Abstract
Ureaplasma parvum is a commensal bacterium in the female reproductive tract but has been associated with pregnancy complications such as preterm prelabor rupture of membranes and preterm birth (PTB). However, the pathologic effects of U. parvum in the cervix, that prevents ascending infections during pregnancy, are still poorly understood. To determine the impact of U. parvum on the cervix, ectocervical (ecto) and endocervical (endo) epithelial and stromal cells were incubated with U. parvum. Macrophages were also tested as a proxy for cervical macrophages to determine the antigenicity of U. parvum. The effects of U. parvum, including influence on cell cycle and cell death, antimicrobial peptide production, epithelial-to-mesenchymal transition (EMT), and inflammatory cytokine levels, were assessed. U. parvum colonized cervical epithelial and stromal cells 4 hours post-infection. Like uninfected control, U. parvum neither inhibited cell cycle progression and nor caused cell death in cervical epithelial and stromal cells. U. parvum increased the production of the antimicrobial peptides (AMPs) cathelicidin and human β-defensin 3 and exhibited weak signs of EMT evidenced by decreased cytokeratin 18 and increased vimentin expression in cervical epithelial cells. U. parvum induced a pro-inflammatory environment (cytokines) and increased MMP-9 in cervical epithelial cells but promoted pro- and anti-inflammatory response in cervical stromal cells and macrophages. U. parvum may colonize the cervical epithelial layer, but induction of AMPs and anti-inflammatory response may protect the cervix and may prevent ascending infections that can cause preterm birth. These findings suggest that U. parvum is a weak inducer of inflammation in the cervix.
Keywords: antimicrobial peptide, cell death, cervical remodeling, epithelial-to-mesenchymal transition, infection, pregnancy
Introduction
The cervix protects the developing fetus from pathogenic microorganisms present in the vagina (Nott et al., 2016; Vink and Feltovich, 2016). To maintain this function, the cervix undergoes extensive remodeling throughout pregnancy, starting as a closed, firm structure, which softens throughout pregnancy and shortens and dilates during labor and delivery. (Sennstrom, 2000; Vink and Myers, 2018). Infection and inflammation in the cervix may compromise its integrity leading to premature cervical remodeling and preterm birth (PTB) (Read et al., 2007; Holt et al., 2011; Vink and Feltovich, 2016; Vink and Mourad, 2017).
Ascending infections from the vagina to the uterine cavity account for almost 40% of preterm births (Goldenberg et al., 2008). Intrauterine infections are usually polymicrobial in nature. However, the most isolated and detected microorganisms in the amniotic cavity are genital mycoplasmas particularly Ureaplasma parvum and Ureaplasma urealyticum (Sweeney et al., 2017; Tantengco and Yanagihara, 2019; Sprong et al., 2020). These bacteria have been associated with poor maternal outcomes such as preterm premature rupture of membranes (PPROM) and preterm birth (PTB) (Kacerovský et al., 2009; Viscardi, 2010; Musilova et al., 2016; Rittenschober-Böhm et al., 2018). However, it is still unclear if Ureaplasma spp. are a cause of PPROM and PTB or if they simply are associated. This inconsistency on the role of Ureaplasma spp. in PPROM and PTB may be due to the methodological differences in the previous studies. Several clinical studies detect Ureaplasma spp., while others specifically detect U. parvum and U. urealyticum. The use of different species, serovars, dose, and site of inoculation in animal studies could have also confounded the association between Ureaplasma spp. infection and PTB. In vivo mice studies have shown that vaginal inoculation of U. parvum in an otherwise healthy cervix/vagina resulted in PTB rates (~10%) similar to that in women, however the PTB rates increased when the cervix was damaged or when Ureaplasma was directly inoculated inside the amniotic cavity (Motomura et al., 2020; Pavlidis et al., 2020). These reports suggest that damage to the cervical epithelial barrier can promote the ascent of Ureaplasma spp.
The lower rate of PTB in mice with an intact, healthy cervix, when compared to those with a damaged cervix, suggests that Ureaplasma spp. alone do not elicit a sufficient pro-inflammatory response to promote preterm labor-associated changes in cervical cells (Pavlidis et al., 2020). Although previous animal and clinical studies have shown that U. parvum infection may promote an inflammatory response in the cervix (Horowitz et al., 1995; Choi and Roh, 2014; Liu et al., 2014; Motomura et al., 2020; Pavlidis et al., 2020), most of these studies focused primarily on pro-inflammatory cytokines and did not investigate the anti-inflammatory cytokine production of the cervix in response to Ureaplasma spp. infection. In a retrospective PPROM cohort study, Kacerovsky et al reported that Ureaplasma spp. colonization is associated with a significant increase in both pro- and anti-inflammatory cytokines in the amniotic fluid suggestive of a balanced immune response in the intrauterine cavity. Moreover, Ureaplasma spp. colonization has not been associated with decreased gestational age at delivery (Kacerovsky et al., 2013).
Previous studies have shown that different cellular mechanisms, such as inhibition of cell cycle progression and cell death, alteration of antimicrobial defense mechanisms, and epithelial-to-mesenchymal transition (EMT), can compromise cervical integrity (Allaire et al., 2001; Hassan et al., 2009; Gordon and Mowa, 2019). However, these cellular-level changes and types of innate immune responses have not been thoroughly investigated in the cervix in the presence of a bacterial infection. This study aimed to understand the pathologic effects of U. parvum in the resident cells of the cervix, particularly, epithelial, stromal, and immune cells. We also documented how colonization affects cell cycle, cell death, cellular transition, and antimicrobial response of cervical cells.
Materials and methods
Human ectocervical (ecto) and endocervical (endo) epithelial cell and cervical stromal cell (Stroma) cultures
Previously characterized immortalized ecto and endo cells (HPV E6/E7 cell immortalization system) and cervical stroma cells (hTERT cell immortalization system) from hysterectomy material from women with benign gynecological conditions were used in this study (Herbst-Kralovetz et al., 2008; Tantengco et al., 2021a–c). Ecto and endo cells were cultured in keratinocyte serum‐free medium (KSFM), a culture medium highly selective for epithelial cells, supplemented with bovine pituitary extract (30 μg/mL), epidermal growth factor (0.1 ng/mL), CaCl2 (0.4 mM) and primocin (0.5 mg/mL) (ant-pm-1; Invitrogen; San Diego, CA). The stromal cells were cultured with Dulbecco’s modified Eagle’s medium: Nutrient Mixture F-12 medium (DMEM/F12; Mediatech Inc.) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich), 10% penicillin/streptomycin (Mediatech Inc.), and 10% amphotericin B (Sigma-Aldrich, Inc.), at 37°C and 5% CO2, and grown to 80% confluence. Cell culture protocols have previously been reported in our prior publications (Tantengco et al., 2021b).
Human THP-1 macrophage culture
THP-1 monocytes were obtained from the American Type Culture Collection (ATCC TIB-202) and were cultured in RPMI 1640 Medium (ATCC modification) (A1049101, ThermoFisher Scientific) and supplemented with 0.05 mM 2-mercaptoethanol (21985023, Gibco™, Carlsbad, CA) and 10% FBS (Sigma-Aldrich). The cells were grown until they reach a density of 1×106 cells/ml. The cells were then centrifuged at 1000 rpm for 5 minutes. THP-1 monocytes were seeded in a 48-well plate (50,000 cells per well) and were differentiated to THP-1 macrophages by incubating in culture medium containing 100 mM phorbol 12-myristate 13-acetate for three days at 37°C and 5% CO2 and grown to 80% confluence as described previously (Bowdish et al., 2005).
Bacterial strain and culture conditions
U. parvum (ATCC® 700970™) were obtained from the American Tissue Culture Collection (ATCC). U. parvum was propagated in UMCHs medium (Namba et al., 2010): Mycoplasma broth base (Becton, Dickinson and Co., Baltimore, MD) 1.47% (wt/vol), yeast extract (Becton, Dickinson and Co.) 2.5% (wt/vol), horse serum (Biowhittaker, Walkersville, MD) 20% (vol/vol), urea 0.04% (wt/vol), phenol red 0.001% (wt/vol), l-cysteine hydrochloride 0.01% (wt/vol), and penicillin G 1000 U/mL. U. parvum cultures were incubated for 16 – 18 h to obtain titers of 1 × 109 – 1 × 1011 color-changing units (CCU)/mL of viable bacteria. The corresponding amounts of Ureaplasma DNA were verified using genesig Std Real-time PCR detection kit, Ureaplasma parvum (Z-Path-U.parvum-std, American Research Products Inc., Waltham, MA) and amounted to 3 × 107 – 3 × 108 copy numbers/mL.
Labeling of U. parvum Cells
U. parvum culture harvested after 16 – 18 h incubation in UMCHs medium were concentrated using Amicon® Ultra-15 Centrifugal Filter Units (UFC910024, 100 kDa; Merk Millipore Ltd., Tullagreen, Carrigtwohill, Co. Cork, Ireland) and was centrifuged at 4000 × g for 15 min. The concentrated U. parvum cells were labeled with DiOC18(3) (3,3’-dioctadecyloxacarbocyanine perchlorate – DiO; D275; Invitrogen, Carlsbad, CA, USA) by resuspending the concentrated U. parvum suspension with 100 μL of 100 μM DiO and were incubated at 37 °C for 30 min. To remove excess DiO, the U. parvum suspension was transferred to Amicon® Ultra-15 Centrifugal Filter Units and was centrifuged at 4000 × g for 15 min.
U. parvum Infection Assays
For immunocytochemical staining of the EMT markers cytokeratin 18 (CK-18, epithelial marker) and vimentin (mesenchymal marker) as well as antimicrobial peptides (AMP), ecto and endo cells were seeded at approximately 80% confluence in an eight-well glass slide and incubated at 37°C with 5% CO2 for 24 h. Approximately 250 μL broths containing 109 – 1011 CCU/mL U. parvum were inoculated per milliliter of cell culture medium. The cells were stimulated for 48 h before staining for EMT markers and AMP.
To check for inflammatory cytokine production (ELISA), cell cycle and cell death (flow cytometry) EMT and AMP production (western blot [WB] analyses), ecto, endo, and stroma cells were seeded at approximately 80% confluence in a 6-well plate and incubated at 37°C with 5% CO2 for 24 h. Approximately 250 μL broths containing 109 – 1011 CCU/mL U. parvum were inoculated per milliliter of cell culture medium. The cells were stimulated for 48 h before performing ELISA, flow cytometry, and WB.
Flow cytometry assays
Cell cycle analysis
Cell cycle analysis was performed by measuring DNA content to distinguish between different phases of the cell cycles. Fluorescence intensity, which directly correlated with the amount of DNA contained in a cell, was measured. Concurrent parameter measurements made it possible to discriminate between S, G2, and mitotic cells. As the DNA content doubles during the S phase, the intensity of the fluorescence increases, making it possible to ascertain the effect of U. parvum infection on cell proliferation. Cell cycle analysis was performed using the flow cytometer, as described previously using the Coulter DNA Prep Reagents Kit (Beckman Coulter, Indianapolis, IN) (Tantengco et al., 2021b). Briefly, cells were harvested by trypsinization and centrifuged for 5 min at 3000 × g. Cell pellets were washed with cold 1× PBS and centrifuged at 3000 × g for 5 min. Cell pellets were fixed with 500 μL 70% ethanol for 15 min. Cell pellets were washed with cold 1× PBS and centrifuged at 3000 × g for 5 min. Then, 500 μL of the prep stain was added to the tubes, vortexed, and run immediately on the CytoFlex flow cytometer (Beckman Coulter). After selecting for single cells, gating was set for the control cells and applied to histograms for each of the treatments in different cervical cells using CytExpert (Beckman Coulter).
Apoptosis and necrosis
To determine the population of cells undergoing apoptosis and/or necrosis, cells were stained using the Dead Cell Apoptosis Kit with Annexin V Alexa Fluor 488 & PI (Life Technologies, Carlsbad, CA) as reported previously (Tantengco et al., 2021b). Briefly, cells were harvested after 48 h of U. parvum infection by trypsinization and centrifuged for 5 min at 3000 × g. Cell pellets were washed with cold 1× PBS and centrifuged at 3000 × g for 5 min. Pellets were resuspended in 100 μL 1× annexin binding buffer supplemented with 5 μL Alexa Fluor 488 Annexin V and 1 μL 100 μg/mL propidium iodide (PI). After a 15 min incubation, 400 μL annexin binding buffer was added, and samples were run immediately on the CytoFlex flow cytometer (Beckman Coulter). Unstained cervical cells were used as negative controls for gating. Data were analyzed using CytExpert software (Beckman Coulter).
Immunocytochemical staining of EMT markers (intermediate filaments CK-18 and vimentin) and antimicrobial peptides (cathelicidin, elafin, and human β-defensin 3)
Human, animal, and in vitro cell culture studies showed evidence of transition of cervical epithelial cells into a more mesenchymal phenotype. This transition is characterized by decreased expression of CK-18 and E-cadherin and increased expression of vimentin and N-cadherin and pro-inflammatory changes (Hassan et al., 2009; Gordon and Mowa, 2019; Tantengco et al., 2021c, d). Immunocytochemical staining for CK-18 (ab668; Abcam), vimentin (ab92547; Abcam, Cambridge, MA), cathelicidin (NBP1–76864, Novus Biologicals Inc., Littleton, CO), beta-Defensin 3 (NB200–117, Novus Biologicals Inc.), or elafin (MAB1747, R&D Systems, Inc., Minneapolis, MN) were performed 48 h post-infection with U. parvum. The dilution factor for primary antibodies was 1:500 for CK-18, 1:300 for vimentin, and 1:200 for cathelicidin, elafin, and human β-defensin 3. After each time point, cells were fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X, and blocked with 3% bovine serum albumin in PBS before incubation with primary antibodies overnight at 4°C. This protocol is adequate to remove non-specific binding of primary antibodies in our system, based on our previous studies (Tantengco et al., 2021c, a). After washing with PBS, slides were incubated with Alexa Fluor 488- and Alexa Fluor 594-conjugated secondary antibodies (Life Technologies, Carlsbad, CA) and diluted 1:400 in PBS for 1 h in the dark. Slides were washed with PBS, treated with NucBlue Fixed Cell Stains ReadyProbes Reagent (R37606; Thermo Fisher Scientific, Watham, MA), and then mounted using Mowiol 4 to 88 mounting medium (475904–100GM-M; Sigma-Aldrich, Inc.).
Western blot
Ecto and endo cells were infected with U. parvum for 48 h before lysis in RIPA lysis buffer with freshly added protease and phosphatase inhibitors (0.01%). The cell lysate was collected after scraping the culture plate, and the insoluble material was removed by centrifugation at 10,000 g for 20 minutes at 4°C. The concentration of protein in each cell lysate was determined using the BCA protein assay kit (Pierce BCA Protein Assay Kit, Thermo Scientific, Waltham, MA, USA). Equal amounts of protein (8 μg) from each sample were loaded onto a 10% SDS-PAGE gel and electrophoresed at 120 V. The resolved proteins were transferred to a polyvinylidene difluoride membrane using the iBlot transfer apparatus (Bio-Rad Laboratories, Hercules, CA, USA). The membranes were blocked in Tris-buffered saline (TBS) containing 0.1% Tween-20 (TBS-T) and 5% skim milk for 2 h at room temperature. Blots were incubated separately with antibodies against, β-actin (Sigma-Aldrich, A5441), CK-18 (Abcam, ab668), cathelicidin (NBP1–76864, Novus Biologicals Inc.), vimentin (Abcam, ab92547) at 4°C and shaken overnight. Blots were washed three times with TBS-T and incubated with an appropriate peroxidase-conjugated IgG secondary antibody for 1 h at room temperature. All blots were developed using chemiluminescence reagents (ECL Western Blotting Detection System, Amersham Piscataway, NJ, USA) in accordance with the manufacturer’s recommendations, followed by autoradiography. Densitometry was performed to normalize the data for statistical analysis.
Enzyme-linked immunosorbent assay for GM-CSF, IL-6, IL-8, IL-10, and TNFα
Culture media collected from ecto, endo, stroma, and THP-1 macrophages 48 h post-infection were tested for human GM-CSF, IL-6, IL-8, IL-10, and TNFα using the BD OptEIA™ Human GM-CSF ELISA Set (555126, BD Biosciences, San Jose, CA), BD OptEIA™ Human IL-6 ELISA Set (555220, BD Biosciences), BD OptEIA™ Human IL-8 ELISA Set (555244, BD Biosciences), BD OptEIA™ Human IL-10 ELISA Set (555157, BD Biosciences), and Human TNF ELISA Set (555212, BD Biosciences), respectively. Standard curves were developed using duplicate samples of known-quantity recombinant proteins that were provided by the manufacturer. Sample concentrations were determined by relating the absorbance of the samples to the standard curve using linear regression analysis.
Statistical Analysis
Data were analyzed for significant differences using GraphPad Prism software version 7 (GraphPad Software, San Diego, CA). The Shapiro-Wilk test for normality was performed to check for the normality of the data. Parametric tests including one-way analysis of variance followed by the Tukey multiple comparison posthoc test and the t-test were used for comparison of the results for normally distributed data. Non-parametric tests, namely the Kruskal-Wallis test with Dunn’s multiple comparison test and the Mann-Whitney U test were used for comparison of the results for data that were not normally distributed. Statistically significant differences are indicated by p < 0.05.
Results
U. parvum can be internalized by cervical epithelial and stromal cells
To determine if U. parvum can infect cervical epithelial and stromal cells, intracellular localization of DiO-labeled U. parvum was evaluated using immunocytochemical staining. CK-18 was used as marker for ecto and endo cells while vimentin was used for stroma. At 4 h post-infection, DiO-labeled U. parvum was observed in the cytosolic and perinuclear region with a clustered pattern in ecto, endo, and stroma cells (Fig. 1). These results showed that U. parvum can infect cervical epithelial and stromal cells in vitro.
Fig 1. U. parvum can colonize cervical epithelial and stromal cells.
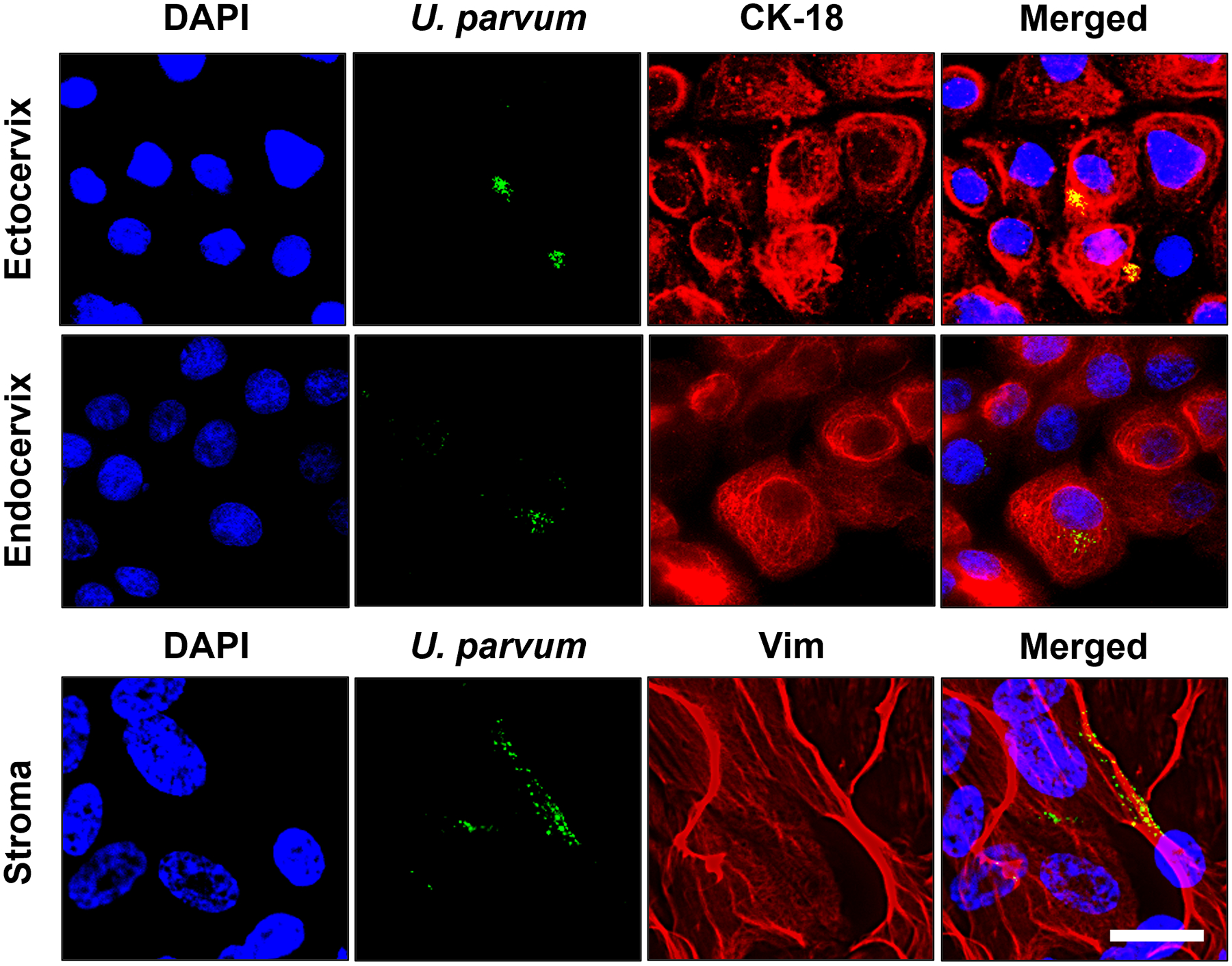
Fluorescence microscopy imaging showing DiO-labeled U. parvum (green) in human cervical epithelial and stromal cells 4 h post-infection. CK-18 (red) was used as a marker for ectocervical and endocervical epithelial cells while vimentin (red) was used as marker for cervical stromal cells. Nuclei are stained with DAPI, n=3 technical replicates. Scale bar, 25 μm.
U. parvum elicits antimicrobial response in cervical epithelial cells
Cervical epithelial cells were previously shown to produce AMP, including cathelicidin, elafin, and human β-defensin 3, in response to bacterial infection (Frew and Stock; Yarbrough et al., 2015). It was also reported that AMP production is increased in patients with cervical shortening and preterm birth (Tribe, 2015; Hezelgrave et al., 2020), therefore the AMP production of ecto and endo cells was quantified after 48 h infection with U. parvum. Immunocytochemical staining (ICC) and WB analysis showed a significant increase in cathelicidin staining in ecto cells infected with U. parvum (p < 0.05) (Fig. 2A, B, C). An increasing trend for cathelicidin was also observed in endo cells; however, it did not reach statistical significance (Fig. 2D, E, F). ICC staining also showed an increased human β-defensin 3 expression in ecto and endo cells. Elafin expression was increased in ecto but was decreased in endo cells (Fig. S1). These results demonstrate that cervical epithelial cells can increase AMP production in response to U. parvum infection.
Fig 2. U. parvum increases antimicrobial peptide production in cervical epithelilal cells.
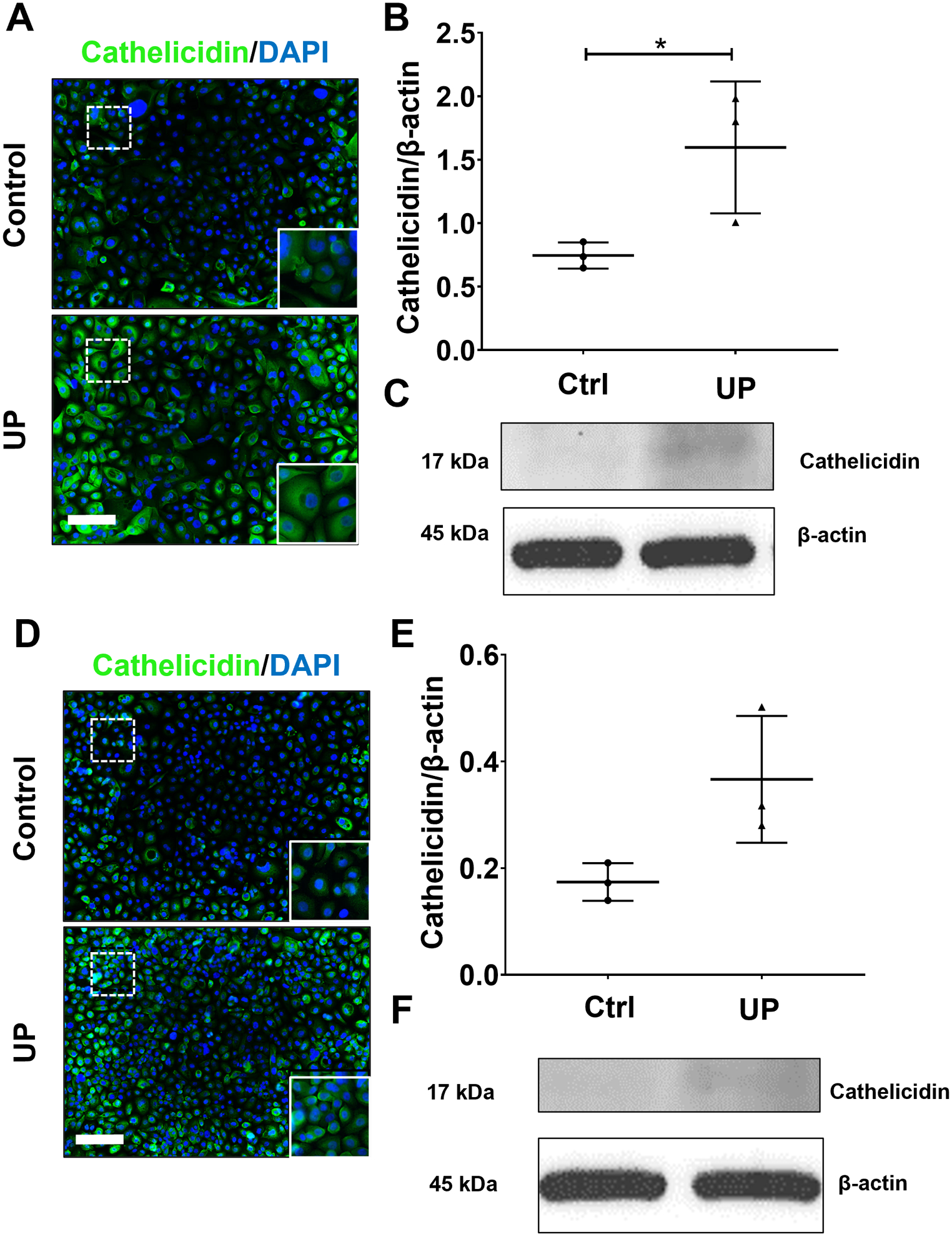
Fluorescence microscopy imaging and western blot analyses showing cathelicidin in uninfected and U. parvum-infected human ectocervical (A – C) and endocervical epithelial cells (D – F). Nuclei are stained with DAPI, n=3 technical replicates. Scale bar, 100 μm. Western blot analysis and quantification of cathelicidin in ectocervical (B – C) and endocervical epithelial cells (E – F). β-actin is used as loading control. Error bars represent mean ± SEM, n=3 technical replicates. Linear adjustment of contrast and brightness has been applied to all fluorescent images throughout the figure. *, p < 0.05.
U. parvum does not promote cell cycle arrest and cell death in cervical epithelial and stromal cells
Cell cycle arrest and cell death are important mechanisms that can prevent persistent bacterial infection, and this can be considered as an innate resistance by cells to minimize microbial spread. However, bacteria have developed mechanisms that can manipulate host cell death to allow bacterial colonization (Ashida et al., 2014; Sixt, 2021), therefore, the effects of U. parvum colonization and cell cycle progression and cell death in cervical epithelial and stroma cells were analyzed using flow cytometry. U. parvum colonization promoted G1-S transition as shown by the significant increase in the population of ecto, endo, and stroma cells in S phase (Fig. 3A–C). U. parvum colonization did not induce apoptosis or necrosis in ecto and stroma but significantly decreased live endo cells (p < 0.0001) and significantly increased necrotic endo cells (p < 0.0001). However, necrotic cells only accounted for ~2% of the total endo cell population, which is negligible (Fig. 3 D–F). Collectively, these data showed that U. parvum did promote cell cycle arrest and did not induce cell death in cervical epithelial and stromal cells.
Fig 3. U. parvum increases G1-S phase transition and does not promote cell death in cervical epithelial and stromal cells.
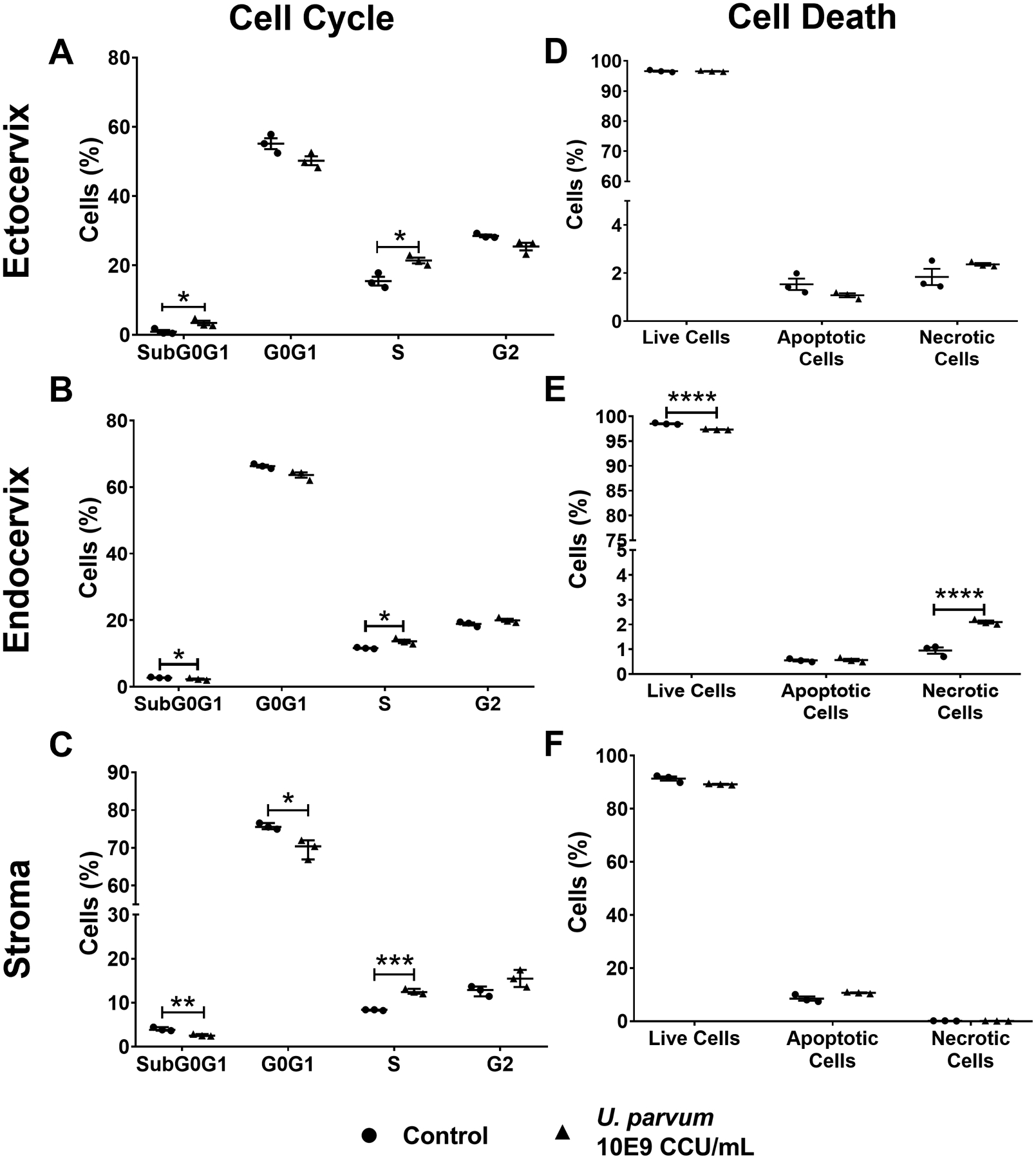
Quantification of flow cytometric analysis of cell cycle progression of ectocervical (A) and endocervical epithelial cells (B), and cervical stromal cells (C) after 48 h of U. parvum infection. Error bars represent mean % cells ± SEM, n=3 technical replicates. Flow cytometric analysis of Annexin V/PI-stained, apoptotic, and necrotic ectocervical (D) and endocervical epithelial cells (E), and cervical stromal cells (F). Error bars represent mean % cells ± SEM (A, B, D, E, and F) or median % cells ± SD (C), n=3 technical replicates. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001
U. parvum induces minimal EMT in cervical epithelial cells
We previously reported that infectious and inflammatory stimuli may promote EMT in cervical epithelial cells (Tantengco et al., 2021a, d, c), which may potentially compromise the cervical epithelial barrier functions and promote premature cervical remodeling due to inflammation and increased presence of pro-inflammatory mesenchymal cells. EMT in ecto and endo cells was evaluated after U. parvum colonization using ICC and WB for CK-18 and vimentin. ICC analysis showed that U. parvum infection induced higher expression of vimentin and lower CK-18 expression in ecto (Fig 4A). WB analyses showed CK-18 and vimentin expression remain unchanged in ecto after U. parvum infection (p < 0.05) (Fig 4B–D). On the other hand, endo cells infected with U. parvum appeared to have lower expression of CK-18 and higher expression of vimentin compared to uninfected control (Fig. 5A, B). But densitometric analysis of WB did not show a statistically significant difference (Fig. 5C, D). These results suggested that U. parvum infection is a weak inducer of EMT and transitions are cell-type dependent. Ecto cells are more susceptible to EMT compared to endo cells at the dose of U. parvum used for stimulation in this study.
Fig 4. U. parvum promotes EMT in ectocervical epithelial cells.
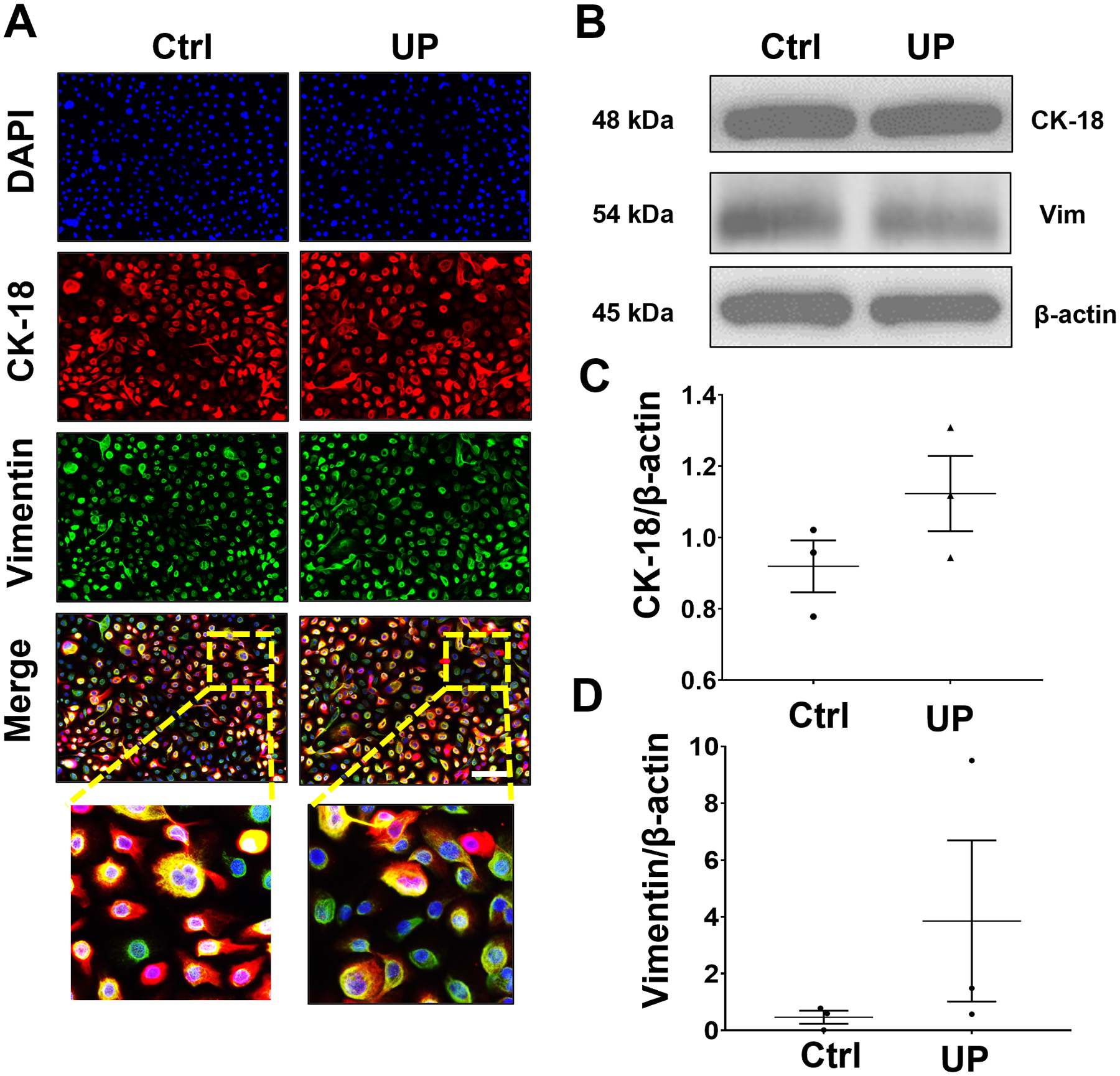
(A) Fluorescence microscopy imaging showing CK-18 and vimentin in uninfected and U. parvum-infected human ectocervical epithelial cells. Nuclei are stained with DAPI, n=3 technical replicates. Scale bar, 100 μm. Western blot analysis and quantification of CK-18 (B, C) and vimentin (B, D) in ectocervical epithelial cells. β-actin is a loading control. Error bars represent mean ± SEM, n=3 technical replicates. Linear adjustment of contrast and brightness has been applied to all fluorescent images throughout the figure. *, p < 0.05.
Fig 5. U. parvum promotes EMT in endocervical epithelial cells.
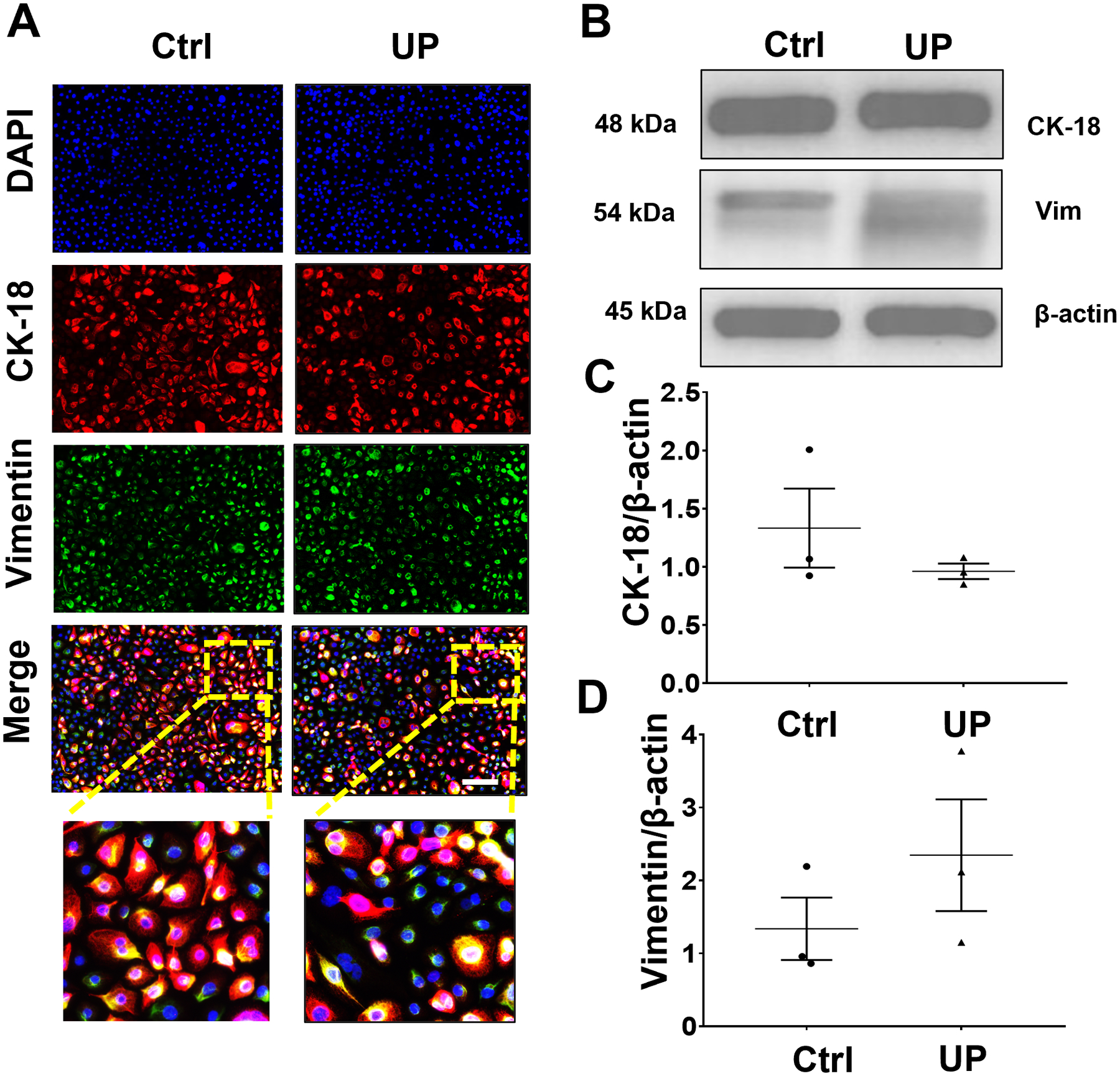
(A) Fluorescence microscopy imaging showing CK-18 and vimentin in uninfected and U. parvum-infected human endocervical epithelial cells. Nuclei are stained with DAPI, n=3 technical replicates. Scale bar, 100 μm. Western blot analysis and quantification of CK-18 (B, C) and vimentin (B, D) in endocervical epithelial cells. β-actin is a loading control. Error bars represent mean ± SEM, n=3 technical replicates. Linear adjustment of contrast and brightness has been applied to all fluorescent images throughout the figure.
U. parvum promotes different inflammatory responses in cervical epithelial and stromal cells, and macrophages
Previous studies have shown that U. parvum infection can elicit inflammatory responses in maternal and fetal cells (Aaltonen et al., 2007; Noda-Nicolau et al., 2016; Potts et al., 2016; Glaser et al., 2017; Polettini et al., 2018; Pavlidis et al., 2020). The inflammatory response of cervical epithelial and stromal cells and macrophages was determined after infection with U. parvum for 48 h. In ecto cells, the increasing trend with MMP-9 after U. parvum infection did not reach statistical significance (Fig 6A, B). However, MMP-9 production by endo cells was significantly increased by U. parvum infection (Fig. 6 C, D). Pro-inflammatory markers were increased in ecto and endo cells after UP infection, with significantly increased IL-6 (p < 0.05) and IL-8 (p < 0.05) levels in ecto cells and GM CSF, IL-6, and IL-8 levels (p < 0.0001) in endo cells (Fig. 7)
Fig 6. U. parvum increases MMP-9 in cervical epithelial cells.
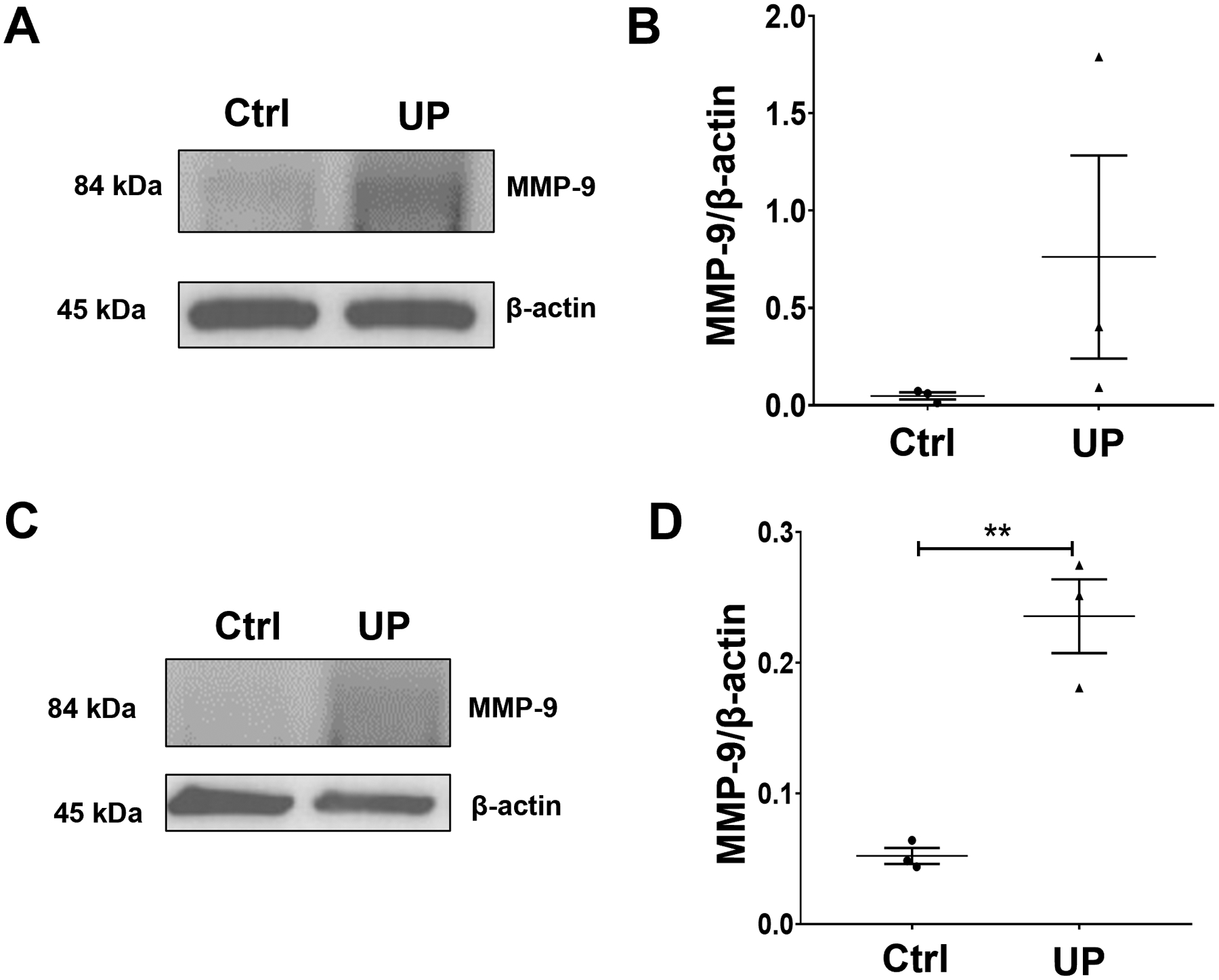
Western blot analysis and quantification of MMP-9 in ectocervical (A, B) and endocervical epithelial cells (C, D). β-actin is a loading control. Error bars represent mean ± SEM, n=3 technical replicates. **, p < 0.01.
Fig 7. U. parvum promotes differential inflammatory responses in cervical epithelial and stromal cells.
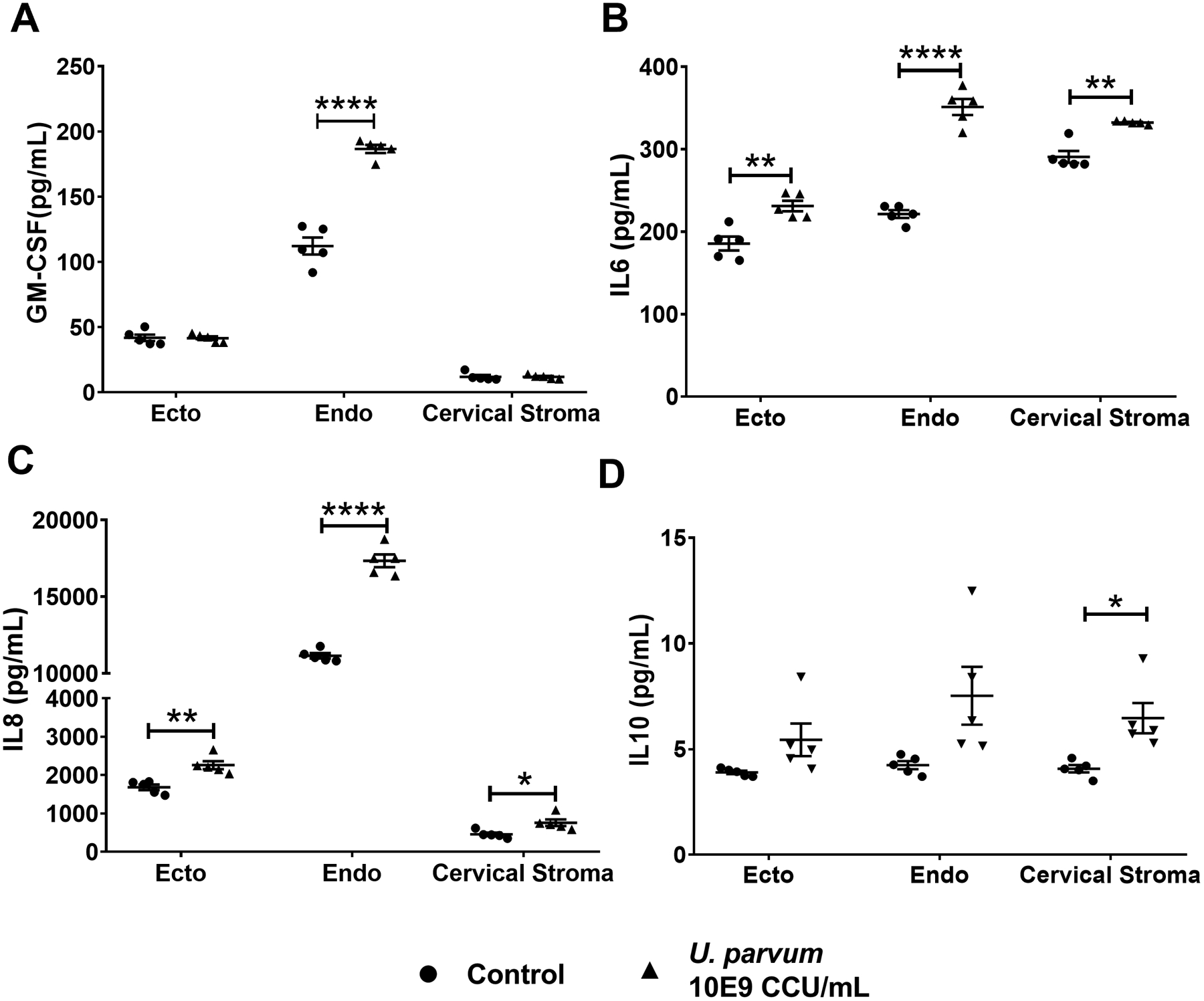
Pro-inflammatory cytokines, human GM-CSF (A), IL-6 (B), and IL-8 (C), and anti-inflammatory cytokine IL-10 (D) levels in culture medium collected from ectocervical and endocervical epithelial cells, and cervical stromal cells after 48 h of U. parvum infection. Error bars represent mean concentration ± SEM, n=5 technical replicates. *, p < 0.05; **, p < 0.01; ****, p < 0.0001.
In stroma cells, there was a mixed response with an increase in the proinflammatory cytokines IL-6 (p < 0.01) and IL-8 (p < 0.05), but also an increase in the anti-inflammatory IL-10 levels (Fig. 7). Similarly, in macrophages, exposure to U. parvum resulted in a significant increase in the pro-inflammatory cytokines IL-8 (p < 0.05) and TNFα (p < 0.01) while the anti-inflammatory cytokine IL-10 (p < 0.05) was also increased (Fig. 8). These results showed a cell-specific inflammatory response of cervical and immune cells after U. parvum infection.
Fig 8. U. parvum infection promotes both pro- and anti-inflammatory responses in human macrophages.
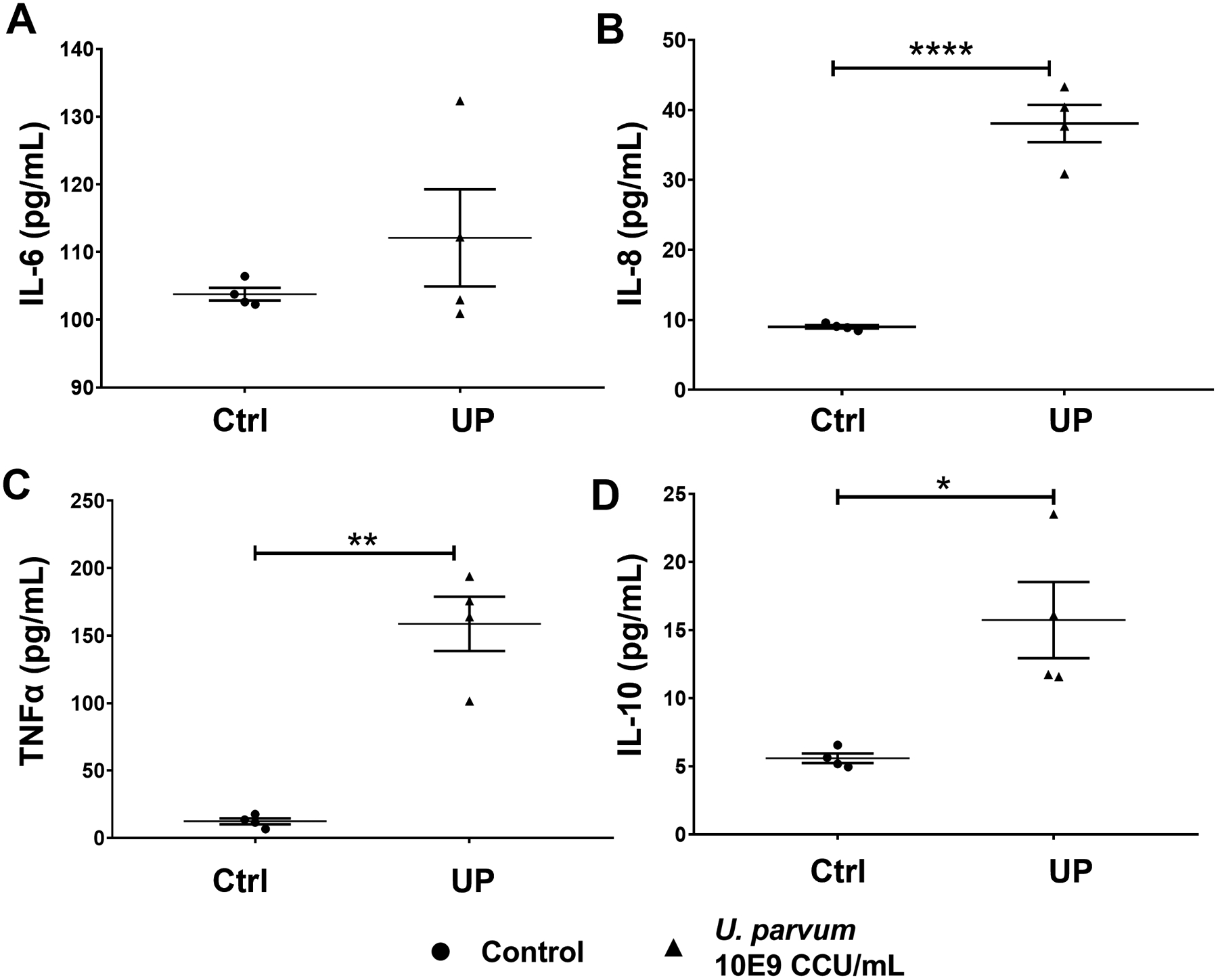
Pro-inflammatory cytokines human IL-6 (A), IL-8 (B), and TNFα (C), and anti-inflammatory cytokine IL-10 (D) levels in culture medium collected from THP-1 macrophages after 48 h of U. parvum infection. Error bars represent mean concentration ± SEM, n=5 technical replicates. *, p < 0.05; **, p < 0.01; ****, p < 0.0001.
Discussion
Cervical remodeling is a vital process that maintains pregnancy until term delivery (Yellon, 2017, 2020). Cervical infections are hypothesized to promote pathologic processes that may compromise the cervix, hasten the cervical ripening process, and predispose the tissue to preterm birth (Word et al., 2007). We investigated these pathological processes in cervical cells in response to U. parvum infection. Our main findings include the following: 1) U. parvum can colonize cervical epithelial and stromal cells; 2) U. parvum colonization increased cathelicidin production in ecto and endo cells 3) U. parvum did not promote cell cycle arrest nor cause cell death in cervical cells; 4) EMT was not pronounced although there was a mild form EMT in ecto cells; 5) U. parvum induced a pro-inflammatory environment including an increase in matrix degrading MMP-9 levels in cervical epithelial cells but promoted a mixed pro- and anti-inflammatory response in cervical stromal cells and human macrophages. In summary, U. parvum can colonize cervical cells and induce an inflammatory response; however, its antigenicity may not be sufficient to independently cause preterm birth.
Our study showed that U. parvum colonization does not result in cell death and may promote cervical cells to proliferate as signified by increased G1-S phase transition. Previous studies on Ureaplasma spp. showed similar results; Wolfs et al showed there was no induction of apoptosis in enterocytes while Silwedel et al reported inhibition of apoptosis in pulmonary endothelial cells (Wolfs et al., 2013; Silwedel et al., 2019). Several intracellular bacteria inhibit host cell apoptosis so they can persistently colonize the cells, which can facilitate their dissemination (Behar and Briken, 2019; FitzGerald et al., 2020). The lack of apoptosis and necrosis in cervical cells is expected since Ureaplasma spp. are known to be part of the commensal microbiota in the cervicovaginal space (Kallapur et al., 2013). However, this may also be a way for U. parvum to persistently colonize these cells for further dissemination in other gestational tissues.
U. parvum colonization increased the production of cathelicidin and human β-defensin 3 in cervical epithelial cells. Xiao et al. demonstrated that cathelicidin and human β -defensin 3 has antimicrobial activity against Ureaplasma spp. (Xiao et al., 2014). Frew et al. showed that cathelicidin concentration is increased in cervicovaginal secretions of women with bacterial vaginosis. Increased cathelicidin production is expected to protect the cervix by killing the bacteria or by modulating the innate immune response (Frew et al., 2014). Suff et al. showed that cervical gene delivery of human β-defensin 3 may boost innate immunity in the cervix and prevent Escherichia coli ascending infection-associated preterm birth (Suff et al., 2020). Overall, the increase in AMP production in cervical epithelial cells may protect the cervix from U. parvum infection.
U. parvum infection may compromise the integrity of the cervical epithelial barrier by inducing EMT and inflammation in ecto and endo cells. EMT in the cervix has been shown to be involved in cervical remodeling during pregnancy. Parturition is associated with an increase in the expression of mesenchymal markers such as vimentin and N-cadherin, and a decrease in epithelial markers such as cytokeratin and E-cadherin (Hassan et al., 2009; Gordon and Mowa, 2019). Our previous in vitro studies have shown that infection and inflammation cause EMT in cervical epithelial cells (Tantengco et al., 2021d, c). However, the evidence of induction of EMT by U. parvum is not as strong as we have seen in our prior reports with LPS and TNFα. It is likely that U. parvum may promote EMT in ecto and endo cells but the constant exposure of cervical cells to high levels of progesterone during pregnancy may not allow full transition of these cells into proinflammatory mesenchymal cells to prevent cellular damage and inflammation (Tantengco et al., 2021c). Our study also showed that U. parvum increased the levels of MMP-9 and pro-inflammatory cytokines, particularly IL-8, IL-6, and GM-CSF, in ecto and endo cells. This increase in collagenolytic enzymes and pro-inflammatory cytokines are hallmarks of cervical ripening (Sennstrom, 2000; Kelly, 2002; Choi et al., 2009). These responses are likely not an effect of EMT but innate immune responses by cervical epithelial cells.
The cervical stromal cells and macrophages elicited both pro- and anti-inflammatory responses to U. parvum colonization. While these cells increased the production of pro-inflammatory cytokines, they also increased the IL-10 production in response to U. parvum colonization. IL-10 counteracts pro-inflammatory responses and modulates intracellular bacterial infections (Cyktor and Turner, 2011). It inhibits excessive Th1 and CD8+ T cell responses that contribute to tissue damage (Couper et al., 2008). IL-10 can also reduce the production of pro-inflammatory cytokines (including IL-1α and β, IL-6, IL-12, IL-18, and TNF-α) (Moore et al., 2001). Altogether, our data suggest that the increased IL-10 levels in cervical stromal cells and macrophages may counteract the inflammatory milieu and prevent further inflammation in the cervix.
This study had several strengths and limitations. This study showed the differential effects of U. parvum infection in cell cycle, cell death, antimicrobial and inflammatory (both pro- and anti-inflammatory) responses in ecto- and endocervical epithelial and stromal cells, and macrophages. Limitations of this study include the use of a single dose of U. parvum for all experiments and the use of one time point in assessing the cellular effects of U. parvum in cervical cells. However, the dose used in this study was within the pathologic dose of Ureaplasma spp. recovered from the cervical fluid of pregnancies complicated by preterm labor <37 weeks (Kacerovsky et al., 2014; Musilova et al., 2016). Additionally, this in vitro monoculture set-up does not fully mimic the in vivo tissue microenvironment where all cells are in physical contact and can communicate with each other. The effects observed in this experiment may change when these cells are allowed to communicate in culture together such as in microfluidic organ-on-a-chip or cervix tissue explants (Richardson et al., 2020).
Overall, our results may explain the relatively low PTB rate in when Ureaplasma spp. is inoculated intravaginally in mice with a healthy cervix (Motomura et al., 2020; Pavlidis et al., 2020). An intact cervix has mechanisms to protect itself from premature cervical ripening by preventing inflammation and cell death induced by infection. We propose that U. parvum is not a strong inducer of inflammation in the cervix. It may compromise the integrity of the cervical epithelial barrier, but the cervix can mount innate immune responses such as antimicrobial peptide and anti-inflammatory cytokine responses that may counter the pathologic effects of U. parvum in the cervix. In summary, U. parvum infection alone is not enough to compromise an intact cervix and cause significant increase in preterm birth. We hypothesize that it may render the cervical cells more susceptible to infection by more antigenic pathogens such as Escherichia coli and Gardnerella vaginalis. This polymicrobial infection can fully compromise the cervix and ultimately result in microbial invasion of the amniotic cavity. When U. parvum invades the amniotic cavity, it can promote preterm birth and neonatal morbidity including bronchopulmonary dysplasia, intraventricular hemorrhage, necrotizing enterocolitis, and sepsis (Katz et al., 2005; Viscardi and Kallapur, 2015).
Supplementary Material
Funding
Ourlad Alzeus G. Tantengco is an MD-PhD trainee in the MD-PhD in Molecular Medicine Program, supported by the Philippine Council for Health Research and Development, Department of Science and Technology, Republic of the Philippines and administered through the University of the Philippines Manila. This study was supported by 1R01HD100729 (NIH/NICHD) to Dr. Ramkumar Menon.
Footnotes
Declaration of interest
The authors declare no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Aaltonen R, Heikkinen J, Vahlberg T, Jensen JS and Alanen A (2007) Local inflammatory response in choriodecidua induced by Ureaplasma urealyticum. BJOG: An International Journal of Obstetrics and Gynaecology 114 1432–1435. [DOI] [PubMed] [Google Scholar]
- Allaire AD, D’Andrea N, Truong P, McMahon MJ and Lessey BA (2001) Cervical stroma apoptosis in pregnancy. Obstetrics and Gynecology 97 399–403. [DOI] [PubMed] [Google Scholar]
- Ashida H, Kim M and Sasakawa C (2014) Manipulation of the host cell death pathway by Shigella. Cellular Microbiology 16 1757–1766. [DOI] [PubMed] [Google Scholar]
- Behar SM and Briken V (2019) Apoptosis inhibition by intracellular bacteria and its consequence on host immunity. Current Opinion in Immunology 60 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowdish DMW, Davidson DJ, Scott MG and Hancock REW (2005) Immunomodulatory Activities of Small Host Defense Peptides. Antimicrobial Agents and Chemotherapy 49 1727–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y and Roh J (2014) Cervical cytopathological findings in women with Chlamydia trachomatis, ureaplasma urealyticum and mycoplasma hominis infections. The Scientific World Journal 25 62–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SJ, Jung KL, Oh S young, Kim JHand Roh CR (2009) Cervicovaginal matrix metalloproteinase-9 and cervical ripening in human term parturition. European Journal of Obstetrics and Gynecology and Reproductive Biology 142 43–47. [DOI] [PubMed] [Google Scholar]
- Couper KN, Blount DG and Riley EM (2008) IL-10: The Master Regulator of Immunity to Infection. The Journal of Immunology 180 5771 LP–5777. [DOI] [PubMed] [Google Scholar]
- Cyktor JC and Turner J (2011) Interleukin-10 and immunity against prokaryotic and eukaryotic intracellular pathogens. Infection and Immunity 79 2964–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald ES, Luz NF and Jamieson AM (2020) Competitive Cell Death Interactions in Pulmonary Infection: Host Modulation Versus Pathogen Manipulation. Frontiers in Immunology 11 814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frew L and Stock SJ Antimicrobial peptides and pregnancy. REPRODUCTION 141 725–735. [DOI] [PubMed] [Google Scholar]
- Frew L, Makieva S, McKinlay ATM, McHugh BJ, Doust A, Norman JE, Davidson DJ and Stock SJ (2014) Human Cathelicidin Production by the Cervix. PLOS ONE 9 e103434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser K, Silwedel C, Fehrholz M, Waaga-Gasser AM, Henrich B, Claus H and Speer CP (2017) Ureaplasma Species differentially modulate pro- and anti-inflammatory cytokine responses in newborn and adult human monocytes pushing the state toward pro-inflammation. Frontiers in Cellular and Infection Microbiology 7 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD and Romero R (2008) Epidemiology and causes of preterm birth. Lancet (London, England) 371 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J and Mowa CN (2019) Mechanobiology of mice cervix: expression profile of mechano-related molecules during pregnancy. Cell and Tissue Research 376 443–456. [DOI] [PubMed] [Google Scholar]
- Hassan SS, Romero R, Tarca AL, Nhan-Chang C-L, Vaisbuch E, Erez O, Mittal P, Kusanovic JP, Mazaki-Tovi S, Yeo L et al. (2009) The transcriptome of cervical ripening in human pregnancy before the onset of labor at term: identification of novel molecular functions involved in this process. The Journal of Maternal-Fetal & Neonatal Medicine : The Official Journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians 22 1183–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst-Kralovetz MM, Quayle AJ, Ficarra M, Greene S, Rose WA, Chesson R, Spagnuolo RA and Pyles RB (2008) Quantification and comparison of toll-like receptor expression and responsiveness in primary and immortalized human female lower genital tract epithelia. American Journal of Reproductive Immunology 59 212–224. [DOI] [PubMed] [Google Scholar]
- Hezelgrave NL, Seed PT, Chin-Smith EC, Ridout AE, Shennan AH and Tribe RM (2020) Cervicovaginal natural antimicrobial expression in pregnancy and association with spontaneous preterm birth. Scientific Reports 10 12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt R, Timmons BC, Akgul Y, Akins ML and Mahendroo M (2011) The molecular mechanisms of cervical ripening differ between term and preterm birth. Endocrinology 152 1036–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz S, Horowitz J, Mazor M, Porath A and Glezerman M (1995) Ureaplasma urealyticum cervical colonization as a marker for pregnancy complications. International Journal of Gynecology and Obstetrics 48 15–19. [DOI] [PubMed] [Google Scholar]
- Kacerovský M, Pavlovský M and Tosner J (2009) Preterm premature rupture of the membranes and genital mycoplasmas. Acta Medica (Hradec Kralove) 52 117–120. [DOI] [PubMed] [Google Scholar]
- Kacerovsky M, Celec P, Vlkova B, Skogstrand K, Hougaard DM, Cobo T and Jacobsson B (2013) Amniotic fluid protein profiles of intraamniotic inflammatory response to Ureaplasma spp. and other bacteria. PLoS ONE 8 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacerovsky M, Pliskova L, Menon R, Kutova R, Musilova I, Maly J and Andrys C (2014) Microbial load of umbilical cord blood Ureaplasma species and Mycoplasma hominis in preterm prelabor rupture of membranes. Journal of Maternal-Fetal and Neonatal Medicine 27 1627–1632. [DOI] [PubMed] [Google Scholar]
- Kallapur SG, Kramer BW and Jobe AH (2013) Ureaplasma and BPD. Seminars in Perinatology 37 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Schelonka RL and Waites KB (2005) Mycoplasmas and Ureaplasmas as neonatal pathogens. Clinical Microbiology Reviews 18 757–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RW (2002) Inflammatory mediators and cervical ripening. Journal of Reproductive Immunology 57 217–224. [DOI] [PubMed] [Google Scholar]
- Liu L, Cao G, Zhao Z, Zhao F and Huang Y (2014) High bacterial loads of Ureaplasma may be associated with non-specific cervicitis. Scandinavian Journal of Infectious Diseases 46 637–641. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL and O’Garra A (2001) Interleukin-10 and the interleukin-10 receptor. Annual Review of Immunology 19 683–765. [DOI] [PubMed] [Google Scholar]
- Motomura K, Romero R, Xu Y, Theis KR, Galaz J, Winters AD, Slutsky R, Garcia-Flores V, Zou C, Levenson D et al. (2020) Intra-Amniotic Infection with Ureaplasma parvum Causes Preterm Birth and Neonatal Mortality That Are Prevented by Treatment with Clarithromycin. MBio 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musilova I, Pliskova L, Kutova R, Hornychova H, Jacobsson B and Kacerovsky M (2016) Ureaplasma species and Mycoplasma hominis in cervical fluid of pregnancies complicated by preterm prelabor rupture of membranes. Journal of Maternal-Fetal and Neonatal Medicine 29 1–7. [DOI] [PubMed] [Google Scholar]
- Namba F, Hasegawa T, Nakayama M, Hamanaka T, Yamashita T, Nakahira K, Kimoto A, Nozaki M, Nishihara M, Mimura K et al. (2010) Placental features of chorioamnionitis colonized with Ureaplasma species in preterm delivery. Pediatric Research 67 166–172. [DOI] [PubMed] [Google Scholar]
- Noda-Nicolau NM, Polettini J, Peltier MR, da Silva MG and Menon R (2016) Combinations and loads of bacteria affect the cytokine production by fetal membranes: An in vitro study. American Journal of Reproductive Immunology 76 504–511. [DOI] [PubMed] [Google Scholar]
- Nott JP, Bonney EA, Pickering JD and Simpson NAB (2016) The structure and function of the cervix during pregnancy. Translational Research in Anatomy 2 1–7. [Google Scholar]
- Pavlidis I, Spiller OB, Sammut Demarco G, MacPherson H, Howie SEM, Norman JE and Stock SJ (2020) Cervical epithelial damage promotes Ureaplasma parvum ascending infection, intrauterine inflammation and preterm birth induction in mice. Nature Communications 11 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polettini J, Peltier MR, Noda-Nicolau NM, Menon R and da Silva MG (2018) Polybacterial stimulation suggests discrete IL-6/IL-6R signaling in human fetal membranes: Potential implications on IL-6 bioactivity. Journal of Reproductive Immunology 126 60–68. [DOI] [PubMed] [Google Scholar]
- Potts LC, Feng L, Seed PC, Jayes FL, Kuchibhatla M, Antczak B, Nazzal MK and Murtha AP (2016) Inflammatory Response of Human Gestational Membranes to Ureaplasma parvum Using a Novel Dual-Chamber Tissue Explant System1. Biology of Reproduction 94 1–8. [DOI] [PubMed] [Google Scholar]
- Read CP, Word RA, Ruscheinsky MA, Timmons BC and Mahendroo MS (2007) Cervical remodeling during pregnancy and parturition: molecular characterization of the softening phase in mice. Reproduction 134 327–340. [DOI] [PubMed] [Google Scholar]
- Richardson L, Kim S, Menon R and Han A (2020) Organ-On-Chip Technology: The Future of Feto-Maternal Interface Research? Frontiers in Physiology 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenschober-Böhm J, Waldhoer T, Schulz SM, Stihsen B, Pimpel B, Goeral K, Hafner E, Sliutz G, Kasper DC, Witt A et al. (2018) First Trimester Vaginal Ureaplasma Biovar Colonization and Preterm Birth: Results of a Prospective Multicenter Study. Neonatology 113 1–6. [DOI] [PubMed] [Google Scholar]
- Sennstrom MB (2000) Human cervical ripening, an inflammatory process mediated by cytokines. Molecular Human Reproduction 6 375–381. [DOI] [PubMed] [Google Scholar]
- Silwedel C, Fehrholz M, Speer CP, Ruf KC, Manig S and Glaser K (2019) Differential modulation of pulmonary caspases: Is this the key to Ureaplasma-driven chronic inflammation? PLOS ONE 14 e0216569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sixt BS (2021) Host cell death during infection with Chlamydia: a double-edged sword. FEMS Microbiology Reviews 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprong KE, Mabenge M, Wright CA and Govender S (2020) Ureaplasma species and preterm birth: current perspectives. Critical Reviews in Microbiology 46 169–181. [DOI] [PubMed] [Google Scholar]
- Suff N, Karda R, Diaz JA, Ng J, Baruteau J, Perocheau D, Taylor PW, Alber D, Buckley SMK, Bajaj-Elliott M et al. (2020) Cervical Gene Delivery of the Antimicrobial Peptide, Human β-Defensin (HBD)-3, in a Mouse Model of Ascending Infection-Related Preterm Birth. Frontiers in Immunology 11 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney EL, Dando SJ, Kallapur SG and Knox L (2017) The human Ureaplasma species as causative agents of chorioamnionitis. Clinical Microbiology Reviews 30 349–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantengco OAG and Yanagihara I (2019) Current understanding and treatment of intra‐amniotic infection with Ureaplasma spp. Journal of Obstetrics and Gynaecology Research jog.14052. [DOI] [PubMed] [Google Scholar]
- Tantengco OAG, Richardson LS and Menon R (2021a) Effects of a gestational level of estradiol on cellular transition, migration, and inflammation in cervical epithelial and stromal cells. American Journal of Reproductive Immunology 85 e13370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantengco OAG, Vink J, Medina PMB and Menon R (2021b) Oxidative stress promotes cellular damages in the cervix: implications for normal and pathologic cervical function in human pregnancy. Biology of Reproduction 105 204–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantengco OAG, Richardson LS, Vink J, Kechichian T, Medina PMB, Pyles RB and Menon R (2021c) Progesterone alters human cervical epithelial and stromal cell transition and migration: Implications in cervical remodeling during pregnancy and parturition. Molecular and Cellular Endocrinology 529 111276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantengco OAG, Richardson LS, Medina PMB, Han A and Menon R (2021d) Organ-on-chip of the cervical epithelial layer: A platform to study normal and pathological cellular remodeling of the cervix. The FASEB Journal 35 e21463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribe RM (2015) Small Peptides with a Big Role: Antimicrobial Peptides in the Pregnant Female Reproductive Tract. American Journal of Reproductive Immunology 74 123–125. [DOI] [PubMed] [Google Scholar]
- Vink J and Feltovich H (2016) Cervical etiology of spontaneous preterm birth. Seminars in Fetal and Neonatal Medicine 21 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink J and Mourad M (2017) The pathophysiology of human premature cervical remodeling resulting in spontaneous preterm birth: Where are we now? Seminars in Perinatology 41 427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink J and Myers K (2018) Cervical alterations in pregnancy. Best Practice and Research: Clinical Obstetrics and Gynaecology 52 88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscardi RM (2010) Ureaplasma species: Role in diseases of prematurity. Clinics in Perinatology 37 393–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscardi RM and Kallapur SG (2015) Role of Ureaplasma Respiratory Tract Colonization in Bronchopulmonary Dysplasia Pathogenesis: Current Concepts and Update. Clinics in Perinatology 42 719–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfs TGAM, Kallapur SG, Knox CL, Thuijls G, Nitsos I, Polglase GR, Collins JJP, Kroon E, Spierings J, Shroyer NF et al. (2013) Antenatal ureaplasma infection impairs development of the fetal ovine gut in an IL-1-dependent manner. Mucosal Immunology 6 547–556. [DOI] [PubMed] [Google Scholar]
- Word RA, Li XH, Hnat M and Carrick K (2007) Dynamics of cervical remodeling during pregnancy and parturition: Mechanisms and current concepts. Seminars in Reproductive Medicine 25 69–79. [DOI] [PubMed] [Google Scholar]
- Xiao L, Crabb DM, Dai Y, Chen Y, Waites KB and Atkinson TP (2014) Suppression of antimicrobial peptide expression by ureaplasma species. Infection and Immunity 82 1657–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarbrough VL, Winkle S and Herbst-Kralovetz MM (2015) Antimicrobial peptides in the female reproductive tract: A critical component of the mucosal immune barrier with physiological and clinical implications. Human Reproduction Update 21 353–377. [DOI] [PubMed] [Google Scholar]
- Yellon SM (2017) Contributions to the dynamics of cervix remodeling prior to term and preterm birth†. Biology of Reproduction 96 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellon SM (2020) Immunobiology of Cervix Ripening. Frontiers in Immunology 10 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


