Abstract
Due to the large number of patients with severe coronavirus disease 2019 (COVID-19), many were treated outside the traditional walls of the intensive care unit (ICU), and in many cases, by personnel who were not trained in critical care. The clinical characteristics and the relative impact of caring for severe COVID-19 patients outside the ICU is unknown. This was a multinational, multicentre, prospective cohort study embedded in the International Severe Acute Respiratory and Emerging Infection Consortium World Health Organization COVID-19 platform. Severe COVID-19 patients were identified as those admitted to an ICU and/or those treated with one of the following treatments: invasive or noninvasive mechanical ventilation, high-flow nasal cannula, inotropes or vasopressors. A logistic generalised additive model was used to compare clinical outcomes among patients admitted or not to the ICU. A total of 40 440 patients from 43 countries and six continents were included in this analysis. Severe COVID-19 patients were frequently male (62.9%), older adults (median (interquartile range (IQR), 67 (55–78) years), and with at least one comorbidity (63.2%). The overall median (IQR) length of hospital stay was 10 (5–19) days and was longer in patients admitted to an ICU than in those who were cared for outside the ICU (12 (6–23) days versus 8 (4–15) days, p<0.0001). The 28-day fatality ratio was lower in ICU-admitted patients (30.7% (5797 out of 18 831) versus 39.0% (7532 out of 19 295), p<0.0001). Patients admitted to an ICU had a significantly lower probability of death than those who were not (adjusted OR 0.70, 95% CI 0.65–0.75; p<0.0001). Patients with severe COVID-19 admitted to an ICU had significantly lower 28-day fatality ratio than those cared for outside an ICU.
Short abstract
Countries and hospitals need to identify strategies to increase their ICU capacity (i.e. trained personnel, ICU beds and monitoring systems) to treat patients presenting to the hospital with severe #COVID19 rather than provide such care outside of the ICU https://bit.ly/3xh9A6M
Background
The clinical presentation of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection varies from asymptomatic to severe respiratory failure [1]. Severely ill patients may require advanced respiratory support (e.g. invasive or noninvasive mechanical ventilation or high-flow nasal cannula (HFNC)) or extra-respiratory support (e.g. vasopressors, inotropes or renal replacement therapy) [2]. Severe coronavirus disease 2019 (COVID-19), defined as requiring intensive care unit (ICU) admission or advanced ventilatory support, occurs in 15–30% of hospitalised individuals, with in-hospital fatality ratios ranging from 30% to 70%, depending on various factors including patients’ age, comorbidities and access to medical interventions [3, 4]. High-quality supportive care remains the standard of care for these patients [5, 6].
During the SARS-CoV-2 pandemic, many international healthcare systems became overwhelmed, requiring medical interventions traditionally restricted to delivery in an ICU by specially trained personnel to be delivered in other hospital areas, sometimes by healthcare workers without equivalent training [7]. Thus, invasive and noninvasive mechanical ventilation, HFNCs and treatment with inotropes or vasopressors, have been used outside of the ICU due to the acute surge in cases and lack of ICU capacity [8]. Several strategies have been proposed to rapidly train non-ICU personnel and to optimise resources during the pandemic [9]. However, the impact of these strategies is unknown.
Most studies describing the clinical characteristics and outcomes of COVID-19 patients admitted to the ICU are limited to a few centres within a single country and have not evaluated the impact of ICU-level treatment on clinical outcomes [1, 10, 11]. Moreover, available studies have not evaluated the outcomes of severely ill patients cared for outside the ICU environment [1, 10, 11]. Moreover, there is a growing concern about whether available data characterising patients with severe COVID-19 are generalisable to other regions of the world and whether severe COVID-19 patients can safely be cared for outside of an ICU [12]. Here, we describe a global population of patients with severe COVID-19, both those with and without ICU admissions during their hospital stay. In addition, we describe outcomes in patients with severe COVID-19 inside and outside the ICU to determine the potential impact of ICU admission.
Methods
The International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC)-World Health Organization (WHO) Clinical Characterisation Protocol for Severe Emerging Infections provided a framework for prospective observational data collection on hospitalised patients. The study information is available in the supplementary material and the protocol, case report forms and consent forms are available on the ISARIC website (https://isaric.tghn.org). This is a standardised protocol for investigating severe acute infections of pathogens of public health interest with tiered data collection tailored to a range of resource settings. Investigators from 43 countries collected prospective data using the ISARIC case report form built on Research Electronic Data Capture (REDCap, version 8.11.11; Vanderbilt University, Nashville, TN, USA) hosted by the University of Oxford (Oxford, UK). Other investigators collected data on a variety of locally hosted data systems and submitted data for centralised mapping to the ISARIC dataset. All investigators retain full rights to their data.
This observational study required no change to clinical management and permitted patient enrolment in other research projects. The ISARIC-WHO Clinical Characterisation Protocol was approved by the WHO ethics review committee (RPC571 and RPC572). Local ethics approval was obtained for each participating country and site according to local requirements.
Study population
Hospital-admitted patients included in the ISARIC database between 17 January and 31 December 2020; this analysis was limited to those with laboratory-confirmed SARS-CoV-2 infection detected by reverse-transcriptase (RT)-PCR in a respiratory sample analysed according to the sites’ local diagnostic methods and protocols and classified as severe COVID-19. We used a modified WHO severity criterion [5] to categorise severe COVID-19 using the following criteria: patients treated with invasive or noninvasive mechanical ventilation, those treated with HFNC, and/or patients treated with vasopressors or inotropes and/or patients treated within the ICU. Patients in whom >30% of the required clinical data variables were missing were excluded from the analysis.
Outcomes
The primary outcome of this analysis was 28-day fatality ratio (from hospital admission date). The secondary outcomes were 90-day fatality ratio and hospital length of stay (LOS).
Variables and measurement
Variables used in this analysis were age, sex, ethnicity (i.e. White, Black, Latino, Asian, Arab, other), symptoms, comorbidities, vital signs on admission, systemic complications, date of hospital admission, date of ICU admission, date of death, the requirement of advanced ventilatory support, treatment with vasopressors or inotropes, country of recruitment and its income classification according to the World Bank (https://data.worldbank.org/country). All the variables are listed in the study protocol and the supplementary material. To study fatality ratios, only patients with a reported date of death were classified as dead. Patients that were still admitted at the point of data extraction were not included in the denominator to calculate the fatality ratio or other clinical outcomes. Only patients with a reported hospital discharge were included in calculating the hospital LOS. All study variables were pre-defined in the ISARIC study protocol and case report form completion guide available online (https://isaric.tghn.org/research/covid-19-clinical-research-resources/covid-19-crf). The number of COVID-19 cases per million were obtained from the website Our World in Data [13].
Study definitions
The complete definitions of all variables were pre-determined in the study protocol and are available in the supplementary material. However, some definitions are provided here.
ICU admission: patients admited to an intensive care, intensive therapy, intermediate care or high dependency unit. This variable was collected independent of treatments received and was reported by each centre.
High-flow nasal cannula: respiratory support continuously applied through large-bore nasal prongs using a gas flow heated and humidified at initial flow greater of 20 L·min−1 (or up to 80 L·min−1) and fraction of inspiratory oxygen of up to 1.0.
Statistical methods
Data were converted to Study Data Tabulation Model standards (version 1.7; Clinical Data Interchange Standards Consortium, Austin, TX, USA) to integrate data collected on locally hosted databases with data collected on the ISARIC database. A bivariate analysis was initially carried out to compare the quantitative variables according to their distribution by other factors. If data were normally distributed, the t-test was applied for independent samples; if the variable data were not normally distributed, the Mann–Whitney U-test was used. Categorical variables were compared by a Chi-squared test. Variables were analysed by age, sex, date of hospital admission and ICU admission. A logistic generalised additive model (GAM) was fitted to assess the association of being admitted to the ICU with 28-day fatality ratio, adjusting for demographics (i.e. sex and number of comorbidities); age, treated as a nonlinear continuous measure using a cubic spline; physiological variables on admission (i.e. heart rate, respiratory rate and systolic and diastolic blood pressure); advanced ventilatory support (i.e. HFNC, noninvasive mechanical ventilation or invasive mechanical ventilation); treatment with vasopressors or inotropes; the development of acute respiratory distress syndrome (ARDS) during hospital stay; month of admission; country income classification; and new cases per million people per country at the moment of hospital admission.
To further assess the nonlinear associations of age, calendar time and per-capita number of COVID-19 cases within a country with fatality ratio, a logistic GAM was fitted using 28-day fatality ratio as a dichotomous outcome. Further nonlinear terms for age, comorbidities, calendar day and COVID-19 cases per million in the affected country were modelled as cubic splines. A sensitivity analysis was constructed using the above GAM and excluding patients enrolled in the United Kingdom (UK) (the majority of paints included in the analysis were registered in this country) to control for the centre effect. A further sensitivity analysis was constructed by excluding all patients identified as admitted to ICU without any other advanced ventilator support or vasopressor. All data processing and statistical analysis were performed using Python version 4.0 with the following data packages: Pandas version 1.2.4, Tidyverse version 1.3.0, Bioconductor version 3.12. In addition, we used R version 4.0.4 and SPSS 27 for Mac.
Results
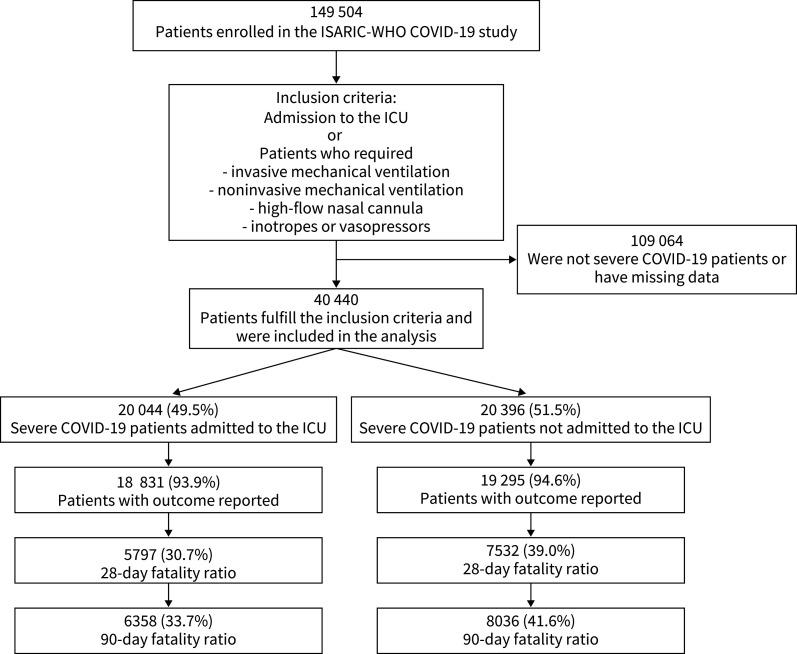
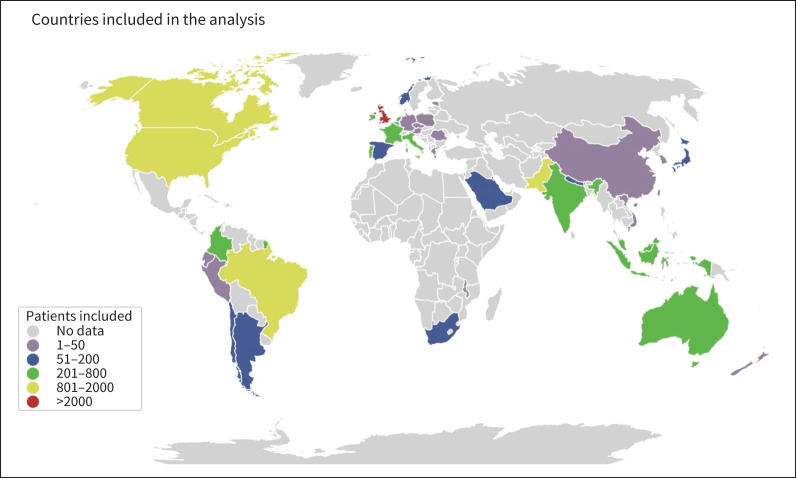
149 504 patients with RT-PCR-confirmed SARS-CoV-2 infection were screened for the study. After applying the enrolment criteria, 40 440 severe COVID-19 patients fulfilled the inclusion criteria and were included in the analysis (figure 1). Patients were enrolled in six continents and 43 countries. The majority of patients were enrolled in high-income countries (88.9%, 35 956 out of 40 440); however, 4472 patients were enrolled in upper middle-income and lower middle-income countries (figure 2, table 1). Importantly, 85.2% of patients were enrolled in Europe (figure 2, table 1).
FIGURE 1.
Study flow chart. ISARIC: International Severe Acute Respiratory and Emerging Infection Consortium; WHO: World Health Organization; COVID-19: coronavirus disease 2019; ICU: intensive care unit.
FIGURE 2.
Patients with severe coronavirus disease 2019 were enrolled in six continents. The colour scale shows the number of patients included in each country. Grey shading represents countries with no patients included in this analysis.
TABLE 1.
Baseline characteristics of patients with confirmed severe acute respiratory syndrome coronavirus 2 infection who developed severe coronavirus disease 2019 stratified by patients admitted to the intensive care unit (ICU)
| All | Patients admitted to the ICU | p-value | ||
| Yes | No | |||
| Patients, n | 40 440 | 20 044 | 20 396 | |
| Demographics | ||||
| Age, median (IQR) | 67 (55–78) | 61 (50–70) | 75 (62–84) | <0.0001 |
| Female, n (%) | 14 939 (36.9) | 6473 (32.2) | 8466 (41.5) | <0.0001 |
| Chronic comorbidities, n (%) | ||||
| Number of comorbidities, median (IQR) | 2.0 (0.0–3.0) | 1.0 (0.0–2.0) | 2.0 (0.0–4.0) | <0.0001 |
| Chronic arterial hypertension | 11 910 (29.5) | 5712 (28.5) | 6198 (30.4) | <0.0001 |
| Chronic cardiac disease | 8161 (20.2) | 2493 (12.4) | 5668 (27.8) | <0.0001 |
| Chronic cardiac arrhythmia | 38 (0.1) | 34 (0.2) | 4 (0.0) | <0.0001 |
| Chronic pulmonary disease | 4848 (12.0) | 1423 (7.1) | 3425 (16.8) | <0.0001 |
| Asthma | 3920 (9.7) | 1857 (9.3) | 2063 (10.1) | <0.0001 |
| Other chronic respiratory disease | 873 (2.2) | 148 (0.7) | 725 (3.6) | <0.0001 |
| Chronic neurological disorder | 2898 (7.2) | 798 (4.0) | 2100 (10.3) | <0.0001 |
| Chronic rheumatic disorder | 2736 (6.8) | 794 (4.0) | 1942 (9.5) | <0.0001 |
| Chronic kidney disease | 4151 (10.3) | 1252 (6.2) | 2899 (14.2) | <0.0001 |
| Mild liver disease | 413 (1.0) | 187 (0.9) | 226 (1.1) | 0.08 |
| Moderate or severe liver disease | 454 (1.1) | 163 (0.8) | 291 (1.4) | <0.0001 |
| Diabetes mellitus | 9141 (22.6) | 4343 (21.7) | 4798 (23.5) | <0.0001 |
| Chronic haematological disorder | 1069 (2.6) | 354 (1.8) | 715 (3.5) | <0.0001 |
| Chronic immunosuppressive disorders | 44 (0.1) | 44 (0.2) | 0 (0.0) | <0.0001 |
| Chronic immunosuppressive medication | 537 (1.3) | 194 (1.0) | 343 (1.7) | <0.0001 |
| Cancer | 680 (1.7) | 197 (1.0) | 483 (2.4) | <0.0001 |
| Malignant neoplasm | 2453 (6.1) | 797 (4.0) | 1656 (8.1) | <0.0001 |
| Solid organ transplant recipient | 192 (0.5) | 76 (0.4) | 116 (0.6) | 0.006 |
| AIDS/HIV | 178 (0.4) | 123 (0.6) | 55 (0.3) | <0.0001 |
| Asplenia | 46 (0.1) | 45 (0.2) | 1 (0.0) | <0.0001 |
| Dementia | 2774 (6.9) | 188 (0.9) | 2586 (12.7) | <0.0001 |
| Obesity | 4623 (11.4) | 2802 (14.0) | 1821 (8.9) | <0.0001 |
| Malnutrition | 628 (1.6) | 210 (1.0) | 418 (2.0) | <0.0001 |
| Symptoms on admission, n (%) | ||||
| Fever | 21 115 (52.2) | 10 578 (52.8) | 10 537 (51.7) | 0.02 |
| Abdominal pain | 2386 (5.9) | 1143 (5.7) | 1243 (6.1) | 0.09 |
| Bleeding (haemorrhage) | 463 (1.1) | 173 (0.9) | 290 (1.4) | <0.0001 |
| Fatigue/malaise | 12 391 (30.6) | 6317 (31.5) | 6074 (29.8) | <0.0001 |
| Shortness of breath | 23 955 (59.2) | 11 924 (59.5) | 12 031 (59.0) | 0.30 |
| Sore throat | 2464 (6.1) | 1619 (8.1) | 845 (4.1) | <0.0001 |
| Dry cough | 21 065 (52.1) | 10 230 (51.0) | 10 835 (53.1) | <0.0001 |
| Cough – productive | 6693 (16.6) | 3132 (15.6) | 3561 (17.5) | <0.0001 |
| Cough – with haemoptysis | 804 (2.0) | 444 (2.2) | 360 (1.8) | 0.0001 |
| Wheezing | 2294 (5.7) | 850 (4.2) | 1444 (7.1) | <0.0001 |
| Seizures | 352 (0.9) | 148 (0.7) | 204 (1.0) | 0.005 |
| Altered consciousness/confusion | 5964 (14.7) | 1759 (8.8) | 4205 (20.6) | <0.0001 |
| Disturbance or loss of smell (anosmia) | 1047 (2.6) | 614 (3.1) | 433 (2.1) | <0.0001 |
| Disturbance or loss of taste (ageusia) | 1275 (3.2) | 631 (3.1) | 644 (3.2) | 0.95 |
| Severe dehydration | 1614 (4.0) | 538 (2.7) | 1076 (5.3) | <0.0001 |
| Vomiting/nausea | 5006 (12.4) | 2469 (12.3) | 2537 (12.4) | 0.71 |
| Diarrhoea | 5335 (13.2) | 2872 (14.3) | 2463 (12.1) | <0.0001 |
| Muscle aches (myalgia) | 5562 (13.8) | 3397 (16.9) | 2165 (10.6) | <0.0001 |
| Chest pain | 3823 (9.5) | 1951 (9.7) | 1872 (9.2) | 0.05 |
| Headache | 2918 (7.2) | 1747 (8.7) | 1171 (5.7) | <0.0001 |
| Joint pain (arthralgia) | 1495 (3.7) | 739 (3.7) | 756 (3.7) | 0.91 |
| Skin ulcers | 419 (1.0) | 77 (0.4) | 342 (1.7) | <0.0001 |
| Lower chest wall indrawing | 528 (1.3) | 334 (1.7) | 194 (1.0) | <0.0001 |
| Skin rash | 304 (0.8) | 140 (0.7) | 164 (0.8) | 0.24 |
| Conjunctivitis | 110 (0.3) | 72 (0.4) | 38 (0.2) | 0.0001 |
| Runny nose (rhinorrhoea) | 927 (2.3) | 665 (3.3) | 262 (1.3) | <0.0001 |
| Ear pain | 95 (0.2) | 49 (0.2) | 46 (0.2) | 0.69 |
| Lymphadenopathy | 170 (0.4) | 59 (0.3) | 111 (0.5) | <0.0001 |
| Inability to walk | 279 (0.7) | 226 (1.1) | 53 (0.3) | <0.0001 |
| Anorexia | 349 (0.9) | 285 (1.4) | 64 (0.3) | <0.0001 |
| Asymptomatic | 272 (0.7) | 93 (0.5) | 179 (0.9) | <0.0001 |
| Physiological parameters on admission, median (IQR) | ||||
| Temperature, °C | 37.3 (36.7–38.2) | 37.4 (36.7–38.3) | 37.3 (36.6–38.1) | <0.0001 |
| Heart rate, beats·min−1 | 93 (8–108) | 96 (84–110) | 91 (79–105) | <0.0001 |
| Respiratory rate, breaths·min−1 | 24 (20–28) | 24 (20–30) | 22 (19–28) | <0.0001 |
| Systolic blood pressure, mmHg | 130 (114–145) | 130 (115–144) | 130 (114–146) | 0.02 |
| Diastolic blood pressure, mmHg | 74 (65–83) | 75 (65–83) | 74 (64–84) | 0.0001 |
| Continent of admission, n (%) | ||||
| Europe | 34 456 (85.2) | 12 427 (61.9) | 20 029 (98.2) | <0.0001 |
| Asia | 3292 (8.1) | 3140 (15.6) | 152 (0.7) | <0.0001 |
| South America | 1408 (3.5) | 1308 (6.5) | 100 (0.5) | <0.0001 |
| North America | 2614 (6.4) | 2505 (12.5) | 109 (0.5) | <0.0001 |
| Africa | 164 (0.4) | 159 (0.6) | 5 (0.0) | <0.0001 |
| Oceania | 506 (1.3) | 505 (2.5) | 1 (0.0) | <0.0001 |
| Regional income stratification, n (%) | ||||
| High-income country | 35 956 (88.9) | 15 810 (78.8) | 20 146 (98.7) | <0.0001 |
| Upper middle-income country | 1976 (4.8) | 1821 (9.1) | 155 (0.8) | <0.0001 |
| Lower middle-income country | 2496 (6.2) | 2401 (11.9) | 96 (0.5) | <0.0001 |
| Clinical outcomes | n=38 126 | n=18 831 | n=19 295 | |
| Hospital LOS, median (IQR) | 10 (5–19) | 12 (6–23) | 8 (4–15) | <0.0001 |
| 28-day fatality ratio, n (%) | 13 329 (34.9) | 5797 (30.7) | 7532 (39.0) | <0.0001 |
| 90-day fatality ratio, n (%) | 14 394 (37.7) | 6358 (33.7) | 8036 (41.6) | <0.0001 |
IQR: interquartile range; LOS: length of stay.
Demographic and clinical characteristics
Most of the patients were male (62.9%, 25 459 out of 40 440), with a median (interquartile range (IQR)) age of 67 (55–78) years. The race of patients was most frequently recorded as White (53.0%, 21 460 out of 40 440), followed by Asian (7.6%, 3080 out of 40 440), Black (4.0%, 1631 out of 40 440) and Latino (1.0%, 425 out of 40 440). At least one comorbidity was reported in 63.2% (25 591 out of 40 440) of patients (table 1); the most frequently identified comorbidity was chronic arterial hypertension (29.5%, 11 910 out of 40 404), followed by chronic cardiac disease (20.2%, 8161 out of 40 440), chronic pulmonary diseases (12.0%, 4848 out of 40 440), obesity (11.4%, 4623 out of 40 440) and chronic kidney disease (10.3%, 4151 out of 40 440). ICU patients were younger (61 (50–70) years versus 75 (62–84) years, p<0.0001), more frequently male (67.7% (13 571 out of 20 044) versus 58.4% (11 930 out of 20 396), p<0.0001) and had fewer comorbidities (1 (0–2) versus 2 (0–4), p<0.0001) than non-ICU patients (table 1).
Regarding symptoms on the day of hospital admission, the most frequently reported were shortness of breath (59.2%, 23 955 out of 40 440), fever (52.2%, 21 115 out of 40 440), dry cough (52.1%, 21 065 out of 40 440) and fatigue/malaise (30.6%, 12 391 out of 40 440). Regarding physiological parameters on hospital admission, the median (IQR) temperature was 37.3°C (36.7–38.2°C); patients were tachycardic (93 (81–108) beats·min−1) and tachypnoeic (24 (20–28) breaths·min−1) on admission (table 1). Other differences were observed in the physiological variables between patients admitted and not admitted to the ICU (table 1).
In-hospital treatments and systemic complications
The most frequently administered treatments were systemic antibiotics (87.3%, 35 316 out of 40 440), systemic corticosteroids (28.3%, 11 435 out of 40 440) and antivirals (19.8%, 8025 out of 40 440). Among the whole cohort, 62.9% (25 433 out of 40 440) of patients were treated with HFNC, 38.4% (15 522 out of 40 440) with noninvasive mechanical ventilation and 40.7% (16 542 out of 40 440) were treated with inotropes or vasopressors (table 2, supplementary figures S1 and S2). Invasive mechanical ventilation was more frequently applied in ICU compared to non-ICU patients (59.6% (11 957 out of 20 044) versus 2.5% (505 out of 19 891), p<0.0001); in contrast, non-ICU patients were more frequently treated with HFNC (81.1% (16 542 out of 19 819) versus 44.4% (8891 out of 20 044), p<0.0001) (table 2). Moreover, patients admitted to the ICU were more frequently treated with prone positioning (36.9% (7390 out of 20 044) versus 2.1% (277 out of 20 396), p<0.0001), systemic corticosteroids (38.1% (7627 out of 20 044) versus 18.7% (3808 out of 20 396), p<0.0001) and haemodialysis (14.7% (2953 out of 20 044) versus 1.2% (238 out of 20 396), p<0.0001) than non-ICU patients (table 2).
TABLE 2.
Treatments stratified by patients admitted to the intensive care unit (ICU)
| All | Patients admitted to the ICU | p-value | ||
| Yes | No | |||
| Patients, n | 40 440 | 20 044 | 20 396 | |
| Invasive mechanical ventilation | 12 462 (30.8) | 11 957 (59.6) | 505 (2.5) | <0.0001 |
| Noninvasive mechanical ventilation | 15 522 (38.4) | 9127 (45.5) | 6395 (31.4) | <0.0001 |
| High-flow nasal cannula | 25 433 (62.9) | 8891 (44.4) | 16 542 (81.1) | <0.0001 |
| Inotropes or vasopressors | 16 542 (40.7) | 8375 (41.8) | 175 (0.9) | <0.0001 |
| Antibiotics | 35 316 (87.3) | 17 800 (88.8) | 17 516 (85.9) | <0.0001 |
| Prone positioning | 7825 (19.3) | 7390 (36.9) | 435 (2.1) | <0.0001 |
| Neuraminidase inhibitors | 231 (0.6) | 118 (0.6) | 113 (0.6) | 0.69 |
| Neuromuscular blocking agents | 3647 (9.0) | 3597 (17.9) | 50 (0.2) | <0.0001 |
| Dialysis/haemofiltration | 3191 (7.9) | 2953 (14.7) | 238 (1.2) | <0.0001 |
| Corticosteroids | 11 435 (28.3) | 7627 (38.1) | 3808 (18.7) | <0.0001 |
| Antivirals | 8025 (19.8) | 5925 (29.6) | 2100 (10.3) | <0.0001 |
| Tracheostomy | 2461 (6.1) | 2422 (12.1) | 39 (0.2) | <0.0001 |
| ECMO | 706 (1.7) | 706 (3.5) | 0 (0.0) | <0.0001 |
| ACE inhibitors | 4399 (10.9) | 1991 (9.9) | 2408 (11.8) | <0.0001 |
| Antifungal | 3200 (7.9) | 2384 (11.9) | 816 (4.0) | <0.0001 |
| Angiotensin II receptor blockers | 2865 (7.1) | 1674 (8.4) | 1191 (5.8) | <0.0001 |
| Therapeutic anticoagulants | 532 (1.3) | 510 (2.5) | 22 (0.1) | <0.0001 |
| Lopinavir/ritonavir | 651 (1.6) | 397 (2.0) | 254 (1.2) | <0.0001 |
| Nonsteroidal anti-inflammatory | 2317 (5.7) | 1191 (5.9) | 1126 (5.5) | 0.07 |
| Inhaled nitric oxide | 505 (1.2) | 491 (2.4) | 14 (0.1) | <0.0001 |
| Chloroquine/hydroxychloroquine | 946 (2.3) | 718 (3.6) | 228 (1.1) | <0.0001 |
| Convalescent plasma | 398 (1.0) | 248 (1.2) | 150 (0.7) | <0.0001 |
| Macrolides | 83 (0.2) | 66 (0.3) | 17 (0.1) | <0.0001 |
| Dexamethasone | 4174 (10.3) | 1992 (9.9) | 2182 (10.7) | 0.01 |
| Interferon-β | 131 (0.3) | 118 (0.6) | 13 (0.1) | <0.0001 |
| Oral steroids | 311 (0.8) | 275 (1.4) | 36 (0.2) | <0.0001 |
| Remdesivir | 2009 (5.0) | 1092 (5.4) | 917 (4.5) | <0.0001 |
| IL-6 inhibitor | 122 (0.3) | 90 (0.4) | 32 (0.2) | <0.0001 |
| Tocilizumab | 51 (0.1) | 42 (0.2) | 9 (0.0) | <0.0001 |
Data are presented as n (%), unless otherwise stated. ECMO: extracorporeal membrane oxygenation; ACE: angiotensin-converting enzyme; IL: interleukin.
Systemic complications were reported in the majority of patients (64.4%, 26 068 out of 40 440); 19.6% (7928 out of 28 182) of patients developed ARDS, being more frequent in patients admitted to the ICU (28.7% (5747 out of 20 044) versus 10.5% (2181 out of 20 396), p<0.0001). Moreover, acute kidney injury was documented more frequently in ICU-admitted patients (20.1% (4030 out of 20 044) versus 12.4% (2536 out of 20 396)). Bacterial pneumonia was more frequent in patients admitted to the ICU (12.9% (2591 out of 20 044) versus 10.3% (2104 out of 20 396), p<0.0001). Other systemic complications are reported in table 3.
TABLE 3.
Patients with severe coronavirus disease 2019 who developed complications stratified by patients admitted to the intensive care unit (ICU)
| All | Patients admitted to the ICU | p-value | ||
| Yes | No | |||
| Patients, n | 40 440 | 20 044 | 20 396 | |
| Neurological, n (%) | ||||
| Seizures | 393 (1.0) | 240 (1.2) | 153 (0.8) | <0.0001 |
| Stroke | 489 (1.2) | 281 (1.4) | 208 (1.0) | <0.0001 |
| Meningitis or encephalitis | 113 (0.3) | 93 (0.5) | 20 (0.1) | <0.0001 |
| Other neurological complications | 490 (1.2) | 228 (1.1) | 262 (1.3) | 0.19 |
| Cardiovascular, n (%) | ||||
| Congestive heart failure | 1183 (2.9) | 432 (2.2) | 751 (3.7) | <0.0001 |
| Endocarditis, myocarditis, pericarditis | 223 (0.6) | 191 (1.0) | 32 (0.2) | <0.0001 |
| Cardiac arrhythmia | 2992 (7.4) | 1995 (10.0) | 997 (4.9) | <0.0001 |
| Cardiac arrest | 1457 (3.6) | 1012 (5.0) | 445 (2.2) | <0.0001 |
| Cardiac ischaemia | 556 (1.4) | 303 (1.5) | 253 (1.2) | 0.021 |
| Cardiomyopathy | 195 (0.5) | 138 (0.7) | 57 (0.3) | <0.0001 |
| Myocardial infarction | 34 (0.1) | 31 (0.2) | 3 (0.0) | <0.0001 |
| Pulmonary, n (%) | ||||
| Bacterial pneumonia | 4695 (11.6) | 2591 (12.9) | 2104 (10.3) | <0.0001 |
| Acute respiratory distress syndrome | 7928 (19.6) | 5747 (28.7) | 2181 (10.7) | <0.0001 |
| Pneumothorax | 555 (1.4) | 447 (2.2) | 108 (0.5) | <0.0001 |
| Pleural effusion | 2198 (5.4) | 1093 (5.5) | 1105 (5.4) | 0.89 |
| Pulmonary embolism | 272 (0.7) | 237 (1.2) | 35 (0.2) | <0.0001 |
| Cryptogenic organising pneumonia | 122 (0.3) | 95 (0.5) | 27 (0.1) | <0.0001 |
| Gastrointestinal, n (%) | ||||
| Pancreatitis | 138 (0.3) | 103 (0.5) | 35 (0.2) | <0.0001 |
| Liver dysfunction | 2385 (5.9) | 1703 (8.5) | 682 (3.3) | <0.0001 |
| Gastrointestinal haemorrhage | 393 (1.0) | 221 (1.1) | 172 (0.8) | 0.008 |
| Renal, n (%) | ||||
| Acute kidney injury | 6566 (16.2) | 4030 (20.1) | 2536 (12.4) | <0.0001 |
| Metabolic, n (%) | ||||
| Hyperglycaemia | 3232 (8.0) | 2265 (11.3) | 967 (4.7) | <0.0001 |
| Hypoglycaemia | 757 (1.9) | 355 (1.8) | 402 (2.0) | 0.14 |
| Haematological, n (%) | ||||
| Anaemia | 5057 (12.5) | 3203 (16.0) | 1854 (9.1) | <0.0001 |
| Disseminated intravascular coagulation | 1296 (3.2) | 844 (4.2) | 452 (2.2) | <0.0001 |
| Bleeding | 84 (0.2) | 80 (0.4) | 4 (0.0) | <0.0001 |
| Others, n (%) | ||||
| Other complication | 6321 (15.6) | 2883 (14.4) | 3438 (16.9) | <0.0001 |
| Bacteraemia | 1848 (4.6) | 1366 (6.8) | 482 (2.4) | <0.0001 |
| Rhabdomyolysis or myositis | 246 (0.6) | 185 (0.9) | 61 (0.3) | <0.0001 |
Clinical outcomes
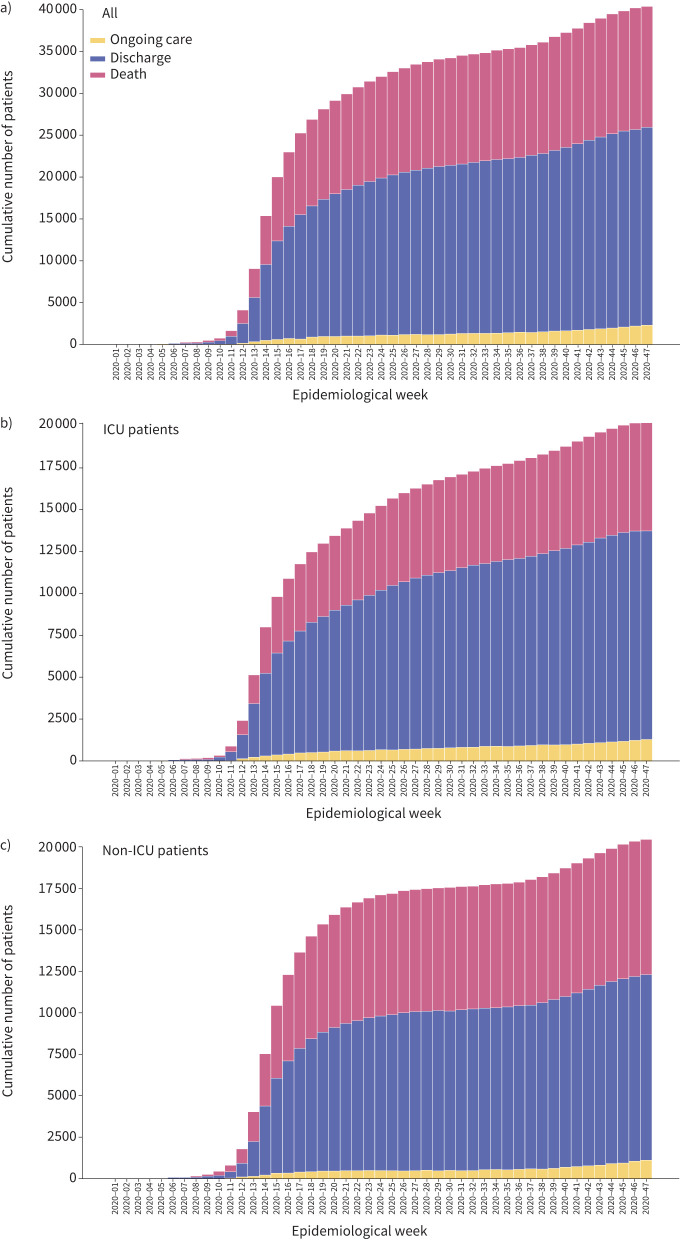
The overall 28-day fatality ratio in our cohort was 34.9% (13 329 out of 38 126), and 37.7% (14 394 out of 38 126) for 90-day fatality ratio (figure 1, table 1, supplementary figure S5). The 28-day fatality ratio was 30.7% (5797 out of 18 831) in patients admitted to the ICU and 39.0% (7532 out of 19 295) in patients cared for exclusively outside the ICU. The 90-day fatality ratio was 33.7% (6358 out of 18 831) in those admitted to the ICU and 41.6% (8036 out of 19 295) in those cared for outside the ICU (figure 3). The median (IQR) overall hospital LOS was 10 (5–19) days, which was longer in ICU patients (12 (6–23) days versus 8 (4–15) days, p<0.0001) when compared to non-ICU patients (table 1). Finally, the hospital LOS in survivors was 11 (5–21) days, and it was longer in ICU-admitted patients (13 (6–27) days versus 9 (5–17) days, p<0.0001).
FIGURE 3.
Patients admitted to the intensive care unit (ICU) have lower cumulative deaths over time. a) The cumulative number of cases included in the study, stratified by b) patients admitted to the ICU and c) patients who were not admitted to the ICU. Proportions of patients still hospitalised at the moment of data extraction, discharged alive and reported dead are shown.
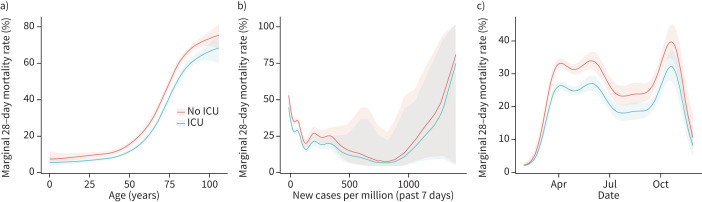
A biphasic distribution of the number of cases and deaths was identified, with peaks between April and May 2020 and between October and November 2020 (supplementary figures S3 and S4). Patients aged >65 years were frequently diagnosed with severe COVID-19 and had a higher fatality ratio (supplementary figures S3 and S4). A clear relationship between age and the probability of death was observed (figure 4 and supplementary figure S5). Over the study period, fatality ratio in patients with severe COVID-19 decreased over time, being higher during April and lower by the end of August (figure 4 and supplementary figure S5).
FIGURE 4.
Estimation of the non-linear effect on 28-day fatality ratio using a generalised additive model in patients with severe coronavirus disease 2019 (COVID-19) stratified by intensive care unit (ICU) admission. a) Age is shown to be an essential factor for higher fatality ratio in patients with severe COVID-19; however, patients admitted to the ICU have a lower fatality ratio independent of age. Even after adjusting by b) the number of new cases per million at the moment of hospital admission and c) the interaction over time, the marginal 28-day fatality ratio was lower in patients admitted to the ICU.
Association of ICU admission with 28-day and 90-day fatality ratios
After adjusting for confounding variables (i.e. age, number of comorbidities, sex, presence of ARDS, treatments (inotropes, vasopressors, HFNC, invasive and noninvasive mechanical ventilation), country's income classification, number of new cases per day in the relevant country and physiological variables (heart rate, respiratory rate, systolic or diastolic blood pressure, and temperature)) on hospital admission, patients admitted to the ICU had a lower 28-day fatality ratio (OR 0.70, 95% CI 0.65–0.75, p<0.0001) (table 4) and 90-day fatality ratio (OR 0.69, 95% CI 0.64–0.74, p<0.0001) (supplementary table S1 and supplementary figure S5). In addition, patients enrolled later in the pandemic (i.e. after the first peak in May–June 2020) were less likely to die, regardless of ICU admission (OR 0.98, 95% CI 0.96–0.99, p<0.0001). Previously documented risk factors for death, including age, sex, prior comorbidities and ARDS were confirmed in our study (table 4).
TABLE 4.
Generalised additive model fitted to assess the association of being admitted to the intensive care unit (ICU) with 28-day fatality ratio
| OR (95% CI) | Estimated marginal mean (range) | p-value | |
| ICU admission | 0.70 (0.65–0.75) | <0.0001 | |
| High-flow nasal cannula | 1.05 (1.00–1.11) | 0.052 | |
| Noninvasive respiratory support | 1.37 (1.31–1.44) | <0.0001 | |
| Invasive mechanical ventilation | 1.97 (1.81–2.14) | <0.0001 | |
| Vasopressors or inotropes | 1.56 (1.44–1.70) | <0.0001 | |
| Number of comorbidities | 1.07 (1.06–1.09) | <0.0001 | |
| Temperature on hospital admission | 0.92 (0.90–0.94) | <0.0001 | |
| Heart rate on hospital admission | 1.01 (1.00–1.01) | <0.0001 | |
| Respiratory rate on hospital admission | 1.03 (1.02–1.03) | <0.0001 | |
| Systolic blood pressure on hospital admission | 1.00 (1.00–1.01) | <0.0001 | |
| Diastolic blood pressure on hospital admission | 0.99 (0.99–0.99) | <0.0001 | |
| Acute respiratory distress syndrome | 1.47 (1.39–1.56) | <0.0001 | |
| Male | 1.25 (1.19–1.31) | <0.0001 | |
| Month of hospital admission | 0.98 (0.96–0.99) | <0.0001 | |
| New cases per million | 1.00 (1.00–1.00) | <0.0001 | |
| Age (years) | |||
| 20 | 7.1% (5.8–8.7%) | ||
| 40 | 9.1% (8.3–9.9%) | ||
| 60 | 19.8% (19.0–20.6%) | ||
| 80 | 51.8% (50.7–52.9%) |
To test these results, we performed a sensitivity analysis with ethnicity (supplementary table S3), patient enrolment in the UK, sex and age revealed a similar protective effect of ICU admission on 28-day and 90-day fatality ratios (table 4, figure 4, supplementary figure S5). Then, we performed a supplementary sensitivity analysis to control for centre effect by removing patients enrolled in the UK, finding lower fatality ratios in patients admitted to the ICU (supplementary table S2). Finally, we removed patients admitted to ICU who did not need advanced ventilatory support (n=2716; supplementary figure S1), confirming that patients admitted to the ICU had lower likelihood of dying.
Discussion
This analysis describes the clinical characteristics, symptoms and outcomes from the largest prospective multinational cohort of hospitalised patients with severe COVID-19. Worse clinical outcomes were seen in older, male, obese patients with comorbidities and those who developed ARDS. Fatality ratios in patients hospitalised with severe COVID-19 changed over time, being higher during the initial weeks of the pandemic's first wave. Finally, we identified that severe COVID-19 patients admitted to ICU had lower 28-day and 90-day fatality ratios, independent of age, disease severity, number of comorbidities, country's income classification, healthcare system saturation (i.e. number of new cases per day) and treatments received when compared with patients that were cared for outside ICU.
Previous studies have found that ∼30% of patients infected with SARS-CoV-2 can develop severe disease requiring admission to an ICU or advanced ventilatory support, such as invasive or noninvasive mechanical ventilation or HFNC [10, 14–17]. Our study found that 32% of patients who required hospital admission developed severe COVID-19, which is in alignment with prior published data. Regarding the clinical characteristics of patients with severe COVID-19, Grasselli et al. [11] reported that 82% of patients hospitalised in Italian hospitals were male, with a median age of 63 years, and frequently had several comorbidities. Xie et al. [10] reported that severely ill patients in China had a median age of 63 years; 65% were male and had a past medical history of cardiovascular diseases, specifically hypertension. Moreover, several studies have also confirmed the associations between age, sex and comorbidities and severe COVID-19 disease [2, 3, 18, 19]. Our findings are in concordance with our results, where the median admission age was 67.5 years, and the majority of patients were male. We also found that most patients who developed severe COVID-19 had at least one comorbidity, with hypertension, chronic cardiac diseases and chronic pulmonary diseases being most frequent.
During the pandemic, several pharmacological and nonpharmacological interventions have been used to treat patients with COVID-19 [5, 6, 20]. The primary approach was to identify effective treatments by repurposing antiviral agents, immunomodulatory drugs and medications with theoretical antiviral properties. However, this approach led to excessive use of interventions without evidence-based support, and the data supporting these interventions are controversial [12, 21]. To date, the only medications that have been consistently demonstrated to reduce fatality ratios in COVID-19 patients requiring oxygen supplementation are corticosteroids, specifically dexamethasone [22, 23]. In our study, patients received several medications and non-pharmacological interventions. Notably, we found that most patients were treated with systemic antibiotics and many other interventions, as reported previously in other studies [24]. This high antibiotic usage in patients with severe COVID-19 is concerning, because bacterial co-infection was not frequent in our cohort. Moreover, it is essential to highlight that only 28.3% of patients were treated with systemic corticosteroids, being more frequently used in patients admitted to the ICU. This might be explained by the fact that the RECOVERY trial results were published 6 months after the beginning of the pandemic [23, 25], and pre-pandemic data did not support the use of corticosteroids by many international guidelines in patients with severe viral pneumonia [26, 27].
The COVID-19 pandemic has brought unprecedented challenges to healthcare systems around the world [28]. In many countries, ICU capacity was overwhelmed, requiring severely ill patients to be treated in non-ICU settings [8, 9]. Other studies have evaluated the utility of using noninvasive respiratory support in the general wards, showing that this treatment can be safely delivered outside of the ICU [8], although outcomes were not compared with similar patients treated in an ICU. We found that treating patients in the ICU was associated with a lower fatality ratio after adjusting for a number of possible confounders, including the severity of illness. There is an important selection bias of patients admitted to ICU because clinicians tend to admit patients with a better survival probability. However, our results build on previous work that personnel trained in ICU care and the presence of a higher nurse-to-patient ratio may significantly impact the fatality ratio in severely ill patients more than access to specific organ support interventions [29].
An interpretation regarding why the fatality ratio is different among patients admitted or not to the ICU is required. These data are novel and have not been reported previously in patients with severe COVID-19. However, in Hong Kong, during the SARS 2003 pandemic, it was reported that expanding ICU settings was associated with higher fatality ratios [30]. Intensive care is often defined by the nurse-to-patient ratio and monitoring by trained staff, which may be a determinant of our findings [31]. Notably, we do not have information regarding personnel training, the monitoring systems used or the staff ratios caring for these patients.
Our study has strengths and limitations that are important to acknowledge. First, the ISARIC COVID-19 dataset is composed mainly of patients enrolled in high-income countries. However, this is a large prospectively collected cohort of patients enrolled in >40 countries worldwide, strengthening the generalisability of these results. We performed a sensitivity analysis which showed no difference across income groupings. Second, the decision not to admit a patient to the ICU may be based on several factors, including treatment restriction orders and the clinician's estimation of survival; however, this information is not easily collected and was not available in our dataset. Not having these data limits the conclusions that may be drawn about the impact of ICU admission on clinical outcomes as it is a residual selection bias. Third, the definitions of what constituted an ICU varies per country and centre. We assumed that similar treatment and care were offered to all COVID-19 patients upon ICU admission. Notably, the identified protective effect of ICU admission was consistent in the entire cohort, even after sensitivity analyses. Fourth, several variants have emerged during the pandemic in different parts of the world. These variants have different disease severities, and vaccines might have different protective effects, affecting overall mortality. However, we did not have information regarding the virus identified, and thus, we did not control for this, which is an important limitation. However, we did control for admission date, which might indirectly control for these variables that appeared worldwide over the pandemic. Fifth, oxygen saturation and blood arterial gases are frequently used to determine severity and guide treatment in patients with COVID-19. However, in our study, we do not have these data in all patients, which is a limitation we need to recognise. Importantly, we have vital signs and systemic complications that allowed us to assess disease severity. Finally, as our study included patients from several countries and centres with high caseloads and resourced limitations, the analysis was limited by missing data, possibly biasing the ultimate results.
Conclusions
More than 30% of hospitalised SARS-CoV-2-infected patients develop severe COVID-19, with high fatality ratios. These patients frequently require advanced supportive treatments, which has imposed an unprecedented burden on healthcare systems worldwide. Providing high-quality care to severely ill patients is a complex endeavour that requires trained personnel, a designated setting, monitoring equipment and specialised management. The results presented in this study warrant caution about treating severely ill patients outside of an ICU and encourage hospitals to find strategies for severely ill patients to be treated in an ICU by personnel trained for the role.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00552-2021.supplement (381.4KB, pdf)
Case report form 00552-2021.CRF (2.5MB, pdf)
Protocol 00552-2021.protocol (773KB, pdf)
Acknowledgements
This work uses data or material provided by patients and collected by the National Health Service as part of their care and support. The data/material used for this research were obtained from ISARIC4C. The COVID-19 Clinical Information Network data were collated by ISARIC4C Investigators. This work was possible due to the dedication and hard work of the Norwegian SARS-CoV-2 study team; the Groote Schuur Hospital COVID Intensive Care Unit Team, supported by the Groote Schuur nursing and University of Cape Town registrar bodies coordinated by the Division of Critical Care at the University of Cape Town; and supported by the COVID clinical management team, AIIMS, Rishikesh, India.
ISARIC Clinical Characterisation Group: Conceptualisation (this analysis): Reyes, Luis Felipe; Murthy, Srinivas; Garcia-Gallo, Esteban; Irvine, Mike; Merson, Laura; Martin-Loeches, Ignacio; Rello, Jordi; Taccone, Fabio S.; Webb, Steve A.; Fowler, Robert A.; Docherty, Annemarie B; Donnelly, Christl A.; Kartsonaki, Christiana; Aragao, Irene; Barrett, Peter W.; Beane, Abigail; Broadley, Tessa; Cheng, Matthew Pellan; Christian, Michael D.; Cidade, Jose Pedro; Citarella, Barbara Wanjiru; Fernandes, Susana; Haniffa, Rashan; Harrison, Ewen M.; Ho, Antonia Ying Wai; Joseph, Mark; Khan, Irfan; Kho, Michelle E; Kildal, Anders Benjamin; Kutsogiannis, Demetrios; Lamontagne, François; Lee, Todd C.; Li Bassi, Gianluigi; Lopez Revilla, Jose Wagner; Marquis, Catherine; Millar, Jonathan; Neto, Raul; Nichol, Alistair; Olliaro, Piero L.; Parke, Rachael; Pereira, Rui; Poli, Sergio; Povoa, Pedro; Ramanathan, Kollengode; Rello, Jordi; Rewa, Oleksa; Riera, Jordi; Rojek, Amanda; Shrapnel, Sally; Silva, Maria Joao; Trapani, Tony; Udy, Andrew; Uyeki, Timothy; Wils, Evert-Jan. Methodology: Reyes, Luis Felipe; Garcia-Gallo, Esteban; Murthy, Srinivas; Irvine, Mike; Merson, Laura. Software and formal analysis: Reyes, Luis Felipe; Garcia-Gallo, Esteban; Irvine, Mike. Data curation: Citarella, Barbara Wanjiru; Kelly, Sadie; Kennon, Kalynn; Lee, James; Merson, Laura; Plotkin, Daniel; Smith, Sue; Strudwick, Samantha. Administration: Citarella, Barbara Wanjiru. Writing – original draft: Reyes, Luis Felipe; Garcia-Gallo, Esteban; Murthy, Srinivas; Irvine, Mike; Merson, Laura. Visualisation: Reyes, Luis Felipe; Garcia-Gallo, Esteban; Irvine, Mike. Writing – review and editing: all authors. Data contributors, including investigation, supervision, resources, project administration and funding acquisition: Abdukahil, Sheryl Ann; Abe, Ryuzo; Abel, Laurent; Absil, Lara; Acharya, Subhash; Acker, Andrew; Adachi, Shingo; Adam, Elisabeth; Adrião, Diana; Ageel, Saleh Al; Ain, Quratul; Ainscough, Kate; Ait Hssain, Ali; Ait Tamlihat, Younes; Akimoto, Takako; Akmal, Ernita; Al Qasim, Eman; Alalqam, Razi; Alam, Tanvir; Al-dabbous, Tala; Alegre, Cynthia; Alex, Beatrice; Alexandre, Kévin; Al-Fares, Abdulrahman; Alfoudri, Huda; Ali Shah, Naseem; Alidjnou, Kazali Enagnon; Aliudin, Jeffrey; Allavena, Clotilde; Alves, João Melo; Alves, João; Alves, Rita; Amaral, Maria; Ampaw, Phoebe; Andini, Roberto; Andrejak, Claire; Angheben, Andrea; Angoulvant, François; Ansart, Séverine; Antonelli, Massimo; Antunes de Brito, Carlos Alexandre; Apriyana, Ardiyan; Arabi, Yaseen; Aragao, Irene; Arancibia, Francisco; Arcadipane, Antonio; Archambault, Patrick; Arenz, Lukas; Arlet, Jean-Benoît; Arnold-Day, Christel; Arora, Lovkesh; Arora, Rakesh; Artaud-Macari, Elise; Aryal, Diptesh; Asensio, Angel; Assie, Jean Baptiste; Atique, Anika; Attanyake, A.M. Udara Lakshan; Auchabie, Johann; Aumaitre, Hugues; Azemar, Laurène; Azoulay, Cecile; Bach, Benjamin; Bachelet, Delphine; Badr, Claudine; Baillie, J. Kenneth; Bak, Erica; Bakakos, Agamemnon; Bal, Andriy; Bani-Sadr, Firouzé; Barrasa, Helena; Barbalho, Renata; Barclay, Wendy S.; Barnikel, Michaela; Barrigoto, Cleide; Bartoli, Marie; Bartone, Cheryl; Baruch, Joaquín; Basmaci, Romain; Bauer, Jules; Bautista, Diego; Beane, Abigail; Behilill, Sylvie; Beljantsev, Aleksandr; Bellemare, David; Beltrame, Anna; Beluze, Marine; Benech, Nicolas; Benkerrou, Dehbia; Bennett, Suzanne; Bento, Luís; Berdal, Jan-Erik; Bergeaud, Delphine; Bertolino, Lorenzo; Bessis, Simon; Bevilcaqua, Sybille; Bhatt, Amar; Bhavsar, Krishna; Bianchi, Isabella; Bianco, Claudia; Bikram Singh, Moirangthem; Bin Humaid, Felwa; Biston, Patrick; Bitker, Laurent; Blanco-Schweizer, Pablo; Blandino Ortiz, Aaron; Blier, Catherine; Blot, Mathieu; Boccia, Filomena; Bodenes, Laetitia; Bogaert, Debby; Bolze, Pierre-Adrien; Bompart, François; Borges, Diogo; Borie, Raphaël; Bosse, Hans Martin; Botelho-Nevers, Elisabeth; Bouadma, Lila; Bouchaud, Olivier; Bouchez, Sabelline; Bouhmani, Dounia; Bouhour, Damien; Bouiller, Kévin; Bouillet, Laurence; Bouisse, Camile; Boureau, Anne-Sophie; Bouscambert, Maude; Bouziotis, Jason; Boxma, Bianca; Boyer-Besseyre, Marielle; Boylan, Maria; Bozza, Fernando Augusto; Brack, Matthew; Braga, Cynthia; Brandenburger, Timo; Brás Monteiro, Filipa; Brazzi, Luca; Breen, Dorothy; Breen, Patrick; Brickell, Kathy; Broadley, Tessa; Browne, Alex; Brozzi, Nicolas; Buchtele, Nina; Buesaquillo, Christian; Buisson, Marielle; Burhan, Erlina; Burrell, Aidan; Bustos, Ingrid G.; Cabie, André; Cabral, Susana; Caceres, Eder; Cadoz, Cyril; Calligy, Kate; Calvache, Jose Andres; Camões, João; Campana, Valentine; Campbell, Paul; Canepa, Cecilia; Cantero, Mireia; Caraux-Paz, Pauline; Cárcel, Sheila; Cardellino, Chiara; Cardoso, Filipa; Cardoso, Filipe; Cardoso, Nelson; Cardoso, Sofia; Carelli, Simone; Carlier, Nicolas; Carney, Gayle; Carpenter, Chloe; Carret, Marie-Christine; Carrier, François Martin; Carson, Gail; Casanova, Maire-Laure; Cascão, Mariana; Casimiro, José; Cassandra, Bailey; Castañeda, Silvia; Castanheira, Nidyanara; Castor-Alexandre, Guylaine; Castrillón, Henry; Castro, Ivo; Catarino, Ana; Catherine, François-Xavier; Cavalin, Roberta; Cavayas, Alexandros; Ceccato, Adrian; Cervantes-Gonzalez, Minerva; Chair, Anissa; Chakveatze, Catherine; Chan, Adrienne; Chand, Meera; Chantalat Auger, Christelle; Chapplain, Jean-Marc; Chaudary, Mobin; Chen, Anjellica; Chen, Yih-Sharng; Cheng, Matthew Pellan; Cheret, Antoine; Chiarabini, Thibault; Chica, Julian; Chirouze, Catherine; Chiumello, Davide; Cho, Hwa Jin; Cho, Sung Min; Cholley, Bernard; Chopin, Marie-Charlotte; Cidade, Jose Pedro; Citarella, Barbara Wanjiru; Ciullo, Anna; Clarke, Jennifer; Clohisey, Sara; Coca, Necsoi; Codan, Cassidy; Cody, Caitriona; Coelho, Alexandra; Colin, Gwenhaël; Collins, Michael; Colombo, Sebastiano Maria; Combs, Pamela; Connor, Marie; Conrad, Anne; Contreras, Sofía; Cooke, Graham S.; Copland, Mary; Cordel, Hugues; Corley, Amanda; Cormican, Sarah; Cornelis, Sabine; Corpuz, Arianne Joy; Corvaisier, Grégory; Couffignal, Camille; Couffin-Cadiergues, Sandrine; Courtois, Roxane; Cousse, Stéphanie; Croonen, Sabine; Crowl, Gloria; Crump, Jonathan; Cruz, Claudina; Csete, Marc; Cullen, Caroline; Cummings, Matthew; Curley, Gerard; Curlier, Elodie; Custodio, Paula; da Silva Filipe, Ana; Da Silveira, Charlene; Dabaliz, Al-Awwab; Dagens, Andrew; Dalton, Heidi; Dalton, Jo; Daneman, Nick; Dankwa, Emmanuelle; Dantas, Jorge; D'Aragon, Frédérick; de Mendoza, Diego; De Montmollin, Etienne; de Oliveira França, Rafael Freitas; De Pablo, Raúl; de Pinho Oliveira, Ana Isabel; De Rosa, Rosanna; de Silva, Thushan; De Vries, Peter; Deacon, Jillian; Dean, David; Debard, Alexa; Debray, Marie-Pierre; DeCastro, Nathalie; Dechert, William; Deconninck, Lauren; Decours, Romain; Delacroix, Isabelle; Delfos, Nathalie M.; Deligiannis, Ionna; Dell'Amore, Andrea; Delmas, Christelle; Delobel, Pierre; Denis, Emmanuelle; Deplanque, Dominique; Depuydt, Pieter; Desai, Mehul; Descamps, Diane; Desvallée, Mathilde; Dewayanti, Santi; Diallo, Alpha; Diamantis, Sylvain; Diaz, Rodrigo; Diaz, Juan Jose; Diaz, Priscila; Didier, Kévin; Diehl, Jean-Luc; Dieperink, Wim; Dimet, Jérôme; Dinot, Vincent; Diop, Fara; Diouf, Alphonsine; Dishon, Yael; Djossou, Félix; Docherty, Annemarie B.; Dondorp, Arjen M; Donnelly, Christl A.; Donnelly, Maria; Donohue, Chloe; Dorival, Céline; D'Ortenzio, Eric; Douglas, James Joshua; Douma, Renee; Dournon, Nathalie; Downer, Triona; Downing, Mark; Drake, Tom; Driscoll, Aoife; Duarte Fonseca, Claudio; Dubos, François; Duculan, Toni; Dudman, Susanne; Dunning, Jake; Duplaix, Mathilde; Durante-Mangoni, Emanuele; Durham III, Lucian; Duthoit, Juliette; Duval, Xavier; Dyrhol-Riise, Anne Margarita; Echeverria-Villalobos, Marco; Egan, Siobhan; Eira, Carla; El Sanharawi, Mohammed; Elapavaluru, Subbarao; Elharrar, Brigitte; Ellerbroek, Jacobien; Ellis, Rachael; Eloy, Philippine; Elshazly, Tarek; Enderle, Isabelle; Engelmann, Ilka; Enouf, Vincent; Epaulard, Olivier; Escher, Martina; Esperatti, Mariano; Esperou, Hélène; Esposito-Farese, Marina; Estevão, João; Etienne, Manuel; Ettalhaoui, Nadia; Evers, Mirjam; Fabre, Isabelle; Faheem, Amna; Fahy, Arabella; Fairfield, Cameron J.; Faria, Pedro; Farooq, Ahmed; Farshait, Nataly; Fatoni, Arie Zainul; Faure, Karine; Favory, Raphaël; Fayed, Mohamed; Feely, Niamh; Fernandes, Jorge; Fernandes, Marília; Fernandes, Susana; Ferrand Devouge, Eglantine; Ferrão, Joana; Ferraz, Mário; Ferrer-Roca, Ricard; Ferriere, Nicolas; Figueiredo-Mello, Claudia; Fiorda, Juan; Flament, Thomas; Flateau, Clara; Fletcher, Tom; Florio, Letizia Lucia; Flynn, Brigid; Foley, Claire; Fonseca, Tatiana; Fontela, Patricia; Forsyth, Simon; Foster, Denise; Foti, Giuseppe; Fourn, Erwan; Fowler, Robert A.; Franch-Llasat, Diego; Fraser, Christophe; Fraser, John; Freire, Marcela Vieira; Freitas Ribeiro, Ana; Friedrich, Caren; Fritz, Ricardo; Fry, Stéphanie; Fuentes, Nora; Fukuda, Masahiro; Gaborieau, Valérie; Gaci, Rostane; Gagliardi, Massimo; Gagneux-Brunon, Amandine; Gaião, Sérgio; Gail Skeie, Linda; Gallagher, Phil; Gamble, Carrol; Garan, Arthur; Garcia, Rebekha; Garcia-Gallo, Esteban; Garot, Denis; Garrait, Valérie; Gault, Nathalie; Gavin, Aisling; Gaymard, Alexandre; Gebauer, Johannes; Gerbaud Morlaes, Louis; Germano, Nuno; Ghosn, Jade; Giani, Marco; Gibson, Jess; Gigante, Tristan; Gilg, Morgane; Giordano, Guillermo; Girvan, Michelle; Gissot, Valérie; Giwangkancana, Gezy; Gnall, Eric; Goco, Geraldine; Goehringer, François; Goepel, Siri; Goffard, Jean-Christophe; Golob, Jonathan; Gómez-Junyent, Joan; Gonzalez Gonzalez, Alicia; Gorenne, Isabelle; Goubert, Laure; Goujard, Cécile; Goulenok, Tiphaine; Grable, Margarite; Graf, Jeronimo; Grandin, Edward Wilson; Grasselli, Giacomo; Grazioli, Lorenzo; Green, Christopher A.; Greenhalf, William; Grieco, Domenico Luca; Griffee, Matthew; Griffiths, Fiona; Grigoras, Ioana; Groenendijk, Albert; Gruner, Heidi; Gu, Yusing; Guedj, Jérémie; Guellec, Dewi; Guerguerian, Anne-Marie; Guerreiro, Daniela; Guery, Romain; Guillaumot, Anne; Guilleminault, Laurent; Guimard, Thomas; Haber, Daniel; Hadri, Nadir; Hakak, Sheeba; Hall, Matthew; Hall, Adam; Halpin, Sophie; Hamer, Ansley; Hamidfar, Rebecca; Hammond, Terese; Haniffa, Rashan; Hardwick, Hayley; Harrison, Ewen M.; Harrison, Janet; Hayat, Muhammad; Hays, Leanne; Heerman, Jan; Heggelund, Lars; Hendry, Ross; Hennessy, Martina; Henriquez-Trujillo, Aquiles; Hentzien, Maxime; Herekar, Fivzia; Hernandez-Montfort, Jaime; Hershey, Andrew; Hesstvedt, Liv; Hidayah, Astarini; Higgins, Eibhilin; Higgins, Dawn; Hinton, Samuel; Hipólito-Reis, Ana; Hiraiwa, Hiroaki; Ho, Antonia Ying Wai; Hoctin, Alexandre; Hoffmann, Isabelle; Hoiting, Oscar; Holt, Rebecca; Holter, Jan Cato; Horby, Peter; Horcajada, Juan Pablo; Hoshino, Koji; Houas, Ikram; Hough, Catherine L.; Hsu, Jimmy Ming-Yang; Hulot, Jean-Sébastien; Ijaz, Samreen; Illes, Hajnal-Gabriela; Imbert, Patrick; Inácio, Hugo; Isgett, Sarah; Ishani, Palliya Guruge Pramodya Ishani; Isidoro, Tiago; Isnard, Margaux; Itai, Junji; Ivulich, Daniel; Jaafar, Danielle; Jaafoura, Salma; Jackson, Clare; Jacquet, Pierre; Jamieson, Nina; Jaureguiberry, Stéphane; Jawad, Issrah; Jayakumar, Devachandran; Jego, Florence; Jenum, Synne; Jimbo-Sotomayor, Ruth; Jorge García, Ruth Noemí; Joseph, Cédric; Joseph, Mark; Joshi, Swosti; Jourdain, Mercé; Jouvet, Philippe; Jung, Hanna; Juzar, Dafsah; Kafif, Ouifiya; Kaguelidou, Florentia; Kali, Sabina; Kalomoiri, Smaragdi; Kambiya, Paul; Kandamby, Darshana Hewa; Kandel, Chris; Kant, Ravi; Kartsonaki, Christiana; Kasugai, Daisuke; Kataria, Anant; Katz, Kevin; Kaur Johal, Simreen; Kay, Christy; Keating, Sean; Kelly, Andrea; Kelly, Sadie; Kennedy, Ryan; Kennon, Kalynn; Kerroumi, Younes; Kestelyn, Evelyne; Khalid, Imrana; Khalil, Antoine; Khan, Coralie; Khan, Irfan; Khanal, Sushil; Kho, Michelle E; Khoo, Saye; Khoso, Nasir; Kida, Yuri; Kiiza, Peter; Kildal, Anders Benjamin; Kim, Jae Burm; Kimmoun, Antoine; Kindgen-Milles, Detlef; Kitamura, Nobuya; Klenerman, Paul; Kloumann Bekken, Gry; Knight, Stephen; Kodippily, Chamira; Kohns Vasconcelos, Malte; Koirala, Sabin; Komatsu, Mamoru; Kosgei, Caroline; Kpangon, Arsène; Krawczyk, Karolina; Kumar, Ashok; Kumar, Deepali; Kumar, Mukesh; Kumar Vecham, Pavan; Kurtzman, Ethan; Kusumastuti, Neurinda Permata; Kutsogiannis, Demetrios; Kyriakoulis, Konstantinos; Lachatre, Marie; Laffey, John G.; Laine, Fabrice; Lambert, Marc; Lamontagne, François; Langelot-Richard, Marie; Langlois, Vincent; Lantang, Eka Yudha; Lanza, Marina; Laouénan, Cédric; Laribi, Samira; Lariviere, Delphine; Launay, Odile; Law, Andrew; Lawrence, Cassie; Le, Minh; Le Bris, Cyril; Le Falher, Georges; Le Fevre, Lucie; Le Hingrat, Quentin; Le Maréchal, Marion; Le Mestre, Soizic; Le Nagard, Hervé; Le Turnier, Paul; Lee, Su Hwan; Lee, James; Lee, Todd C.; Leeming, Gary; Lefebvre, Bénédicte; Lefevre, Benjamin; LeGac, Sylvie; Lelievre, Jean-Daniel; Lellouche, François; Lemaignen, Adrien; Lemee, Véronique; Lemeur, Anthony; Lemmink, Gretchen; León, Rafael; Leone, Michela; Lepiller, Quentin; Lescure, François-Xavier; Lesens, Olivier; Lesouhaitier, Mathieu; Levy, Bruno; Levy, Yves; Levy-Marchal, Claire; L'Her, Erwan; Li Bassi, Gianluigi; Liang, Janet; Liaquat, Ali; Liegeon, Geoffrey; Lim, Wei Shen; Lima, Chantre; Lina, Bruno; Lind, Andreas; Lingas, Guillaume; Link, Linda; Lion-Daolio, Sylvie; Lissauer, Samantha; Liu, Keibun; Livrozet, Marine; Lolong, Navy; Lopes, Diogo; Lopez-Colon, Dalia; Loschner, Anthony L.; Loufti, Bouchra; Louis, Guillame; Lourenco, Silvia; Lucet, Jean Christophe; Luna, Carlos M.; Lungu, Olguta; Luong, Liem; Luton, Dominique; Lwin, Nilar; Lyons, Ruth; Maasikas, Olavi; Mabiala, Oryane; Macheda, Gabriel; Macias Sanchez, Juan; Madhok, Jai; Madiha, Hashmi; Mahy, Sophie; Maia, Raquel; Maier, Lars Siegfried; Maillet, Mylène; Maitre, Thomas; Malfertheiner, Maximilian; Malik, Nadia; Malvy, Denis; Manda, Victoria; Mandei, Jose M.; Mandelbrot, Laurent; Mankikian, Julie; Manning, Edmund; Manuel, Aldric; Maria Sant`Ana Malaque, Ceila; Marino, Daniel; Marino, Flávio; Mariz, Carolline de Araújo; Markowicz, Samuel; Marques, Ana; Marquis, Catherine; Marsh, Brian; Marsh, Laura; Marshall, John; Martelli, Celina Turchi; Martin, Emily; Martin-Blondel, Guillaume; Martinelli, Alessandra; Martin-Loeches, Ignacio; Martins, Ana; Martins, João; Martins, Nuno; Martins Rego, Caroline; Martucci, Gennaro; Marwali, Eva Miranda; Maslove, David; Mason, Sabina; Mat Nor, Basri; Mathieu, Daniel; Mattei, Mathieu; Matulevics, Romans; May, Jennifer; Maynar, Javier; Mazzoni, Thierry; Mc Evoy, Natalie; McCarthy, Aine; McCarthy, Anne; McCloskey, Colin; McConnochie, Rachael; McConnochie, Rachael; McDermott, Sherry; McDonald, Sarah; McElwee, Samuel; McGeer, Allison; McKay, Chris; McKeown, Johnny; McLean, Kenneth A.; McNicholas, Bairbre; Meaney, Edel; Mear-Passard, Cécile; Mechlin, Maggie; Mele, Ferruccio; Melo, Luis; Mendes, Joao Joao; Menkiti, Ogechukwu; Menon, Kusum; Mentré, France; Mentzer, Alexander J.; Mercier, Emmanuelle; Mercier, Noémie; Mergeay-Fabre, Mayka; Mergler, Blake; Merson, Laura; Mesquita, António; Meybeck, Agnès; Meyer, Dan; Meynert, Alison M.; Meysonnier, Vanina; Meziane, Amina; Mezidi, Mehdi; Michelet, Isabelle; Mihelis, Efstathia; Mihnovitš, Vladislav; Miranda-Maldonado, Hugo; Moin, Asma; Molina, David; Molinos, Elena; Mone, Mary; Monteiro, Agostinho; Montes, Claudia; Montrucchio, Giorgia; Moore, Sarah; Moore, Shona C.; Morales Cely, Lina; Moro, Lucia; Morton, Ben; Motherway, Catherine; Motos, Ana; Mouquet, Hugo; Mouton Perrot, Clara; Moyet, Julien; Mullaert, Jimmy; Müller, Fredrik; Müller, Karl Erik; Muneeb, Syed; Murris, Marlène; Murthy, Srinivas; Myrodia, Dimitra Melia; Nagpal, Dave; Nagrebetsky, Alex; Narasimhan, Mangala; Nasim Khan, Rashid; Neant, Nadège; Neb, Holger; Neto, Raul; Neumann, Emily; Ng, Wing Yiu; Ng, Pauline Yeung; Nguyen, Duc; Ni Choileain, Orna; Nichol, Alistair; Nonas, Stephanie; Noret, Marion; Norman, Lisa; Notari, Alessandra; Noursadeghi, Mahdad; Nseir, Saad; Nunez, Jose I; Nurnaningsih, Nurnaningsih; Occhipinti, Giovanna; O'Donnell, Max; Ogston, Tawnya; Ogura, Takayuki; Oh, Tak-Hyuk; O'Hearn, Katie; Ohshimo, Shinichiro; Oinam, Budhacharan Singh; Oliveira, João; Oliveira, Larissa; Olliaro, Piero L.; O'Neil, Conar; Ong, David S.Y.; Oosthuyzen, Wilna; Opavsky, Anne; Openshaw, Peter; Orakzai, Saijad; Orozco-Chamorro, Claudia Milena; Ortoleva, Jamel; Osatnik, Javier; Ouamara, Nadia; Ouissa, Rachida; Owyang, Clark; Oziol, Eric; Pabasara, H.M. Upulee; Pagadoy, Maïder; Pages, Justine; Palacios, Mario; Palacios, Amanda; Palmarini, Massimo; Panarello, Giovanna; Panda, Prasan Kumar; Paneru, Hem; Panigada, Mauro; Papadopoulos, Aurélie; Parker, Melissa; Parra, Briseida; Pasquier, Jérémie; Patauner, Fabian; Patel, Junaid; Patrão, Luís; Patricio, Patricia; Patrier, Juliette; Patterson, Lisa; Paul, Christelle; Paul, Mical; Paulos, Jorge; Paxton, William A.; Payen, Jean-François; Peek, Giles; Peelman, Florent; Peiffer-Smadja, Nathan; Peigne, Vincent; Pejkovska, Mare; Peltan, Ithan D.; Pereira, Rui; Perez, Daniel; Periel, Luis; Perpoint, Thomas; Pesenti, Antonio; Pestre, Vincent; Petroušová, Lenka; Petrov-Sanchez, Ventzislava; Pettersen, Frank Olav; Peytavin, Gilles; Pharand, Scott; Piagnerelli, Michael; Picard, Walter; Picone, Olivier; Pierobon, Carola; Pimentel, Carlos; Piroth, Lionel; Pius, Riinu; Piva, Simone; Plantier, Laurent; Plotkin, Daniel; Poissy, Julien; Pokeerbux, Ryadh; Poli, Sergio; Pollakis, Georgios; Ponscarme, Diane; Post, Andra-Maris; Postma, Douwe F.; Povoa, Pedro; Póvoas, Diana; Powis, Jeff; Prapa, Sofia; Preau, Sébastien; Prebensen, Christian; Preiser, Jean-Charles; Prinssen, Anton; Pritchard, Mark; Priyadarshani, Gamage Dona Dilanthi; Proença, Lúcia; Puéchal, Oriane; Pulicken, Matthew; Purcell, Gregory; Quesada, Luisa; Quinones-Cardona, Vilmaris; Quist-Paulsen, Else; Quraishi, Mohammed; Rabaud, Christian; Rafael, Aldo; Rafiq, Marie; Rahutullah, Arsalan; Rainieri, Fernando; Ralib, Azrina; Ramakrishnan, Nagarajan; Ramos, Grazielle Viana; Rana, Asim; Rashan, Aasiyah; Rashan, Thalha; Rasmin, Menaldi; Rätsep, Indrek; Real, Andre; Rebaudet, Stanislas; Reeve, Brenda; Rehan, Ali; Rehman, Attaur; Reid, Liadain; Reikvam, Dag Henrik; Reis, Renato; Remppis, Jonathan; Remy, Martine; Ren, Hongru; Renk, Hanna; Resende, Liliana; Revest, Matthieu; Rewa, Oleksa; Reyes, Luis F.; Reyes, Tiago; Ribeiro, Maria Ines; Richardson, David; Richardson, Denise; Richier, Laurent; Riera, Jordi; Rios, Ana Lúcia; Rishu, Asgar; Rizer, Nicholas; Roberto, André; Roberts, Stephanie; Robertson, David L.; Robineau, Olivier; Roche-Campo, Ferran; Rodari, Paola; Rodeia, Simão; Roger, Pierre-Marie; Rojek, Amanda; Romaru, Juliette; Roncon-Albuquerque Jr, Roberto; Rosa-Calatrava, Manuel; Rose, Michael; Rosenberger, Dorothea; Rossanese, Andrea; Rossetti, Matteo; Rossignol, Bénédicte; Rossignol, Patrick; Rössler, Bernhard; Rousset, Stella; Roy, Carine; Roze, Benoît; Rusmawatiningtyas, Desy; Russell, Clark D.; Ryckaert, Steffi; Rygh Holten, Aleksander; Saba, Isabela; Sadaf, Sairah; Sadat, Musharaf; Sahraei, Valla; Saint-Gilles, Maximilien; Salahuddin, Nawal; Salazar, Leonardo; Sales, Gabriele; Sallaberry, Stéphane; Salmon Gandonniere, Charlotte; Salvator, Hélène; Sanchez, Olivier; Sanchez-Miralles, Angel; Sancho-Shimizu, Vanessa; Sandhu, Gyan; Sandrine, Pierre-François; Sandulescu, Oana; Santos, Marlene; Sarfo-Mensah, Shirley; Sarmiento, Iam Claire E.; Sarton, Benjamine; Satyapriya, Sree; Saviciute, Egle; Schaffer, Justin; Schermer, Tjard; Scherpereel, Arnaud; Schneider, Marion; Schroll, Stephan; Schwameis, Michael; Scott, Janet T.; Scott-Brown, James; Sedillot, Nicholas; Seitz, Tamara; Semaille, Caroline; Semple, Malcolm G.; Senneville, Eric; Sepulveda, Claudia; Sequeira, Filipa; Sequeira, Tânia; Serpa Neto, Ary; Shadowitz, Ellen; Shamsah, Mohammad; Sharma, Pratima; Shaw, Catherine A.; Shaw, Victoria; Sheharyar, Ashraf; Shi, Haixia; Shiekh, Mohiuddin; Shime, Nobuaki; Shimizu, Hiroaki; Shimizu, Keiki; Shimizu, Naoki; Shrapnel, Sally; Shum, Hoi Ping; Si Mohammed, Nassima; Sibiude, Jeanne; Siddiqui, Atif; Sigfrid, Louise; Sillaots, Piret; Silva, Catarina; Silva, Maria Joao; Silva, Rogério; Sin, Wai Ching; Skogen, Vegard; Smith, Sue; Smood, Benjamin; Smyth, Michelle; Snacken, Morgane; So, Dominic; Solis, Monserrat; Solomon, Joshua; Solomon, Tom; Somers, Emily; Sommet, Agnès; Song, Myung Jin; Song, Rima; Song, Tae; Sonntagbauer, Michael; Soum, Edouard; Sousa, Ana Chora; Sousa, Marta; Sousa Uva, Maria; Souza-Dantas, Vicente; Sperry, Alexandra; Sri Darshana, B.P. Sanka Ruwan; Sriskandan, Shiranee; Stabler, Sarah; Staudinger, Thomas; Stecher, Stephanie-Susanne; Stienstra, Ymkje; Stiksrud, Birgitte; Streinu-Cercel, Anca; Streinu-Cercel, Adrian; Strudwick, Samantha; Stuart, Ami; Stuart, David; Suen, Jacky Y.; Suen, Gabriel; Sultana, Asfia; Summers, Charlotte; Surovcová, Magdalena; Syrigos, Konstantinos; Sztajnbok, Jaques; Szuldrzynski, Konstanty; Tabrizi, Shirin; Taccone, Fabio; Tagherset, Lysa; Taleb, Sara; Talsma, Jelmer; Tan, Le Van; Tanaka, Hiroyuki; Tanaka, Taku; Taniguchi, Hayato; Tanveer, Hussain; Tardivon, Coralie; Tattevin, Pierre; Taufik, M. Azhari; Tedder, Richard S.; Teixeira, João; Tejada, Sofia; Tellier, Marie-Capucine; Teotonio, Vanessa; Téoulé, François; Terpstra, Pleun; Terrier, Olivier; Terzi, Nicolas; Tessier-Grenier, Hubert; Thibault, Vincent; Thill, Benoît; Thompson, Shaun; Thomson, Emma C.; Thomson, David; Thuy, Duong Bich; Thwaites, Ryan S.; Timsit, Jean-François; Tirupakuzhi Vijayaraghavan, Bharath Kumar; Tissot, Noémie; Toki, Maria; Tolppa, Timo; Tonby, Kristian; Torres, Antoni; Torres-Zevallos, Hernando; Trapani, Tony; Treoux, Théo; Trieu, Huynh Trung; Tromeur, Cécile; Trontzas, Ioannis; Troost, Jonathan; Trouillon, Tiffany; Truong, Jeanne; Tual, Christelle; Tubiana, Sarah; Tuite, Helen; Turmel, Jean-Marie; Turtle, Lance C.W.; Twardowski, Pawel; Uchiyama, Makoto; Udayanga, P.G. Ishara; Udy, Andrew; Ullrich, Roman; Uribe, Alberto; Usman, Asad; Val-Flores, Luís; Valran, Amélie; Van De Velde, Stijn; van den Berge, Marcel; Van der Feltz, Machteld; Van Der Vekens, Nicky; Van der Voort, Peter; Van Der Werf, Sylvie; van Gulik, Laura; Van Hattem, Jarne; van Lelyveld, Steven; van Netten, Carolien; van Twillert, G; Vanel, Noémie; Vanoverschelde, oHenk; Vauchy, Charline; Veislinger, Aurélie; Ventura, Sara; Verbon, Annelies; Vidal, José Ernesto; Vieira, César; Villanueva, Joy Ann; Villar, Judit; Villeneuve, Pierre-Marc; Villoldo, Andrea; Vinh Chau, Nguyen Van; Visseaux, Benoit; Visser, Hannah; Vitiello, Chiara; Vuotto, Fanny; Wang, Chih-Hsien; Wei, Jia; Weil, Katharina; Wesselius, Sanne; Wham, Murray; Whelan, Bryan; White, Nicole; Wicky, Paul Henri; Wiedemann, Aurélie; Wille, Keith; Williams, Virginie; Wils, Evert-Jan; Wolf, Timo; Xynogalas, Ioannis; Yacoub, Sophie; Yamazaki, Masaki; Yazdanpanah, Yazdan; Yelnik, Cécile; Yerkovich, Stephanie; Yokoyama, Toshiki; Yonis, Hodane; Yuliarto, Saptadi; Zaaqoq, Akram; Zabbe, Marion; Zacharowski, Kai; Zahran, Maram; Zambon, Maria; Zambrano, Miguel; Zanella, Alberto; Zoufaly, Alexander; Zucman, David.
Data sharing: The dataset is available through the Infectious Disease Data Observatory website (https://www.iddo.org).
Provenance: Submitted article, peer reviewed.
This article has supplementary material available from openres.ersjournals.com
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of this manuscript. The corresponding author (LFR) had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Conflict of interest: L.F. Reyes has nothing to disclose.
Conflict of interest: S. Murthy declares receiving salary support from the Health Research Foundation and Innovative Medicines Canada Chair in Pandemic Preparedness Research.
Conflict of interest: E. Garcia-Gallo has nothing to disclose.
Conflict of interest: M. Irvine has nothing to disclose.
Conflict of interest: L. Merson has nothing to disclose.
Conflict of interest: I. Martin-Loeches declares consulting fees for Gilead outside of the submitted work.
Conflict of interest: J. Rello has nothing to disclose.
Conflict of interest: F.S. Taccone has nothing to disclose.
Conflict of interest: R.A. Fowler has nothing to disclose.
Conflict of interest: A.B. Docherty has nothing to disclose.
Conflict of interest: C. Kartsonaki has nothing to disclose.
Conflict of interest: I. Aragao has nothing to disclose.
Conflict of interest: P.W. Barrett has nothing to disclose.
Conflict of interest: A. Beane has nothing to disclose.
Conflict of interest: A. Burrell has nothing to disclose.
Conflict of interest: M.P. Cheng has nothing to disclose.
Conflict of interest: M.D. Christian has nothing to disclose.
Conflict of interest: J.P. Cidade has nothing to disclose.
Conflict of interest: B.W. Citarella has nothing to disclose.
Conflict of interest: C.A. Donnelly has nothing to disclose.
Conflict of interest: S.M. Fernandes has nothing to disclose.
Conflict of interest: C. French has nothing to disclose.
Conflict of interest: R. Haniffa has nothing to disclose.
Conflict of interest: E.M. Harrison has nothing to disclose.
Conflict of interest: A.Y.W. Ho declares grant funding from Medical Research Council UK, Scottish Funding Council – Grand Challenges Research Fund and the Wellcome Trust, outside this submitted work.
Conflict of interest: M. Joseph has nothing to disclose.
Conflict of interest: I. Khan has nothing to disclose.
Conflict of interest: M.E. Kho has nothing to disclose.
Conflict of interest: A.B. Kildal has nothing to disclose.
Conflict of interest: D. Kutsogiannis declares personal fees for a lecture from Tabuk Pharmaceuticals and the Saudi Critical Care Society.
Conflict of interest: F. Lamontagne has nothing to disclose.
Conflict of interest: T.C. Lee declares research salary support from les Fonds de recherche du Québec – Santé.
Conflict of interest: G.L. Bassi has nothing to disclose.
Conflict of interest: J.W. Lopez Revilla has nothing to disclose.
Conflict of interest: C. Marquis has nothing to disclose.
Conflict of interest: J. Millar has nothing to disclose.
Conflict of interest: R. Neto has nothing to disclose.
Conflict of interest: A. Nichol declares a grant from the Health Research Board of Ireland to support data collection in Ireland (CTN-2014-012).
Conflict of interest: R. Parke has nothing to disclose.
Conflict of interest: R. Pereira has nothing to disclose.
Conflict of interest: S. Poli has nothing to disclose.
Conflict of interest: P. Povoa declares personal fees (for lectures and advisory boards) from MSD, Technophage, Sanofi and Gilead.
Conflict of interest: K. Ramanathan has nothing to disclose.
Conflict of interest: O. Rewa declares consulting fees from Baxter Inc.
Conflict of interest: J. Riera has nothing to disclose.
Conflict of interest: S. Shrapnel participated as an investigator for an observational study analysing intensive care unit patients with COVID-19 (for the Critical Care Consortium including ECMOCARD) funded by The Prince Charles Hospital Foundation during the conduct of this study. S. Shrapnel reports in kind support from the Australian Research Council Centre of Excellence for Engineered Quantum Systems (CE170100009).
Conflict of interest: M.J. Silva has nothing to disclose.
Conflict of interest: A. Udy has nothing to disclose.
Conflict of interest: T. Uyeki has nothing to disclose.
Conflict of interest: S.A. Webb has nothing to disclose.
Conflict of interest: E-J. Wils has nothing to disclose.
Conflict of interest: A. Rojek has nothing to disclose.
Conflict of interest: P.L. Olliaro has nothing to disclose.
Support statement: This work was supported by the UK Foreign, Commonwealth and Development Office and Wellcome (215091/Z/18/Z), the Bill and Melinda Gates Foundation (OPP1209135), Canadian Institutes of Health Research Coronavirus Rapid Research Funding Opportunity OV2170359, grants from Rapid European COVID-19 Emergency Response Research (Horizon 2020 project 101003589), the European Clinical Research Alliance on Infectious Diseases (965313), The Imperial National Institute for Health Research (NIHR) Biomedical Research Centre, and The Cambridge NIHR Biomedical Research Centre; and endorsed by the Irish Critical Care Clinical Trials Group, co-ordinated in Ireland by the Irish Critical Care Clinical Trials Network at University College Dublin and funded by the Health Research Board of Ireland (CTN-2014-12). Data and Material provision was supported by grants from: the NIHR (award CO-CIN-01), the Medical Research Council (grant MC_PC_19059), the NIHR Health Protection Research Unit (HPRU) in Emerging and Zoonotic Infections at University of Liverpool in partnership with Public Health England (PHE) (award 200907), Wellcome Trust (Turtle, Lance-fellowship 205228/Z/16/Z), NIHR HPRU in Respiratory Infections at Imperial College London with PHE (award 200927), Liverpool Experimental Cancer Medicine Centre (grant C18616/A25153), NIHR Biomedical Research Centre at Imperial College London (award IS-BRC-1215-20013), and NIHR Clinical Research Network providing infrastructure support. This work was by Research Council of Norway grant number 312780, and a philanthropic donation from Vivaldi Invest A/S owned by Jon Stephenson von Tetzchner. Funding information for this article has been deposited with the Crossref Funder Registry.
Contributor Information
ISARIC Clinical Characterisation Group:
Luis Felipe Reyes, Srinivas Murthy, Esteban Garcia-Gallo, Mike Irvine, Laura Merson, Ignacio Martin-Loeches, Jordi Rello, Fabio S. Taccone, Steve A. Webb, Robert A. Fowler, Annemarie B Docherty, Christl A. Donnelly, Christiana Kartsonaki, Irene Aragao, Peter W. Barrett, Abigail Beane, Tessa Broadley, Matthew Pellan Cheng, Michael D. Christian, Jose Pedro Cidade, Barbara Wanjiru Citarella, Susana Fernandes, Rashan Haniffa, Ewen M. Harrison, Antonia Ying Wai Ho, Mark Joseph, Irfan Khan, Michelle E Kho, Anders Benjamin Kildal, Demetrios Kutsogiannis, François Lamontagne, Todd C. Lee, Gianluigi Li Bassi, Jose Wagner Lopez Revilla, Catherine Marquis, Jonathan Millar, Raul Neto, Alistair Nichol, Piero L. Olliaro, Rachael Parke, Rui Pereira, Sergio Poli, Pedro Povoa, Kollengode Ramanathan, Jordi Rello, Oleksa Rewa, Jordi Riera, Amanda Rojek, Sally Shrapnel, Maria Joao Silva, Tony Trapani, Andrew Udy, Timothy Uyeki, Evert-Jan Wils, Luis Felipe Reyes, Esteban Garcia-Gallo, Srinivas Murthy, Mike Irvine, Laura Merson, Luis Felipe Reyes, Esteban Garcia-Gallo, Mike Irvine, Barbara Wanjiru Citarella, Sadie Kelly, Kalynn Kennon, James Lee, Laura Merson, Daniel Plotkin, Sue Smith, Samantha Strudwick, Barbara Wanjiru Citarella, Luis Felipe Reyes, Esteban Garcia-Gallo, Srinivas Murthy, Mike Irvine, Laura Merson, Luis Felipe Reyes, Esteban Garcia-Gallo, Mike Irvine, Sheryl Ann Abdukahil, Ryuzo Abe, Laurent Abel, Lara Absil, Subhash Acharya, Andrew Acker, Shingo Adachi, Elisabeth Adam, Diana Adrião, Saleh Al Ageel, Quratul Ain, Kate Ainscough, Ali Ait Hssain, Younes Ait Tamlihat, Takako Akimoto, Ernita Akmal, Eman Al Qasim, Razi Alalqam, Tanvir Alam, Tala Al-dabbous, Cynthia Alegre, Beatrice Alex, Kévin Alexandre, Abdulrahman Al-Fares, Huda Alfoudri, Naseem Ali Shah, Kazali Enagnon Alidjnou, Jeffrey Aliudin, Clotilde Allavena, João Melo Alves, João Alves, Rita Alves, Maria Amaral, Phoebe Ampaw, Roberto Andini, Claire Andrejak, Andrea Angheben, François Angoulvant, Séverine Ansart, Massimo Antonelli, Carlos Alexandre Antunes de Brito, Ardiyan Apriyana, Yaseen Arabi, Irene Aragao, Francisco Arancibia, Antonio Arcadipane, Patrick Archambault, Lukas Arenz, Jean-Benoît Arlet, Christel Arnold-Day, Lovkesh Arora, Rakesh Arora, Elise Artaud-Macari, Diptesh Aryal, Angel Asensio, Jean Baptiste Assie, Anika Atique, A.M. Udara Lakshan Attanyake, Johann Auchabie, Hugues Aumaitre, Laurène Azemar, Cecile Azoulay, Benjamin Bach, Delphine Bachelet, Claudine Badr, J. Kenneth Baillie, Erica Bak, Agamemnon Bakakos, Andriy Bal, Firouzé Bani-Sadr, Helena Barrasa, Renata Barbalho, Wendy S. Barclay, Michaela Barnikel, Cleide Barrigoto, Marie Bartoli, Cheryl Bartone, Joaquín Baruch, Romain Basmaci, Jules Bauer, Diego Bautista, Abigail Beane, Sylvie Behilill, Aleksandr Beljantsev, David Bellemare, Anna Beltrame, Marine Beluze, Nicolas Benech, Dehbia Benkerrou, Suzanne Bennett, Luís Bento, Jan-Erik Berdal, Delphine Bergeaud, Lorenzo Bertolino, Simon Bessis, Sybille Bevilcaqua, Amar Bhatt, Krishna Bhavsar, Isabella Bianchi, Claudia Bianco, Moirangthem Bikram Singh, Felwa Bin Humaid, Patrick Biston, Laurent Bitker, Pablo Blanco-Schweizer, Aaron Blandino Ortiz, Catherine Blier, Mathieu Blot, Filomena Boccia, Laetitia Bodenes, Debby Bogaert, Pierre-Adrien Bolze, François Bompart, Diogo Borges, Raphaël Borie, Hans Martin Bosse, Elisabeth Botelho-Nevers, Lila Bouadma, Olivier Bouchaud, Sabelline Bouchez, Dounia Bouhmani, Damien Bouhour, Kévin Bouiller, Laurence Bouillet, Camile Bouisse, Anne-Sophie Boureau, Maude Bouscambert, Jason Bouziotis, Bianca Boxma, Marielle Boyer-Besseyre, Maria Boylan, Fernando Augusto Bozza, Matthew Brack, Cynthia Braga, Timo Brandenburger, Filipa Brás Monteiro, Luca Brazzi, Dorothy Breen, Patrick Breen, Kathy Brickell, Tessa Broadley, Alex Browne, Nicolas Brozzi, Nina Buchtele, Christian Buesaquillo, Marielle Buisson, Erlina Burhan, Aidan Burrell, Ingrid G. Bustos, André Cabie, Susana Cabral, Eder Caceres, Cyril Cadoz, Kate Calligy, Jose Andres Calvache, João Camões, Valentine Campana, Paul Campbell, Cecilia Canepa, Mireia Cantero, Pauline Caraux-Paz, Sheila Cárcel, Chiara Cardellino, Filipa Cardoso, Filipe Cardoso, Nelson Cardoso, Sofia Cardoso, Simone Carelli, Nicolas Carlier, Gayle Carney, Chloe Carpenter, Marie-Christine Carret, François Martin Carrier, Gail Carson, Maire-Laure Casanova, Mariana Cascão, José Casimiro, Bailey Cassandra, Silvia Castañeda, Nidyanara Castanheira, Guylaine Castor-Alexandre, Henry Castrillón, Ivo Castro, Ana Catarino, François-Xavier Catherine, Roberta Cavalin, Alexandros Cavayas, Adrian Ceccato, Minerva Cervantes-Gonzalez, Anissa Chair, Catherine Chakveatze, Adrienne Chan, Meera Chand, Christelle Chantalat Auger, Jean-Marc Chapplain, Mobin Chaudary, Anjellica Chen, Yih-Sharng Chen, Matthew Pellan Cheng, Antoine Cheret, Thibault Chiarabini, Julian Chica, Catherine Chirouze, Davide Chiumello, Hwa Jin Cho, Sung Min Cho, Bernard Cholley, Marie-Charlotte Chopin, Jose Pedro Cidade, Barbara Wanjiru Citarella, Anna Ciullo, Jennifer Clarke, Sara Clohisey, Necsoi Coca, Cassidy Codan, Caitriona Cody, Alexandra Coelho, Gwenhaël Colin, Michael Collins, Sebastiano Maria Colombo, Pamela Combs, Marie Connor, Anne Conrad, Sofía Contreras, Graham S. Cooke, Mary Copland, Hugues Cordel, Amanda Corley, Sarah Cormican, Sabine Cornelis, Arianne Joy Corpuz, Grégory Corvaisier, Camille Couffignal, Sandrine Couffin-Cadiergues, Roxane Courtois, Stéphanie Cousse, Sabine Croonen, Gloria Crowl, Jonathan Crump, Claudina Cruz, Marc Csete, Caroline Cullen, Matthew Cummings, Gerard Curley, Elodie Curlier, Paula Custodio, Ana da Silva Filipe, Charlene Da Silveira, Al-Awwab Dabaliz, Andrew Dagens, Heidi Dalton, Jo Dalton, Nick Daneman, Emmanuelle Dankwa, Jorge Dantas, Frédérick D'Aragon, Diego de Mendoza, Etienne De Montmollin, Rafael Freitas de Oliveira França, Raúl De Pablo, Ana Isabel de Pinho Oliveira, Rosanna De Rosa, Thushan de Silva, Peter De Vries, Jillian Deacon, David Dean, Alexa Debard, Marie-Pierre Debray, Nathalie DeCastro, William Dechert, Lauren Deconninck, Romain Decours, Isabelle Delacroix, Nathalie M. Delfos, Ionna Deligiannis, Andrea Dell'Amore, Christelle Delmas, Pierre Delobel, Emmanuelle Denis, Dominique Deplanque, Pieter Depuydt, Mehul Desai, Diane Descamps, Mathilde Desvallée, Santi Dewayanti, Alpha Diallo, Sylvain Diamantis, Rodrigo Diaz, Juan Jose Diaz, Priscila Diaz, Kévin Didier, Jean-Luc Diehl, Wim Dieperink, Jérôme Dimet, Vincent Dinot, Fara Diop, Alphonsine Diouf, Yael Dishon, Félix Djossou, Annemarie B. Docherty, Arjen M Dondorp, Christl A. Donnelly, Maria Donnelly, Chloe Donohue, Céline Dorival, Eric D'Ortenzio, James Joshua Douglas, Renee Douma, Nathalie Dournon, Triona Downer, Mark Downing, Tom Drake, Aoife Driscoll, Claudio Duarte Fonseca, François Dubos, Toni Duculan, Susanne Dudman, Jake Dunning, Mathilde Duplaix, Emanuele Durante-Mangoni, Lucian Durham III, Juliette Duthoit, Xavier Duval, Anne Margarita Dyrhol-Riise, Marco Echeverria-Villalobos, Siobhan Egan, Carla Eira, Mohammed El Sanharawi, Subbarao Elapavaluru, Brigitte Elharrar, Jacobien Ellerbroek, Rachael Ellis, Philippine Eloy, Tarek Elshazly, Isabelle Enderle, Ilka Engelmann, Vincent Enouf, Olivier Epaulard, Martina Escher, Mariano Esperatti, Hélène Esperou, Marina Esposito-Farese, João Estevão, Manuel Etienne, Nadia Ettalhaoui, Mirjam Evers, Isabelle Fabre, Amna Faheem, Arabella Fahy, Cameron J. Fairfield, Pedro Faria, Ahmed Farooq, Nataly Farshait, Arie Zainul Fatoni, Karine Faure, Raphaël Favory, Mohamed Fayed, Niamh Feely, Jorge Fernandes, Marília Fernandes, Susana Fernandes, Eglantine Ferrand Devouge, Joana Ferrão, Mário Ferraz, Ricard Ferrer-Roca, Nicolas Ferriere, Claudia Figueiredo-Mello, Juan Fiorda, Thomas Flament, Clara Flateau, Tom Fletcher, Letizia Lucia Florio, Brigid Flynn, Claire Foley, Tatiana Fonseca, Patricia Fontela, Simon Forsyth, Denise Foster, Giuseppe Foti, Erwan Fourn, Robert A. Fowler, Diego Franch-Llasat, Christophe Fraser, John Fraser, Marcela Vieira Freire, Ana Freitas Ribeiro, Caren Friedrich, Ricardo Fritz, Stéphanie Fry, Nora Fuentes, Masahiro Fukuda, Valérie Gaborieau, Rostane Gaci, Massimo Gagliardi, Amandine Gagneux-Brunon, Sérgio Gaião, Linda Gail Skeie, Phil Gallagher, Carrol Gamble, Arthur Garan, Rebekha Garcia, Esteban Garcia-Gallo, Denis Garot, Valérie Garrait, Nathalie Gault, Aisling Gavin, Alexandre Gaymard, Johannes Gebauer, Louis Gerbaud Morlaes, Nuno Germano, Jade Ghosn, Marco Giani, Jess Gibson, Tristan Gigante, Morgane Gilg, Guillermo Giordano, Michelle Girvan, Valérie Gissot, Gezy Giwangkancana, Eric Gnall, Geraldine Goco, François Goehringer, Siri Goepel, Jean-Christophe Goffard, Jonathan Golob, Joan Gómez-Junyent, Alicia Gonzalez Gonzalez, Isabelle Gorenne, Laure Goubert, Cécile Goujard, Tiphaine Goulenok, Margarite Grable, Jeronimo Graf, Edward Wilson Grandin, Giacomo Grasselli, Lorenzo Grazioli, Christopher A. Green, William Greenhalf, Domenico Luca Grieco, Matthew Griffee, Fiona Griffiths, Ioana Grigoras, Albert Groenendijk, Heidi Gruner, Yusing Gu, Jérémie Guedj, Dewi Guellec, Anne-Marie Guerguerian, Daniela Guerreiro, Romain Guery, Anne Guillaumot, Laurent Guilleminault, Thomas Guimard, Daniel Haber, Nadir Hadri, Sheeba Hakak, Matthew Hall, Adam Hall, Sophie Halpin, Ansley Hamer, Rebecca Hamidfar, Terese Hammond, Rashan Haniffa, Hayley Hardwick, Ewen M. Harrison, Janet Harrison, Muhammad Hayat, Leanne Hays, Jan Heerman, Lars Heggelund, Ross Hendry, Martina Hennessy, Aquiles Henriquez-Trujillo, Maxime Hentzien, Fivzia Herekar, Jaime Hernandez-Montfort, Andrew Hershey, Liv Hesstvedt, Astarini Hidayah, Eibhilin Higgins, Dawn Higgins, Samuel Hinton, Ana Hipólito-Reis, Hiroaki Hiraiwa, Antonia Ying Wai Ho, Alexandre Hoctin, Isabelle Hoffmann, Oscar Hoiting, Rebecca Holt, Jan Cato Holter, Peter Horby, Juan Pablo Horcajada, Koji Hoshino, Ikram Houas, Catherine L. Hough, Jimmy Ming-Yang Hsu, Jean-Sébastien Hulot, Samreen Ijaz, Hajnal-Gabriela Illes, Patrick Imbert, Hugo Inácio, Sarah Isgett, Palliya Guruge Pramodya Ishani Ishani, Tiago Isidoro, Margaux Isnard, Junji Itai, Daniel Ivulich, Danielle Jaafar, Salma Jaafoura, Clare Jackson, Pierre Jacquet, Nina Jamieson, Stéphane Jaureguiberry, Issrah Jawad, Devachandran Jayakumar, Florence Jego, Synne Jenum, Ruth Jimbo-Sotomayor, Ruth Noemí Jorge García, Cédric Joseph, Mark Joseph, Swosti Joshi, Mercé Jourdain, Philippe Jouvet, Hanna Jung, Dafsah Juzar, Ouifiya Kafif, Florentia Kaguelidou, Sabina Kali, Smaragdi Kalomoiri, Paul Kambiya, Darshana Hewa Kandamby, Chris Kandel, Ravi Kant, Christiana Kartsonaki, Daisuke Kasugai, Anant Kataria, Kevin Katz, Simreen Kaur Johal, Christy Kay, Sean Keating, Andrea Kelly, Sadie Kelly, Ryan Kennedy, Kalynn Kennon, Younes Kerroumi, Evelyne Kestelyn, Imrana Khalid, Antoine Khalil, Coralie Khan, Irfan Khan, Sushil Khanal, Michelle E Kho, Saye Khoo, Nasir Khoso, Yuri Kida, Peter Kiiza, Anders Benjamin Kildal, Jae Burm Kim, Antoine Kimmoun, Detlef Kindgen-Milles, Nobuya Kitamura, Paul Klenerman, Gry Kloumann Bekken, Stephen Knight, Chamira Kodippily, Malte Kohns Vasconcelos, Sabin Koirala, Mamoru Komatsu, Caroline Kosgei, Arsène Kpangon, Karolina Krawczyk, Ashok Kumar, Deepali Kumar, Mukesh Kumar, Pavan Kumar Vecham, Ethan Kurtzman, Neurinda Permata Kusumastuti, Demetrios Kutsogiannis, Konstantinos Kyriakoulis, Marie Lachatre, John G. Laffey, Fabrice Laine, Marc Lambert, François Lamontagne, Marie Langelot-Richard, Vincent Langlois, Eka Yudha Lantang, Marina Lanza, Cédric Laouénan, Samira Laribi, Delphine Lariviere, Odile Launay, Andrew Law, Cassie Lawrence, Minh Le, Cyril Le Bris, Georges Le Falher, Lucie Le Fevre, Quentin Le Hingrat, Marion Le Maréchal, Soizic Le Mestre, Hervé Le Nagard, Paul Le Turnier, Su Hwan Lee, James Lee, Todd C. Lee, Gary Leeming, Bénédicte Lefebvre, Benjamin Lefevre, Sylvie LeGac, Jean-Daniel Lelievre, François Lellouche, Adrien Lemaignen, Véronique Lemee, Anthony Lemeur, Gretchen Lemmink, Rafael León, Michela Leone, Quentin Lepiller, François-Xavier Lescure, Olivier Lesens, Mathieu Lesouhaitier, Bruno Levy, Yves Levy, Claire Levy-Marchal, Erwan L'Her, Gianluigi Li Bassi, Janet Liang, Ali Liaquat, Geoffrey Liegeon, Wei Shen Lim, Chantre Lima, Bruno Lina, Andreas Lind, Guillaume Lingas, Linda Link, Sylvie Lion-Daolio, Samantha Lissauer, Keibun Liu, Marine Livrozet, Navy Lolong, Diogo Lopes, Dalia Lopez-Colon, Anthony L. Loschner, Bouchra Loufti, Guillame Louis, Silvia Lourenco, Jean Christophe Lucet, Carlos M. Luna, Olguta Lungu, Liem Luong, Dominique Luton, Nilar Lwin, Ruth Lyons, Olavi Maasikas, Oryane Mabiala, Gabriel Macheda, Juan Macias Sanchez, Jai Madhok, Hashmi Madiha, Sophie Mahy, Raquel Maia, Lars Siegfried Maier, Mylène Maillet, Thomas Maitre, Maximilian Malfertheiner, Nadia Malik, Denis Malvy, Victoria Manda, Jose M. Mandei, Laurent Mandelbrot, Julie Mankikian, Edmund Manning, Aldric Manuel, Ceila Maria Sant`Ana Malaque, Daniel Marino, Flávio Marino, Carolline de Araújo Mariz, Samuel Markowicz, Ana Marques, Catherine Marquis, Brian Marsh, Laura Marsh, John Marshall, Celina Turchi Martelli, Emily Martin, Guillaume Martin-Blondel, Alessandra Martinelli, Ignacio Martin-Loeches, Ana Martins, João Martins, Nuno Martins, Caroline Martins Rego, Gennaro Martucci, Eva Miranda Marwali, David Maslove, Sabina Mason, Basri Mat Nor, Daniel Mathieu, Mathieu Mattei, Romans Matulevics, Jennifer May, Javier Maynar, Thierry Mazzoni, Natalie Mc Evoy, Aine McCarthy, Anne McCarthy, Colin McCloskey, Rachael McConnochie, Rachael McConnochie, Sherry McDermott, Sarah McDonald, Samuel McElwee, Allison McGeer, Chris McKay, Johnny McKeown, Kenneth A. McLean, Bairbre McNicholas, Edel Meaney, Cécile Mear-Passard, Maggie Mechlin, Ferruccio Mele, Luis Melo, Joao Joao Mendes, Ogechukwu Menkiti, Kusum Menon, France Mentré, Alexander J. Mentzer, Emmanuelle Mercier, Noémie Mercier, Mayka Mergeay-Fabre, Blake Mergler, Laura Merson, António Mesquita, Agnès Meybeck, Dan Meyer, Alison M. Meynert, Vanina Meysonnier, Amina Meziane, Mehdi Mezidi, Isabelle Michelet, Efstathia Mihelis, Vladislav Mihnovitš, Hugo Miranda-Maldonado, Asma Moin, David Molina, Elena Molinos, Mary Mone, Agostinho Monteiro, Claudia Montes, Giorgia Montrucchio, Sarah Moore, Shona C. Moore, Lina Morales Cely, Lucia Moro, Ben Morton, Catherine Motherway, Ana Motos, Hugo Mouquet, Clara Mouton Perrot, Julien Moyet, Jimmy Mullaert, Fredrik Müller, Karl Erik Müller, Syed Muneeb, Marlène Murris, Srinivas Murthy, Dimitra Melia Myrodia, Dave Nagpal, Alex Nagrebetsky, Mangala Narasimhan, Rashid Nasim Khan, Nadège Neant, Holger Neb, Raul Neto, Emily Neumann, Wing Yiu Ng, Pauline Yeung Ng, Duc Nguyen, Orna Ni Choileain, Alistair Nichol, Stephanie Nonas, Marion Noret, Lisa Norman, Alessandra Notari, Mahdad Noursadeghi, Saad Nseir, Jose I Nunez, Nurnaningsih Nurnaningsih, Giovanna Occhipinti, Max O'Donnell, Tawnya Ogston, Takayuki Ogura, Tak-Hyuk Oh, Katie O'Hearn, Shinichiro Ohshimo, Budhacharan Singh Oinam, João Oliveira, Larissa Oliveira, Piero L. Olliaro, Conar O'Neil, David S.Y. Ong, Wilna Oosthuyzen, Anne Opavsky, Peter Openshaw, Saijad Orakzai, Claudia Milena Orozco-Chamorro, Jamel Ortoleva, Javier Osatnik, Nadia Ouamara, Rachida Ouissa, Clark Owyang, Eric Oziol, H.M. Upulee Pabasara, Maïder Pagadoy, Justine Pages, Mario Palacios, Amanda Palacios, Massimo Palmarini, Giovanna Panarello, Prasan Kumar Panda, Hem Paneru, Mauro Panigada, Aurélie Papadopoulos, Melissa Parker, Briseida Parra, Jérémie Pasquier, Fabian Patauner, Junaid Patel, Luís Patrão, Patricia Patricio, Juliette Patrier, Lisa Patterson, Christelle Paul, Mical Paul, Jorge Paulos, William A. Paxton, Jean-François Payen, Giles Peek, Florent Peelman, Nathan Peiffer-Smadja, Vincent Peigne, Mare Pejkovska, Ithan D. Peltan, Rui Pereira, Daniel Perez, Luis Periel, Thomas Perpoint, Antonio Pesenti, Vincent Pestre, Lenka Petroušová, Ventzislava Petrov-Sanchez, Frank Olav Pettersen, Gilles Peytavin, Scott Pharand, Michael Piagnerelli, Walter Picard, Olivier Picone, Carola Pierobon, Carlos Pimentel, Lionel Piroth, Riinu Pius, Simone Piva, Laurent Plantier, Daniel Plotkin, Julien Poissy, Ryadh Pokeerbux, Sergio Poli, Georgios Pollakis, Diane Ponscarme, Andra-Maris Post, Douwe F. Postma, Pedro Povoa, Diana Póvoas, Jeff Powis, Sofia Prapa, Sébastien Preau, Christian Prebensen, Jean-Charles Preiser, Anton Prinssen, Mark Pritchard, Gamage Dona Dilanthi Priyadarshani, Lúcia Proença, Oriane Puéchal, Matthew Pulicken, Gregory Purcell, Luisa Quesada, Vilmaris Quinones-Cardona, Else Quist-Paulsen, Mohammed Quraishi, Christian Rabaud, Aldo Rafael, Marie Rafiq, Arsalan Rahutullah, Fernando Rainieri, Azrina Ralib, Nagarajan Ramakrishnan, Grazielle Viana Ramos, Asim Rana, Aasiyah Rashan, Thalha Rashan, Menaldi Rasmin, Indrek Rätsep, Andre Real, Stanislas Rebaudet, Brenda Reeve, Ali Rehan, Attaur Rehman, Liadain Reid, Dag Henrik Reikvam, Renato Reis, Jonathan Remppis, Martine Remy, Hongru Ren, Hanna Renk, Liliana Resende, Matthieu Revest, Oleksa Rewa, Luis F. Reyes, Tiago Reyes, Maria Ines Ribeiro, David Richardson, Denise Richardson, Laurent Richier, Jordi Riera, Ana Lúcia Rios, Asgar Rishu, Nicholas Rizer, André Roberto, Stephanie Roberts, David L. Robertson, Olivier Robineau, Ferran Roche-Campo, Paola Rodari, Simão Rodeia, Pierre-Marie Roger, Amanda Rojek, Juliette Romaru, Roberto Roncon-Albuquerque Jr, Manuel Rosa-Calatrava, Michael Rose, Dorothea Rosenberger, Andrea Rossanese, Matteo Rossetti, Bénédicte Rossignol, Patrick Rossignol, Bernhard Rössler, Stella Rousset, Carine Roy, Benoît Roze, Desy Rusmawatiningtyas, Clark D. Russell, Steffi Ryckaert, Aleksander Rygh Holten, Isabela Saba, Sairah Sadaf, Musharaf Sadat, Valla Sahraei, Maximilien Saint-Gilles, Nawal Salahuddin, Leonardo Salazar, Gabriele Sales, Stéphane Sallaberry, Charlotte Salmon Gandonniere, Hélène Salvator, Olivier Sanchez, Angel Sanchez-Miralles, Vanessa Sancho-Shimizu, Gyan Sandhu, Pierre-François Sandrine, Oana Sandulescu, Marlene Santos, Shirley Sarfo-Mensah, Iam Claire E. Sarmiento, Benjamine Sarton, Sree Satyapriya, Egle Saviciute, Justin Schaffer, Tjard Schermer, Arnaud Scherpereel, Marion Schneider, Stephan Schroll, Michael Schwameis, Janet T. Scott, James Scott-Brown, Nicholas Sedillot, Tamara Seitz, Caroline Semaille, Malcolm G. Semple, Eric Senneville, Claudia Sepulveda, Filipa Sequeira, Tânia Sequeira, Ary Serpa Neto, Ellen Shadowitz, Mohammad Shamsah, Pratima Sharma, Catherine A. Shaw, Victoria Shaw, Ashraf Sheharyar, Haixia Shi, Mohiuddin Shiekh, Nobuaki Shime, Hiroaki Shimizu, Keiki Shimizu, Naoki Shimizu, Sally Shrapnel, Hoi Ping Shum, Nassima Si Mohammed, Jeanne Sibiude, Atif Siddiqui, Louise Sigfrid, Piret Sillaots, Catarina Silva, Maria Joao Silva, Rogério Silva, Wai Ching Sin, Vegard Skogen, Sue Smith, Benjamin Smood, Michelle Smyth, Morgane Snacken, Dominic So, Monserrat Solis, Joshua Solomon, Tom Solomon, Emily Somers, Agnès Sommet, Myung Jin Song, Rima Song, Tae Song, Michael Sonntagbauer, Edouard Soum, Ana Chora Sousa, Marta Sousa, Maria Sousa Uva, Vicente Souza-Dantas, Alexandra Sperry, B.P. Sanka Ruwan Sri Darshana, Shiranee Sriskandan, Sarah Stabler, Thomas Staudinger, Stephanie-Susanne Stecher, Ymkje Stienstra, Birgitte Stiksrud, Anca Streinu-Cercel, Adrian Streinu-Cercel, Samantha Strudwick, Ami Stuart, David Stuart, Jacky Y. Suen, Gabriel Suen, Asfia Sultana, Charlotte Summers, Magdalena Surovcová, Konstantinos Syrigos, Jaques Sztajnbok, Konstanty Szuldrzynski, Shirin Tabrizi, Fabio Taccone, Lysa Tagherset, Sara Taleb, Jelmer Talsma, Le Van Tan, Hiroyuki Tanaka, Taku Tanaka, Hayato Taniguchi, Hussain Tanveer, Coralie Tardivon, Pierre Tattevin, M. Azhari Taufik, Richard S. Tedder, João Teixeira, Sofia Tejada, Marie-Capucine Tellier, Vanessa Teotonio, François Téoulé, Pleun Terpstra, Olivier Terrier, Nicolas Terzi, Hubert Tessier-Grenier, Vincent Thibault, Benoît Thill, Shaun Thompson, Emma C. Thomson, David Thomson, Duong Bich Thuy, Ryan S. Thwaites, Jean-François Timsit, Bharath Kumar Tirupakuzhi Vijayaraghavan, Noémie Tissot, Maria Toki, Timo Tolppa, Kristian Tonby, Antoni Torres, Hernando Torres-Zevallos, Tony Trapani, Théo Treoux, Huynh Trung Trieu, Cécile Tromeur, Ioannis Trontzas, Jonathan Troost, Tiffany Trouillon, Jeanne Truong, Christelle Tual, Sarah Tubiana, Helen Tuite, Jean-Marie Turmel, Lance C.W. Turtle, Pawel Twardowski, Makoto Uchiyama, P.G. Ishara Udayanga, Andrew Udy, Roman Ullrich, Alberto Uribe, Asad Usman, Luís Val-Flores, Amélie Valran, Stijn Van De Velde, Marcel van den Berge, Machteld Van der Feltz, Nicky Van Der Vekens, Peter Van der Voort, Sylvie Van Der Werf, Laura van Gulik, Jarne Van Hattem, Steven van Lelyveld, Carolien van Netten, G van Twillert, Noémie Vanel, oHenk Vanoverschelde, Charline Vauchy, Aurélie Veislinger, Sara Ventura, Annelies Verbon, José Ernesto Vidal, César Vieira, Joy Ann Villanueva, Judit Villar, Pierre-Marc Villeneuve, Andrea Villoldo, Nguyen Van Vinh Chau, Benoit Visseaux, Hannah Visser, Chiara Vitiello, Fanny Vuotto, Chih-Hsien Wang, Jia Wei, Katharina Weil, Sanne Wesselius, Murray Wham, Bryan Whelan, Nicole White, Paul Henri Wicky, Aurélie Wiedemann, Keith Wille, Virginie Williams, Evert-Jan Wils, Timo Wolf, Ioannis Xynogalas, Sophie Yacoub, Masaki Yamazaki, Yazdan Yazdanpanah, Cécile Yelnik, Stephanie Yerkovich, Toshiki Yokoyama, Hodane Yonis, Saptadi Yuliarto, Akram Zaaqoq, Marion Zabbe, Kai Zacharowski, Maram Zahran, Maria Zambon, Miguel Zambrano, Alberto Zanella, Alexander Zoufaly, and David Zucman
References
- 1.Richards-Belle A, Orzechowska I, Gould DW, et al. . COVID-19 in critical care: epidemiology of the first epidemic wave across England, Wales and Northern Ireland. Intensive Care Med 2020; 46: 2035–2047. doi: 10.1007/s00134-020-06267-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinha P, Calfee CS, Cherian S, et al. . Prevalence of phenotypes of acute respiratory distress syndrome in critically ill patients with COVID-19: a prospective observational study. Lancet Respir Med 2020; 8: 1209–1218. doi: 10.1016/S2213-2600(20)30366-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quah P, Li A, Phua J. Mortality rates of patients with COVID-19 in the intensive care unit: a systematic review of the emerging literature. Crit Care 2020; 24: 285. doi: 10.1186/s13054-020-03006-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodríguez A, Ruiz-Botella M, Martín-Loeches I, et al. . Deploying unsupervised clustering analysis to derive clinical phenotypes and risk factors associated with mortality risk in 2022 critically ill patients with COVID-19 in Spain. Crit Care 2021; 25: 63. doi: 10.1186/s13054-021-03487-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coopersmith CM, Antonelli M, Bauer SR, et al. . The Surviving Sepsis campaign: research priorities for coronavirus disease 2019 in critical illness. Crit Care Med 2021; 49: 598–622. doi: 10.1097/CCM.0000000000004895 [DOI] [PubMed] [Google Scholar]
- 6.Kalil AC. Treating COVID-19-off-label drug use, compassionate use, and randomised clinical trials during pandemics. JAMA 2020; 323: 1897–1898. doi: 10.1001/jama.2020.4742 [DOI] [PubMed] [Google Scholar]
- 7.Wu G, Yang P, Xie Y, et al. . Development of a clinical decision support system for severity risk prediction and triage of COVID-19 patients at hospital admission: an international multicentre study. Eur Respir J 2020; 56: 2001104. doi: 10.1183/13993003.01104-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franco C, Facciolongo N, Tonelli R, et al. . Feasibility and clinical impact of out-of-ICU noninvasive respiratory support in patients with COVID-19-related pneumonia. Eur Respir J 2020; 56: 2002130. doi: 10.1183/13993003.02130-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aziz S, Arabi YM, Alhazzani W, et al. . Managing ICU surge during the COVID-19 crisis: rapid guidelines. Intensive Care Med 2020; 46: 1303–1325. doi: 10.1007/s00134-020-06092-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie J, Wu W, Li S, et al. . Clinical characteristics and outcomes of critically ill patients with novel coronavirus infectious disease (COVID-19) in China: a retrospective multicenter study. Intensive Care Med 2020; 46: 1863–1872. doi: 10.1007/s00134-020-06211-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grasselli G, Zangrillo A, Zanella A, et al. . Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020; 323: 1574–1581. doi: 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azoulay E, de Waele J, Ferrer R, et al. . International variation in the management of severe COVID-19 patients. Crit Care 2020; 24: 486. doi: 10.1186/s13054-020-03194-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Our World in Data . Coronavirus (COVID-19) Cases. https://ourworldindata.org/covid-cases/ 2020. Date last accessed: 12 April 2021.
- 14.Ranzani OT, Bastos LSL, Gelli JGM, et al. . Characterisation of the first 250,000 hospital admissions for COVID-19 in Brazil: a retrospective analysis of nationwide data. Lancet Respir Med 2021; 9: 407–418. doi: 10.1016/S2213-2600(20)30560-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X, Yu Y, Xu J, et al. . Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8: 475–481. doi: 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrando C, Suarez-Sipmann F, Mellado-Artigas R, et al. . Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med 2020; 46: 2200–2211. doi: 10.1007/s00134-020-06192-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson S, Hirsch JS, Narasimhan M, et al. . Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalised with COVID-19 in the New York City area. JAMA 2020; 323: 2052–2059. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.COVID-ICU Group on behalf of the REVA network and the COVID-ICU investigators . Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med 2021; 47: 60–73. doi: 10.1007/s00134-020-06294-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta RK, Harrison EM, Ho A, et al. . Development and validation of the ISARIC 4C deterioration model for adults hospitalised with COVID-19: a prospective cohort study. Lancet Respir Med 2021; 9: 349–359. doi: 10.1016/S2213-2600(20)30559-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waterer GW, Rello J, Wunderink RG. COVID-19: first do no harm. Am J Respir Crit Care Med 2020; 201: 1324–1325. doi: 10.1164/rccm.202004-1153ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angus DC. Optimising the trade-off between learning and doing in a pandemic. JAMA 2020; 323: 1895–1896. doi: 10.1001/jama.2020.4984 [DOI] [PubMed] [Google Scholar]
- 22.Sterne JAC, Murthy S, Diaz JV, et al. . Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 2020; 324: 1330–1341. doi: 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horby P, Lim WS, Emberson JR, et al. . Dexamethasone in hospitalised patients with Covid-19. N Engl J Med 2021; 384: 693–704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langford BJ, So M, Raybardhan S, et al. . Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect 2020; 26: 1622–1629. doi: 10.1016/j.cmi.2020.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Águas R, Mahdi A, Shretta R, et al. . Potential health and economic impacts of dexamethasone treatment for patients with COVID-19. Nat Commun 2021; 12: 915. doi: 10.1038/s41467-021-21134-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metlay JP, Waterer GW, Long AC, et al. . Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200: e45–e67. doi: 10.1164/rccm.201908-1581ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreno G, Rodríguez A, Reyes LF, et al. . Corticosteroid treatment in critically ill patients with severe influenza pneumonia: a propensity score matching study. Intensive Care Med 2018; 44: 1470–1482. doi: 10.1007/s00134-018-5332-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grasselli G, Pesenti A, Cecconi M. Critical care utilisation for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA 2020; 323: 1545–1546. doi: 10.1001/jama.2020.4031 [DOI] [PubMed] [Google Scholar]
- 29.McHugh MD, Aiken LH, Sloane DM, et al. . Effects of nurse-to-patient ratio legislation on nurse staffing and patient mortality, readmissions, and length of stay: a prospective study in a panel of hospitals. Lancet 2021; 397: 1905–1913. doi: 10.1016/S0140-6736(21)00768-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomersall CD, Tai DY, Loo S, et al. . Expanding ICU facilities in an epidemic: recommendations based on experience from the SARS epidemic in Hong Kong and Singapore. Intensive Care Med 2006; 32: 1004–1013. doi: 10.1007/s00134-006-0134-5 [DOI] [PubMed] [Google Scholar]
- 31.Shrestha GS, Kwizera A, Lundeg G, et al. . International Surviving Sepsis campaign guidelines 2016: the perspective from low-income and middle-income countries. Lancet Infect Dis 2017; 17: 893–895. doi: 10.1016/S1473-3099(17)30453-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00552-2021.supplement (381.4KB, pdf)
Case report form 00552-2021.CRF (2.5MB, pdf)
Protocol 00552-2021.protocol (773KB, pdf)