Abstract
The antifibrotic therapies nintedanib and pirfenidone were first approved by the United States for the treatment of idiopathic pulmonary fibrosis in 2014. In 2020, nintedanib received U.S. Food and Drug Administration (FDA) approval for the treatment of all progressive fibrosing interstitial lung disease (ILD). Given that a major cause of mortality and morbidity in the idiopathic inflammatory myopathies (IIM) is progressive interstitial lung disease and respiratory failure, antifibrotic therapies may be useful as adjuvant to traditional immunosuppression. However, randomized controlled trials of antifibrotic therapies in IIM are lacking. The purpose of this review is to (1) summarize the mechanism of action of nintedanib and pirfenidone in ILD with possible role in IIM-ILD, (2) review the clinical data supporting their use in interstitial lung disease in general, and more specifically in connective tissue disease associated ILD, and (3) discuss the evidence and remaining challenges for using antifibrotic therapies in IIM-ILD.
Keywords: antifibrotic therapies, antisynthetase syndrome, dermatomyositis, idiopathic inflammatory myopathies, interstitial lung disease, polymyositis
Introduction
Introduction to idiopathic inflammatory myopathies (IIM)-associated interstitial lung disease (ILD)
The idiopathic inflammatory myopathies encompass a group of heterogeneous systemic autoimmune disorders, including dermatomyositis (DM), polymyositis (PM), connective tissue disease associated myositis (overlap myositis), and the anti-tRNA synthetase syndrome (ARS), which affect the skin, muscle, and lungs in variable combinations. These disorders are uncommon, although the prevalence does vary by geographic region. In the United States and Sweden, the prevalence has been estimated to be 10–20 per 100,000 persons.1,2 A Korean study reported a slightly lower incidence of 2.3–4.0 per 100,000 persons. 3 Several myositis-specific and associated autoantibodies have been identified, and each of these autoantibodies is associated with unique clinical phenotype and prognosis. 4
Interstitial lung disease (ILD) is a prevalent end-organ manifestation of IIM (30–40%) and is serologically strongly associated with two types of myositis-specific antibodies—anti-synthetase anti-bodies (Jo-1, PL-7, PL-12, EJ, OJ) and anti-melanoma-associated protein 5 (MDA5)—as well as various myositis-associated autoantibodies such as anti-PmScl, anti-Ku, anti-SSA52, and anti-U1RNP.4–6 IIM-ILD is associated with a high mortality rate.7–9 One study estimated that 71% of the mortality in clinically amyopathic dermatomyositis and 60% of mortality in primary dermatomyositis was attributable to ILD.10–14 Historically, immunosuppressive agents such as azathioprine (AZA), 15 intravenous immunoglobulin,16,17 mycophenolate mofetil (MMF), 15 rituximab,18,19 and tacrolimus20–22 have been used to treat IIM-ILD with variable efficacy. For MDA5 disease, rapidly progressive ILD is a feared and often fatal manifestation. 23 Both tofacitinib 24 and calcineurin inhibitors20,25,26 have been reported to improve outcomes in MDA5-associated RP-ILD. Nonetheless, some patients still have progressive disease despite immunosuppression leading to hypoxic respiratory failure and death.
The antifibrotic therapies nintedanib and pirfenidone were approved in the United States for the treatment of idiopathic pulmonary fibrosis (IPF) in 2014, and these medications improved survival for IPF patients.27,28 In March 2020, the FDA approved nintedanib to treat all patients with progressive fibrosing interstitial lung disease (PF-ILD). This approval was based on the phase III double-blind, randomized controlled INBUILD trial, which defined PF-ILD as (1) having a forced vital capacity (FVC) decline of >10% predicted, (2) an FVC decline of 5–10% plus either worsening symptoms or radiographic progression, or (3) worsening symptoms with radiographic progression. 29 Notably, nearly 25% of INBUILD participants carried a connective tissue disease (CTD) ILD diagnosis, including 13.4% rheumatoid arthritis (RA) ILD and 5.9% systemic sclerosis (SSc) ILD. Until this approval, patients with CTD-ILD were managed with immunosuppression alone,30–36 and patients with idiopathic pulmonary fibrosis (IPF) were managed with the antifibrotic therapies pirfenidone 37 and nintedanib. 38 The question then arose, what is the role for combining antifibrotic therapies with immunosuppression in CTD-ILD. Nintedanib was proven to be safe and efficacious in SSc-associated ILD in the phase III double-blind, randomized controlled, SENSCIS trial, leading to FDA approval for this indication. 39 In addition, nintedanib seems to have an additive benefit to mycophenolate mofetil in patients with systemic sclerosis-associated ILD. 40 However, the role of antifibrotic in IIM-ILD remains unclear due to lack of clinical trials. It is also unclear when in the disease course antifibrotics should be initiated. The purpose of this narrative review is to summarize the mechanism of action, clinical trials supporting use of antifibrotics in ILD, and discuss the potential role for antifibrotics in IIM-ILD.
Mechanisms of action
Pirfenidone
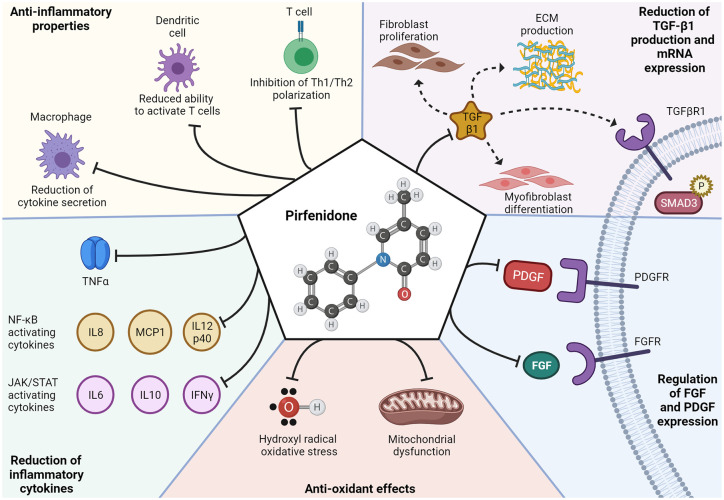
Pirfenidone was initially developed as an immunomodulatory agent with anti-inflammatory effects, but its antifibrotic effects strongly contribute to the efficacy in ILD. In animal models, pirfenidone reduces T cell activation by dendritic cells, 41 suppresses macrophage-mediated inflammation, 42 and reduces neutrophil influx.43,44 Pirfenidone also suppresses inflammatory cytokine production 45 to restore the Th1/Th2 balance through reduction of interferon-γ. Through cytokine modulation, pirfenidone could impact the JAK-STAT 46 and NF-κB 47 pathways that are activated in IIM. The mechanistic effects of pirfenidone are reviewed in detail by Ruwanpura et al. 48 (Figure 1).
Figure 1.
Proposed mechanisms of action for pirfenidone. Pirfenidone’s most recognized mechanism of action is the reduction of transforming growth factor (TGF)-β1, which leads to a subsequent reduction in TGF-β1-mediated fibroblast proliferation, myofibroblast differentiation, TGFβR1 signaling/SMAD3 phosphorylation, and extracellular matrix production. Pirfenidone has also been proposed to (1) reduce platelet derived growth factor (PDGF) and fibroblast growth factor (FGF) thereby decreasing PDGF/FGF signaling; (2) inhibit redox reactions to relieve oxidative stress from hydroxyl radicals and mitochondrial dysfunction; (3) reduce expression of cytokines tumor necrosis factor alpha (TNF-α), interleukin-6, interleukin-8 (IL-8), interleukin 10 (IL-10), interleukin 12 subunit p40 (IL12p40), interferon gamma (IFN-γ), and monocyte chemoattractant protein-1 (MCP1); and (4) modulate the cellular immune system by reducing macrophage cytokine secretion, reducing dendritic cell mediated T cell activation, and inhibiting Th1/Th2 polarization. 48
The antifibrotic effects of pirfenidone have also been investigated using human lung fibroblasts. Pirfenidone decreased human lung fibroblasts proliferation and reduced mRNA and protein levels of α-smooth muscle actin and procollagen B. Transforming growth factor (TGF)-β-mediated myofibroblast differentiation was also suppressed by inhibiting Smad3, Akt, and p38 phosphorylation. 49 TGF-β induced expression of Hsp47, a key chaperone for collagen secretion, is also inhibited by pirfenidone. 50 Pirfenidone inhibited the proliferation, invasion, and migration of RA-ILD fibroblasts derived from patient lung biopsies as well as myofibroblast differentiation. 51 As myofibroblasts are commonly observed in CTD-ILD,52–54 inhibition of myofibroblast differentiation is likely also important in IIM-ILD.
Nintedanib
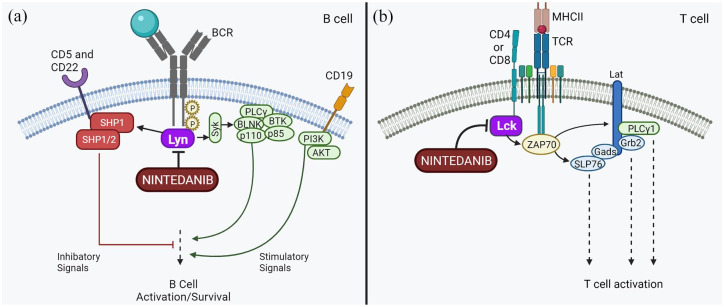
Nintedanib is a promiscuous tyrosine kinase inhibitor that inhibits Lck, Lyn, and other Src family kinases in addition to its well-recognized inhibition of platelet-derived growth factor receptor (PDGFR), fibroblast growth factor receptor (FGFR), and vascular endothelial growth factor receptor (VEGFR). 55 The oft overlooked Lyn and Lck inhibition may give nintedanib important immunomodulatory effects (Figure 2). Lyn activates B cells stimulated through the BCR and provides negative feedback to prevent B cell overactivation. 56 Lck is critically involved in T cell activation. When an antigen binds the T cell receptor (TCR), Lck phosphorylates the cytoplasmic tail of the TCR allowing ZAP70 to bind. Lck then also phosphorylates ZAP70, which triggers further T cell activation. Lck inhibition by calcineurin inhibitors is beneficial in the treatment of graft versus host disease. 57 Pre-clinical data also support nintedanib as a potential immunomodulatory agent. Treatment of healthy T cells with nintedanib inhibited Lck-Y394 phosphorylation to reduce anti-CD3/anti-CD28 activation and secretion of IFN-γ, IL-2, IL-4, IL-5, IL-10, and IL-12. Interestingly, proliferation was unaffected.56–59 Additional work is needed to understand whether nintedanib is immunomodulatory in vivo.
Figure 2.
Possible immunomodulatory effects of nintedanib. (a) Nintedanib inhibits Lyn, which is involved in both activation and inhibition of B cell signaling after B cell receptor (BCR) ligation. (b) Nintedanib inhibits Lck, which phosphorylates ZAP70 and leads to T cell activation.
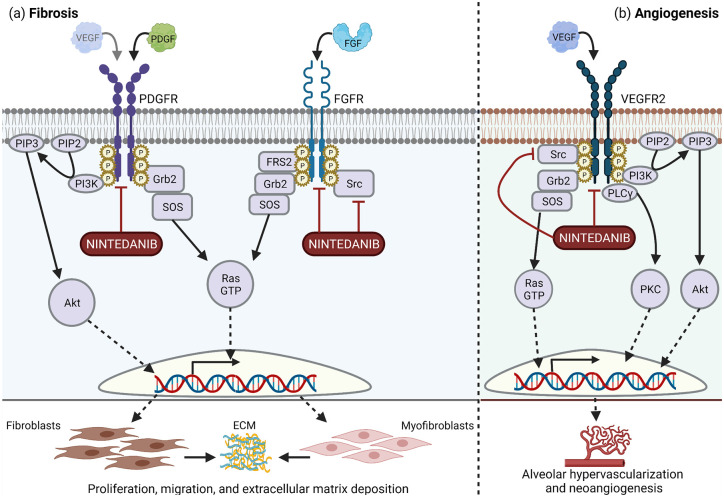
The antifibrotic mechanisms of nintedanib are more clearly elucidated. Wollin et al. 60 have reviewed how nintedanib modulates platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and vascular endothelial growth factor (VEGF) signaling in IPF to ameliorate pulmonary fibrosis (Figure 3). Both VEGF and PDGF are important in IIM pathogenesis. Higher levels of PDGF have been linked to lower lung volumes in juvenile dermatomyositis, 61 and VEGF is upregulated in the muscle of IIM patients.62,63 While no in vitro studies of IIM fibroblasts have been reported, the effects of nintedanib on SSc fibroblasts have been described. Nintedanib inhibited SSc-lung fibroblasts proliferation and migration in a dose-dependent manner and reduced secretion of type I collagen and fibronectin. Nintedanib also decreased the contractility of SSc-lung fibroblasts. Interestingly, nintedanib’s cross-inhibition of other tyrosine kinases may improve its antifibrotic effects. In a study of human dermal fibroblasts from healthy controls and SSc patients, selective inhibitors of PDGFR, FGFR, and VEGFR were less effective than nintedanib at inhibiting fibroblast migration. 64 Given the clinical and pathogenic similarities between SSc-ILD and IIM-ILD, the effects of antifibrotics on IIM-fibroblasts are likely similar to those on SSc-fibroblasts.
Figure 3.
Nintedanib inhibits platelet-derived growth factor receptor (PDGFR), fibroblast growth factor receptor (FGFR), and vascular endothelial growth factor receptor (VEGFR)-2 to impair pulmonary fibrosis. (a) Nintedanib directly antagonizes PDGFR signaling, which is triggered by binding of PDGF or possibly to a lesser extent FGF. Nintedanib inhibits FGFR directly and inhibits its signaling molecule, Src. Inhibition of PDGFR and FGFR decreases proliferation and migration of fibroblasts and myofibroblasts and subsequent extracellular matrix (ECM) deposition. (b) Nintedanib inhibits both VEGFR2 directly and its signaling molecule, Src, to mitigate alveolar hypervascularization and angiogenesis. 60
Clinical evidence for the use of antifibrotics in IPF
The first evidence of the use of antifibrotics in interstitial lung disease is from IPF. IPF is a specific form of chronic progressive fibrosing interstitial lung disease that predominantly affects older adults. IPF is histologically characterized by temporal heterogeneity, fibroblastic foci, and honeycomb scar in the absence of interstitial inflammation, commonly refers to as usual interstitial pneumonia (UIP). 65 After the PANTHER-IPF trial that demonstrated the clear harm of immunosuppression in IPF, 66 there were no pharmacologic treatments for IPF until the approval of the antifibrotic agents pirfenidone and nintedanib. The only potential option was lung transplantation.
Pirfenidone
Initial data for pirfenidone were mixed, which led to a delay in regulatory approval. Pirfenidone was first shown to slow IPF in 2010, 67 but the CAPACITY study of two paired phase III trials of 779 patients had heterogeneous results. While one trial demonstrated that pirfenidone reduced the rate of FVC decline at all timepoints beyond 24 weeks, the other showed an initial decline that was lost at weeks 60 and 72. 68 This discrepancy necessitated the ASCEND study of 555 patients, where pirfenidone was associated with a reduced decline in FVC (−164 mL versus −280 mL) and improved progression-free survival. Pooled data from CAPACITY and ASCEND showed an overall mortality benefit of pirfenidone in IPF, 37 and pirfenidone was approved by the FDA for IPF in 2014.
Nintedanib
The efficacy for nintedanib in IPF was demonstrated in the paired INPULSIS-1 and INPULSIS-2 trials containing 1066 patients. Patients on nintedanib had a slower rate of FVC decline compared to placebo in both trials (−115 mL versus −240 mL and −114 mL versus −207 mL), and patients on nintedanib were less likely to have a >5% decline in FVC. 38 While mortality was not evaluated as part of the initial trial, a subsequent analysis of six pooled clinical trials did show improved survival with nintedanib. 27 Nintedanib was also found to have a decreased frequency of acute exacerbations in INPULSIS-2 and a pre-specified pooled analysis, but INPULSIS-1 did not show a decrease in acute exacerbations in the initial report. 38 The discrepancy in reduced acute exacerbations between INPULSIS-1 and INPULSIS-2 was subsequently found to be related to how acute exacerbations were being reported and analyzed. The initial analysis used investigator reported acute exacerbations rather than adjudicated acute exacerbations. When only adjudicated acute exacerbations were analyzed, both INPULSIS studies had a reduced frequency of acute exacerbations on nintedanib. 69 Thus, nintedanib reduces both the rate of FVC decline and frequency of acute exacerbations in IPF. It was FDA approved for this indication in 2014.
Combination therapy
Given mechanistic differences in pirfenidone and nintedanib (Table 1), there has been some thought that these medicines could work synergistically to slow fibrosis. Two small studies have been conducted to evaluate the safety and efficacy of combination pirfenidone and nintedanib in IPF. In a phase IV study evaluating the safety and tolerability of nintedanib added to background pirfenidone, 82% of patients completed 24 weeks of combination treatment. The most common causes for early discontinuation of combination therapy were nausea (4.5%), diarrhea (4.5%), fatigue (2.2%), and weight loss (2.2%). Two patients had a serious treatment-related adverse events. While efficacy data are difficult to interpret given the lack of a control group, a preliminary efficacy analysis found an FVC decline of 0.4% and diffusion capacity of the lung for carbon dioxide (DLCO) decline of 1.9% over the 24-week study period. 70
Table 1.
Proposed mechanisms of action for pirfenidone and nintedanib.
| Pirfenidone 48 | Nintedanib 60 | |
|---|---|---|
| TGF-β1 | Reduces protein production and mRNA expression | None |
| FGF/FGFR | Regulation of FGF expression | Inhibits FGFR signaling |
| PDGF/PDGFR | Regulation of PDGF expression | Inhibits PDGFR signaling |
| VEGF/VEGFR | None | Inhibits VEGFR signaling |
| Modulation of cellular immunity | Reduction of macrophage cytokine secretion Reduction of T cell activation by dendritic cells Inhibition of Th1/Th2 polarization |
Modulation of BCR signaling via Lyn inhibition Inhibition of T cell activation via Lck inhibition |
| ECM deposition | Decreased due to lower levels of TGF-β1 | Decreased due to PDGFR/FGFR inhibition |
| Cytokine modulation | Reduced IL-6, IL-8, IL-10, IL-12p40, IFN-γ, TNFα, and MCP1 | None |
| Antioxidant effects | Reduction of hydroxyl radical oxidative stress Improved mitochondrial function |
None |
BCR, B cell receptor; ECM, extra cellular matrix; FGF, fibroblast growth factor; FGFR, fibroblast growth factor receptor; IFN, interferon; IL, interleukin; MCP1, monocyte chemoattractant protein; PDGF, platelet-derived growth factor; PDGFR, platelet-derived growth factor receptor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; TGFβ1, transforming growth factor β1.
The INJOURNEY study evaluated the effects of pirfenidone added to background nintedanib. In this study, 35.8% of patients were unable to tolerate combination therapy. While a similar number of patients reported any adverse event in the combination and nintedanib-only groups (88.7% versus 88.2%), combination therapy was associated with more nausea (41.5% versus 11.8%), vomiting (28.3% versus 11.8%), upper abdominal pain (13.2% versus 7.8%), fatigue (18.9% versus 11.8%), and elevated transaminases to three times the upper limit of normal (5.7% versus 0%). 71 This study did find, however, that combination therapy was associated with a smaller decline in FVC at 12 weeks compared to nintedanib alone (−13.3 mL versus −41 mL). While promising, additional work is required to evaluate the long-term tolerance and efficacy of combined antifibrotic therapy.
Evidence for antifibrotics in all chronic progressive ILD
Even if the underlying cause of fibrosis varies in IIM-ILD and IPF, lung scaring and fibrosis may occur through shared pathways. As reviewed by Distler et al., 72 the core pathways of fibrosis are TGF-β, WNT, hedgehog, and PDGF signaling pathways. Once triggered, these pathways lead to increased extracellular matrix deposition, myofibroblast differentiation, and scar. This concept of shared fibrotic pathway is further supported by a study of lung explants, which found no differences in the levels of PDGF, FGF-2, and VEGF in IPF, SSc-ILD, or other PF-ILD patients. We will now review the data from the two major trials of antifibrotics in non-IPF progressive ILD. 73
Pirfenidone
A phase II clinical trial outside the United States evaluated the benefit of pirfenidone in chronic progressive unclassifiable interstitial lung disease. 74 Thirteen percent of these patients met classification criteria for interstitial pneumonia with autoimmune features, 75 and approximately half of the patients were on mycophenolate mofetil during the trial. This study found that patients receiving pirfenidone had a smaller decrease in FVC; the between group difference was 95.3 mL (95% CI: 35.9–154.6 mL, p = 0.002). Patients on pirfenidone were less likely to have a 10% decline in FVC (OR: 0.42, 95% CI: 0.23–0.84, p = 0.01) or 15% decline in DLCO (OR: 0.25, 95% CI: 0.07–0.93, p = 0.04). As seen in previous studies, 13% of patients discontinued pirfenidone due to treatment-related adverse events. 74 There has been no phase III clinical trial of pirfenidone in PF-ILD.
Nintedanib
The efficacy of nintedanib in chronic progressive interstitial lung disease was assessed in the phase III INBUILD trial, which included 663 patients in 15 countries. Patients were stratified during randomization for the presence of usual interstitial pneumonia, and most patients had a UIP-like fibrotic pattern. A significant number of patients did have CTD-ILD (13.4% RA, 5.9% SSc, 2.9% mixed connective tissue disease, 3.4% other), but immunosuppression was not permitted. A 4-week washout was required for AZA, cyclosporin A, MMF, tacrolimus, prednisone >20 mg/day. Nintedanib was beneficial for both UIP-like and other PF-ILD. The overall between group difference was 107 mL (95% CI: 65.4–148.5, p < 0.001). While there was a trend toward decreased acute exacerbations in the nintedanib group, this did not reach statistical significance (HR: 0.68, 95% CI: 0.46–1.01). 29 A subsequent subgroup analysis specifically evaluated the effect of nintedanib in autoimmune-ILD. This subgroup analysis found the for 170 autoimmune-ILD patients, the between group difference in FVC decline was 104.0 mL, 95% CI: 21.1–186.9. For patients with idiopathic nonspecific interstitial pneumonia, some of whom may have had a myositis-spectrum disease, the between-group difference was even larger at 141.6 mL, 95% CI: 46.0–237.2 mL. 76 Based on above results, FDA granted an approval for use of nintedanib in chronic progressive interstitial lung disease, which is applicable to many CTD-ILD patients including IIM-ILD.
Evidence for antifibrotic therapies in SSc-ILD
Pirfenidone
Pending results of two large randomized controlled trials evaluating pirfenidone in RA-ILD (NCT02808871) and SSc-ILD (NCT03221257), data supporting the use of pirfenidone in CTD-ILD are sparse. A small single-center randomized trial of 34 patients with SSc-ILD did not detect a difference in either the % predicted FVC or 6-min walk test, and the majority of patients were taking MMF, AZA, or methotrexate. 77 A phase II safety and tolerability study in SSc-ILD reported stable FVC and DLCO at 16 weeks, but interpretation is limited due to the lack of a control group and short follow-up interval. 78
Nintedanib
The safety and efficacy of nintedanib in SSc-ILD was demonstrated by the SENSCIS trial. This phase III trial of 576 patients with SSc-ILD did allow for concomitant use of methotrexate or mycophenolate mofetil provided the dose was stable for the antecedent 6 months. Nintedanib was associated with slower rate of FVC decline and between group difference of 41.0 mL/year (95% CI: 2.9–79, p = 0.04). Patients on nintedanib were less likely to suffer a >5% change in percent predicted FVC (20.6% versus 28.5%, OR: 0.65, 95% CI: 0.44–0.96). While 75% of patients receiving nintedanib reported diarrhea, only 16% of patients discontinued therapy compared to 9% in the placebo group. 39 There was no increase in adverse events when nintedanib was combined with other immunosuppressive agents or glucocorticoids. 79 Approximately half of the patients in both groups were on MMF, and a subgroup analysis found that patients on combination of MMF and nintedanib were less likely to have an absolute decline in FVC >5% at 52 weeks compared to those on MMF alone (15% versus 26%, OR: 0.52, 95% CI: 0.29–0.95). Furthermore, patients on combination therapy had an absolute annual rate of FVC decline comparable to the absolute annual rate of FVC decline in healthy adults. 40 While these studies support the use of combination therapy in SSc-ILD, they could not assess whether initial combination therapy or step-up therapy was preferred. Nevertheless, the study led to FDA approval of nintedanib in SSc-ILD.
Evidence supporting the use of antifibrotics in IIM-ILD
While no large, randomized trials have assessed the efficacy of antifibrotics in IIM-ILD, smaller series do exist and imply that antifibrotics may have benefit in IIM-ILD.
Pirfenidone
The efficacy of pirfenidone in addition to immunosuppression was assessed in one open-label study of 27 patients with rapidly progressive IIM-ILD compared to historical controls. While pirfenidone did not improve overall survival, a subgroup analyses revealed that patients with subacute ILD (3–6 months duration) had improved survival compared to historical controls (44% versus 90%, p = 0.045). Patients with acute ILD of less than 3 months duration did not have a survival benefit. No radiographic differences were observed among survivors treated with pirfenidone versus immunosuppression alone, and the authors were unable to assess changes in FVC due to missing data and patient acuity at time of enrollment. Notably, three of the survivors did have to discontinue pirfenidone due to rash, elevated LFTs, and diarrhea, but pirfenidone was otherwise reasonably well tolerated. 80
Nintedanib
Nintedanib was evaluated in a retrospective study of IIM-ILD comparing 36 patients treated with nintedanib plus immunosuppression to 115 patients (historical controls) managed with immunosuppression alone. Included patients had a wide range of positive serologies including 25.0% + MDA5, 13.9% + Jo1, 25.0% + non-Jo1 ARS, and + 58.3% Ro-52. After propensity matching to adjust for age and sex, there were no differences in the baseline FVC or DLCO between the treatment groups. Nintedanib was protective for the development of rapidly progressive (RP)-ILD (OR: 0.09, 95% CI: 0.15–0.55) and was associated with improved survival (HR: 0.26, 95% CI: 0.09–0.75). 81
Safety and tolerability of antifibrotic therapies
Both pirfenidone and nintedanib are taken orally. Pirfenidone is available in both 267 and 801 mg tablets. Typically, patients are started on one 267 mg tablet three times daily with food. The dose is titrated up every week to 534 mg three times daily during the second week and 801 mg three times daily during the third week, if tolerated. Nintedanib is available in both 100 and 150 mg tablets. Most patients are started at 150 mg twice daily with food, and the dose can be reduced to 100 mg twice daily for intolerance. Pirfenidone and nintedanib require monthly liver function test monitoring for the first 3 months and every 3 months thereafter.
While adverse events are common with both pirfenidone and nintedanib, only 10–20% of patients discontinue these medications due to adverse events. Table 2 summarizes the frequency of adverse events in several representative clinical trials of both pirfenidone and nintedanib. The most common adverse events for both agents are nausea, vomiting, and diarrhea. In many causes, taking these medications with food can improve GI tolerance. Mild to moderate diarrhea can frequently be improved with antidiarrheal medications such as loperamide. Should moderate diarrhea persist despite antidiarrheal medicines (>4 bowel movements per day), dose reduction can be considered. Significant elevation of transaminases does occur in a minority of patients, so routine monitoring with liver function tests is important. While diarrhea is common with both mycophenolate mofetil and nintedanib, the SENSCIS trial showed a similar frequency of diarrhea treated with either combination or nintedanib monotherapy. Given these data, safety and tolerability of these agents seem reasonable, especially as a treatment for life-threatening progressive ILD.
Table 2.
Safety and tolerability of antifibrotic agents in selective clinical trials.
| ASCEND
37
|
INBUILD
29
|
SENCIS
40
|
INJOURNEY
71
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pl | Pi | Pl | Ni | Pl | MMF | Ni | Ni + MMF | Ni | Ni + Pi | |
| Any AE† | n.r. | n.r. | 89.4% | 95.5% | 95% | 96% | 99% | 98% | 88.2% | 88.7% |
| Nausea | 13.4% | 36.0% | 9.4% | 28.9% | 11% | 16% | 32% | 31% | 11.8% | 41.5% |
| Vomiting | 8.7% | 12.9% | 5.1% | 18.4% | 9% | 12% | 26% | 23% | 11.8% | 28.3% |
| Diarrhea | 21.7% | 22.3% | 23.9% | 66.9% | 29% | 34% | 75% | 76% | 31.4% | 37.7% |
| Abdominal pain | n.r. | n.r. | 2.4% | 10.2% | 10% | 4% | 13% | 10% | 7.8% | 13.2% |
| Fatigue | 17.3% | 20.9% | n.r. | n.r. | 4% | 10% | 8% | 14% | 11.8% | 18.9% |
| Headache | 23.1% | 25.9% | 6.9% | 10.5% | 6% | 11% | 7% | 12% | 2.0% | 13.2% |
| Weight loss | 7.9% | 12.6% | 3.3% | 12.3% | 5% | 3% | 16% | 7% | n.r. | n.r. |
| SAE | 24.9% | 19.8% | 32.2% | 32.2% | 27% | 13% | 22% | 26% | 9.8% | 3.8% |
| Transaminitis ‡ | 0.9% | 2.7% | 3.6% | 13.0% | n.r. | n.r. | n.r. | n.r. | 0 | 5.7% |
| Treatment discontinuation | 10.8% | 14.4% | 10.3% | 19.6% | 11% | 6% | 21% | 11% | 21.4% | 35.8% § |
AE, adverse event; MMF, mycophenolate mofetil; Ni, nintedanib; n.r., not reported; Pi, Pirfenidone; Pl, placebo; SAE, serious adverse event.
Adverse events were attributed by adjudicators to being due to pirfenidone, nintedanib, or combination therapy.
Greater than three-fold the upper limit of normal.
Unable to tolerate combination therapy.
Remaining challenges and unanswered questions
The antifibrotic agents pirfenidone and nintedanib have proven efficacy in idiopathic pulmonary fibrosis,37,38 and nintedanib is safe and effective in chronic progressive ILD and specifically in SSc-ILD. 39 In addition, subgroup analyses demonstrated that nintedanib had additive benefit to MMF in SSc-ILD. 40 Given the evidence for shared fibrotic pathways in progressive interstitial lung disease irrespective of the disease type,72,73 antifibrotic therapies are promising therapeutic adjuncts in IIM-ILD, but several considerations remain.
First, the mechanisms of action are different for pirfenidone and nintedanib, and these medications may not be interchangeable. We should gain additional insight into the efficacy of pirfenidone in CTD-ILD once the trials in RA-ILD (NCT02808871) and SSc-ILD (NCT03221257) are reported. Nintedanib has shown efficacy in a dedicated randomized trial in systemic sclerosis and in a subset of CTD-ILD patients taking part in a larger study on chronic progressive ILD.
Second, and most importantly, immunosuppression can improve lung function in IIM-ILD15,17–20,22 and should remain first-line therapy. In clinical trials, antifibrotic agents slow the decline of FVC loss, but have not demonstrated any significant improvement in pulmonary function. For this reason, patients with IIM should currently be treated aggressively with immunosuppression regardless of the initiation of antifibrotic agents. A key unanswered question is whether early antifibrotic therapy would benefit any subsets of IIM-ILD or should antifibrotic be reserved in IIM-ILD patients with progressive disease despite aggressive immunosuppression. For example, patients with honeycomb changes on CT scan at presentation may benefit from up-front antifibrotic therapy in addition to immunosuppressive therapies. Would patients with MDA-5 who are prone to RP-ILD benefit from concomitant therapy with nintedanib since this agent may reduce the risk of RP-ILD? Should patients presenting with chronic respiratory failure requiring supplemental oxygen be simultaneously treated with immunosuppression and antifibrotics given advanced fibrosis and lack of pulmonary reserve? While Figure 4 shows potential roles of antifibrotics in IIM-ILD, additional studies are needed to determine how antifibrotics should be employed in clinical practice and whether a particular antifibrotic is best for certain clinical scenario.
Figure 4.
Possible role of antifibrotics in the management of IIM-ILD. Schematic of how antifibrotics might be employed in the management of IIM-ILD. Key outstanding questions are (1) efficacy of upfront antifibrotic therapy in all patients both in the mild-moderate category to improve outcomes, (2) efficacy of early initiation of antifibrotics to prevent ILD in high-risk patients (e.g. +MDA5 or Ro52), (3) efficacy in severe/rapidly progressing ILD to improve survival, and (4) at failure of induction or maintenance therapy for additive benefit and to slow progression.
*Tacrolimus could be considered as a first-line agent for the management of MDA5 + dermatomyositis with ILD in the outpatient setting; tofacitinib could be considered as early therapy in life-threatening MDA5+ disease.
Conclusion
Pirfenidone and nintedanib have proven efficacy in IPF, and nintedanib has proven efficacy in chronic progressive ILD and specifically in SSc-ILD. While antifibrotics are promising adjuvants in IIM-ILD, additional studies are needed to elucidate the timing and ideal patient population for these medications.
Acknowledgments
The authors gratefully acknowledge the assistance of Camille Ivey, who aided EMW with the designing and performing the PubMed search utilized for this study. Figures 1–3 were created with BioRender.com.
Footnotes
Author contributions: EMW designed the study, performed the literature review, and wrote the initial draft. RA designed the study and substantially revised the manuscript. Both authors have read and approved the final manuscript.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: EMW receives research funding from Boehringer-Ingelheim and was a member of their myositis ILD advisory board. RA has served on the myositis ILD advisory board for Boehringer-Ingelheim and received consulting fees for the same.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: EMW acknowledges funding from the National Institutes of Health (KL2TR002245). She also receives research funding from Boehringer-Ingelheim for an unrelated investigator-initiated study. RA acknowledges funding from the National Institutes of Health (R01AR071659). The contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
ORCID iD: Erin M. Wilfong  https://orcid.org/0000-0002-6584-1578
https://orcid.org/0000-0002-6584-1578
Contributor Information
Erin M. Wilfong, Divisions of Rheumatology and Immunology & Allergy, Pulmonary, and Critical Care Medicine, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, 37232 USA.
Rohit Aggarwal, Division of Rheumatology, Department of Medicine, University of Pittsburgh Medical Center, Pittsburgh, PA, USA.
References
- 1. Furst DE, Amato AA, Iorga S¸R, et al. Epidemiology of adult idiopathic inflammatory myopathies in a U.S. managed care plan. Muscle Nerve 2012; 45: 676–683. [DOI] [PubMed] [Google Scholar]
- 2. Svensson J, Arkema EV, Lundberg IE, et al. Incidence and prevalence of idiopathic inflammatory myopathies in Sweden: a nationwide population-based study. Rheumatology 2017; 56: 802–810. [DOI] [PubMed] [Google Scholar]
- 3. Cho S-K, Kim H, Myung J, et al. Incidence and prevalence of idiopathic inflammatory myopathies in Korea: a nationwide population-based study. J Korean Med Sci 2019; 34: e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Betteridge Z, Tansley S, Shaddick G, et al. Frequency, mutual exclusivity and clinical associations of myositis autoantibodies in a combined European cohort of idiopathic inflammatory myopathy patients. J Autoimmun 2019; 101: 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lega JC, Fabien N, Reynaud Q, et al. The clinical phenotype associated with myositis-specific and associated autoantibodies: a meta-analysis revisiting the so-called antisynthetase syndrome. Autoimmun Rev 2014; 13: 883–891. [DOI] [PubMed] [Google Scholar]
- 6. Marie I, Hachulla E, Cherin P, et al. Interstitial lung disease in polymyositis and dermatomyositis. Arthritis Rheum 2002; 47: 614–622. [DOI] [PubMed] [Google Scholar]
- 7. Johnson C, Pinal-Fernandez I, Parikh R, et al. Assessment of mortality in autoimmune myositis with and without associated interstitial lung disease. Lung 2016; 194: 733–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marie I, Hatron PY, Dominique S, et al. Short-term and long-term outcomes of interstitial lung disease in polymyositis and dermatomyositis: a series of 107 patients. Arthritis Rheum 2011; 63: 3439–3447. [DOI] [PubMed] [Google Scholar]
- 9. Nuno-Nuno L, Joven BE, Carreira PE, et al. Mortality and prognostic factors in idiopathic inflammatory myositis: a retrospective analysis of a large multicenter cohort of Spain. Rheumatol Int 2017; 37: 1853–1861. [DOI] [PubMed] [Google Scholar]
- 10. Yamasaki Y, Yamada H, Ohkubo M, et al. Long term survival and associated risk factors in patients with adult-onset idiopathic inflammatory myopathies and amyopathic dermatomyositis: experience in a single institute in Japan. J Rheumatol 2011; 38: 1636–1643. [DOI] [PubMed] [Google Scholar]
- 11. Marie I, Josse S, Decaux O, et al. Clinical manifestations and outcome of anti-PL7 positive patients with antisynthetase syndrome. Eur J Intern Med 2013; 24: 474–479. [DOI] [PubMed] [Google Scholar]
- 12. Moghadam-Kia S, Oddis CV, Sato S, et al. Antimelanoma differentiation-associated gene 5 antibody: expanding the clinical spectrum in North American patients with dermatomyositis. J Rheumatol 2017; 44: 319–325. [DOI] [PubMed] [Google Scholar]
- 13. Hoa S, Troyanov Y, Fritzler MJ, et al. Describing and expanding the clinical phenotype of anti-MDA5-associated rapidly progressive interstitial lung disease: case series of nine Canadian patients and literature review. Scand J Rheumatol 2018; 47: 210–224. [DOI] [PubMed] [Google Scholar]
- 14. Tsuji H, Nakashima R, Imura Y, et al. Efficacy and safety of combined immunosuppressive therapy with high-dose glucocorticoid, tacrolimus, and cyclophosphamide in interstitial lung disease accompanied by anti-MDA5-positive-dermatomyositis – a multicenter prospective study. Chicago, IL: American College of Rheumatology, 2018. [DOI] [PubMed] [Google Scholar]
- 15. Huapaya JA, Silhan L, Pinal-Fernandez I, et al. Long-term treatment with azathioprine and mycophenolate mofetil for myositis-related interstitial lung disease. Chest 2019; 156: 896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ji S-y, Zeng F-q, Guo Q, et al. Predictive factors and unfavourable prognostic factors of interstitial lung disease in patients with polymyositis or dermatomyositis: a retrospective study. Chin Med J 2010; 123: 517–522. [PubMed] [Google Scholar]
- 17. Suzuki Y, Hayakawa H, Miwa S, et al. Intravenous immunoglobulin therapy for refractory interstitial lung disease associated with polymyositis/dermatomyositis. Lung 2009; 187: 201–206. [DOI] [PubMed] [Google Scholar]
- 18. Doyle TJ, Dhillon N, Madan R, et al. Rituximab in the treatment of interstitial lung disease associated with antisynthetase syndrome: a multicenter retrospective case review. J Rheumatol 2018; 45: 841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Andersson H, Sem M, Lund MB, et al. Long-term experience with rituximab in anti-synthetase syndrome-related interstitial lung disease. Rheumatology 2015; 54: 1420–1428. [DOI] [PubMed] [Google Scholar]
- 20. Kurita T, Yasuda S, Oba K, et al. The efficacy of tacrolimus in patients with interstitial lung diseases complicated with polymyositis or dermatomyositis. Rheumatology 2015; 54: 39–44. [DOI] [PubMed] [Google Scholar]
- 21. Suzuka T, Kotani T, Takeuchi T, et al. Efficacy and safety of oral high-trough level tacrolimus in acute/subacute interstitial pneumonia with dermatomyositis. Int J Rheum Dis 2019; 22: 303–313. [DOI] [PubMed] [Google Scholar]
- 22. Sharma N, Putman MS, Vij R, et al. Myositis-associated interstitial lung disease: predictors of failure of conventional treatment and response to tacrolimus in a US cohort. J Rheumatol 2017; 44: 1612–1618. [DOI] [PubMed] [Google Scholar]
- 23. Allenbach Y, Uzunhan Y, Toquet S, et al. Different phenotypes in dermatomyositis associated with anti-MDA5 antibody. Neurology 2020; 95: e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen Z, Wang X, Ye S. Tofacitinib in amyopathic dermatomyositis–associated interstitial lung disease. N Engl J Med 2019; 381: 291–293. [DOI] [PubMed] [Google Scholar]
- 25. Abe Y, Matsushita M, Tada K, et al. Clinical characteristics and change in the antibody titres of patients with anti-MDA5 antibody-positive inflammatory myositis. Rheumatology 2017; 56: 1492–1497. [DOI] [PubMed] [Google Scholar]
- 26. Romero-Bueno F, Diaz Del Campo P, Trallero-Araguás E, et al. Recommendations for the treatment of anti-melanoma differentiation-associated gene 5-positive dermatomyositis-associated rapidly progressive interstitial lung disease. Semin Arthritis Rheum 2020; 50: 776–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lancaster L, Crestani B, Hernandez P, et al. Safety and survival data in patients with idiopathic pulmonary fibrosis treated with nintedanib: pooled data from six clinical trials. BMJ Open Respir Res 2019; 6: e000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vasakova M, Sterclova M, Mogulkoc N, et al. Long-term overall survival and progression-free survival in idiopathic pulmonary fibrosis treated by pirfenidone or nintedanib or their switch. Real world data from the EMPIRE registry. Eur Respir J 2019; 54: PA4720. [Google Scholar]
- 29. Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med 2019; 381: 1718–1727. [DOI] [PubMed] [Google Scholar]
- 30. Tashkin DP, Elashoff R, Clements PJ, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med 2006; 354: 2655–2666. [DOI] [PubMed] [Google Scholar]
- 31. Tashkin DP, Roth MD, Clements PJ, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med 2016; 4: 708–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Md Yusof MY, Kabia A, Darby M, et al. Effect of rituximab on the progression of rheumatoid arthritis–related interstitial lung disease: 10 years’ experience at a single centre. Rheumatology 2017; 56: 1348–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vadillo C, Nieto MA, Romero-Bueno F, et al. Efficacy of rituximab in slowing down progression of rheumatoid arthritis–related interstitial lung disease: data from the NEREA Registry. Rheumatology 2020; 59: 2099–2108. [DOI] [PubMed] [Google Scholar]
- 34. Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum 2013; 65: 314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fernández-Díaz C, Castañeda S, Melero-González RB, et al. Abatacept in interstitial lung disease associated with rheumatoid arthritis: national multicenter study of 263 patients. Rheumatology 2020; 59: 3906–3916. [DOI] [PubMed] [Google Scholar]
- 36. Chung L, Spino C, McLain R, et al. Safety and efficacy of abatacept in early diffuse cutaneous systemic sclerosis (ASSET): open-label extension of a phase 2, double-blind randomised trial. Lancet Rheumatol 2020; 2: e743–e753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. King TE, Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2083–2092. [DOI] [PubMed] [Google Scholar]
- 38. Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2071–2082. [DOI] [PubMed] [Google Scholar]
- 39. Distler O, Highland KB, Gahlemann M, et al. Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med 2019; 380: 2518–2528. [DOI] [PubMed] [Google Scholar]
- 40. Highland KB, Distler O, Kuwana M, et al. Efficacy and safety of nintedanib in patients with systemic sclerosis-associated interstitial lung disease treated with mycophenolate: a subgroup analysis of the SENSCIS trial. Lancet Respir Med 2021; 9: 96–106. [DOI] [PubMed] [Google Scholar]
- 41. Bizargity P, Liu K, Wang L, et al. Inhibitory effects of pirfenidone on dendritic cells and lung allograft rejection. Transplantation 2012; 94: 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Toda M, Mizuguchi S, Minamiyama Y, et al. Pirfenidone suppresses polarization to M2 phenotype macrophages and the fibrogenic activity of rat lung fibroblasts. J Clin Biochem Nutr 2018; 63: 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Iyer SN, Hyde DM, Giri SN. Anti-inflammatory effect of pirfenidone in the bleomycin-hamster model of lung inflammation. Inflammation 2000; 24: 477–491. [DOI] [PubMed] [Google Scholar]
- 44. Trivedi R, Redente EF, Thakur A, et al. Local delivery of biodegradable pirfenidone nanoparticles ameliorates bleomycin-induced pulmonary fibrosis in mice. Nanotechnology 2012; 23: 505101. [DOI] [PubMed] [Google Scholar]
- 45. Visner GA, Liu F, Bizargity P, et al. Pirfenidone inhibits T-cell activation, proliferation, cytokine and chemokine production, and host alloresponses. Transplantation 2009; 88: 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kahn JS, Deverapalli SC, Rosmarin DM. JAK-STAT signaling pathway inhibition: a role for treatment of discoid lupus erythematosus and dermatomyositis. Int J Dermatol 2018; 57: 1007–1014. [DOI] [PubMed] [Google Scholar]
- 47. Yan W, Fan W, Chen C, et al. IL-15 up-regulates the MMP-9 expression levels and induces inflammatory infiltration of macrophages in polymyositis through regulating the NF-kB pathway. Gene 2016; 591: 137–147. [DOI] [PubMed] [Google Scholar]
- 48. Ruwanpura SM, Thomas BJ, Bardin PG. Pirfenidone: molecular mechanisms and potential clinical applications in lung disease. Am J Respir Cell Mol Biol 2020; 62: 413–422. [DOI] [PubMed] [Google Scholar]
- 49. Conte E, Gili E, Fagone E, et al. Effect of pirfenidone on proliferation, TGF-β-induced myofibroblast differentiation and fibrogenic activity of primary human lung fibroblasts. Eur J Pharm Sci 2014; 58: 13–19. [DOI] [PubMed] [Google Scholar]
- 50. Nakayama S, Mukae H, Sakamoto N, et al. Pirfenidone inhibits the expression of HSP47 in TGF-β1-stimulated human lung fibroblasts. Life Sci 2008; 82: 210–217. [DOI] [PubMed] [Google Scholar]
- 51. Wu C, Lin H, Zhang X. Inhibitory effects of pirfenidone on fibroblast to myofibroblast transition in rheumatoid arthritis-associated interstitial lung disease via the downregulation of activating transcription factor 3 (ATF3). Int Immunopharmacol 2019; 74: 105700. [DOI] [PubMed] [Google Scholar]
- 52. Niimi T, Yoshinouchi T, Ohtsuki Y, et al. Myofibroblasts proliferation of idiopathic and collagen vascular disorders associated nonspecific interstitial pneumonia. Acta Med Okayama 2003; 57: 33–38. [DOI] [PubMed] [Google Scholar]
- 53. Beon M, Harley RA, Wessels A, et al. Myofibroblast induction and microvascular alteration in scleroderma lung fibrosis. Clin Exp Rheumatol 2004; 22: 733–742. [PubMed] [Google Scholar]
- 54. Yoshinouchi T, Ohtsuki Y, Ueda R, et al. Myofibroblasts and S-100 protein positive cells in idiopathic pulmonary fibrosis and rheumatoid arthritis-associated interstitial pneumonia. Eur Respir J 1999; 14: 579–584. [DOI] [PubMed] [Google Scholar]
- 55. Hilberg F, Roth GJ, Krssak M, et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res 2008; 68: 4774–4782. [DOI] [PubMed] [Google Scholar]
- 56. Hibbs ML, Harder KW, Armes J, et al. Sustained activation of Lyn tyrosine kinase in vivo leads to autoimmunity. J Exp Med 2002; 196: 1593–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Otsuka S, Melis N, Gaida MM, et al. Calcineurin inhibitors suppress acute graft-versus-host disease via NFAT-independent inhibition of T cell receptor signaling. J Clin Invest 2021; 131: e147683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ubieta K, Thomas MJ, Wollin L. The effect of nintedanib on T-cell activation, subsets and functions. Drug Des Devel Ther 2021; 15: 997–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Talab F, Allen JC, Thompson V, et al. LCK is an important mediator of B-cell receptor signaling in chronic lymphocytic leukemia cells. Mol Cancer Res 2013; 11: 541–554. [DOI] [PubMed] [Google Scholar]
- 60. Wollin L, Wex E, Pautsch A, et al. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur Respir J 2015; 45: 1434–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Marstein H, Schwartz T, Aaløkken TM, et al. Novel associations between cytokines and pulmonary involvement in juvenile dermatomyositis – a cross-sectional study of long-term disease. Rheumatology 2020; 59: 1862–1870. [DOI] [PubMed] [Google Scholar]
- 62. Volpi N, Pecorelli A, Lorenzoni P, et al. Antiangiogenic VEGF isoform in inflammatory myopathies. Mediators Inflamm 2013; 2013: 219313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Grundtman C, Tham E, Ulfgren A-K, et al. Vascular endothelial growth factor is highly expressed in muscle tissue of patients with polymyositis and patients with dermatomyositis. Arthritis Rheum 2008; 58: 3224–3238. [DOI] [PubMed] [Google Scholar]
- 64. Huang J, Beyer C, Palumbo-Zerr K, et al. Nintedanib inhibits fibroblast activation and ameliorates fibrosis in preclinical models of systemic sclerosis. Ann Rheum Dis 2016; 75: 883–890. [DOI] [PubMed] [Google Scholar]
- 65. Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2018; 198: e44–e68. [DOI] [PubMed] [Google Scholar]
- 66. Idiopathic Pulmonary Fibrosis Clinical Research N, Raghu G, Anstrom KJ, et al. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med 2012; 366: 1968–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Taniguchi H, Ebina M, Kondoh Y, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J 2010; 35: 821–829. [DOI] [PubMed] [Google Scholar]
- 68. Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 2011; 377: 1760–1769. [DOI] [PubMed] [Google Scholar]
- 69. Suissa S, Ernst P. The INPULSIS trials of idiopathic pulmonary fibrosis treatment: explaining further discrepancies on exacerbations. Eur Respir J 2016; 47: 344–345. [DOI] [PubMed] [Google Scholar]
- 70. Flaherty KR, Fell CD, Huggins JT, et al. Safety of nintedanib added to pirfenidone treatment for idiopathic pulmonary fibrosis. Eur Respir J 2018; 52: 1800230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vancheri C, Kreuter M, Richeldi L, et al. Nintedanib with add-on pirfenidone in idiopathic pulmonary fibrosis. Results of the INJOURNEY trial. Am J Respir Crit Care Med 2018; 197: 356–363. [DOI] [PubMed] [Google Scholar]
- 72. Distler JHW, Györfi A-H, Ramanujam M, et al. Shared and distinct mechanisms of fibrosis. Nat Rev Rheumatol 2019; 15: 705–730. [DOI] [PubMed] [Google Scholar]
- 73. Hoffmann-Vold A-M, Weigt SS, Saggar R, et al. Endotype–phenotyping may predict a treatment response in progressive fibrosing interstitial lung disease. EBioMedicine 2019; 50: 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Maher TM, Corte TJ, Fischer A, et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med 2020; 8: 147–157. [DOI] [PubMed] [Google Scholar]
- 75. Fischer A, Antoniou KM, Brown KK, et al. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J 2015; 46: 976–987. [DOI] [PubMed] [Google Scholar]
- 76. Wells AU, Flaherty KR, Brown KK, et al. Nintedanib in patients with progressive fibrosing interstitial lung diseases-subgroup analyses by interstitial lung disease diagnosis in the INBUILD trial: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Respir Med 2020; 8: 453–460. [DOI] [PubMed] [Google Scholar]
- 77. Acharya N, Sharma SK, Mishra D, et al. Efficacy and safety of pirfenidone in systemic sclerosis-related interstitial lung disease – a randomised controlled trial. Rheumatol Int 2020; 40: 703–710. [DOI] [PubMed] [Google Scholar]
- 78. Khanna D, Albera C, Fischer A, et al. An open-label, phase II study of the safety and tolerability of pirfenidone in patients with scleroderma-associated interstitial lung disease: the LOTUSS trial. J Rheumatol 2016; 43: 1672–1679. [DOI] [PubMed] [Google Scholar]
- 79. Cottin V, Richeldi L, Rosas I, et al. Nintedanib and immunomodulatory therapies in progressive fibrosing interstitial lung diseases. Respir Res 2021; 22: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Li T, Guo L, Chen Z, et al. Pirfenidone in patients with rapidly progressive interstitial lung disease associated with clinically amyopathic dermatomyositis. Sci Rep 2016; 6: 33226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Liang J, Cao H, Yang Y, et al. Efficacy and tolerability of nintedanib in idiopathic-inflammatory-myopathy-related interstitial lung disease: a pilot study. Front Med 2021; 8: 626953. [DOI] [PMC free article] [PubMed] [Google Scholar]