Abstract
Exposure of rats to the pesticide and complex I inhibitor rotenone reproduces features of Parkinson’s disease, including selective nigrostriatal dopaminergic degeneration and α-synuclein-positive cytoplasmic inclusions (Betarbet et al., 2000; Sherer et al., 2003). Here, we examined mechanisms of rotenone toxicity using three model systems. In SK-N-MC human neuroblastoma cells, rotenone (10 nM to 1μM) caused dose-dependent ATP depletion, oxidative damage, and death. To determine the molecular site of action of rotenone, cells were transfected with the rotenone-insensitive single-subunit NADH dehydrogenase of Saccharomyces cerevisiae (NDI1), which incorporates into the mammalian ETC and acts as a “replacement” for endogenous complex I. In response to rotenone, NDI1-transfected cells did not show mitochondrial impairment, oxidative damage, or death, demonstrating that these effects of rotenone were caused by specific interactions at complex I. Although rotenone caused modest ATP depletion, equivalent ATP loss induced by 2-deoxyglucose was without toxicity, arguing that bioenergetic defects were not responsible for cell death. In contrast, reducing oxidative damage with antioxidants, orbyNDI1 transfection, blocked cell death. To determine the relevance of rotenone-induced oxidative damage to dopaminergic neuronal death, we used a chronic midbrain slice culture model. In this system, rotenone caused oxidative damage and dopaminergic neuronal loss, effects blocked by α-tocopherol. Finally, brains from rotenone-treated animals demonstrated oxidative damage, most notably in midbrain and olfactory bulb, dopaminergic regions affected by Parkinson’s disease. These results, using three models of increasing complexity, demonstrate the involvement of oxidative damage in rotenone toxicity and support the evaluation of antioxidant therapies for Parkinson’s disease.
Keywords: mitochondria, oxidative stress, NADH dehydrogenase, dopamine, organotypic, α-tocopherol
Introduction
Parkinson’s disease (PD) is a late-onset, progressive neurodegenerative disorder characterized by relatively selective nigrostriatal dopaminergic degeneration and the development of fibrillar cytoplasmic inclusions containing α-synuclein and ubiquitin (Spillantini et al., 1997; Wooten, 1997). The etiology of PD is not completely understood, but it is believed to involve an interaction between genetic and environmental factors (Sherer et al., 2002a). Epidemiological studies suggest that exposure to environmental agents, such as pesticides, may increase PD risk (Gorell et al., 1998; Menegon et al., 1998). Mitochondrial dysfunction has also been linked to PD. Specifically, there are systemic reductions in the activity of complex I of the mitochondrial electron transfer chain (ETC) in PD brain, muscle, and platelets (Mizuno et al., 1989; Parker et al., 1989; Schapira et al., 1989; Cardellach et al., 1993; Haas et al., 1995). Additional evidence for mitochondrial impairment in PD comes from the finding that MPP (1-methyl-4-phenyl-2,3-dihydropyridinium), the active metabolite of the parkinsonism toxin N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), acts as a complex I inhibitor (Nicklas et al., 1985).
Recently, we developed the rotenone model of PD, which sub-stantiated involvement of pesticide exposure and systemic complex I dysfunction in PD etiology. In addition to being a common pesticide, rotenone is a high-affinity inhibitor of complex I of the mitochondrial ETC. Although rotenone caused uniform complex I inhibition throughout the brain, rotenone-treated rats demonstrated many characteristics of PD, including selective nigrostriatal dopaminergic degeneration, formation of ubiquitin-and α-synuclein-positive nigral inclusions, and motor deficits (Betarbet et al., 2000; Alam and Schmidt, 2002; Sherer et al., 2003). Although the rotenone model demonstrated the potential relevance of complex I defects to PD pathogenesis, the mechanisms through which systemic complex I dysfunction produce neurotoxicity are unknown. Because the doses of rotenone used did not alter mitochondrial oxygen consumption in isolated brain mitochondria (Betarbet et al., 2000), it did not appear that a bioenergetic defect could account for rotenone-induced neurodegeneration.
Instead, rotenone toxicity may result from oxidative stress. Brains of PD patients show evidence of oxidative stress, including decreased levels of reduced glutathione and oxidative modifications to DNA, lipids, and proteins (Dexter et al., 1989;Alam et al., 1997; Pearce et al., 1997; Floor and Wetzel, 1998), and oxidative damage is hypothesized to contribute to the neurodegenerative process in PD (Jenner,1998). The source of this oxidative damage is unknown. Reactive oxygen species (ROS) are generated during dopamine metabolism and by mitochondrial respiration. Within complex I, upstream of the rotenone-binding site, is a site of electron leakage that produces ROS (Hensley et al., 1998; Kushnareva et al., 2002), and impaired complex I activity enhances ROS formation (Cassarino et al., 1997; Barrientos and Moraes, 1999; Kushnareva et al., 2002).
Here, we investigated oxidative stress as a mechanism for rotenone toxicity in three in vitro and in vivo models relevant to PD. We report that rotenone-induced ATP depletion per se does not cause cell death, but pharmacological or genetic manipulations that attenuate oxidative damage are protective. Results from organotypic midbrain slice cultures and from rotenone-treated animals also support the conclusion that complex-I-mediated oxidative damage is responsible for rotenone-induced degeneration of dopaminergic neurons. Thus, moderate complex I impairment causes dopaminergic neurodegeneration via oxidative stress rather than a bioenergetic defect.
Materials and Methods
Cell culture.
SK-N-MC human neuroblastoma cells were cultured in minimum essential medium (MEM) with Earle’s salts containing 5 mM glucose (Mediatech, Herndon, VA), 15% fetal bovine serum (Invitrogen, Carlsbad, CA), 50 U/ml penicillin and streptomycin, 5 mM sodium pyruvate, and nonessential amino acid solutions for MEM (Mediatech). For routine culture, cells were grown in 100 mm plates, fed two times per week, and passaged twice per week on reaching confluence.
Midbrain slice cultures.
Our protocol is modified from Stoppini et al. (1991). Lewis rat pups (postnatal day 10) were fully anesthetized with isoflurane. Brains were rapidly removed and transferred to a sterile hood to cold sterile chopping buffer containing the following (in mM): 110 sucrose, 60 NaCl, 3 KCl, 1.25 NaH2PO4, 28 NaHCO3, 5 D-glucose, 0.5 CaCl2, 7 MgCl2, and 0.6 ascorbate. Brains were rapidly blocked, glued to a slicer chuck (OTS-4000 tissue slicer; FHC Inc, Bowdoinham, ME), and transferred to the slicer basin, which was filled with cold, oxygenated chopping buffer. Coronal slices were cut at 300 μm and transferred to a cold dissection medium (Gey’s balanced salt solution with glucose and KCl). Midbrain slices were isolated under a dissection microscope, and each slice was transferred onto a Millicell-CM membrane insert (Millipore, Bedford, MA) set in a six well plate on 1 ml of OPTI-MEM (Invitrogen, Rockville, MD)-based serum-containing medium. Plates were kept in a 37°C tissue culture incubator. After 2–4 d, the medium was changed to Neurobasal (Invitrogen)-based serum-free medium with B-27 and L-glutamine. Medium was changed three times per week. Slices were grown for 10 d before drug treatments.
For treatments, coronal sections were cut in half, separating the two hemispheres into two different slices. The two half slices were used as a matched pair, one receiving drug treatments and the other serving as an internal control. Parallel slices received either 50 nM rotenone or vehicle (ethanol) in the presence of 100 μM α-tocopherol (vitamin E; Avocado Research Chemicals) or vehicle (DMSO) for 1 week.
Surgeries and animal care.
All animal use was in accordance with National Institutes of Health guidelines and was approved by the Emory University Institutional Animal Care and Use Committee. Animal surgery was performed as described previously (Sherer et al., 2003). Briefly, male Lewis rats (300–350 gm) received 3.0 mg · kg−1 · d−1 rotenone (Sigma, St. Louis, MO) for up to 5 weeks after the subcutaneous implantation of osmotic minipumps (Alzet Corporation, Palo Alto, CA). Control rats received vehicle only: DMSO:polyethylene glycol (PEG, 1:1) (PEG-300, Sigma). After surgery, rats were monitored daily for behavior and over all health. Diet was supplemented with Nutrical (Evsco Pharmaceuticals, Buena, NJ) when necessary. Subcutaneous lactated Ringer’s injection U.S. Pharmacopeial solution (Baxter Healthcare Corporation, Deerfield, IL) was given when rats showed signs of dehydration. Rotenone-infused rats were killed at the time of severe systemic illness, characterized by rigidity and akinesia that prevented adequate feeding and grooming. Control rats were killed at similar time intervals.
Cell death assays.
Cell death was determined using Sytox green (Molecular Probes, Eugene, OR). Sytox green intercalates into the DNA of dead cells but is excluded from live cells; it was detected with excitation at 485 nm and emission at 538 nm with a fluorescence microplate system (Molecular Devices, Sunnyvale, CA). Cells were grown on 96 well plates, loaded with 1 μM Sytox green for 10 min, and treated with varying concentrations of rotenone or 2-deoxy-D-glucose (2-DG; both from Sigma). Control wells received equal concentrations of solvent (DMSO for rotenone). Treatments were done in triplicate, unless stated otherwise. Using the plate reader, cell death was followed continuously for 24–48 hr during treatments. For pretreatment studies, cells were exposed to 62.5 or 125 μM α-tocopherol (Avocado Research Chemicals) or 12.5 M coenzyme Q10 (Sigma) for 24 hr before Sytox green loading and rotenone exposure. Fluorescence readings were taken once per hour and normalized to initial plating density after fixation with 4% para formal dehydefor 45 min at 4°C.
rAAV-NDI1 infection.
Construction and preparation of rAAV-NDI1 (a recombinant adeno-associated virus containing the NDI1 gene) and the infection protocol were described previously (Seo et al., 2000, 2002; Bai et al., 2001). NDI1-expressing cells were selected by culturing in MEM containing 5 mM galactose and 10% FBS in the presence of 100 nM rotenone for 1 week. Under these conditions (in which the glycolytic pathway is unavailable) nontransduced cells die within a few days, because rotenone inhibits respiration. For cell death studies, NDI1transduced cells and control SK-N-MC cells were plated on the same 96 well plate. Cell death was analyzed using the Sytox green assay after a 48 hr exposure to 100 nM rotenone, 1 mM azide, or 300 M hydrogen peroxide.
Oxygen consumption.
Oxygen consumption experiments used digitonin-permeabilized cells as described previously (Seo et al., 2000, 2002; Bai et al., 2001).
ATP measurements.
Cellular ATP levels were analyzed using the ATP lite kit (PerkinElmer Life Sciences, Emeryville, CA) that is based on the luciferase assay. Briefly, cells were grown in a 96 well plate, media was removed, and cells were rinsed two times with HBSS containing calcium, magnesium, and glucose. For pretreatment studies, cells were exposed to α-tocopherol 24 hr before rotenone treatment. ATP measurements followed the manufacturer’s protocol and were calculated based on a standard curve and expressed as percentage of ATP in solvent-treated wells.
Detection of protein carbonyls.
For determination of protein carbonyls in SK-N-MC human neuroblastoma cells, cells were grown on 100 mm plates and treated with rotenone for 24 hr. Control cells received DMSO. For pretreatment studies, cells were exposed to 125 μM α-tocopherol 24 hr before exposure to rotenone. Cells were washed two times with PBS and incubated in 400 μl of cell lysis buffer (Promega, Madison, WI) containing protease inhibitors (Roche, Indianapolis, IN), 700 U/ml DNase I, and 1% β-mercaptoethanol (Sigma) for 15 min at room temperature. Cells were scraped and lysate was centrifuged at 10,000 × g for 10min. Supernatant was collected as the soluble protein fraction. Protein levels were determined using the Bio-Rad Protein Assay (Bio-Rad, Hercules, CA).
For the determination of protein carbonyl levels in organotypic midbrain slice cultures, slices were rinsed briefly in PBS (three times) to remove media and then cut out of the membrane inserts. Two slices were pooled for protein isolation. Slices were homogenized in TPER Tissue Protein Extraction Reagent (Pierce, Rockford, IL) supplemented with protease inhibitors. The homogenate was centrifuged for 5 min at 10,000 rpm at 4°C. The supernatant was collected as soluble protein. Protein assays were performed using the Bio-Rad Protein Assay.
For the determination of protein carbonyls in tissue extracts, vehicle and rotenone-infused rats were killed, and brains were removed without previous perfusion and dissected midsagittally into two halves. One hemisphere was fixed in 4% paraformaldehyde and processed for TH immunocytochemistry. From the remaining hemisphere, specific brain regions were rapidly dissected and frozen on dry ice. Tissue was homogenized in TPER Tissue Protein Extraction Reagent (Pierce) supplemented with protease inhibitors. The homogenate was centrifuged for 5 min at 10,000 rpm at 4°C. The supernatant was collected as soluble protein. The pellet was resuspended in 12% SDS and used as the insoluble fraction. Protein assays were performed using the Bio-Rad Protein Assay and Bio-Rad Dc Protein Assay.
Protein carbonyl levels were determined using dot blots and the Oxyblot Protein Oxidation Detection Kit (Serologicals, Atlanta, GA) according to the manufacturer’s protocol and as described previously (Sherer et al., 2002b). Briefly, in this protocol, protein carbonyls are derivatized to 2,4-dinitrophenylhydrazone (DNP) by reaction with 2,4-dinitrophenylhydrazine. DNP-derivatized protein samples were analyzed using dot blots and antibodies against DNP.
Glutathione levels.
Cells were grown on 100 mm culture plates and treated with rotenone or solvent for 24 hr. Cells were scraped and collected by centrifugation. The cell pellet was homogenized in 50mM 2-(N-morpholino) ethanosulfonic acid, containing 1 mM EDTA. The supernatant was deproteinated by adding an equal volume of 10% metaphosphoric acid (Sigma), incubating at room temperature for 5 min, and centrifuging for 5 min at 5000 × g. Total glutathione was determined using a glutathione assay kit (Cayman Chemical, Ann Arbor, MI), which is based on the glutathione reductase enzymatic recycling method. Glutathione levels were normalized to cellular protein and expressed as percentage of glutathione levels in solvent-treated cells.
Immunocytochemistry.
We used 40 μm paraformaldehyde-fixed brain sections. Organotypic midbrain slices were also fixed with 4% paraformaldehyde overnight, rinsed in PBS, and stored at 4°C in PBS. Sections were washed six times for 10 min each in Tris-buffered saline (TBS), treated for 10 min with 10% hydrogen peroxide (Sigma), washed three times for 10 min each in TBS, and blocked for 1 hr with 10% normal goat serum (Invitrogen). Sections were incubated overnight in primary antibody, washed three times for 10 min each in TBS, and incubated for 1 hr with biotinylated secondary antibody. The avidin–biotin complex method was used to detect the antigen signal (ABC Elite Kit; Vector Laboratories, Burlingame, CA), and 3,3’-diaminobenzidine tetrachloride (Sigma) was used to visualize the final product. Primary antibody was a monoclonal mouse antibody against TH (1:2000; Chemicon, Temecula, CA). Secondary antibody was biotinylated goat anti-mouse IgG (1:200; Jackson ImmunoResearch, West Grove, PA). We examined immunostained sections using bright-field microscopy and Zeiss (Oberkochen, Germany) Axiovision 3.0 software. For final output, images were processed identically using Adobe Photoshop 6.0 (Adobe Systems, San Jose, CA) software.
Statistical analysis.
Statistical analysis used multivariate ANOVA or Student’s t test for independent samples. Significance was set at p ≤ 0.05. Values shown represent means ± SEM unless otherwise stated.
Results
Rotenone causes dose-dependent cell death in SK-N-MC neuroblastoma cells
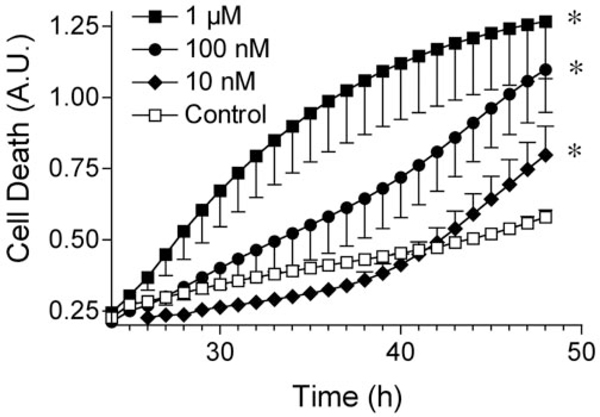
Rotenone exposure in rats reproduces many features of PD (Betarbet et al., 2000; Sherer et al., 2003). To analyze mechanisms of rotenone toxicity, we used an in vitro system. SK-N-MC neuroblastoma cells were exposed to increasing doses of rotenone for 48 hr, and cell death was determined using Sytox green fluorescence. Because rotenone did not cause substantial cell death during the initial 24 hr period, we focused on the period 24–48 hr after rotenone exposure. Dose-dependent cell death was observed after exposure to rotenone (Fig. 1). Cell death occurred after exposure to 1 μM, 100 nM, and 10 nM rotenone; lower doses of rotenone (100 pM to 1 nM) did not cause cell death over this period.
Figure1.
Dose-dependent cell death in response to rotenone exposure. Cells were exposed to rotenone for 48 hr, and cell death was monitored between 24 and 48 hr using Sytox green fluorescence. Rotenone treatment at 1 μM, 100 nM, and 10 nM caused toxicity. Results show means ± SEM of six independent experiments. *p 0.05. A.U., Arbitrary units.
Rotenone toxicity is caused by complex I inhibition
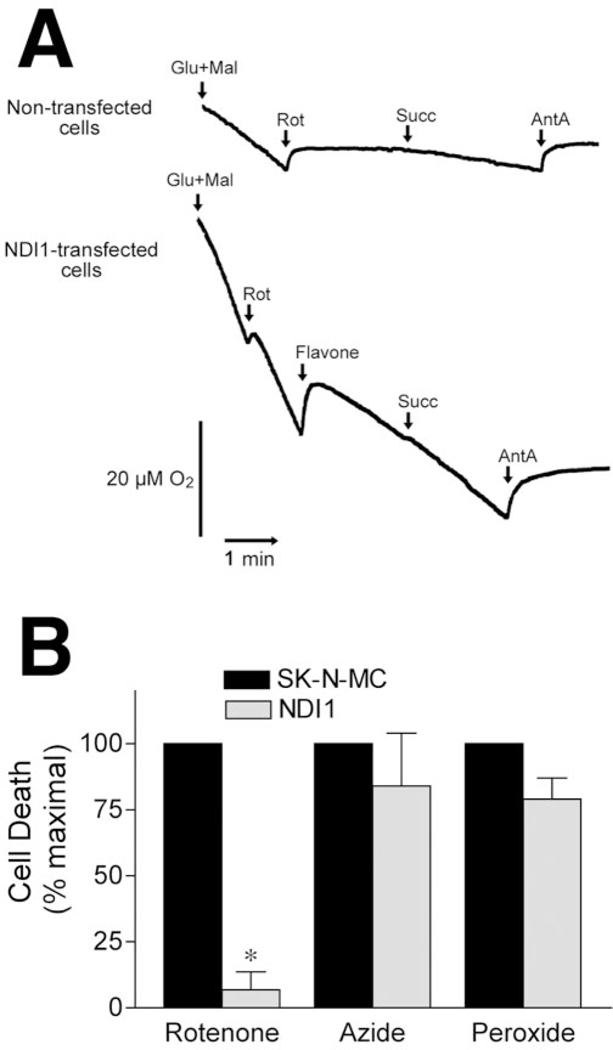
Rotenone is believed to be a specific inhibitor of complex I of the mitochondrial ETC. To determine whether rotenone toxicity depended on interaction with complex I, we analyzed toxicity in SK-N-MC cells expressing the rotenone-insensitive single-subunit NADH dehydrogenase of Saccharomyces cerevisiae (NDI1), which acts as a “replacement” for the entire complex I in mammalian cells (Seo et al., 2000, 2002; Bai et al., 2001). Malate-glutamate-driven mitochondrial respiration was increased twofoldtothreefoldinNDI-1-transducedcells.Oxygen utilization in NDI1-transduced cells, using complex I substrates (malate and glutamate), was rotenone-insensitive, but remained sensitive to the complex III inhibitor antimycin A, indicating that NDI1 integrated into the mitochondrial ETC and donated electrons appropriately to complex III (Fig. 2A).
Figure 2.
Rotenone toxicity requires electron transfer through complex I. A, Cells infected with rAAV-NDI1 are resistant to rotenone inhibition of oxygen utilization. Where indicated, 5 mM glutamate (Glu), 5 mM malate (Mal), 5μM rotenone (Rot), 500μM flavone, 5 mM succinate (Succ), and 5 μM antimycin (AntA) were added. Note that oxygen utilization in NDI1 transfected cells was not sensitive to rotenone inhibition but that antimycin A still inhibited oxygen consumption. B, NDI1-transduced cells were resistant to rotenone (100 nM) toxicity but remained sensitive to hydrogen peroxide (300 μM) and azide (1 mM). Control and NDI1transduced cells were grown in 96 well plates, and cell death was analyzed 48hr after exposure to compounds. Results represent means ± SEM for three independent experiments.
Control and NDI1-transduced cells were exposed to rotenone (100 nM), and toxicity was analyzed at 48 hr. Untransduced SKN-MC cells exhibited substantial death in response to rotenone (Figs. 1, 2B), but NDI1-transduced cells were completely resistant to rotenone-induced cell death, indicating that the site of action of rotenone was complex I (Fig. 2B). As expected, NDI1transduced cells remained sensitive to the oxidative stressor, 300 μM hydrogen peroxide, and to the complex IV inhibitor, 1 mM azide (Fig. 2B).
Rotenone toxicity does not result solely from ATP depletion
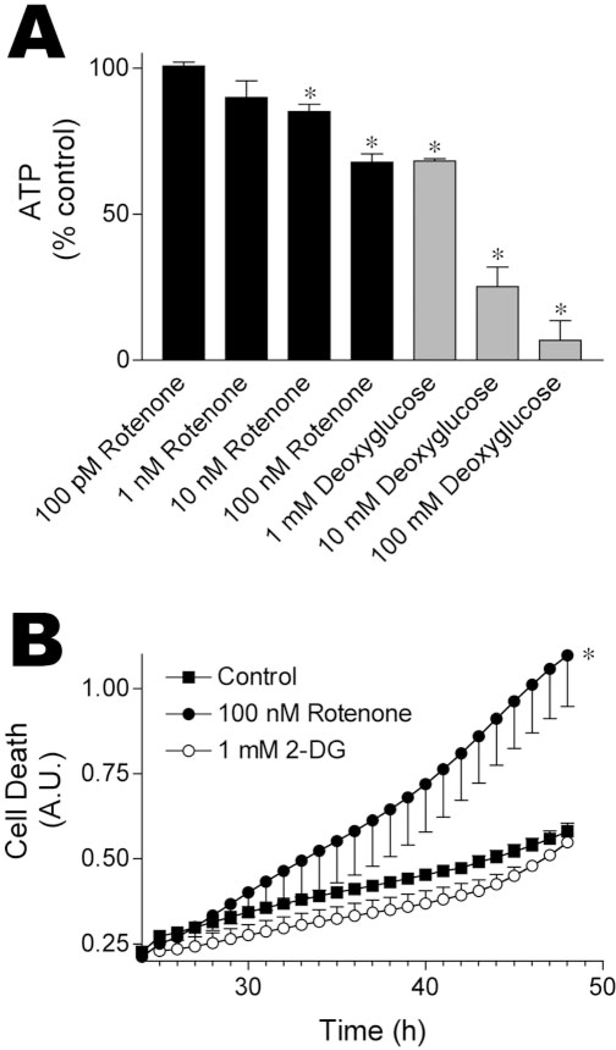
Complex I inhibition after rotenone exposure can have several cellular consequences, including depletion of cellular ATP. Rotenone treatment (6–8 hr) mildly depleted cellular ATP levels (32.2 ± 2.9% decrease at 100nM; 15.0 ± 2.5% at 10nM) (Fig.3A). Similar results were observed at 20 hr (data not shown). To determine whether rotenone toxicity resulted solely from ATP depletion, we examined the effects of 2-DG exposure. 2-DG caused dose-dependent ATP depletion, with 1mM2-DG causing an ATP depletion similar to 100 nM rotenone (Fig. 3A). Despite causing energy impairment similar to 100 nM rotenone, 2-DG exposure did not cause toxicity (Fig. 3B).
Figure3.
Rotenone toxicity does not result solely from ATP depletion. A, ATP depletion after exposure to rotenone or 2-DG. Note that rotenone (100 nM) and 2-DG (1 mM) caused similar reductions in cellular ATP levels. Cells were treated for 6–8 hr before ATP measurements; similar results were obtained at 20 hr. Data represent means ± SEM for three to five independent experiments. *p 0.05 versus ATP levels in vehicle-treated wells. B, Although rotenone (100nM) and 2-DG(1mM) caused similar bioenergetic defects, only rotenone (100nM) was toxic. Cells were exposed to rotenone or 2-DG for 48 hr, and cell death was determined using Sytox green fluorescence. Results show means ± SEM of three independent experiments, six replicates per experiment. *p 0.05 versus death in vehicle-treated wells.
Rotenone exposure causes oxidative damage in vitro
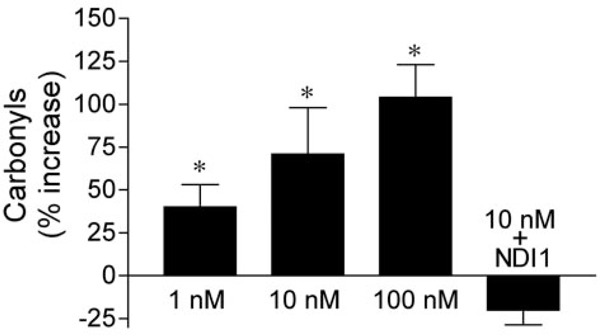
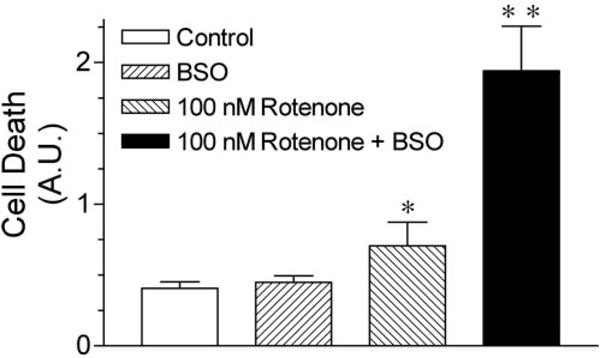
Another potential consequence of complex I impairment is oxidative stress. Rotenone exposure caused dose-dependent elevations in oxidative damage, indicated by increased protein carbonyl levels (Fig. 4). 2-DG (1 mM) did not elevate protein carbonyl levels (data not shown). Rotenone treatment did not cause oxidative damage in NDI1-transduced cells, suggesting that electron transfer through complex I is required for rotenone to induce oxidative stress (Fig. 4). As another measure of oxidative damage, we measured total cellular glutathione levels. Cellular glutathione levels were 4.97 ± 1.2 nmol/mg protein in control cells. Rotenone treatment (10 nM for 24 hr) reduced total cellular glutathionelevelsby57 ± 14%.Thus,10nM rotenone has a much larger effect on glutathione than on ATP levels. At 1 nM, rotenone did not alter total cellular glutathione levels over the same period. To further determine the relationship between oxidative stress and rotenone toxicity, we depleted cellular glutathione with 10 μM buthionine sulfoximine (BSO) (Jha et al., 2000) before rotenone exposure. Although BSO was not toxic by itself, the pretreatment of cells with BSO potentiated rotenone-induced cell death (Fig. 5).
Figure4.
Rotenone-induced oxidative damage is dose-dependent and depends on electron transfer through complex I. Untransfected SK-N-MCandNDI1-transfected cells were exposed to rotenone for 24 hr, and then protein carbonyls were measured. Protein carbonyl levels are expressed as a percentage change from levels in solvent-treated cultures. NDI1-transfectedcells received 10 nM rotenone for 24 hr. Data shown are means ± SEM and represent five to six independent experiments. *p 0.05 versus solvent-treated controls.
Figure5.
Glutathione depletion potentiates rotenone toxicity. Cells were exposed to 10μM BSO for 24 hr to deplete cellular glutathione before treatment with 10 nM rotenone. Cell death was then analyzed using Sytox green fluorescence, and death at 36hr after rotenone treatment is shown. Results shown are means ± SEM for three independent experiments. *p < 0.05 versus solvent-treated controls; **p < 0.05 versus rotenone-treated wells.
Antioxidants protect against rotenone toxicity and oxidative damage
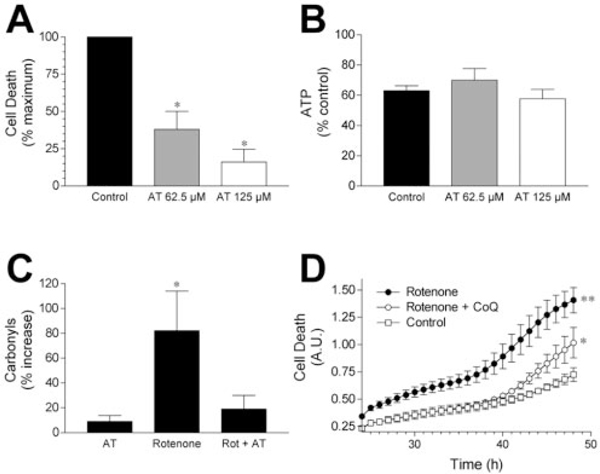
To further test the relevance of rotenone-induced oxidative damage to cell death, we investigated the effects of antioxidants. Rotenone-induced death was reduced by pretreatment with α-tocopherol in a dose-dependent manner (Fig. 6A). After α-tocopherol (62.5 μM) pretreatment, cell death induced by 100 nM rotenone was attenuated by 72 ± 10%, and that caused by 10 nM rotenone was reduced by 87 ± 5%. α-Tocopherol treatment alone did not influence cell survival (data not shown). The beneficial effect of α-tocopherol on survival was not attributable to the preservation of ATP, because the rotenone-induced drop in ATP was unaffected by the antioxidant (Fig. 6B). Finally, although α-tocopherol pretreatment alone did not alter baseline protein carbonyl levels, it markedly attenuated rotenone induced elevations in protein carbonyls (Fig. 6B). Coenzyme Q10 (12.5 μM) pretreatment also reduced rotenone-induced cell death (Fig.6D). Coenzyme Q10 treatment alone did not influence cell survival, and lower doses of coenzyme Q10 failed to protect against rotenone toxicity.
Figure 6.
Antioxidants protect cells from rotenone toxicity. A, α-Tocopherol pretreatment [62.5 μM (gray column) or 125 μM (white column)] attenuated rotenone toxicity. Death was expressed as a percentage of maximal cell death after rotenone treatment. Results represent means ± SEM for four to five independent experiments. *p 0.05 versus rotenone-induced death (black column). B, α-Tocopherol pretreatment did not prevent ATP depletions after rotenone exposure. Cells were exposed to solvent or α-tocopherol (62.5 or 125 μM) before rotenone exposure, and ATP levels were measured. Column colors are as in A. Data shown are means ± SEM and represent three independent experiments. C, α-Tocopherol pretreatment prevented rotenone-induced oxidative damage. Cells were exposed to solvent or 125 μM α-tocopherol for 24 hr before rotenone (10 nM) treatment and before protein carbonyls were measured. Carbonyls are expressed as a percentage change from levels in solvent-treated cultures. Data shown are means ± SEM and represent five independent experiments. *p 0.05 versus solvent-treated controls. D, Coenzyme Q10 (CoQ) pretreatment attenuated rotenone toxicity. Cells were pretreated for 24 hr with 12.5 μM coenzyme Q10 before exposure to 10 nM rotenone. Cell death was followed for 48 hr after rotenone treatment, and the last 24 hr period is shown. **p < 0.05 versus rotenone-treated cells in the presence of CoQ and versus solventtreated cells; *p < 0.05 versus solvent-treated cells.
Effects of rotenone on dopaminergic midbrain neurons
To determine the relevance of rotenone-induced oxidative damage to dopaminergic neuronal toxicity, we used a chronic midbrain slice culture model. This system used a longer rotenone exposure to more closely mimic the in vivo rotenone model. Mid-brain slice cultures were exposed to 50 nM rotenone for 1 week in the presence of 100 μM α-tocopherol or vehicle. Rotenone exposure increased protein carbonyls in midbrain slices by 23.4 ± 6.5% (Fig. 7A) and damaged midbrain dopaminergic neurons, as determined by TH immunocytochemistry (Figs. 7B,C). In rotenone-treated slice cultures, there was marked pruning and loss of dendritic and axonal processes in dopaminergic neurons (Figs. 7B,C). The toxic effects of rotenone on dopaminergic mid-brain neurons were attenuated by treatment with α-tocopherol (Figs. 7B–D). α-Tocopherol treatment also prevented rotenone induced oxidative damage in midbrain slice cultures (Fig. 7A).
Figure 7.
α-Tocopherol protected dopaminergic neurons in midbrain slice cultures from rotenone oxidative damage and toxicity. A, Rotenone caused oxidative damage to midbrain slice cultures that was prevented by α-tocopherol treatment. Slice cultures were exposed to rotenone (50nM) for 7d in the presence of vehicle or-tocopherol (100M).Data are expressed as a percentage increase in protein carbonyl levels in rotenone-treated cultures. *p 0.05 versus solvent-treated cells. B–D, Rotenone toxicity in midbrains lice cultures was prevented by α-tocopherol. Midbrain slice cultures were exposed to vehicle (B) or 50 nM rotenone for 7 d in the presence of vehicle (C) or -tocopherol (100 M) (D). Rotenone exposure markedly reduced the number of TH processes in midbrain dopaminergic neurons (compare B and C). -Tocopherol treatment reduced the effects of rotenone on these degenerative changes (D).
Chronic systemic rotenone exposure causes oxidative damage in vivo
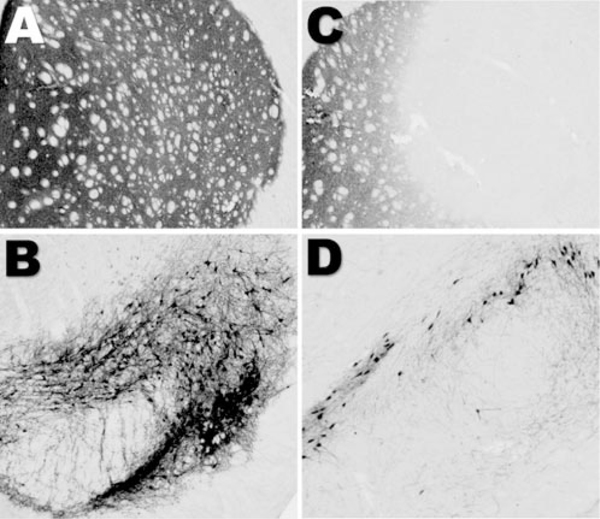
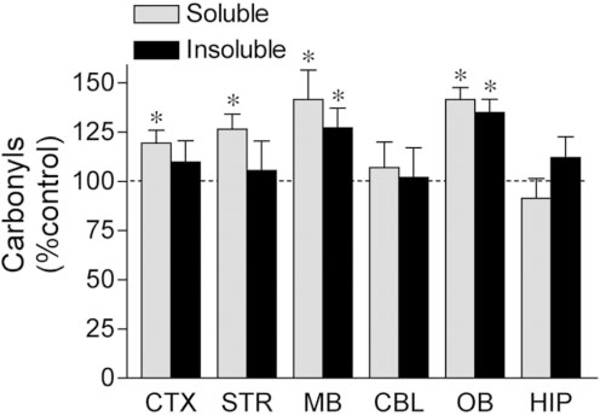
Finally, we assessed oxidative damage in the in vivo rotenone model. We demonstrated previously that systemic rotenone infusion to Lewis rats causes selective nigrostriatal dopaminergic degeneration (Betarbet et al., 2000; Sherer et al., 2003). An example of dopaminergic degeneration in a rotenone-treated animal is shown in Fig. 8. In this rotenone-treated lesioned rat, there was a striatal region devoid of TH immunoreactivity (Fig. 8A,B) and decreased TH immunoreactivity in the substantia nigra (Figs. 8C,D). As demonstrated previously, rotenone-induced loss of TH expression in the nigrostriatal pathway represents true neurodegeneration (Betarbet et al., 2000; Sherer et al., 2003). We analyzed protein carbonyl levels as a measure of oxidative damage in various brain regions of control and rotenone-treated rats. No differences were observed in basal levels of protein carbonyls across brain regions in control animals. Despite causing uniform complex I inhibition throughout the brain (Betarbet et al., 2000), systemic rotenone exposure caused selective oxidative protein damage (Fig. 9). Systemic rotenone infusion modestly elevated soluble protein carbonyls in the cortex by 19 ± 6% and in the striatum by 27 ± 8%, whereas it had no effect in the cerebellum and hippocampus. The largest elevations in soluble protein carbonyls occurred in dopaminergic areas, the midbrain (41 ± 15%), and the olfactory bulb (41 ± 6%). Rotenone-induced elevations in insoluble protein carbonyls were even more selective, with increases occurring only in the midbrain (27 ± 10%) and olfactory bulb (35 ± 7%). The extent of oxidative damage did not differ between lesioned and non lesioned rotenone-infused animals.
Figure 8.
Rotenone-induced loss of TH immunoreactivity in the nigrostriatal pathway. TH immunocytochemistry in the striatum (A) and substantia nigra (B) of a vehicle-treated rat is shown. Rotenone treatment reduced TH immunore activity in both the striatum(C) and the substantia nigra (D).The rotenone-treated animal received 3.0mg/kg/d rotenone, subcutaneously, for 5d.
Figure 9.
Chronic rotenone infusion induced selective oxidative damage, with the greatest damage in the midbrain and the olfactory bulb. Chronic rotenone infusion increased protein carbonyl levels in soluble protein isolated from the striatum (n = 18), midbrain (n = 18), cortex (n 11), and olfactory bulb (n 12) but not the cerebellum (n = 10) or hippocampus (n = 10). Rotenone exposure increased insoluble protein carbonyl levels only in mid brain (n = 18) and olfactory bulb (n = 12). Data are expressed as a percentage of protein carbonyl levels in vehicle-treated animals. Results represent means ± SEM. *p < 0.05 compared with control.
Discussion
Mitochondrial dysfunction, particularly complex I impairment, has been implicated in PD pathogenesis (Schapira, 1999). PD patients demonstrate systemic reductions in complex I activity and MPP, the active metabolite of the parkinsonism toxin, MPTP, acts as a complex I inhibitor (Nicklas et al., 1985; Mizuno et al., 1989; Parker et al., 1989; Schapira et al., 1989; Cardellach et al., 1993; Haas et al., 1995). The mitochondrial hypothesis for PD was bolstered by the discovery that rotenone, a highly selective complex I inhibitor, reproduced features of PD when administered to rats using a chronic treatment regimen (Betarbet et al., 2000; Sherer et al., 2003). Although systemic rotenone infusion caused uniform complex I inhibition throughout the brain (Betarbet et al., 2000), rotenone exposure caused selective degeneration to the nigrostriatal dopaminergic pathway. In the present study, using three model systems of increasing complexity, we determined that rotenone toxicity involved an oxidative mechanism. In neuroblastoma cells, rotenone toxicity depended on an interaction with complex I. Antioxidants attenuated rotenone induced death, suggesting a causal role for oxidative damage. Using a chronic mid brain slice model, oxidative damage was similarly implicated in rotenone-induced dopaminergic neuronal death. Finally, systemic in vivo rotenone exposure caused relatively selective oxidative damage to midbrain and olfactory bulb, regions marked by selective neurodegeneration after rotenone treatment (Betarbet et al., 2000; Sherer et al., 2001, 2003). Together, these results substantiate involvement of oxidative damage in rotenone toxicity.
Rotenone toxicity depended on a direct interaction with complex I, because cells expressing the rotenone-insensitive, single subunit NADH dehydrogenase of yeast, NDI1, were resistant to rotenone toxicity. In this system, electrons from complex I substrates (e.g., glutamate) are shunted through NDI1 into downstream portions of the ETC, thereby allowing mitochondrial respiration to bypass complex I inhibition. Thus, the effects of rotenone observed in our system (modest ATP depletion, oxidative damage, and cell death) can be entirely explained by the inhibition of complex I. Increasing evidence suggests that complex I may be a source of ROS. There is a site of electron leak age in complex I upstream of the rotenone-binding site; partial inhibition of complex I, as produced by rotenone or as reported in PD, can enhance ROS production (Cassarino et al., 1997; Hensley et al., 1998; Barrientos and Moraes, 1999; Kushnareva et al., 2002).
Our results indicate that rotenone toxicity did not result solely from a bioenergetic defect. Brain levels of rotenone after chronic rotenone exposure were not sufficient to alter mitochondrial respiration in isolated brain mitochondria (Betarbet et al., 2000). In addition, although both rotenone (100 nM) and 2-DG (1 mM) caused similar ATP depletion, only rotenone exposure was toxic, suggesting that rotenone toxicity resulted from additional mechanisms. Thus, instead of a bioenergetic defect per se, rotenone toxicity appears to result primarily from oxidative damage. In neuroblastoma cells, rotenone caused dose-dependent oxidative damage, and rotenone toxicity was prevented by two antioxidants, -tocopherol and coenzyme Q10. The oxidative damage hypothesis is further supported by the fact that -tocopherol pretreatment prevented rotenone-induced oxidative damage but did not attenuate rotenone-induced ATP depletions.
Using a chronic midbrain slice model, rotenone-induced oxidative damage and toxicity to mature dopaminergic neurons were also attenuated by antioxidant treatment. Because oxidative damage appeared to be important in the degeneration of dopaminergic neurons in vitro, it was of interest to determine whether rotenone-induced oxidative damage was relevant to the in vivo model of rotenone exposure. Systemic rotenone infusion, which caused uniform complex I inhibition throughout the brain (Betarbet et al., 2000), was marked by selective oxidative damage and neurodegeneration. The regions demonstrating the greatest oxidative protein damage, the midbrain and olfactory bulb, are rich in dopaminergic neuronal elements, and both regions degenerate after rotenone exposure (Betarbet et al., 2000; Sherer et al., 2001, 2003). It has also been reported that brain mitochondria isolated from rotenone-treated animals show enhanced superoxide production (Cormier et al., 2003). Because oxidative damage occurred before the development of nigrostriatal dopaminergic lesions, we hypothesize that oxidative damage may play a primary role in rotenone-induced neurodegeneration.
Rotenone infusion replicates the oxidative damage observed in the brains of PD patients, in which there is increased oxidative damage to lipids, DNA, and protein (Dexter et al., 1989; Yoritaka et al., 1996; Alam et al., 1997; Pearce et al., 1997; Floor and Wetzel, 1998). Other experimental models of PD, including 6-hydroxydopamine, paraquat, and MPTP, also implicate oxidative stress in PD (Roghani and Behzadi, 2001; McCormack et al., 2002; Mollace et al., 2003). For example, MPTP toxicity is attenuated by antioxidants or spin trap agents (Matthews et al., 1999).
Because in vivo rotenone infusion resulted in uniform complex I inhibition across brain regions (Betarbet et al., 2000), ROS production by complex I dysfunction cannot fully explain the selectivity of oxidative damage resulting from rotenone exposure. Nevertheless, rotenone infusion caused relatively selective oxidative damage to certain dopamine-rich brain regions known to degenerate in PD. We hypothesize that there may be an interaction between ROS synthesized during rotenone-induced mitochondrial inhibition and ROS produced during dopamine metabolism. Dopaminergic neurons are believed to exist normally in a state of compensated oxidative stress because they contain dopamine. Normal metabolism of dopamine by monoamine oxidase produces H2O2 (Maker et al., 1981), which is sufficient to increase levels of oxidized glutathione (Maker et al., 1981; Spina and Cohen, 1988), and which suggests ongoing oxidative stress. In addition, in the presence of iron (abundant in the substantia nigra), H2O2 is converted to the reactive and highly damaging hydroxyl radical. Furthermore, dopamine may be oxidized nonenzymatically to form reactive dopamine semiquinones (Hastings et al., 1996). For these reasons, we propose that the greatest oxidative damage after rotenone exposure may occur in dopaminergic neurons. If so, because protein from dopaminergic neurons comprised only a small percentage of the total midbrain homogenate, the extent of oxidative damage in dopaminergic neurons may be much greater than that observed for the entire homogenate.
Oxidative damage after rotenone exposure may explain, in part, the aggregation of α-synuclein caused by rotenone (Betarbet et al., 2000). In the PD brain, α-synuclein becomes oxidatively modified and insoluble, leading to aggregation (Giasson et al., 2000). In vitro, rotenone-induced -synuclein aggregation (Lee et al., 2001) is correlated with elevations in insoluble protein carbonyls (Lee et al., 2001; Sherer et al., 2002b). In addition, in vivo rotenone infusion results in selective upregulation of α-synuclein in the substantia nigra and the increased occurrence of higher-molecular-weight, aggregated forms of α-synuclein (Betarbet et al., 2000, 2002; Höglinger et al., 2003). An elevation in insoluble protein carbonyls in the midbrains of rotenone treated animals suggests that rotenone treatment may cause oxidative modifications to α-synuclein that make it more likely to aggregate (Giasson et al., 2000; Krishnan et al., 2003). On the other hand, elevated α-synuclein expression can itself cause oxidative damage and render cells more vulnerable to oxidative insults (Hsu et al., 2000; Kanda et al., 2000; Ko et al., 2000). We are currently investigating whether rotenone treatment causes oxidative modifications to α-synuclein.
Both coenzyme Q10 and α-tocopherol were protective in our in vitro systems, and both are currently being discussed as potential therapies for PD. Recently, a small, randomized, placebo controlled, double-blind study suggested that coenzyme Q10 may slow PD progression (Shults et al., 2002). The initial studies examining α-tocopherol as a disease-modifying therapy for PD have not been as encouraging, but this is likely because of the low brain penetrance of α-tocopherol at the doses used (Parkinson Study Group, 1993). Indeed, in preliminary studies, 2 months of continuous, systemic, high-dose α-tocopherol treatment in rats had only marginal effects on brain levels of the antioxidant (our unpublished results). Nevertheless, α-tocopherol prevents oxidative damage in culture and has neuroprotective properties against 6-hydroxydopamine and paraquat toxicity (Storch et al., 2000; Roghani and Behzadi, 2001; Choi et al., 2003; Mollace et al., 2003). In our study, α-tocopherol exerted its protection by reducing oxidative damage without affecting rotenone-induced ATP depletion. Future studies will determine whether high-dose α-tocopherol, given more long term, is neuroprotective in vivo.
The rotenone model of PD substantiated the involvement of mitochondrial dysfunction and environmental exposures in PD. The current study demonstrates (1) that rotenone acts specifically at complex I, and (2) that oxidative damage, which has been strongly implicated in PD pathogenesis, plays a pivotal role in rotenone toxicity both in vivo and in vitro. Future studies will focus on the ability of antioxidants to attenuate rotenone toxicity in vivo. A better understanding of the mechanisms through which antioxidants provide neuroprotection may uncover novel avenues for PD therapeutics.
Acknowledgments
This work was supported by National Institutes of Health Grants NS38899 (J.T.G.), ES012068 (J.T.G. and G.W.M.), T32NS07480 (J.R.R.), and DK53244 and NS43776 (A.M.-Y. and T.Y.).T.B.S. is a Michael J. Fox Foundation Fellow. We thank Dr. Terence R. Flotte for construction of the rAAV-NDI1 and Serena Lund and Hye Mee Na for their technical assistance.
References
- Alam A, Schmidt WJ (2002) Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav Brain Res 136. [DOI] [PubMed] [Google Scholar]
- Alam ZI, Daniel SE, Lees AJ, Marsden DC, Jenner P, Halliwell B (1997) A generalised increase in protein carbonyls in the brain in Parkinson’s but not incidental Lewy body disease. J Neurochem 69:1326–1329. [DOI] [PubMed] [Google Scholar]
- Bai Y, Hajek P, Chomyn A, Chan E, Seo BB, Matsuno-Yagi A, Yagi T, Attardi G (2001) Lack of complex I activity in human cells carrying a mutation in MtDNA-encoded ND4 subunit is corrected by the Saccharomyces cerevisiae NADH-quinone oxidoreductase (NDI1) gene. J Biol Chem 276:38808–38813. [DOI] [PubMed] [Google Scholar]
- Barrientos A, Moraes CT (1999) Titrating the effects of mitochondrial complex I impairment in the cell physiology. J Biol Chem 274:16188–16197. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000) Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3:1301–1306. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, Kim JH, Baptista M, Cookson MR, Greenamyre JT (2002) Rotenone models of Parkinson’s disease: altered alpha-synuclein expression following chronic inhibition of complex I. Soc Neurosci Abstr 386.316. [Google Scholar]
- Cardellach F, Marti MJ, Fernandez-Sola J, Marin C, Hoek JB, Tolosa E, Urbano-Marquez A (1993) Mitochondrial respiratory chain activity in skeletal muscle from patients with Parkinson’s disease. Neurology 43:2258–2262. [DOI] [PubMed] [Google Scholar]
- Cassarino DS, Fall CP, Swerdlow RH, Smith TS, Halvorsen EM, Miller SW, Parks JP, Parker WD Jr, Bennett JP Jr (1997) Elevated reactive oxygen species and antioxidant enzyme activities in animal and cellular models of Parkinson’s disease. Biochim Biophys Acta 1362:77–86. [DOI] [PubMed] [Google Scholar]
- Choi J, Conrad CC, Dai R, Malakowsky CA, Talent JA, Carroll CA, Weintraub ST, Gracy RW (2003) Vitamin E prevents oxidation of antiapoptotic proteins in neuronal cells. Proteomics 3:73–77. [DOI] [PubMed] [Google Scholar]
- Cormier A, Morin C, Zini R, Tillement JP, Lagrue G (2003) Nicotine protects rat brain mitochondria against experimental injuries. Neuropharmacology 44:642–652. [DOI] [PubMed] [Google Scholar]
- Dexter DT, Carter CJ, Wells FR, Javoy-Agid F, Agid Y, Lees A, Jenner P, Marsden CD (1989) Basal lipid peroxidation in substantia nigra is increased in Parkinson’s disease. J Neurochem 52:381–389. [DOI] [PubMed] [Google Scholar]
- Floor E, Wetzel MG (1998) Increased protein oxidation in human substantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J Neurochem 70:268–275. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM (2000) Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science 290:985–989. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Richardson RJ (1998) The risk of Parkinson’s disease with exposure to pesticides, farming, well water, and rural living. Neurology 50:1346–1350. [DOI] [PubMed] [Google Scholar]
- Haas RH, Nasirian F, Nakano K, Ward D, Pay M, Hill R, Shults CW (1995) Low platelet mitochondrial complex I and complex II/III activity in early untreated Parkinson’s disease. Ann Neurol 37:714–722. [DOI] [PubMed] [Google Scholar]
- Hastings TG, Lewis DA, Zigmond MJ (1996) Reactive dopamine metabolites and neurotoxicity: implications for Parkinson’s disease. Adv Exp Med Biol 387:97–106. [DOI] [PubMed] [Google Scholar]
- Hensley K, Pye QN, Maidt ML, Stewart CA, Robinson KA, Jaffrey F, Floyd RA (1998) Interaction of alpha-phenyl-N-tert-butyl nitrone and alternative electron acceptors with complex I indicates a substrate reduction site upstream from the rotenone binding site. J Neurochem 71:2549–2557. [DOI] [PubMed] [Google Scholar]
- Höglinger GU, Feger J, Prigent A, Michel PP, Parain K, Champy P, Ruberg M, Oertel WH, Hirsch EC (2003) Chronic systemic complex I inhibition induces a hypokinetic multisystem degeneration in rats. J Neurochem 84:491–502. [DOI] [PubMed] [Google Scholar]
- Hsu LJ, Sagara Y, Arroyo A, Rockenstein E, Sisk A, Mallory M, Wong J, Takenouchi T, Hashimoto M, Masliah E (2000) alpha-synuclein promotes mitochondrial deficit and oxidative stress. Am J Pathol 157:401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner P (1998) Oxidative mechanisms in nigral cell death in Parkinson’s disease. Mov Disord 13:24–34. [PubMed] [Google Scholar]
- Jha N, Jurma O, Lalli G, Liu Y, Pettus EH, Greenamyre JT, Liu RM, Forman HJ, Andersen JK (2000) Glutathione depletion in PC12 results in selective inhibition of mitochondrial complex I activity. Implications for Parkinson’s disease. J Biol Chem 275:26096–26101. [DOI] [PubMed] [Google Scholar]
- Kanda S, Bishop JF, Eglitis MA, Yang Y, Mouradian MM (2000) Enhanced vulnerability to oxidative stress by alpha-synuclein mutations and C-terminal truncation. Neuroscience 97:279–284. [DOI] [PubMed] [Google Scholar]
- Ko L, Mehta ND, Farrer M, Easson C, Hussey J, Yen S, Hardy J, Yen SH (2000) Sensitization of neuronal cells to oxidative stress with mutated human alpha-synuclein. J Neurochem 75:2546–2554. [DOI] [PubMed] [Google Scholar]
- Krishnan S, Chi EY, Wood SJ, Kendrick BS, Li C, Garzon-Rodriguez W, Wypych J, Randolph TW, Narhi LO, Biere AL, Citron M, Carpenter JF (2003) Oxidative dimer formation is the critical rate-limiting step for Parkinson’s disease alpha-synuclein fibrillogenesis. Biochemistry 42:829–837. [DOI] [PubMed] [Google Scholar]
- Kushnareva Y, Murphy AN, Andreyev A (2002) Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P) oxidation-reduction state. Biochem J 368:545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Shin SY, Choi C, Lee YH, Lee SJ (2001) Formation and removal of alpha-synuclein aggregates in cells exposed to mitochondrial inhibitors. J Biol Chem 27:27. [DOI] [PubMed] [Google Scholar]
- Maker HS, Weiss C, Silides DJ, Cohen G (1981) Coupling of dopamine oxidation (monoamine oxidase activity) to glutathione oxidation via the generation of hydrogen peroxide in rat brain homogenates. J Neurochem 36:589–593. [DOI] [PubMed] [Google Scholar]
- Matthews RT, Klivenyi P, Mueller G, Yang L, Wermer M, Thomas CE, Beal MF (1999) Novel free radical spin traps protect against malonate and MPTP neurotoxicity. Exp Neurol 157:120–126. [DOI] [PubMed] [Google Scholar]
- McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, Di Monte DA (2002) Environmental risk factors and Parkinson’s disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis 10:119–127. [DOI] [PubMed] [Google Scholar]
- Menegon A, Board PG, Blackburn AC, Mellick GD, Le Couteur DG (1998) Parkinson’s disease, pesticides, and glutathione transferase polymorphisms. Lancet 352:1344–1346. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Ohta S, Tanaka M, Takamiya S, Suzuki K, Sato T, Oya H, Ozawa T, Kagawa Y (1989) Deficiencies in complex I subunits of the respiratory chain in Parkinson’s disease. Biochem Biophys Res Commun 163:1450–1455. [DOI] [PubMed] [Google Scholar]
- Mollace V, Iannone M, Muscoli C, Palma E, Granato T, Rispoli V, Nistico R, Rotiroti D, Salvemini D (2003) The role of oxidative stress in paraquatinduced neurotoxicity in rats: protection by non peptidyl superoxide dismutase mimetic. Neurosci Lett 335:163–166. [DOI] [PubMed] [Google Scholar]
- Nicklas WJ, Vyas I, Heikkila RE (1985) Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci 36:2503–2508. [DOI] [PubMed] [Google Scholar]
- Parker WD Jr, Boyson SJ, Parks JK (1989) Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Ann Neurol 26:719–723. [DOI] [PubMed] [Google Scholar]
- Parkinson Study Group (1993) Effects of tocopherol and deprenyl on the progression of disability in early Parkinson’s disease. N Engl J Med 328:176–183. [DOI] [PubMed] [Google Scholar]
- Pearce RK, Owen A, Daniel S, Jenner P, Marsden CD (1997) Alterations in the distribution of glutathione in the substantia nigra in Parkinson’s disease. J Neural Transm 104:661–677. [DOI] [PubMed] [Google Scholar]
- Roghani M, Behzadi G (2001) Neuroprotective effect of vitamin E on the early model of Parkinson’s disease in rat: behavioral and histochemical evidence. Brain Res 892:211–217. [DOI] [PubMed] [Google Scholar]
- Schapira AH (1999) Mitochondrial involvement in Parkinson’s disease, Huntington’s disease, hereditary spastic paraplegia and Friedreich’s ataxia. Biochim Biophys Acta 1410:159–170. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD (1989) Mitochondrial complex I deficiency in Parkinson’s disease. Lancet 1:1269. [DOI] [PubMed] [Google Scholar]
- Seo BB, Wang J, Flotte TR, Yagi T, Matsuno-Yagi A (2000) Use of the NADH-quinone oxidoreductase (NDI1) gene of Saccharomyces cerevisiae as a possible cure for complex I defects in human cells. J Biol Chem 275:37774–37778. [DOI] [PubMed] [Google Scholar]
- Seo BB, Nakamaru-Ogiso E, Flotte TR, Yagi T, Matsuno-Yagi A (2002) A single-subunit NADH-quinone oxidoreductase renders resistance to mammalian nerve cells against complex I inhibition. Mol Ther 6:336–341. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Greenamyre JT (2001) The rotenone model of Parkinson’s disease in vivo: selective striatal oxidative damage and caspase-3 activation in nigrostriatal neurons. Soc Neurosci Abstr 27:653.2. [Google Scholar]
- Sherer TB, Betarbet R, Greenamyre JT (2002a) Environment, mitochondria, and Parkinson’s disease. Neuroscientist 8:192–197. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Stout AK, Lund S, Baptista M, Panov AV, Cookson MR, Greenamyre JT (2002b) An in vitro model of Parkinson’s disease: linking mitochondrial impairment to altered -synuclein metabolism and oxidative damage. J Neurosci 22:7006–7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer TB, Kim JH, Betarbet R, Greenamyre JT (2003) Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp Neurol 179:9–16. [DOI] [PubMed] [Google Scholar]
- Shults CW, Oakes D, Kieburtz K, Beal MF, Haas R, Plumb S, Juncos JL, Nutt J, Shoulson I, Carter J, Kompoliti K, Perlmutter JS, Reich S, Stern M, Watts RL, Kurlan R, Molho E, Harrison M, Lew M (2002) Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol 59:1541–1550. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388:839–840. [DOI] [PubMed] [Google Scholar]
- Spina MB, Cohen G (1988) Exposure of striatal synaptosomes to L-dopa increases levels of oxidized glutathione. J Pharmacol Exp Ther 247:502–507. [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D (1991) A simple method for organotypic cultures of nervous tissue. J Neurosci Methods 37:173–182. [DOI] [PubMed] [Google Scholar]
- Storch A, Kaftan A, Burkhardt K, Schwarz J (2000) 6-Hydroxydopamine toxicity towards human SH-SY5Y dopaminergic neuroblastoma cells: independent of mitochondrial energy metabolism. J Neural Transm 107:281–293. [DOI] [PubMed] [Google Scholar]
- Wooten GF (1997) Neurochemistry and neuropharmacology of Parkinson’s disease. In: Movement disorders: neurologic principles and practice (Watts, Koller WC, eds), pp 153–160. New York: McGraw-Hill. [Google Scholar]
- Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y (1996) Immunohistochemicaldetectionof4-hydroxynonenalproteinadductsin Parkinson disease. Proc Natl Acad Sci USA 93:2696–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]