Abstract
In Saccharomyces cerevisiae, rapid degradation of nonsense-containing mRNAs requires the decapping enzyme Dcp1p, the 5′-to-3′ exoribonuclease Xrn1p, and the three nonsense-mediated mRNA decay (NMD) factors, Upf1p, Nmd2p, and Upf3p. To identify specific functions for the NMD factors, we analyzed the mRNA decay phenotypes of yeast strains containing deletions of DCP1 or XRN1 and UPF1, NMD2, or UPF3. Our results indicate that Upf1p, Nmd2p, and Upf3p regulate decapping and exonucleolytic degradation of nonsense-containing mRNAs. In addition, we show that these factors also regulate the same processes in the degradation of wild-type mRNAs. The participation of the NMD factors in general mRNA degradation suggests that they may regulate an aspect of translation termination common to all transcripts.
mRNA degradation, an important aspect of gene expression, is a regulated process that is often linked to mRNA translation (30, 59). Specific pathways of mRNA decay have been studied in several experimental systems and have been most extensively characterized in the yeast Saccharomyces cerevisiae (5, 30). In yeast, wild-type mRNAs are primarily degraded by a 5′-to-3′ deadenylation-dependent mechanism in which the initial nucleolytic event is the shortening of the poly(A) tail to an oligo(A) length of 10 to 15 nucleotides. After poly(A) shortening, transcripts are decapped by the product of the DCP1 gene (Dcp1p) and digested exonucleolytically by the 5′-to-3′ exoribonuclease, Xrn1p (6, 9, 37). Although this decay pathway appears to comprise the predominant mode of mRNA degradation, recent evidence indicates that mRNAs can also be degraded by a 3′-to-5′ mechanism that requires the products of the SKI2, SKI3, and SKI8 genes, as well as Ski6p (also known as Rrp41) and Rrp4p, two 3′-to-5′ exonucleases of the exosome (46, 29, 67).
mRNAs containing a premature termination codon are degraded by the nonsense-mediated mRNA decay pathway (26, 31, 54). This type of mRNA decay has been observed in all eukaryotic cells so far examined and may, in part, serve as a surveillance mechanism that eliminates aberrant mRNAs (14, 22, 50, 57). Degradation of nonsense-containing mRNAs is deadenylation independent, proceding from decapping by Dcp1p to Xrn1p-catalyzed 5′-to-3′ decay without prior poly(A) shortening (6, 21, 47, 65). Nonsense-mediated mRNA decay also requires at least three additional trans-acting factors: Upf1p, Nmd2p (Upf2p), and Upf3p. Mutations or deletions of one or more of the genes encoding these factors (UPF1, NMD2 [UPF2], and UPF3) generally lead to the same phenotype: the selective stabilization of nonsense-containing mRNAs with no apparent effect on the stability of most wild-type mRNAs (10, 23, 38, 40–42, 52, 54, 68).
The UPF1, NMD2, and UPF3 genes and their products, have been characterized extensively. UPF1 encodes a 109-kDa protein that has two putative Zn fingers near its N terminus and seven conserved motifs common to the members of RNA/DNA helicase superfamily 1 (1, 36, 41). Upf1p has been purified from yeast cells and shown to possess nucleic acid-binding activity as well as nucleic acid-dependent ATPase and helicase activities (12). Nmd2p, a 127-kDa acidic protein, and Upf3p, a 45-kDa basic protein, both contain putative bipartite nuclear localization signals (23, 38). However, both proteins, as well as Upf1p, are primarily localized in the cytoplasm and appear to be polyribosome associated (3, 4, 23, 44, 53; F. He and A. Jacobson, unpublished data). Nmd2p interacts with Upf3p and Upf1p, and the latter, in turn, can also interact with itself, the release factors Sup35p and Sup45p, and Nmd1p, Nmd3p, and Dbp2p (7, 13, 23–25; A. Bond, D. Mangus, F. He, and A. Jacobson, submitted for publication; F. He and A. Jacobson, submitted for publication).
Although the identification and characterization of Upf1p, Nmd2p, and Upf3p have provided insight into the mechanism of nonsense-mediated mRNA decay, the precise functions of these factors remain unknown. For example, a role in regulating translational termination and fidelity is suggested by the interaction of Upf1p with Sup35p and Sup45p (13) and by the occurrence of allosuppression, omnipotent suppression, and −1 frameshifting phenotypes in upf1, nmd2, and upf3 mutants (11, 17, 39, 43, 61; He and Jacobson, submitted). Whether the translational functions of these proteins dictate their mRNA decay functions (or vice versa) remains to be determined, but it is noteworthy that the two roles of Upf1p have been separated by distinct mutations (69, 70; He and Jacobson, submitted) and by overexpression (43; He and Jacobson, submitted). Likewise, little is known about the epistatic and regulatory interactions of the respective factors. DCP1, XRN1, UPF1, NMD2, and UPF3 are all required for degradation of nonsense-containing mRNAs, but the only dissection of their regulatory interactions has been a study demonstrating that Nmd2p and Upf3p regulate Upf1p's activity in nonsense suppression (43).
To address these basic issues, we constructed a set of yeast strains that contain single or multiple deletions of the DCP1, XRN1, UPF1, NMD2, and UPF3 genes and analyzed mRNA decay phenotypes in these strains. We show that (i) deletions of UPF1, NMD2, or UPF3 lead to increased accumulation of capped nonsense-containing mRNAs, regardless of Xrn1p function; (ii) deletions of these genes in xrn1Δ cells differentially affect the accumulation of decapped nonsense-containing mRNAs, as well as capped and decapped wild-type mRNAs; and (iii) deletions of these genes in dcp1Δ cells differentially affect the accumulation of capped nonsense-containing and wild-type mRNAs. Our data indicated that Upf1p, Nmd2p, and Upf3p can regulate decapping and exonucleolytic degradation of both nonsense-containing mRNAs and wild-type mRNAs and suggest that these effects may be a consequence of the roles played by these factors in regulating a general aspect of translation termination.
MATERIALS AND METHODS
General methods.
Preparation of standard yeast media and methods of cell culture were as described (58). Transformation of yeast was done by the high-efficiency method (63). DNA manipulations were performed according to standard techniques (62). All PCR amplifications were performed with Taq DNA polymerase (71). Plasmid DNAs were prepared from Escherichia coli DH5α. The oligonucleotides used in this study were obtained from Operon, Inc.
Yeast strains.
The yeast strains used in this study are listed in Table 1. Strains containing deletions of XRN1 or DCP1 were constructed by gene replacement (60). A NotI-SalI fragment containing the xrn1::ADE2 allele isolated from pHF2095 or a DraI-ClaI fragment containing the dcp1::URA3 allele isolated from pRP716 was used for yeast transformation. ADE+ or URA+ transformants were selected, and the disruption was confirmed by PCR analysis of genomic DNA.
TABLE 1.
Yeast strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| HFY1200 | MATaade2-1 his3-11, 15 leu2-3, 112 trp1-1 ura3-1 can1-100 UPF1 NMD2 UPF3 | 23 |
| HFY871 | MATaade2-1 his3-11, 15 leu2-3, 112 trp1-1 ura3-1 can1-100 upf1::HIS3 NMD2 UPF3 | 25 |
| HFY1300 | MATα ade2-1 his3-11, 15 leu2-3, 112 trp1-1 ura3-1 can1-100 UPF1 nmd2::HIS3 UPF3 | 23 |
| HFY861 | MATaade2-1 his3-11, 15 leu2-3, 112 trp1-1 ura3-1 can1-100 UPF1 NMD2 upf3::HIS3 | 25 |
| HFY3000 | MATα ade2-1 his3-11, 15 leu2-3, 112 trp1-1 ura3-1 can1-100 upf1::URA3 nmd2::HIS3 UPF3 | 23 |
| HFY872 | MATaade2-1 his3-11, 15 leu2-3, 112 trp1-1 ura3-1 can1-100 upf1-1::URA3 NMD2 upf3::HIS3 | 25 |
| HFY874 | MATaade2-1 his3-11, 15 leu2-3, 112 trp1-1 ura3-1 can1-100 UPF1 nmd2::URA3 upf3::HIS3 | 25 |
| HFY883 | MATaade2-1 his3-11, 15 leu2-3, 112 trp1-1 ura3-1 can1-100 upf1::LEU2 nmd2::URA3 upf3::HIS3 | 25 |
| HF1081 | MATaade2-1 his3-11, 15 leu2-3, 112 trp1-1 ura3-1 can1-100 UPF1 NMD2 UPF3 xrn1::ADE2 | This study |
| HF1083 | MATaade2-1 his3-11, 15 leu2-3, 112 trp1-1 ura3-1 can1-100 upf1::HIS3 NMD2 UPF3 xrn1::ADE2 | This study |
| HF1085 | MATα ade2-1 his3-11, 15 leu2-3, 112 trp1-1 ura3-1 can1-100 UPF1 nmd2::HIS3 UPF3 xrn1::ADE2 | This study |
| HF1087 | MATaade2-1 his3-11, 15 leu2-3, 112 trp1-1 ura3-1 can1-100 UPF1 NMD2 upf3::HIS3 xrn1::ADE2 | This study |
| HF1089 | MATα ade2-1 his3-11, 15 leu2-3, 112 trp1-1 ura3-1 can1-100 upf1::URA3 nmd2::HIS3 UPF3 xrn1::ADE2 | This study |
| HF1091 | MATaade2-1 his3-11, 15 leu2-3, 112 trp1-1 ura3-1 can1-100 upf1-1::URA3 NMD2 upf3::HIS3 xrn1::ADE2 | This study |
| HF1093 | MATaade2-1 his3-11, 15 leu2-3, 112 trp1-1 ura3-1 can1-100 UPF1 nmd2::URA3 upf3::HIS3 xrn1::ADE2 | This study |
| HF1095 | MATaade2-1 his3-11, 15 leu2-3, 112 trp1-1 ura3-1 can1-100 upf1::LEU2 nmd2::URA3 upf3::HIS3 xrn1::ADE2 | This study |
| HF1112 | MATaade2-1 his3-11, 15 leu2-3, 112 trp1-1 ura3-1 can1-100 UPF1 NMD2 UPF3 xrn1::ADE2 dcp1::URA3 | This study |
| HFY1067 | MATaade2-1 his3-11, 15 leu2-3, 112 trp1-1 ura3-1 can1-100 UPF1 NMD2 UPF3 dcp1::URA3 | This study |
| HFY1070 | MATaade2-1 his3-11, 15 leu2-3, 112 trp1-1 ura3-1 can1-100 upf1::HIS3 NMD2 UPF3 dcp1::URA3 | This study |
| HFY1073 | MATα ade2-1 his3-11, 15 his4-38 leu2-3, 112 trp1-1 ura3-1 can1-100 UPF1 nmd2::HIS3 UPF3 dcp1::URA3 | This study |
| HFY1076 | MATaade2-1 his3-11, 15 his4-38 leu2-3, 112 trp1-1 ura3-1 can1-100 UPF1 NMD2 upf3::HIS3 dcp1::URA3 | This study |
| DAt1-1 | MATα ura3-52 trp1-Δ63 leu2-Δ1 rat1-1 | 2 |
| HFY1101 | MATα ura3-52 trp1-Δ63 leu2-Δ1 rat1-1 [pRS316] | This study |
| HFY1102 | MATα ura3-52 trp1-Δ63 leu2-Δ1 rat1-1 xrn1::URA3 | This study |
Plasmids.
Plasmids used in this study included (i) pHF2095, which contains the xrn1::ADE2 allele in pBluescript KSII (+); (ii) pRP716 (a gift from Roy Parker, University of Arizona), which contains the dcp1::URA3 allele in pBluescript; (iii) pHF2105, which contains the MER2 gene in YEplac112; and (iv) pHF1083, pHF1085, and pHF1463, which contain the ADH1-HA-UPF1, -NMD2, or -UPF3 alleles in YEplac112, respectively.
Construction of the xrn1::ADE2 allele.
The plasmid pHF2095, which carries the xrn1::ADE2 allele, was constructed in two steps. First, a 514-bp PCR-derived NotI-BglII fragment containing the promoter and 5′ untranslated region and a 425-bp PCR-derived BglII-SalI fragment containing sequences 3′ to the translational stop codon of XRN1 were ligated into Bluescript digested previously by NotI and SalI in a three-fragment ligation reaction, generating pHF2095a. The oligonucleotide pairs XRN1-DS1 (AAAAGCGGCCGCCAACAGAGACAAACAAGAAGAGGTTA) and XRN1-DS2 (AAA AGATCTACCGTACTGATATATATTTGTTGCTGC) and XRN1-DS3 (AAAAGATCTACCGTACTGATATATATTTGTTGCTGC) and XRN1-DS4 (AAAGTCGACAGAAGACCCTGCAATAACATTTACACA) were used for PCR amplification of both fragments, respectively. Second, a 2,526-bp BglII-BglII ADE2 fragment was ligated into pHF2095a digested previously by BglII. This led to a replacement of the entire XRN1 coding region by the ADE2 gene.
RNA analysis.
RNA isolation, Northern blotting, and primer extension analyses were performed as described previously (23). The DNA fragments used for Northern blot analysis included a 0.6-kb EcoRI-HindIII fragment of CYH2, a 0.7-kb BglII-XbaI fragment of MER2, a 0.8-kb EcoRI-HindIII fragment of TCM1, a 0.8-kb ClaI-EcoRI fragment of ADE3, a 2.0-kb Asp718-EcoRI fragment of URA5, a 0.65-kb Asp718-BglII fragment of PGK1, a 3.0-kb EcoRI-SalI fragment of GCN4, a 1.3-kb BamHI-BamHI fragment of CUP1, a 0.7-kb PCR fragment of PYK1 amplified by using PYK1-1 (AAAGTCGACCCAGTTATATCATGGTCCCCTTTCAAA) and PYK1-2 (TGACACCCTTGTGGGAACAGATCTTACCGG), and a 0.4-kb fragment of SCR1 amplified by PCR using SCR1-1 (AGGCTGTAATGGCTTTCTGGTGGGATGGGA) and SCR1-2 (GATATGTGCTATCCCGGCCGCCTCCATCAC). The oligonucleotides used for primer extension analysis included CYH2-IN4 (ATATACACACGA CATATTGGTTGCACAACA), which hybridizes to a region in the CYH2 pre-mRNA intron from nucleotides 59 to 88 downstream of the 5′ splicing site; MER2-2 (CATCAACGAGTGTTCAGAATTAGCCTCTGAAAC), which hybridizes to a region in the first exon of MER2 pre-mRNA from nucleotides +40 to +72 downstream of the translation initiation codon; ADH1-1 (TATCCTTGTGTTCCAATTTACCGTGG), which hybridizes to a region in the ADH1 mRNA from nucleotides +45 to +70 downstream of the translation initiation codon; CUP1-1 (GGCATTGGCACTCATGACCTTC), which hybridizes to a region in the CUP1 mRNA from nucleotides +31 to +52 downstream of the translation initiation codon; and URA5-1 (AGTAGTATATCGCAGAAGTAATGCTTTATG), which hybridizes to a region in the URA5 mRNA from nucleotides −85 to −114 upstream of the translation initiation codon. RNA immunoprecipitations with polyclonal anti-m7 G antibodies were performed as described (48, 72).
RESULTS
Nonsense-containing mRNAs that accumulate in upf1Δ, nmd2Δ, or upf3Δ cells are full-length and capped.
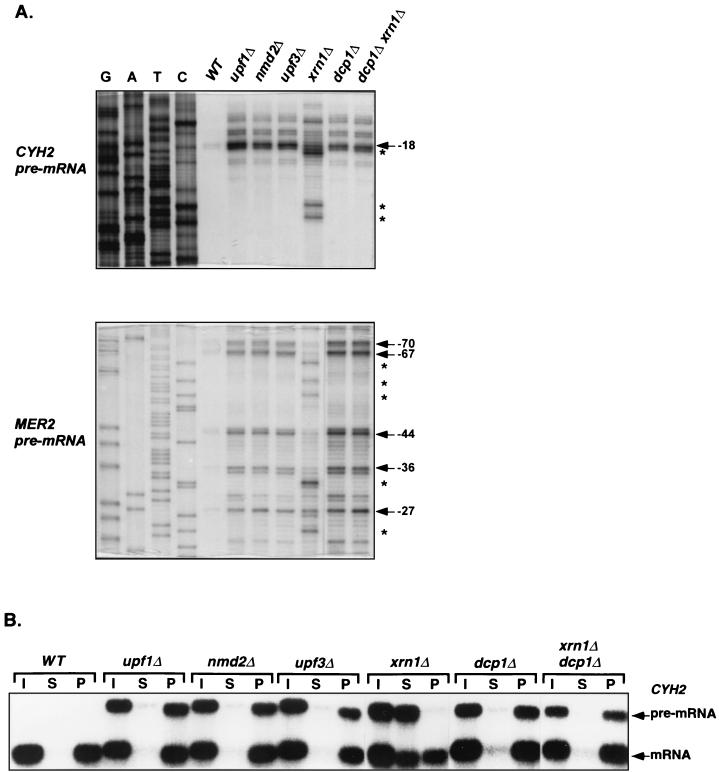
To define the functional roles and interrelationships of Upf1p, Nmd2p, Upf3p, Xrn1p, and Dcp1p, we first determined the 5′ ends and cap status of two nonsense-containing mRNAs that were stabilized in otherwise isogenic cells harboring deletions of the genes encoding these factors. Primer extension and anti-m7 G immunoprecipitation assays were utilized to characterize the CYH2 and MER2 pre-mRNAs, two endogenous substrates of the nonsense-mediated mRNA decay pathway (22). These pre-mRNAs are normally very unstable, and their 5′ ends were barely detectable in wild-type cells (Fig. 1A). However, CYH2 pre-mRNA transcripts accumulated in the upf1Δ, nmd2Δ, upf3Δ, and dcp1Δ strains, and all had identical 5′ ends, including a major transcript that initiated at nucleotide −18 and two minor species with ends at nucleotides −22 and −27 (Fig. 1A). In contrast, the predominant transcripts that accumulated in xrn1Δ cells had 5′ ends at nucleotides +1, −3, and −16. Comparable results were obtained with the MER2 pre-mRNA. This transcript exhibited identical 5′ ends at nucleotides −27, −36, −44, −67, and −70 in upf1Δ, nmd2Δ, upf3Δ, and dcp1Δ cells but had 5′ ends at nucleotides −23, −33, −55, −59, and −64 in xrn1Δ cells (Fig. 1A).
FIG. 1.
Analysis of the 5′ ends and cap status of nonsense-containing mRNAs that accumulate in yeast strains defective in nonsense-mediated mRNA decay. (A) Analysis of the 5′ ends of the CYH2 and MER2 pre-mRNAs by primer extension. Total RNA was isolated from yeast strains of the indicated genotypes. Radiolabeled primers (CYH2-IN4 or MER2-2) were annealed to aliquots (20 μg) of each RNA sample and extended by avian myeloblastosis virus reverse transcriptase. DNA sequencing reactions with the same primers (run in lanes G, A, T, and C) were used to determine the positions of the primer extension products. The major transcriptional start sites (positions noted are relative to the initiation codon) for both pre-mRNAs are indicated by arrows. The atypical extension products detected in RNA from xrn1Δ cells are denoted by asterisks. (B) Analysis of the 5′ cap status of the CYH2 pre-mRNA by anti-m7 G immunoprecipitation. Aliquots (10 μg) of total RNA isolated from the indicated yeast strains were immunoprecipitated using polycolonal anti-m7 G antibodies. RNA comprising the supernatant (S) (uncapped) and pellet (P) (capped) fractions, as well as an aliquot of the input RNA (I), were analyzed by Northern blotting, using a CYH2 probe. The positions of the CYH2 pre-mRNA and mRNA are indicated. Quantitation of this experiment is summarized in Table 2. WT, wild type.
Since the CYH2 and MER2 pre-mRNAs present in upf1Δ, nmd2Δ, or upf3Δ cells all had the same 5′ ends as those in the dcp1Δ strain, it seemed likely that they accumulated as capped species. To test this directly, anti-m7 G antibodies were used to separate the CYH2 and MER2 pre-mRNAs into capped and uncapped fractions that were subsequently analyzed by Northern blotting. These experiments showed that approximately 90% of the CYH2 pre-mRNA present in cells harboring a dcp1Δ mutation or upf1Δ, nmd2Δ, or upf3Δ mutations was in the capped fraction (Fig. 1B and Table 2). In xrn1Δ cells, however, only 10% of the CYH2 pre-mRNA transcripts were in the capped fraction and the remainder were in the decapped fraction (Fig. 1B and Table 2). Analyses of the cap status of the MER2 pre-mRNA in the same strains provided identical results (data not shown). These data indicate that full-length, capped CYH2 and MER2 pre-mRNAs accumulate in cells lacking Upf1p, Nmd2p, or Upf3p and suggest that these factors promote efficient decapping of nonsense-containing transcripts.
TABLE 2.
Effects of single or multiple deletions of the UPF1, NMD2, and UPF3 genes on the accumulation of total, capped, and decapped CYH2 pre-mRNA and mRNAa
| Strain | Pre-mRNA
|
mRNA
|
||||
|---|---|---|---|---|---|---|
| Totalb | Cappedc | Uncappedc | Total | Capped | Uncapped | |
| Wild type | 1.00 | NAd | NA | 1.00 | 0.90 | 0.10 |
| upf1Δ | 24.40 | 21.50 | 2.90 | 1.10 | 1.00 | 0.10 |
| nmd2Δ | 25.80 | 22.20 | 3.60 | 1.10 | 1.00 | 0.10 |
| upf3Δ | 23.70 | 20.20 | 3.50 | 1.10 | 1.00 | 0.10 |
| xrn1Δ | 37.70 | 3.80 | 33.90 | 1.40 | 0.70 | 0.70 |
| xrn1Δ upf1Δ | 37.20 | 13.40 | 23.80 | 1.50 | 0.86 | 0.64 |
| xrn1Δ nmd2Δ | 20.00 | 10.00 | 10.00 | 1.00 | 0.80 | 0.20 |
| xrn1Δ upf3Δ | 28.30 | 10.20 | 18.10 | 1.20 | 0.72 | 0.48 |
| xrn1Δ upf1Δ nmd2Δ | 37.80 | 12.90 | 24.90 | 1.50 | 0.85 | 0.65 |
| xrn1Δ upf1Δ upf3Δ | 37.70 | 13.20 | 24.50 | 1.50 | 0.90 | 0.60 |
| xrn1Δ nmd2Δ upf3Δ | 28.60 | 10.60 | 18.00 | 1.30 | 0.80 | 0.50 |
| xrn1Δ upf1Δ nmd2Δ upf3Δ | 35.60 | 12.10 | 23.50 | 1.50 | 0.90 | 0.60 |
The northern blot in Fig. 4C was used to measure the levels of the CYH2 pre-mRNA and mRNA in each sample. Northern blots in Fig. 1B and 4A were used to determine the percentage of capped and uncapped transcripts.
Numbers represent the relative abundance of the transcripts and are the ratios of RNA in each mutant strain to that in the wild-type strain, after normalizing to the SCR1 internal control. The level of transcripts in the wild-type strain was arbitrarily defined as 1 unit.
Relative levels of capped and decapped transcripts and were obtained by multiplying the corresponding number in the Total column by the percentage of capped or decapped transcripts, respectively.
NA, not applicable.
Nonsense-containing mRNAs that accumulate in xrn1Δ cells are decapped, shortened at their 5′ ends, and generated by partial Rat1p digestion.
As shown in Fig. 1, the CYH2 and MER2 pre-mRNAs that accumulate in xrn1Δ cells are uncapped and shortened at their 5′ ends. The structure of these RNAs suggests that they may be decay intermediates, possibly arising by either of two general mechanisms. In the first, decapping could proceed by the usual Dcp1p-mediated event, but be followed by inefficient 5′-to-3′ exonucleolytic digestion by a nuclease other than Xrn1p. In the second, the premature nonsense codon could trigger decapping by a 5′-proximal Dcp1p-independent endonucleolytic cleavage. To distinguish between these possibilities, we first determined whether the appearance of the atypical RNA species is dependent on Dcp1p. To this end, we constructed a dcp1Δ xrn1Δ double mutant and examined the 5′ ends and 5′-cap status of the CYH2 and MER2 pre-mRNAs that accumulated in this strain. As shown in Fig. 1A, in dcp1Δ xrn1Δ cells all putative decay intermediates are absent and both pre-mRNAs have the same 5′ ends as do their counterparts in dcp1Δ cells. Moreover, approximately 90% of the CYH2 and MER2 pre-mRNAs present in dcp1Δ xrn1Δ cells are capped (Fig. 1B and data not shown). These results indicate that the RNA species peculiar to xrn1Δ cells must arise from decapping by Dcp1p, thereby ruling out the second possibility. To test the first possibility directly, we sought to identify a 5′-to-3′ exonuclease that might generate the respective truncated RNAs.
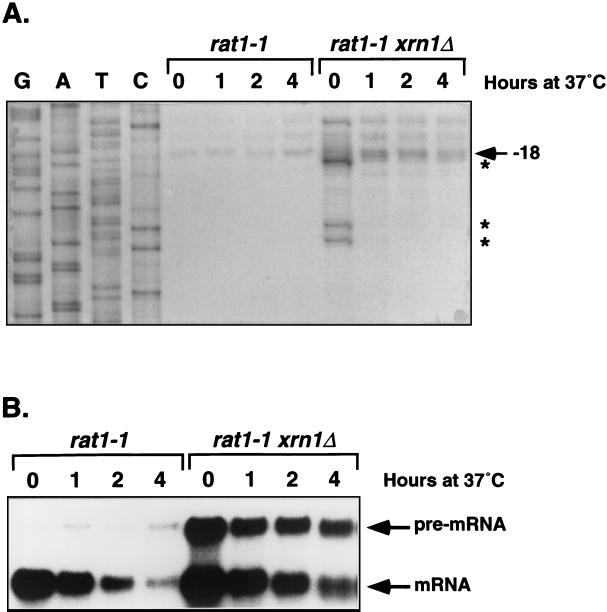
Biochemical analyses of 5′-to-3′ exonuclease activities in yeast identified only two such activities. One, as noted above, is encoded by the XRN1 gene (37), and the other is encoded by the RAT1 (also known as KEM1) gene (2, 35). Since sequence analyses indicated that Rat1p is the only protein in the yeast genome that shares significant homology with Xrn1p (data not shown), we reasoned that Rat1p might be responsible for the production of the putative decay intermediates in xrn1Δ cells. To test this notion, we used the temperature-sensitive rat1-1 allele and constructed a rat1-1 xrn1Δ strain. Due to loss of Rat1p function, this strain ceased growth within 2 h after being shifted to 37°C (2). The effect of inactivation of Rat1p on the production of mRNA decay intermediates in xrn1Δ cells was analyzed by Northern blotting and primer extension. The results presented in Fig. 2A indicate that, when grown at 24°C, rat1-1 xrn1Δ cells accumulate the same shortened species of CYH2 pre-mRNA as do xrn1Δ cells (i.e., RNA species with 5′ ends at nucleotides +1, −3, and −16 [Fig. 2A, lane 0]). However, when shifted to 37°C for 1, 2, or 4 h, the rat1-1 xrn1Δ cells (i) stabilized the CYH2 pre-mRNA (Fig. 2B); (ii) accumulated full-length CYH2 pre-mRNAs with 5′ ends at nucleotides −18, −22, and −27 (Fig. 2A); and (iii) greatly reduced the levels of shortened RNA species (Fig. 2A). These results demonstrate that Rat1p is responsible for the production of the unusual CYH2 and MER2 RNAs detected in xrn1Δ cells. Taken together, the data indicate that inactivation of Xrn1p leads to the accumulation of decay intermediates for nonsense-containing mRNAs and that these decay intermediates arise from decapping by Dcp1p and incomplete 5′-to-3′ exonucleolytic digestion by Rat1p.
FIG. 2.
Inactivation of Rat1p inhibits accumulation of CYH2 pre-mRNA decay intermediates in xrn1Δ cells. rat1-1 and rat1-1 xrn1Δ cells were grown at 24°C and then shifted to 37°C for 1, 2, or 4 h. Total RNA was isolated from each culture at the indicated time points and used for primer extension analysis of the 5′ ends (A) or Northern analysis of the levels of the CYH2 pre-mRNA (B), as in Fig. 1.
In xrn1Δ cells, incomplete Rat1p digestion also leads to the accumulation of decay intermediates of wild-type mRNAs.
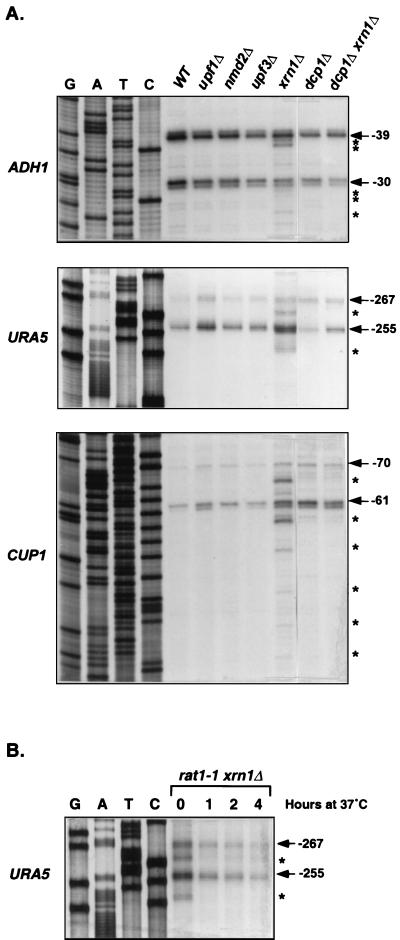
Decapped and 5′ shortened species of wild-type PGK1 and RP51A mRNAs have been identified in xrn1Δ cells previously (28, 49). Since Xrn1p plays a general role in mRNA degradation, we reasoned that the accumulation of decay intermediates in xrn1Δ cells might be a general phenomenon. To evaluate this possibility further, we analyzed the 5′ ends and cap status of additional wild-type transcripts that accumulated in xrn1Δ cells. As shown in Fig. 3A, ADH1, URA5, and CUP1 mRNAs that accumulated in wild-type; upf1Δ, nmd2Δ, upf3Δ, or dcp1Δ strains all had identical 5′ ends. The ADH1, URA5, and CUP1 mRNAs had major transcription start sites at nucleotides −39 and −30, −267 and −255, and −70 and −61, respectively. In contrast, novel species of each of these mRNAs accumulated in xrn1Δ cells, including those with 5′ ends at nucleotides −38, −37, −28, −27, and −23 for ADH1 mRNA, nucleotides −260 and −248 for URA5 mRNA, and nucleotides −66, −57, −51, −43, −37, and −32 for CUP1 mRNA. Consistent with these primer extension data, anticap immunoprecipitation experiments showed that transcripts accumulating in the wild-type, upf1Δ, nmd2Δ, upf3Δ, and dcp1Δ strains were largely in the capped fraction and those accumulating in xrn1Δ cells were predominantly in the decapped fraction (data not shown; see also Fig. 6). Two observations indicate that these shortened and decapped species of wild-type mRNAs arise in xrn1Δ cells by the same mechanism that generated decay intermediates of nonsense-containing mRNAs. First, the 5′-shortened species of ADH1, URA5, and CUP1 mRNAs detected in the xrn1Δ strain are absent in dcp1Δ xrn1Δ cells (Fig. 3A). Second, although rat1-1 xrn1Δ cells grown at the permissive temperature accumulated 5′-shortened species of URA5 mRNA, these cells did not accumulate the shortened transcripts when shifted to the nonpermissive temperature for 1, 2, or 4 h (Fig. 3B).
FIG. 3.
Formation of decay intermediates of wild-type mRNAs in xrn1Δ cells requires the decapping enzyme Dcp1p and the 5′-to-3′ exoribonuclease Rat1p. (A) Inactivation of Dcp1p inhibits the formation of mRNA decay intermediates in xrn1Δ cells. Total RNA was isolated from yeast strains of the indicated genotypes and the 5′ ends of wild-type ADH1, URA5, and CUP1 mRNAs were analyzed by primer extension, as in Fig. 1A. Radiolabeled primers (ADH1-1, URA5-1, and CUP1-1) were used for both reverse transcription and DNA sequencing reactions. The major transcriptional start sites (position noted is relative to the translation initiation codon) for each mRNA are indicated by arrows. mRNA decay intermediates that accumulate in xrn1Δ cells are marked by asterisks. WT, wild type. (B) Inactivation of Rat1p inhibits the formation of mRNA decay intermediates in xrn1Δ cells. rat1-1 xrn1Δ cells were grown at 24°C and then shifted to 37°C. Total RNA was isolated from cells at the indicated time points, and the 5′ ends of the URA5 mRNA were analyzed by primer extension as in panel A.
FIG. 6.
Effects of single or mutiple deletions of UPF1, NMD2, UPF3, and XRN1 on the accumulation of capped and decapped wild-type mRNAs. (A and B) Analysis of the levels of capped and decapped wild-type (WT) mRNAs by anti-m7 G immunoprecipitation. Total RNA was isolated from yeast strains of the indicated genotypes, and anticap immunoprecipitation was carried out as in Fig. 1B. DNA probes specific for URA5, TCM1, and PGK1 were used for Northern analysis of the respective RNA fractions. Lanes I, S, and P represent input, supernant, and pellet, respectively. Quantitation of this experiment is summarized in Table 3. (C) Analysis of the levels of URA5 mRNA decay intermediates. Primer extension analysis of the URA5 mRNA was performed on total RNA from each indicated yeast strain, as in Fig. 3A. Total RNA isolated from the upf3Δ strain was used as a control. The major transcriptional start sites of the URA5 mRNA and the 5′ ends of its decay intermediates are indicated by arrows and asterisks, respectively.
Cells harboring both xrn1Δ and upf1Δ, nmd2Δ, or upf3Δ mutations accumulate nonsense-containing mRNAs that are full length and capped.
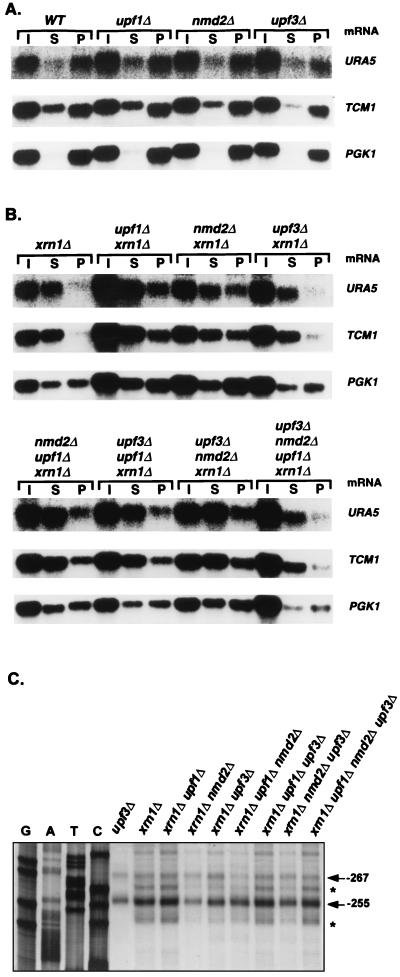
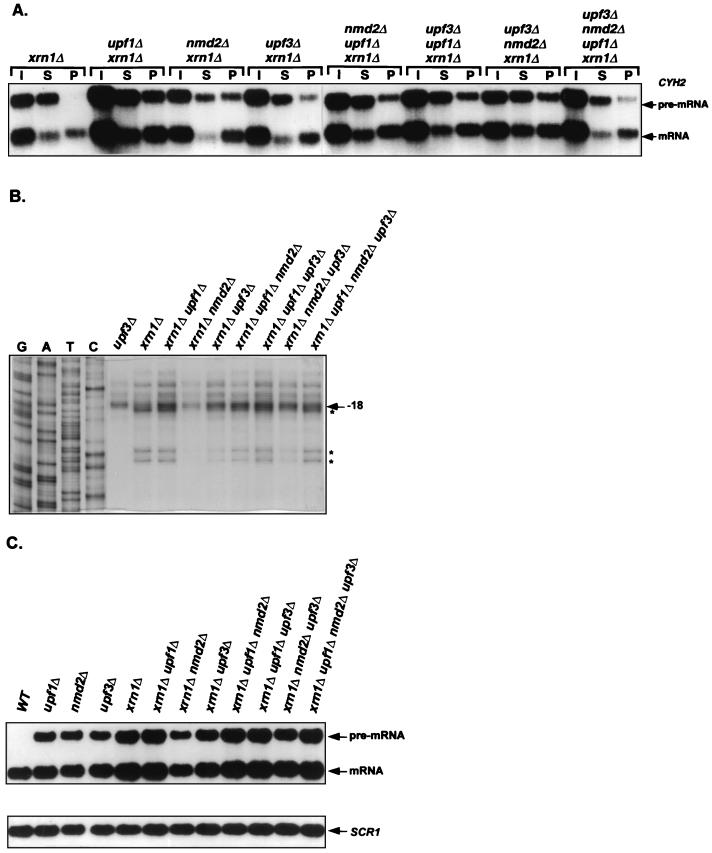
The experiments shown in Fig. 1 indicated that Upf1p, Nmd2p, and Upf3p may play a role in the decapping of nonsense-containing mRNAs (see above). To test this idea further, we constructed xrn1Δ upf1Δ, xrn1Δ nmd2Δ, and xrn1Δ upf3Δ double mutants and analyzed the 5′ ends and relative abundance of capped and uncapped CYH2 pre-mRNAs in these strains. In contrast to xrn1Δ cells, in which the CYH2 pre-mRNA was principally present as an uncapped species, xrn1Δ upf1Δ, xrn1Δ nmd2Δ, and xrn1Δ upf3Δ cells all exhibited substantially increased levels of this RNA in the capped fraction (Fig. 4A and Table 2). Consistent with their increased levels of capped CYH2 pre-mRNAs, the doubly mutant strains also contained higher levels of full-length transcripts (Fig. 4B). However, in contrast to xrn1Δ dcp1Δ cells, which accumulated only the full-length and capped transcripts (Fig. 1), the xrn1Δ upf1Δ, xrn1Δ nmd2Δ, and xrn1Δ upf3Δ strains also retained significant amounts of decapped and 5′-shortened CYH2 pre-mRNA (Fig. 4A and B and Table 2). Since inactivation of Upf1p, Nmd2p, or Upf3p in xrn1Δ cells leads to an increased accumulation of full-length and capped nonsense-containing mRNAs without completely eliminating the accumulation of decapped and 5′-shortened RNAs, it is likely that these factors regulate but do not catalyze decapping of nonsense-containing transcripts.
FIG. 4.
In xrn1Δ cells, single or multiple deletions of UPF1, NMD2, or UPF3 differentially affect the levels of decapped nonsense-containing transcripts. (A) Analysis of the levels of CYH2 pre-mRNAs by anti-m7 G immunoprecipitation. Total RNA was isolated from yeast strains of the indicated genotypes, capped mRNAs were immunoprecipitated as in Fig. 1B, and each sample was analyzed by Northern blotting, using a CYH2 probe. Lanes I, S, and P designate input, supernatant, and pellet, respectively. (B) Analysis of the levels of CYH2 pre-mRNA decay intermediates. Primer extension analysis of the CYH2 pre-mRNA was performed on total RNA from each yeast strain as in Fig. 1A. The major transcriptional start sites of the CYH2 pre-mRNA and the 5′ ends of its decay intermediates are indicated by arrows and asterisks, respectively. Total RNA from the upf3Δ strain was used as a control. (C) Northern analysis of the steady-state levels of CYH2 pre-mRNA. Total RNA was isolated from yeast strains of the indicated genotypes and analyzed by Northern hybridization. The SCR1 RNA (20), which is transcribed by RNA polymerase III, was used as an internal control. WT, wild type. In panels A and C, the CYH2 DNA probe used was the same as in Fig. 1B. Quantitation of this experiment is summarized in Table 2.
In xrn1Δ cells, deletions of UPF1, NMD2, or UPF3 differentially affect the accumulation of decapped nonsense-containing mRNAs.
As shown in Fig. 4C, comparable amounts of the CYH2 pre-mRNA accumulated in XRN1 cells that contain deletions of UPF1, NMD2, or UPF3. However, in an xrn1Δ background, deletions of the same genes affected the levels of the CYH2 pre-mRNA to different extents. The level of the CYH2 pre-mRNA was highest in the xrn1Δ upf1Δ strain, intermediate in the xrn1Δ upf3Δ strain, and lowest in the xrn1Δ nmd2Δ strain (Fig. 4C and Table 2). Analyses of anticap immunoprecipitation assays indicate that these differences are largely a reflection of variations in the accumulation of decapped transcripts, i.e., xrn1Δ upf1Δ, xrn1Δ nmd2Δ, and xrn1Δ upf3Δ strains accumulated levels of capped CYH2 pre-mRNA that differed by at most 30%, but varied more than twofold in their levels of the decapped version of the same transcript (Fig. 4A and Table 2). Much like the variations seen in the distribution of unfractionated CYH2 pre-mRNA, the level of decapped transcripts was highest in xrn1Δ upf1Δ cells, intermediate in xrn1Δ upf3Δ cells, and lowest in xrn1Δ nmd2Δ cells. Consistent with these variations in the levels of decapped transcripts, primer extension analyses revealed the same relationships between strains for the accumulation of CYH2 pre-mRNA decay intermediates (Fig. 4B). Comparable analyses of the levels of total, capped, and decapped MER2 pre-mRNA in these strains yielded essentially identical results (data not shown). Since deletions of UPF1, NMD2, and UPF3 in xrn1Δ cells have differential effects on the accumulation of decapped nonsense-containing mRNAs, it appears that Upf1p, Nmd2p, and Upf3p may also regulate the degradation of decapped nonsense-containing mRNAs.
In xrn1Δ cells, deletions of UPF1, NMD2, or UPF3 also have a differential effect on the accumulation of capped and decapped wild-type mRNAs.
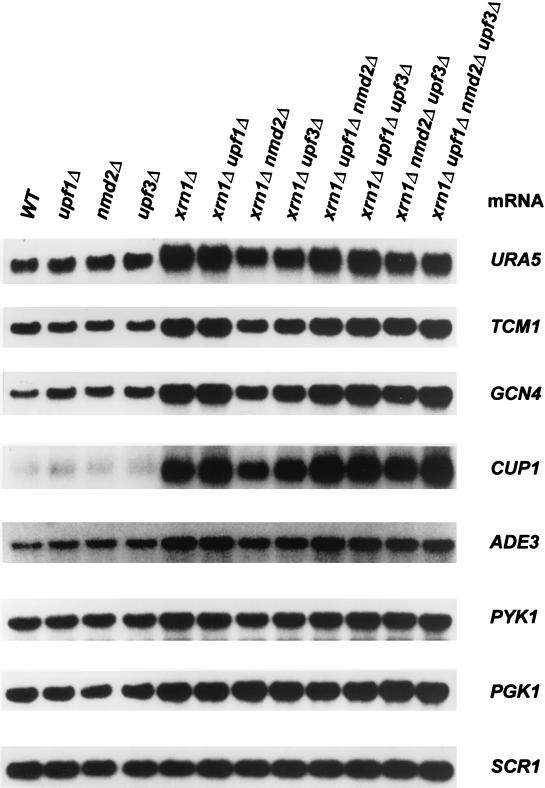
In our analyses of xrn1Δ cells it became apparent that deletions of UPF1, NMD2, and UPF3 also had differential effects on the steady-state level of the CYH2 mRNA. These differences were comparable to the effects seen with the CYH2 pre-mRNA, such that levels of the mature mRNA were highest in xrn1Δ upf1Δ cells, intermediate in xrn1Δ upf3Δ cells, and lowest in xrn1Δ nmd2Δ cells (Fig. 4C). To determine whether the variations seen in these strains were restricted to the CYH2 mRNA, we examined the accumulation of seven other wild-type mRNAs that represented a broad range of inherent stabilities. As shown in Fig. 5, for all but the PGK1 mRNA (see below), levels were highest in the xrn1Δ upf1Δ strain, intermediate in the xrn1Δ upf3Δ strain, and lowest in the xrn1Δ nmd2Δ strain. The level of the PGK1 mRNA, in contrast, was highest in the xrn1Δ nmd2Δ strain, intermediate in the xrn1Δ upf3Δ strain, and lowest in the xrn1Δ upf1Δ strain (Fig. 5 and Table 3). These results indicate that, in xrn1Δ cells, deletions of UPF1, NMD2, or UPF3 affect the accumulation of wild-type mRNAs differentially and in an mRNA-specific manner.
FIG. 5.
Single or multiple deletions of UPF1, NMD2, or UPF3 in xrn1Δ cells differentially affect the levels of wild-type (WT) mRNAs. Total RNA was isolated from yeast strains of the indicated genotypes and analyzed by Northern blotting, using the SCR1 RNA as an internal control. Quantitation of the results for the URA5, TCM1, and PGK1 mRNAs is summarized in Table 3.
TABLE 3.
Effects of single or mutiple deletions of UPF1, NMD2, and UPF3 on the accumulation of total, capped, and decapped wild-type mRNAsa
| Strain | mRNA
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
URA5
|
TCM1
|
PGK1
|
|||||||
| Total | Capped | Uncapped | Total | Capped | Uncapped | Total | Capped | Uncapped | |
| Wild type | 1.00 | 0.84 | 0.16 | 1.00 | 0.66 | 0.34 | 1.00 | 0.95 | 0.05 |
| upf1Δ | 1.10 | 0.83 | 0.27 | 0.90 | 0.67 | 0.33 | 0.98 | 0.90 | 0.10 |
| nmd2Δ | 1.20 | 0.90 | 0.30 | 1.00 | 0.69 | 0.31 | 1.05 | 0.95 | 0.10 |
| upf3Δ | 1.20 | 0.84 | 0.36 | 0.96 | 0.70 | 0.26 | 0.95 | 0.90 | 0.05 |
| xrn1Δ | 2.50 | 0.38 | 2.12 | 2.10 | 0.30 | 1.80 | 1.70 | 0.95 | 0.75 |
| xrn1Δ upf1Δ | 2.40 | 0.72 | 1.68 | 1.80 | 0.50 | 1.30 | 1.40 | 0.76 | 0.64 |
| xrn1Δ nmd2Δ | 1.20 | 0.53 | 0.67 | 1.20 | 0.50 | 0.70 | 1.90 | 1.37 | 0.53 |
| xrn1Δ upf3Δ | 1.60 | 0.40 | 1.20 | 1.30 | 0.36 | 0.94 | 1.60 | 0.93 | 0.67 |
| xrn1Δ upf1Δ nmd2Δ | 2.50 | 0.75 | 1.75 | 1.70 | 0.50 | 1.20 | 1.40 | 0.76 | 0.64 |
| xrn1Δ upf1Δ upf3Δ | 2.40 | 0.72 | 1.68 | 1.70 | 0.50 | 1.20 | 1.40 | 0.81 | 0.59 |
| xrn1Δ nmd2Δ upf3Δ | 1.70 | 0.50 | 1.20 | 1.50 | 0.45 | 1.05 | 1.80 | 1.00 | 0.80 |
| xrn1Δ upf1Δ nmd2Δ upf3Δ | 2.35 | 0.70 | 1.65 | 1.70 | 0.50 | 1.20 | 1.40 | 0.81 | 0.59 |
The Northern blot in Fig. 5 was used to measure the total levels of the URA5, TCM1, and PGK1 mRNAs in each sample. Northern blots in Fig. 6A and B were used to determine the percentage of capped and uncapped transcripts. The relative levels of total, capped, and decapped RNAs were calculated as explained in footnotes b and c to Table 2.
To determine whether the differences in the levels of wild-type mRNAs reflected selective effects on capped or uncapped transcripts, we characterized the respective RNA samples by anticap immunoprecipitation and primer extension. Control experiments demonstrated that deletion of UPF1, NMD2, or UPF3 in XRN1 cells had no significant consequences for the accumulation of capped or decapped wild-type mRNAs (Fig. 6). The parental strains (XRN1, UPF1, NMD2, and UPF3), as well as the individual upf1Δ, nmd2Δ, and upf3Δ strains, accumulated primarily capped transcripts for each mRNA examined. For example, in both wild-type and single-deletion strains, approximately 90% of the CYH2 and PGK1 mRNAs, and 70% of the URA5 and TCM1 mRNAs, were in the capped fraction (Fig. 1A and 6A; Tables 2 and 3). As expected, deletion of only the XRN1 gene led to substantial increases in the levels of decapped transcripts for all mRNAs examined and, for all but the PGK1 mRNA, large decreases in the levels of capped transcripts (Fig. 4A and 6B; Tables 2 and 3).
In contrast to the effects of the single deletions, inactivation of both XRN1 and UPF1, NMD2, or UPF3 affected the accumulation of capped wild-type transcripts differentially and in an mRNA-specific manner. Deletions of UPF1 or NMD2 in xrn1Δ cells led to increases in the accumulation of capped URA5, TCM1, and CYH2 mRNAs. However, the levels of capped PGK1 transcripts decreased in xrn1Δ upf1Δ cells and increased in xrn1Δ nmd2Δ cells. In all cases, simultaneous deletion of UPF3 and XRN1 did not affect the levels of capped wild-type transcripts significantly (Fig. 4A and 6B; Tables 2 and 3).
Deletions of XRN1 and UPF1, NMD2, or UPF3 also had differential effects on the accumulation of decapped wild-type mRNAs. The levels of decapped CYH2, URA5, and TCM1 mRNAs were highest in xrn1Δ upf1Δ cells, intermediate in xrn1Δ upf3Δ cells, and lowest in xrn1Δ nmd2Δ cells (Fig. 4A and 6B; Tables 2 and 3). These observations were consistent with primer extension analyses which demonstrated that the levels of mRNA decay intermediates paralleled the levels of decapped transcripts in the respective strains (Fig. 6C and data not shown). Unlike the results obtained with the CYH2, URA5, and TCM1 mRNAs, the levels of decapped PGK1 transcripts did not vary substantially in the double mutant strains (Fig. 6B and Table 3).
Collectively, the differential effects of xrn1Δ upf1Δ, xrn1Δ nmd2Δ, and xrn1Δ upf3Δ mutations on the levels of capped and decapped CYH2, URA5, TCM1, and PGK1 mRNAs indicate that, in addition to their functions in nonsense-mediated mRNA decay, Upf1p, Nmd2p, and Upf3p also have roles in regulating the decapping and degradation of wild-type mRNAs.
The function of Upf1p is epistatic to those of Nmd2p and Upf3p in the degradation of nonsense-containing and wild-type mRNAs.
Cells harboring xrn1Δ upf1Δ, xrn1Δ nmd2Δ, or xrn1Δ upf3Δ mutations contained similar levels of capped but different levels of decapped CYH2 pre-mRNA (Fig. 4A and Table 2). By constructing a set of xrn1Δ strains harboring double and triple deletions of UPF1, NMD2, and UPF3, we were able to exploit the differences in the levels of decapped CYH2 pre-mRNA to determine the epistatic relationships of Upf1p, Nmd2p, and Upf3p. These experiments showed that all strains containing a upf1Δ allele (i.e., xrn1Δ upf1Δ nmd2Δ, xrn1Δ upf1Δ upf3Δ, and xrn1Δ upf1Δ nmd2Δ upf3Δ strains) accumulated the same level of decapped transcripts as did the xrn1Δ upf1Δ strain. The xrn1Δ nmd2Δ upf3Δ strain accumulated the same level of decapped transcripts as the xrn1Δ upf3Δ strain but differed from the level in the xrn1Δ nmd2Δ strain (Fig. 4A and Table 2). Primer extension analyses showed the same relationships among UPF1, NMD2, and UPF3 mutations for accumulation of CYH2 pre-mRNA decay intermediates (Fig. 4B). These results indicate that, at least with regard to effects on the abundance of decapped nonsense-containing mRNAs in xrn1Δ cells, the function of Upf1p is epistatic to Upf3p, and that of Upf3p is epistatic to Nmd2p.
Similar analyses with the CYH2, URA5, PGK1, and TCM1 mRNAs allowed us to examine the epistatic relationships of these factors that pertained to effects on the levels of capped and decapped wild-type mRNAs (Fig. 5 and 6 and Table 3). These studies showed that, in the regulation of the levels of capped wild-type mRNAs, the function of Upf1p is always epistatic to Nmd2p and Upf3p. However, the epistatic relationships of Nmd2p and Upf3p are mRNA specific. For the CYH2, URA5, and TCM1 mRNAs, the function of Nmd2p is epistatic to Upf3p, but for the PGK1 mRNA, the function of Upf3p is epistatic to Nmd2p (Fig. 4A and 6B; Tables 2 and 3). With regard to the effects on decapped mRNAs, the function of Upf1p was found to be epistatic to Upf3p and Upf3p was found to be epistatic to Nmd2p (Fig. 4A and 6B; Tables 2 and 3).
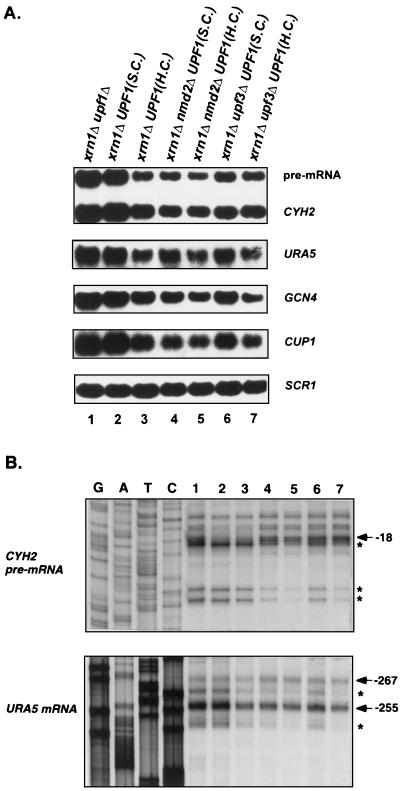
Overexpression of UPF1 in xrn1Δ cells decreases the levels of mRNA decay intermediates.
As described above, Upf1p, Nmd2p, and Upf3p influence the steady-state levels of decapped nonsense-containing and wild-type mRNAs in xrn1Δ cells. Of the three factors, Upf1p appears to play the most significant role in this regulatory event because all xrn1Δ strains in which Upf1p is retained (i.e., xrn1Δ nmd2Δ, xrn1Δ upf3Δ, and xrn1Δ nmd2Δ upf3Δ strains) accumulate lower levels of total and decapped transcripts than the xrn1Δ upf1Δ strain (Fig. 4C and 5; Tables 2 and 3). One possible explanation for this observation is that Upf1p promotes exonucleolytic degradation of decapped mRNAs. To test this idea, we examined the consequences of UPF1 overexpression on the accumulation of total mRNA and mRNA decay intermediates in xrn1Δ cells. These experiments showed that introduction of a high-copy-number plasmid expressing the UPF1 gene into xrn1Δ cells, xrn1Δ nmd2Δ cells, or xrn1Δ upf3Δ cells leads to reductions in (i) the abundance of all mRNAs examined (Fig. 7A) and (ii) the levels of decay intermediates for the CYH2 pre-mRNA and URA5 mRNA (Fig. 7B, compare lanes 2 and 3, 4 and 5, and 6 and 7). These results support the hypothesis that, in xrn1Δ cells, Upf1p can stimulate the degradation of decapped transcripts and suggest that this stimulation most likely affects the efficiency of 5′-to-3′ decay and can occur independent of NMD2 or UPF3 function.
FIG. 7.
In xrn1Δ cells, overexpression of the UPF1 gene decreases total mRNA levels as well as the levels of mRNA decay intermediates. (A) Northern analysis of total mRNA. xrn1Δ upf1Δ, xrn1Δ nmd2Δ, and xrn1Δ upf3Δ strains were transformed with a single-copy (S.C.) or a high-copy-number (H.C.) plasmid harboring the UPF1 gene. Total RNAs isolated from the resulting yeast strains, as well as the xrn1Δupf1Δ strain, were analyzed by Northern hybridization, using probes for the CYH2, URA5, GCN4, and CUP1 mRNAs. The SCR1 RNA was used as an internal control. (B) Analysis of mRNA decay intermediates. Primer extension analysis of the CYH2 pre-mRNA and the URA5 mRNA was performed on each of the RNAs used in panel A. Radiolabeled primers CYH2-IN4 and URA5-1 were used for both reverse transcription and DNA sequencing reactions. The major transcriptional start sites and the 5′ ends of decay intermediates are indicated by arrows and asterisks, respectively. The yeast strains labeled 1 to 7 correspond to those in panel A.
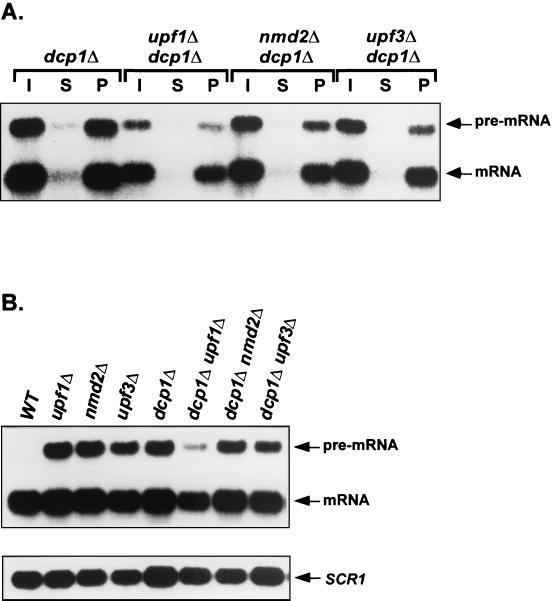
In dcp1Δ cells, deletions of UPF1, NMD2, and UPF3 differentially affect the accumulation of capped nonsense-containing and wild-type mRNAs.
Experiments described above indicated that Upf1p, Nmd2p, and Upf3p promote, but do not catalyze, decapping of nonsense-containing mRNAs and also regulate the degradation of decapped transcripts of any type. Our epistasis analyses, as well as the experiments of Fig. 7, demonstrated that the latter effect could be attributable to increased efficiency of 5′-to-3′ exonucleolytic digestion. Since exonucleolytic digestion of decapped transcripts has also been shown to occur by a 3′-to-5′ mechanism (29) we sought to determine whether Upf1p, Nmd2p, and Upf3p have a role in modulating this pathway. Accordingly, we constructed dcp1Δupf1Δ, dcp1Δ nmd2Δ, and dcp1Δupf3Δ strains, rationalizing that the inhibition of decapping that would occur in such strains would eliminate 5′-to-3′ decay. Indeed, all of the transcripts that accumulated in these strains were capped (Fig. 8A and data not shown). Northern analyses of RNAs isolated from these strains demonstrated that the abundance of the nonsense-containing CYH2 pre-mRNA and the wild-type CYH2 and TCM1 mRNAs were uniformly lowest in the dcp1Δ upf1Δ strain, intermediate in the dcp1Δ upf3Δ and dcp1Δ strains, and highest in the dcp1Δ nmd2Δ strain (Fig. 8B, Table 4, and data not shown). These results imply that Upf1p, Nmd2p, and Upf3p can also regulate 3′-to-5′ exonucleolytic decay differentially, with Upf1p having apparent negative regulatory capability in dcp1Δ cells.
FIG. 8.
Deletion of UPF1, NMD2, or UPF3 in dcp1Δ cells differentially affects the levels of the CYH2 pre-mRNA and mRNA. (A) Analysis of 5′ cap status. Total RNA was isolated from yeast strains of the indicated genotypes and anti-m7 G immunoprecipitation was performed as in Fig. 1B. Lanes I, S, and P represent input, supernatant, and pellet samples, respectively. (B) Northern analysis of total mRNA. Total RNA from yeast strains of the indicated genotypes was isolated and analyzed by Northern hybridization, using the SCR1 RNA as an internal control. WT, wild type. In both panels A and B, the CYH2 probe used was the same as in Fig. 1B. Quantitation of this experiment is summarized in Table 4.
TABLE 4.
Effects of deletions of UPF1, NMD2, or UPF3 on the accumulation of CYH2 pre-mRNA and mRNA in dcp1Δ cellsa
| Strain | Pre-mRNA
|
mRNA
|
||||
|---|---|---|---|---|---|---|
| Total | Capped | Uncapped | Total | Capped | Uncapped | |
| Wild type | 1.00 | NAb | NA | 1.00 | 0.90 | 0.10 |
| dcp1Δ | 14.20 | 12.10 | 2.10 | 0.75 | 0.65 | 0.10 |
| dcp1Δ upf1Δ | 8.30 | 7.60 | 0.70 | 0.60 | 0.50 | 0.10 |
| dcp1Δ nmd2Δ | 20.90 | 17.80 | 3.10 | 1.05 | 0.93 | 0.12 |
| dcp1Δ upf3Δ | 12.20 | 10.60 | 1.60 | 0.70 | 0.60 | 0.10 |
DISCUSSION
Upf1p, Nmd2p, and Upf3p regulate decapping of nonsense-containing and wild-type mRNAs.
In the yeast S. cerevisiae the rapid degradation of nonsense-containing mRNAs proceeds from deadenylation-independent removal of the 5′ cap by the decapping enzyme, Dcp1p, to 5′→3′ digestion of the remainder of the mRNA by the exoribonuclease Xrn1p (6, 21, 47). This decay pathway also requires the activities of three additional trans-acting factors, Upf1p, Nmd2p, and Upf3p (10, 22, 23, 25, 38, 40, 41, 52, 54). Previous studies showed that mutations in any of the respective UPF or NMD genes led to the selective stabilization of nonsense-containing mRNAs but did not identify a mechanistic basis for such stabilization. Here, we show that loss of Upf1p, Nmd2p, or Upf3p inhibits the decapping of nonsense-containing mRNAs. This conclusion follows from experiments showing that inactivation of any or all of these factors, in XRN1 or xrn1Δ cells, leads to the accumulation of capped CYH2 and MER2 pre-mRNAs (Fig. 1, 2, and 4). Moreover, since dcp1Δ xrn1Δ cells accumulate only capped nonsense-containing transcripts, and all xrn1Δ strains containing single or multiple deletions of UPF1, NMD2, or UPF3 still accumulate some decapped transcripts, our data indicate that Upf1p, Nmd2p, and Upf3p regulate but do not catalyze decapping of nonsense-containing mRNAs.
Although Upf1p, Nmd2p, and Upf3p were originally identified as factors that only regulated nonsense-containing mRNAs, our data indicate that these factors can, under some circumstances, also affect decapping of wild-type mRNAs. Several observations support this conclusion. First, in xrn1Δ cells, inactivation of these factors differentially alters the levels of all capped wild-type transcripts examined (Fig. 4A and 6B; Tables 2 and 3). Second, the effects of inactivation of Upf1p, Nmd2p, and Upf3p on the accumulation of capped transcripts in xrn1Δ cells are mRNA specific, suggesting that certain mRNA features can influence the activities of these factors. Third, even in XRN1 cells, deletions of these genes lead to small but reproducible increases in the levels of some wild-type mRNAs (Fig. 5).
Although decapping of nonsense-containing and wild-type mRNAs requires the same decapping enzyme, our data also indicate that the functions of Upf1p, Nmd2p, and Upf3p affect decapping of both classes of mRNAs differently. For example, Upf1p, Nmd2p, and Upf3p are all required to promote efficient decapping of nonsense-containing mRNAs. In contrast, in xrn1Δ cells, it appears that Upf1p and Nmd2p affect normal decapping of wild-type mRNAs, while Upf3p seems to have no effect on this activity. In addition, inactivation of Upf1p, Nmd2p, and Upf3p affects the levels of capped nonsense-containing transcripts dramatically but only affects the levels of capped wild-type transcripts modestly.
Rat1p functions in cytoplasmic mRNA degradation.
Rat1p, one of two 5′-to-3′ exoribonucleases in yeast, is predominantly localized to the nucleus in the steady state and has an essential nuclear function (33, 35). Consistent with these characteristics, the protein is involved in the formation of the 5′ ends of 5.8S rRNA and some snoRNAs (45, 55). Here, we have demonstrated that Rat1p also functions in 5′-to-3′ exonucleolytic degradation of decapped yeast mRNAs, at least in the absence of Xrn1p. Inactivation of Xrn1p leads to the accumulation of nonsense-containing and wild-type mRNAs that lacked the cap structure and several 5′ nucleotides. Two key observations indicate that these mRNA decay intermediates arise from decapping by Dcp1p and 5′ trimming by Rat1p. First, in contrast to the xrn1Δ strain, the dcp1Δ xrn1Δ strain accumulated only full-length, capped mRNAs, indicating that formation of the decay intermediates in xrn1Δ cells requires the activity of Dcp1p. Second, mRNA decay intermediates present in rat1-1 xrn1Δ cells grown at the permissive temperature disappeared after a shift to the nonpermissive temperature, indicating that the formation of these decay intermediates in xrn1Δ cells also requires the activity of Rat1p.
The ability of Rat1p to degrade cytoplasmic mRNAs in a 5′-to-3′ direction in the absence of Xrn1p is consistent with two earlier observations. Muhlrad and Parker (47) showed that xrn1Δ cells can still accumulate low levels of 5′-to-3′ decay intermediates of the nonsense-containing and wild-type PGK1 mRNAs. Further, Johnson (33) identified several dominant alleles of the RAT1 gene that cause mislocalization of Rat1p to the cytoplasm and complement the mRNA turnover defect of xrn1Δ cells. Surprisingly, however, while the 5′ ends of the mRNA decay intermediates generated by Rat1p in vivo suggest a distributive activity for this enzyme, earlier in vitro analyses indicated that Rat1p had processive activity (56).
Upf1p, Nmd2p, and Upf3p regulate exonucleolytic degradation of nonsense-containing and wild-type mRNAs.
After decapping, the remainder of a nonsense-containing or wild-type transcript is eliminated by exonucleolytic digestion. In wild-type cells, decapped transcripts are principally degraded in a 5′-to-3′ direction by Xrn1p (28, 47, 48). However, in xrn1Δ cells, decapped transcripts are degraded in both the 5′-to-3′ and 3′-to-5′ directions (47, 49). Our observation that inactivation of Rat1p in xrn1Δ cells eliminates the formation of 5′-to-3′ mRNA decay intermediates but does not increase the levels of all mRNAs examined (Fig. 2 and 3) supports this conclusion further. Our analyses of xrn1Δ cells provide several lines of evidence that Upf1p, Nmd2p, and Upf3p can also regulate exonucleolytic degradation of decapped nonsense-containing and wild-type mRNAs, including the following: (i) inactivation of Upf1p, Nmd2p, or Upf3p differentially affects total mRNA levels only in xrn1Δ cells, but not in XRN1 cells (Fig. 4 and 5); (ii) inactivation of these factors differentially affects the accumulation of decapped transcripts and mRNA decay intermediates; and (iii) the phenotypes caused by inactivation of these factors exhibit epistatic relationships.
Our epistatic analysis indicates that Upf1p plays a positive role in promoting exonucleolytic degradation and that Nmd2p and Upf3p function by regulating the activity of Upf1p (see below). However, in the experiments reported here, the xrn1Δ strain always accumulated higher levels of decapped transcripts than the xrn1Δ upf1Δ strain. This indicates that Upf1p can also play a negative role in exonucleolytic degradation. The dual roles of Upf1p may reflect differential regulation of two different pathways: positively on 5′-to-3′ decay and negatively on 3′-to-5′ decay. Two observations support this conclusion. First, when 5′-to-3′ exonucleolytic degradation is partially blocked by inactivation of Xrn1p, overexpression of Upf1p reduces the accumulation of 5′-to-3′ mRNA decay intermediates (Fig. 7B). Second, when 5′-to-3′ exonucleolytic degradation is completely blocked by inactivation of Dcp1p, inactivation of Upf1p leads to decreased accumulation of capped mRNAs (Fig. 8A and Table 4). These apparently conflicting roles of Upf1p could be explained if the positive function reflected an indirect consequence of enhancing ribosome release at termination codons (31, 43) and the negative function reflected a regulatory interaction with a component(s) of the 3′→5′ pathway (see below).
Functional relationships of Upf1p, Nmd2p, and Upf3p.
The differential effects on the accumulation of decapped transcripts engendered by inactivation of Upf1p, Nmd2p, or Upf3p in xrn1Δ cells not only led us to conclude that these factors have different roles in regulating exonucleolytic degradation but also allowed us to determine their respective functional relationships. Our data indicate that the function of Upf1p is epistatic to Nmd2p and Upf3p and that the function of Upf3p is epistatic to that of Nmd2p. We interpret these relationships to suggest that Nmd2p and Upf3p regulate the activity of Upf1p, a conclusion consistent with our earlier analyses of nonsense suppression in upf and nmd cells (43) and with several observations in this study. Here, we show that (i) in an xrn1Δ background, all strains that contain UPF1, except the UPF or NMD wild-type strain, accumulate lower levels of decapped transcripts than strains lacking UPF1, indicating that Upf1p plays a more direct role in regulating exonucleolytic degradation than Nmd2p or Upf3p; (ii) the xrn1Δ upf1Δ nmd2Δ, xrn1Δ upf1Δ upf3Δ, and xrn1Δ upf1Δ nmd2Δ upf3Δ mutant strains accumulate the same level of decapped transcripts as an xrn1Δ upf1Δ strain, indicating that, in the absence of Upf1p, the presence of Nmd2p, Upf3p, or both, has no additional effects and that the functions of Nmd2p and Upf3p must operate through Upf1p; (iii) UPF or NMD wild-type, xrn1Δ nmd2Δ, xrn1Δ upf3Δ, and xrn1Δ nmd2Δ upf3Δ strains accumulate different levels of decapped transcripts, demonstrating that the presence of Nmd2p, Upf3p, or both, has different effects on the activity of Upf1p; (iv) xrn1Δ nmd2Δ cells accumulate a lower level of decapped transcripts than xrn1Δ nmd2Δ upf3Δ cells, indicating that, in the absence of Nmd2p, Upf3p enhances the function of Upf1p; and (v) xrn1Δ nmd2Δ upf3Δ cells accumulate the same level of decapped transcripts as xrn1Δ upf3Δ cells but a lower level than UPF or NMD wild-type cells. This last observation establishes that Nmd2p has no effect on Upf1p in the absence of Upf3p and that, in the presence of Upf3p, Nmd2p negatively regulates Upf1p.
In xrn1Δ cells, inactivation of the UPF and NMD factors also had differential effects on the accumulation of capped wild-type transcripts. Using these phenotypes to determine the functional relationships of Upf1p, Nmd2p, and Upf3p, we found the same functional relationships among the factors, i.e., that the function of Upf1p was epistatic to Nmd2p and Upf3p for all mRNAs examined. However, these analyses revealed that the epistatic relationships of Nmd2p and Upf3p are mRNA-specific. Interestingly, our data indicate that the functional relationships of these factors in controlling the accumulation of capped PGK1 mRNA are the same as those regulating exonucleolytic degradation. It remains to be determined what these two sets of events have in common.
Reconciliation of the diverse functions of Upf1p, Nmd2p, and Upf3p.
The data presented in this paper demonstrate that Upf1p, Nmd2p, and Upf3p function in the regulation of mRNA decapping and in both modes of exonucleolytic decay. These mRNA degradative events involve multiple factors, including Dcp1p, Xrn1p, Rat1p, and the components of the exosome (6, 28, 29, 46, 48), none of which have been shown to have significant physical interactions with the products of the UPF and NMD genes. How, then, could Upf1p, Nmd2p, and Upf3p function as such general regulators of mRNA decay? Since it is unlikely that these factors regulate the activities of all of the degradative enzymes directly, it seems reasonable to consider the possibility that they exert their regulatory effects by controlling substrate availability to the decay pathways.
Other than regulating the stability of nonsense-containing mRNAs, the principal function ascribed to the UPF and NMD gene products has been the regulation of translation termination fidelity and/or efficiency. This conclusion follows from experiments showing that (i) mutations or deletions of the UPF1, NMD2, and UPF3 genes promote omnipotent nonsense suppression and allosuppression (10, 11, 41, 43, 69, 70); (ii) nonsense suppression in upf and nmd mutants is directly attributable to effects on translation termination, not mRNA decay (43, 69, 70); and (iii) Upf1p interacts with the polypeptide release factors, Sup35p and Sup45p, both in vitro and in vivo (13). While translation termination is generally defined as release of the completed polypeptide from the peptidyl-tRNA in response to a stop codon, it is clear, at least in prokaryotes, that the event is considerably more complex and must include at least one more step in which ribosomes are dissociated from the mRNA (32). Moreover, the participation of the initiation factors eIF3 and IF3 in the dissociation process (18, 34, 66) suggests that disassembly of the termination complex may prepare the ribosome for recycling to the next round of translation initiation on the same mRNA or a different mRNA. The possibility that this event may influence the subsequent translation or stability of the mRNA in question is suggested by experiments showing that (i) mutations in eIF3 can lead to the selective stabilization of nonsense-containing mRNAs (68); (ii) premature translation termination can decrease the translational efficiency of an mRNA (51); and (iii) Upf1p interacts with Nmd3p, a 60S ribosome-associated factor that may have a role in subunit association and dissociation (7, 15, 16, 18, 27, 73).
These observations, and the suggestion that proper termination of translation can only occur in the context of interactions between a terminating ribosome and a specific RNP domain or set of factors localized 3′ to a normal stop codon (8, 26, 31), lead us to propose that the direct regulatory effects of Upf1p, Nmd2p, and Upf3p on translation termination can explain at least some of their effects on mRNA decay. In this model, Upf1p is thought to utilize its ATPase and helicase activities to promote ribosome release or a conformational change among the components of the termination complex (26, 31). If interactions with factors bound 3′ to the termination site influence Upf1p's activity, then the efficiency of the latter event, and/or the subsequent translational competence of the ribosome, may differ with normal versus premature stop codons. In turn, the altered competence of the ribosome for an additional round of initiation may render the mRNA more susceptible to decapping (51) and inefficient ribosome release may decrease the efficiency of 5′→3′ exonucleolytic decay. This model does not accommodate our observations on the effects of upf and nmd mutations on 3′→5′ decay, leading us to suggest further that the apparatus involved in the latter mode of decay may be influenced by factors involved in effecting proper termination at the normal end of an open reading frame. As presented, this model also does not explain why inactivation of Xrn1p renders several of the decay phenotypes detectable or more pronounced. One plausible explanation is that inactivation of Xrn1p leads to increased accumulation of decapped transcripts for most mRNAs and these decapped transcripts may sequester some component(s) involved in translation initiation and/or termination, thereby making decapping of wild-type mRNAs more dependent on the function of Upf1p, Nmd2p, and Upf3p.
ACKNOWLEDGMENTS
This work was supported by a grant (GM27757) to A.J. from the National Institutes of Health.
We thank Elsebet Lund for anticap antibodies, Roy Parker for one plasmid, and members of the Jacobson laboratory for their helpful editorial comments.
REFERENCES
- 1.Altamura N, Groudinsky O, Dujardin G, Slonimski P P. NAM7 nuclear gene encodes a novel member of a family of helicases with a Zn-ligand motif and is involved in mitochondrial functions in Saccharomyces cerevisiae. J Mol Biol. 1992;224:575–587. doi: 10.1016/0022-2836(92)90545-u. [DOI] [PubMed] [Google Scholar]
- 2.Amberg D C, Goldstein A L, Cole C N. Isolation and characterization of RAT1: an essential gene of Saccharomyces cerevisiae required for the efficient nucleocytoplasmic trafficking of mRNA. Genes Dev. 1992;6:1173–1189. doi: 10.1101/gad.6.7.1173. [DOI] [PubMed] [Google Scholar]
- 3.Atkin A L, Schenkman L R, Eastham M, Dahlseid J N, Lelivelt M J, Culbertson M R. Relationship between yeast polyribosomes and Upf proteins required for nonsense mRNA decay. J Biol Chem. 1997;272:22163–22172. doi: 10.1074/jbc.272.35.22163. [DOI] [PubMed] [Google Scholar]
- 4.Atkin A L, Altamura N, Leeds P, Culbertson M R. The majority of yeast UPF1 co-localizes with polyribosomes in the cytoplasm. Mol Biol Cell. 1995;6:611–625. doi: 10.1091/mbc.6.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beelman C A, Parker R. Degradation of mRNA in eukaryotes. Cell. 1995;81:179–183. doi: 10.1016/0092-8674(95)90326-7. [DOI] [PubMed] [Google Scholar]
- 6.Beelman C A, Stevens A, Caponigro G, LaGrandeur T E, Hatfield L, Fortner D M, Parker R. An essential component of the decapping enzyme required for normal rates of mRNA turnover. Nature. 1996;382:642–646. doi: 10.1038/382642a0. [DOI] [PubMed] [Google Scholar]
- 7.Belk J P, He F, Jacobson A. Overexpression of truncated Nmd3p inhibits protein synthesis in yeast. RNA. 1999;5:1055–1070. doi: 10.1017/s1355838299990027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonetti B, Fu L, Moon J, Bedwell D M. The efficiency of translation termination is determined by a synergistic interplay between upstream and downstream sequences in Saccharomyces cerevisiae. J Mol Biol. 1995;251:334–345. doi: 10.1006/jmbi.1995.0438. [DOI] [PubMed] [Google Scholar]
- 9.Caponigro G, Parker R. Multiple functions for the poly(A)-binding protein in mRNA decapping and deadenylation in yeast. Genes Dev. 1995;9:2421–2432. doi: 10.1101/gad.9.19.2421. [DOI] [PubMed] [Google Scholar]
- 10.Cui Y, Hagan K W, Zhang S, Peltz S W. Identification and characterization of genes that are required for the accelerated degradation of mRNAs containing a premature translational termination codon. Genes Dev. 1995;9:423–436. doi: 10.1101/gad.9.4.423. [DOI] [PubMed] [Google Scholar]
- 11.Culbertson M, Underbrink K M, Fink G R. Frameshift suppression in Saccharomyces cerevisiae. II. Genetic properties of group II suppressors. Genetics. 1980;95:833–853. doi: 10.1093/genetics/95.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czaplinski K, Weng Y, Hagan K W, Peltz S W. Purification and characterization of the Upf1 protein: a factor involved in translation and mRNA degradation. RNA. 1995;1:610–623. [PMC free article] [PubMed] [Google Scholar]
- 13.Czaplinski K, Ruiz-Echevarria M J, Paushkin S V, Han X, Weng Y, Perlick H A, Dietz H C, Ter-Avanesyan M D, Peltz S W. The surveillance complex interacts with the translation release factors to enhance termination and degrade aberrant mRNAs. Genes Dev. 1998;12:1665–1677. doi: 10.1101/gad.12.11.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das B, Guo Z, Russo P, Chartrand P, Sherman F. The role of nuclear cap binding protein Cbc1p of yeast in mRNA termination and degradation. Mol Cell Biol. 2000;20:2827–2838. doi: 10.1128/mcb.20.8.2827-2838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dick F A, Karamanou S, Trumpower B L. QSR1, an essential yeast gene with a genetic relationship to a subunit of the mitochondrial cytochrome bc1 complex, codes for a 60 S ribosomal subunit protein. J Biol Chem. 1997;16:13372–13379. doi: 10.1074/jbc.272.20.13372. [DOI] [PubMed] [Google Scholar]
- 16.Dick F A, Trumpower B L. Heterologous complementation reveals that mutant alleles of QSR1 render 60S ribosomal subunits unstable and translationally inactive. Nucleic Acids Res. 1998;26:2442–2448. doi: 10.1093/nar/26.10.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dinman J D, Wickner R B. Translational maintenance of frame: mutants of Saccharomyces cerevisiae with altered -1 ribosomal frameshifting efficiencies. Genetics. 1994;136:75–86. doi: 10.1093/genetics/136.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisinger D P, Dick F A, Trumpower B L. Qsr1p, a 60S ribosomal subunit protein, is required for joining of 40S and 60S subunits. Mol Cell Biol. 1997;17:5136–5145. doi: 10.1128/mcb.17.9.5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feinberg B, McLaughlin C S, Moldave K. Analysis of temperature-sensitive mutant ts 187 of Saccharomyces cerevisiae altered in a component required for the initiation of protein synthesis. J Biol Chem. 1982;257:10846–10851. [PubMed] [Google Scholar]
- 20.Felici F, Cesareni G, Hughes J M. The most abundant small cytoplasmic RNA of Saccharomyces cerevisiae has an important function required for normal cell growth. Mol Cell Biol. 1989;9:3260–3268. doi: 10.1128/mcb.9.8.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatfield L, Beelman C A, Stevens A, Parker R. Mutations in trans-acting factors affecting mRNA decapping in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:5830–5838. doi: 10.1128/mcb.16.10.5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He F, Peltz S W, Donahue J L, Rosbash M, Jacobson A. Stabilization and ribosome association of unspliced pre-mRNAs in a yeast upf1 mutant. Proc Natl Acad Sci USA. 1993;90:7034–7039. doi: 10.1073/pnas.90.15.7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He F, Jacobson A. Identification of a novel component of the nonsense-mediated mRNA decay pathway by use of an interacting protein screen. Genes Dev. 1995;9:437–454. doi: 10.1101/gad.9.4.437. [DOI] [PubMed] [Google Scholar]
- 24.He F, Brown A H, Jacobson A. Interaction between Nmd2p and Upf1p is required for activity but not for dominant-negative inhibition of the nonsense-mediated mRNA decay pathway in yeast. RNA. 1996;2:153–170. [PMC free article] [PubMed] [Google Scholar]
- 25.He F, Brown A H, Jacobson A. Upf1p, Nmd2p, and Upf3p are interacting components of the yeast nonsense-mediated mRNA decay pathway. Mol Cell Biol. 1997;17:1580–1594. doi: 10.1128/mcb.17.3.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hilleren P, Parker R. Mechanisms of mRNA surveillance in eukaryotes. Annu Rev Genet. 1999;33:229–260. doi: 10.1146/annurev.genet.33.1.229. [DOI] [PubMed] [Google Scholar]
- 27.Ho J H, Johnson A W. NMD3 encodes an essential cytoplasmic protein required for stable 60S ribosomal subunits in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:2389–2399. doi: 10.1128/mcb.19.3.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu C, Stevens A. Yeast cells lacking 5′→3′ exoribonuclease 1 contain mRNA species that are poly(A) deficient and partially lack the 5′ cap structure. Mol Cell Biol. 1993;13:4826–4835. doi: 10.1128/mcb.13.8.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobs-Anderson S J, Parker R. The 3′ to 5′ degradation of yeast mRNAs is a general mechanism for mRNA turnover that requires the SKI2 DEVH box protein and 3′ to 5′ exonucleases of the exosome complex. EMBO J. 1998;17:1497–1506. doi: 10.1093/emboj/17.5.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobson A, Peltz S W. Interrelationships of the pathways of mRNA decay and translation in eukaryotic cells. Annu Rev Biochem. 1996;65:693–739. doi: 10.1146/annurev.bi.65.070196.003401. [DOI] [PubMed] [Google Scholar]
- 31.Jacobson, A., and S. W. Peltz. Destabilization of nonsense-containing transcripts in Saccharomyces cerevisiae. In N. Sonenberg, J. Hershey, and M. Mathews (ed.), Translational control, vol. 2, in press. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 32.Janosi L, Hara H, Zhang S, Kaji A. Ribosome recycling by ribosome recycling factor (RRF)—an important but overlooked step of protein biosynthesis. Adv Biophys. 1996;32:121–201. doi: 10.1016/0065-227x(96)84743-5. [DOI] [PubMed] [Google Scholar]
- 33.Johnson A W. Rat1p and Xrn1p are functionally interchangeable exoribonucleases that are restricted to and required in the nucleus and cytoplasm, respectively. Mol Cell Biol. 1997;17:6122–6130. doi: 10.1128/mcb.17.10.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karimi R, Pavlov M Y, Buckingham R H, Ehrenberg M. Novel roles for classical factors at the interface between translation termination and initiation. Mol Cell. 1999;3:601–609. doi: 10.1016/s1097-2765(00)80353-6. [DOI] [PubMed] [Google Scholar]
- 35.Kenna M, Stevens A, McCammon M, Douglas M G. An essential yeast gene with homology to the exonuclease-encoding XRN1/KEM1 gene also encodes a protein with exoribonuclease activity. Mol Cell Biol. 1993;13:341–350. doi: 10.1128/mcb.13.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koonin E V. A new group of putative RNA helicases. Trends Biochem Sci. 1992;17:495–497. doi: 10.1016/0968-0004(92)90338-a. [DOI] [PubMed] [Google Scholar]
- 37.Larimer F W, Hsu C L, Maupin M K, Stevens A. Characterization of the XRN1 gene encoding a 5′→3′ exoribonuclease:sequence data and analysis of disparate protein and mRNA levels of gene-disrupted yeast cells. Gene. 1992;120:51–57. doi: 10.1016/0378-1119(92)90008-d. [DOI] [PubMed] [Google Scholar]
- 38.Lee B-S, Culbertson M R. Identification of an additional gene required for eukaryotic nonsense mRNA turnover. Proc Natl Acad Sci USA. 1995;92:10354–10358. doi: 10.1073/pnas.92.22.10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee S I, Umen J G, Varmus H E. A genetic screen identifies cellular factors involved in retroviral -1 frameshifting. Proc Natl Acad Sci USA. 1995;92:6587–6591. doi: 10.1073/pnas.92.14.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leeds P, Peltz S W, Jacobson A, Culbertson M R. The product of the yeast UPF1 gene is required for rapid turnover of mRNAs containing a premature translational termination codon. Genes Dev. 1991;5:2303–2314. doi: 10.1101/gad.5.12a.2303. [DOI] [PubMed] [Google Scholar]
- 41.Leeds P, Wood J M, Lee B-S, Culbertson M R. Gene products that promote mRNA turnover in Saccharomyces cerevisiae. Mol Cell Biol. 1992;12:2165–2177. doi: 10.1128/mcb.12.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lelivelt M J, Culbertson M R. Yeast Upf proteins required for RNA surveillance affect global expression of the yeast transcriptome. Mol Cell Biol. 1999;19:6710–6719. doi: 10.1128/mcb.19.10.6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maderazo A B, He F, Mangus D A, Jacobson A. Upf1p control of nonsense mRNA translation is regulated by Nmd2p and Upf3p. Mol Cell Biol. 2000;20:4591–4603. doi: 10.1128/mcb.20.13.4591-4603.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mangus D A, Jacobson A. Linking mRNA turnover and translation: assessing the polyribosome association of mRNA decay factors and degradative intermediates. Methods. 1999;17:28–37. doi: 10.1006/meth.1998.0704. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell P, Petfalski E, Tollervey D. The 3′ end of yeast 5.8S rRNA is generated by an exonuclease processing mechanism. Genes Dev. 1996;10:502–513. doi: 10.1101/gad.10.4.502. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell P, Petfalski E, Shevchenko A, Mann M, Tollervey D. The exosome: a conserved eukaryotic RNA processing complex containing multiple 3′→5′ exoribonucleases. Cell. 1997;91:457–466. doi: 10.1016/s0092-8674(00)80432-8. [DOI] [PubMed] [Google Scholar]
- 47.Muhlrad D, Parker R. Premature translational termination triggers mRNA decapping. Nature. 1994;370:578–581. doi: 10.1038/370578a0. [DOI] [PubMed] [Google Scholar]
- 48.Muhlrad D, Decker C J, Parker R. Deadenylation of the unstable mRNA encoded by the yeast MFA2 gene leads to decapping followed by 5′→3′ digestion of the transcript. Genes Dev. 1994;8:855–866. doi: 10.1101/gad.8.7.855. [DOI] [PubMed] [Google Scholar]
- 49.Muhlrad D, Decker C J, Parker R. Turnover mechanisms of the stable yeast PGK1 mRNA. Mol Cell Biol. 1995;15:2145–2156. doi: 10.1128/mcb.15.4.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muhlrad D, Parker R. Aberrant mRNAs with extended 3′ UTRs are substrates for rapid degradation by mRNA surveillance. RNA. 1999;5:1299–1307. doi: 10.1017/s1355838299990829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muhlrad D, Parker R. Recognition of yeast mRNAs as “nonsense containing” leads to both inhibition of mRNA translation and mRNA degradation: implications for the control of mRNA decapping. Mol Biol Cell. 1999;10:3971–3988. doi: 10.1091/mbc.10.11.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peltz S W, Brown A H, Jacobson A. mRNA destabilization triggered by premature translational termination depends on at least three cis-acting sequence elements and one trans-acting factor. Genes Dev. 1993;7:1737–1754. doi: 10.1101/gad.7.9.1737. [DOI] [PubMed] [Google Scholar]
- 53.Peltz S W, Trotta C, He F, Brown A, Donahue J, Welch E, Jacobson A. Identification of the cis-acting sequences and trans-acting factors involved in nonsense-mediated mRNA decay. In: Tuite M, McCarthy J, Sherman F, editors. Protein synthesis and targetting in yeast. Berlin, Germany: Springer-Verlag; 1993. pp. 1–10. [Google Scholar]
- 54.Peltz S W, He F, Welch E, Jacobson A. Nonsense-mediated mRNA decay in yeast. Prog Nucleic Acid Res Mol Biol. 1994;47:271–297. doi: 10.1016/s0079-6603(08)60254-8. [DOI] [PubMed] [Google Scholar]
- 55.Petfalski E, Dandekar T, Henry Y, Tollervey D. Processing of the precursors to small nucleolar RNAs and rRNAs requires common components. Mol Cell Biol. 1998;18:1181–1189. doi: 10.1128/mcb.18.3.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poole T L, Stevens A. Comparison of features of the RNase activity of 5′-exonuclease-1 and 5′-exonuclease-2 of Saccharomyces cerevisiae. Nucleic Acids Symp Ser. 1995;33:79–81. [PubMed] [Google Scholar]
- 57.Pulak R, Anderson P. mRNA surveillance by the Caenorhabditis elegans smg genes. Genes Dev. 1993;7:1885–1897. doi: 10.1101/gad.7.10.1885. [DOI] [PubMed] [Google Scholar]
- 58.Rose M D, Winston F, Hieter P. Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1990. [Google Scholar]
- 59.Ross J. mRNA stability in mammalian cells. Microbiol Rev. 1995;59:423–450. doi: 10.1128/mr.59.3.423-450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rothstein R. Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Methods Enzymol. 1991;194:281–301. doi: 10.1016/0076-6879(91)94022-5. [DOI] [PubMed] [Google Scholar]
- 61.Ruiz-Echevarria M J, Yasenchak J M, Han X, Dinman J D, Peltz S W. The UPF3 protein is a component of the surveillance complex that monitors both translation and mRNA turnover and affects viral propagation. Proc Natl Acad Sci USA. 1998;95:8721–8726. doi: 10.1073/pnas.95.15.8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 63.Schiestl R H, Gietz R D. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989;16:339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- 64.Shirley R L, Lelivelt M J, Schenkman L R, Dahlseid J N, Culbertson M R. A factor required for nonsense-mediated mRNA decay in yeast is exported from the nucleus to the cytoplasm by a nuclear export signal sequence. J Cell Sci. 1998;111:3129–3143. doi: 10.1242/jcs.111.21.3129. [DOI] [PubMed] [Google Scholar]
- 65.Shyu A B, Belasco J G, Greenberg M E. Two distinct destabilizing elements in the c-fos message trigger deadenylation as a first step in rapid mRNA decay. Genes Dev. 1991;5:221–231. doi: 10.1101/gad.5.2.221. [DOI] [PubMed] [Google Scholar]
- 66.Trachsel H, Staehelin T. Initiation of mammalian protein synthesis. The multiple functions of the initiation factor eIF-3. Biochim Biophys Acta. 1979;565:305–314. doi: 10.1016/0005-2787(79)90207-7. [DOI] [PubMed] [Google Scholar]
- 67.van Hoof A, Parker R. The exosome: a proteasome for RNA? Cell. 1999;99:347–350. doi: 10.1016/s0092-8674(00)81520-2. [DOI] [PubMed] [Google Scholar]
- 68.Welch E M, Jacobson A. An internal open reading frame triggers nonsense-mediated mRNA decay of the yeast SPT10 mRNA. EMBO J. 1999;18:6134–6145. doi: 10.1093/emboj/18.21.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weng Y, Czaplinski K, Peltz S W. Genetic and biochemical characterization of mutations in the ATPase and helicase regions of the Upf1 protein. Mol Cell Biol. 1996;16:5477–5490. doi: 10.1128/mcb.16.10.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weng Y, Czaplinski K, Peltz S W. Identification and characterization of mutations in the UPF1 gene that affect nonsense suppression and the formation of the Upf protein complex but not mRNA turnover. Mol Cell Biol. 1996;16:5491–5506. doi: 10.1128/mcb.16.10.5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.White T J, Arnheim N, Erlich H A. The polymerase chain reaction. Trends Genet. 1989;5:185–189. doi: 10.1016/0168-9525(89)90073-5. [DOI] [PubMed] [Google Scholar]
- 72.Zuk D, Jacobson A. A single amino acid substitution in yeast eIF-5A results in mRNA stabilization. EMBO J. 1998;17:2914–2925. doi: 10.1093/emboj/17.10.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zuk D, Belk J P, Jacobson A. Temperature-sensitive mutations in the yeast MRT4, GRC5, SLA2, and THS1 genes result in defects in mRNA turnover. Genetics. 1999;153:35–47. doi: 10.1093/genetics/153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]