary RLN palsy caused by a non-transection RLN injury, Chiang et al. (12) reported that VFM usually recovers within 3 days to 4 months (mean, 30.7 days) after surgery. However, the course of VFM recovery depends on the nerve injury mechanism (13). Thermal injuries are associated with a long recovery time and severe histological disturbance in the endoneurium whereas mechanical injuries are associated with distorted epineuria and perineuria (13, 14). After RLN injury, natural neuromuscular compensation mechanisms may yield outcomes that are acceptable to the patient, including RLN function outcomes and voice outcomes (15). However, early assessment of voice performance is important for deciding subsequent voice intervention (16). There is evidence that voice interventions 3 months after RLN injury can greatly improve voice outcome after thyroid surgery (10, 17, 18).
Impaired VFM after thyroid surgery is considered evidence of RLN injury. Unrecovered VFM and subjective voice impairment degrade quality of life and satisfaction with treatment, which causes extreme distress in patients. For surgeons, treating these poor outcomes is also extremely challenging. To enable early identification and treatment of VFM impairment and to improve patient satisfaction with surgery outcomes, this study evaluated prognostic indicators of non-transection RLN injury and VFM impairment after thyroid surgery. Correlations between IONM findings and perioperative voice parameters were also investigated.
Materials and Methods
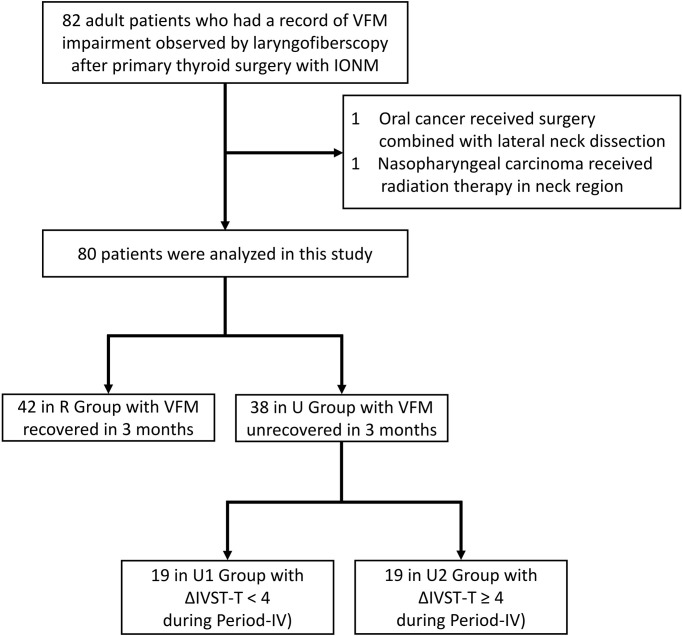
The subjects of this study were adult patients who had received IONM-assisted primary thyroid surgery by “IONM team” at a single institution (Kaohsiung Medical University Hospital, Taiwan) from June, 2013, to June, 2019. All patients had normal and symmetric VFM before surgery and had VFM impairment within 2 weeks after surgery. The total number of surgeries performed in this period was 1033, and the number of surgeries without VFM impairment was 951. The analysis included 82 (7.9%) patients after exclusion of one oral cancer patient who had received cancer surgery combined with lateral neck dissection and one nasopharyngeal carcinoma patient who had received radiation therapy in the neck region. The flowchart in Figure 1 depicts the procedure for inclusion and exclusion of patients in this study. Ethical approval of this study was obtained from the Kaohsiung Medical University Hospital Institutional Review Board (KMUHIRB-E(I)-20200359). In all patients, vagus nerve function and RLN function were routinely evaluated using the standard four-step vagus nerve and RLN stimulation (V1-R1-R2-V2) procedure under IONM and the evoked electromyography (EMG) amplitudes were obtained and recorded (3, 19). The RLN EMG decrease was defined and calculated as the decrease in the EMG amplitude of the post-dissection R2 signal from the EMG amplitude of the pre-dissection R1 signal. No patient who had received bilateral thyroidectomy in this study had RLN signal >50% decreases on both sides. All exposed RLNs, including injury mechanisms (transection, mechanical, or thermal), were documented by photograph. Mechanical injury was classified as type 1 (segmental injury) or type 2 (global injury) according to the criteria proposed by Chiang et al. (3) and the INMSG (6). All patients received surgery by “IOINM team” routinely received a VFM survey. Laryngofiberscopy was documented by video in all patients before surgery and within 2 weeks after surgery. All patients with VFM impairment 2 weeks after surgery underwent additional monthly examinations until VFM recovery. Patients were divided into two groups according to VFM recovery: a recovered VFM group (R Group) and an unrecovered VFM group (U Group). The R Group criterion was laryngofiberscopic evidence of VFM impairment recovery within 3 months after thyroidectomy; the U group criterion was persistent VFM impairment more than 3 months after thyroidectomy. Patient information, including gender, age, surgery type (unilateral versus bilateral), and pathology results (benign versus malignant) was recorded and compared between groups.
Figure 1.
Flow diagram for inclusion and exclusion of patients. VFM, vocal fold motion; IONM, intraoperative neuromonitoring; IVST, Index of Voice and Swallowing Handicap of Thyroidectomy.
Objective and Subjective Voice Analysis
All patients underwent both subjective and objective voice analyses in four periods: period-I (preoperative period, within 2 months before surgery), period-II (immediate postoperative period, median duration of 3 days, range of 1-7 days), period-III (short-term postoperative period, median duration of 12 days, range of 7-30 days), and period-IV (long-term postoperative period, median duration of 40 days, range of 30-90 days).
All objective voice analyses in all subjects were performed by a single experienced speech-language pathologist (WHV. Y) using the Multidimensional Voice Program (model 5105, version 3.1.7; KayPENTAX, USA) and the Voice Range Profile (model 4326, version 3.3.0; KayPENTAX, USA). Multidimensional Voice Program analyses included mean fundamental frequency (mean F0), jitter, shimmer and noise-to-harmonic ratio whereas Voice Range Profile analyses included maximum pitch frequency (Fmax), minimum pitch frequency (Fmin), and pitch range (PR). The PR was defined as the number of semitones between Fmax and Fmin.
All preoperative and postoperative subjective voice analyses were performed using the 10-item Index of Voice and Swallowing Handicap of Thyroidectomy (IVST) ( Supplemental Table 1 ), which provides an index of the main symptoms observed in the patient. Each item in this subjective assessment is scored from 0-2 (never, sometimes, and always), respectively. The two domains of the IVST are the voice domain (IVST-V; items 1-7; score range 0-14) and the swallowing domain (IVST-S; items 8-10; score range 0-6). Thus, the score range for the total IVST (IVST-T) is 0 to 20.
The equation for calculating postoperative change in objective voice analysis data was Δ= (B - A)/A, and the equation for calculating postoperative change in subjective voice analysis data was Δ= B – A, where A and B are preoperative and postoperative values, respectively.
Long-term postoperative subjective voice impairment was also evaluated in patients with unrecovered VFM (U Group). The U group was divided into patients with no/mild subjective voice impairment (ΔIVST-T < 4) in Period-IV (U1 Group) and patients with moderate/severe subjective voice impairment (ΔIVST-T ≥ 4) in Period-IV (U2 Group).
Statistical Analysis
To analyze the variables, independent t test and Pearson chi-square test were performed using R software (version-3.4). A two-tailed p value less than 0.05 was considered statistically significant.
Results
Demographic Characteristics of R Group and U Group
Table 1 compares the 42 (52.5%) patients in R Group and the 38 (47.5%) patients in U Group. Age, gender, surgical extent, and pathology results did not significantly differ between groups. The proportions of patients with <50%, 50-90%, >90% EMG decreases in the injured RLN were 0.0%, 83.3%, and 16.7% in R Group, respectively; the corresponding proportions in U Group were 0.0%, 44.7%, and 55.3%, respectively. That is, the proportions of patients with >90% EMG decreases were significantly larger in U Group compared to R Group (p<0.001).
Table 1.
Demographic characteristics of Recovered VFM Group (R Group) and Unrecovered VFM Group (U Group).
| R Group | U Group | p value | |
|---|---|---|---|
| Number (%) | 42 (52.5%) | 38 (47.5%) | |
| Age (mean ± SD) | 50.7 ± 13.7 | 52.7 ± 12.4 | 0.497 |
| Gender | 0.073 | ||
| Male (%) | 11 (26.2%) | 5 (13.2%) | |
| Female (%) | 31 (73.8%) | 33 (86.8%) | |
| Surgical extent | 0.086 | ||
| Unilateral (%) | 7 (16.7%) | 1 (2.6%) | |
| Bilateral (%) | 35 (83.3%) | 37 (97.4%) | |
| Pathology | 0.069 | ||
| Benign (%) | 24 (57.1%) | 14 (36.8%) | |
| Malignant (%) | 18 (42.9%) | 24 (73.2%) | |
| EMG decrease in the injured RLN | <0.001 | ||
| <50% decreased (%) | 0 (0.0%) | 0 (0.0%) | |
| 50-90% decreased (%) | 35 (83.3%) | 17 (44.7%) | |
| >90% decreased(%) | 7 (16.7%) | 21 (55.3%) | |
| RLN Injury mechanism and type | 0.002 | ||
| Transection (%) | 0 (0.0%) | 0 (0.0%) | |
| Mechanical (%) | 42 (100.0%) | 30 (78.9%) | |
| Type 1 | 33 | 27 | |
| Type 2 | 9 | 3 | |
| Thermal (%) | 0 (0.0%) | 8 (21.1%) | |
| Subjective voice impairment during period-IV | 0.028* | ||
| No/Mild (ΔIVST-T <4) (%) | 31 (73.8%) | 19 (50.0%) | |
| Moderate/Severe (ΔIVST-T ≥4) (%) | 11 (26.2%) | 19 (50.0%) | |
| RLN reinnervation or nerve grafting | 0 (0.0%) | 0 (0.0%) |
VFM, vocal fold motion; SD, standard deviation; EMG, electromyography; RLN, recurrent laryngeal nerve; IVST, Index of Voice and Swallowing Handicap of Thyroidectomy; IVST-T, Total IVST score; period IV, Long-term postoperative period (range of 30-90 days).
*p value <0.05, showed significant difference.
No patients had transection injury in either the R Group or the U Group. All 42 (100.0%) patients in the R Group had mechanical injury (type 1 in 33 patients and type 2 in 9 patients). Among the 38 patients in U Group, 30 (78.9%) patients had mechanical injury, 27 had type 1 injury, 3 had type 2 injury; and 8 (21.1%) had thermal injury. The percentage of patients with thermal injury was significantly higher in U Group compared to R Group (p=0.002). The proportions of patients with no/mild (ΔIVST-T <4) and moderate/severe (ΔIVST-T ≥4) subjective voice impairment in period-IV were 73.8% and 26.2% in R Group, respectively, versus 50.0% and 50.0% in U Group, respectively. In period-IV, U group also had significantly more patients with moderate/severe subjective voice impairment compared to R Group (p=0.028). No patients in this study had received RLN reinnervation or nerve grafting.
Voice Parameter Changes (Δ) Associated With Different VFM Outcomes
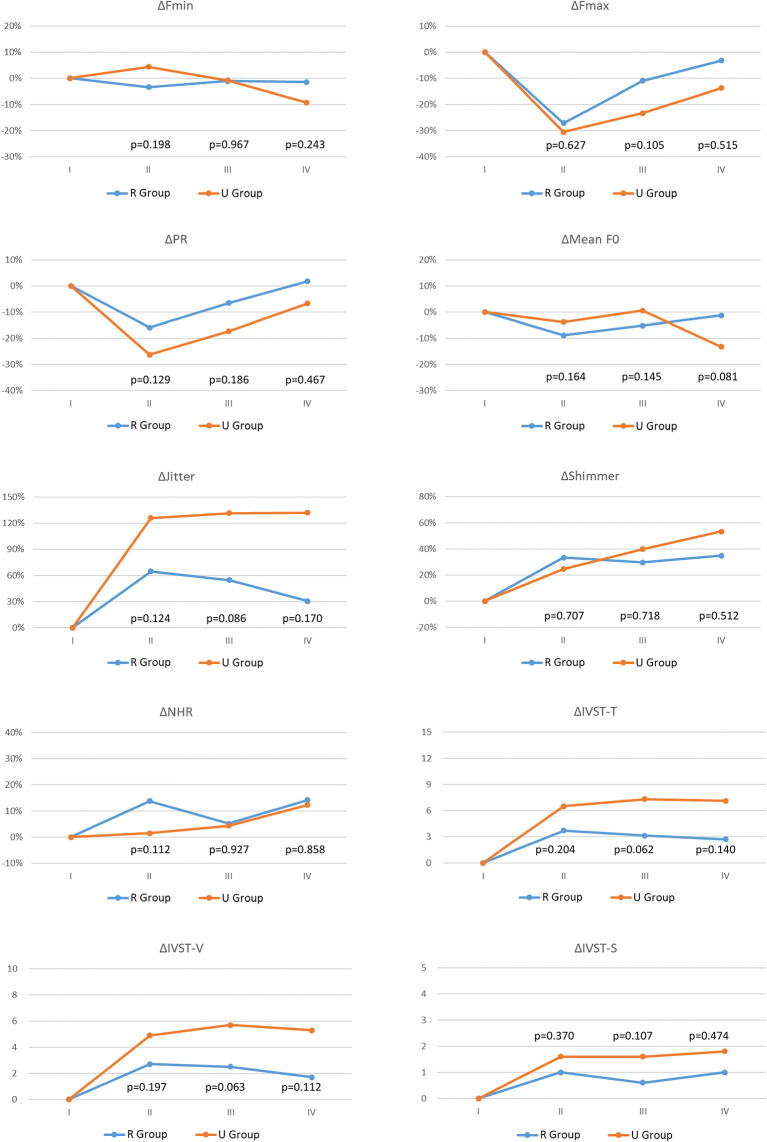
In Supplemental Table 2 , the detailed voice parameters, voice parameter changes (Δ), and p values are compared between R Group and U Group in each follow-up period. Voice parameter comparisons between the two groups revealed significant differences in preoperative Fmax and preoperative PR. Postoperative parameters did not significantly differ. To minimize the influence of preoperative differences, voice parameter changes (Δ) were calculated and compared. In period-IV the U group had a ΔIVST-T score of 7.1 ± 6.8.
Figure 2 compares voice parameter changes (Δ) between the R Group and the U Group in each follow-up period. Due to large standard deviations, the two groups did not significantly differ in objective or subjective voice parameters in all follow-up periods. However, U Group showed larger impairments of voice parameters compared to R Group, especially in ΔFmax, ΔPR, ΔJitter, ΔIVST-T, ΔIVST-V, and ΔIVST-S.
Figure 2.
Voice parameter changes (Δ) with different vocal cord movement (VFM) outcomes: R Group (Blue line) and U Group (Red line). Fmax, Maximum pitch frequency; Fmin, Minimum pitch frequency; PR, Pitch range; Mean F0, mean fundamental frequency; NHR, noise-to-harmonic ratio; IVST, Index of Voice and Swallowing Handicap of Thyroidectomy; IVST-T, Total IVST score; IVST-V, IVST score of voice domain score; IVST-S, IVST score of swallowing domain. period I, Preoperative period (within 2 months before surgery); period II, Immediate postoperative period (median duration of 3 days; range of 1-7 days); period III, Short-term postoperative period (median duration of 12 days; range of7-30 days); period IV, Long-term postoperative period (median duration of 40 days, range of 30-90 days). The equation for calculating postoperative change in objective voice analysis (Fmax, Fmin, PR, Mean F0, Jitter, Shimmer, NHR) data was Δ= (B - A)/A, the unit is %; the equation for calculating postoperative change in subjective voice analysis (IVST-T, IVST-V, IVST-S) data was Δ= B – A, the unit is score. Where A and B are preoperative and postoperative values, respectively. The preoperative Δ is 0 in all the voice parameter. p value <0.05, showed significant difference.
Demographic Characteristics and Objective Voice Parameter Changes (Δ) in U Group According to Severity of Subjective Voice Impairment in Long-Term Postoperative Period
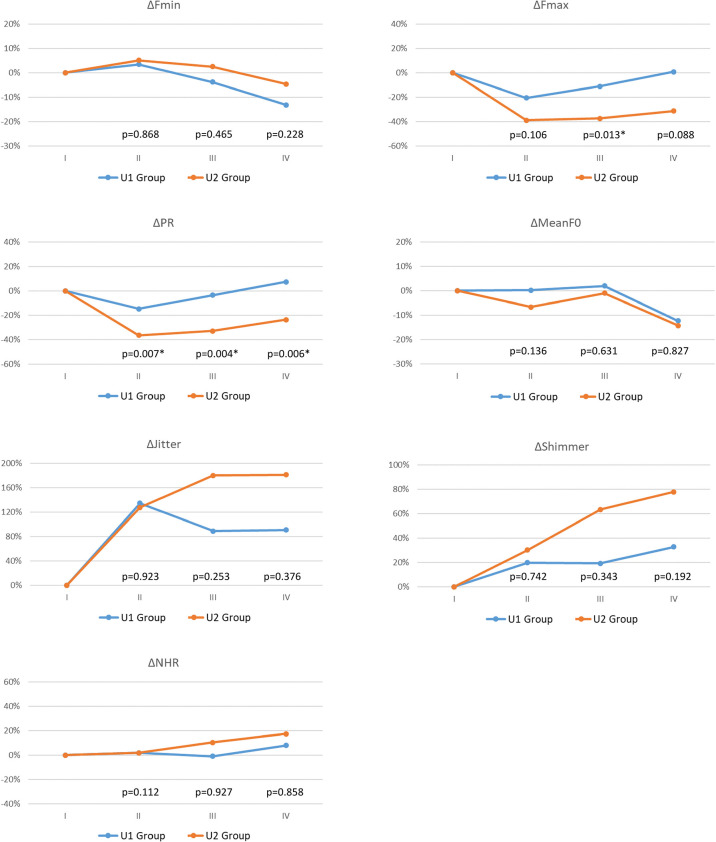
To evaluate the wide range of subjective voice impairment within U Group, Table 2 shows the demographic characteristics of the U1 Group (no/mild subjective voice impairment) and U2 Group (moderate/severe subjective voice impairment). Compared to the U1 Group the U2 Group had significantly larger proportions of patients with EMG decrease >90% in the injured RLN (p=0.022) and patients with thermal injury as the primary RLN injury mechanism (p=0.017). Figure 3 compares objective voice parameter changes (Δ) in the U1 Group and U2 Group in each follow-up period. In all postoperative periods, impairments in objective voice parameters were much larger in U2 Group compared to the U1 group. In period-II, ΔPR significantly p=0.007) differed between the U1 and U2 groups. In period-III, ΔFmax (p=0.013) and ΔPR (p=0.004) differed between the U1 and U2 groups; ΔJitter and ΔShimmer were larger in U2 Group compared to U1 Group. In period-IV, ΔPR (p=0.006) also significantly differed between groups; ΔFmax, ΔJitter, and ΔShimmer were larger in U2 Group compared to U1 Group.
Table 2.
Demographic characteristic of patients in U Group with different subjective voice outcomes during long-term postoperative period (Period-IV).
| U1 Group (ΔIVST-T < 4 during Period-IV) | U2 Group (ΔIVST-T ≥ 4 during Period-IV) | p value | |
|---|---|---|---|
| Case number | 19 patients (50.0%) | 19 patients (50.0%) | |
| Age | 50.5 ± 10.9 | 54.9 ± 11.8 | 0.240 |
| Gender | 0.150 | ||
| Male (%) | 4 (21.1%) | 1 (5.3%) | |
| Female (%) | 15 (78.8%) | 18 (94.7%) | |
| Surgical extent | 0.311 | ||
| Unilateral (%) | 1 (5.3%) | 0 (0.0%) | |
| Bilateral (%) | 18 (94.7%) | 19 (100.0%) | |
| Pathology | 0.179 | ||
| Benign (%) | 5 (26.3%) | 9 (47.4%) | |
| Malignant (%) | 14 (73.7%) | 10 (52.6%) | |
| EMG decrease in the injured RLN | 0.022* | ||
| 50-90% decreased (%) | 12 (63.2%) | 5 (26.3%) | |
| >90% decreased (%) | 7 (36.8%) | 14 (73.7%) | |
| RLN injury mechanism | 0.017* | ||
| Mechanical (%) | 18 (94.7%) | 12 (63.2%) | |
| Thermal (%) | 1 (5.3%) | 7 (36.8%) |
IVST, Index of Voice and Swallowing Handicap of Thyroidectomy; IVST-T, Total IVST score; EMG, electromyography; RLN, recurrent laryngeal nerve.
Period IV (range of 30-90 days).
*p value <0.05, showed significant difference.
Figure 3.
Objective voice parameter changes (Δ) with different subjective voice outcomes during long-term postoperative period (Period-IV): U1 Group (Blue line, ΔIVST-T< 4 during Period-IV) and U2 Group (Red line, ΔIVST-T ≥ 4 during Period-IV). The abbreviations of objective/subjective voice parameters, the definition of follow-up periods (I/II/III/IV), and the equation for calculating postoperative change in objective voice analysis are same in Figure 2 . The preoperative Δ is 0 in all the voice parameter. p value <0.05, showed significant difference.
Discussion
This study is the first to investigate correlations between IONM findings and voice outcomes in patients with impaired VFM after thyroid surgery. This analysis showed that, compared to the R Group, the U Group (particularly the U2 Group) had larger proportions of patients with EMG decrease >90% in the injured RLN and patients with thermal injury as the RLN injury mechanism ( Tables 1 , 2 ). Voice parameter changes were much larger in the U Group compared to the R Group, but the change did not reach statistical significance ( Figure 2 ). A large pitch range decrease in period-II is a prognostic indicator of moderate/severe long-term postoperative subjective voice impairment ( Figure 3 ). Therefore, surgeons should make every effort to avoid severe type RLN injury during thyroid surgery and should utilize voice analysis to enable early identification of patients who may be experiencing distress and low satisfaction caused by poor surgical outcomes and to enable early initiation of intervention therapy to maintain quality of life in these patients.
The EMG data in revealed four interesting findings regarding injury mechanisms and postoperative VFM outcomes ( Table 1 ). 1) No patient with EMG <50% decrease had postoperative asymmetric VFM. This finding indicates that preserving RLN function during surgery is the key factor in VFM preservation after surgery. Accurately recording all data for asymmetric VFM before surgery is also essential for avoiding misjudgment of vocal cord function and potential legal liability (20). 2) Patients with EMG 50-90% decrease in the injured RLN may not achieve total recovery from asymmetric VFM. Using continuous IONM can minimize complications by avoiding surgical procedures that can cause mechanical injury (21). Continuous IONM prevents most mechanical injuries with visually intact RLN, thereby enabling modification of injury-causing surgical procedures in 80% of cases (22). The advancement of continuous IONM technology can make a breakthrough in VFM evaluation after thyroidectomy (23). 3) All patients with thermal RLN injury in this study had unrecovered VFM (U Group). RLN thermal injury is difficult to be detected visually and the risk of permanent palsy is high, surgeons should maintain vigilance in modern thyroid surgery that increases the use of energy-based devices (24). Compared to relatively mild mechanical injuries, thermal injuries sustained during surgery may require earlier voice intervention (25) performed under comprehensive postoperative laryngofiberscopy and voice analysis. 4) The R Group and the U Group in this study did not significantly differ in Type 1 and Type 2 mechanical injury. In severe Type 2 (global) mechanical injury, VFM may have chance to be unrecovered.
In current study, patients in U group had higher proportion of malignant pathologic report than patients in R group (73.2% vs. 42.9%). For malignant disease, surgeons tend to dissect a longer segment in proximal end of RLN and perform central neck dissection; and tend to dissect the most distal end of RLN near laryngeal entry to decrease the tissue remnant in malignant disease. According to a large international registry database study with 1,000 RLNs at risks enrolled, the abnormal RLN trajectory (23%) was higher than surgeon expected, and 34% of RLN with loss of signal following an abnormal trajectory, for instance, fixed/splayed/entrapped RLN at the ligament of Berry, extensive RLN dissection, cases of cancer invasion or when lateral lymph node dissection (26). In this manner, the risk of RLN mechanical and thermal injury rate can be higher in malignant disease.
Some of the patients who revealed asymmetric VFM under laryngofiberscopy still had good subjective voice performance, which has been mentioned previously (27, 28). Reiter et al. (29) reported that voice parameters did not significantly differ between patients with and patients without recovery from vocal cord paralysis at the end of a 12-month follow up. In the current study, the R Group and the U Group did not significantly differ in objective or subjective voice parameters in all follow-up periods. However, U Group had much larger impairments compared to R Group, especially in ΔFmax, ΔPR, ΔJitter, ΔIVST-T, ΔIVST-V, and ΔIVST-S in Figure 2 . The likely explanation for the lack of significant between-group differences was the large within-group differences in subjective voice outcomes. As Table 2 indicates, the U1 and U2 Groups significantly differed in the incidence of severe RLN injury (i.e., EMG >90% decrease in injured RLN) and in RLN injury mechanism (thermal injury). Therefore, thyroid surgeons should make every effort to avoid severe type RLN injury. In the literature, several technical maneuvers had been reported for rescuing RLN function, including intravenous steroid (30), nimodipine (31), cold dextrose solution irrigation (32), etc., but the effectiveness of these methods remain uncertain. Besides of RLN factors, sufficient neuromuscular compensation plays an important role in reducing voice impairment after thyroid surgery (15, 33). Therefore, for good long-term postoperative voice outcomes after thyroid surgery, surgeons should prioritize restoration of neuromuscular compensation. Achieving the maximum neuromuscular compensation requires a multidisciplinary approach, especially with the participation of a speech-language pathologist to perform speech and dysphagia therapy (34). Other advanced interventions such as augmentation laryngoplasty and nerve reinnervation also have important rehabilitative roles in patients with voice recovery failure (35, 36).
In many institutions, the clinical practice environment may not include a thyroid surgeon with a laryngology background and may not have well-established procedures for routine cooperation between surgeons and speech-language pathologists (34). Under these conditions, compensation for voice impairment after thyroid surgery may not occur until long after surgery, which complicates detection of complications and other outcomes. Inadequate personnel and practices such as these may also mislead surgeons to believe that follow-up laryngofiberscopy and voice analysis are unnecessary. Early identification of voice impairment caused by VFM asymmetry can reduce the number of patient consultations needed to address psychological distress caused by poor voice outcomes and related concerns. It is worth mentioning that the voice impairment related to external branch of superior laryngeal nerve (EBSLN) injury is less significant than that of iatrogenic RLN injury. However, the stable EBSLN IONM (37) and careful analysis of high-pitched voice change (16) after thyroidectomy will largely improve life quality of the patients.
In terms of screening and interpreting voice outcomes, subjective voice analysis using IVST still has advantages over objective voice analysis. First, since a normal range of objective voice parameters has not been established, objective voice analysis requires time-consuming calculations of voice parameter changes (Δ) in individual patients whereas subjective voice analysis avoids this limitation. Second, for classifying patients with a wide range of long-term postoperative subjective voice impairments, e.g., the U Group in this study, IVST-T is simpler and more acceptable than objective voice parameters. Nevertheless, comprehensive objective voice analysis is still preferable for collecting and analyzing detailed voice data and for using these data as prognostic indicators of subjective voice impairment ( Figure 3 ). Third, in patients with vocal cord paralysis, change in voice quality and loudness are among the most common complaints (38). Since objective voice recording method was used in the current study, loudness adjustments were made during recording, which limited objective assessments of loudness changes. Such changes could only be represented by IVST scores.
Several limitations of this study should be mentioned. First, demographic characteristics that may affect preoperative objective voice parameters differed between R Group and U Group. Future research may investigate novel preoperative objective voice parameters with less demographic variation and define “normal” ranges for such parameters. Second, no system for classifying nerve reinnervation or grafting has been established. Therefore, future works should consider how to account for the wide variation in nerve function outcomes after these procedures. Last, a longer observation time (e.g., 1 year) may be needed to observe voice compensation processes. However, the current data were sufficient for initial identification of trends in voice parameter changes after thyroidectomy. A future long-term post-thyroidectomy voice study should discuss and compare voice interventions (e.g., voice therapy, augmentation laryngoplasty, and medialization thyroplasty) and their effects.
Conclusion
This study is the first to evaluate correlations between IONM findings and voice outcomes in patients with VFM impairment after thyroid surgery. In addition to assessing EMG status and RLN injury mechanisms, thyroid surgeons should routinely include laryngofiberscopy and subjective/objective voice analyses as standard tools for evaluation and diagnosis before and after thyroid surgery. Thyroid surgeons should also make every effort to avoid severe type (EMG >90% decrease, thermal-related) RLN injury, which is associated with a high risk of unrecovered VFM and with a high risk of moderate-to-severe long-term postoperative subjective voice impairment. Using objective voice parameters (e.g., pitch range) as prognostic indicators only enables surgeons to earlier identify patients who have low voice satisfaction after surgery, it also enables early implementation of intervention therapy (e.g., speech therapy, augmentation laryngoplasty, and nerve reinnervation), which can maximize neuromuscular compensation in these patients and maintain their quality of life.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Ethics Statement
Ethical approval of this study was obtained from the Kaohsiung Medical University Hospital Institutional Review Board (KMUHIRB-E(I)-20200359). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Supervision – F-YC, C-WW, K-WL, and S-HL. Materials – T-YH, W-HY, F-YC, and C-WW. Data Collection and Processing – T-YH, W-HY, and S-HL. Analysis and Interpretation- T-YH, S-CF, A-ST, and S-HL. Literature Search - T-YH, W-HY, Y-CL, H-YT, and S-HL. Writing Manuscript – All authors. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from Kaohsiung Medical University Hospital, Kaohsiung Medical University (KMUH109-9M44), Kaohsiung Municipal Siaogang Hospital/Kaohsiung Medical University Research Center grants (KMHK-DK(C)110009, I-109-04, H-109-05, I-108-02), and Ministry of Science and Technology (MOST 108-2628-B-037-006, MOST 109-2628-B-037-014, MOST 110-2636-B-009-008, MOST 110-2314-B-037-104-MY2, MOST 110-2314-B-037-120), Taiwan.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank all authors of the papers included in this study, as well as participants in the contributing studies.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.755231/full#supplementary-material
References
- 1. Rosenthal LHS, Benninger MS, Deeb RH. Vocal Fold Immobility: A Longitudinal Analysis of Etiology Over 20 Years. Laryngoscope (2007) 117(10):1864–70. doi: 10.1097/MLG.0b013e3180de4d49 [DOI] [PubMed] [Google Scholar]
- 2. Dralle H, Lorenz K, Machens A. Verdicts on Malpractice Claims After Thyroid Surgery: Emerging Trends and Future Directions. Head Neck (2012) 34(11):1591–6. doi: 10.1002/hed.21970 [DOI] [PubMed] [Google Scholar]
- 3. Chiang FY, Lu IC, Kuo WR, Lee KW, Chang NC, Wu CW. The Mechanism of Recurrent Laryngeal Nerve Injury During Thyroid Surgery–the Application of Intraoperative Neuromonitoring. Surgery (2008) 143(6):743–9. doi: 10.1016/j.surg.2008.02.006 [DOI] [PubMed] [Google Scholar]
- 4. Snyder SK, Lairmore TC, Hendricks JC, Roberts JW. Elucidating Mechanisms of Recurrent Laryngeal Nerve Injury During Thyroidectomy and Parathyroidectomy. J Am Coll Surg (2008) 206(1):123–30. doi: 10.1016/j.jamcollsurg.2007.07.017 [DOI] [PubMed] [Google Scholar]
- 5. Chiang FY, Lee KW, Chen HC, Chen HY, Lu IC, Kuo WR, et al. Standardization of Intraoperative Neuromonitoring of Recurrent Laryngeal Nerve in Thyroid Operation. World J Surg (2010) 34(2):223–9. doi: 10.1007/s00268-009-0316-8 [DOI] [PubMed] [Google Scholar]
- 6. Randolph GW, Dralle H, Abdullah H, Barczynski M, Bellantone R, Brauckhoff M, et al. Electrophysiologic Recurrent Laryngeal Nerve Monitoring During Thyroid and Parathyroid Surgery: International Standards Guideline Statement. Laryngoscope (2011) 121 Suppl 1:S1–16. doi: 10.1002/lary.21119 [DOI] [PubMed] [Google Scholar]
- 7. Jeannon JP, Orabi A, Bruch G, Abdalsalam H, Simo R. Diagnosis of Recurrent Laryngeal Nerve Palsy After Thyroidectomy: A Systematic Review. Int J Clin Pract (2009) 63(4):624–9. doi: 10.1111/j.1742-1241.2008.01875.x [DOI] [PubMed] [Google Scholar]
- 8. Leow C, Webb A. The Lateral Thyroid Ligament of Berry. Int Surg (1998) 83(1):75–8. [PubMed] [Google Scholar]
- 9. Yang S, Zhou L, Lu Z, Ma B, Ji Q, Wang Y. Systematic Review With Meta-Analysis of Intraoperative Neuromonitoring During Thyroidectomy. Int J Surg (2017) 39:104–13. doi: 10.1016/j.ijsu.2017.01.086 [DOI] [PubMed] [Google Scholar]
- 10. Chandrasekhar SS, Randolph GW, Seidman MD, Rosenfeld RM, Angelos P, Barkmeier-Kraemer J, et al. Clinical Practice Guideline: Improving Voice Outcomes After Thyroid Surgery. Otolaryngology—Head Neck Surg (2013) 148(6_suppl):S1–S37. doi: 10.1177/0194599813487301 [DOI] [PubMed] [Google Scholar]
- 11. Crumley RL. Unilateral Recurrent Laryngeal Nerve Paralysis. J voice (1994) 8(1):79–83. doi: 10.1016/S0892-1997(05)80323-6 [DOI] [PubMed] [Google Scholar]
- 12. Chiang F-Y, Wang L-F, Huang Y-F, Lee K-W, Kuo W-R. Recurrent Laryngeal Nerve Palsy After Thyroidectomy With Routine Identification of the Recurrent Laryngeal Nerve. Surgery (2005) 137(3):342–7. doi: 10.1016/j.surg.2004.09.008 [DOI] [PubMed] [Google Scholar]
- 13. Dionigi G, Wu C-W, Kim HY, Rausei S, Boni L, Chiang F-Y. Severity of Recurrent Laryngeal Nerve Injuries in Thyroid Surgery. World J Surg (2016) 40(6):1373–81. doi: 10.1007/s00268-016-3415-3 [DOI] [PubMed] [Google Scholar]
- 14. Wu CW, Dionigi G, Sun H, Liu X, Kim HY, Hsiao PJ, et al. Intraoperative Neuromonitoring for the Early Detection and Prevention of RLN Traction Injury in Thyroid Surgery: A Porcine Model. Surgery (2014) 155(2):329–39. doi: 10.1016/j.surg.2013.08.015 [DOI] [PubMed] [Google Scholar]
- 15. Dewan K, Vahabzadeh-Hagh A, Soofer D, Chhetri DK. Neuromuscular Compensation Mechanisms in Vocal Fold Paralysis and Paresis. Laryngoscope (2017) 127(7):1633–8. doi: 10.1002/lary.26409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang T-Y, Yu W-HV, Chiang F-Y, Wu C-W, Fu S-C, Tai A-S, et al. Correlation Between Objective and Subjective High-Pitched Voice Impairment in Patients After Thyroid Surgery. Front Endocrinol (2021) 12:788878(1514). doi: 10.3389/fendo.2021.788878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Friedman AD, Burns JA, Heaton JT, Zeitels SM. Early Versus Late Injection Medialization for Unilateral Vocal Cord Paralysis. Laryngoscope (2010) 120(10):2042–6. doi: 10.1002/lary.21097 [DOI] [PubMed] [Google Scholar]
- 18. Yung KC, Likhterov I, Courey MS. Effect of Temporary Vocal Fold Injection Medialization on the Rate of Permanent Medialization Laryngoplasty in Unilateral Vocal Fold Paralysis Patients. Laryngoscope (2011) 121(10):2191–4. doi: 10.1002/lary.21965 [DOI] [PubMed] [Google Scholar]
- 19. Chiang F-Y, Lu I-C, Chen H-C, Chen H-Y, Tsai C-J, Hsiao P-J, et al. Anatomical Variations of Recurrent Laryngeal Nerve During Thyroid Surgery: How to Identify and Handle the Variations With Intraoperative Neuromonitoring. Kaohsiung J Med Sci (2010) 26(11):575–83. doi: 10.1016/S1607-551X(10)70089-9 [DOI] [PubMed] [Google Scholar]
- 20. Heuer RJ, Sataloff RT, Emerich K, Rulnick R, Baroody M, Spiegel JR, et al. Unilateral Recurrent Laryngeal Nerve Paralysis: The Importance of “Preoperative” Voice Therapy. J voice (1997) 11(1):88–94. doi: 10.1016/S0892-1997(97)80028-8 [DOI] [PubMed] [Google Scholar]
- 21. Dionigi G, Donatini G, Boni L, Rausei S, Rovera F, Tanda ML, et al. Continuous Monitoring of the Recurrent Laryngeal Nerve in Thyroid Surgery: A Critical Appraisal. Int J Surg (2013) 11:S44–S6. doi: 10.1016/S1743-9191(13)60014-X [DOI] [PubMed] [Google Scholar]
- 22. Schneider R, Randolph GW, Barczynski M, Dionigi G, Wu C-W, Chiang F-Y, et al. Continuous Intraoperative Neural Monitoring of the Recurrent Nerves in Thyroid Surgery: A Quantum Leap in Technology. Gland Surg (2016) 5(6):607. doi: 10.21037/gs.2016.11.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim DH, Kim SW, Hwang SH. Intraoperative Neural Monitoring for Early Vocal Cord Function Assessment After Thyroid Surgery: A Systematic Review and Meta-Analysis. World J Surg (2021) 45(11):3320–7. doi: 10.1007/s00268-021-06225-x [DOI] [PubMed] [Google Scholar]
- 24. Liu C-H, Wang C-C, Wu C-W, Lin Y-C, Lu I, Chang P-Y, et al. Comparison of Surgical Complications Rates Between LigaSure Small Jaw and Clamp-And-Tie Hemostatic Technique in 1,000 Neuro-Monitored Thyroidectomies. Front Endocrinol (2021) 12:313. doi: 10.3389/fendo.2021.638608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leclerc A-A, Smith LJ. Early Management of Unilateral Vocal Fold Paralysis. In: Amin M, Johnson M, (eds). Decision Making in Vocal Fold Paralysis (2019) 29–39. doi: 10.1007/978-3-030-23475-1_3 [DOI] [Google Scholar]
- 26. Liddy W, Wu C-W, Dionigi G, Donatini G, Giles Senyurek Y, Kamani D, et al. Varied RLN Course Is Associated With Increased Risk of Nerve Dysfunction During Thyroidectomy: Results of the Surgical Anatomy of the RLN in Thyroid Surgery (SAR) Study, an International Multicenter Prospective Anatomic and Electrophysiologic Study of 1000 Monitored Nerves at Risk From the International Neural Monitoring Study Group (INMSG). Thyroid (2021) 31(11):1730–40. doi: 10.1089/thy.2021.0155 [DOI] [PubMed] [Google Scholar]
- 27. Christakis I, Klang P, Talat N, Galata G, Schulte K-M. Long-Term Quality of Voice Is Usually Acceptable After Initial Hoarseness Caused by a Thyroidectomy or a Parathyroidectomy. Gland Surg (2019) 8(3):226. doi: 10.21037/gs.2018.09.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sorensen JR, Printz T, Iwarsson J, Grøntved ÅM, Døssing H, Hegedüs L, et al. The Impact of Post-Thyroidectomy Paresis on Quality of Life in Patients With Nodular Thyroid Disease. Otolaryngology–Head Neck Surg (2019) 161(4):589–97. doi: 10.1177/0194599819855379 [DOI] [PubMed] [Google Scholar]
- 29. Reiter R, Heyduck A, Hoffmann TK, Brosch S, Buchberger MA, Schorer K, et al. Quality of Voice and Prognostic Markers for the Recovery of Vocal Fold Paralysis After Thyroid Surgery. Ann Otol Rhinol Laryngol (2019) 128(12):1104–10. doi: 10.1177/0003489419858629 [DOI] [PubMed] [Google Scholar]
- 30. Wang L-F, Lee K-W, Kuo W-R, Wu C-W, Lu S-P, Chiang F-Y. The Efficacy of Intraoperative Corticosteroids in Recurrent Laryngeal Nerve Palsy After Thyroid Surgery. World J Surg (2006) 30(3):299–303. doi: 10.1007/s00268-005-0399-9 [DOI] [PubMed] [Google Scholar]
- 31. Hydman J, Björck G, Persson JK, Zedenius J, Mattsson P. Diagnosis and Prognosis of Iatrogenic Injury of the Recurrent Laryngeal Nerve. Ann Otol Rhinol Laryngol (2009) 118(7):506–11. doi: 10.1177/000348940911800709 [DOI] [PubMed] [Google Scholar]
- 32. Chung SR, Baek JH, Choi YJ, Lee JH. Management Strategy for Nerve Damage During Radiofrequency Ablation of Thyroid Nodules. Int J Hyperthermia (2019) 36(1):203–9. doi: 10.1080/02656736.2018.1554826 [DOI] [PubMed] [Google Scholar]
- 33. Borel F, Christou N, Marret O, Mathonnet M, Caillard C, Bannani S, et al. Long-Term Voice Quality Outcomes After Total Thyroidectomy: A Prospective Multicenter Study. Surgery (2018) 163(4):796–800. doi: 10.1016/j.surg.2017.09.023 [DOI] [PubMed] [Google Scholar]
- 34. Wu C-W, Hao M, Tian M, Dionigi G, Tufano RP, Kim HY, et al. Recurrent Laryngeal Nerve Injury With Incomplete Loss of Electromyography Signal During Monitored Thyroidectomy—Evaluation and Outcome. Langenbeck’s Arch Surg (2017) 402(4):691–9. doi: 10.1007/s00423-016-1381-8 [DOI] [PubMed] [Google Scholar]
- 35. Lee SW, Kim JW, Chung CH, Mok JO, Shim SS, Koh YW, et al. Utility of Injection Laryngoplasty in the Management of Post-Thyroidectomy Vocal Cord Paralysis. Thyroid (2010) 20(5):513–7. doi: 10.1089/thy.2009.0397 [DOI] [PubMed] [Google Scholar]
- 36. Lee SW, Park KN. A Long-Term Comparative Prospective Study Between Reinnervation and Injection Laryngoplasty. Laryngoscope (2018) 128(8):1893–7. doi: 10.1002/lary.27140 [DOI] [PubMed] [Google Scholar]
- 37. Zhang D, Pino A, Caruso E, Dionigi G, Sun H. Neural Monitoring in Thyroid Surgery Is Here to Stay. Gland Surg (2020) 9(Suppl 1):S43. doi: 10.21037/gs.2019.10.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Siu J, Tam S, Fung K. A Comparison of Outcomes in Interventions for Unilateral Vocal Fold Paralysis: A Systematic Review. Laryngoscope (2016) 126(7):1616–24. doi: 10.1002/lary.25739 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.