Abstract
Introduction:
Whether the incidence of reflux esophagitis (RE) increases after the eradication of Helicobacter pylori (H. pylori) is controversial. Few reports have evaluated the presence or absence of RE after a long period of time, taking into account the degree of atrophy and/or administration of acid secretion inhibitors. We investigated the relationship between H. pylori and RE taking into account these factors.
Methods:
This was a retrospective cohort study with approval by the Ethics Committee. Patients who succeeded in H. pylori eradication treatment, and in whom there were images of the gastroesophageal junction on endoscopic examinations within 1 year before eradication treatment and more than 3 years after eradication were included. The degrees of RE and atrophy were retrospectively determined from the endoscopic images. The prevalence of RE before and after eradication and the incidence of newly developed RE after eradication between patients with or without atrophy improvement were compared using Fisher’s exact test.
Results:
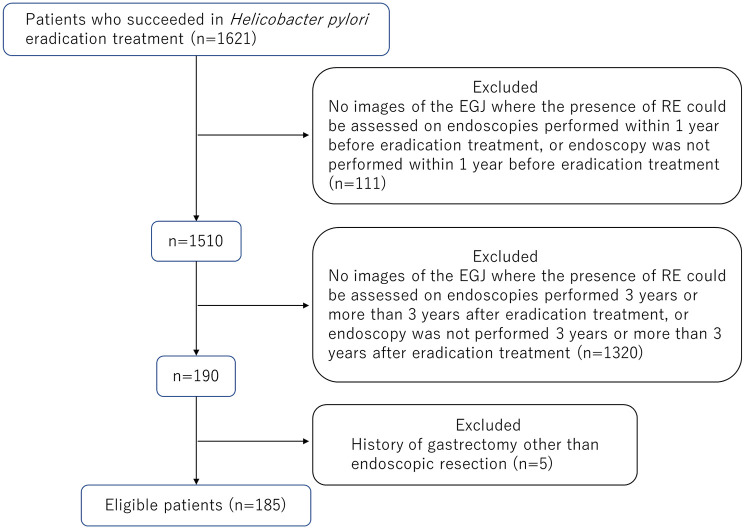
A total of 185 cases (male:female = 104:81; mean age, 63.5 years; mean observation period, 6.4 years) were examined. The prevalence of RE before and after eradication was 1.6% (3/185) and 7.0% (13/185), respectively (P = 0.019). RE was present in 8 (7.5%) of 106 cases with closed-type atrophy and in 5 (6.3%) of 79 cases with open-type atrophy after eradication (P = 0.75). Atrophy improved after eradication in 56 cases, of whom 4 (7.1%) had new onset of RE; the degree of atrophy did not improve in 126 cases, of whom 7 (5.4%) had new onset of RE (P = 0.74). There was no difference between the percentage of cases who took acid secretion inhibitors before and after eradication (P = 0.14).
Conclusion:
The prevalence of RE increased a long time after eradication, even in patients who were taking an acid secretion inhibitor. The prevalence of RE was not related to the degree of atrophy or change in atrophy.
Keywords: eradication, Helicobacter pylori, reflux esophagitis
Introduction
Reflux esophagitis (RE) is one of the most common upper gastrointestinal disorders worldwide.1,2 The prevalence of RE in Japan is less than that in Europe and the United States, but it has been increasing in Japan in recent years. 3 Various factors such as westernization of the diet and obesity are thought to have contributed to the increase, and the decrease in the number of people infected with Helicobacter pylori (H. pylori) is also associated with the increase in prevalence of RE. 4 H. pylori infection causes chronic active gastritis in the stomach,5,6 and infiltrated inflammatory cells secrete inflammatory cytokines such as interleukin-1β and tumor necrosis factor-α, resulting in direct suppression of gastric acid secretion.7,8 In addition, the long-term presence of H. pylori in the fundic gland region causes fundic gland atrophy and reduces gastric acid secretion.9,10 The gastric acid secretion capacity of H. pylori-infected persons is often lower than that of uninfected persons, and the H. pylori infection rate is said to be inversely correlated with the prevalence of RE.11–14 Eradication treatment of H. pylori results in elimination of inflammatory cell infiltration and may lead to improvement of atrophy. 6 It has been reported that elimination of inflammation and improvement of atrophy restore gastric acid secretion.15,16 On the contrary, when H. pylori is present in the antrum, greater acid output is often caused; after eradication, acid output decreases and becomes normal.17,18 The ammonia produced by H. pylori acts as a powerful acid neutralizer in the esophagus and disappears after eradication. 19
Some studies reported that the incidence of RE increases while other studies reported that it does not change after H. pylori eradication. 20 Most of these reports evaluated the presence or absence of RE 1 to 2 years after eradication, and few reports evaluated it a long period of time after eradication. 21 Although gastric acid secretion capacity is expected to differ depending on the degree of atrophy, few reports have investigated whether the degree of atrophy is related to the incidence of RE after H. pylori eradication. 22 In patients who are infected with H. pylori, gastric acid secretion inhibitors are often administered to prevent gastric ulcer and/or duodenal ulcer formation. 23 In many cases, these drugs continue to be administered even after H. pylori has been eradicated. The onset of RE is also affected by the use of such acid secretion inhibitors. However, few reports have investigated the prevalence of RE after eradication of H. pylori in patients who are or are not taking a proton pump inhibitor (PPI) or histamine 2 receptor antagonist (H2RA). 22
We investigated the relationship between H. pylori infection and RE by investigating the prevalence of RE before H. pylori eradication and the prevalence of RE 3 years or more after H. pylori eradication, considering the effects of the degree of atrophy and the use of acid secretion inhibitors.
Methods
Study design
This study was a retrospective cohort study. The protocol used for this study was reviewed and approved by the Juntendo University Ethics Committee (20-050). The reporting of this study conforms to the STROBE statement. 24
Subjects
Patients who underwent H. pylori eradication treatment in our department between 2008 and 2013 and in whom H. pylori was successfully eradicated were enrolled in this study. A 13C-urea breath test result of 2.5‰ or less (Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan), or negative result on the stool H. pylori antigen test 6 weeks after the end of eradication treatment (SRL, Inc., Tokyo, Japan) was considered as successful eradication of H. pylori. Inclusion criteria were the following: patients who underwent endoscopy within 1 year before the start of eradication treatment and had images of the gastroesophageal junction that could be used to determine the presence or absence of RE, and who underwent endoscopy 3 years or more than 3 years after eradication treatment and had the same images as those described above. Patients who had undergone gastrectomy other than endoscopic mucosal resection or endoscopic submucosal dissection were excluded.
Endoscopic findings
Endoscopic images that had been taken by white light endoscopy and image-enhanced endoscopy, if any, were retrospectively viewed, and the presence and degree of RE, the presence and the degree of atrophy, the presence of a gastric and/or duodenal ulcer including ulcer scar, and the presence of esophageal hiatal hernia were determined. The LA-A classification was used to determine the degree of RE. 25 Atrophy was defined as the presence of discoloration of the mucosa, a visible capillary network, low niveau, and fold disappearance in the corpus area.26,27 The degree of atrophy was evaluated by the Kimura-Takemoto classification, and classified into two types based on the location of the endoscopic atrophic border which was endoscopically recognized by discriminating differences in color and height of the gastric mucosa: closed type (C-1, C-2, and C-3) and open type (O-1, O-2, and O-3). 28 No atrophy was set as C-0 and was included in the closed-type group. If the degree of atrophy decreased by one grade or more, it was judged that the atrophy was improved. A distance of 2 cm or greater between the lower margin of the palisade vessels and the hiatus of diaphragm was considered to indicate the presence of esophageal hiatal hernia. Among the images of the gastroesophageal junction and esophagus where the presence or absence of RE could be determined, we selected the images obtained during the endoscopic examination at the time closest to and prior to the eradication treatment, and the images obtained during the endoscopic examination with the longest post-eradication time at the time of the search on 28 February 2019. These endoscopic images that had been obtained at the two time points before and after eradication were examined for determination of the presence or absence of RE, atrophy, and esophageal hiatal hernia. The endoscopic images were judged independently by two experienced endoscopists who were board-certified by the Japan Gastroenterological Endoscopy Society (MH and TT), and when the judgments differed, the judgments were unified by discussion. The judgments of the two endoscopists were consistent except for the judgments of the images of four patients.
Acid secretion inhibitors
The presence or absence of administration of acid secretion inhibitors such as PPIs including a potassium-competitive acid blocker, vonoprazan, and H2RAs at the time of endoscopy was investigated in the medical records.
Statistical analysis
Analyses were performed with BellCurve for Excel (Social Survey Research Information Co., Ltd., Tokyo, Japan). The prevalences of RE between two groups were compared using Fisher’s exact test. The percentages of patients with administration of acid secretion inhibitors before and after eradication were compared using Fisher’s exact test. P values of less than 0.05 were considered to be statistically significant.
Results
Between 2008 and 2013, 1621 patients underwent successful H. pylori eradication, and 1436 patients were excluded according to the inclusion and exclusion criteria. A total of 185 patients were finally included in the analyses (Figure 1). The characteristics of the 185 patients in this study and their observation period, which was defined as the interval between the two endoscopic examinations before and after H. pylori eradication whose images we examined in each patient, are summarized in Table 1. The male-to-female ratio was 104:81, and their age at the time of endoscopy before H. pylori eradication whose images were examined in this study was 63.5 ± 9.6 years (mean ± standard deviation (SD)). Before H. pylori eradication, three patients had RE (two patients with LA-A and one patient with LA-B). Before eradication, there were 77 patients with closed-type atrophy, 104 patients with open-type atrophy, and four patients without atrophy. Twenty-three patients had esophageal hiatal hernia. Twenty-four patients had a gastric ulcer including ulcer scar, and 50 patients had a duodenal ulcer. The mean observation period of the 185 patients was 6.4 ± 1.9 (SD) years.
Figure 1.
The flow chart of study participants. Some patients who had undergone endoscopy at a different clinic and found to be infected with H. pylori, were referred to our department for only H. pylori eradication treatment.
GEJ, gastroesophageal junction; RE, reflux esophagitis.
Table 1.
Baseline characteristics of the patients who underwent successful H. pylori eradication and their observation period.
| Cases (n) | 185 |
|---|---|
| Gender (male/female (n)) | 104/81 |
| Age at the time of endoscopy before H. pylori eradication whose images were examined in this study (mean ± SD (years)) | 63.5 ± 9.6 |
| RE before eradication of H. pylori (LA-A/LA-B/LA-C/LA-D (n)) | 2/1/0/0 |
| Atrophy a before eradication of H. pylori (closed type/open type (n)) | 81/104 |
| Ulcer including scar before eradication of H. pylori (gastric ulcer/duodenal ulcer (n)) | 24/50 |
| HH before eradication of H. pylori (n) | 23 |
| Observation period b (mean ± SD (years)) | 6.4 ± 1.9 |
HH, hiatal hernia; H. pylori, Helicobacter pylori; LA, Los Angeles classification; n, number of patients; RE, reflux esophagitis; SD, standard deviation.
Gastric mucosal atrophy was evaluated according to the Kimura–Takemoto classification and was classified by degree into two grades of closed type and open type. No atrophy (n = 4) was included as the closed type.
Observation period, the interval between the two endoscopic examinations before and after H. pylori eradication whose images were examined in this study.
The prevalence of RE before H. pylori eradication was 1.6% (3/185 cases), and the prevalence of RE after eradication was 7.0% (13/185 cases), showing a significant increase (P = 0.019). Before eradication, there were two patients with LA-A and one patient with LA-B, as mentioned above, and after eradication, there were seven patients with LA-A and six patients with LA-B; all cases of RE before and after eradication were mild esophagitis (Figure 2). Among the two patients with LA-A esophagitis before eradication, RE disappeared after eradication in one patient and LA-A esophagitis was observed after eradication in the other patient. The patient with LA-B esophagitis before eradication showed LA-B esophagitis even after eradication. Among the three patients with pre-eradication esophagitis, two patients had closed-type atrophy, and one patient had open-type atrophy. Among the 13 patients with esophagitis after eradication, 5 patients had closed-type atrophy and 8 patients had open-type atrophy before eradication. One (33%) of the three patients who had esophagitis before eradication was taking a PPI, and six (46%) of the 13 patients who had esophagitis after eradication were taking a PPI (Table 2).
Figure 2.
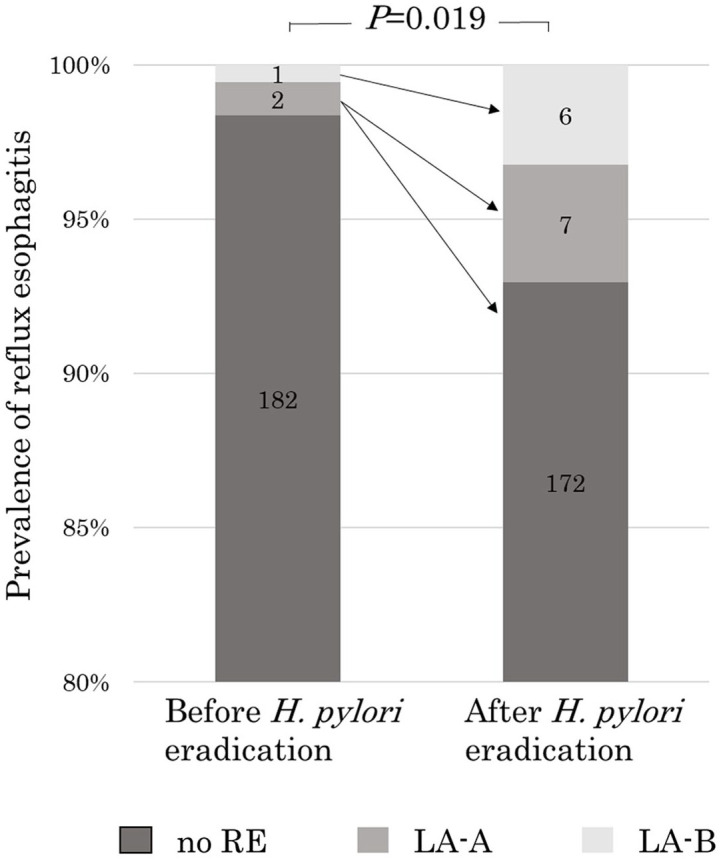
Prevalence of reflux esophagitis before and after eradication of Helicobacter pylori. The prevalence of reflux esophagitis was significantly higher after H. pylori eradication than before eradication (7.0% (13/185) vs 1.6% (3/185), P = 0.019).
LA, Los Angeles classification; RE, reflux esophagitis.
Table 2.
Characteristics of the patients with reflux esophagitis before and/or after H. pylori eradication.
| Degree of RE before eradication | Degree of atrophy a before eradication | Use of acid secretion inhibitors before eradication | Degree of RE after eradication | Degree of atrophy after eradication | Use of acid secretion inhibitor after eradication | Hiatal hernia | Observation period b (years) |
|---|---|---|---|---|---|---|---|
| LA-A | C-2 | – | – | C-2 | – | – | 6.7 |
| LA-A | O-3 | PPI | LA-A | C-1 | PPI | + | 8.3 |
| LA-B | C-1 | – | LA-B | C-1 | PPI | + | 3.8 |
| – | O-2 | – | LA-B | O-1 | PPI | – | 9.7 |
| – | O-1 | PPI | LA-B | C-2 | PPI | – | 3.1 |
| – | C-2 | – | LA-B | C-1 | PPI | + | 7.3 |
| – | O-2 | – | LA-A | C-1 | – | – | 10.5 |
| – | O-3 | – | LA-A | O-3 | – | – | 5.8 |
| – | O-3 | – | LA-A | O-3 | – | – | 3.6 |
| – | O-3 | – | LA-A | O-3 | – | – | 5.7 |
| – | O-2 | PPI | LA-B | O-2 | – | – | 4.9 |
| – | C-2 | – | LA-A | C-2 | PPI | – | 9.3 |
| – | C-2 | – | LA-A | C-2 | – | – | 7.9 |
| – | C-1 | – | LA-B | C-1 | – | – | 5.8 |
H. pylori, Helicobacter pylori; LA, Los Angeles classification; PPI, proton pump inhibitor; RE, reflux esophagitis.
Gastric mucosal atrophy was evaluated according to the Kimura–Takemoto classification and was classified by degree into two grades of open type (O) and closed type (C).
Observation period, the interval between the two endoscopic examinations before and after H. pylori eradication whose images were examined in this study.
RE was present in 8 (7.5%) of the 106 cases with closed-type atrophy after eradication and in 5 (6.3%) of the 79 cases with open-type atrophy after eradication (P = 1.00) (Figure 3). Among the 182 patients who did not have RE before H. pylori eradication, atrophy improved between before and after eradication in 56 patients and atrophy did not improve in 126 patients. Four (7.1%) of the 56 patients with atrophy improvement had new onset of RE after eradication, as did 7 (5.4%) of the 126 patients without atrophy improvement (Figure 4). There was no significant difference in the prevalence rate of newly developed RE between patients with atrophy improvement and patients without atrophy improvement (P = 0.74).
Figure 3.
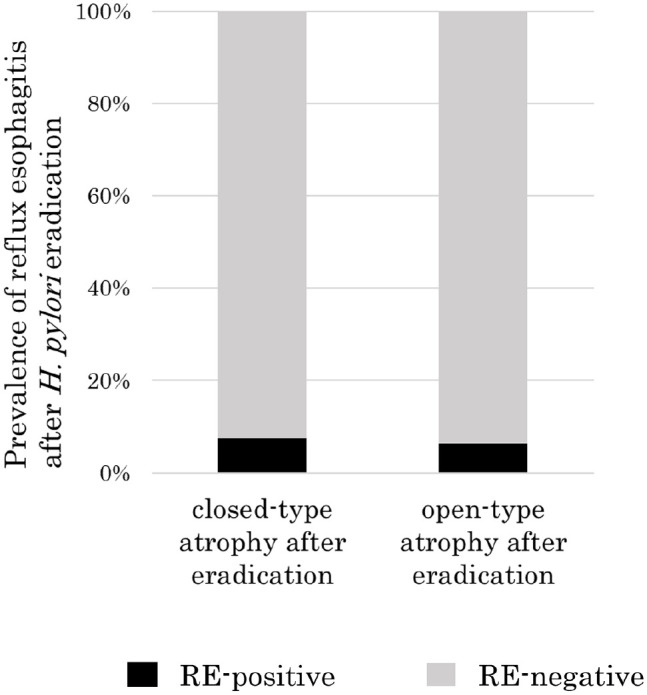
Prevalence of reflux esophagitis after eradication in patients with the closed-type or open-type atrophy after eradication of Helicobacter pylori. There was no significant difference in the prevalence of reflux esophagitis after H. pylori eradication between patients with closed-type atrophy and those with open-type atrophy after eradication (7.5% (8/106) vs 6.3% (5/79), P = 1.00).
RE, reflux esophagitis.
Figure 4.
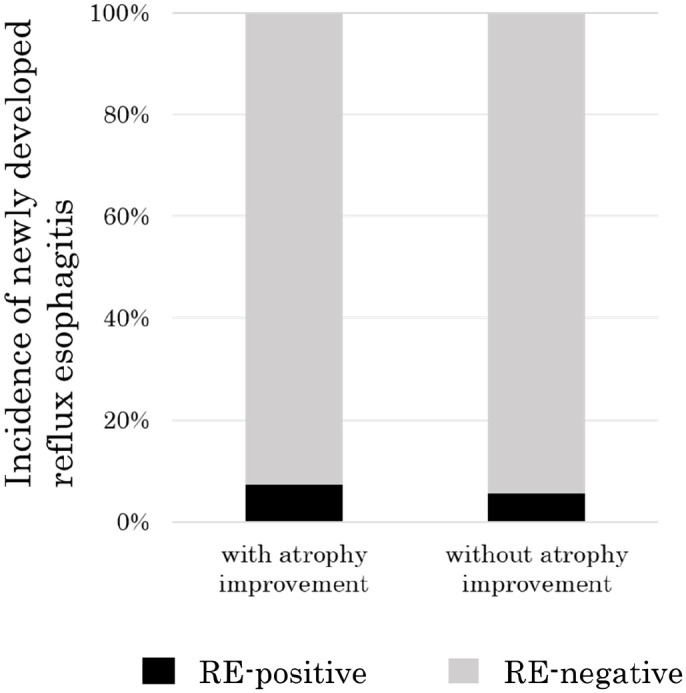
Incidence rate of newly developed reflux esophagitis after Helicobacter pylori eradication between patients with or without improvement of atrophy. The three patients with reflux esophagitis before H. pylori eradication were excluded from this analysis. There was no significant difference in the incidence of newly developed reflux esophagitis after eradication between patients with or without atrophy improvement (7.1% (4/56) vs 5.4% (7/126), P = 0.74).
RE, reflux esophagitis.
After eradication, RE was present in 2 (8.3%) of the 24 cases with gastric ulcer before eradication and in 11 (6.8%) of the 161 cases without gastric ulcer before eradication, showing no significant difference (P = 0.68). RE was present in 5 (10%) of the 50 cases with duodenal ulcer before eradication and in 8 (5.9%) of the 135 cases without duodenal ulcer before eradication, also showing no significant difference (P = 0.34).
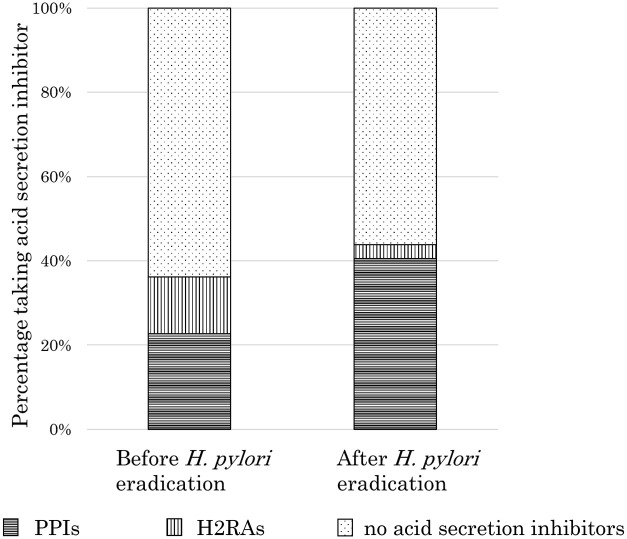
Sixty-seven patients (PPI 42 patients, H2RA 25 patients) were taking acid secretion inhibitors before eradication, and 81 patients (PPI 75 patients, H2RA 6 patients) were taking acid secretion inhibitors after eradication. The percentage of patients who were taking an acid secretion inhibitor before and after eradication was 36.2% (67/185) and 43.8% (81/185), respectively, showing no significant difference (P = 0.14) (Figure 5).
Figure 5.
Comparison of the percentage of patients who were taking acid secretion inhibitors before and after eradication of Helicobacter pylori. There was no significant difference in the percentage of patients who were taking acid secretion inhibitors before and after eradication of H. pylori (36.2% (67/185) vs 43.8% (81/185), P = 0.14).
H2RA, histamine H2-receptor antagonist; PPI, proton pump inhibitor.
Discussion
Our study showed that the prevalence of RE significantly increased after H. pylori eradication even if some of the subjects were taking acid secretion inhibitors. Moreover, our study showed that the prevalence of RE may not depend on the degree of atrophy.
Many studies have evaluated the prevalence of esophagitis after eradication during a relatively short follow-up period of 1–2 years after eradication, but there are few reports in which patients were evaluated over a long period of time after eradication.20,21 We evaluated patients before and after a long period of 3 years or more after H. pylori eradication. As a result, we found that the prevalence of RE of 1.6% before eradication increased to 7% 3 years or more after eradication. The prevalence of RE in the Japanese adult population is reported to be about 10%. 29 It may be more appropriate to consider that the prevalence of RE after eradication in our study is closer to the prevalence of RE in the general population than to think that its prevalence increased after eradication.
The H. pylori infection rate is inversely correlated with the prevalence of RE because inflammatory cytokines suppress gastric acid secretion and because of fundic gland atrophy.7–10 After eradication, polymorphonuclear neutrophil activity and chronic inflammation improve, and atrophy may also improve.6,30,31 It has been reported that elimination of inflammation and improvement of atrophy restore gastric acid secretion.15,16 Therefore, it is expected that the degree of atrophy and changes in atrophy after eradication affect the prevalence of RE. 32 However, in this study, no associations were found between the degree of atrophy or the degree of change in atrophy after eradication and the prevalence of RE. It was considered that factors other than gastric acid secretion were involved in the development of RE. Ammonia produced by H. pylori acts protectively against RE and eradication may eliminate the protective effect and cause esophagitis.19,22 Kyphosis and hernia may occur over the long term after eradication. 33 Patients’ body mass index sometimes increases after eradication.34,35 Therefore, these factors might be involved in the development of RE after eradication. The prevalence of esophagitis is affected by acid secretion inhibitors. In this study, there was no difference in the proportion of patients taking acid secretion inhibitors before and after eradication.
One limitation of this study is that the optimal sample size was not calculated. This study was a retrospective study, and the number of cases could not be increased. Therefore, since the prevalence of RE and the incidence of newly developed esophagitis were low, there may be β-errors among the items that were judged to be not significantly different. Also, in this study, endoscopic atrophy was not confirmed by histological examination. However, it has been reported that there was a strong correlation between endoscopic diagnosis of gastric atrophy according to the Kimura–Takemoto classification and histological diagnosis of gastric atrophy.27,36 Moreover, because of the retrospective nature of this study, the medication history may be inaccurate. Information on body mass index and symptoms was unknown and could not be considered. Since no patient had undergone measurement of gastric pH, it was not possible to know how much gastric acid was actually secreted.
In this study, it was shown that the prevalence of RE increased a long period of time after H. pylori eradication, even among subjects who were taking acid secretion inhibitors. The prevalence of RE after eradication and incidence of new onset of RE after eradication were not related to the degree of atrophy or changes in atrophy.
In the future, it is necessary to investigate factors related to the occurrence of RE other than atrophy and to examine changes in gastroesophageal reflux symptoms before and after eradication of H. pylori over a long period of time.
Footnotes
Author contributions: MH and AN designed the study. MH, KU YA, HU, YS, and DA acquired data. MH and TT judged endoscopic images. MH analyzed and interpreted the data, and drafted and revised the manuscript. All authors reviewed the manuscript and approved the final version of the manuscript.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Mariko Hojo  https://orcid.org/0000-0001-9671-5585
https://orcid.org/0000-0001-9671-5585
Contributor Information
Mariko Hojo, Department of Gastroenterology, Juntendo University School of Medicine, 2-1-1 Hongo Bunkyo-ku, Tokyo 113-8421, Japan.
Kumiko Ueda, Department of Gastroenterology, Juntendo University School of Medicine, Tokyo, Japan.
Tsutomu Takeda, Department of Gastroenterology, Juntendo University School of Medicine, Tokyo, Japan.
Yoichi Akazawa, Department of Gastroenterology, Juntendo University School of Medicine, Tokyo, Japan.
Hiroya Ueyama, Department of Gastroenterology, Juntendo University School of Medicine, Tokyo, Japan.
Yuji Shimada, Department of Gastroenterology, Juntendo University School of Medicine, Tokyo, Japan.
Daisuke Asaoka, Department of Gastroenterology, Juntendo University School of Medicine, Tokyo, Japan.
Akihito Nagahara, Department of Gastroenterology, Juntendo University School of Medicine, Tokyo, Japan.
References
- 1. Savarino E, de Bortoli N, De Cassan C, et al. The natural history of gastro-esophageal reflux disease: a comprehensive review. Dis Esophagus 2017; 30: 1–9. [DOI] [PubMed] [Google Scholar]
- 2. El-Serag HB, Sweet S, Winchester CC, et al. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2014; 63: 871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fujiwara Y. [Recent epidemiology of GERD in the Japanese population]. Nihon Shokakibyo Gakkai Zasshi 2017; 114: 1781–1789. [DOI] [PubMed] [Google Scholar]
- 4. Fujiwara Y, Arakawa T. Epidemiology and clinical characteristics of GERD in the Japanese population. J Gastroenterol 2009; 44: 518–534. [DOI] [PubMed] [Google Scholar]
- 5. Asaka M, Kato M, Kudo M, et al. Atrophic changes of gastric mucosa are caused by Helicobacter pylori infection rather than aging: studies in asymptomatic Japanese adults. Helicobacter 1996; 1: 52–56. [DOI] [PubMed] [Google Scholar]
- 6. Hojo M, Miwa H, Ohkusa T, et al. Alteration of histological gastritis after cure of Helicobacter pylori infection. Aliment Pharmacol Ther 2002; 16: 1923–1932. [DOI] [PubMed] [Google Scholar]
- 7. Calam J. Helicobacter pylori modulation of gastric acid. Yale J Biol Med 1999; 72: 195–202. [PMC free article] [PubMed] [Google Scholar]
- 8. Beales IL, Calam J. Interleukin 1 beta and tumour necrosis factor alpha inhibit acid secretion in cultured rabbit parietal cells by multiple pathways. Gut 1998; 42: 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. El-Omar EM, Oien K, El-Nujumi A, et al. Helicobacter pylori infection and chronic gastric acid hyposecretion. Gastroenterology 1997; 113: 15–24. [DOI] [PubMed] [Google Scholar]
- 10. Furuta T, Baba S, Takashima M, et al. Effect of Helicobacter pylori infection on gastric juice pH. Scand J Gastroenterol 1998; 33: 357–363. [DOI] [PubMed] [Google Scholar]
- 11. Kinoshita Y, Kawanami C, Kishi K, et al. Helicobacter pylori independent chronological change in gastric acid secretion in the Japanese. Gut 1997; 41: 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Delaney B, McColl K. Review article: Helicobacter pylori and gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2005; 22(Suppl. 1): 32–40. [DOI] [PubMed] [Google Scholar]
- 13. Wu JC. Does Helicobacter pylori infection protect against esophageal diseases in Asia? Indian J Gastroenterol 2011; 30: 149–153. [DOI] [PubMed] [Google Scholar]
- 14. Gunji T, Sato H, Iijima K, et al. Risk factors for erosive esophagitis: a cross-sectional study of a large number of Japanese males. J Gastroenterol 2011; 46: 448–455. [DOI] [PubMed] [Google Scholar]
- 15. Iijima K, Ohara S, Sekine H, et al. Changes in gastric acid secretion assayed by endoscopic gastrin test before and after Helicobacter pylori eradication. Gut 2000; 46: 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tucci A, Biasco G, Paparo GF. Effect of eradication of Helicobacter pylori in patients with fundic atrophic gastritis. N Engl J Med 1997; 336: 957–958. [DOI] [PubMed] [Google Scholar]
- 17. el-Omar EM, Penman ID, Ardill JE, et al. Helicobacter pylori infection and abnormalities of acid secretion in patients with duodenal ulcer disease. Gastroenterology 1995; 109: 681–691. [DOI] [PubMed] [Google Scholar]
- 18. Haruma K, Mihara M, Okamoto E, et al. Eradication of Helicobacter pylori increases gastric acidity in patients with atrophic gastritis of the corpus-evaluation of 24-h pH monitoring. Aliment Pharmacol Ther 1999; 13: 155–162. [DOI] [PubMed] [Google Scholar]
- 19. Labenz J, Blum AL, Bayerdörffer E, et al. Curing Helicobacter pylori infection in patients with duodenal ulcer may provoke reflux esophagitis. Gastroenterology 1997; 112: 1442–1447. [DOI] [PubMed] [Google Scholar]
- 20. Sugimoto M, Murata M, Mizuno H, et al. Endoscopic reflux esophagitis and reflux-related symptoms after Helicobacter pylori eradication therapy: meta-analysis. J Clin Med 2020; 9: 3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Take S, Mizuno M, Ishiki K, et al. Helicobacter pylori eradication may induce de novo, but transient and mild, reflux esophagitis: prospective endoscopic evaluation. J Gastroenterol Hepatol 2009; 24: 107–113. [DOI] [PubMed] [Google Scholar]
- 22. Hamada H, Haruma K, Mihara M, et al. High incidence of reflux oesophagitis after eradication therapy for Helicobacter pylori: impacts of hiatal hernia and corpus gastritis. Aliment Pharmacol Ther 2000; 14: 729–735. [DOI] [PubMed] [Google Scholar]
- 23. Satoh K, Yoshino J, Akamatsu T, et al. Evidence-based clinical practice guidelines for peptic ulcer disease 2015. J Gastroenterol 2016; 51: 177–194. [DOI] [PubMed] [Google Scholar]
- 24. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 25. Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999; 45: 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Toyoshima O, Nishizawa T, Koike K. Endoscopic Kyoto classification of Helicobacter pylori infection and gastric cancer risk diagnosis. World J Gastroenterol 2020; 26: 466–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kono S, Gotoda T, Yoshida S, et al. Can endoscopic atrophy predict histological atrophy? Historical study in United Kingdom and Japan. World J Gastroenterol 2015; 21: 13113–13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kimura KTT. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy 1969; 1: 87–97. [Google Scholar]
- 29. Iwakiri K, Kinoshita Y, Habu Y, et al. Evidence-based clinical practice guidelines for gastroesophageal reflux disease 2015. J Gastroenterol 2016; 51: 751–767. [DOI] [PubMed] [Google Scholar]
- 30. Kodama M, Murakami K, Okimoto T, et al. Ten-year prospective follow-up of histological changes at five points on the gastric mucosa as recommended by the updated Sydney system after Helicobacter pylori eradication. J Gastroenterol 2012; 47: 394–403. [DOI] [PubMed] [Google Scholar]
- 31. Ohkusa T, Fujiki K, Takashimizu I, et al. Improvement in atrophic gastritis and intestinal metaplasia in patients in whom Helicobacter pylori was eradicated. Ann Intern Med 2001; 134: 380–386. [DOI] [PubMed] [Google Scholar]
- 32. Nakajima S, Hattori T. Active and inactive gastroesophageal reflux diseases related to Helicobacter pylori therapy. Helicobacter 2003; 8: 279–293. [DOI] [PubMed] [Google Scholar]
- 33. Yoshimura M, Nagahara A, Ohtaka K, et al. Presence of vertebral fractures is highly associated with hiatal hernia and reflux esophagitis in Japanese elderly people. Intern Med 2008; 47: 1451–1455. [DOI] [PubMed] [Google Scholar]
- 34. Lender N, Talley NJ, Enck P, et al. Review article: associations between Helicobacter pyloriand obesity – an ecological study. Aliment Pharmacol Ther 2014; 40: 24–31. [DOI] [PubMed] [Google Scholar]
- 35. Azuma T, Suto H, Ito Y, et al. Eradication of Helicobacter pylori infection induces an increase in body mass index. Aliment Pharmacol Ther 2002; 16(Suppl. 2): 240–244. [DOI] [PubMed] [Google Scholar]
- 36. Takao T, Ishikawa T, Ando T, et al. Multifaceted assessment of chronic gastritis: a study of correlations between serological, endoscopic, and histological diagnostics. Gastroenterol Res Pract 2011; 2011: 631461. [DOI] [PMC free article] [PubMed] [Google Scholar]