Abstract
Background
Diabetes and vascular disease are the leading causes of lower limb amputation. Currently, 463 million adults are living with diabetes, and 202 million with peripheral vascular disease, worldwide. When a lower limb amputation is considered, preservation of the knee in a below‐knee amputation allows for superior functional recovery when compared with amputation at a higher level. When a below‐knee amputation is not feasible, the most common alternative performed is an above‐knee amputation. Another possible option, which is less commonly performed, is a through‐knee amputation which may offer some potential functional benefits over an above‐knee amputation.
Objectives
To assess the effects of through‐knee amputation compared to above‐knee amputation on clinical and rehabilitation outcomes and complication rates for all patients undergoing vascular and non‐vascular major lower limb amputation.
Search methods
The Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE, Embase, and CINAHL databases; the World Health Organization International Clinical Trials Registry Platform; and the ClinicalTrials.gov trials register to 17 February 2021.
We undertook reference checking, citation searching, and contact with study authors to identify additional studies.
Selection criteria
Published and unpublished randomised controlled trials (RCTs) comparing through‐knee amputation and above‐knee amputation were eligible for inclusion in this study. Primary outcomes were uncomplicated primary wound healing and prosthetic limb fitting. Secondary outcomes included time taken to achieve independent mobility with a prosthesis, health‐related quality of life, walking speed, pain, and 30‐day survival.
Data collection and analysis
Two review authors independently reviewed all records identified by the search. Data collection and extraction were planned in line with recommendations outlined in the Cochrane Handbook for Systematic Reviews of Interventions. We planned to assess the certainty of evidence using the GRADE approach.
Main results
We did not identify RCTs that met the inclusion criteria for this review.
Authors' conclusions
No RCTs have been conducted to determine comparative clinical or rehabilitation outcomes of through‐knee amputation and above‐knee amputation, or complication rates. It is unknown whether either of these approaches offers improved outcomes for patients. RCTs are needed to guide practice and to ensure the best outcomes for this patient group.
Plain language summary
Does surgical removal of the lower leg (amputation) through the knee offer patients improved surgical recovery and better rehabilitation than amputation above the knee?
Background
Each year, thousands of people worldwide need to have their lower leg surgically removed (lower limb amputation) due to problems such as blockages in blood vessels (vascular disease), diabetes, and injury. When an amputation is planned, a surgeon needs to decide how high up the leg to go, and therefore how much leg to leave behind. This decision is based on a balance between leaving as much of the leg as possible to improve a person’s ability to walk with an artificial leg (prosthesis) and removing anything that will not survive or go on to heal. If possible, a surgeon will prefer to preserve the knee, as having a working knee of one's own ensures a person’s best chance of walking. In some cases, this is not possible, and currently almost all people in this situation will have an amputation in the middle of the thigh (above the knee). However, another option is an amputation that can be performed through the knee joint itself. This carries potential advantages, as all of the muscles controlling movements of the thighbone are undamaged. A longer remaining leg would be expected to act as a lever to reduce the effort of swinging a prosthetic limb during walking and to aid sitting balance and transfer from bed to chair. By avoiding cutting the muscles, it is possible to minimise the physical trauma of surgery, allowing a procedure with reduced blood loss and less procedure time. In addition, the end of the thighbone and in some cases the knee cap remain. These bones can support the body’s weight at the end of the remaining limb through the same mechanism as kneeling down. On the other hand, some surgeons think that problems with healing may be more common with this approach. It is unclear whether amputation through the knee may therefore be a better operation, allowing improved recovery, greater likelihood of being able to walk with an artificial leg, and better quality of life, or whether it is associated with worse outcomes due to wound healing failure and the need for further surgery. The aim of this review was to look at the best available evidence to see how these operations compare.
This review searched for studies that looked at whether through‐the‐knee or above‐the‐knee amputation resulted in better wound healing after amputation, improved patient survival, and reduced pain (clinical outcomes), as well as better rates of prosthesis use, walking speed, and quality of life (rehabilitation outcomes).
Study characteristics and key results
A thorough search of the available literature was performed (up to 17 February 2021) to find studies comparing through‐knee with above‐knee amputation. We identified no studies comparing these two procedures.
Certainty of the evidence
We were unable to assess the certainty of evidence because of the absence of studies included in this review.
Conclusion
Due to a lack of randomised trials, we are unable to determine if through‐knee amputations have different outcomes from above‐knee amputations. High‐quality randomised controlled trials are required to provide evidence on this topic.
Background
Description of the condition
Diabetes and vascular disease are the leading causes of lower limb amputation. Currently, 463 million adults are living with diabetes, and 202 million with peripheral vascular disease, worldwide (Behrendt 2018; International Diabetes Federation 2017). For every 100,000 people in Europe and Australia, between 7 and 41 persons undergo a major amputation every year due to diabetes or vascular disease (Behrendt 2018).
It is estimated that between 30 and 40 million people are living with total or partial limb loss in low‐income countries internationally (WHO 2005). The leading cause of amputation in these countries, and the second most common cause in the rest of the world, is severe traumatic injury (Ajibade 2013; Nwosu 2017). The population of people with limb loss due to trauma is large, as they tend to be young with a long life expectancy (Perkins 2012). Diabetes and vascular disease are associated with significant morbidity and mortality. There is a strong link between diabetes and vascular disease, as hyperglycaemia is one of the causal factors for vascular dysfunction (Kirpichnikov 2001). Furthermore, cigarette smoking has been reported to significantly increase rates of vascular disease (Liu 2018). Vascular complications that can lead to amputation include progressive infection, major tissue loss due to infection, ischaemia, loss of limb function, or intractable pain. Lower limb amputation places a significant economic burden on the individual and on healthcare services. The estimated yearly cost of inpatient amputation care from the United Kingdom (UK) National Health Service (NHS) is more than GBP 40 million (Kerr 2019). In 2007, lifetime healthcare costs for a prosthetic user were estimated to be USD 509,275 (MacKenzie 2007).
There are four main levels of major lower limb amputation: below‐knee, through‐knee, above‐knee, and through‐hip. These four types of amputation have been shown to provide the best chance of using a prosthesis, which is why amputation is not performed directly at the level of the most distal viable tissue (BSRM 2018). Instead, the level of amputation is chosen to best facilitate primary healing and to optimise rehabilitation potential. Quality of life (QoL) outcomes are reported to be inversely correlated with the level of amputation up the limb. Studies have always considered below‐knee versus above‐knee amputation in this regard, but a direct comparison of the outcomes of through‐knee versus above‐knee amputation has never been done (Davie‐Smith 2017; Murakami 2016).
Description of the intervention
The level of amputation affects patients' postoperative outcomes. It is accepted that below‐knee amputation has preferable outcomes to above‐knee amputation (Tisi 2014). People with below‐knee amputation achieve a higher level of mobility with an artificial limb (prosthesis), and they report better QoL, compared to those with above‐knee amputation (Aulivola 2004; Davie‐Smith 2017; Vogel 2014). However, below‐knee amputation is not always possible, and a more proximal amputation is sometimes required. In these instances, an above‐knee amputation is routinely performed (Aulivola 2004; Kidmas 2004; Yusof 2007).
Above‐knee amputation, also referred to as 'transfemoral amputation', is an amputation of the leg through the femur, above the level of the condyles, with removal of the patella. Soft tissue flaps are fashioned by using the muscle from the front and back of the leg to cover the transected bone (Woodburn 2009). Above‐knee amputation offers a good chance of primary healing and an even appearance with a prosthesis. However, the transected femur is associated with worse functionality for prosthetic limb users. They cannot use their transected femur as an effective physical endpoint for load‐bearing through a prosthesis, and sometimes may need to wear an additional suspension strap to hold the prosthesis in place, to compensate for the short length of the residuum (Gholizadeh 2014). To use a prosthesis safely and effectively following above‐knee amputation, the individual must have sufficient cardiovascular fitness and strength, good balance and dexterity, and good cognitive function. Achieving prosthetic ambulation, therefore, becomes more challenging with age (Bowrey 2018). In addition, the above‐knee amputation prosthesis is not comfortable to sit in. If a person spends more time sitting than standing and mobilising, he or she most likely will abandon the prosthesis in favour of a wheelchair. For these reasons, prosthetic fitting post above‐knee amputation is not always appropriate for the geriatric population (Bowrey 2018; Davies 2003).
Through‐knee amputation is an alternative to above‐knee amputation. Conflicting evidence surrounding through‐knee amputation describes varying postoperative levels of success. Earlier papers recommended above‐knee amputation for the best chance of primary healing (Chilvers 1971; Jamieson 1976), as delayed wound healing increases length of hospital stay, increases time taken to achieve mobility with a prosthesis, and decreases level of mobility achieved (Nijmeijer 2017). However, recent advancements in surgical techniques for through‐knee amputation are improving patient outcomes for survival, morbidity, infection, and dehiscence rates (Lim 2018; Nijmeijer 2017).
A true through‐knee amputation, also referred to as 'knee disarticulation', consists of surgical removal of the lower half of the leg through the knee joint with the femur left intact. Revised design of this amputation has improved healing rates and prosthetic fit, and variations of the through‐knee amputation have been developed (Murakami 2016). Modified techniques, such as that of Mazet, Burgess, and Youkey, involve removing the patella and trimming the femoral condyles to achieve a less bulbous residual end (Burgess 1977; Mazet 1966). With Gritti‐Stokes and Nellis/Van De Water amputations, the patella is attached to the distal end of the femur (Middleton 1962; Nellis 2002).
Both above‐knee and through‐knee surgeries are appropriate for people requiring a major lower limb amputation. However, surgeons tend to perform an above‐knee amputation despite the potential functional advantages that a longer, more powerful, end weight‐bearing, through‐knee residuum offers to a prosthetic or a non‐prosthetic user. As a result, through‐knee amputations represent less than 2% of all amputations in the United States (US) (Albino 2014; Lim 2018), and less than 1% in the UK (Moxey 2010).
How the intervention might work
Although both through‐knee and above‐knee amputations are suitable for most patients, an above‐knee amputation is often the standard method of treatment, and the small numbers of through‐knee amputations performed are reflected in the sample sizes of retrospective evidence (Bae 2007; Moxey 2010). Thus, there is no consensus regarding who makes a good or a bad candidate for through‐knee amputation.
Despite this, through‐knee amputation may offer the following potential advantages for patients over above‐knee amputation.
The surgery is less traumatic and the cartilage barrier is maintained, which reduces the risk of infection or bone spurs (Bowker 2000; Jensen 1996; Pinzur 2004).
The long end‐bearing lever arm creates a strong residual limb with reduced propensity to develop hip flexion contracture (Bowker 2000; Hughes 1983; Persson 2001; Smith 2004).
The longer residuum provides a stable sitting platform, more efficient transfers, and reduced energy requirements (Pinzur 1992; Pinzur 2004; Siev‐Ner 2000).
The residuum supports superior ambulatory stability, prosthetic sockets are more comfortable, and pressure inside the socket is reduced (Hughes 1983; Pinzur 2004; Smith 2004).
However, through‐knee amputation may involve the following potential disadvantages for patients when compared to above‐knee amputation.
The prosthesis can have a poor cosmetic finish, and issues with socket fit can occur (Jensen 1996; Persson 2001; Smith 2004).
Positioning of the prosthetic knee when it is attached to the end of the socket may cause asymmetrical knee levels (Hagberg 1992; Smith 2004).
This procedure has a reputation for delayed wound healing despite documented evidence of successful healing (Ten Duis 2009).
Why it is important to do this review
People live with postoperative limitations after both through‐knee and above‐knee amputation procedures. The incidence of return to theatre and of revision to above‐knee amputation remains an issue with through‐knee amputation (Lim 2018), and it is unknown how all postoperative outcomes compare between above‐knee and through‐knee amputation. An earlier systematic review compared through‐knee techniques to establish whether through‐knee amputation is suitable for the dysvascular patient (Murakami 2016). Murakami 2016 reported that a more substantial body of evidence would be necessary to establish the effects of different surgical techniques for mobility outcomes and gait biomechanics to determine whether through‐knee amputation is a useful treatment option for dysvascular patients. Murakami 2016 recommended that future research should compare through‐knee and above‐knee amputation in the dysvascular population over a suitable follow‐up period. Retrospective data suggest that reamputation or revision surgery rates range from 0% to 21% for through‐knee cases (Murakami 2016), compared to 8% to 12% for above‐knee amputations (Conte 2019). However, poor rehabilitation outcomes are common amongst people with above‐knee amputation, with less than 30% able to mobilise with a prosthesis outdoors (Davies 2003), whereas through‐knee amputation mobility rates have been reported to range from 13% to 75% (Murakami 2016). Sufficient wound healing is essential for successful prosthetic rehabilitation, and these factors must be considered carefully when the level of amputation is decided (Conte 2019). The ability to mobilise with a prosthesis has a direct impact on a person's QoL (Agrawal 2017; Davie‐Smith 2017). People with through‐knee amputation theoretically have gait biomechanical benefits, although some of these are potentially mitigated by the shorter lower leg segment, limited prosthetic knee joint options, and lack of prosthetist experience (Schuett 2018; Smith 2004). Recent global vascular guidelines for the management of chronic limb‐threatening ischaemia set a research priority to determine whether primary healing rates, postoperative mobility and prosthetic use, and quality of life data justify through‐knee rather than above‐knee amputation (Conte 2019). A recent patient and public involvement (PPI) group conducted by authors of the current review confirmed that QoL, time taken to achieve independent mobility, and level of walking ability are considered research priorities by people post amputation and by their family members. The review authors attended a UK artificial limb centre and spoke with service users in the waiting room to better understand these research priorities. The National Institute for Health Research (NIHR) recommends using PPI to improve the quality and relevance of research (NIHR INVOLVE).
For these reasons, it is important to determine which level of amputation provides a lower complication rate (in terms of delayed wound healing, pain, and patient survival), alongside improved postoperative, rehabilitation, and QoL outcomes. The aim of this Cochrane Review was to compare clinical and rehabilitation outcomes and complication rates of through‐knee amputation with those of above‐knee amputation. We collated and evaluated evidence to facilitate discussions and shared decision‐making between physicians and patients about which level of amputation offers improved healing rates, a better chance of survival, and better QoL, and improves the potential for successful rehabilitation outcomes. We aimed to present available evidence supporting decision‐making for each clinical or patient group. We anticipated that evidence suitable for inclusion in this review would serve as a base on which both amputation levels can be incorporated into a body of consensus guidelines, such as the Vascular Society of Great Britain and Northern Ireland (VSGBI), the British Association of Chartered Physiotherapists in Amputation Rehabilitation (BACPAR), the British Orthopaedic Association (BOA), and the British Association of Plastic Reconstructive and Aesthetic Surgeons (BAPRAS).
Objectives
To assess the effects of through‐knee amputation compared to above‐knee amputation on clinical and rehabilitation outcomes and complication rates for all patients undergoing vascular and non‐vascular major lower limb amputation.
Methods
Criteria for considering studies for this review
Types of studies
We aimed to include only randomised controlled trials (RCTs) that compare through‐knee amputation with above‐knee amputation for all aetiologies. We planned to incorporate studies that include amputations at all above‐knee levels if through‐knee outcomes were reported separately. We planned to exclude studies for which we were unable to obtain separate through‐knee amputation data.
Types of participants
We aimed to include participants of both sexes and all ages undergoing major unilateral lower limb amputation at or above the knee (between but not including the levels of below‐knee and through‐hip) in all countries of origin. We aimed to include participants with any level of preexisting function and comorbidities, with the exception of major lower limb amputation on the contralateral side. Indications for amputation included vascular or diabetic indications, such as infection, tissue loss, pain, and ischaemia, as well as non‐vascular indications, such as trauma, malignancy, and congenital malformation. Participants may have had previous major or minor lower limb surgery, including salvage attempts, limb reconstruction, revascularisation, or below‐knee or other distal lower limb amputations.
Types of interventions
We aimed to include RCTs that compared through‐knee amputation versus above‐knee amputation. We planned to use the umbrella term 'through‐knee amputation' to refer to all variations including:
standard through‐knee (through‐knee, knee disarticulation);
modified through‐knee (Mazet, Burgess, Youkey); and
Gritti‐Stokes and Nellis/Van De Water.
Above‐knee amputations are amputations at all levels through the femur for all aetiologies. We excluded below‐knee and through‐hip amputations (hip disarticulation). We planned to compare any variation on a through‐knee amputation with any above‐knee amputation.
Types of outcome measures
Primary outcomes
Limb‐fitted and not limb‐fitted: measured as whether patients are referred for limb fitting and are successfully fitted with a prosthetic limb, or are not fitted with a prosthetic limb
Uncomplicated primary wound healing (30 days)
Secondary outcomes
Time taken to achieve independent mobility with a prosthesis, with or without use of a walking aid
Health‐related QoL: reported by a validated QoL outcome measure, including those relevant to life as a prosthetic limb user, such as the 36‐Item Short Form Survey (SF‐36), the EuroQol‐5D (EQ‐5D), or the Prosthetic Patient Satisfaction Survey
Walking speed: measured as the distance walked during a time period divided by the time taken to walk that distance. This will be converted and reported as metres per second (m/s) (Peel 2012)
Pain (postoperative, phantom limb, and pain associated with prosthetic limb‐wearing) reported on a validated pain measure such as the visual analogue scale (VAS) or the Short Form McGill Pain Questionnaire
30‐Day patient survival
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Information Specialist conducted systematic searches of the following databases for RCTs and controlled clinical trials without language, publication year, or publication status restrictions.
Cochrane Vascular Specialised Register, via the Cochrane Register of Studies (CRS‐Web) (searched 17 February 2021).
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 1), in the Cochrane Library, via the Cochrane Register of Studies Online (CRSO).
MEDLINE (Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations; Ovid MEDLINE Daily; and Ovid MEDLINE) (searched 17 February 2021).
Embase Ovid (searched 17 February 2021).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) Ebsco (searched 17 February 2021).
The Information Specialist developed search strategies for other databases from the search strategy designed for MEDLINE. When appropriate, these were combined with adaptations of the highly sensitive search strategy designed by Cochrane for identifying RCTs and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 4; Lefebvre 2021). Search strategies for major databases are provided in Appendix 1.
The Information Specialist searched the following trials registries on 17 February 2021.
World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch).
ClinicalTrials.gov (clinicaltrials.gov).
Searching other resources
Two review authors (HC, GB) independently checked the bibliographies of included trials and non‐Cochrane systematic reviews for further references of interest. When necessary, we contacted study authors to request any unpublished data that may be available. We also canvassed professionals within relevant specialities to identify any as yet unpublished RCTs.
Data collection and analysis
Selection of studies
Two review authors (HC, GB) independently reviewed the titles and abstracts and determined which studies were eligible for inclusion, discussing any conflicts with the review team to reach consensus when necessary. This process was repeated with full texts of studies that were initially evaluated as appropriate for inclusion, with illustration of the study selection process in a PRISMA diagram (Liberati 2009). Records of all articles excluded after full‐text assessment were reported in a 'Characteristics of excluded studies' table along with reasons for their exclusion.
Data extraction and management
We planned for two review authors (HC, GB) to independently extract and collect relevant data from the included studies using a data extraction form provided by Cochrane Vascular. We planned to contact study authors to request raw data if through‐knee amputation outcomes were used within the population but were not reported. We aimed to resolve any disagreement by discussion within the review team. We planned to extract the following information.
Publication details: year, country, study authors.
Methods: study design, randomisation, total duration of study, number of study centres and locations, study settings, withdrawals, dates of study.
Participants: number, setting, demographic characteristics, aetiology, presence or absence of multi‐morbidities, previous lower limb surgery, inclusion criteria, exclusion criteria.
Interventions: amputation level, surgical technique.
Outcomes: primary and secondary outcomes specified and collected, time points reported.
Assessment of risk of bias in included studies
We planned for two review authors (HC, GB) to independently assess the included studies for risk of bias using Cochrane's 'Risk of bias' tool, as defined in Section 8.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We aimed to rate each domain as having low risk, high risk, or unclear risk of bias. Again, we planned to resolve any disagreements between two review authors by discussion and, if necessary, by consultation with the wider review team. We intended for a senior author to review a random subset of papers (10% to 20%) for risk of bias, as quality control.
We planned to assess risk of bias in the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective reporting.
Other sources of bias.
We aimed to report the judgement for each individual study in 'Risk of bias' tables located in the 'Characteristics of included studies' section. We planned to contact study authors for further clarification if required.
Measures of treatment effect
We aimed to calculate and report odds ratios (ORs) with 95% confidence interval (CIs) to investigate the pooled estimate of effect for dichotomous data (limb‐fitted and not limb‐fitted, uncomplicated primary wound healing, and 30‐day patient survival). We intended to calculate mean differences (MDs) between treatment groups with 95% CIs for continuous outcome measures (health‐related QoL, walking speed, and pain). We planned to use standardised mean differences (SMDs) if different scales were used to measure the same concept. We aimed to calculate time‐to‐event outcomes (time to achieve independent mobility) as hazard ratios (HRs) with 95% CIs. If sufficient data were not reported, we planned to contact study authors.
Unit of analysis issues
We planned to consider the unit of analysis within each trial to be the participant. If a trial allowed participants who have a through‐knee amputation that is reamputated to an above‐knee amputation in the same admission to remain in the trial, they would also be included.
Dealing with missing data
We planned to analyse all available data and to contact study authors to request any missing data, allowing six weeks for response before treating the data as missing. We aimed to perform an intention‐to‐treat analysis and to report incidents of loss to follow‐up.
Assessment of heterogeneity
We planned to consider clinical, methodological, and statistical heterogeneity of included studies. We planned to assess heterogeneity using Chi² and I², and to use the below guidance for interpretation as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: showing considerable heterogeneity.
Assessment of reporting biases
Reporting biases may occur when dissemination of research findings is influenced by the nature and direction of results (Higgins 2011). We aimed to examine small‐study effects by using funnel plots and to seek statistical advice for their interpretation for outcomes with more than 10 studies (Higgins 2011).
Data synthesis
We planned to synthesise data using Review Manager 5 software (Review Manager 2014), and to use statistical analysis in agreement with statistical guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We aimed to use the fixed‐effect model of meta‐analysis when there was minimal or no heterogeneity. When there was a high level of heterogeneity, we aimed to use a random‐effects model. We aimed to adopt a narrative approach if it was not possible to perform a meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We aimed to perform the following subgroup analyses if sufficient data were available.
Aetiology. We anticipated that participants' surgical, QoL, and mobility outcomes may differ between underlying causes of amputation. The large range of reamputation rates for through‐knee amputation (0% to 21%) is due to differences between aetiologies from studies using single aetiology samples (Murakami 2016). Similarly, we expected that participants would be more likely to experience delayed wound healing or to require reamputation if the presenting cause for the amputation was diabetes or a vascular cause rather than a non‐vascular cause or trauma. We aimed to investigate any effects on outcomes from vascular or non‐vascular causes of amputation by using subgroup analysis when possible.
Gender (Heidari 2016). It has been reported that a female person with an amputation is less likely to mobilise with a prosthesis, and that they are less satisfied with the cosmetic appearance of a through‐knee amputation (Singh 2008). However, Davie‐Smith 2017 described being male as one of the most significant factors to negatively affect QoL post major lower limb amputation. We aimed to carry out subgroup analysis to provide evidence for any gender impact.
Age. It is claimed that through‐knee amputation is more suitable for paediatric patients to retain growth plates (Smith 2004). We aimed to investigate any effects on outcomes due to age of participants at the time of amputation by comparing participants under age 18 with those age 18 years and older (Le 2015; NHS 2013; Rijnders 2000).
Surgical technique. We aimed to use subgroup analyses to investigate any effect differences between through‐knee, modified through‐knee, Gritti‐Stokes, and Nellis/Van De Water amputation techniques.
Sensitivity analysis
We aimed to use sensitivity analysis to investigate the robustness of findings for primary and secondary outcomes by excluding studies that we judge to have high risk of methodological bias. We aimed to classify trials as being at high risk of methodological bias if they were at high risk of bias for random sequence generation and allocation concealment.
Summary of findings and assessment of the certainty of the evidence
We aimed to present review findings in a 'Summary of findings' table, based on the methods presented in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We aimed to create a table for the comparison 'Through‐knee versus above‐knee amputation for vascular and non‐vascular major lower limb amputations'. If sufficient data were available, we aimed to create separate tables for specific through‐knee variations (such as Gritti‐Stokes, etc.) versus any above‐knee amputation. We aimed to include the following outcomes in each table: limb‐fitted or not limb‐fitted, uncomplicated primary wound healing within 30 days, time taken to achieve independent mobility, QoL, walking speed, pain, and 30‐day patient survival. We planned to assess the certainty of evidence for each outcome by using the GRADE approach (Atkins 2004; Higgins 2011). We aimed to assign the certainty of evidence as high, moderate, low, or very low based on overall risk of bias, inconsistency, indirectness, imprecision, and publication bias. We aimed to prepare 'Summary of findings' tables by using GRADEpro GDT software (GRADEpro GDT 2015). A draft 'Summary of findings' table can be viewed in Table 1.
1. Example Summary of findings table.
| Through‐knee amputation compared with above‐knee amputation for vascular and non‐vascular major lower limb amputations | ||||||
|
Patient or population: participants with through‐knee or above‐knee amputation Settings: all settings (surgical wards, rehabilitation centres, artificial limb units, community settings, etc.) Intervention: through‐knee amputation Comparison: above‐knee amputation | ||||||
| Outcomes | Anticipated absolute effects * | Relative effect (95% CI) | No. of participants (studies) | Certainty of evidence (GRADE) | Comments | |
| Risk with above‐knee amputation | Risk with through‐knee amputation | |||||
|
Limb‐fitted and not limb‐fitted (follow‐up: upon completion of prosthetic rehabilitation) |
Study population | OR [value] ([value] to [value]) | [value] ([value]) | ⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
| [value] per 1000 |
[value] per 1000 ([value] to [value]) |
|||||
|
Uncomplicated primary wound healing (follow‐up: within 30 days) |
Study population | OR [value] ([value] to [value]) | [value] ([value]) | ⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
| [value] per 1000 | [value] per 1000 ([value] to [value]) | |||||
|
Time to achieve independent mobility (follow‐up) |
Study population | HR [value] ([value] to [value]) | [value] ([value]) | ⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
| [value] per 1000 | [value] per 1000 ([value] to [value]) | |||||
|
Health‐related QoL (any validated QoL outcome measure) (follow‐up) |
Mean [outcome] ranged across control groups from [value] [measure] | Mean [outcome] in the intervention groups was [value] [lower/higher] [(value to value lower/higher)] | [value] ([value]) | ⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
|
Walking speed (m/s) (follow‐up: upon completion of prosthetic rehabilitation) |
Mean [outcome] ranged across control groups from [value] [measure] | Mean [outcome] in the intervention groups was [value] [lower/higher] [(value to value lower/higher)] | [value] ([value]) | ⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
|
Pain (any validated pain measure) (follow‐up) |
Mean [outcome] ranged across control groups from [value] [measure] | Mean [outcome] in the intervention groups was [value] [lower/higher] [(value to value lower/higher)] | [value] ([value]) | ⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
|
Patient survival (follow‐up: within 30 days) |
Study population | OR [value] ([value] to [value]) | [value] ([value]) | ⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
| [value] per 1000 | [value] per 1000 ([value] to [value]) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval;HR: hazard ratio; OR: odds ratio; QoL: quality of life. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
Results
Description of studies
Results of the search
The PRISMA flow diagram (Figure 1) shows the number of studies assessed within this review. The search strategy identified 3493 references. From these, we removed 973 duplicates, and we screened 2520 studies by title and abstract. We obtained the full text for 12 studies; however we subsequently excluded all and noted reasons for exclusion in the Characteristics of excluded studies table.
1.
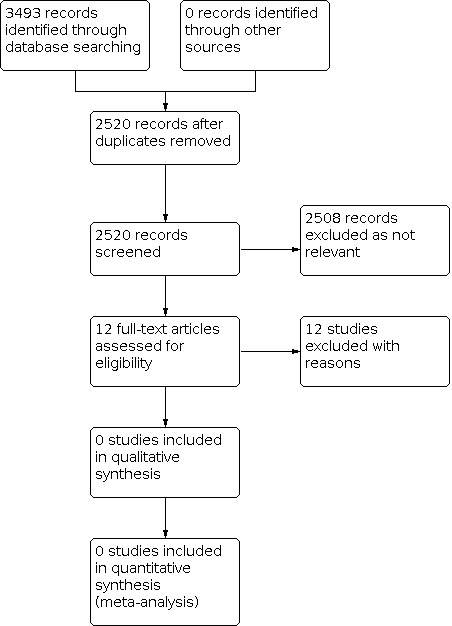
Figure 1: PRISMA flow diagram.
Included studies
We identified no eligible studies for inclusion.
Excluded studies
We excluded the 12 full‐text studies for the following reasons: two studies included an incorrect intervention (NCT03900845; NCT04023045); eight used an incorrect design (Anderson 2005; Baumgartner 1979; Dustmann 1985; Hagberg 1992; Houghton 1989; Jeans 2011; Knahr 1979; NCT04120558); one used the wrong comparators (Campbell 1987); and the full text of one study could not be obtained (Kahle 2016), despite attempts to contact the study author; therefore it was excluded.
Risk of bias in included studies
No studies met the inclusion criteria.
Effects of interventions
No studies met the inclusion criteria.
Discussion
Summary of main results
Despite the potential benefits of through‐knee amputation, we identified no RCTs comparing through‐knee amputation with above‐knee amputation; therefore there we can make no conclusions regarding the comparative performance of these two procedures for people requiring lower limb amputation at a site more proximal to the below‐knee level. As a result of this lack of evidence, people undergoing above‐knee amputation as “standard care” may be missing out on potentially superior rehabilitation outcomes of through‐knee amputation, and people undergoing through‐knee amputation performed by enthusiasts for this procedure may be at risk of increased complication rates and revisional surgery than if they had received the default “standard care” of above‐knee amputation.
Overall completeness and applicability of evidence
Currently no high‐quality evidence compares through‐knee amputation and above‐knee amputation in the literature.
Quality of the evidence
Currently no high‐quality evidence compares through‐knee amputation and above‐knee amputation in the literature.
Potential biases in the review process
The risk of bias in this review was minimised. The Cochrane Information Specialist conducted a comprehensive search of the literature. Two review authors independently reviewed all study titles found by the searches against the inclusion and exclusion criteria that had been prospectively published in the peer‐reviewed protocol (Crane 2021). We planned to resolve disagreement by consensus or with the arbitration of a third review author. This was not necessary, and both review authors' findings were consistent.
Agreements and disagreements with other studies or reviews
We found no other reviews or non‐randomised studies comparing outcomes of through‐knee versus above‐knee amputation. Previous randomised studies have involved individuals with through‐knee and above‐knee amputations (Hargrove 2015; Highsmith 2012; Prinsen 2015; Prinsen 2017; Seelen 2009). However, the aim of these studies was to compare differences between a microprocessor‐controlled prosthetic knee and a non‐microprocessor‐controlled prosthetic knee. One RCT examined differences between primary and delayed wound closure but did not include through‐knee amputation (Katiyar 2020). Another RCT investigated wound healing in Gritti‐Stokes and knee disarticulation amputations but did not include above‐knee amputation as a comparator group (Campbell 1987).
Authors' conclusions
Implications for practice.
For planning a lower limb amputation in a clinical situation in which a below‐knee amputation is unsuitable, no RCT evidence is available to inform the decision as to whether a through‐knee or an above‐knee amputation will offer the best outcomes for a patient. Clinicians need to rely on clinical experience in the absence of high‐quality evidence on which to base practice. It is therefore possible that there is a significant risk that patients may not be offered the best operation for them, and their clinical and rehabilitation outcomes may suffer as a result.
Implications for research.
We found no RCTs from which we could draw conclusions regarding the review objectives. High‐quality research is needed to inform clinical decision‐making for this group of patients to ensure optimal outcomes, maximise quality of life for patients and their families, and ensure the best use of both health and social care expenditures. Although studies have recruited participants with through‐knee amputation and above‐knee amputation, no high‐certainty evidence is available to support surgical practice of one type over the other. RCTs are required to determine whether a through‐knee amputation offers any advantage for patient outcomes compared to an above‐knee amputation. Future RCTs for patients who are eligible for either a through‐knee amputation or an above‐knee amputation would help to determine the true differences in outcomes between these two levels of amputation.
History
Protocol first published: Issue 1, 2021
Notes
Parts of the Methods section of the protocol are based on a standard template established by Cochrane Vascular.
Acknowledgements
The review authors and the Cochrane Vascular Editorial base are grateful to the following peer reviewers of the protocol for their time and comments: Richard A Frieden, MD, MS, Medical Director, Amputation Specialty Program Medical Director, Comprehensive Integrated Inpatient Rehabilitation Program, Department of Rehabilitation Medicine, New York City, USA; Mark E Huang, MD, Shirley Ryan AbilityLab, Chicago, USA.
Appendices
Appendix 1. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| VASCULAR REGISTER IN CRSW | #1 Knee* AND INREGISTER #2 above‐knee AND INREGISTER #3 Through‐knee AND INREGISTER #4 #1 OR #2 OR #3 #5 Amput* AND INREGISTER #6 stump* AND INREGISTER #7 #5 OR #6 #8 #4 AND #7 |
Feb 2021: 116 |
| CENTRAL via CRSW | #1 MESH DESCRIPTOR Knee EXPLODE ALL WITH QUALIFIER SU AND CENTRAL:TARGET #2 MESH DESCRIPTOR Knee Joint EXPLODE ALL WITH QUALIFIER SU AND CENTRAL:TARGET #3 Knee* AND CENTRAL:TARGET #4 "above‐knee" AND CENTRAL:TARGET #5 "Through‐knee" AND CENTRAL:TARGET #6 "trans femoral" AND CENTRAL:TARGET #7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 #8 MESH DESCRIPTOR Amputation EXPLODE ALL AND CENTRAL:TARGET #9 MESH DESCRIPTOR Amputation Stumps EXPLODE ALL AND CENTRAL:TARGET #10 MESH DESCRIPTOR Amputees EXPLODE ALL AND CENTRAL:TARGET #11 "residua* limb*" AND CENTRAL:TARGET #12 (phantom adj6 limb*) AND CENTRAL:TARGET #13 amput* AND CENTRAL:TARGET #14 disarticulat* AND CENTRAL:TARGET #15 exarticulat* AND CENTRAL:TARGET #16 postamputation* AND CENTRAL:TARGET #17 post‐amputation* AND CENTRAL:TARGET #18 stump* AND CENTRAL:TARGET #19 Gritti‐Stokes AND CENTRAL:TARGET #20 Mazet AND CENTRAL:TARGET #21 Burgess AND CENTRAL:TARGET #22 #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #7 AND #22 |
Feb 2021: 509 |
| Clinicaltrials.gov | Amputation OR amputate OR amputee OR stump | Knee OR above‐knee OR Through‐knee | Feb 2021: 142 |
| ICTRP search portal | Amputation OR amputate OR amputee OR stump | Knee OR above‐knee OR Through‐knee | Feb 2021: 0 |
| MEDLINE (Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations; Ovid MEDLINE Daily; and Ovid MEDLINE) 1946 to present | 1 exp Knee/su [Surgery] 2 exp Knee Joint/su [Surgery] 3 Knee*.ti,ab. 4 "above‐knee".ti,ab. 5 "Through‐knee".ti,ab. 6 "trans femoral".ti,ab. 7 or/1‐6 8 exp Amputation/ 9 exp Amputation Stumps/ 10 exp Amputees/ 11 "residua* limb*".ti,ab. 12 (phantom adj6 limb*).ti,ab. 13 amput*.ti,ab. 14 disarticulat*.ti,ab. 15 exarticulat*.ti,ab. 16 postamputation*.ti,ab. 17 post‐amputation*.ti,ab. 18 stump*.ti,ab. 19 Gritti‐Stokes.ti,ab. 20 Mazet.ti,ab. 21 Burgess.ti,ab. 22 or/8‐21 23 7 and 22 24 randomized controlled trial.pt. 25 controlled clinical trial.pt. 26 randomized.ab. 27 placebo.ab. 28 drug therapy.fs. 29 randomly.ab. 30 trial.ab. 31 groups.ab. 32 or/24‐31 33 exp animals/ not humans.sh. 34 32 not 33 35 23 and 34 |
Feb 2021: 942 |
| Embase | 1 exp knee/su [Surgery] 2 exp knee/su [Surgery] 3 Knee*.ti,ab. 4 "above‐knee".ti,ab. 5 "Through‐knee".ti,ab. 6 "trans femoral".ti,ab. 7 or/1‐6 8 exp amputation/ 9 exp amputation stump/ 10 exp amputee/ 11 "residua* limb*".ti,ab. 12 (phantom adj6 limb*).ti,ab. 13 amput*.ti,ab. 14 disarticulat*.ti,ab. 15 exarticulat*.ti,ab. 16 postamputation*.ti,ab. 17 post‐amputation*.ti,ab. 18 stump*.ti,ab. 19 Gritti‐Stokes.ti,ab. 20 Mazet.ti,ab. 21 Burgess.ti,ab. 22 or/8‐21 23 7 and 22 24 randomized controlled trial/ 25 controlled clinical trial/ 26 random$.ti,ab. 27 randomization/ 28 intermethod comparison/ 29 placebo.ti,ab. 30 (compare or compared or comparison).ti. 31 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 32 (open adj label).ti,ab. 33 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 34 double blind procedure/ 35 parallel group$1.ti,ab. 36 (crossover or cross over).ti,ab. 37 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 38 (assigned or allocated).ti,ab. 39 (controlled adj7 (study or design or trial)).ti,ab. 40 (volunteer or volunteers).ti,ab. 41 trial.ti. 42 or/24‐41 43 23 and 42 |
Feb 2021: 1377 |
| CINAHL | S38 MH "Random Assignment" S37 S22 AND S36 S36 S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 S35 MH "Random Assignment" S34 MH "Triple‐Blind Studies" S33 MH "Double‐Blind Studies" S32 MH "Single‐Blind Studies" S31 MH "Crossover Design" S30 MH "Factorial Design" S29 MH "Placebos" S28 MH "Clinical Trials" S27 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" S26 TX crossover OR "cross‐over" S25 AB placebo* S24 TX random* S23 TX "latin square" S22 S6 AND S21 S21 S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 S20 TX Burgess S19 TX Mazet S18 TX Gritti‐Stokes S17 TX stump* S16 TX post‐amputation* S15 TX postamputation* S14 TX exarticulat* S13 TX disarticulat* S12 TX amput* S11 TX phantom N6 limb* S10 TX "residua* limb*" S9 (MH "Amputees") S8 (MH "Amputation Stumps") S7 (MH "Amputation+") S6 S1 OR S2 OR S3 OR S4 OR S5 S5 TX "trans femoral" S4 TX "Through‐knee" S3 TX "above‐knee" S2 TX Knee* S1 (MH "Knee Joint+/SU") |
Feb 2021: 407 |
| TOTAL before de‐duplication | 3493 | |
| TOTAL after de‐duplication | 2520 | |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anderson 2005 | Wrong study design ‐ not an RCT |
| Baumgartner 1979 | Wrong study design ‐ not an RCT |
| Campbell 1987 | Wrong comparator ‐ not above‐knee amputation |
| Dustmann 1985 | Wrong study design ‐ not an RCT |
| Hagberg 1992 | Wrong study design ‐ not an RCT |
| Houghton 1989 | Wrong study design ‐ not an RCT |
| Jeans 2011 | Wrong study design ‐ not an RCT |
| Kahle 2016 | Unable to locate full text |
| Knahr 1979 | Wrong study design ‐ not an RCT |
| NCT03900845 | Wrong intervention ‐ not through‐knee amputation |
| NCT04023045 | Wrong intervention ‐ not through‐knee amputation |
| NCT04120558 | Wrong study design ‐ not an RCT |
RCT: randomised controlled trial
Differences between protocol and review
None.
Contributions of authors
HC: protocol drafting, acquisition of trial reports, trial selection, data extraction, data analysis, data interpretation, review drafting and future review updates, guarantor of the review.
GB: protocol drafting, acquisition of trial reports, trial selection, data extraction, data analysis, data interpretation, review drafting and future review updates, guarantor of the review.
DC: protocol drafting, acquisition of trial reports, trial selection, data extraction, data analysis, data interpretation, review drafting and future review updates.
NV: protocol drafting, acquisition of trial reports, trial selection, data extraction, data analysis, data interpretation, review drafting and future review updates.
MT: protocol drafting, acquisition of trial reports, trial selection, data extraction, data analysis, data interpretation, review drafting and future review updates.
GES: protocol drafting, acquisition of trial reports, trial selection, data extraction, data analysis, data interpretation, review drafting and future review updates.
Sources of support
Internal sources
No sources of support provided
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
HC: has declared that her institution received a research bursary of £2000 from the British Association of Chartered Physiotherapists in Amputee Rehabilitation (BACPAR). This was solely for participant costs for qualitative interviews conducted with through‐knee and above‐knee amputees for her PhD.
GB: has declared that her institution received £1000 from Help for Health solely to cover the costs of participant incentives and their travel costs when completing data collection for her PhD.
DC: has received consultancy fees from Medtronic related to venous treatment policy, and travel expenses from All Party Parliamentary Group on Vascular Disease, NICE, MHRA, to provide expert advice.
NV: has received payment for Editorial board membership (Associate Editor) for Journal of Sports Sciences. She is Chief Investigator on a study funded by the National Institute for Health Research (PB‐PG‐0816‐20029). This study is conducted to assess the feasibility of conducting an RCT about the effectiveness and cost‐effectiveness of a novel prosthesis for older patients with vascular‐related below‐the‐knee amputations and multi‐morbidities. It is not relevant to this review.
MT: none known.
GES: has in the past received consulting fees from BSN Medical for consulting on NICE technology appraisal application, speakers fees from BSN Medical and Bayer with regard to presenting research related to their products. GES reports that in the past, his institution has received unconditional funding from Diomed/Angiodynamics. This was used to help fund a research nurse to assist with objective assessments in the context of randomised controlled trials.
New
References
References to studies excluded from this review
Anderson 2005 {published data only}
- Anderson KM. Knee disarticulation and above-knee amputation. Operative Techniques in General Surgery 2005;7(2):90-5. [Google Scholar]
Baumgartner 1979 {published data only}
- Baumgartner RF. Knee disarticulation versus above-knee amputation. Prosthetics & Orthotics International 1979;3(1):15-9. [DOI] [PubMed] [Google Scholar]
Campbell 1987 {published data only}
- Campbell WB, Morris PJ. A prospective randomized comparison of healing in Gritti-Stokes and through-knee amputations. Annals of the Royal College of Surgeons of England 1987;69(1):1-4. [PMC free article] [PubMed] [Google Scholar]
Dustmann 1985 {published data only}
- Dustmann HO, Munny G. The advantages of the knee joint exarticulation compared to thigh amputation. Orthopadische Praxis 1985;21(11):883-6. [Google Scholar]
Hagberg 1992 {published data only}
- Hagberg E, Berlin OK, Renstrom P. Function after through-knee compared with below-knee and above-knee amputation. Prosthetics and Orthotics International 1992;16(3):168-73. [DOI] [PubMed] [Google Scholar]
Houghton 1989 {published data only}
- Houghton A, Allen A, Luff R, McColl I. Rehabilitation after lower limb amputation: a comparative study of above-knee, through-knee and Gritti-Stokes amputations. British Journal of Surgery 1989;76(6):622-4. [DOI] [PubMed] [Google Scholar]
Jeans 2011 {published data only}
- Jeans KA, Browne RH, Karol LA. Effect of amputation level on energy expenditure during overground walking by children with an amputation. Journal of Bone & Joint Surgery - American Volume 2011;93(1):49-56. [DOI] [PubMed] [Google Scholar]
Kahle 2016 {published data only}
- Kahle JT, Highsmith MJ. Anatomy of a clinical trial. InMotion 2016;26(5):38-41. [Google Scholar]
Knahr 1979 {published data only}
- Knahr K, Kristen H. Disarticulation in the knee joint [Die Exartikulation im Kniegelenk]. Langenbecks Archiv fűr Chirurgie 1979;350(1):1-11. [DOI] [PubMed] [Google Scholar]
NCT03900845 {published data only}
- NCT03900845. Adherence and perspiration while wearing lower limb prostheses. ClinicalTrials.gov/show/NCT03900845 (first received 3 April 2019).
NCT04023045 {published data only}
- NCT04023045. Assist-Knee: energy-harvesting knee prosthesis. ClinicalTrials.gov/show/NCT04023045 (first received 17 July 2019).
NCT04120558 {published data only}
- NCT04120558. Comparing OutcomeS of Through Knee and Above Knee Amputation. ClinicalTrials.gov/show/NCT04120558 (first received 9 December 2019).
Additional references
Agrawal 2017
- Agrawal M, Kalra A, Joshi M. Correlation of ambulation potential with quality of life in lower limb amputees. International Journal of Community Medicine and Public Health 2017;4(11):4259-65. [Google Scholar]
Ajibade 2013
- Ajibade A, Akinniyi OT, Okoye CS. Indications and complications of major limb amputations in Kano, Nigeria. Ghana Medical Journal 2013;47(4):185-8. [PMC free article] [PubMed] [Google Scholar]
Albino 2014
- Albino FP, Seidel R, Brown BJ, Crone CG, Attinger CE. Through knee amputation: technique modifications and surgical outcomes. Archives of Plastic Surgery 2014;41(5):562-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aulivola 2004
- Aulivola B, Hile CN, Hamdan AD, Sheahan MG, Veraldi JR, Skillman JJ, et al. Major lower extremity amputation: outcome of a modern series. Archives of Surgery 2004;139(4):395-9. [DOI] [PubMed] [Google Scholar]
Bae 2007
- Bae TS, Choi K, Hong D, Mun M. Dynamic analysis of above-knee amputee gait. Clinical Biomechanics 2007;22(5):557-66. [DOI] [PubMed] [Google Scholar]
Behrendt 2018
- Behrendt CA, Sigvant B, Szeberin Z, Beiles B, Eldrup N, Thomson IA, et al. International variations in amputation practice: a VASCUNET report. European Journal of Vascular and Endovascular Surgery 2018;56:391-9. [DOI] [PubMed] [Google Scholar]
Bowker 2000
- Bowker JH, San Giovanni TP, Pinzur MS. North American experience with knee disarticulation with use of a posterior myofasciocutaneous flap. Healing rate and functional results in seventy-seven patients. Journal of Bone & Joint Surgery - American Volume 2000;82(11):1571-4. [DOI] [PubMed] [Google Scholar]
Bowrey 2018
- Bowrey S, Naylor H, Russell P, Thompson J. Development of a scoring tool (BLARt score) to predict functional outcome in lower limb amputees. Disability and Rehabilitation 2018;41(19):2324-32. [DOI] [PubMed] [Google Scholar]
BSRM 2018
- British Society of Rehabilitation Medicine. Amputee and Prosthetic Rehabilitation – Standards and Guidelines. 3rd edition. Report of the Working Party (Co-Chairs: Hanspal RS, Sedki I). British Society of Rehabilitation Medicine: London, 2018. [Google Scholar]
Burgess 1977
- Burgess EM. Disarticulation of the knee: a modified technique. Archives of Surgery 1977;112(10):1250-5. [DOI] [PubMed] [Google Scholar]
Chilvers 1971
- Chilvers AS, Briggs J, Browse L, Kinmonth JB. Below- and through-knee amputations in ischaemic disease. British Journal of Surgery 1971;58(11):824-6. [DOI] [PubMed] [Google Scholar]
Conte 2019
- Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. European Journal of Vascular and Endovascular Surgery 2019;58(1):S1-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davies 2003
- Davies B, Datta D. Mobility outcome following unilateral lower limb amputation. Prosthetics and Orthotics International 2003;27(3):186-90. [DOI] [PubMed] [Google Scholar]
Davie‐Smith 2017
- Davie-Smith F, Coulter E, Kennon B, Wyke S, Paul L. Factors influencing quality of life following lower limb amputation for peripheral arterial occlusive disease: a systematic review of the literature. Prosthetics and Orthotics International 2017;41(6):537-47. [DOI] [PubMed] [Google Scholar]
Gholizadeh 2014
- Gholizadeh H, Abu Osman NA, Eshraghi A, Ali S. Transfemoral prosthesis suspension systems: a systematic review of the literature. American Journal of Physical Medicine and Rehabilitation 2014;93(9):809-23. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available from gradepro.org.
Hargrove 2015
- Hargrove LJ, Young AJ, Simon AM, Fey NP, Lipschutz RD, Finucane SB, et al. Intuitive control of a powered prosthetic leg during ambulation: a randomized clinical trial. Journal of the American Medical Association 2015;313(22):2244-52. [DOI] [PubMed] [Google Scholar]
Heidari 2016
- Heidari S, Babor TF, De Castro P, Tort S, Curno M. Sex and gender equity in research: rationale for the SAGER guidelines and recommended use. Research Integrity and Peer Review 2016;1(2):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Highsmith 2012
- Highsmith MJ. Comparative outcomes assessment of the C-Leg and X2 knee prosthesis. Graduate Theses and Dissertations 2012.
Hughes 1983
- Hughes J. Biomechanics of the through-knee prosthesis. Prosthetics and Orthotics International 1983;7(2):96-9. [DOI] [PubMed] [Google Scholar]
International Diabetes Federation 2017
- International Diabetes Federation. IDF Diabetes Atlas, 8th edition. www.diabetesatlas.org (accessed 8 September 2019).
Jamieson 1976
- Jamieson C, Hill D. Amputation for vascular disease. British Journal of Surgery 1976;63(9):683-90. [DOI] [PubMed] [Google Scholar]
Jensen 1996
- Jensen JS. Surgical techniques of knee disarticulation and femoral transcondylar amputations. In: Murdoch G, Bennett Wilson A, editors(s). Amputation Surgical Practice and Patient Management. Oxford: Butterworth-Heinemann, 1996:127-34. [Google Scholar]
Katiyar 2020
- Katiyar AK, Agarwal H, Priyadarshini P, Kumar A, Kumar S, Gupta A, et al. Primary vs delayed primary closure in patients undergoing lower limb amputation following trauma: a randomised control study. International Wound Journal 2020;17(2):419-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerr 2019
- Kerr M, Barron E, Chadwick P, Evans T, Kong WM, Rayman G, et al. The cost of diabetic foot ulcers and amputations to the National Health Service in England. Diabetic Medicine 2019;36(8):995-1002. [DOI] [PubMed] [Google Scholar]
Kidmas 2004
- Kidmas AT, Nwadiaro CH, Igun GO. Lower limb amputation in Jos, Nigeria. East African Medical Journal 2004;81(8):427-9. [DOI] [PubMed] [Google Scholar]
Kirpichnikov 2001
- Kirpichnikov D, Sowers JR. Diabetes mellitus and diabetes-associated vascular disease. Trends in Endocrinology and Metabolism 2001;12(5):223-30. [DOI] [PubMed] [Google Scholar]
Le 2015
- Le JT, Scott-Wyard PR. Pediatric limb differences and amputations. Physical Medicine and Rehabilitation Clinics of North America 2015;26(1):95-108. [DOI] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4. Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lim 2018
- Lim S, Javorski MJ, Halandras PM, Aulivola B, Crisostomo PR. Through-knee amputation is a feasible alternative to above-knee amputation. Journal of Vascular Surgery 2018;68(1):197-203. [DOI] [PubMed] [Google Scholar]
Liu 2018
- Liu M, Zhang W, Yan Z, Xiangzhen Y. Smoking increases the risk of diabetic foot amputation: a meta‑analysis. Experimental and Therapeutic Medicine 2018;15:1680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacKenzie 2007
- MacKenzie E, Jones A, Bosse M, Castillo R, Pollak A, Webb L, et al. Health-care costs associated with amputation or reconstruction of a limb-threatening injury. Journal of Bone & Joint Surgery 2007;89(8):1685-92. [DOI] [PubMed] [Google Scholar]
Mazet 1966
- Mazet RJ, Hennessy CA. Knee disarticulation: a new technique and a new knee-joint mechanism. Journal of Bone & Joint Surgery 1966;48:126-39. [Google Scholar]
Middleton 1962
- Middleton MD, Webster CU. Clinical review of the gritti-stokes amputation. British Medical Journal 1962;574(2):574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moxey 2010
- Moxey PW, Hofman D, Hinchliffe RJ, Jones K, Thompson MM, Holt PJ. Epidemiological study of lower limb amputation in England between 2003 and 2008. British Journal of Surgery 2010;97(9):1348-53. [DOI] [PubMed] [Google Scholar]
Murakami 2016
- Murakami T, Murray K. Outcomes of knee disarticulation and the influence of surgical techniques in dysvascular patients: a systematic review. Prosthetics and Orthotics International 2016;40(4):423-35. [DOI] [PubMed] [Google Scholar]
Nellis 2002
- Nellis N, Van De Water JM. Through-the-knee amputation: an improved technique. The American Surgeon 2002;68(5):466-9. [PubMed] [Google Scholar]
NHS 2013
- NHS England. NHS standard contract for paediatric surgery: surgery (and surgical pathology, anaesthesia and pain). www.england.nhs.uk/wp-content/uploads/2013/06/e02-paed-surg-surgi-path-anaes.pdf (accessed 15 April 2020).
NIHR INVOLVE
- NIHR INVOLVE. Briefing notes for researchers. www.invo.org.uk (last accessed 26 March 2020).
Nijmeijer 2017
- Nijmeijer R, Voesten HG, Geertzen JH, Dijkstra PU. Disarticulation of the knee: analysis of an extended database on survival, wound healing, and ambulation. Journal of Vascular Surgery 2017;66(3):866-74. [DOI] [PubMed] [Google Scholar]
Nwosu 2017
- Nwosu C, Babaloa MO, Ibrahim MH, Suleiman SI. Major limb amputations in a tertiary hospital in North Western Nigeria. African Health Sciences 2017;17(2):508-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peel 2012
- Peel NM, Kuys SS, Klein K. Gait speed as a measure in geriatric assessment in clinical settings: a systematic review. Journals of Gerontology: Medical Sciences 2012;68(1):39-46. [DOI] [PubMed] [Google Scholar]
Perkins 2012
- Perkins Z, De'Ath H, Sharp G, Tai N. Factors affecting outcome after traumatic limb amputation. British Journal of Surgery 2012;99(1):75-86. [DOI] [PubMed] [Google Scholar]
Persson 2001
- Persson B. Lower limb amputation. Part 1: amputation methods – a 10 year literature review. Prosthetics and Orthotics International 2001;25(1):7-13. [DOI] [PubMed] [Google Scholar]
Pinzur 1992
- Pinzur MS, Gold J, Schwartz D, Gross N. Energy demands for walking in dysvascular amputees as related to the level of amputation. Orthopedics 1992;15(9):1033-7. [DOI] [PubMed] [Google Scholar]
Pinzur 2004
- Pinzur MS. Knee disarticulation: surgical management. In: Smith DG, Michael JW, Bowker JH, editors(s). Atlas of Amputations and Limb Deficiencies: Surgical, Prosthetic and Rehabilitation Principles. 3rd edition. Rosemont, IL: American Academy of Orthopaedic Surgeons, 2004:517-23. [Google Scholar]
Prinsen 2015
- Prinsen EC, Nederhand MJ, Olsman J, Rietman JS. Influence of a user-adaptive prosthetic knee on quality of life, balance confidence, and measures of mobility: a randomised cross-over trial. Clinical Rehabilitation 2015;29(6):581-91. [DOI] [PubMed] [Google Scholar]
Prinsen 2017
- Prinsen EC, Nederhand MJ, Sveinsdottir HS, Prins MR, Meer F, Koopman HF, et al. The influence of a user-adaptive prosthetic knee across varying walking speeds: a randomized cross-over trial. Gait and Posture 2017;51:254-60. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rijnders 2000
- Rijnders l, Boonstra A, Groothoff J, Cornel M, Eisma W. Lower limb deficient children in the Netherlands: epidemiological aspects. Prosthetics and Orthotics International 2000;24(1):13-8. [DOI] [PubMed] [Google Scholar]
Schuett 2018
- Schuett D, Wyatt MP, Kingsbury T, Thesing N, Dromsky DM, Kuhn KM. Are gait parameters for through-knee amputees different from matched transfemoral amputees. Clinical Orthopaedics and Related Research 2018;477(4):821-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seelen 2009
- Seelen HA, Hemmen B, Schmeets AJ, Ament AJ, Evers SM. Costs and consequences of a prosthesis with an electronically stance and swing phase controlled knee joint. Technology and Disability 2009;21(1-2):25-34. [Google Scholar]
Siev‐Ner 2000
- Siev-Ner I, Heim M, Wershavski M, Adunsky A, Azariat M. Why knee disarticulation (through-knee-amputation) is appropriate for nonambulatory patients. Disability and Rehabilitation 2000;15(22):862-4. [DOI] [PubMed] [Google Scholar]
Singh 2008
- Singh R, Hunter J, Philip A, Tyson S. Gender differences in amputation outcome. Disability and Rehabilitation 2008;30(2):122-5. [DOI] [PubMed] [Google Scholar]
Smith 2004
- Smith D. The knee disarticulation: it's better when it's better and it's not when it's not. inMotion 2004;14(1):56-62. [Google Scholar]
Ten Duis 2009
- Ten Duis K, Bosmans J, Voesten H, Geertzen J, Dijkstra P. Knee disarticulation: survival, wound healing and ambulation. A historic cohort study. Prosthetics and Orthotics International 2009;33(1):52-60. [DOI] [PubMed] [Google Scholar]
Tisi 2014
- Tisi P, Than M. Type of incision for below knee amputation. Cochrane Database of Systematic Reviews 2014, Issue 4. Art. No: CD003749. [DOI: 10.1002/14651858.CD003749.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vogel 2014
- Vogel TR, Petroski GF, Kruse RL. Impact of amputation level and comorbidities on functional status of nursing home residents after lower extremity amputation. Journal of Vascular Surgery 2014;59(5):1323-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2005
- World Health Organization. Guidelines for training personnel in developing countries for prosthetics and orthotics services. apps.who.int/iris/handle/10665/43127 2005.
Woodburn 2009
- Woodburn K, Lindsay B. Above knee amputation. In: Hallett JW, Mills JL, Earnshaw J, Reekers JA, Rooke T, editors(s). Comprehensive Vascular and Endovascular Surgery. Second edition. Philadelphia: Mosby Elselvier, 2009:229-39. [Google Scholar]
Yusof 2007
- Yusof MI, Sulaiman AR, Muslim DA. Diabetic foot complications: a two-year review of limb amputation in a Kelantanese population. Singapore Medical Journal 2007;48(8):729-32. [PubMed] [Google Scholar]
References to other published versions of this review
Crane 2021
- Crane H, Boam G, Carradice D, Vanicek N, Twiddy M, Smith GE. Through‐knee versus above‐knee amputation for vascular and non‐vascular major lower limb amputations. Cochrane Database of Systematic Reviews 2021, Issue 1. Art. No: CD013839. [DOI: 10.1002/14651858.CD013839] [DOI] [PMC free article] [PubMed] [Google Scholar]


